Evaluation of ZIF-8 and ZIF-90 as Heat Storage Materials by Using Water, Methanol and Ethanol as Working Fluids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZIF-8
2.3. Synthesis of ZIF-90
2.4. Characterisation
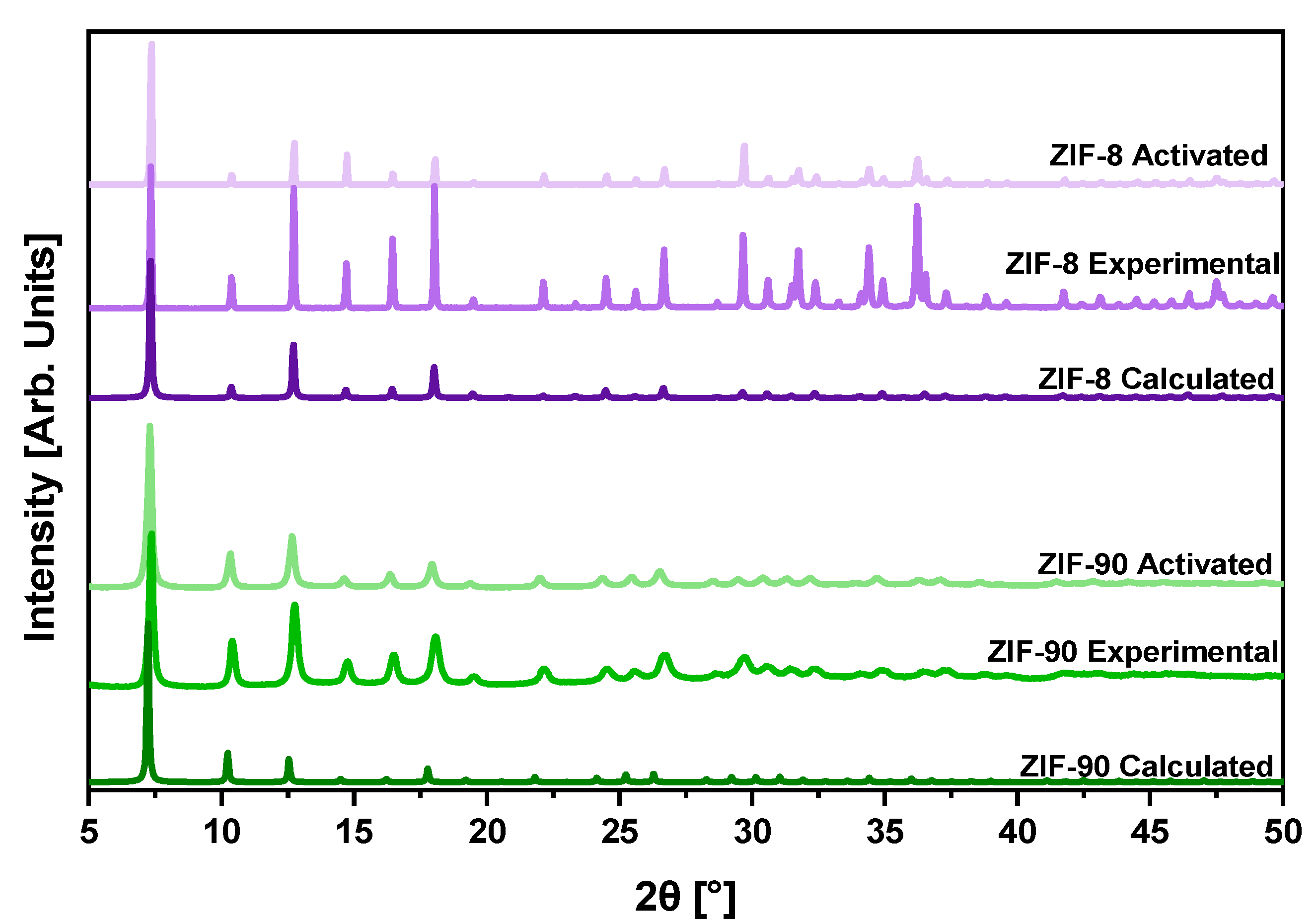
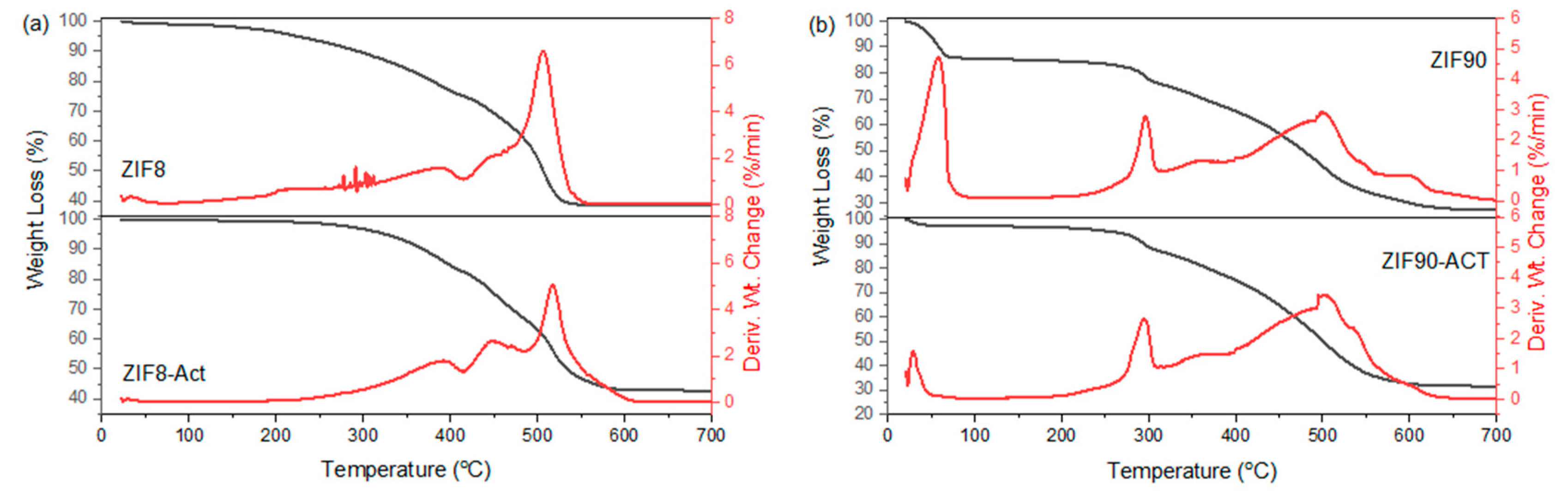
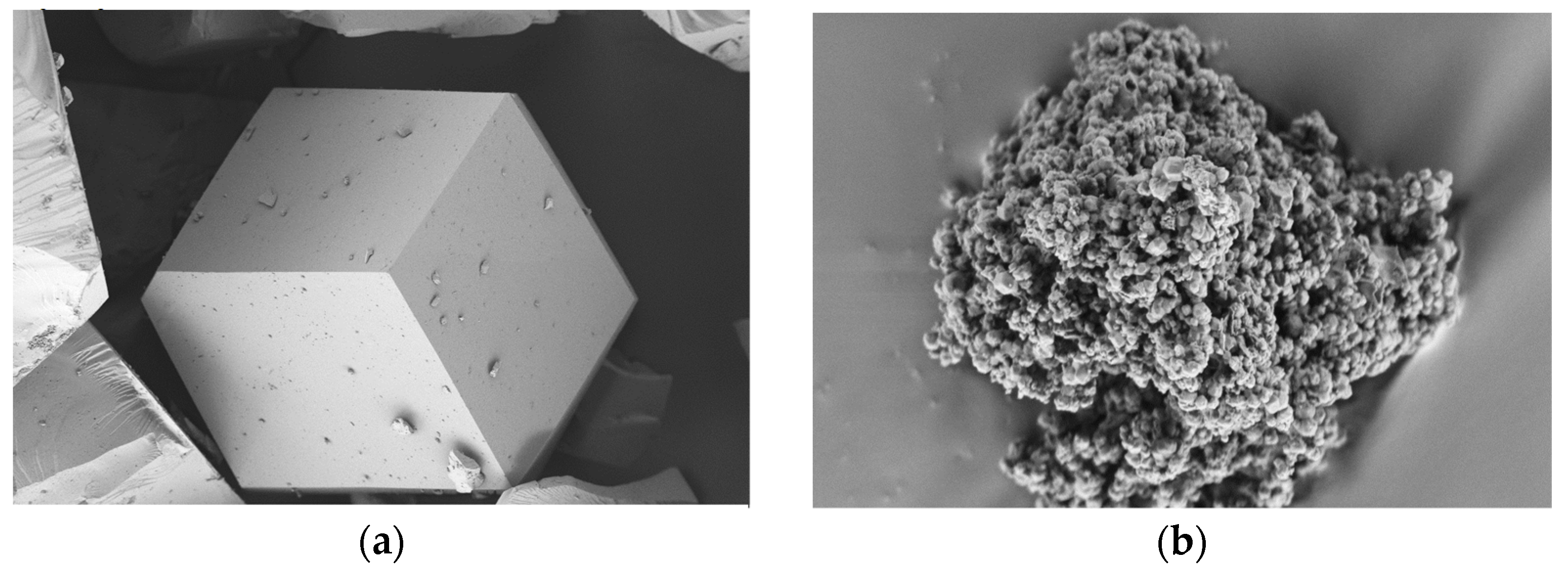
2.4.1. Structural Analysis
2.4.2. Adsorption and Desorption Enthalpy Studies
3. Results
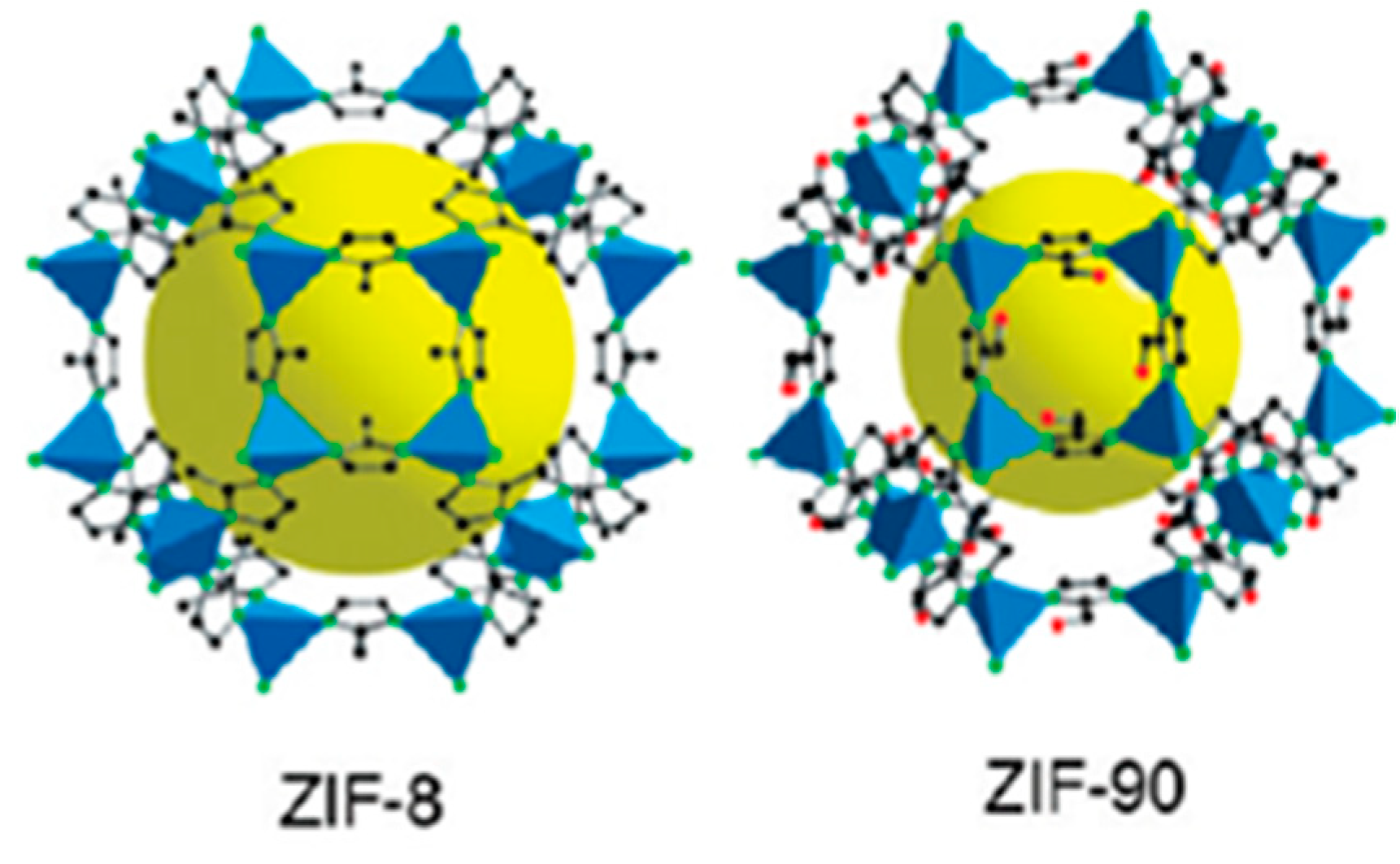
3.1. Structural and Physicochemical Properties of ZIFs
3.2. MeOH, EtOH and H2O Adsorption and DSC Analysis of ZIFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ristić, A.; Logar, N.Z. New composite water sorbents CaCl2-PHTS for low-temperature sorption heat storage: Determination of structural properties. Nanomaterials 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Henninger, S.K.; Ernst, S.J.; Gordeeva, L.; Bendix, P.; Fröhlich, D.; Grekova, A.D.; Bonaccorsi, L.; Aristov, Y.; Jaenchen, J. New materials for adsorption heat transformation and storage. Renew. Energy 2017, 110, 59–68. [Google Scholar] [CrossRef]
- Krajnc, A.; Varlec, J.; Mazaj, M.; Ristić, A.; Logar, N.Z.; Mali, G. Superior Performance of Microporous Aluminophosphate with LTA Topology in Solar-Energy Storage and Heat Reallocation. Adv. Energy Mater. 2017, 7, 1601815. [Google Scholar] [CrossRef]
- Ban, Y.; Peng, Y.; Zhang, Y.; Jin, H.; Jiao, W.; Guo, A.; Wang, P.; Li, Y.; Yang, W. Dual-ligand zeolitic imidazolate framework crystals and oriented films derived from metastable mono-ligand ZIF-108. Microporous Mesoporous Mater. 2016, 219, 190–198. [Google Scholar] [CrossRef]
