Abstract
Two new nonmetal cation tetrafluoroborate phases [H3tren](BF4)3 (I) and [H3tren](BF4)3 HF (II) were synthesized by microwave-assisted solvothermal and characterized by single crystal X-ray diffraction, IR spectroscopy and thermal analysis DTA-TGA. [H3tren](BF4)3 is cubic (P213) with unit cell parameter a = 11.688(1) Å. [H3tren](BF4)3•HF is trigonal (R3c) with unit cell parameters a = 15.297(6) Å and c = 12.007(2) Å. Both (I) and (II) structures can be described from isolated tetrafluoroborate BF4- anions, triprotonated tris-(2-aminoethyl)amine (tren) [H3tren]3+. Phase (II) contains disordered BF4- tetrahedron and hydrofluoric acid.
1. Introduction
Hybrid materials are chemical compounds consisting of the association of organic moiety and inorganic portion. The inorganic part generally comprises of metal or nonmetal cations surrounded by electron donor atoms such as nitrogen, oxygen, fluorine, etc. Organic molecules are highly diverse and may contain functions such as carboxylates, phosphonates and amines. The idea behind this type of material is to couple specific properties of the various components within a single entity. The assembly of the properties of both components can be established in two ways: first, the entity simply presents the final properties of the two components regarding simple apposition, or second, the combination of two properties induces a strong synergistic interaction, allowing the creation of a new property resulting from the association of specific physical properties, such as optical, mechanical, magnetic and/or electrical properties.
Hybrid materials can be classified according to the dimensionality of the network. The dimensionality of the network can be 0D formed by isolated polyanions [1], one dimensional (1D) chains [2], two-dimensional (2D) formed by layers [3,4], and three-dimensional (3D) [5]. The cohesion between organic and inorganic moieties are provided by weak forces (hydrogen bonding and Van der Waals forces) or strong forces (covalent bonds).
Hybrid materials have applications in various domains such as gas molecule storage: CO2 for its conversion ability [6], H2 for fuel cells that provide a solution to the energy crisis [7], catalysis [8], and petroleum cracking [9]. Other applications related to the physical properties of these materials are possible in the field of Non-Linear Optical (NLO) [10,11], luminescence [12] or ferroelectricity [13].
Nonmetal cation oxoborates or tetrafluoroborates can be considered as a branch of hybrid solid in which the inorganic moiety does not contain metals. Nonmetal cation borates have received considerable attention due to their structural architecture which make them candidates to interesting applications in many domains such a: catalysis [14,15], ion exchange [16,17], Non-Linear Optical (NLO) [18] etc. Recently, H.-X. Liu et al. [19] reported nonmetal borates [C6H13N2][B5O6(OH)4] with interesting NLO properties. This compound presents a Second Harmonic Generation (SHG) efficiency nearly 0.9 times that of standard potassium dehydrogenates phosphate (KDP). V. Gieu et al. [20] and J. Ramajouti et al. [21] reported examples of tetrafluoroborates showing motivating NLO properties. In addition to their optical properties, nonmetal cation tetrafluoroborates are largely used as friendly solvent ionic liquids [22,23,24]. These ionic liquids are widely used for applications in separation, selective adsorption performed under humid conditions or catalysis in water. Great efforts are given to the synthesis and the crystal structure study of these materials since their physical properties are strongly dependent to their structures.
In this paper, we describe the solvothermal synthesis, single crystal X-ray measurements, thermal behaviour and IR spectroscopy of two nonmetal cation tetrafluoroborates [H3tren](BF4)3 (I) and [H3tren](BF4)3 HF (II).
