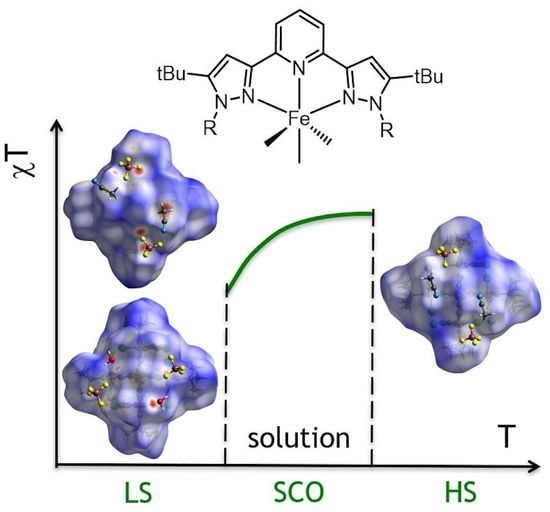
Spin State Behavior of A Spin-Crossover Iron(II) Complex with N,N′-Disubstituted 2,6-bis(pyrazol-3-yl)pyridine: A Combined Study by X-ray Diffraction and NMR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of the Ligand L
2.1.2. Synthesis of the Complex [Fe(L)2](BF4)2
2.2. X-ray Crystallography
2.3. NMR Spectroscopy
2.3.1. Evans Method
2.3.2. Temperature-Dependence of Chemical Shifts
2.3.3. Analysis of Theoretical Chemical Shifts
2.4. Quantum Chemistry
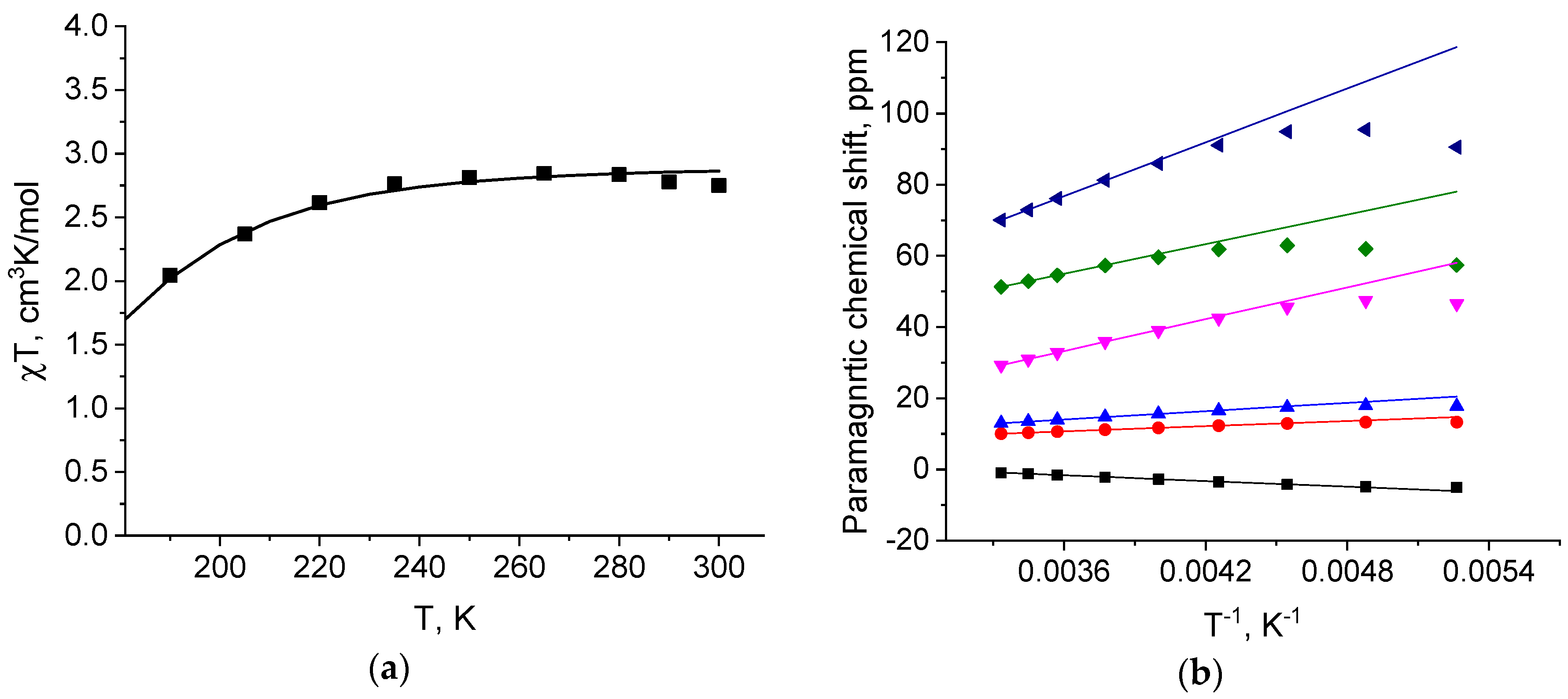
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cambi, L.; Szego, L. Uber die magnetische Susceptibilitat der komplexen Verbindungen. Ber. Dtsch. Chem. Ges. 1931, 64, 2591–2598. [Google Scholar] [CrossRef]
- Gütlich, P. Spin Crossover—Quo Vadis? Eur. J. Inorg. Chem. 2013, 2013, 581–591. [Google Scholar] [CrossRef]
- Halcrow, M.A. Spin-Crossover Materials: Properties and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Molnár, G.; Rat, S.; Salmon, L.; Nicolazzi, W.; Bousseksou, A. Spin Crossover Nanomaterials: From Fundamental Concepts to Devices. Adv. Mater. 2017, 30, 1703862. [Google Scholar]
- Senthil Kumar, K.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Gaudette, A.I.; Harris, T.D. Spin-crossover and high-spin iron(II) complexes as chemical shift 19F magnetic resonance thermometers. Chem. Sci. 2017, 8, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Gaudette, A.I.; Thorarinsdottir, A.E.; Harris, T.D. pH-Dependent spin state population and 19F NMR chemical shift via remote ligand protonation in an iron(II) complex. Chem. Commun. 2017, 53, 12962–12965. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gondrand, C.; Touti, F.; Hasserodt, J. A pair of highly biotolerated diamagnetic and paramagnetic iron(II) complexes displaying electroneutrality. Dalton Trans. 2015, 44, 15391–15395. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S. Spin crossover with thermal hysteresis: Practicalities and lessons learnt. Chem. Soc. Rev. 2015, 44, 2880–2892. [Google Scholar] [CrossRef]
- Halcrow, M.A. Spin-crossover Compounds with Wide Thermal Hysteresis. Chem. Lett. 2014, 43, 1178–1188. [Google Scholar] [CrossRef]
- Gutlich, P.; Garcia, Y.; Goodwin, H.A. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar] [CrossRef]
- Halcrow, M.A. The synthesis and coordination chemistry of 2,6-bis(pyrazolyl)pyridines and related ligands—Versatile terpyridine analogues. Coord. Chem. Rev. 2005, 249, 2880–2908. [Google Scholar] [CrossRef]
- Halcrow, M.A. Iron(II) complexes of 2,6-di(pyrazol-1-yl)pyridines—A versatile system for spin-crossover research. Coord. Chem. Rev. 2009, 253, 2493–2514. [Google Scholar] [CrossRef]
