Thermal Energy Storage Materials (TESMs)—What Does It Take to Make Them Fly?
Abstract
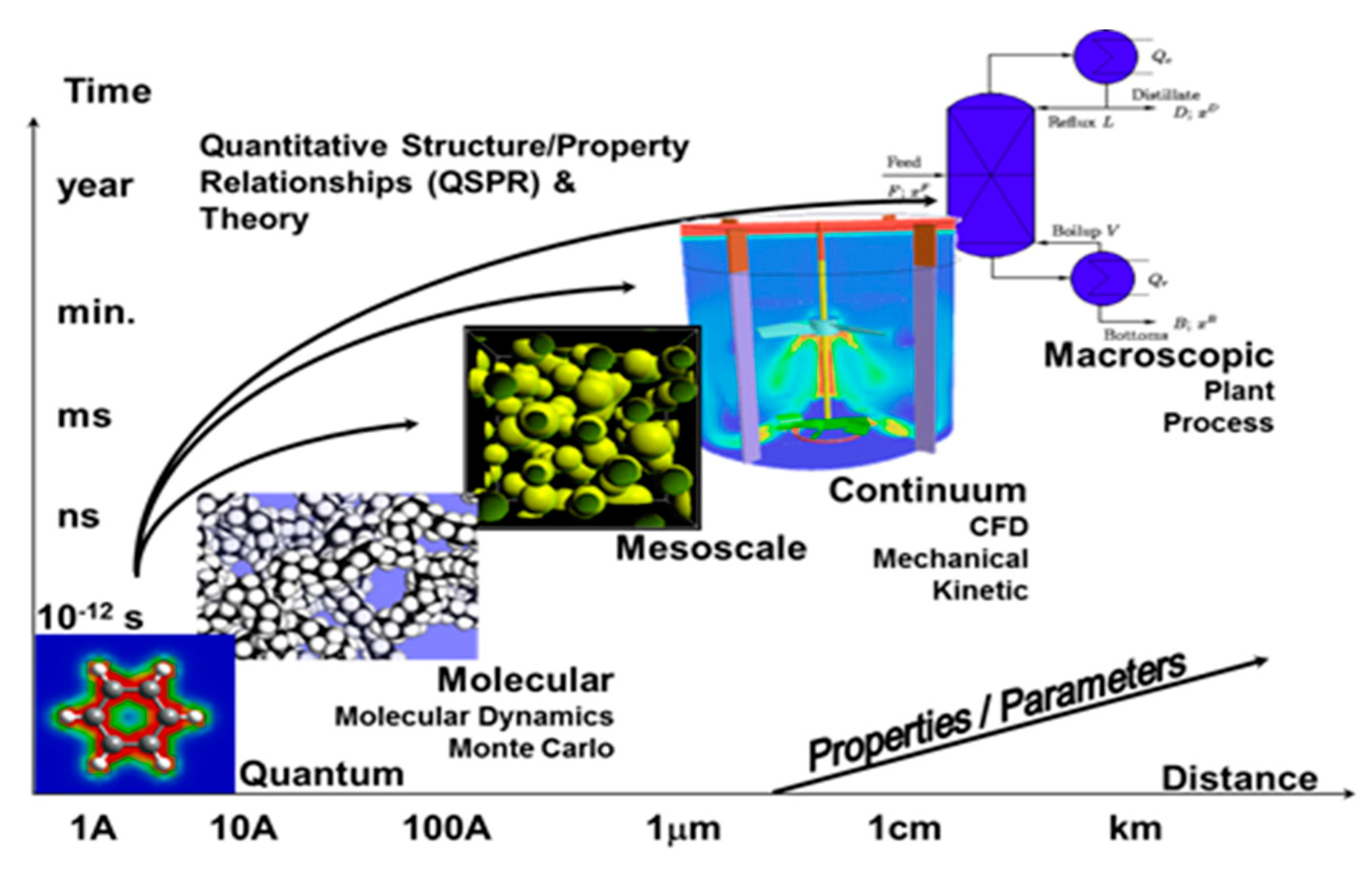
:1. Introduction and Gaps in a Nutshell
1.1. Objectives and Scope
- Discuss the evolution of TESMs using historical milestones and background;
- Illustrate the current context of TESMs in terms of awareness, state-of-the-art, and trends via a bibliometric analysis combined with our own experience and literature;
- Identify and discuss the material and non-material challenges, barriers and missing links from fundamentals to applications, which are the likely reasons why TESMs are not flying on the market;
- Explain the gaps: why TES development is highly customized, what consequences this has for TES material development, and that the market success of compact TES is therefore still low;
- Identify and discuss the essential elements from a materials perspective to bring TES technologies to the market, i.e., to close the gaps;
- Propose the key actions that are crucial to make TES materials “fly” on the market.
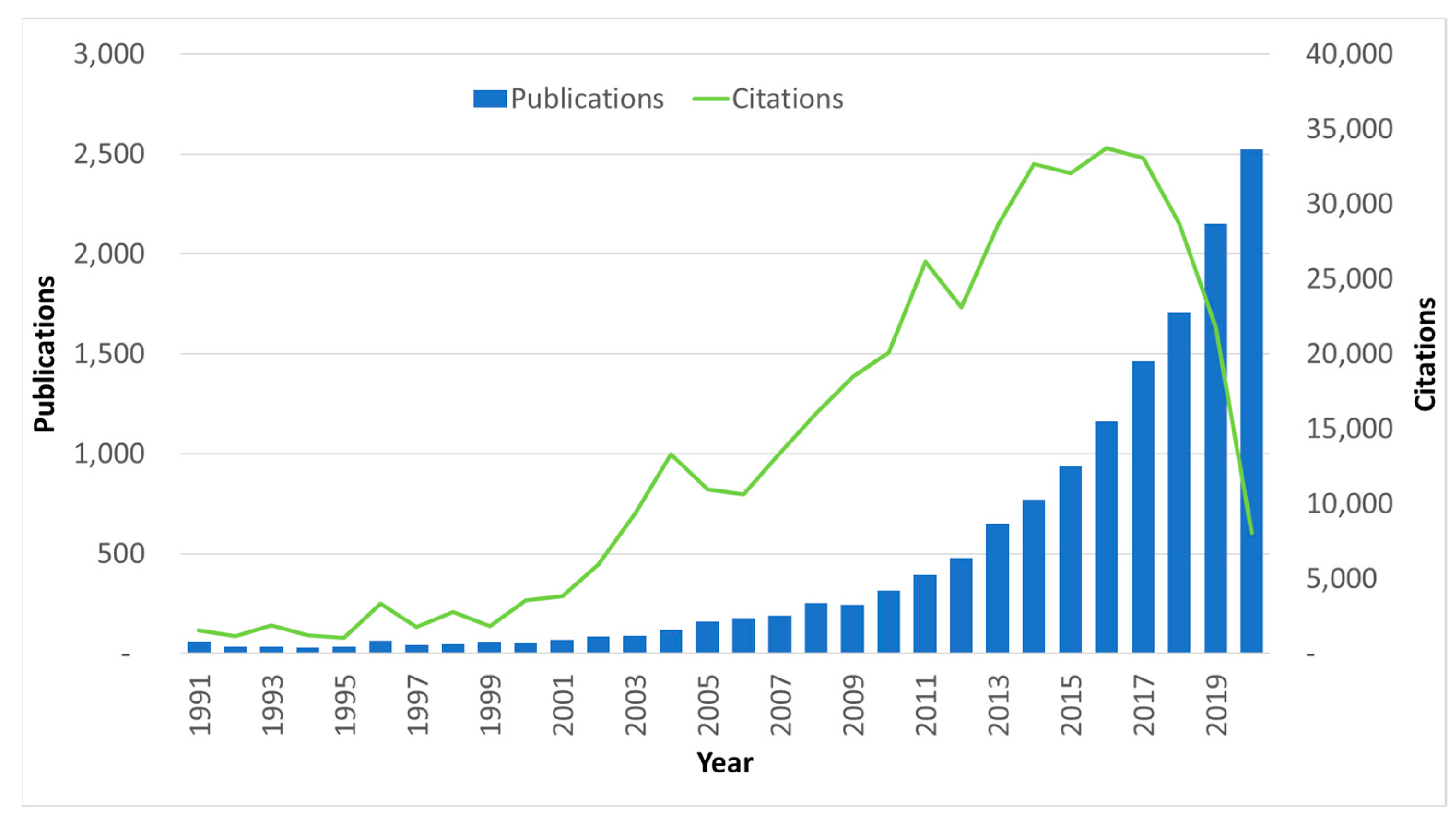
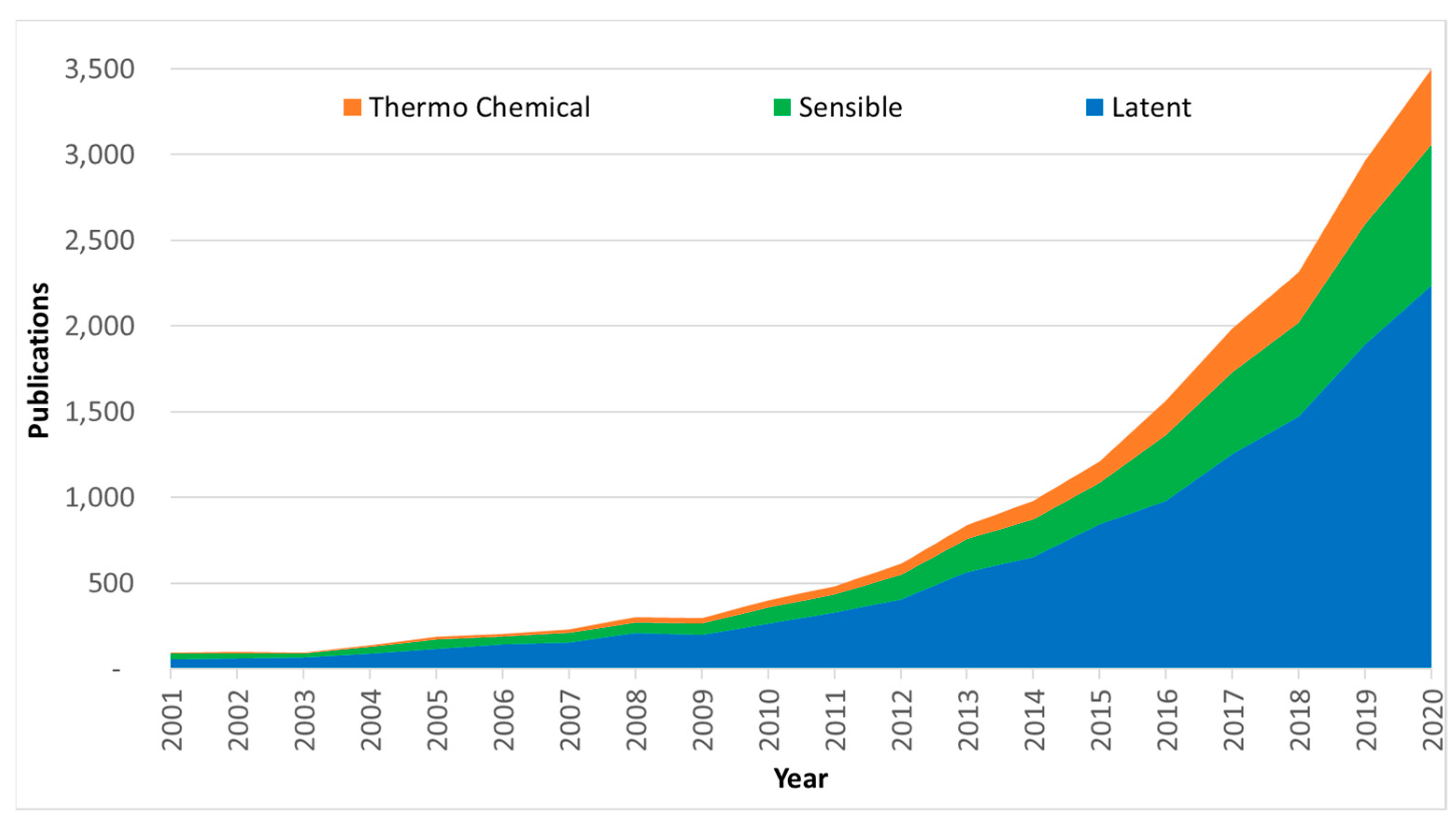
1.2. The ‘How’ and the Bibliometric Analysis
1.3. Outline
2. History and Background
2.1. STESMs—Evolution and Categorization
2.2. PCMs—Evolution, Categorization and Key Fundamentals
2.3. TCMs—Evolution and Categorization
3. TESMs Today? Trends, Gaps, Barriers and Missing Links
3.1. A Holistic View
3.2. Specific Trends and Gaps
3.2.1. STESMs
3.2.2. PCMs
3.2.3. TCMs
3.2.4. Common TESM Trends
3.2.5. Barriers and Missing Links from the Laboratory to Application
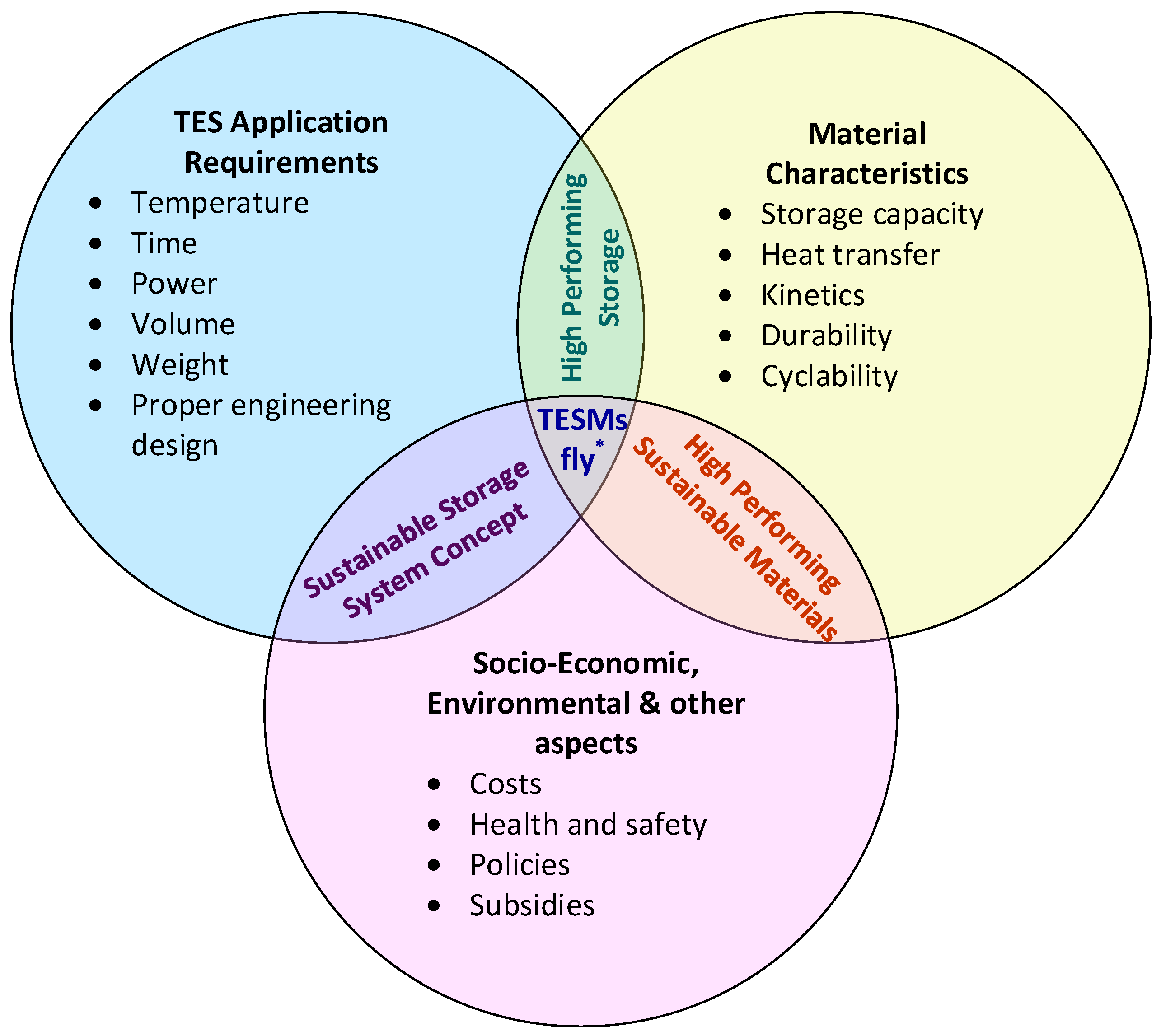
4. Concluding Remarks—What Do We Really Need to Do to Make TESM Fly?
- Determine the theoretical limits of the PCMs’ (and TCMs’) thermo-physical properties;
- Achieve molecular-level accurate prediction of crystallization and melting behavior;
- Demonstrate through pilot projects, tailor-made energy storage materials that conform to the user requirements, show socio-economic soundness and contribute to technical advancement.
- Synthesis of new TCM adsorbents with appropriate chemical composition and pore sizes in accordance with cost-efficient and green principles (used reagents, solvents, etc.) and without hysteresis during sorption process;
- Detailed microscopic, spectroscopic, and diffraction-based structure characterization. The exact knowledge about the structure is an enabling tool for a targeted synthesis of new materials and processes, i.e., structure-property relationship.
- 3.
- Evaluation of sorption mechanism, thermophysical properties and numerical modelling (i.e., interactions of materials with working fluids, the reaction dynamics) for further optimization of synthesis;
- 4.
- Improvement of the thermophysical properties to increase sorption performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- REN21. Renewables 2020 Global Status Report; REN21 Secretariat; United Nations Environment Programme: Paris, France, 2020; Available online: http://www.ren21.net/resources/publications/2 (accessed on 2 April 2021).
- Bloess, A.; Schill, W.-P.; Zerrahn, A. Power-to-heat for renewable energy integration: A review of technologies, modeling approaches, and flexibility potentials. Appl. Energy 2018, 212, 1611–1626. [Google Scholar] [CrossRef]
- Böttger, D.; Götz, M.; Lehr, N.; Kondziella, H.; Bruckner, T. Potential of the Power-to-Heat Technology in District Heating Grids in Germany. 8th International Renewable Energy Storage Conference and Exhibition, IRES 2014. Energy Procedia 2014, 46, 246–253. [Google Scholar] [CrossRef] [Green Version]
- WINDNODE. Balancing the power grid by generating heat and cold. In Balancing the Power Grid by Generating Heat and Cold; 2021. Available online: https://www.windnode.de/en/windnode-spotlight/heat-cold-power-grid/ (accessed on 12 April 2021).
