Optimization Preparation of Indium Tin Oxide Nanoparticles via Microemulsion Method Using Orthogonal Experiment
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
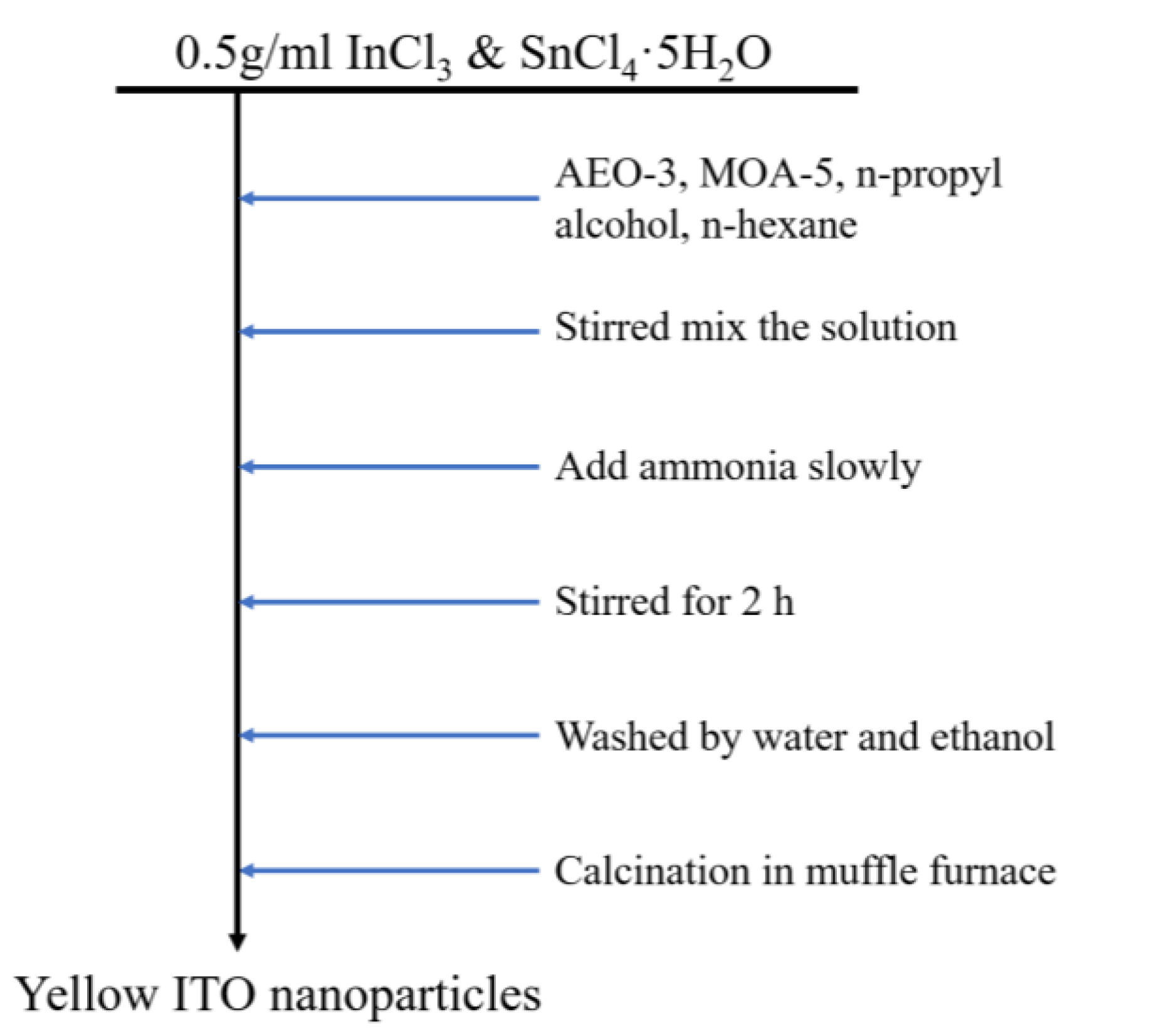
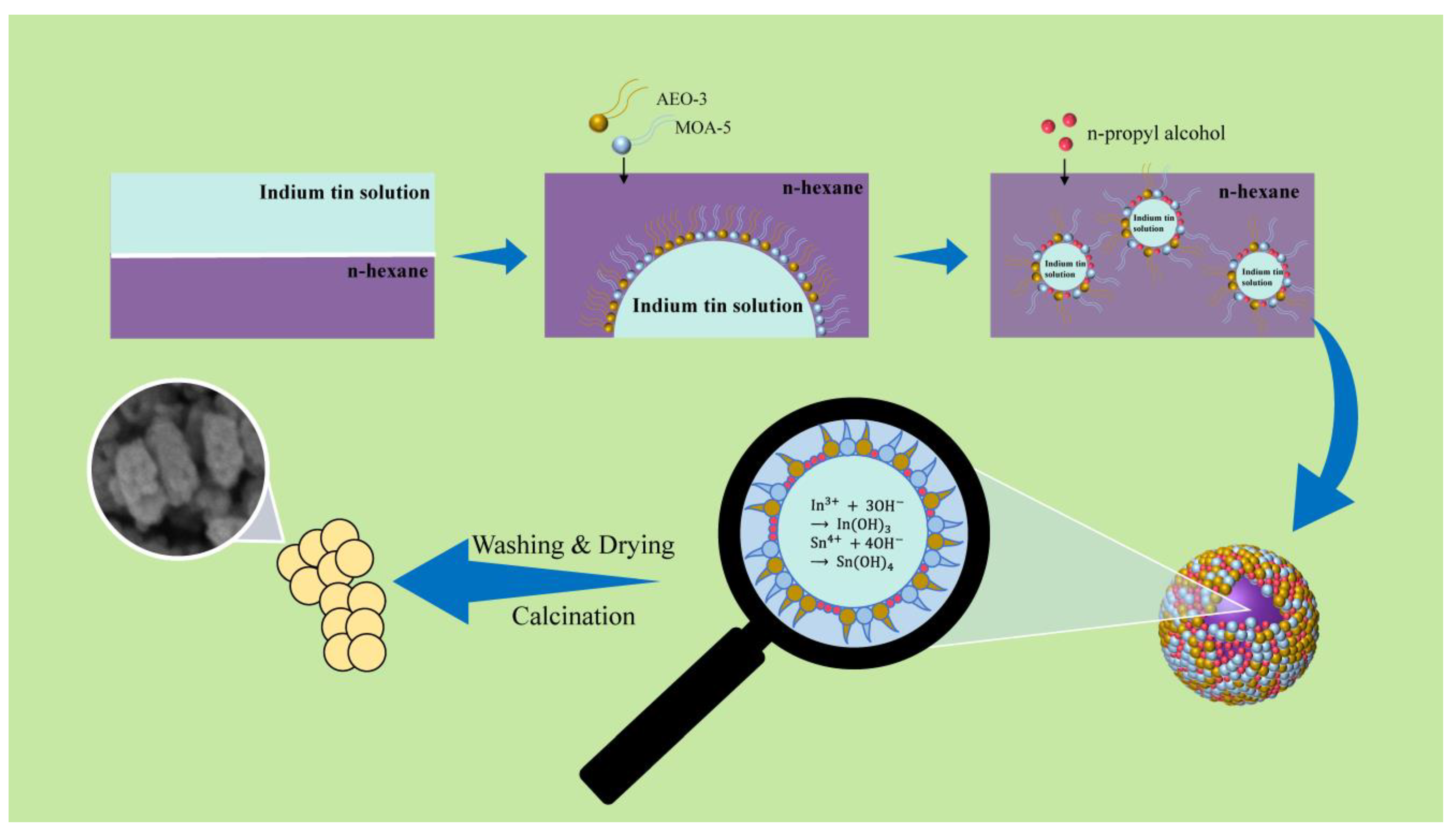
2.2. Preparation of ITO Nanoparticles
2.3. Characterization
3. Results and Discussion
3.1. Orthogonal Experiment Optimization of ITO Nanoparticles Preparation Process
3.1.1. Orthogonal Experiment Design and Data
3.1.2. Range Analysis of the Orthogonal Experiment Results
3.1.3. Variance Analysis of Orthogonal Experiment Results
3.1.4. The Influence of Various Factors on Resistivity
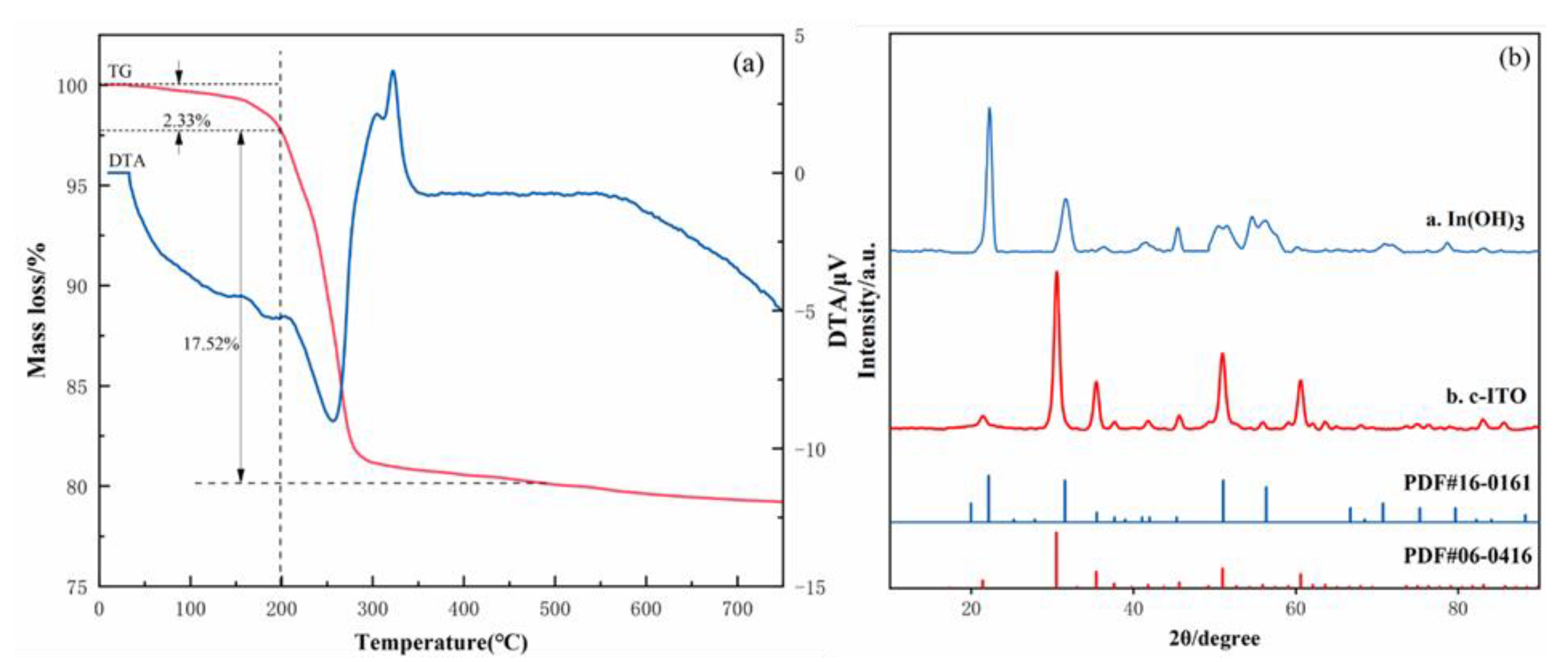
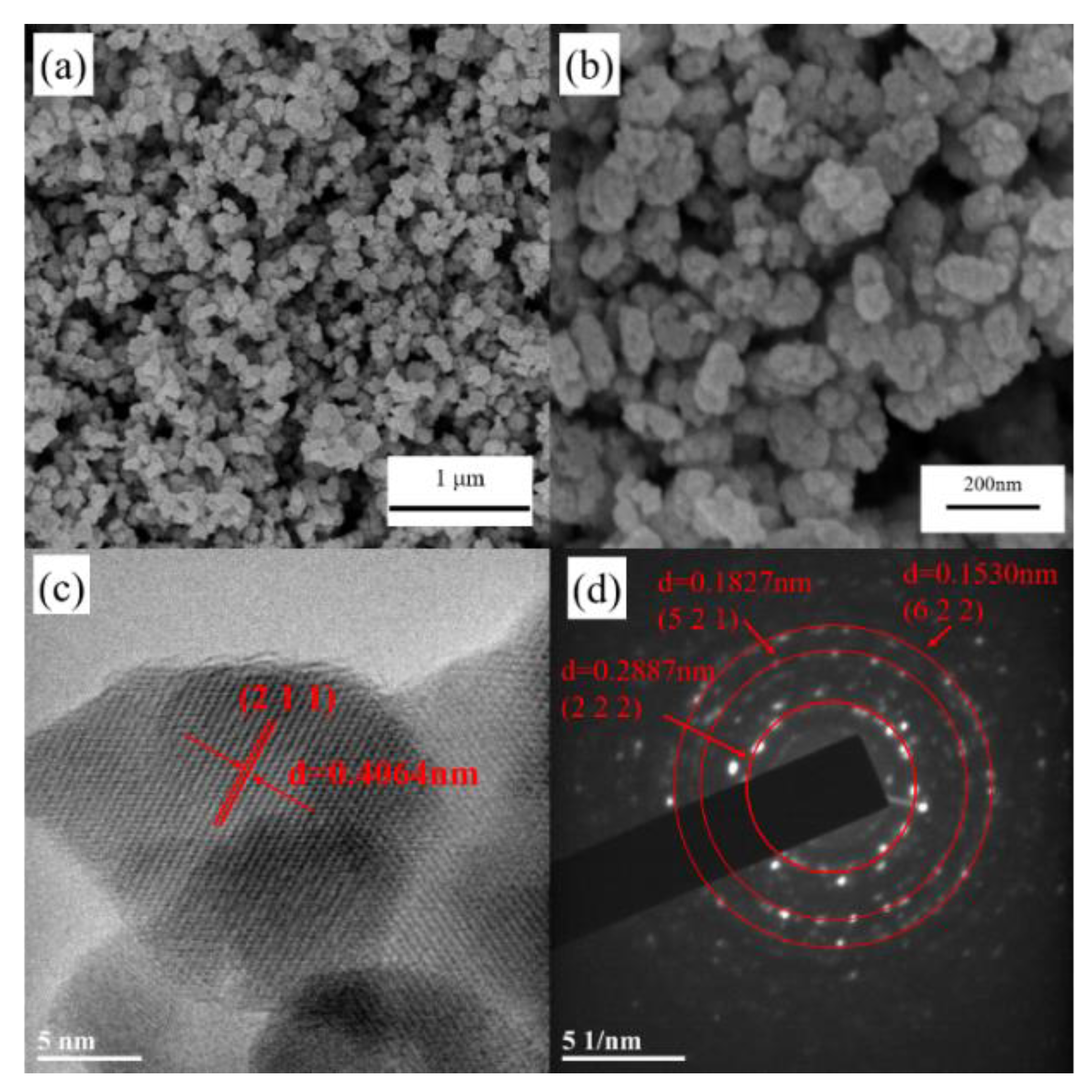
3.2. Characterization of ITO Nanoparticles
4. Preparation Mechanism of Crystalline ITO Nanoparticles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aziz, A.; Zahir, H.; Shaikh, M.N.; Al-Betar, A.-R.; Oyama, M.; Sulaiman, K.O. Hydrothermal synthesis of tin-doped indium oxide nanoparticles using pamoic acid as an organic additive and their photoluminescence properties. J. Mater. Sci. Mater. Electron. 2017, 28, 3226–3233. [Google Scholar] [CrossRef]
- Yang, S.; Sun, B.; Liu, Y.; Zhu, J.; Song, J.; Hao, Z.; Zeng, X.; Zhao, X.; Shu, Y.; Chen, J.; et al. Effect of ITO target crystallinity on the properties of sputtering deposited ITO films. Ceram. Int. 2020, 46, 6342–6350. [Google Scholar] [CrossRef]
- Mei, F.; Yuan, T.; Li, R.; Qin, K.; Zhou, L.; Wang, W. Micro-structure of ITO ceramics sintered at different temperatures and its effect on the properties of deposited ITO films. J. Eur. Ceram. Soc. 2018, 38, 521–533. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, T.; Mei, F.; Li, R. Effects of cerium oxide doping on the microstructure and properties of ITO targets and the photoelectric properties of the films. J. Mater. Sci. Mater. Electron. 2019, 30, 15469–15481. [Google Scholar] [CrossRef]