2. Materials and Methods
2.1. Synthesis of [H3tren](BF4)3 (I) and [H3tren](BF4)3•HF (II)
[H3tren](BF4)3 (I) and [H3tren](BF4)3 HF (II) were synthesized by the hydrothermal synthesis method using microwave heating (oven MDS2100). Mixture of 0.5g (8.1 mmol) of boric acid B(OH)3, 1.3 mL (8.1 mmol) of tris-(2-aminoethyl)amine (tren) (95%) were dissolved in 10 mL of ethanol and introduced in Teflon vessel. Hydrofluoric acid HF (40%, Riedel-de Haën) was then added: 0.71 mL for phase (I) and 3.55 mL for phase (II). The mixture is heated at 190 °C for 1 h. Crystals of (I) and (II) can be obtained with high crystallinity using Teflon lined PARR autoclaves at 190 °C for 48 h.
2.2. Single-Crystal Data Collection and Structure Determination
Single crystal X-ray diffraction data were obtained on a SIEMENS AED2 four-circle diffractometer at room temperature using ω-2θ scans. The structure determinations were carried out with SHELXS-97 [25], SHELXL-97 [26] programs within the WINGX package [27]. Positions and anisotropic displacement parameters for all non-hydrogen atoms are freely refined without any constraints. Hydrogen atoms bound to carbon and nitrogen atoms are fixed geometrically using AFIX23 and AFIX137 for CH2 and CH3 groups, respectively. Experimental powder X-ray diffraction patterns of the two compounds are in good agreement with those calculated using full pattern matching mode simulations (Figure S2 for (I) and S5 for (II) in the ESI).
2.2.1. Structure Determination of [H3tren](BF4)3 (I)
The extinction conditions have led to the P213 non-centrosymetric space group. The structure of [H3tren](BF4)3 was solved by the TREF option in SHELXS-97 program. Three boron and two fluorine atoms were first located at 4a crystallographic sites. The remaining non-hydrogen atoms: fluorine, nitrogen and carbon were localized from Fourier difference map and were distinguished from distance criteria [28]. Three primary amines of tren molecule are found to be protonated unlike tertiary amine. Final refinements of atomic positions, anisotropic displacement parameters (ADP) and secondary extinction converged to R = 0.062 and Rw = 0.190 (1241 independent reflections and 79 parameters).
2.2.2. Structure Determination of [H3tren](BF4)3 HF (II)
All attempts to solve the structure in the centrosymmetric space group (R-3c) have been unsuccessful. The agreement factors are high, and no acceptable structural model has been proposed. This prompted us to determine the structure in the non-centrosymmetric space groupR3c. TREF option in SHELXS-97 program was used to get the preliminary solution. The five atoms found were attributed arbitrarily to carbon atoms. The visualization of the arrangement of these atoms, using the Cameron graphic tool included in the Wingx package, helps to distinguish between carbon, nitrogen and fluorine atoms. At this step, all the atoms were located in general position except N(1) in special crystallographic site 6a. The remaining fluorine, boron and carbon atoms were localized from Fourier difference maps and were distinguished from distance criteria. Unbounded fluorine atoms are attributed to hydrofluoric acid. The hydrogen atom is easily located using the difference Fourier map.
The atomic displacement parameters of the fluorine atoms F(2) and F(4) were found to be abnormally high. These two fluorine atoms are disordered, and hence each atom is equitably positioned in two general positions with an occupation factor equal to 50%. Similarly to phase (I), the three primary amines of tren molecule are found to be protonated, unlike tertiary amine. The refinement of all atomic positions and anisotropic atomic displacement parameters (ADP) for all atoms except hydrogen atoms leads to confidence factors R1 = 0.07 and Rw = 0.21 (825 independent reflections and 99 parameters).
The conditions of data collection for both phases are shown in Table 1.

Table 1.
Crystallographic data of [H3tren](BF4)3 (I) and [H3tren](BF4)3 HF (II).
3. Results
3.1. Structure Description of [H3tren](BF4)3 (I)
The asymmetric unit of [H3tren](BF4)3, drawn using the ORTEP program [29], contains three independent boron atoms, six fluorine anion, two nitrogen atoms, and two carbon atoms. Ellipsoids are given with a probability of 50% and show an abnormally high thermal agitation for some fluorine atoms (Figure S1, left in the Supplementary).