- IEA TCP ES Annex 35. IEA ES–Flexible Sector Coupling Annex 35. 2021. Available online: https://iea-eces.org/annex-35 (accessed on 22 March 2021).
- Gur, I.; Sawyer, K.; Prasher, R. Searching for a Better Thermal Battery. Science 2012, 335, 1454–1455. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L.F. Heat and Cold Storage with PCM: An up to Date Introduction into Basics and Applications; Heat and Mass Transfer; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Raoux, S. Phase Change Materials. Annu. Rev. Mater. Res. 2009, 39, 25–48. [Google Scholar] [CrossRef]
- Almendros-Ibáñez, J.A.; Fernández-Torrijos, M.; Díaz-Heras, M.; Belmonte, J.F.; Sobrino, C. A review of solar thermal energy storage in beds of particles: Packed and fluidized beds. Sol. Energy 2019, 192, 193–237. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal Energy Storage Materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Koçak, B.; Paksoy, H. Using demolition wastes from urban regeneration as sensible thermal energy storage material. Int. J. Energy Res. 2019, 43, 6454–6460. [Google Scholar] [CrossRef]
- Ravotti, R.; Worlitschek, J.; Pulham, C.R.; Stamatiou, A. Triglycerides as Novel Phase-Change Materials: A Review and Assessment of Their Thermal Properties. Molecules 2020, 25, 5572. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Martin, V.; Chiu, J.N. Phase equilibrium in the design of phase change materials for thermal energy storage: State-of-the-art. Renew. Sustain. Energy Rev. 2017, 73, 558–581. [Google Scholar] [CrossRef]
- Wu, H.; Salles, F.; Zajac, J. A Critical Review of Solid Materials for Low-Temperature Thermochemical Storage of Solar Energy Based on Solid-Vapour Adsorption in View of Space Heating Uses. Molecules 2019, 24, 945. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.; Knoll, C.; Gravogl, G.; Jordan, C.; Eitenberger, E.; Friedbacher, G.; Artner, W.; Welch, J.M.; Werner, A.; Harasek, M.; et al. Medium-temperature thermochemical energy storage with transition metal ammoniates–A systematic material comparison. Appl. Energy 2021, 285, 116470. [Google Scholar] [CrossRef]
- Hauer, A.; Fischer, F.; Rathgeber, C. 4-Temperatures Approach: Testing Thermochemical Heat Storage Materials Under Application Conditions. Chem. Ing. Tech. 2021, 93, 618–623. [Google Scholar] [CrossRef]
- Köll, R.; van Helden, W.; Engel, G.; Wagner, W.; Dang, B.; Jänchen, J.; Kerskes, H.; Badenhop, T.; Herzog, T. An experimental investigation of a realistic-scale seasonal solar adsorption storage system for buildings. Sol. Energy 2017, 155, 388–397. [Google Scholar] [CrossRef]
- Mustapha, A.N.; Onyeaka, H.; Omoregbe, O.; Ding, Y.; Li, Y. Latent heat thermal energy storage: A bibliometric analysis explicating the paradigm from 2000–2019. J. Energy Storage 2021, 33, 102027. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Chiu, J.N.; Martin, V.; Hedström, P. The experimental phase diagram study of the binary polyols system erythritol-xylitol. Sol. Energy Mater. Sol. Cells 2018, 174, 248–262. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Ignatowicz, M.; Chiu, J.N.; Martin, V. Thermal conductivity measurement of erythritol, xylitol, and their blends for phase change material design: A methodological study. Int. J. Energy Res. 2019, 43, 1785–1801. [Google Scholar] [CrossRef]
- Abdi, A.; Ignatowicz, M.; Gunasekara, S.N.; Chiu, J.N.; Martin, V. Experimental investigation of thermo-physical properties of n-octadecane and n-eicosane. Int. J. Heat Mass Transf. 2020, 161, 120285. [Google Scholar] [CrossRef]
- Hauer, A.; Fumey, B.; Gschwander, S.; Lager, D.; Lázaro, A.; Rathgeber, C.; Ristić, A.; van Helden, W. IEA-ES-TCP-Annex-33-Executive-Summary. 2020. Available online: https://iea-eces.org/wp-content/uploads/public/IEA-ES-TCP-Annex-33-Executive-Summary_revised.pdf (accessed on 13 April 2021).