- Solieman, A.; Moharram, A.H.; Aegerter, M.A. Patterning of nanoparticulate transparent conductive ITO films using UV light irradiation and UV laser beam writing. Appl. Surf. Sci. 2010, 256, 1925–1929. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Liu, H.; Seo, D.-S. Super fast switching and low operating of liquid crystals sandwiched between ion beam-spurted ITO thin layers. Liq. Cryst. 2018, 46, 1052–1059. [Google Scholar] [CrossRef]
- Krajangsang, T.; Thongpool, V.; Piromjit, C.; Sriprapha, K. Development of indium tin oxide stack layer using oxygen and argon gas mixture for crystalline silicon heterojunction solar cells. Opt. Mater. 2020, 101, 109743. [Google Scholar] [CrossRef]
- Abdulkadir, A.; Aziz, A.A.; Pakhuruddin, M.Z. Properties of indium tin oxide on black silicon after post-deposition annealing for heterojunction solar cells. Results Phys. 2020, 19, 103405. [Google Scholar] [CrossRef]
- Janković, B. Isothermal thermo-analytical study and decomposition kinetics of non-activated and mechanically activated indium tin oxide (ITO) scrap powders treated by alkaline solution. Trans. Nonferrous Met. Soc. China 2015, 25, 1657–1676. [Google Scholar] [CrossRef]
- Xu, S.; Shi, Y. Low temperature high sensor response nano gas sensor using ITO nanofibers. Sens. Actuators B Chem. 2009, 143, 71–75. [Google Scholar] [CrossRef]
- Honga, J.-W.; Guo, Y.-W.; Shin, J.-Y.; Kim, T.W. Performance Enhancement of Organic Light-emitting Diodes with an Electron-transport Layer of Bathocuproine. Trans. Electr. Electron. Mater. 2016, 17, 37–40. [Google Scholar] [CrossRef][Green Version]
- Besbes, S.; Ben Ouada, H.; Davenas, J.; Ponsonnet, L.; Jaffrezic, N.; Alcouffe, P. Effect of surface treatment and functionalization on the ITO properties for OLEDs. Mater. Sci. Eng. C 2006, 26, 505–510. [Google Scholar] [CrossRef]