- Craig, G.A.; Roubeau, O.; Aromí, G. Spin state switching in 2,6-bis(pyrazol-3-yl)pyridine (3-bpp) based Fe(II) complexes. Coord. Chem. Rev. 2014, 269, 13–31. [Google Scholar] [CrossRef]
- Kershaw Cook, L.J.; Mohammed, R.; Sherborne, G.; Roberts, T.D.; Alvarez, S.; Halcrow, M.A. Spin state behavior of iron(II)/dipyrazolylpyridine complexes. New insights from crystallographic and solution measurements. Coord. Chem. Rev. 2015, 289–29, 2–12. [Google Scholar] [CrossRef]
- Kershaw Cook, L.J.; Kulmaczewski, R.; Mohammed, R.; Dudley, S.; Barrett, S.A.; Little, M.A.; Deeth, R.; Halcrow, M.A. A Unified Treatment of the Relationship Between Ligand Substituents and Spin State in a Family of Iron(II) Complexes. Angew. Chem. Int. Ed. 2016, 55, 4327–4331. [Google Scholar] [CrossRef]
- Halcrow, M.A.; Capel Berdiell, I.; Pask, C.M.; Kulmaczewski, R. Relationship between the Molecular Structure and Switching Temperature in a Library of Spin-Crossover Molecular Materials. Inorg. Chem. 2019, 58, 9811–9821. [Google Scholar] [CrossRef]
- Nikovskiy, I.; Polezhaev, A.V.; Novikov, V.V.; Aleshin, D.; Pavlov, A.A.; Saffiulina, E.; Aysin, R.R.; Dorovatovskii, P.; Nodaraki, L.; Tuna, F.; et al. Towards molecular design of spin-crossover complexes of 2,6-bis(pyrazol-3-yl)pyridines. Chem. Eur. J. 2020, 26, 5629–5638. [Google Scholar] [CrossRef]
- Barrett, S.A.; Halcrow, M.A. Anion-dependent spin crossover in solution for an iron(II) complex of a 1H-pyrazolyl ligand. RSC Adv. 2014, 4, 11240–11243. [Google Scholar] [CrossRef]
- Barrett, S.A.; Kilner, C.A.; Halcrow, M.A. Spin-crossover in [Fe(3-bpp)2][BF4]2 in different solvents—A dramatic stabilisation of the low-spin state in water. Dalton Trans. 2011, 40, 12021–12024. [Google Scholar] [CrossRef]
- Halcrow, M.A. The Effect of Ligand Design on Metal Ion Spin State—Lessons from Spin Crossover Complexes. Crystals 2016, 6, 58. [Google Scholar] [CrossRef]
- Nelyubina, Y.V.; Polezhaev, A.V.; Pavlov, A.A.; Aleshin, D.; Savkina, S.A.; Efimov, N.N.; Aliev, T.; Novikov, V.V. Intramolecular Spin State Locking in Iron(II) 2,6-Di(pyrazol-3-yl)pyridine Complexes by Phenyl Groups: An Experimental Study. Magnetochemistry 2018, 4, 46. [Google Scholar] [CrossRef]
- Bartual-Murgui, C.; Vela, S.; Darawsheh, M.; Diego, R.; Teat, S.J.; Roubeau, O.; Aromi, G. A probe of steric ligand substituent effects on the spin crossover of Fe(II) complexes. Inorg. Chem. Front. 2017, 4, 1374–1383. [Google Scholar] [CrossRef]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. Structure: Function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef] [PubMed]