- Simó-Solsona, M.; Palumbo, M.; Bosch, M.; Fernandez, A.I. Why it’s so hard? Exploring social barriers for the deployment of thermal energy storage in Spanish buildings. Energy Res. Soc. Sci. 2021, 76, 102057. [Google Scholar] [CrossRef]
- Müller, D.; Knoll, C.; Ruh, T.; Artner, W.; Welch, J.M.; Peterlik, H.; Eitenberger, E.; Friedbacher, G.; Harasek, M.; Blaha, P.; et al. Calcium Doping Facilitates Water Dissociation in Magnesium Oxide. Adv. Sustain. Syst. 2018, 2, 1700096. [Google Scholar] [CrossRef]
- Gravogl, G.; Knoll, C.; Welch, J.; Artner, W.; Freiberger, N.; Nilica, R.; Eitenberger, E.; Friedbacher, G.; Harasek, M.; Werner, A.; et al. Cycle Stability and Hydration Behavior of Magnesium Oxide and Its Dependence on the Precursor-Related Particle Morphology. Nanomaterials 2018, 8, 795. [Google Scholar] [CrossRef] [Green Version]
- Krajnc, A.; Varlec, J.; Mazaj, M.; Ristić, A.; Logar, N.Z.; Mali, G. Superior Performance of Microporous Aluminophosphate with LTA Topology in Solar-Energy Storage and Heat Reallocation. Adv. Energy Mater. 2017, 7, 1601815. [Google Scholar] [CrossRef]
- Ristić, A.; Logar, N.Z.; Henninger, S.K.; Kaučič, V. The Performance of Small-Pore Microporous Aluminophosphates in Low-Temperature Solar Energy Storage: The Structure-Property Relationship. Adv. Funct. Mater. 2012, 22, 1952–1957. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; Wiley: New York, NY, USA, 1973; ISBN 978-0-471-09985-7. [Google Scholar]
- Müller, D.; Knoll, C.; Gravogl, G.; Artner, W.; Welch, J.M.; Eitenberger, E.; Friedbacher, G.; Schreiner, M.; Harasek, M.; Hradil, K.; et al. Tuning the performance of MgO for thermochemical energy storage by dehydration–From fundamentals to phase impurities. Appl. Energy 2019, 253, 113562. [Google Scholar] [CrossRef]
- Centre for Science and Technology Studies–Leiden University. In VOSviewer—Visualizing Scientific Landscape; VOSviewer, 2021; Available online: http://www.vosviewer.com/ (accessed on 13 April 2021).
- Complexity Lab Barcelona. Complexity Lab Barcelona-Home Page. Available online: http://complex.ffn.ub.es (accessed on 17 March 2021).
- Fernández, A.I.; Barreneche, C.; Miró, L.; Brückner, S.; Cabeza, L.F.; Fernández, A.I.; Barreneche, C.; Miró, L.; Cabeza, L.F.; Brückner, S. Waste heat recovery using thermal energy storage. In Advances in Thermal Energy Storage Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 639–653. ISBN 978-0-12-819885-8. [Google Scholar]
- Alnaimat, F.; Rashid, Y. Thermal Energy Storage in Solar Power Plants: A Review of the Materials, Associated Limitations, and Proposed Solutions. Energies 2019, 12, 4164. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Steven Tay, N.H.; Bell, S.; Belusko, M.; Jacob, R.; Will, G.; Saman, W.; Bruno, F. Review on concentrating solar power plants and new developments in high temperature thermal energy storage technologies. Renew. Sustain. Energy Rev. 2016, 53, 1411–1432. [Google Scholar] [CrossRef]
- Trevisan, S.; Guédez, R. Thermodynamic Analysis of a High-Temperature Multi-Layered Sensible-Latent Thermal Energy Storage. AIP Conf. Proc. 2020, 2303, 190030. [Google Scholar]
- Trevisan, S. Literature Survey–Course MJ 3123; 3rd Cycle Course on Literature Survey; KTH Royal Institute of Technology, Department of Energy Technology: Stockholm, Sweden, 2021; p. 28. [Google Scholar]
- Konuklu, Y.; Şahan, N.; Paksoy, H. 2.14 Latent Heat Storage Systems. In Comprehensive Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 396–434. ISBN 978-0-12-814925-6. [Google Scholar]
- Lane, G.A. Solar Heat Storage: Latent Heat Material; CRC Press Inc.: Boca Raton, FL, USA, 1983; Volume 1. [Google Scholar]
- Telkes, M. Thermal energy storage in salt hydrates. Sol. Energy Mater. 1980, 2, 381–393. [Google Scholar] [CrossRef]
- Shahid, U.B.; Abdala, A. A critical review of phase change material composite performance through Figure-of-Merit analysis: Graphene vs. Boron Nitride. Energy Storage Mater. 2021, 34, 365–387. [Google Scholar] [CrossRef]
- Whittingham, M.S. History, Evolution, and Future Status of Energy Storage. Proc. IEEE 2012, 100, 1518–1534. [Google Scholar] [CrossRef]
- United Nations Climate Agreement. The Paris Agreement. 2021. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 19 May 2021).
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
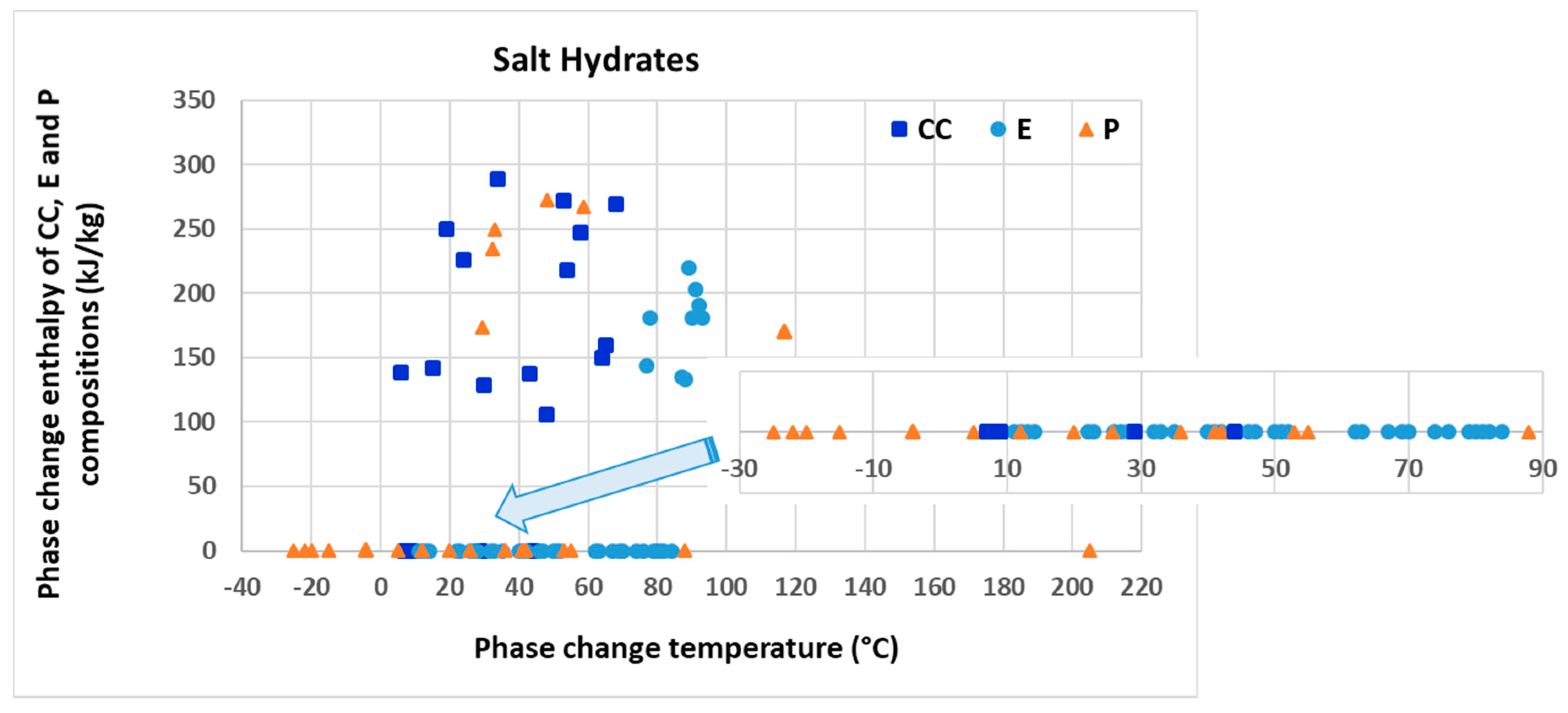
- Purohit, B.K.; Sistla, V.S. Inorganic salt hydrate for thermal energy storage application: A review. Energy Storage 2021, 3, e212. [Google Scholar] [CrossRef]
- Jankowski, N.R.; McCluskey, F.P. A review of phase change materials for vehicle component thermal buffering. Appl. Energy 2014, 113, 1525–1561. [Google Scholar] [CrossRef]
- Mandelcorn, L. Clathrates. Chem Rev. 1959, 59, 827–839. [Google Scholar] [CrossRef]
- IEA ECES Annex 29 and SHC Task 42. Task 42–Annex 29 Thermal Material Database–Wiki PCM. 2021. Available online: https://thermalmaterials.org/ (accessed on 10 February 2021).
- Callister, W.D. Materials Science and Engineering: An Introduction, 7th ed.; John Wiley & Sons: New York, NY, USA, 2007; ISBN 978-0-471-73696-7. [Google Scholar]
- Gunasekara, S.N. Phase Equilibrium-aided Design of Phase Change Materials from Blends. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2017. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:kth:diva-212440 (accessed on 18 February 2021).