The structure of [H3tren](BF4)3 can be described as a distorted BF4 tetrahedron and [H3tren]3+ triprotonated amine (Figure 1). In each BF4- tetrahedron, the boron atom is linked to three equivalent fluorine atoms and one other fluorine atom. The average of the distances B(1)-F and B(2)-F are strictly identical and are equal to 1.37 Å, while the mean B(3)-F distance is 1.34 Å. In each tetrahedron, two F-B-F angles are observed, ranging from 107 to 111° with an average of 109.5°. These two angles are close to being the same for the B(2)F4 tetrahedron. The values of F-B-F angles and B-F distances in phase (I) are comparable to those found in other nonmetal cation tetrafluoroborates encountered in the literature. The N-C distances are slightly smaller than the C-C distances in the organic part. These interatomic distances and angles are habitually observed in hybrid compounds.
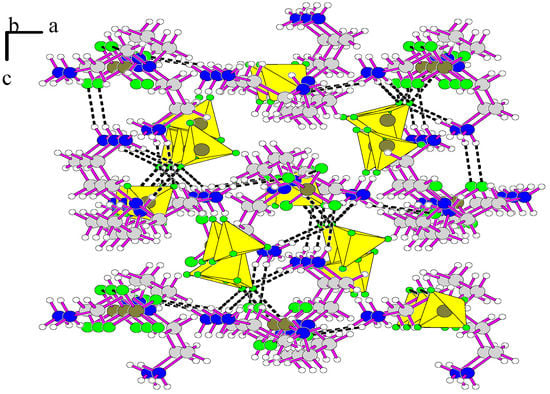
Figure 1.
View of the structure of [H3tren](BF4)3.
Strong hydrogen bonds are established between fluorine atoms of B(1)F4 and B(2)F4 with hydrogen atoms of terminal nitrogen atoms of the organic molecule and form 3 dimensional network. One can note that B(3)F4 is free, and that no hydrogen bonds exist between fluorine atoms and hydrogen atoms of terminal amine groups.
The terminal N(2)H3 groups established hydrogen bonds (dN-F < 2.94 Å) with fluoride ligands, whereas central nitrogen Nc atoms are distant from fluoride anions (d > 3.03 Å). The three equivalent fluorine atoms F(4) of the B(2)F4 tetrahedron link three amine groups [H3tren]3+. Fluorine ion F(1) of the B(1)F4 tetrahedron established three hydrogen bonds with three hydrogen atoms of the three terminal atoms of the same triprotonated amine molecule (Figure 2). The [H3tren]3+ cation adopts a “spider”-shaped configuration. It should be considered that tren amines cannot be tetra protonated, as proved from the values of the Nc-Nt distances from central nitrogen atoms Nc to terminal nitrogen atoms Nt [30,31,32], and Nt-Nt distances. Only the scorpion configuration corresponds to a tetra protonated amine (Figure 3).
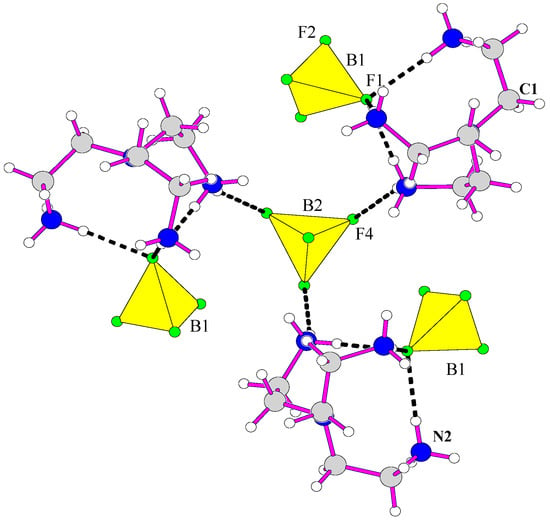
Figure 2.
Environment of the BF4 tetrahedron and [H3tren] cation in the structure of [H3tren](BF4)3.