- Craig, G.A.; Costa, J.S.; Roubeau, O.; Teat, S.J.; Aromí, G. Local Coordination Geometry and Spin State in Novel FeII Complexes with 2,6-Bis(pyrazol-3-yl)pyridine-Type Ligands as Controlled by Packing Forces: Structural Correlations. Chem. Eur. J. 2012, 18, 11703–11715. [Google Scholar] [CrossRef]
- Evans, D.F. The determination of the paramagnetic susceptibility of substances in solution by nuclear magnetic resonance. J. Chem. Soc. 1959, 2003–2005. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Denisov, G.L.; Kiskin, M.A.; Nelyubina, Y.V.; Novikov, V.V. Probing Spin Crossover in a Solution by Paramagnetic NMR Spectroscopy. Inorg. Chem. 2017, 56, 14759–14762. [Google Scholar] [CrossRef]
- Petzold, H.; Djomgoue, P.; Hörner, G.; Lochenie, C.; Weber, B.; Rüffer, T. Bis-meridional Fe2+ spincrossover complexes of phenyl and pyridyl substituted 2-(pyridin-2-yl)-1,10-phenanthrolines. Dalton Trans. 2018, 47, 491–506. [Google Scholar] [CrossRef]
- Ide, Y.; Murai, N.; Ishimae, H.; Suzuki, M.; Mori, S.i.; Takahashi, M.; Nakamura, M.; Yoshino, K.; Ikeue, T. Spin-crossover between high-spin (S = 5/2) and low-spin (S = 1/2) states in six-coordinate iron(III) porphyrin complexes having two pyridine-N oxide derivatives. Dalton Trans. 2017, 46, 242–249. [Google Scholar] [CrossRef]
- Lin, H.-J.; Siretanu, D.; Dickie, D.A.; Subedi, D.; Scepaniak, J.J.; Mitcov, D.; Clérac, R.; Smith, J.M. Steric and Electronic Control of the Spin State in Three-Fold Symmetric, Four-Coordinate Iron(II) Complexes. J. Am. Chem. Soc. 2014, 136, 13326–13332. [Google Scholar] [CrossRef]
- Pankratova, Y.; Aleshin, D.; Nikovskiy, I.; Novikov, V.; Nelyubina, Y. In Situ NMR Search for Spin-Crossover in Heteroleptic Cobalt(II) Complexes. Inorg. Chem. 2020, 59, 7700–7709. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Walker, F.A. Solution NMR studies of iron(II) spin-crossover complexes. Inorg. Chem. 2007, 46, 6794–6803. [Google Scholar] [CrossRef] [PubMed]
- Saalfrank, R.W.; Löw, N.; Trummer, S.; Sheldrick, G.M.; Teichert, M.; Stalke, D. Octanuclear Bis(triple-helical) Metal(II) Complexes. Eur. J. Inorg. Chem. 1998, 1998, 559–563. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Piguet, C. Paramagnetic Susceptibility by NMR: The “Solvent Correction” Removed for Large Paramagnetic Molecules. J. Chem. Educ. 1997, 74, 815. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Nehrkorn, J.; Pankratova, Y.A.; Ozerov, M.; Mikhalyova, E.A.; Polezhaev, A.V.; Nelyubina, Y.V.; Novikov, V.V. Detailed electronic structure of a high-spin cobalt(II) complex determined from NMR and THz-EPR spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 8201–8204. [Google Scholar] [CrossRef]
- Creutz, S.E.; Peters, J.C. Spin-State Tuning at Pseudo-tetrahedral d6 Ions: Spin Crossover in [BP3]FeII–X Complexes. Inorg. Chem. 2016, 55, 3894–3906. [Google Scholar] [CrossRef]