- Lane, G.A. Solar Heat Storage, Latent Heat Materials; CRC Press: Boca Baton, FL, USA, 1986; Volume 2, ISBN 978-1-315-89764-6. [Google Scholar]
- Knoll, C.; Müller, D.; Artner, W.; Welch, J.M.; Werner, A.; Harasek, M.; Weinberger, P. Probing cycle stability and reversibility in thermochemical energy storage–CaC2O4·H2O as perfect match? Appl. Energy 2017, 187, 1–9. [Google Scholar] [CrossRef]
- Müller, D.; Knoll, C.; Gravogl, G.; Lager, D.; Welch, J.M.; Eitenberger, E.; Friedbacher, G.; Werner, A.; Artner, W.; Harasek, M.; et al. CuSO4/[Cu(NH3)4]SO4-Composite Thermochemical Energy Storage Materials. Nanomaterials 2020, 10, 2485. [Google Scholar] [CrossRef] [PubMed]
- Gravogl, G.; Knoll, C.; Artner, W.; Eitenberger, E.; Friedbacher, G.; Werner, A.; Harasek, M.; Weinberger, P.; Müller, D.; Miletich, R. Moisture-triggered ambient-temperature carbonatization of main group II metal oxides under elevated CO2 pressure. In Proceedings of the SWC2017/SHC2017, International Solar Energy Society, Abu Dhabi, United Arab Emirates, 29 October–2 November 2017; pp. 1–12. [Google Scholar]
- Gravogl, G.; Knoll, C.; Artner, W.; Welch, J.M.; Eitenberger, E.; Friedbacher, G.; Harasek, M.; Hradil, K.; Werner, A.; Weinberger, P.; et al. Pressure effects on the carbonation of MeO (Me = Co, Mn, Pb, Zn) for thermochemical energy storage. Appl. Energy 2019, 252, 113451. [Google Scholar] [CrossRef]
- Gravogl, G.; Birkelbach, F.; Müller, D.; Lengauer, C.L.; Weinberger, P.; Miletich, R. Pressure Dependence of the Low Temperature Carbonation Kinetics of Calcium Oxide for Potential Thermochemical Energy Storage Purposes and Sustainable CO2 Fixation. Adv. Sustain. Syst. 2021, 5, 2100022. [Google Scholar] [CrossRef]
- Knoll, C.; Müller, D.; Artner, W.; Welch, J.M.; Eitenberger, E.; Friedbacher, G.; Werner, A.; Weinberger, P.; Harasek, M. Magnesium oxide from natural magnesite samples as thermochemical energy storage material. Energy Procedia 2019, 158, 4861–4869. [Google Scholar] [CrossRef]
- Muthukumar, P.; Groll, M. Metal hydride based heating and cooling systems: A review. Int. J. Hydrog. Energy 2010, 35, 3817–3831. [Google Scholar] [CrossRef]
- Rönnebro, E.; Whyatt, G.; Powell, M.; Westman, M.; Zheng, F.; Fang, Z. Metal Hydrides for High-Temperature Power Generation. Energies 2015, 8, 8406–8430. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, M.; Horvath, F.; Knoll, C.; Lager, D.; Gierl-Mayer, C.; Weinberger, P.; Winter, F. High-Temperature Energy Storage: Kinetic Investigations of the CuO/Cu 2 O Reaction Cycle. Energy Fuels 2017, 31, 2324–2334. [Google Scholar] [CrossRef]
- Müller, D.; Knoll, C.; Artner, W.; Harasek, M.; Gierl-Mayer, C.; Welch, J.M.; Werner, A.; Weinberger, P. Combining in-situ X-ray diffraction with thermogravimetry and differential scanning calorimetry–An investigation of Co3O4, MnO2 and PbO2 for thermochemical energy storage. Sol. Energy 2017, 153, 11–24. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Laios, M.; Karabanova, A.; Martin, V.; Blanchard, D. Design of a bench-scale ammonia-SrCl2 thermochemical storage system using numerical modelling. In Eurotherm Seminar #112–Advances in Thermal Energy Storage; Universitat de Lleida: Lleida, Spain, 2019; pp. 87–97. [Google Scholar]
- Deutsch, M.; Müller, D.; Aumeyr, C.; Jordan, C.; Gierl-Mayer, C.; Weinberger, P.; Winter, F.; Werner, A. Systematic search algorithm for potential thermochemical energy storage systems. Appl. Energy 2016, 183, 113–120. [Google Scholar] [CrossRef]
- Hauer, A.; Fischer, F. Open Adsorption System for an Energy Efficient Dishwasher. Chem. Ing. Tech. 2011, 83, 61–66. [Google Scholar] [CrossRef]
- Krönauer, A.; Lävemann, E.; Brückner, S.; Hauer, A. Mobile Sorption Heat Storage in Industrial Waste Heat Recovery. Energy Procedia 2015, 73, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, J.M.; Salústio, S.; Rocha, J.; Valente, A.A.; Silva, C.M. Adsorption heat pumps for heating applications. Renew. Sustain. Energy Rev. 2020, 119, 109528. [Google Scholar] [CrossRef]
- RAL Quality Association PCM. Phase Change Materials, Phase Change Materials. 2021. Available online: https://www.pcm-ral.org/pcm/en/ (accessed on 29 August 2021).
- Mohan, G.; Venkataraman, M.; Gomez-Vidal, J.; Coventry, J. Thermo-economic analysis of high-temperature sensible thermal storage with different ternary eutectic alkali and alkaline earth metal chlorides. Sol. Energy 2018, 176, 350–357. [Google Scholar] [CrossRef]
- John, E.; Hale, M.; Selvam, P. Concrete as a thermal energy storage medium for thermocline solar energy storage systems. Sol. Energy 2013, 96, 194–204. [Google Scholar] [CrossRef]
- Navarro, M.E.; Martínez, M.; Gil, A.; Fernández, A.I.; Cabeza, L.F.; Olives, R.; Py, X. Selection and characterization of recycled materials for sensible thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 107, 131–135. [Google Scholar] [CrossRef]
- Faik, A.; Guillot, S.; Lambert, J.; Véron, E.; Ory, S.; Bessada, C.; Echegut, P.; Py, X. Thermal storage material from inertized wastes: Evolution of structural and radiative properties with temperature. Sol. Energy 2012, 86, 139–146. [Google Scholar] [CrossRef]
- Motte, F.; Falcoz, Q.; Veron, E.; Py, X. Compatibility tests between Solar Salt and thermal storage ceramics from inorganic industrial wastes. Appl. Energy 2015, 155, 14–22. [Google Scholar] [CrossRef]
- Ozger, O.B.; Girardi, F.; Giannuzzi, G.M.; Salomoni, V.A.; Majorana, C.E.; Fambri, L.; Baldassino, N.; Di Maggio, R. Effect of nylon fibres on mechanical and thermal properties of hardened concrete for energy storage systems. Mater. Des. 2013, 51, 989–997. [Google Scholar] [CrossRef]
- Agalit, H.; Zari, N.; Maaroufi, M. Thermophysical and chemical characterization of induction furnace slags for high temperature thermal energy storage in solar tower plants. Sol. Energy Mater. Sol. Cells 2017, 172, 168–176. [Google Scholar] [CrossRef]
- Ortega-Fernández, I.; Calvet, N.; Gil, A.; Rodríguez-Aseguinolaza, J.; Faik, A.; D’Aguanno, B. Thermophysical characterization of a by-product from the steel industry to be used as a sustainable and low-cost thermal energy storage material. Energy 2015, 89, 601–609. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Wei, G.; Wang, G.; Xu, C.; Ju, X.; Xing, L.; Du, X.; Yang, Y. Selection principles and thermophysical properties of high temperature phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2018, 81, 1771–1786. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Salt hydrates as latent heat storage materials:Thermophysical properties and costs. Sol. Energy Mater. Sol. Cells 2016, 145, 255–286. [Google Scholar] [CrossRef]
- Rubitherm Technologies GmbH. PCM RT SERIES. 2021. Available online: https://www.rubitherm.eu/index.php/produktkategorie/organische-pcm-rt (accessed on 20 July 2021).
- Oliver, D.E.; Bissell, A.J.; Liu, X.; Tang, C.C.; Pulham, C.R. Crystallisation studies of sodium acetate trihydrate–suppression of incongruent melting and sub-cooling to produce a reliable, high-performance phase-change material. Cryst. Eng. Comm. 2021, 23, 700–706. [Google Scholar] [CrossRef]
- Sunamp Ltd. Sunamp, World-Leading Thermal Storage Technologies. 2021. Available online: https://sunamp.com/ (accessed on 20 July 2021).
- Pluss Advanced Technologies. PLUSS–Technology for a Better World; Pluss Advanced Technologies: Gurugram, India, 2021; Available online: https://www.pluss.co.in/index.php (accessed on 20 July 2021).
- Swerod, A.B. Swerod–Moving Energy in Time, Energy Storage on a New Level -Capture Free Energy and Use It When Needed. 2021. Available online: http://www.swerod.com/en/ (accessed on 20 July 2021).
- Gunasekara, S.N.; Kumova, S.; Chiu, J.N.; Martin, V. Experimental phase diagram of the dodecane–tridecane system as phase change material in cold storage. Int. J. Refrig. 2017, 82, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Noël, J.A.; Allred, P.M.; White, M.A. Life cycle assessment of two biologically produced phase change materials and their related products. Int. J. Life Cycle Assess. 2015, 20, 367–376. [Google Scholar] [CrossRef]
- Lizana, J.; Chacartegui, R.; Barrios-Padura, A.; Valverde, J.M.; Ortiz, C. Identification of best available thermal energy storage compounds for low-to-moderate temperature storage applications in buildings. Mater. Constr. 2018, 68, 160. [Google Scholar] [CrossRef] [Green Version]
- Noël, J.A.; Kreplak, L.; Getangama, N.N.; de Bruyn, J.R.; White, M.A. Supercooling and Nucleation of Fatty Acids: Influence of Thermal History on the Behavior of the Liquid Phase. J. Phys. Chem. B 2018, 122, 12386–12395. [Google Scholar] [CrossRef] [PubMed]
- Riemenschneider, W.; Bolt, H.M. Esters, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; ISBN 978-3-527-30673-2. [Google Scholar]
- Stamatiou, A.; Obermeyer, M.; Fischer, L.J.; Schuetz, P.; Worlitschek, J. Investigation of unbranched, saturated, carboxylic esters as phase change materials. Renew. Energy 2017, 108, 401–409. [Google Scholar] [CrossRef]
- Ravotti, R.; Fellmann, O.; Lardon, N.; Fischer, L.; Stamatiou, A.; Worlitschek, J. Investigation of Lactones as Innovative Bio-Sourced Phase Change Materials for Latent Heat Storage. Molecules 2019, 24, 1300. [Google Scholar] [CrossRef] [Green Version]
- Ravotti, R.; Fellmann, O.; Lardon, N.; Fischer, L.; Stamatiou, A.; Worlitschek, J. Synthesis and Investigation of Thermal Properties of Highly Pure Carboxylic Fatty Esters to Be Used as PCM. Appl. Sci. 2018, 8, 1069. [Google Scholar] [CrossRef] [Green Version]
- Ravotti, R.; Fellmann, O.; Lardon, N.; Fischer, L.; Stamatiou, A.; Worlitschek, J. Analysis of Bio-Based Fatty Esters PCM’s Thermal Properties and Investigation of Trends in Relation to Chemical Structures. Appl. Sci. 2019, 9, 225. [Google Scholar] [CrossRef] [Green Version]
- Ravotti, R.; Fellmann, O.; Fischer, L.J.; Worlitschek, J.; Stamatiou, A. Investigation of the Thermal Properties of Diesters from Methanol, 1-Pentanol, and 1-Decanol as Sustainable Phase Change Materials. Materials 2020, 13, 810. [Google Scholar] [CrossRef] [Green Version]
- Burke, R.A. Hazardous Materials Chemistry for Emergency Responders, 3rd ed.; CRC Press: New York, NY, USA, 2013; ISBN 13: 978-1-4398-4986-6. [Google Scholar]
- Schmidt, B.; Buddrus, J. Grundlagen der Organischen Chemie; De Gruyter Studium: Berlin/München, Germany; Boston, MA, USA, 2009; ISBN 978-3-11-030559-3. Available online: http://scripts.iucr.org/cgi-bin/paper?S0108768109046060 (accessed on 7 May 2021).