- Ma, W.H.; Cai, C.L. Studying on Thickness Control of ITO Films Deposited Using RF Magnetron Sputtering. Adv. Mater. Res. 2011, 415–417, 1921–1924. [Google Scholar] [CrossRef]
- Mei, F.; Yuan, T.; Li, R.; Huang, J. A Comparative Study on the Microstructure and Properties of ITO Targets and Thin Films Prepared from Two Different Powders. Acta Met. Sin. (Engl. Lett.) 2021, 34, 675–693. [Google Scholar] [CrossRef]
- Wu, M.; Wang, C.; Zhao, Y.; Xiao, L.; Zhang, C.; Yu, X.; Luo, B.; Hu, B.; Fan, W.; Shi, W. Hydrothermal synthesis of porous rh-In2O3nanostructures with visible-light-driven photocatalytic degradation of tetracycline. Cryst. Eng. Comm. 2015, 17, 2336–2345. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Senthil, K.; Vickraman, P. Microstructural, electrical and optical properties of indium tin oxide (ITO) nanoparticles synthesized by co-precipitation method. Mater. Res. Bull. 2012, 47, 1051–1056. [Google Scholar] [CrossRef]
- Yang, G.J.; Park, S.J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.R.; Fan, T.; Wang, C.A.; Ma, L.G.; Liu, F. Template Induced Synthesis of Nano-Hydroxyapatite by Co-Precipitation Method. Adv. Mater. Res. 2011, 311–313, 1713–1716. [Google Scholar] [CrossRef]
- Wang, G.Y. Research Progress in Solvothermal Synthesis of Nano Functional Materials. Adv. Mater. Res. 2012, 531, 223–226. [Google Scholar] [CrossRef]
- Lou, S.; Zhou, Z.; Gan, W.; Xuan, T.; Bao, Z.; Si, S.; Cao, L.; Li, H.; Xia, Z.; Qiu, J.; et al. In situ synthesis of high-efficiency CsPbBr3/CsPb2Br5 composite nanocrystals in aqueous solution of microemulsion. Gr. Chem. 2020, 22, 5257–5261. [Google Scholar] [CrossRef]
- Chen, J.; Shi, T.; Duan, L.; Sun, Z.; Anthony, E.J. Microemulsion-derived, nanostructured CaO/CuO composites with controllable particle grain size to enhance cyclic CO2 capture performance for combined Ca/Cu looping process. Chem. Eng. J. 2020, 393, 124716. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Wang, S.; He, Z.; Zhou, Y.; Ren, H. Structure, surface tension, and rheological behaviors of hydrophobically associative polyacrylamides by self-emulsified microemulsion polymerization. J. Appl. Polym. Sci. 2020, 137, 49234. [Google Scholar] [CrossRef]