- Brown, A.J.; Johnson, J.R.; Lydon, M.E.; Koros, W.J.; Jones, C.W.; Nair, S. Continuous polycrystalline zeolitic imidazolate framework-90 membranes on polymeric hollow fibers. Angew. Chemie Int. Ed. 2012, 51, 10615–10618. [Google Scholar] [CrossRef]
- De Lange, M.F.; Van Velzen, B.L.; Ottevanger, C.P.; Verouden, K.J.F.M.; Lin, L.C.; Vlugt, T.J.H.; Gascon, J.; Kapteijn, F. Metal-Organic Frameworks in Adsorption-Driven Heat Pumps: The Potential of Alcohols as Working Fluids. Langmuir 2015, 31, 12783–12796. [Google Scholar] [CrossRef]
- Lalonde, M.B.; Mondloch, J.E.; Deria, P.; Sarjeant, A.A.; Al-Juaid, S.S.; Osman, O.I.; Farha, O.K.; Hupp, J.T. Selective Solvent-Assisted Linker Exchange (SALE) in a Series of Zeolitic Imidazolate Frameworks. Inorg. Chem. 2015, 54, 7142–7144. [Google Scholar] [CrossRef]
- Gao, M.; Wang, J.; Rong, Z.; Shi, Q.; Dong, J. A combined experimental-computational investigation on water adsorption in various ZIFs with the SOD and RHO topologies. RSC Adv. 2018, 8, 39627–39634. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Xia, X.; Li, S. Screening of Covalent-Organic Frameworks for Adsorption Heat Pumps. ACS Appl. Mater. Interfaces 2020, 12, 3265–3273. [Google Scholar] [CrossRef]
- Hunter-Sellars, E.; Saenz-Cavazos, P.A.; Houghton, A.R.; McIntyre, S.R.; Parkin, I.P.; Williams, D.R. Sol–Gel Synthesis of High-Density Zeolitic Imidazolate Framework Monoliths via Ligand Assisted Methods: Exceptional Porosity, Hydrophobicity, and Applications in Vapor Adsorption. Adv. Funct. Mater. 2021, 31, 2008357–2008367. [Google Scholar] [CrossRef]