- Alper Aydın, A. High-chain fatty acid esters of 1-octadecanol as novel organic phase change materials and mathematical correlations for estimating the thermal properties of higher fatty acid esters’ homologous series. Sol. Energy Mater. Sol. Cells 2013, 113, 44–51. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Pan, R.; Chiu, J.N.; Martin, V. Polyols as phase change materials for surplus thermal energy storage. Appl. Energy 2016, 162, 1439–1452. [Google Scholar] [CrossRef]
- Croda International Plc. CRODA-Smart Science to improve lives. Product Finder. 2021. Available online: https://www.crodaenergytechnologies.com/en-gb/product-finder?currentPage=1&pageSize=20&sortBy=recommended&lang=en-gb (accessed on 20 July 2021).
- Gunasekara, S.N.; Stalin, J.; Marçal, M.; Delubac, R.; Karabanova, A.; Wei Chiu, J.N.; Martin, V. Erythritol, glycerol, their blends, and olive oil, as sustainable phase change materials. Energy Procedia 2017, 135, 249–262. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Composites ‘salt inside porous matrix’ for adsorption heat transformation: A current state-of-the-art and new trends. Int. J. Low-Carbon Technol. 2012, 7, 288–302. [Google Scholar] [CrossRef] [Green Version]
- Ristić, A.; Logar, N.Z. New Composite Water Sorbents CaCl2-PHTS for Low-Temperature Sorption Heat Storage: Determination of Structural Properties. Nanomaterials 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Permyakova, A.; Wang, S.; Courbon, E.; Nouar, F.; Heymans, N.; D’Ans, P.; Barrier, N.; Billemont, P.; De Weireld, G.; Steunou, N.; et al. Design of salt–metal organic framework composites for seasonal heat storage applications. J. Mater. Chem. A 2017, 5, 12889–12898. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Palomba, V.; Frazzica, A.; Cabeza, L.F. Magnesium sulphate-silicone foam composites for thermochemical energy storage: Assessment of dehydration behaviour and mechanical stability. Sol. Energy Mater. Sol. Cells 2019, 200, 109992. [Google Scholar] [CrossRef]
- Ristić, A.; Maučec, D.; Henninger, S.K.; Kaučič, V. New two-component water sorbent CaCl2-FeKIL2 for solar thermal energy storage. Microporous Mesoporous Mater. 2012, 164, 266–272. [Google Scholar] [CrossRef]
- Jänchen, J.; Schumann, K.; Thrun, E.; Brandt, A.; Unger, B.; Hellwig, U. Preparation, hydrothermal stability and thermal adsorption storage properties of binderless zeolite beads. Int. J. Low-Carbon Technol. 2012, 7, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Fischer, F.; Lutz, W.; Buhl, J.-C.; Laevemann, E. Insights into the hydrothermal stability of zeolite 13X. Microporous Mesoporous Mater. 2018, 262, 258–268. [Google Scholar] [CrossRef]
- Ristić, A.; Fischer, F.; Hauer, A.; Zabukovec Logar, N. Improved performance of binder-free zeolite Y for low-temperature sorption heat storage. J. Mater. Chem. A 2018, 6, 11521–11530. [Google Scholar] [CrossRef] [Green Version]
- Brancato, V.; Frazzica, A. Characterisation and comparative analysis of zeotype water adsorbents for heat transformation applications. Sol. Energy Mater. Sol. Cells 2018, 180, 91–102. [Google Scholar] [CrossRef]
- Henninger, S.K.; Munz, G.; Ratzsch, K.-F.; Schossig, P. Cycle stability of sorption materials and composites for the use in heat pumps and cooling machines. Renew. Energy 2011, 36, 3043–3049. [Google Scholar] [CrossRef]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Janiak, C. MOFs for Use in Adsorption Heat Pump Processes. Eur. J. Inorg. Chem. 2012, 2012, 2625–2634. [Google Scholar] [CrossRef]
- Chen, B.; Kuznik, F.; Horgnies, M.; Johannes, K.; Morin, V.; Gengembre, E. Physicochemical properties of ettringite/meta-ettringite for thermal energy storage: Review. Sol. Energy Mater. Sol. Cells 2019, 193, 320–334. [Google Scholar] [CrossRef]
- SaltX Technology Holding AB. SaltX Technology–Energy Storage with Nano Coated Salt. The Shift to a Sustainable Future is Now Possible–Industrial Scale Energy Storage Green–Clean–Safe. 2021. Available online: https://saltxtechnology.com/ (accessed on 20 July 2021).
- Harasek, M.; Werner, A.; Weinberger, P. SolidHeat; TheoCryst. TU Wien, Institut für Angewandte Synthesechemie: Vienna, Austria, 2015. [Google Scholar]
- Müller, D.; Knoll, C.; Gravogl, G.; Werner, A.; Harasek, M.; Miletich, R.; Weinberger, P. Lab-scale demonstration of thermochemical energy storage with NH3 and impregnated-loaded zeolites. In Proceedings of the SWC2017/SHC2017; International Solar Energy Society, Abu Dhabi, United Arab Emirates, 29 October–2 November 2017; pp. 1–9. [Google Scholar]
- Müller, D.; Knoll, C.; Gravogl, G.; Artner, W.; Werner, A.; Welch, J.M.; Harasek, M.; Miletich, R.; Weinberger, P. Low-temperature carbonatization of metal oxides. Energy Procedia 2019, 158, 4870–4881. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tao, Y.B.; Yu, Y.S. Molecular dynamics simulation of nanoparticle effect on melting enthalpy of paraffin phase change material. Int. J. Heat Mass Transf. 2020, 150, 119382. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Zhang, W.; Huang, X.; Ruan, L.; Wu, L. Experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies of methionine and valine as corrosion inhibitors on carbon steel in phase change materials (PCMs) solution. J. Mol. Liq. 2018, 272, 528–538. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tao, Y.B.; Yu, Y.S. Molecular dynamics simulation of thermal and phonon transport characteristics of nanocomposite phase change material. J. Mol. Liq. 2021, 329, 115448. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Luo, A.; Liu, Z.; Zhang, X. Molecular dynamics simulation on thermophysics of paraffin/EVA/graphene nanocomposites as phase change materials. Appl. Therm. Eng. 2020, 166, 114639. [Google Scholar] [CrossRef]
- Liu, X.; Rao, Z. Molecular dynamics simulations on the heat and mass transfer of hypercrosslinked shell structure of phase change nanocapsules as Thermal Energy Storage Materials. Int. J. Heat Mass Transf. 2019, 132, 362–374. [Google Scholar] [CrossRef]
- Yu, Y.; Tao, Y.; He, Y.-L. Molecular dynamics simulation of thermophysical properties of NaCl-SiO2 based molten salt composite phase change materials. Appl. Therm. Eng. 2020, 166, 114628. [Google Scholar] [CrossRef]
- Göbel, A.; Vidi, S.; Klinker, F.; Hemberger, F.; Brütting, M.; Ebert, H.-P.; Mehling, H. Method for the Thermal Characterization of PCM Systems in the Volume Range from 100 mL to 1000 mL. Int. J. Thermophys. 2017, 38, 67. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Barreneche, C.; Martorell, I.; Miró, L.; Sari-Bey, S.; Fois, M.; Paksoy, H.O.; Sahan, N.; Weber, R.; Constantinescu, M.; et al. Unconventional experimental technologies available for phase change materials (PCM) characterization. Part 1. Thermophysical properties. Renew. Sustain. Energy Rev. 2015, 43, 1399–1414. [Google Scholar] [CrossRef] [Green Version]
- Majó, M.; Sánchez, R.; Barcelona, P.; García, J.; Fernández, A.I.; Barreneche, C. Degradation of Fatty Acid Phase-Change Materials (PCM): New Approach for Its Characterization. Molecules 2021, 26, 982. [Google Scholar] [CrossRef]
- Lovelyn Theresa, I.; Velraj, R. Thermophysical characterization and comparison of PCMs using DSC and T-History experimental setup. Mater. Res. Express 2019, 6, 125527. [Google Scholar] [CrossRef]
- Nkhonjera, L.; Bello-Ochende, T.; John, G.; King’ondu, C.K. A review of thermal energy storage designs, heat storage materials and cooking performance of solar cookers with heat storage. Renew. Sustain. Energy Rev. 2017, 75, 157–167. [Google Scholar] [CrossRef]
- Paksoy, H.Ö. (Ed.) Thermal Energy Storage for Sustainable Energy Consumption; NATO Science Series; Springer: Dordrecht, The Netherlands, 2007; Volume 234, ISBN 978-1-4020-5288-0. [Google Scholar]
- Nie, B.; Zou, B.; She, X.; Zhang, T.; Li, Y.; Ding, Y. Development of a heat transfer coefficient based design method of a thermal energy storage device for transport air-conditioning applications. Energy 2020, 196, 117083. [Google Scholar] [CrossRef]
- Duraković, B. PCM-Based Building Envelope Systems–Innovative Energy Solutions for Passive Design; Faculty of Engineering and Natural Sciences, International University of Sarajevo Sarajevo: Ilidža, Bosnia and Herzegovina, 2020. [Google Scholar]
- Beaupere, N.; Soupremanien, U.; Zalewski, L. Nucleation triggering methods in supercooled phase change materials (PCM), a review. Thermochim. Acta 2018, 670, 184–201. [Google Scholar] [CrossRef]
- Lee, A.Y.; Erdemir, D.; Myerson, A.S. Crystal Polymorphism in Chemical Process Development. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 259–280. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Feeder, N.; Davey, R.J. Open questions in organic crystal polymorphism. Commun. Chem. 2020, 3, 142. [Google Scholar] [CrossRef]
- Kumar, N.; Hirschey, J.; LaClair, T.J.; Gluesenkamp, K.R.; Graham, S. Review of stability and thermal conductivity enhancements for salt hydrates. J. Energy Storage 2019, 24, 100794. [Google Scholar] [CrossRef]
- Rathgeber, C.; Hiebler, S.; Bayón, R.; Cabeza, L.F.; Zsembinszki, G.; Englmair, G.; Dannemand, M.; Diarce, G.; Fellmann, O.; Ravotti, R.; et al. Experimental Devices to Investigate the Long-Term Stability of Phase Change Materials under Application Conditions. Appl. Sci. 2020, 10, 7968. [Google Scholar] [CrossRef]
- Xu, T. Integrating Latent Heat Storage into Residential Heating Systems-A Study from Material and Component Characterization to System Analysis. PhD Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2021. Available online: https://kth.diva-portal.org/smash/record.jsf?dswid=-9366&pid=diva2%3A1548439&c=1&searchType=SIMPLE&language=en&query=tianhao+xu&af=%5B%22publicationTypeCode%3AcomprehensiveDoctoralThesis%22%5D&aq=%5B%5B%5D%5D&aq2=%5B%5B%5D%5D&aqe=%5B%5D&noOfRows=50&sortOrder=author_sort_asc&sortOrder2=title_sort_asc&onlyFullText=false&sf=all (accessed on 30 May 2021).