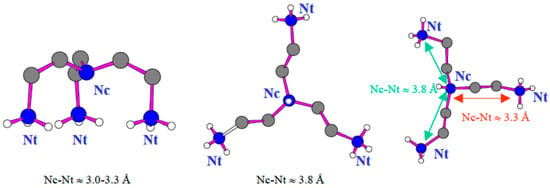
Figure 3.
Configuration spider (left), plane (middle) and scorpion (right) of the tren amine.
3.2. Structure Description of [H3tren](BF4)3 HF (II)
The projection of the structure along the c direction is given in Figure 4. The asymmetric unit of the structure of [H3tren](BF4)3 HF is composed of ethylene diamine groups and distorted BF4-tetrahedron anions. One can see that the disordered fluorine atoms have high atomic displacement parameters. Atomic displacement parameters of terminal nitrogen atoms are almost greater than tertiary amine group (Figure S1, right in the Supplementary).
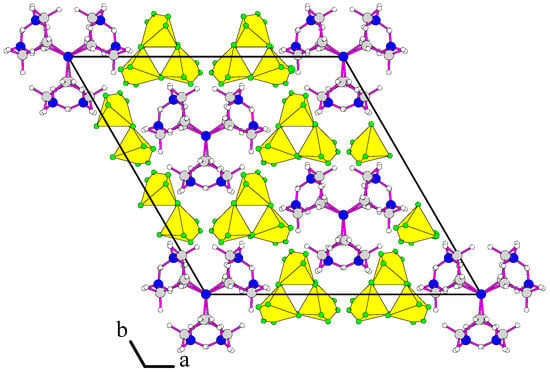
Figure 4.
Projection of the structure of [H3tren](BF4)3 HF along the [001] direction.
In the structure of [H3tren](BF4)3 HF, the environment of boron is disordered. It is composed of four fluorine atoms; among them, two occupy a general position with a 50% occupancy factor. The distances B-F vary between 1.29 and 1.44 Å, with an average of 1.36 Å. Distances between disordered fluorine atoms are 0.76(3) and 0.77(2) for F(2)-F(2)p and F(4)-F(4)p, respectively. Distances between two non-equivalent fluorine atoms vary between 2.17 and 2.3 Å.
Hydrogen bonds are established between fluorine atoms of hydrofluoric acid and three hydrogen atoms, each one belongs to one terminal NH3 groups of the one organic molecules (Figure 5). In addition, each of the organic molecules established via NH3 groups hydrogen bonds with F(2), F(3) and F. A total of 15 hydrogen bonds are observed between organic molecules and inorganic part.
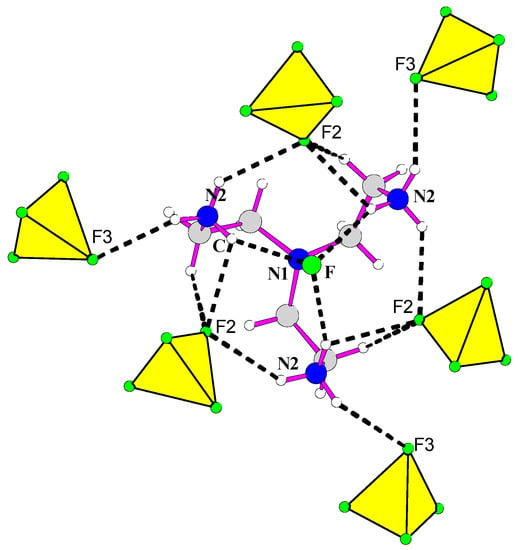
Figure 5.
Hydrogen bonds in [H3tren](BF4)3 HF.
Concerning the organic moiety, the amine is triproptonated [H3tren]3+ and adopts a “spider” type configuration. Indeed, the values of the Nc-Nt distances from central nitrogen atoms Nc to terminal nitrogen atoms Nt (3.147 Å), as well as Nt-Nt distances (4.67 Å), agree with triprotonated amine. The averages of the N-C distances (1.496 Å) and the C-C distances (1.517 Å) are similar to those encountered in other hybrid compounds.