- Gee, J.A.; Chung, J.; Nair, S.; Sholl, D.S. Adsorption and diffusion of small alcohols in zeolitic imidazolate frameworks ZIF-8 and ZIF-90. J. Phys. Chem. C 2013, 117, 3169–3176. [Google Scholar] [CrossRef]
- Gao, C.; Shi, Q.; Dong, J. Adsorptive separation performance of 1-butanol onto typical hydrophobic zeolitic imidazolate frameworks (ZIFs). CrystEngComm 2016, 18, 3842–3849. [Google Scholar] [CrossRef]
- Eum, K.; Jayachandrababu, K.C.; Rashidi, F.; Zhang, K.; Leisen, J.; Graham, S.; Lively, R.P.; Chance, R.R.; Sholl, D.S.; Jones, C.W.; et al. Highly tunable molecular sieving and adsorption properties of mixed-linker zeolitic imidazolate frameworks. J. Am. Chem. Soc. 2015, 137, 4191–4197. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Huang, J.; Li, W.; Peng, H.; Li, S. Impacts of Ethanol and Water Adsorptions on Thermal Conductivity of ZIF-8. J. Phys. Chem. C 2019, 123, 27369–27374. [Google Scholar] [CrossRef]
- Tang, Y.; Dubbeldam, D.; Guo, X.; Rothenberg, G.; Tanase, S. Efficient Separation of Ethanol-Methanol and Ethanol-Water Mixtures Using ZIF-8 Supported on a Hierarchical Porous Mixed-Oxide Substrate. ACS Appl. Mater. Interfaces 2019, 11, 21126–21136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Lively, R.P.; Dose, M.E.; Brown, A.J.; Zhang, C.; Chung, J.; Nair, S.; Koros, W.J.; Chance, R.R. Alcohol and water adsorption in zeolitic imidazolate frameworks. Chem. Commun. 2013, 49, 3245–3247. [Google Scholar] [CrossRef]
- Du, Y.; Mao, K.; Wooler, B.; Sharma, A.K.; Colmyer, D.; Nines, M.; Weston, S.C. Insights into the Flexibility of ZIF-7 and Its Structural Impact in Alcohol Adsorption. J. Phys. Chem. C 2017, 121, 28090–28095. [Google Scholar] [CrossRef]