- Huggins, R. Energy Storage, 2nd ed.; Springer International Publishing: Stanford, CA, USA, 2016; ISBN 978-3-319-21238-8. [Google Scholar]
- IEA SHC. IEA Solar Heating & Cooling Technology Collaboration Programme. 2021. Available online: https://www.iea-shc.org/ (accessed on 19 August 2021).
- IEA ECES. IEA–Technology Collaboration Program–Energy Conservation through Energy Storage. 2021. Available online: https://iea-eces.org/ (accessed on 19 August 2021).
- IEA DHC. International Energy Agency Technology Collaboration Programme On District Heating And Cooling. 2021. Available online: https://www.iea-dhc.org/home (accessed on 19 August 2021).
- IRENA. IRENA–International Renewable Energy Agency. 2020. Available online: https://www.irena.org/ (accessed on 19 August 2021).
- Guion, J.; Teisseire, M. Nucleation of sodium acetate trihydrate in thermal heat storage cycles. Sol. Energy 1991, 46, 97–100. [Google Scholar] [CrossRef]
- Massobrio, C.; Du, J.; Bernasconi, M.; Salmon, P.S. (Eds.) Molecular Dynamics Simulations of Disordered Materials; Springer Series in Materials Science; Springer International Publishing: Cham, Switzerland; Berlin/Heidelberg, Germany, 2015; Volume 215, ISBN 978-3-319-15674-3. [Google Scholar]
- Kalikka, J.; Akola, J.; Jones, R.O. Crystallization processes in the phase change material Ge2Sb2Te5: Unbiased density functional/molecular dynamics simulations. Phys. Rev. B 2016, 94, 134105. [Google Scholar] [CrossRef] [Green Version]
- Kalaiselvam, S.; Parameshwaran, R. Thermal Energy Storage Technologies for Sustainability–Systems Design, Assessment and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-417291-3. [Google Scholar]
- Chiu, J.N.-W. Latent Heat Thermal Energy Storage for Indoor Comfort Control. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2013. [Google Scholar]
- Hassine, I.B.; Helmke, A.; Heß, S.; Krummenacher, P.; Muster, B.; Schmitt, B.; Schnitzer, H. Solar Process Heat for Production and Advanced Applications–Integration Guideline (Deliverable B2). 2015. Available online: http://task49.iea-shc.org/data/sites/1/publications/150218_IEA%20Task%2049_D_B2_Integration_Guideline-final1.pdf (accessed on 23 August 2021).
- Denholm, P.; Jeffrey, C.K.; Kutcher, C.F.; Paul, P.H. Wilson Decarbonizing the electric sector Combining renewable and nuclear energy using thermal storage. Energy Policy 2012, 44, 301–311. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Lai, C.S.; Lai, L.L. A Review on Thermal Energy Modelling for Optimal Microgrids Management. Thermo 2021, 1, 63–76. [Google Scholar] [CrossRef]


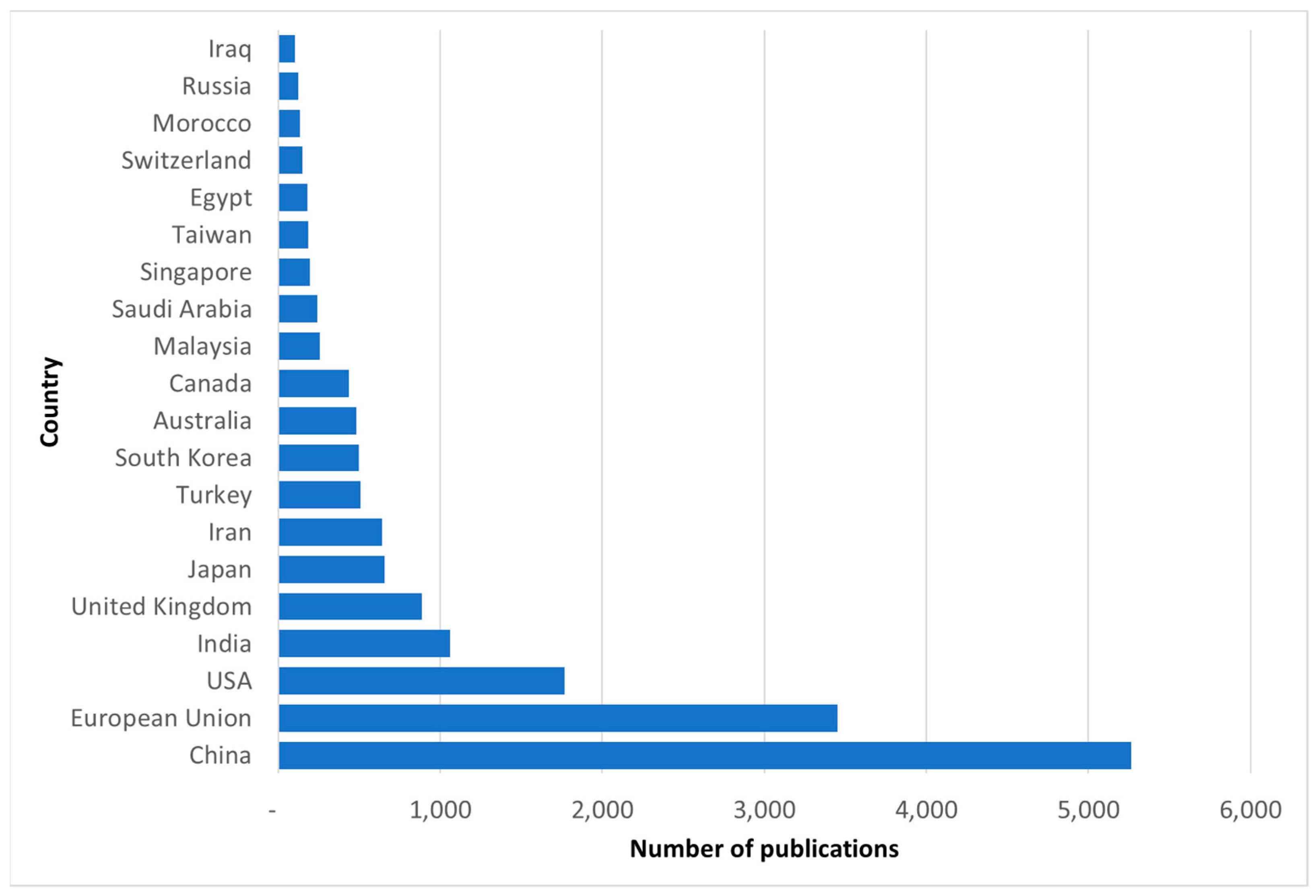
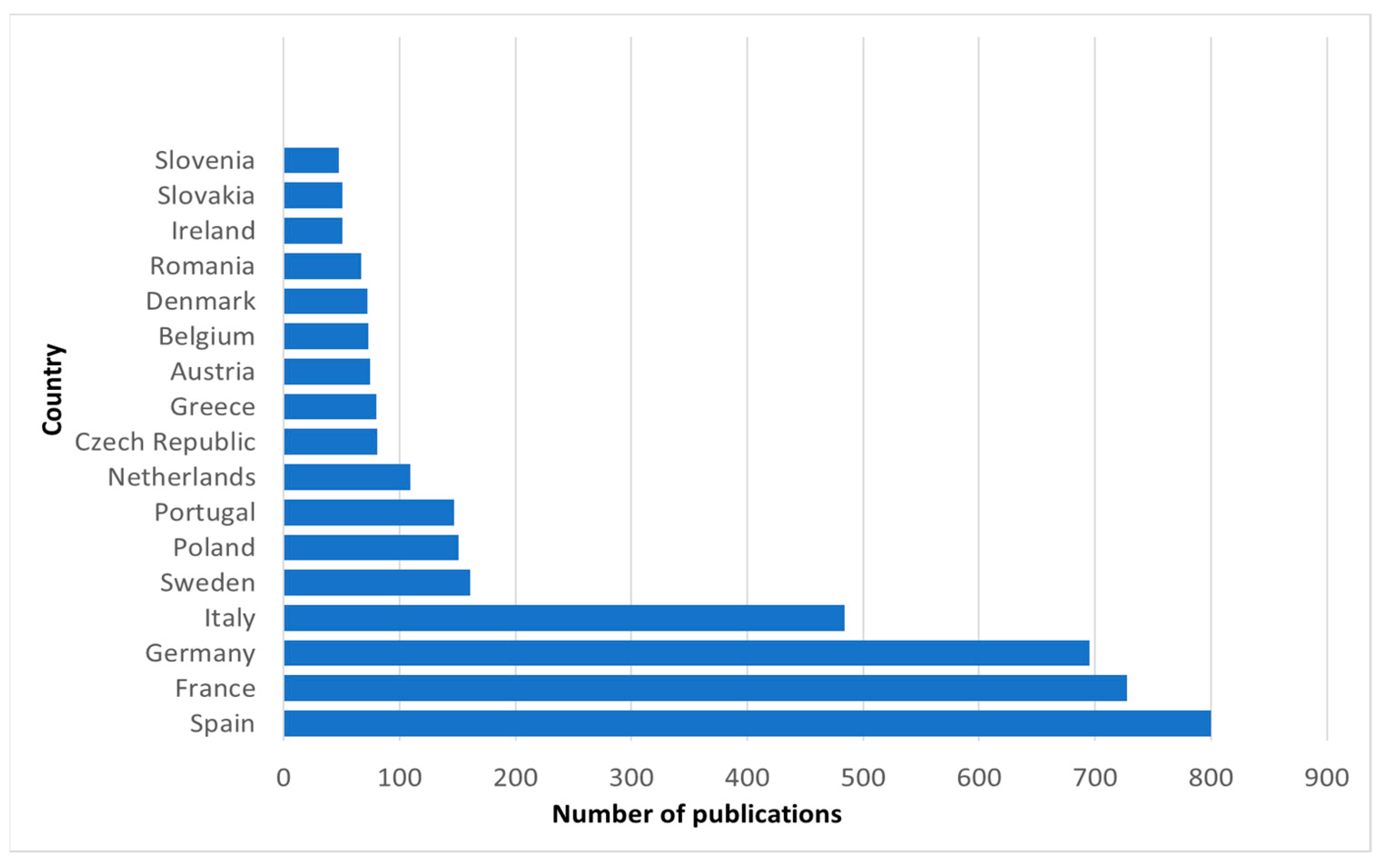

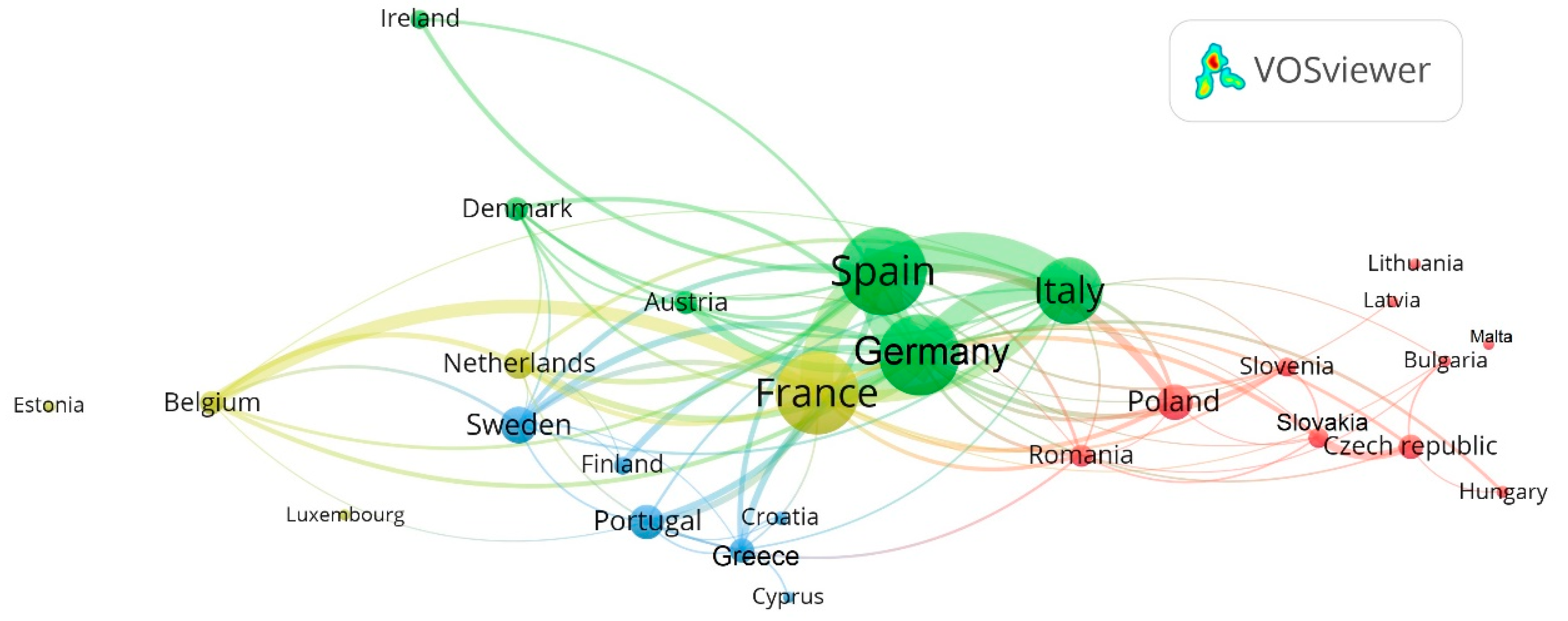
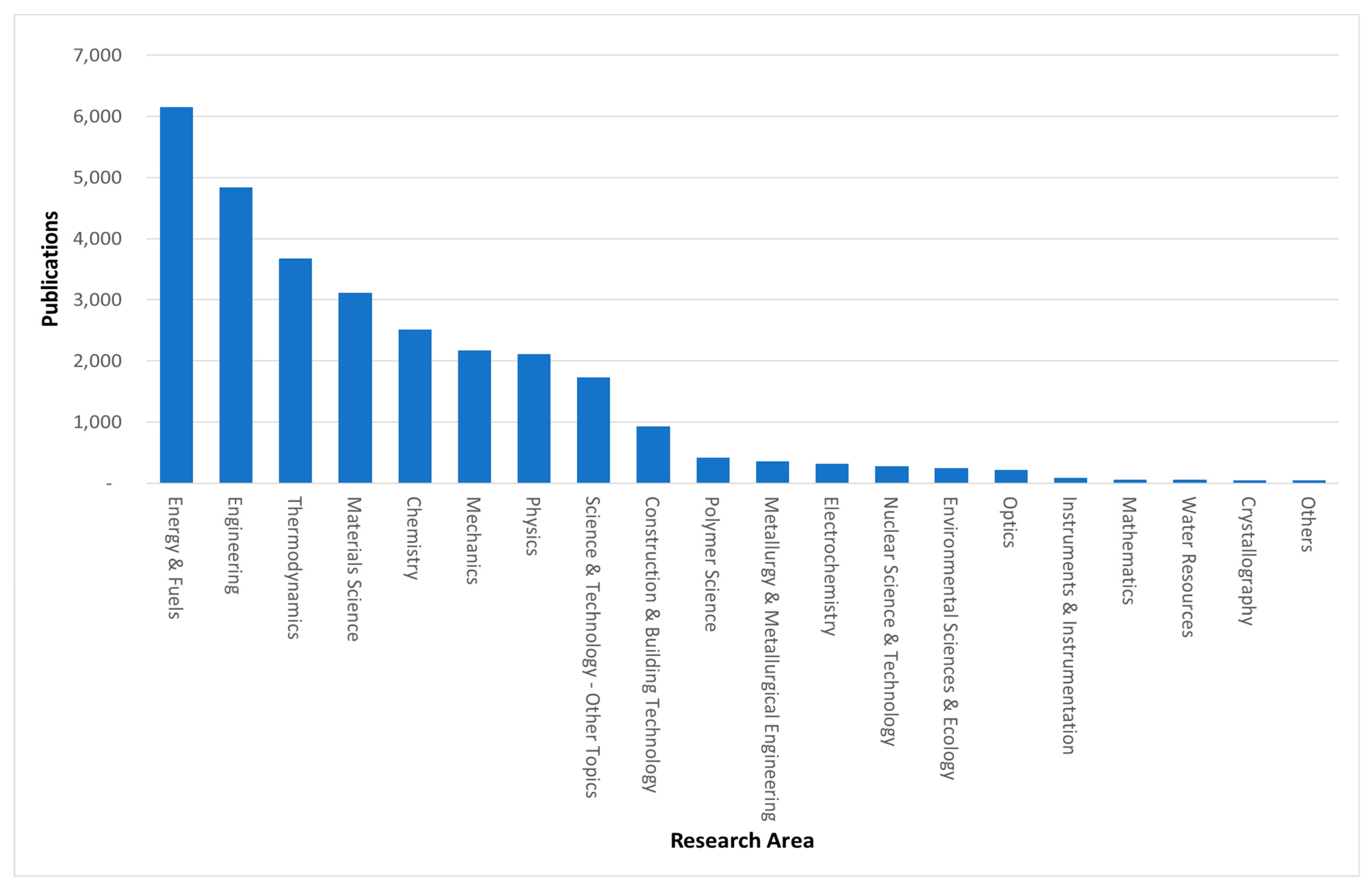
| Exclusion Phrases | Main Phrases | Complementary Phrases | ||||||
|---|---|---|---|---|---|---|---|---|
| thermal storage | ||||||||
| thermal energy | storage | |||||||
| cool storage | thermal | |||||||
| concentrated solar power | ||||||||
| phase change material | ||||||||
| thermochemical storage | ||||||||
| PV | photovoltaic | molten salts | solar | energy | power plant | storage | ||
| cloud | internet | software | CSP | solar | energy | renewable | power | storage |
| heat storage | ||||||||
| latent heat | storage | |||||||
| sensible heat | storage | |||||||
| thermochemical | energy storage | |||||||
| PCM | energy storage | |||||||
| TES Journal | Papers | Citations | PR | TES Materials-Related Journal | Papers | Citations | PR |
|---|---|---|---|---|---|---|---|
| Applied Thermal Engineering | 910 | 28,403 | 31 | Solar Energy Materials And Solar Cells | 521 | 21,356 | 41 |
| Applied Energy | 648 | 31,645 | 49 | Thermochimica Acta | 206 | 6229 | 30 |
| Solar Energy | 547 | 20,497 | 37 | Journal Of Thermal Analysis And Calorimetry | 201 | 2942 | 15 |
| Solar Energy Materials And Solar Cells | 521 | 21,356 | 41 | Construction And Building Materials | 131 | 2132 | 16 |
| Energy Conversion And Management | 486 | 24,668 | 51 | Journal Of Applied Polymer Science | 97 | 2222 | 23 |
| Energy And Buildings | 474 | 19,723 | 42 | Applied Physics Letters | 96 | 2819 | 29 |
| International Journal of Heat And Mass Transfer | 445 | 16,654 | 37 | Materials | 92 | 868 | 9 |
| Energy | 362 | 10,628 | 29 | Journal Of Materials Chemistry A | 90 | 3200 | 36 |
| Renewable Energy | 337 | 10,135 | 30 | Acs Applied Materials & Interfaces | 89 | 1959 | 22 |