3.3. Thermal Analysis
Thermal behavior DTA-TGA was studied using a TA 2960 instrument within a temperature range 25–650 °C with heating rate 10 °C/min in an argon atmosphere (Figures S3 and S6 in the Supplementary). Upon heating, [H3tren]·(BF4)3 exhibited a single weight loss which took place in the interval 200–450 °C, and was attributed to the loss of almost the totality of the sample. This weight loss corresponds to the decomposition of the organic molecules and the departure of BF3. Less than 10% of the residual sample was attributed to black carbon. For [H3tren](BF4)3 HF (II), two weight losses were observed in two steps; the first step was observed in the interval 100–250 °C, and was assigned to the loss of one mole of HF molecules per one mole of (II) (exp./th. = 4.83/4.87) for (II). The second weight loss, which occurred in the interval 250–425 °C, corresponded to the decomposition of the organic molecules and the departure of BF3.
3.4. IR Spectroscopy
Infrared spectroscopy is effectively used to approve the structure determined by X-ray diffraction and to identify functional groups in the obtained material. To qualitatively analyze the main functional groups in these compounds, the infrared spectrum was acquired using a Bomem Michelson MB120 Fourier transform infrared spectrometer with a diamond-anvil cell as a micro sampling device. Infrared data were collected in the range 650–4000 cm−1. Spectral resolution was 4 cm−1.
The infrared spectrum of compounds (I), (II) and tris-(2-aminoethyl)amine are given in the Electronic Supplementary Information ESI (Figures S4, S7 and S8). Infrared spectra of (I) and (II) are quite similar. Absorption peaks at higher wave numbers (3300–3150 cm−1) for (I) and (II) are assigned to N-H symmetric and antisymmetric stretching vibrations of NH3 groups. A clear shift to higher wavenumbers is observed compared to the starting reactant tris-(2-aminoethyl)amine. This shift is attributed to the presence of strong hydrogen bonding N-H.F in (I) and (II). Moreover, hydrogen bonds are stronger in (II) compared to (I), in direct correlation with N-F distances (Tables S3 and S6 in the Supplementary). This may explain the appearance of ν (NH3) at higher wavenumber in (II). Bands observed in the range 2850–2930 cm−1 are assigned to symmetric and antisymmetric stretching of methylene groups. For example, in compound (I), methylene scissoring, wagging, twisting and rocking vibration bands appear, respectively, around 1469, 1323, 1298 and 724 cm−1. C-N and C-C aliphatic groups stretching bands are observed in the range 1220–1020 cm−1 [33]. These results are in good agreement with the literature [34,35].
Tetrafluoroborate anion BF4− is characterized by both symmetric and asymmetric stretching and deformation vibrations. For ideal BF4 tetrahedron, symmetric modes ν1 (symmetric stretching) = 777 cm−1 and ν2 (symmetric deformation) = 360 cm−1 are Raman active whereas ν3 (asymmetric stretching) = 1070 cm−1 and ν4 (asymmetric deformation) = 533 cm−1 are both Raman and IR active [36]. One can note that ν1 (symmetric stretching) appear in both IR spectra of (I) and (II) in spite that this mode is IR inactive for regular BF4 tetrahedron [37]. Broadening of peaks corresponding to BF4 tetrahedron in phase (II) compared to phase (I) may explain the disordered observed for two fluorine atoms belonging to boron atoms. An approximately full assignment of band vibrations is given in Tables S7 and S8 in the Supplementary.
For compound (II), no vibration bands corresponding to H2O are observed, this supports the results obtained by X-ray diffraction and agrees with the thermogravimetric analysis. On the other hand, the formula of (II) with hydrofluoric acid is in good agreement with synthesis condition in high HF ratio.