- Virdis, T.; Danilov, V.; Baron, G.V.; Denayer, J.F.M. Nonideality in the Adsorption of Ethanol/Ethyl Acetate/Water Mixtures on ZIF-8 Metal Organic Framework. Ind. Eng. Chem. Res. 2018, 57, 7040–7047. [Google Scholar] [CrossRef]
- Yin, H.; Lau, C.Y.; Rozowski, M.; Howard, C.; Xu, Y.; Lai, T.; Dose, M.E.; Lively, R.P.; Lind, M.L. Free-standing ZIF-71/PDMS nanocomposite membranes for the recovery of ethanol and 1-butanol from water through pervaporation. J. Memb. Sci. 2017, 529, 286–292. [Google Scholar] [CrossRef]
- Xia, X.; Liu, Z.; Li, S. Adsorption characteristics and cooling/heating performance of COF-5. Appl. Therm. Eng. 2020, 176, 115442–115450. [Google Scholar] [CrossRef]
- Bingel, L.W.; Chen, A.; Agrawal, M.; Sholl, D.S. Experimentally Verified Alcohol Adsorption Isotherms in Nanoporous Materials from Literature Meta-Analysis. J. Chem. Eng. Data 2020, 65, 4970–4979. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-romo, F.J.; Knobler, C.B.; Keeffe, M.O.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, J.; Chen, X. [Zn(bim)2] · (H2O)1.67: A metal-organic open-framework with sodalite topology. Chin. Sci. Bull. 2003, 48, 1531–1534. [Google Scholar] [CrossRef]
- Morris, W.; Doonan, C.J.; Furukawa, H.; Banerjee, R.; Yaghi, O.M. Crystals as molecules: Postsynthesis covalent functionalization of zeolitic imidazolate frameworks. J. Am. Chem. Soc. 2008, 130, 12626–12627. [Google Scholar] [CrossRef] [PubMed]