| Journal Of Energy Storage | 335 | 2633 | 8 | Journal Of Molecular Liquids | 76 | 1510 | 20 |
| Renewable & Sustainable Energy Reviews | 261 | 31,933 | 122 | Journal Of Applied Physics | 70 | 1907 | 27 |
| International Journal Of Energy Research | 231 | 3556 | 15 | Journal Of Physical Chemistry C | 57 | 1571 | 28 |
| Thermochimica Acta | 206 | 6229 | 30 | Journal Of Alloys And Compounds | 51 | 1413 | 28 |
| Energies | 202 | 1311 | 6 | Materials Research Express | 47 | 187 | 4 |
| Journal Of Thermal Analysis And Calorimetry | 201 | 2942 | 15 | Materials Letters | 41 | 1028 | 25 |
| Construction And Building Materials | 131 | 2132 | 16 | Journal Of Materials Science | 40 | 751 | 19 |
| International Journal Of Thermal Sciences | 110 | 3346 | 30 | Materials Chemistry And Physics | 38 | 1247 | 33 |
| International Journal Of Refrigeration | 107 | 2779 | 26 | Nanomaterials | 31 | 159 | 5 |
| Journal Of Solar Energy Engineering | 101 | 2571 | 25 | Advanced Materials | 25 | 1334 | 53 |
| Journal Of Applied Polymer Science | 97 | 2222 | 23 | Fibers And Polymers | 25 | 323 | 13 |
| Category | STESM | Operational Temperature (°C) | Energy Density (kJ m−3 K−1) | Cost (Euro/kg) | Sources |
|---|---|---|---|---|---|
| Waste/By-products | Demolition Waste | <750 | 3500–4000 | <0.001 | [10] |
| Induction furnace slag (IFS) from steel making process | <1000 | 1200–1850 | <0.001 | [73] | |
| Asbestos containing waste (Cofalit) | <1100 | 2490–3220 | <0.001 | [71] | |
| Electric arc furnace slags (EAF) | <1100 | 3200–3400 | <0.001 | [74] | |
| Solid | Concrete | <400 | 1900 | 0.05 | [75] |
| Cast steel | <700 | 4700 | 4 | [75] | |
| Magnesia Fire Brick | <1200 | 3500 | 2 | [75] | |
| NaCl (Solid) | <500 | 1800 | 0.12 | [75] | |
| Metal Alloys | 450–620 | 3000–4500 | NA | [76] | |
| Liquid | Solar Salt (NaNO3 KNO3 (50–50) | <600 | 2800 | 0.4 | [76] |
| HITEC, NaNO3-KNO3-NaNO2 (7–53–40) | <535 | 2560 | 0.5 | [77] | |
| Carbonate Salt | <850 | 3800 | 2.2 | [75] | |
| Nitrate Salt | <565 | 3000 | 0.4 | [76] |
| STESM Category | Typical Temperatures | Advantages | Disadvantages and Challenges | |
|---|---|---|---|---|
| Pure solids | Ice | Subzero | Cheap, abundant, simple, and high TRL, non-toxic, higher heat capacity | |
| Pure liquids | Water | Medium | Cheap, abundant, non-toxic, higher heat capacity, high TRL | For narrow temperature applications, volumetric heat storage density is low |
| Molten salt | High | Commercially available, suitable for high temperature applications up to 600 °C | Corrosion, high cost, higher environmental impacts comparing with natural solids | |
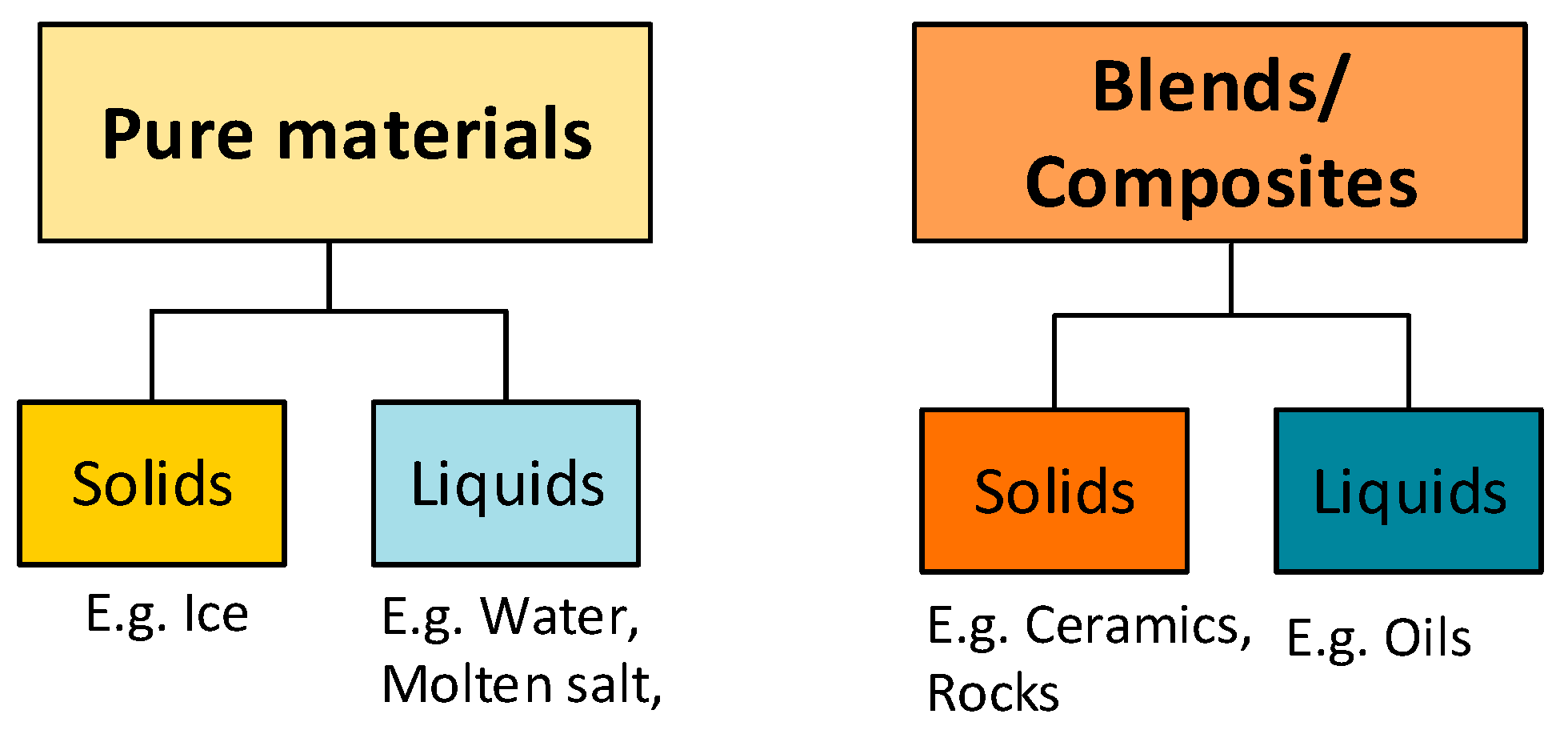
| Blend/composite solids | Ceramics | High | Thermally stable up to 1200 °C, suitable for high temperature applications, cheap | Relative inhomogeneity between different types if come as waste/by-products, brittle |
| Rocks | Medium and high | Good thermal and mechanical stability, suitable for high temperature applications up to 1000 °C, high density, cheap, no corrosive effect | Low heat capacity, depletion of natural sources, low thermal conductivity | |
| Wates | High | Can be derived from waste/inertized materials (such as slags, asbestos and demolition wastes), stable up to 1000 °C (based on its origin), high heat capacity, | Need additional processes to obtain uniform STESM | |
| Liquid blends | Oils (e.g., silicon oil) | Medium and high | Suitable for medium temperature applications up to 400 °C, low freezing point | High cost, do not freeze in the system during the cold weather or nights |
| PCM Category | Typical Temperatures | Advantages | Disadvantages and Challenges | |
|---|---|---|---|---|
| Ice (or snow) | ~0 °C | Cheap, abundant, simple and high TRL, non-toxic, higher volumetric heat storage density | ||
| Inorganics | Salt hydrates | Low to relatively high | Cheap, abundant, some non-toxic, quite high volumetric heat storage density and thermal conductivity | Non-renewable, poor cycling stability if chosen from incongruent compositions, high degree of supercooling, corrosive to metals, some can be toxic |
| Metals and their alloys | Relatively high to high | High volumetric heat storage density and very high thermal conductivity | Expensive, competition against other metal applications, non-renewable | |
| Salt blends | Relatively high to high | Abundant, some non-toxic, quite high volumetric heat storage density and thermal conductivity | Non-renewable, poor cycling stability if chosen from incongruent compositions, corrosive to metals, some can be toxic | |