4. Conclusions
In the chemical system boric acid tris-(2-aminoethyl) amine (tren)-hydrofluoric acid, two new phases were successfully obtained, and their structures were determined by single-crystal X-ray diffraction method. These two obtained phases were characterized by means of various techniques, such as single-crystal and powder X-ray diffraction, IR absorption spectroscopy, and thermal analysis.
The cubic structure of [H3tren](BF4)3 (I) can be described as a distorted BF4 tetrahedron and organic cations [H3tren]3+. The cohesion between the two entities is provided by strong hydrogen bonds. In the structure of [H3tren](BF4)3 HF (II) the B3+ cations environment is disordered: two fluorine atoms of BF4 tetrahedron occupy two positions with occupancy factor of 50%. Compared with previous studies, the amine tren is triprotonated [H3tren]3+ and adopts a spider configuration.
Borate materials, including nonmetal tetrafluoroborates, are candidates in practical applications mainly as second harmonic generating (SHG) optical materials, host materials for fluorescence, piezoelectric materials, and so on. These applications are related to the diversity in the structural architecture of this class of materials.
Supplementary Materials
CSD 2009817 and 2009720 contain the supplementary crystallographic data for (I) and (II) compounds respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Author Contributions
N.O.A. and G.M.A.-E. conceived and designed the experiments; I.A., A.H.A. and A.B. carried out the X-ray diffraction analysis; all authors planned the characterization by IR, absorption spectroscopy. All authors took part in writing and discussion processes. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external.
Acknowledgments
The authors would like to express their gratitude to Vincent Maisonneuve (Le Mans University, France), for X-ray data collection. This work received support from Grant 2020-084-BASRC awarded by the Research Center, Scientific Research Deanship, Imam Abdulrahman Bin Faisal University, KSA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jayasundera, C.A.; Goff, R.J.; Li, J.; Lightfoot, P. Solvothermal indium fluoride chemistry: Syntheses and crystal structures of K5In3F14, β-(NH4)3InF6 and [NH4]3[C6H21N4]2[In4F21]. J. Solid State Chem. 2010, 183, 356. [Google Scholar] [CrossRef]
- Adil, A.; Leblanc, M.; Maisonneuve, V.; Lightfoot, P. Structural chemistry of organically-templated metal fluorides. Dalton Trans. 2010, 39, 5983. [Google Scholar] [CrossRef] [PubMed]
- Lhoste, J.; Rocquefelte, X.; Dessapt, R.; Jobic, S. A New Organic–Inorganic Hybrid Oxyfluorotitanate [Hgua]2·(Ti5O5F12) as a Transparent UV Filter. Inorg. Chem. 2011, 50, 5671. [Google Scholar] [CrossRef] [PubMed]
- Abdi, I.; Lhoste, J.; Leblanc, M.; Maisonneuve, V.; Grenèche, J.-M.; Viau, G.; Ben Ali, A. [H2amtaz]+ iron fluorides: Synthesis, crystal structures, magnetic and Mössbauer studies. J. Fluo. Chem. 2015, 173, 23. [Google Scholar] [CrossRef]
- Cadiau, A.; Martineau, C.; Taulelle, F.; Adil, K. Investigation of the composition space diagram of the ZnF2–3,5-diamino-1,2,4-triazole–HF–H2O chemical system and structural characterization of a new fluorinated guanazolate MOF [Zn3F2]·(Am2TAZ)4. J. Fluorine Chem. 2013, 150, 104. [Google Scholar] [CrossRef]
- Nijem, N.; Wu, H.H.; Canepa, P.; Marti, A. Tuning the Gate Opening Pressure of Metal–Organic Frameworks (MOFs) for the Selective Separation of Hydrocarbons. J. Am. Chem. Soc. 2012, 134, 15201. [Google Scholar] [CrossRef]
- Benaouadj, M.; Aboubou, A.; Ayad, M.Y.; Becherif, M. Nonlinear Flatness Control Applied to Supercapacitors Contribution in Hybrid Power Systems Using Photovoltaic Source and Batteries. Energy Procedia 2014, 50, 333. [Google Scholar] [CrossRef]