- Lin, G.; Cortez-Jugo, C.; Ju, Y.; Besford, Q.A.; Ryan, T.M.; Pan, S.; Richardson, J.J.; Caruso, F. Microemulsion-Assisted Templating of Metal-Stabilized Poly(ethylene glycol) Nanoparticles. Biomacromolecules 2021, 22, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Song, W.; Jiang, D. Preparation of nanometer-sized In2O3 particles by a reverse microemulsion method. J. Colliods Interface Sci. 2004, 271, 366–371. [Google Scholar] [CrossRef]
- Yang, J.; Lin, C.; Wang, Z.; Lin, J. In(OH)3 and In2O3 Nanorod Bundles and Spheres: Microemulsion-Mediated Hydrothermal Synthesis and Luminescence Properties. Inorg. Chem. 2006, 45, 8973–8979. [Google Scholar] [CrossRef]
- Devi, P.S.; Chatterjee, M.; Ganguli, D. Indium tin oxide nano-particles through an emulsion technique. Mater. Lett. 2002, 55, 205–210. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.; Guo, T.; Shu, D.; Shan, W.; Li, G.; Guo, D. Preparation and properties of Eu and Dy co-doped strontium aluminate long afterglow nanomaterials. Ceram. Int. 2018, 44, 7535–7544. [Google Scholar] [CrossRef]
- Tan, Y.; Luo, X.; Mao, M.; Shu, D.; Shan, W.; Li, G.; Guo, D. Optimization red emission of SrMoO4: Eu3+ via hydro-thermal co-precipitation synthesis using orthogonal experiment. Curr. Appl. Phys. 2018, 18, 1403–1409. [Google Scholar] [CrossRef]
- Shan, W.; Wu, L.; Tao, N.; Chen, Y.; Guo, D. Optimization method for green SrAl2O4:Eu2+, Dy3+ phosphors synthesized via co-precipitation route assisted by microwave irradiation using orthogonal experimental design. Ceram. Int. 2015, 41, 15034–15040. [Google Scholar] [CrossRef]
- Fu, J.; Hu, X.; Luan, F.; Li, G.; Guo, D. Optimum synthesis and properties of NaBiF4:Yb/Er upconversion nanoparticles. Ceram. Int. 2019, 45, 24365–24374. [Google Scholar] [CrossRef]