- Škrjanc, A.; Byrne, C.; Zabukovec Logar, N. Green Solvents as an Alternative to DMF in ZIF-90 Synthesis. Molecules 2021, 26, 1573. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Ghahramaninezhad, M.; Mohajer, F.; Niknam Shahrak, M. Improved CO2 capture performances of ZIF-90 through sequential reduction and lithiation reactions to form a hard/hard structure. Front. Chem. Sci. Eng. 2020, 14, 425–435. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, S.; Weidman, J.; Guo, R. Surface modification of ZIF-90 with triptycene for enhanced interfacial interaction in mixed-matrix membranes for gas separation. J. Polym. Sci. 2020, 58, 2675–2687. [Google Scholar] [CrossRef]
- Shieh, F.K.; Wang, S.C.; Leo, S.Y.; Wu, K.C.W. Water-based synthesis of zeolitic imidazolate framework-90 (ZIF-90) with a controllable particle size. Chem. A Eur. J. 2013, 19, 11139–11142. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, C.; Li, F.; Yan, X.; Xi, H. Green and rapid synthesis of hierarchical porous zeolitic imidazolate frameworks for enhanced CO2 capture. Inorganica Chim. Acta 2018, 482, 358–363. [Google Scholar] [CrossRef]
- Thompson, J.A.; Chapman, K.W.; Koros, W.J.; Jones, C.W.; Nair, S. Sonication-induced Ostwald ripening of ZIF-8 nanoparticles and formation of ZIF-8/polymer composite membranes. Microporous Mesoporous Mater. 2012, 158, 292–299. [Google Scholar] [CrossRef]
- Nordin, N.A.H.M.; Ismail, A.F.; Mustafa, A.; Murali, R.S.; Matsuura, T. The impact of ZIF-8 particle size and heat treatment on CO2/CH4 separation using asymmetric mixed matrix membrane. RSC Adv. 2014, 4, 52530–52541. [Google Scholar] [CrossRef]

| ZIF | dag [Å] | dph [Å] | Topology | Ref. |
|---|---|---|---|---|
| ZIF-8 | 3.5 | 11.6 | SOD | [22,23] |
| ZIF-90 | 3.5 | 11.2 | SOD | [22,24] |
| ZIF | Crystalline Size (nm) |
|---|---|
| ZIF-8 | 400 |
| ZIF-90 | 20 |