- Kaupp, M.; Buhl, M.; Malkin, V.G. Calculation of NMR and EPR Parameters. Theory and Applications; WILEY-VCH Verlag GmbH & Co.: Weinheim, 2004. [Google Scholar]
- Bertini, I.; Luchinat, C. NMR of Paramagnetic Substances. Coord. Chem. Rev. 1996, 150, 1–296. [Google Scholar]
- Bertini, I.; Luchinat, C.; Parigi, G. Magnetic susceptibility in paramagnetic NMR. Progr. Nucl. Mag. Res. Spectr. 2002, 40, 249–273. [Google Scholar] [CrossRef]
- Bertini, I.; Luchinat, C.; Parigi, G.; Ravera, E. Solution NMR of Paramagnetic Molecules (Second Edition): Applications to Metallobiomolecules and Models; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the Density Functional Ladder: Nonempirical Meta—Generalized Gradient Approximation Designed for Molecules and Solids. Phys. Rev. Letts 2003, 91, 146401. [Google Scholar] [CrossRef] [PubMed]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative assessment of a new nonempirical density functional: Molecules and hydrogen-bonded complexes. J. Chem. Phys. 2003, 119, 12129–12137. [Google Scholar] [CrossRef]
- Cirera, J.; Via-Nadal, M.; Ruiz, E. Benchmarking Density Functional Methods for Calculation of State Energies of First Row Spin-Crossover Molecules. Inorg. Chem. 2018, 57, 14097–14105. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Roberts, T.D.; Little, M.A.; Kershaw Cook, L.J.; Barrett, S.A.; Tuna, F.; Halcrow, M.A. Iron(II) complexes of 2,6-di(1-alkylpyrazol-3-yl)pyridine derivatives—The influence of distal substituents on the spin state of the iron centre. Polyhedron 2013, 64, 4–12. [Google Scholar] [CrossRef]
- Argent, S.P.; Adams, H.; Harding, L.P.; Riis-Johannessen, T.; Jeffery, J.C.; Ward, M.D. Homo- and heteropolynuclear helicates with a ‘2 + 3 + 2′-dentate compartmental ligand. New J. Chem. 2005, 29, 904–911. [Google Scholar] [CrossRef]
- Barrios, L.A.; Bartual-Murgui, C.; Peyrecave-Lleixa, E.; Le Guennic, B.; Teat, S.J.; Roubeau, O.; Aromi, G. Homoleptic versus Heteroleptic Formation of Mononuclear Fe(II) Complexes with Tris-Imine Ligands. Inorg. Chem. 2016, 55, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Wolny, J.A.; Schünemann, V.; Németh, Z.; Vankó, G. Spectroscopic techniques to characterize the spin state: Vibrational, optical, Mössbauer, NMR, and X-ray spectroscopy. Comptes Rendus Chim. 2018, 21, 1152–1169. [Google Scholar] [CrossRef]
- Pritchard, R.; Kilner, C.A.; Halcrow, M.A. Iron(ii) complexes with a terpyridine embrace packing motif show remarkably consistent cooperative spin-transitions. Chem. Commun. 2007, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Scudder, M.L.; Goodwin, H.A.; Dance, I.G. Crystal supramolecular motifs: Two-dimensional grids of terpy embraces in [ML2] complexes (L = terpy or aromatic N3-tridentate ligand). New J. Chem. 1999, 23, 695–705. [Google Scholar] [CrossRef]