- Igasaki, Y.; Kanma, H. Argon gas pressure dependence of the properties of transparent conducting ZnO:Al films deposited on glass substrates. Appl. Surf. Sci. 2001, 169–170, 508–511. [Google Scholar] [CrossRef]
- Gupta, R.; Ghosh, K.; Patel, R.; Kahol, P. Effect of substrate temperature on opto-electrical properties of Nb-doped In2O3 thin films. J. Cryst. Growth 2008, 310, 4336–4339. [Google Scholar] [CrossRef]
- Zhang, D.H.; Ma, H.L. Scattering mechanisms of charge carriers in transparent conducting oxide films. Appl. Phys. A 1996, 62, 487–492. [Google Scholar] [CrossRef]
- Ganguli, A.K.; Ganguly, A.; Vaidya, S. Microemulsion-based synthesis of nanocrystalline materials. Chem. Soc. Rev. 2010, 39, 474–485. [Google Scholar] [CrossRef]
- Yousefi, S.; Ghasemi, B. Precipitator concentration-dependent opto-structural properties of MgO nanoparticles fabricated using natural brine. SN Appl. Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Ma, Y.; Zhai, X.; Liu, J. Synthesis of hexagonal-phase indium tin oxide nanoparticles by deionized water and glycerol binary solvothermal method and their resistivity. J. Mater. Sci. 2020, 55, 3860–3870. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, P.; Kumar, A.; Meena, R.; Tomar, R.; Chand, F.; Asokan, K. Structural, morphological, electrical and dielectric properties of Mn doped CeO2. J. Alloys Compd. 2016, 672, 543–548. [Google Scholar] [CrossRef]
- Baghi, R.; Zhang, K.; Wang, S.; Hope-Weeks, L.J. Conductivity tuning of the ITO sol-gel materials by adjusting the tin oxide concentration, morphology and the crystalline size. Microporous Mesoporous Mater. 2017, 244, 258–263. [Google Scholar] [CrossRef]