| ZIF | SBET (m2/g) | Vtotal (cm3/g) |
|---|---|---|
| ZIF-8 | 621 | 0.252 |
| ZIF-90 | 1119 | 0.571 |
| ZIF | Solvent | 1 Day | 3 Days | 5 Days |
|---|---|---|---|---|
| ZIF-8 | MeOH EtOH H2O | 0.9% 9.4% 1.7% | 2.6% 12.4% 1.7% | 4.9% 12.4% 1.2% |
| ZIF-90 | MeOH EtOH H2O | 18.9% 20.4% 22.5% | 13.3% 15.8% 10.1% | 14.2% 13.4% 11.9% |
| ZIF | Solvent | 1 Day | 3 Days | 5 Days |
|---|---|---|---|---|
| ZIF-8 | MeOH EtOH H2O | 158.3 117.5 61.9 | 154.3 201.8 52.8 | 199.0 233.3 53.2 |
| ZIF-90 | MeOH EtOH H2O | 291.7 291.6 544.3 | 258.8 268.4 304.0 | 194.0 224.0 255.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrne, C.; Ristić, A.; Mal, S.; Opresnik, M.; Zabukovec Logar, N. Evaluation of ZIF-8 and ZIF-90 as Heat Storage Materials by Using Water, Methanol and Ethanol as Working Fluids. Crystals 2021, 11, 1422. https://doi.org/10.3390/cryst11111422
Byrne C, Ristić A, Mal S, Opresnik M, Zabukovec Logar N. Evaluation of ZIF-8 and ZIF-90 as Heat Storage Materials by Using Water, Methanol and Ethanol as Working Fluids. Crystals. 2021; 11(11):1422. https://doi.org/10.3390/cryst11111422
Chicago/Turabian StyleByrne, Ciara, Alenka Ristić, Suzana Mal, Mojca Opresnik, and Nataša Zabukovec Logar. 2021. "Evaluation of ZIF-8 and ZIF-90 as Heat Storage Materials by Using Water, Methanol and Ethanol as Working Fluids" Crystals 11, no. 11: 1422. https://doi.org/10.3390/cryst11111422
APA StyleByrne, C., Ristić, A., Mal, S., Opresnik, M., & Zabukovec Logar, N. (2021). Evaluation of ZIF-8 and ZIF-90 as Heat Storage Materials by Using Water, Methanol and Ethanol as Working Fluids. Crystals, 11(11), 1422. https://doi.org/10.3390/cryst11111422