- McMurtrie, J.; Dance, I. Alternative metal grid structures formed by [M(terpy)2]2+ and [M(terpyOH)2]2+ complexes with small and large tetrahedral dianions, and by [Ru(terpy)2]0. CrystEngComm 2010, 12, 2700–2710. [Google Scholar] [CrossRef]
- Kershaw Cook, L.J.; Kulmaczewski, R.; Cespedes, O.; Halcrow, M.A. Different Spin-State Behaviors in Isostructural Solvates of a Molecular Iron(II) Complex. Chem. Eur. J. 2015, 22, 1789–1799. [Google Scholar] [CrossRef]
- Vela, S.; Gourlaouen, C.; Fumanal, M.; Ribas-Arino, J. Disclosing the Ligand- and Solvent-Induced Changes on the Spin Transition and Optical Properties of Fe(II)-Indazolylpyridine Complexes. Magnetochemistry 2016, 2, 6. [Google Scholar] [CrossRef]
- Bartual-Murgui, C.; Codina, C.; Roubeau, O.; Aromí, G. A Sequential Method to Prepare Polymorphs and Solvatomorphs of [Fe(1,3-bpp)2](ClO4)2⋅nH2O (n = 0, 1, 2) with Varying Spin-Crossover Behaviour. Chem. Eur. J. 2016, 22, 12767–12776. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Mitchell, A.S.; Spackman, M.A. Hirshfeld Surfaces: A New Tool for Visualising and Exploring Molecular Crystals. Chem. Eur. J. 1998, 4, 2136–2141. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer (Version 3.1); University of Western Australia: Perth, Australia, 2012. [Google Scholar]
- Alvarez, S. Relationships between Temperature, Magnetic Moment, and Continuous Symmetry Measures in Spin Crossover Complexes. J. Am. Chem. Soc. 2003, 125, 6795–6802. [Google Scholar] [CrossRef]
- Ashley, D.C.; Jakubikova, E. Ray-Dutt and Bailar Twists in Fe(II)-Tris(2,2′-bipyridine): Spin States, Sterics, and Fe–N Bond Strengths. Inorg. Chem. 2018, 57, 5585–5596. [Google Scholar] [CrossRef]
- Alvarez, S. Distortion Pathways of Transition Metal Coordination Polyhedra Induced by Chelating Topology. Chem. Rev. 2015, 115, 13447–13483. [Google Scholar] [CrossRef]
- Kershaw Cook, L.J.; Thorp-Greenwood, F.L.; Comyn, T.P.; Cespedes, O.; Chastanet, G.; Halcrow, M.A. Unexpected Spin-Crossover and a Low-Pressure Phase Change in an Iron(II)/Dipyrazolylpyridine Complex Exhibiting a High-Spin Jahn—Teller Distortion. Inorg. Chem. 2015, 54, 6319–6330. [Google Scholar] [CrossRef]
- Hayami, S.; Komatsu, Y.; Shimizu, T.; Kamihata, H.; Lee, Y.H. Spin-crossover in cobalt(II) compounds containing terpyridine and its derivatives. Coord. Chem. Rev. 2011, 255, 1981–1990. [Google Scholar] [CrossRef]
- Guionneau, P. Crystallography and spin-crossover. A view of breathing materials. Dalton Trans. 2014, 43, 382–393. [Google Scholar] [CrossRef]