- Kim, H.; Horwitz, J.S.; Kim, W.H.; Kafafi, Z.H.; Chrisey, U.B. Highly oriented indium tin oxide thin films for organic light-emitting diodes. Int. Symp. Opt. Sci. Technol. 2002, 4464, 76–84. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, G.; Ren, Y.; Chen, D. Microstructure analysis of sol-gel-derived nanocrystalline ITO thin films. Surf. Interface Anal. 2011, 43, 1199–1202. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, Y.; Chen, Y.; Ma, Y.; Liu, J. Controllable phase transition ITO nano powders and temperature-structure sensitivity. Chem. Phys. Lett. 2020, 742, 137174. [Google Scholar] [CrossRef]
- Liang, F.; Liu, J.-X. Photoluminescence properties of hexagonal indium tin oxide nanopowders prepared by solvothermal method. Rare Met. 2017, 37, 47–53. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Ding, H.; Pan, S.; Zhang, Y.; Li, G. Influence of post-annealing on the structure and optical properties of ITO nanocrystals prepared by electrochemical method. Mater. Res. Bull. 2012, 47, 4457–4462. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Vickraman, P.; Jayachandran, M.; Sanjeeviraja, C. Structural and optical properties of indium tin oxide (ITO) thin films with different compositions prepared by electron beam evaporation. Vacuum 2010, 84, 864–869. [Google Scholar] [CrossRef]
- Jeevanandam, P.; Mulukutla, R.S.; Phillips, M.; Chaudhuri, S.; Erickson, L.E.; Klabunde, K.J. Near Infrared Reflectance Properties of Metal Oxide Nanoparticles. J. Phys. Chem. C 2007, 111, 1912–1918. [Google Scholar] [CrossRef]
- Wang, T.; Radovanovic, P.V. Free Electron Concentration in Colloidal Indium Tin Oxide Nanocrystals Determined by Their Size and Structure. J. Phys. Chem. C 2010, 115, 406–413. [Google Scholar] [CrossRef]
- Ray, S.; Banerjee, R.; Basu, N.; Batabyal, A.K.; Barua, A.K. Properties of tin doped indium oxide thin films prepared by magnetron sputtering. J. Appl. Phys. 1983, 54, 3497–3501. [Google Scholar] [CrossRef]
- Shong, B.; Shin, N.; Lee, Y.-H.; Ahn, K.H.; Lee, Y.-W. Synthesis of indium tin oxide (ITO) nanoparticles in supercritical methanol. J. Supercrit. Fluids 2016, 113, 39–43. [Google Scholar] [CrossRef]