- Luz, I.; Corma, A.; Llabres, F.X. Cu-MOFs as active, selective and reusable catalysts for oxidative C–O bond coupling reactions by direct C–H activation of formamides, aldehydes and ethers. Catal. Sci. Technol. 2014, 4, 1829. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232. [Google Scholar] [CrossRef]
- Espa, D.; Pilia, L.; Makedonas, C.; Marchio, L.; Mercuri, M.L. Role of the Acceptor in Tuning the Properties of Metal [M(II) = Ni, Pd, Pt] Dithiolato/Dithione (Donor/Acceptor) Second-Order Nonlinear Chromophores: Combined Experimental and Theoretical Studies. Inorg. Chem. 2014, 53, 1170. [Google Scholar] [CrossRef]
- Zidan, D.; Al-Ktaifani, M.M.; L-Daher, M.S.E.; Allahham, A.; Ghanem, A. Diffraction ring patterns and nonlinear measurements of the Tris(2′,2-bipyridyl)iron(II) tetrafluoroborate. Optics Laser Tech. 2020, 131, 106449. [Google Scholar] [CrossRef]
- Cornu, L.; Gaudon, M.; Jubera, V. ZnAl2O4 as a potential sensor: Variation of luminescence with thermal history. J. Mater. Chem. C 2013, 34, 5419. [Google Scholar] [CrossRef]
- Pardo, E.; Train, C.; Liu, H.; Chamoreau, L.M. Multiferroics by Rational Design: Implementing Ferroelectricity in Molecule-Based Magnets. Angew. Chem. Int. Ed. Engl. 2012, 51, 8356. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Han, W.; Deng, J.; Zhu, W.; Zhang, Y.; Wang, H. Photocatalytic conversion of lignin into chemicals and fuels. ChemSusChem 2020, 13, 1. [Google Scholar]
- Iao, K.; Hagiwara, H.; Yokoyama, C. Acidic ionic liquid modified silica gel as novel solid catalysts for esterification and nitration reactions. J. Mol. Catal. A Chem. 2006, 246, 65. [Google Scholar]
- Wiscons, A.; Zeller, M.; Rowsell, J.L.C. Anion Exchange in Cationic Frameworks: Structures of Channel-Forming Triarylpyrylium Tetrafluoroborate Salts. Cryst. Growth Des. 2016, 16, 4. [Google Scholar] [CrossRef]
- Enayati, M.; Faghihian, H. N-butyl-pyridinium tetrafluoroborate as a highly efficient ionic liquid for removal of dibenzothiophene from organic solutions. J. Fuel Chem. Technol. 2015, 43, 195. [Google Scholar] [CrossRef]
- Fleck, M.; Petrosyan, A.M. Salts of Amino Acids: Crystallization, Structure and Properties; Springer: Dordrecht, The Netherlands, 2014; p. 574. [Google Scholar]
- Liu, H.-X.; Liang, Y.-X.; Jiang, X. Synthesis, crystal structure and NLO property of a nonmetal pentaborate [C6H13N2][B5O6(OH)4]. J. Solid State Chem. 2008, 181, 3243. [Google Scholar] [CrossRef]
- Guieu, V.; Payrastre, C.; Madaule, Y.; Garcia-Alonso, S.; Lacroix, P.G.; Nakatani, K. Large Quadratic Nonlinear Optical Efficiencies in Pseudosymmetric Streptocyanine Dyes. Chem. Mater. 2006, 18, 3674. [Google Scholar] [CrossRef]
- Ramajothi, J.; Dhanuskodi, S. Large Quadratic Nonlinear Optical Efficiencies in Pseudosymmetric Streptocyanine Dyes. Cryst. Res. Technol. 2003, 38, 986. [Google Scholar]
- Rogers, R.D.; Seddon, K.R.; Volkov, S. (Eds.) Green Industrial Applications of Ionic Liquids; NATO Science Series; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Wasserscheid, P.; Welton, T. (Eds.) Ionic Liquids in Synthesis; Wiley VCH: Weinheim, Germany, 2002. [Google Scholar]
- Umebayashi, Y.; Mitsugi, T.; Fukuda, S.; Fujimori, T.; Fujii, K.; Kanzaki, R.; Takeuchi, M.; Ishiguro, S. Lithium Ion Solvation in Room-Temperature Ionic Liquids Involving Bis(trifluoromethanesulfonyl) Imide Anion Studied by Raman Spectroscopy and DFT Calculations. J. Phys. Chem. B 2007, 111, 13028. [Google Scholar] [CrossRef]
- Sheldrick, M. “SHELXS-97”, a Program for Automatic Solution of Crystal Structures, Release 97-2; Göttingen University: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, M. “SHELXL-97”, Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997; Available online: https://www.scienceopen.com/document?vid=527f1309-ef7d-4d71-9985-641965ab49ab (accessed on 13 September 2020).