- Beniwal, S.; Zhang, X.; Mu, S.; Naim, A.; Rosa, P.; Chastanet, G.; Létard, J.F.; Liu, J.; Sterbinsky, G.E.; Arena, D.A.; et al. Surface-induced spin state locking of the [Fe(H2B(pz)2)2(bipy)] spin crossover complex. J. Phys. Condens. Mat. 2016, 28, 206002. [Google Scholar] [CrossRef]
- Gentili, D.; Liscio, F.; Demitri, N.; Schäfer, B.; Borgatti, F.; Torelli, P.; Gobaut, B.; Panaccione, G.; Rossi, G.; Degli Esposti, A.; et al. Surface induces different crystal structures in a room temperature switchable spin crossover compound. Dalton Trans. 2016, 45, 134–143. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Fumanal, M.; Jimenez-Gravalos, F.; Ribas-Arino, J.; Vela, S. Lattice-Solvent Effects in the Spin-Crossover of an Fe(II)-Based Material. The Key Role of Intermolecular Interactions between Solvent Molecules. Inorg. Chem. 2017, 56, 4474–4483. [Google Scholar] [CrossRef]
- Phan, H.; Hrudka, J.J.; Igimbayeva, D.; Lawson Daku, L.M.; Shatruk, M. A Simple Approach for Predicting the Spin State of Homoleptic Fe(II) Tris-diimine Complexes. J. Am. Chem. Soc. 2017, 139, 6437–6447. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, S.; Yang, M.; Stewart, I.; Garden, A.L.; Brooker, S. A Simple Method of Predicting Spin State in Solution. J. Am. Chem. Soc. 2017, 139, 18392–18396. [Google Scholar] [CrossRef]
- Kimura, A.; Ishida, T. Spin-Crossover Temperature Predictable from DFT Calculation for Iron(II) Complexes with 4-Substituted Pybox and Related Heteroaromatic Ligands. ACS Omega 2018, 3, 6737–6747. [Google Scholar] [CrossRef]
- McPherson, J.N.; Elton, T.E.; Colbran, S.B. A Strain-Deformation Nexus within Pincer Ligands: Application to the Spin States of Iron(II) Complexes. Inorg. Chem. 2018, 57, 12312–12322. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Nehrkorn, J.; Zubkevich, S.V.; Fedin, M.V.; Holldack, K.; Schnegg, A.; Novikov, V.V. A Synergy and Struggle of EPR, Magnetometry and NMR: A Case Study of Magnetic Interaction Parameters in a Six-Coordinate Cobalt(II) Complex. Inorg. Chem. 2020. [Google Scholar] [CrossRef]
- Dudkin, S.V.; Belov, A.S.; Nelyubina, Y.V.; Savchuk, A.V.; Pavlov, A.A.; Novikov, V.V.; Voloshin, Y.Z. Synthesis, X-ray structure and electrochemical properties of hybrid binuclear metallophthalocyaninate-capped tris-pyridineoximates. New J. Chem. 2017, 41, 3251–3259. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Savkina, S.A.; Belov, A.S.; Nelyubina, Y.V.; Efimov, N.N.; Voloshin, Y.Z.; Novikov, V.V. Trigonal Prismatic Tris-pyridineoximate Transition Metal Complexes: A Cobalt(II) Compound with High Magnetic Anisotropy. Inorg. Chem. 2017, 56, 6943–6951. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Aleshin, D.Y.; Nikovskiy, I.A.; Polezhaev, A.V.; Efimov, N.N.; Korlyukov, A.A.; Novikov, V.V.; Nelyubina, Y.V. New Spin-Crossover Complexes of Substituted 2,6-Bis(pyrazol-3-yl)pyridines. Eur. J. Inorg. Chem. 2019, 2019, 2819–2829. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Belov, A.S.; Savkina, S.A.; Polezhaev, A.V.; Aleshin, D.Y.; Novikov, V.V.; Nelyubina, Y.V. Synthesis and Spin State of the Cobalt(II) Complexes with Substituted 2,6-Bis(pyrazol-3-yl)pyridine Ligands. Russ. J. Coord. Chem. 2018, 44, 489–495. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Nikovskii, I.A.; Polezhaev, A.V.; Aleshin, D.Y.; Melnikova, E.K.; Pankratova, Y.A.; Nelyubina, Y.V. Spin State of the Iron(II) and Cobalt(II) 2,6-Di(5-Amino-1H-Pyrazol-3-yl)pyridine Complexes in Solution and in Crystal. Russ. J. Coord. Chem. 2019, 45, 402–410. [Google Scholar] [CrossRef]
- Yatsunyk, L.A.; Walker, F.A. Structural, NMR, and EPR Studies of S = 1/2 and S = 3/2 Fe(III) Bis(4-Cyanopyridine) Complexes of Dodecasubstituted Porphyrins. Inorg. Chem. 2004, 43, 757–777. [Google Scholar] [CrossRef]
- Brunker, T.J.; Green, J.C.; O’Hare, D. Electronic Structures of Mixed-Sandwich Complexes of Cyclopentadienyl and Hydrotris(pyrazolyl)borate Ligands with 3d Transition Metals. Inorg. Chem. 2003, 42, 4366–4381. [Google Scholar] [CrossRef]
- Petzold, H.; Djomgoue, P.; Hörner, G.; Speck, J.M.; Rüffer, T.; Schaarschmidt, D. 1H NMR spectroscopic elucidation in solution of the kinetics and thermodynamics of spin crossover for an exceptionally robust Fe2+ complex. Dalton Trans. 2016, 45, 13798–13809. [Google Scholar] [CrossRef]
- Petzold, H.; Djomgoue, P.; Hörner, G.; Heider, S.; Lochenie, C.; Weber, B.; Rüffer, T.; Schaarschmidt, D. Spin state variability in Fe2+ complexes of substituted (2-(pyridin-2-yl)-1,10-phenanthroline) ligands as versatile terpyridine analogues. Dalton Trans. 2017, 46, 6218–6229. [Google Scholar] [CrossRef]
- Galadzhun, I.; Kulmaczewski, R.; Cespedes, O.; Yamada, M.; Yoshinari, N.; Konno, T.; Halcrow, M.A. 2,6-Bis(pyrazol-1-yl)pyridine-4-carboxylate Esters with Alkyl Chain Substituents and Their Iron(II) Complexes. Inorg. Chem. 2018, 57, 13761–13771. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Scudder, M.L.; Craig, D.C.; Goodwin, H.A. Hydrogen bonding influences on the properties of heavily hydrated chloride salts of iron(ii) and ruthenium(ii) complexes of 2,6-bis(pyrazol-3-yl)pyridine, 2,6-bis(1,2,4-triazol-3-yl)pyridine and 2,2′:6′,2″-terpyridine. CrystEngComm 2005, 7, 642–649. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Giménez-López, M.C.; Romero, F.M. Structural, Thermal, and Magnetic Study of Solvation Processes in Spin-Crossover [Fe(bpp)2][Cr(L)(ox)2]2·nH2O Complexes. Inorg. Chem. 2007, 46, 11266–11276. [Google Scholar] [CrossRef]