- Wei, X. Effect of fabrication conditions on the properties of indium tin oxide powders. Chin. Phys. B 2008, 17, 6. [Google Scholar] [CrossRef]
- Patashinski, A.Z.; Orlik, R.; Paclawski, K.; Ratner, M.A.; Grzybowski, B.A. The unstable and expanding interface between reacting liquids: Theoretical interpretation of negative surface tension. Soft Matter 2012, 8, 1601–1608. [Google Scholar] [CrossRef]

| Factors | Ms/Mc (A) | NIn/Na (B) | tc (°C) (C) | th (h) (D) | |
|---|---|---|---|---|---|
| Levels | |||||
| Ⅰ | 5:1 | 1:10 | 500 | 2 | |
| Ⅱ | 5:2 | 1:15 | 600 | 3 | |
| Ⅲ | 5:3 | 1:20 | 700 | 4 | |
| Run | Factor | Resistivity Rik (Ω·cm) | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | 1 | 2 | |
| 1 | 1 | 1 | 1 | 1 | 16.100 | 17.175 |
| 2 | 1 | 2 | 2 | 2 | 7.616 | 6.828 |
| 3 | 1 | 3 | 3 | 3 | 3.710 | 3.310 |
| 4 | 2 | 1 | 2 | 3 | 3.190 | 3.506 |
| 5 | 2 | 2 | 3 | 1 | 2.884 | 3.262 |
| 6 | 2 | 3 | 1 | 2 | 9.766 | 7.760 |
| 7 | 3 | 1 | 3 | 2 | 1.732 | 1.412 |
| 8 | 3 | 2 | 1 | 3 | 7.996 | 8.864 |
| 9 | 3 | 3 | 2 | 1 | 1.868 | 1.470 |
| Run | Factor | Resistivity Rik (Ω·cm) | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | 1 | 2 | |
| 1 | 1 | 1 | 1 | 1 | 16.100 | 17.175 |
| 2 | 1 | 2 | 2 | 2 | 7.616 | 6.828 |
| 3 | 1 | 3 | 3 | 3 | 3.710 | 3.310 |
| 4 | 2 | 1 | 2 | 3 | 3.190 | 3.506 |
| 5 | 2 | 2 | 3 | 1 | 2.884 | 3.262 |
| 6 | 2 | 3 | 1 | 2 | 9.766 | 7.760 |
| 7 | 3 | 1 | 3 | 2 | 1.732 | 1.412 |
| 8 | 3 | 2 | 1 | 3 | 7.996 | 8.864 |
| 9 | 3 | 3 | 2 | 1 | 1.868 | 1.470 |
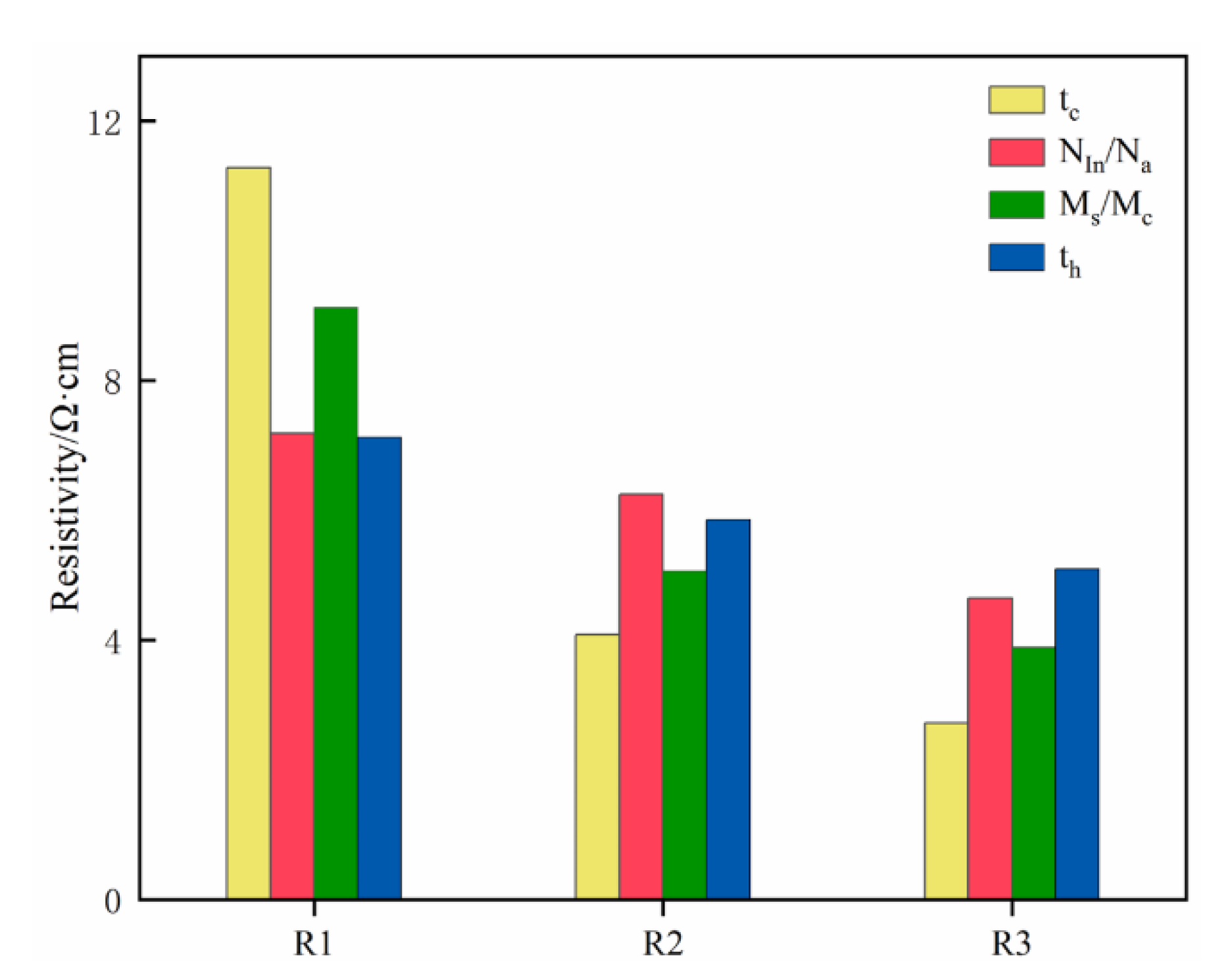
| R1 | 54.739 | 43.115 | 67.661 | 42.759 | ||
| R2 | 30.368 | 37.450 | 24.478 | 35.114 | ||
| R3 | 23.342 | 27.884 | 16.310 | 30.576 | ||
| R | 31.397 | 15.231 | 51.351 | 12.183 | ||
| Run | Factor | Resistivity Rik (Ω·cm) | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | 1 | 2 | |
| 1 | 1 | 1 | 1 | 1 | 16.100 | 17.175 |