| Organics | Alkanes | Subzero and medium | Some non-toxic, relatively high TRL and lower cost | Lower volumetric heat storage density and thermal conductivity, some can be toxic, flammability, non-renewable, corrosion of plastics |
| Fatty acids | Medium and relatively high | Bio-based, from renewable sources, broad range of melting temperatures | Corrosive, less chemically inert, lower volumetric enthalpies compared with salt hydrates, sometimes polymorphism | |
| Polyols | Medium and relatively high | Moderate to high volumetric heat storage densities, bio-based and renewable, non-corrosive, broad range of phase change temperatures, often non-toxic (many even food-grade) | Can be prone to glass transition, polymorphism, metastability, thermally activated change, high degree of supercooling, high costs at high purity (due to niche markets for large-scale production) | |
| Esters | Medium and relatively high | Non corrosive, chemically stable, bio-based, from renewable sources, broad range of melting temperatures | Lack of commercially available pure materials (due to lack of applications), lack of data, lower volumetric enthalpies compared with salt hydrates, some polymorphism | |
| Clathrates | Low | Rather abundant | Non-renewable, corrosive to metals, lower TRL | |
| TCM Mechanism/Material | Typical Temperatures | Advantages | Disadvantages and Challenges | |
|---|---|---|---|---|
| Adsorption | Zeolites | Medium | Good energy storage density, cost, good hydrothermal cycle stability | High desorption temperature, low thermal conductivity |
| Silica gels | Low up to 90 °C | Low desorption temperature, cost | Low energy storage density, low thermal conductivity | |
| Aluminophosphates | Low, 60–90 °C | High energy storage density, low desorption temperature, excellent hydrothermal cycle stability | Low thermal conductivity, cost | |
| Metal organic frameworks (MOFs) | Low up to 90 °C | High energy storage density, low desorption temperature, hydrothermal cycle stability | Low thermal conductivity, cost | |
| Chemical reactions | Salt hydrates | Low to medium | Moderate energy storage density, medium costs, reasonable cycle stability | Low thermal conductivity, corrosion |
| Halide ammines | Medium | High energy storage density, good cycle stability | Costs, reversible mass transport only if on matrix support | |
| Metal carbonates | Medium to high | Low costs, tunability via CO2 pressure | Poor cycle stability, humidity required | |
| Redox reactions | High | High temperature application, tunability via aerobic/anaerobic conditions | High costs | |
| Metal hydrides | High | High energy storage density | Corrosion (of metals) | |
| Absorption | Liquid salt solutions | Low | High TRL, relatively inexpensive chiller solutions | Restricted to cooling applications |
| Adsorption + Chemical reaction + Absorption | Composites of porous matrix and salts/oxides | Low to Medium | High energy storage density, cost, good cycling stability | Low thermal conductivity, corrosion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunasekara, S.N.; Barreneche, C.; Inés Fernández, A.; Calderón, A.; Ravotti, R.; Ristić, A.; Weinberger, P.; Ömur Paksoy, H.; Koçak, B.; Rathgeber, C.; et al. Thermal Energy Storage Materials (TESMs)—What Does It Take to Make Them Fly? Crystals 2021, 11, 1276. https://doi.org/10.3390/cryst11111276
Gunasekara SN, Barreneche C, Inés Fernández A, Calderón A, Ravotti R, Ristić A, Weinberger P, Ömur Paksoy H, Koçak B, Rathgeber C, et al. Thermal Energy Storage Materials (TESMs)—What Does It Take to Make Them Fly? Crystals. 2021; 11(11):1276. https://doi.org/10.3390/cryst11111276
Chicago/Turabian StyleGunasekara, Saman Nimali, Camila Barreneche, A. Inés Fernández, Alejandro Calderón, Rebecca Ravotti, Alenka Ristić, Peter Weinberger, Halime Ömur Paksoy, Burcu Koçak, Christoph Rathgeber, and et al. 2021. "Thermal Energy Storage Materials (TESMs)—What Does It Take to Make Them Fly?" Crystals 11, no. 11: 1276. https://doi.org/10.3390/cryst11111276
APA StyleGunasekara, S. N., Barreneche, C., Inés Fernández, A., Calderón, A., Ravotti, R., Ristić, A., Weinberger, P., Ömur Paksoy, H., Koçak, B., Rathgeber, C., Ningwei Chiu, J., & Stamatiou, A. (2021). Thermal Energy Storage Materials (TESMs)—What Does It Take to Make Them Fly? Crystals, 11(11), 1276. https://doi.org/10.3390/cryst11111276