- Farrugia, J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837. [Google Scholar] [CrossRef]
- Shannon, D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751. [Google Scholar] [CrossRef]
- Johnson, C.K.; Burnett, M.N. ORTEP-III Version 1.0.2.: Oak Ridge, Thermal Ellipsoid Plot Program for Crystal Structure Illustrations, Oak Ridge National Laboratory Report ORNL-6895, USA 1996. Available online: https://digital.library.unt.edu/ark:/67531/metadc678816/ (accessed on 13 September 2020).
- Ali, A.B.; Grenèche, J.-M.; Leblanc, M.; Maisonneuve, V. [H3tren]3+ templated iron fluorides; synthesis, crystal structures and Mössbauer studies. Solid State Sci. 2009, 9, 1631. [Google Scholar] [CrossRef]
- Adil, K.; Saada, M.A.; Ali, A.B.; Body, M.; Dang, M.T.; Hémon-Ribaud, A.; Leblanc, M.; Maisonneuve, V. Hydrogen bonded H3O+, H2O, HF, F− in fluoride metalates (Al, Cr, Fe, Zr, Ta) templated with tren (tris-(2-aminoethyl)amine). J. Fluor. Chem. 2007, 128, 404. [Google Scholar] [CrossRef]
- Adil, K.; Ali, A.B.; Dujardin, G.; Dhal, R.; Leblanc, M.; Maisonneuve, V. Ternary and tetrahedral symmetry in hybrid fluorides, fluoride carbonates and carbonates. J. Fluor. Chem. 2004, 125, 1709. [Google Scholar] [CrossRef]
- Stuart, B. (Ed.) Infrared Spectroscopy: Fundamentals and Applications; Wiley and Sons: Hoboken, NJ, USA, 2004; p. 104. [Google Scholar]
- Abdi, I.; Alzahrany, F.; Lhoste, J.; Greneche, J.-M.; Ben Ali, A. Synthesis, crystal structure and Mössbauer study of new iron fluoride [C2N5H6] 2•(FeF5 (H2O) 2H2O. J. Adv. Chem. 2014, 10, 2617. [Google Scholar]
- Chang, C.; Jiang, J.-C.; Liou, Y.-C.; Hung, C.-H.; Lai, T.-Y.; Lin, S.H. Local Structures of Water in 1-Butyl-3-methylimidazolium Tetrafluoroborate Probed by High-Pressure Infrared Spectroscopy. Anal. Sci. 2008, 24, 1305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamoto, K. (Ed.) Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley and Sons: Hoboken, NJ, USA, 2009; p. 196. [Google Scholar]
- Sukiasyan, P.; Suponitsky, K.Y.; Atanesyan, A.K.; Danghyan, A.A.; Hovhannisyan, A.A.; Petrosyan, A.M. Crystal structures and vibrational spectra of L-argininium(2+) bis(tetrafluoroborate) and L-argininium(2+) bis(perchlorate). Spectrochim. Acta A 2020, 228, 117782. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).