| 2 | 1 | 2 | 2 | 2 | 7.616 | 6.828 |
| 3 | 1 | 3 | 3 | 3 | 3.710 | 3.310 |
| 4 | 2 | 1 | 2 | 3 | 3.190 | 3.506 |
| 5 | 2 | 2 | 3 | 1 | 2.884 | 3.262 |
| 6 | 2 | 3 | 1 | 2 | 9.766 | 7.760 |
| 7 | 3 | 1 | 3 | 2 | 1.732 | 1.412 |
| 8 | 3 | 2 | 1 | 3 | 7.996 | 8.864 |
| 9 | 3 | 3 | 2 | 1 | 1.868 | 1.470 |
| R1 | 54.739 | 43.115 | 67.661 | 42.759 | ||
| R2 | 30.368 | 37.450 | 24.478 | 35.114 | ||
| R3 | 23.342 | 27.884 | 16.310 | 30.576 | ||
| R | 31.397 | 15.231 | 51.351 | 12.183 | ||
| Qj | 90.5045762 | 19.7546652 | 250.213644 | 12.6370112 | Qe = 3.6887865 | |
| fj | 2 | 2 | 2 | 2 | fe = 9 | |
| Fj | 110.407742 | 24.0989802 | 305.238969 | 15.4160588 | F0.05(2,9) = 4.26 | |
| F0.01(2,9) = 8.02 | ||||||
| Peak Position (°) | Β(FWHM) | D (nm) | D Average (nm) |
|---|---|---|---|
| 30.580(222) | 0.762 | 10.80 | |
| 35.466(400) | 0.696 | 11.99 | |
| 45.691(431) | 0.572 | 15.07 | 11.99 |
| 51.037(440) | 0.864 | 10.18 | |
| 60.676(622) | 0.771 | 11.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Liu, T.; Zhai, X.; Liu, J. Optimization Preparation of Indium Tin Oxide Nanoparticles via Microemulsion Method Using Orthogonal Experiment. Crystals 2021, 11, 1387. https://doi.org/10.3390/cryst11111387
Jiang Z, Liu T, Zhai X, Liu J. Optimization Preparation of Indium Tin Oxide Nanoparticles via Microemulsion Method Using Orthogonal Experiment. Crystals. 2021; 11(11):1387. https://doi.org/10.3390/cryst11111387
Chicago/Turabian StyleJiang, Zhucheng, Ting Liu, Xiaoyu Zhai, and Jiaxiang Liu. 2021. "Optimization Preparation of Indium Tin Oxide Nanoparticles via Microemulsion Method Using Orthogonal Experiment" Crystals 11, no. 11: 1387. https://doi.org/10.3390/cryst11111387
APA StyleJiang, Z., Liu, T., Zhai, X., & Liu, J. (2021). Optimization Preparation of Indium Tin Oxide Nanoparticles via Microemulsion Method Using Orthogonal Experiment. Crystals, 11(11), 1387. https://doi.org/10.3390/cryst11111387