- Coronado, E.; Gimenez-Lopez, M.C.; Gimenez-Saiz, C.; Romero, F.M. Spin crossover complexes as building units of hydrogen-bonded nanoporous structures. CrystEngComm 2009, 11, 2198–2203. [Google Scholar] [CrossRef]
- Jornet-Molla, V.; Gimenez-Saiz, C.; Romero, F.M. Synthesis, Structure, and Photomagnetic Properties of a Hydrogen-Bonded Lattice of [Fe(bpp)2]2+ Spin-Crossover Complexes and Nicotinate Anions. Crystals 2018, 8, 439. [Google Scholar] [CrossRef]
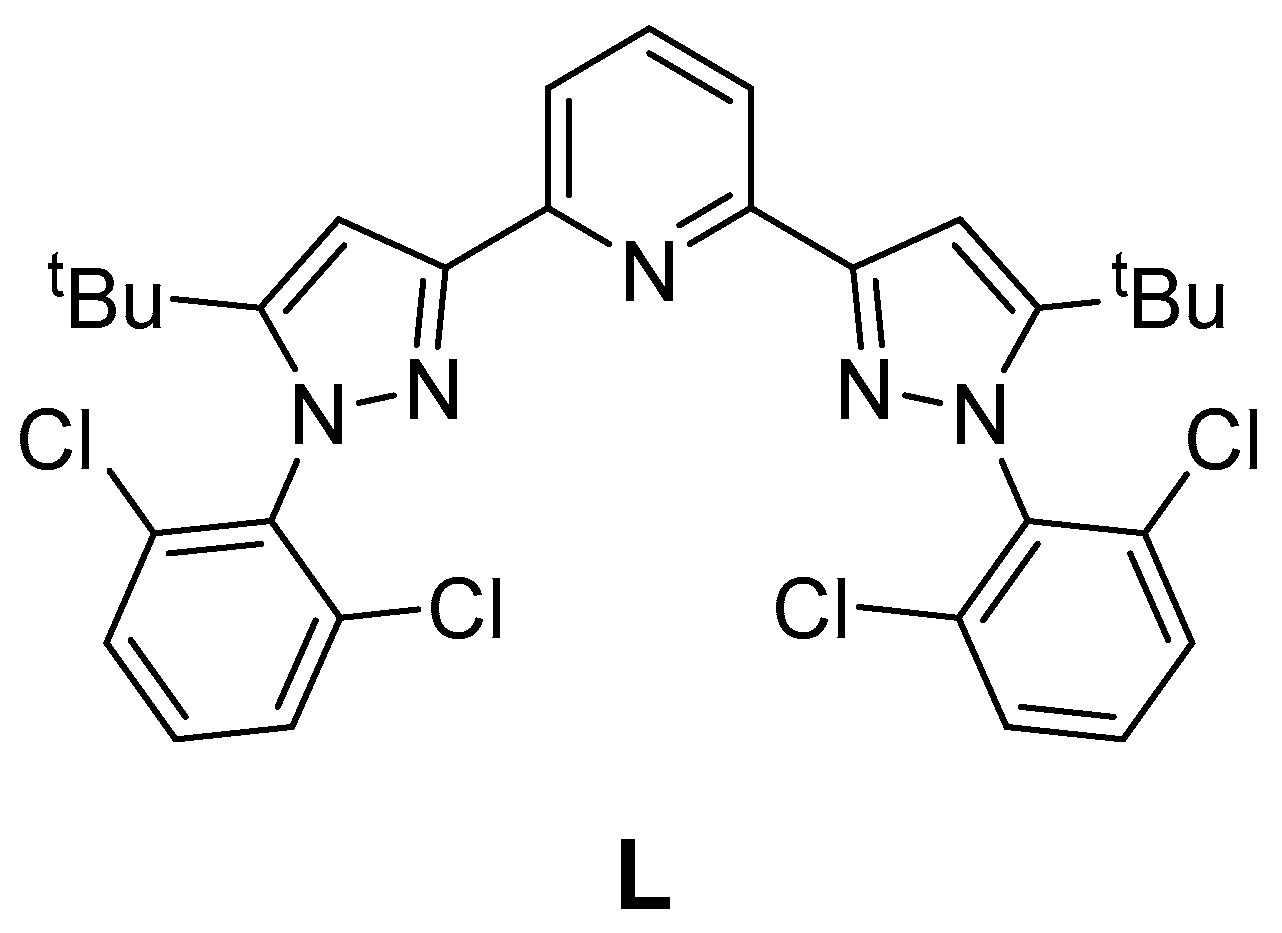
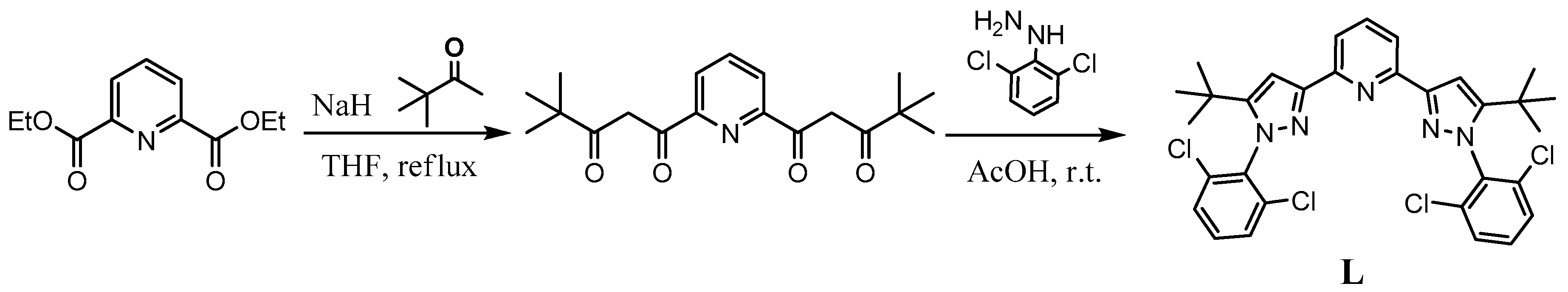
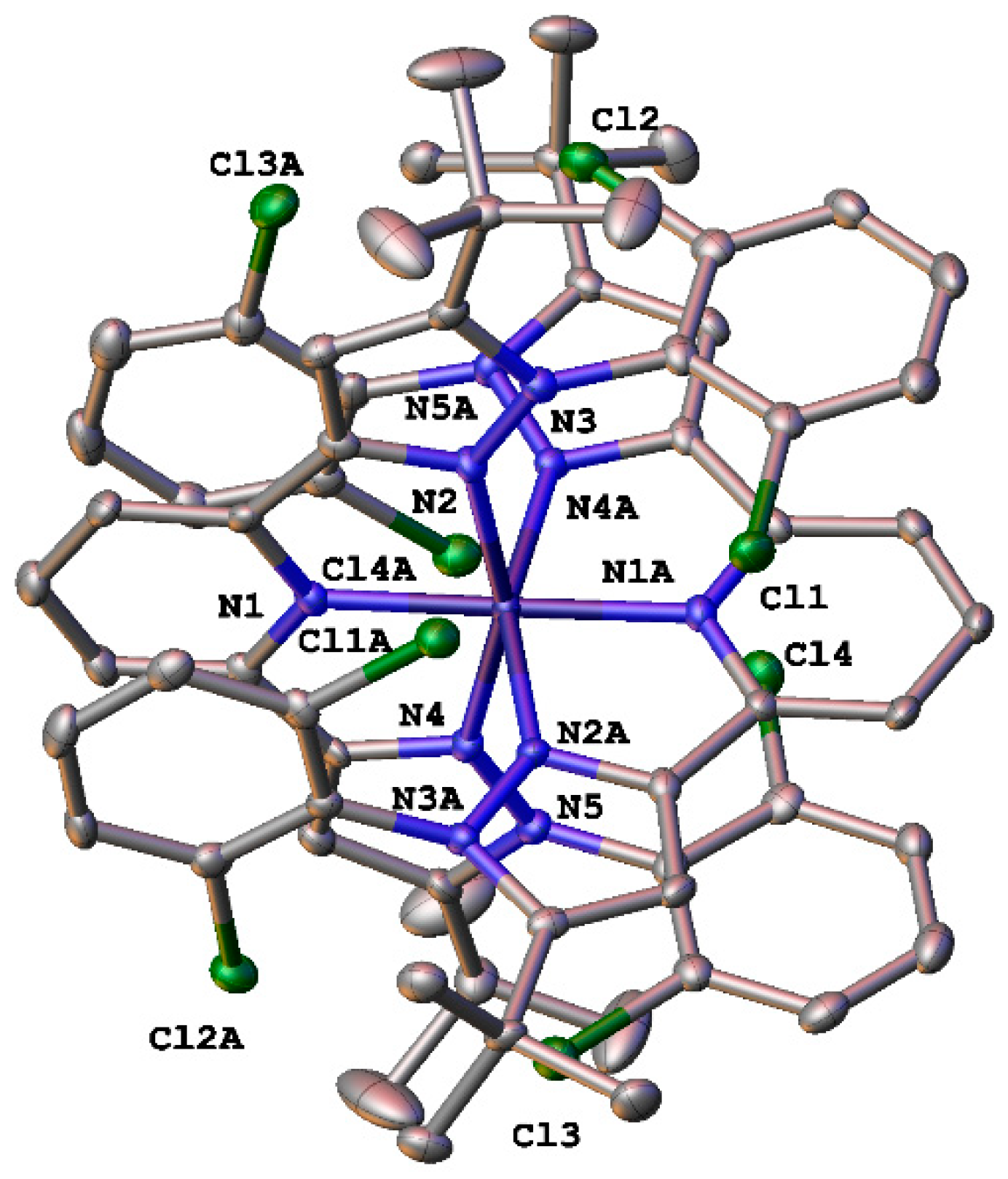
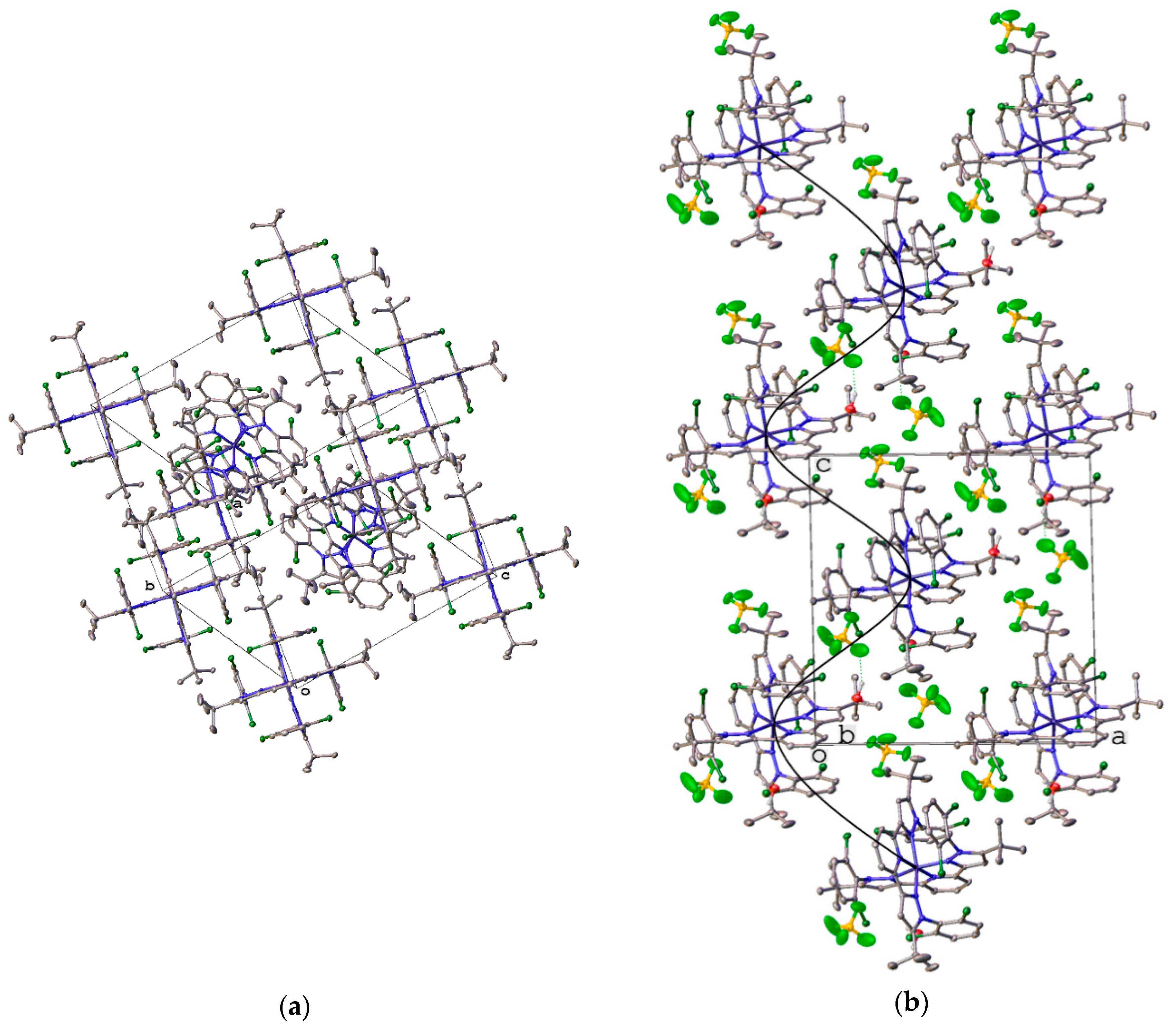
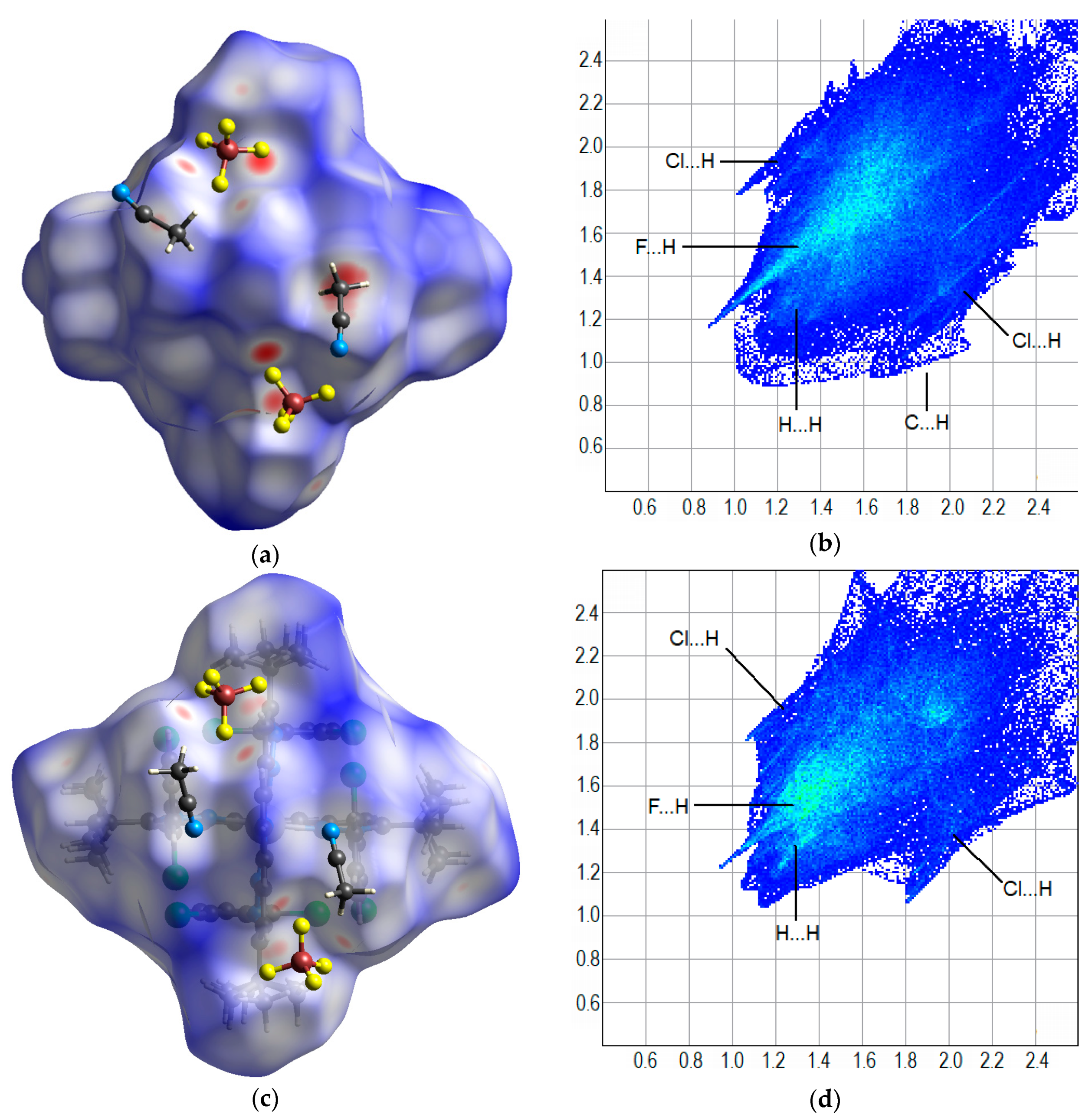

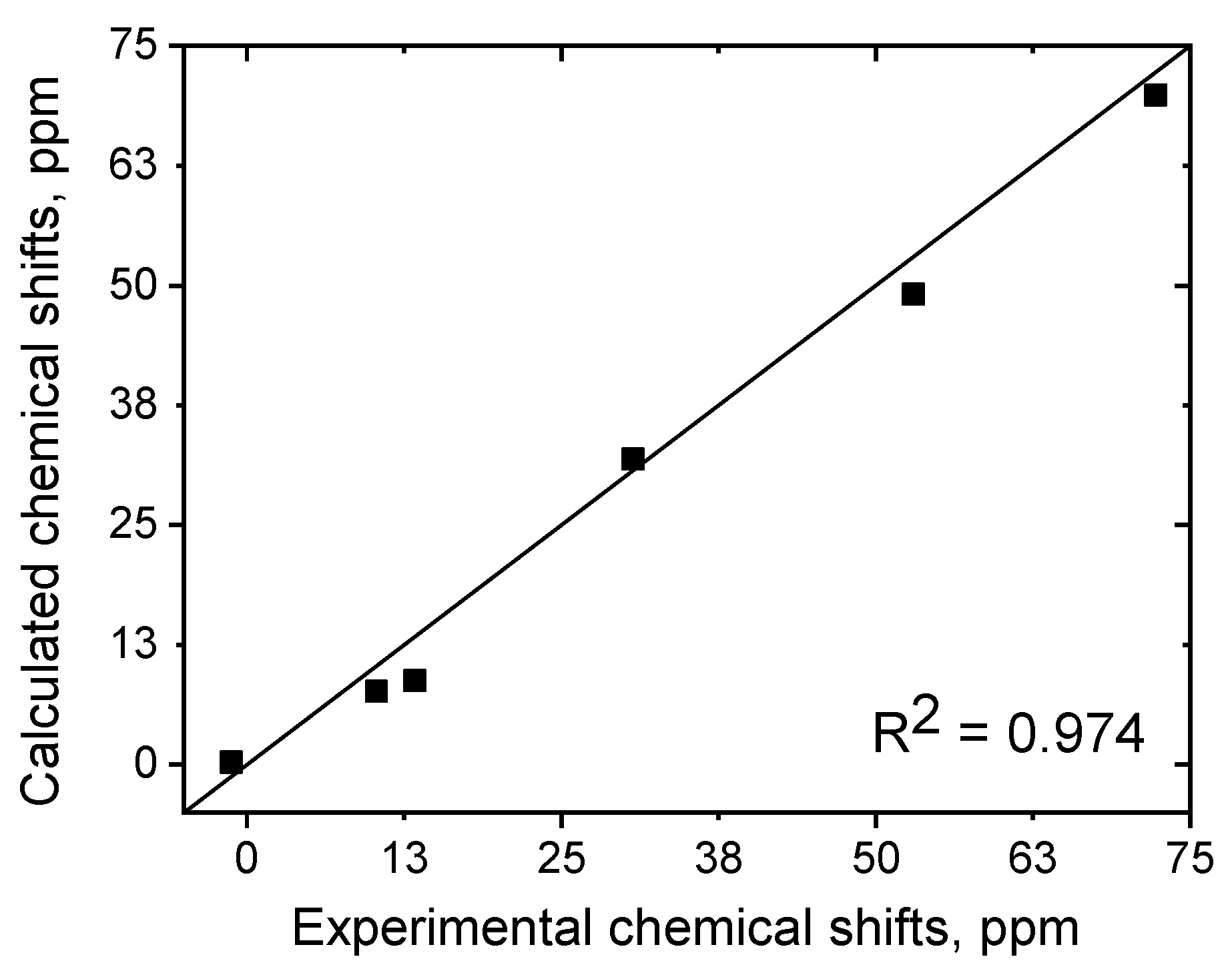
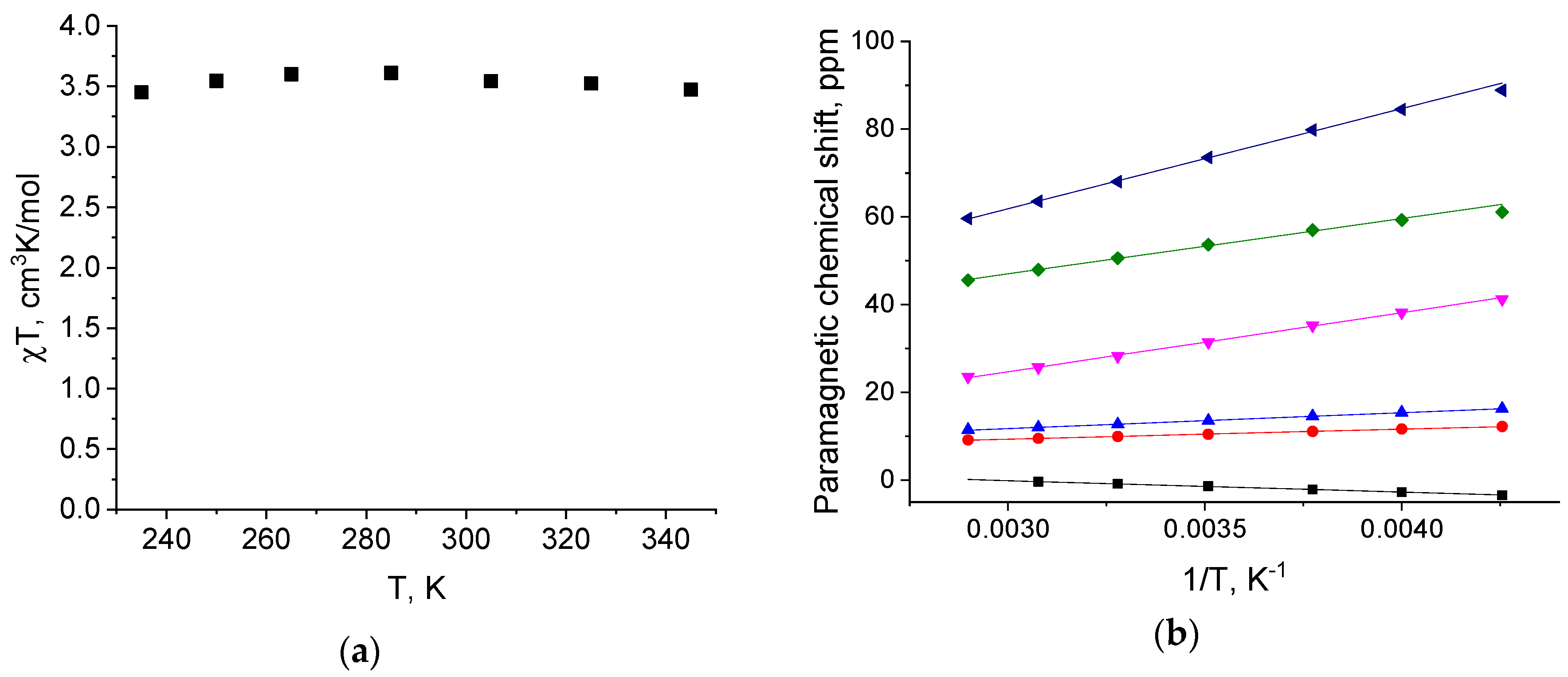
| Parameter | [Fe(L)2](BF4)2·2CH3CN (red) | [Fe(L)2](BF4)2·0.5H2O (red) | [Fe(L)2](BF4)2·2CH3CN (Yellow) | |
|---|---|---|---|---|
| Formula unit | 2(C62H58Cl8FeN10), 4(BF4), 4(C2H3N) | 2C62H58Cl8FeN10, 4BF4, H2O | C62H58Cl8FeN10, 2BF4, 2C2H3N | C62H58Cl8FeN10, 2BF4, 2C2H3N |
| Formula weight | 3088.73 | 2930.52 | 1538.36 | 1539.37 |
| T, K | 120 | 120 | 120 | 293 |
| Crystal system | Orthorhombic | Monoclinic | Orthorhombic | Orthorhombic |
| Space group | Pcbn | P21/n | Fdd2 | Fdd2 |
| Z | 4 | 2 | 8 | 8 |
| a, Å | 45.272(7) | 16.9032(12) | 42.2980(16) | 43.047(14) |
| b, Å | 13.335(2) | 21.2547(15) | 14.6224(6) | 14.742(6) |
| c, Å | 24.665(4) | 17.4043(12) | 22.9540(9) | 23.161(6) |
| β, ° | 90 | 91.033(2) | 90 | 90 |
| V, Å3 | 14,890(4) | 6251.9(8) | 14,197.0(10) | 14,699(8) |
| Dcalc (g cm−1) | 1.378 | 1.557 | 1.439 | 1.390 |
| Linear absorption, μ (cm−1) | 5.58 | 6.59 | 5.85 | 5.65 |
| F(000) | 6328 | 2996 | 6304 | 6304 |
| 2θmax, ° | 52 | 54 | 54 | 54 |
| Reflections measured | 93,601 | 66,188 | 37,118 | 36,822 |
| Independent reflections | 14,645 | 13,643 | 7759 | 8029 |
| Observed reflections [I > 2σ(I)] | 6631 | 7932 | 6893 | 3666 |
| Parameters | 938 | 841 | 509 | 474 |
| R1 | 0.1504 | 0.0521 | 0.0341 | 0.0656 |
| wR2 | 0.4839 | 0.1237 | 0.0776 | 0.1776 |
| GOF | 1.067 | 0.993 | 1.024 | 0.939 |
| Δρmax/Δρmin (e Å−3) | 1.398/−0.664 | 0.753/−0.644 | 0.538/−0.332 | 0.847/−0.544 |
| Parameter | [Fe(L)2](BF4)2·2CH3CN (red) | [Fe(L)2] (BF4)2·0.5H2O (red) | [Fe(L)2](BF4)2·2CH3CN (Yellow) | |
|---|---|---|---|---|
| 120 K | 293 K | |||
| Fe-NPy, Å | 1.901(12)–1.912(13) | 1.910(3)–1.916(3) | 2.044(5)–2.046(5) | 2.056(9)–2.067(12) |
| Fe-NPz, Å | 2.025(12)–2.050(9) | 2.023(3)–2.032(3) | 2.196(3)–2.187(3) | 2.202(6)–2.205(7) |
| θ,° | 89.61(11) | 89.545(1) | 88.882(1) | 90.53(9) |
| φ,° | 179.4(4) | 179.30(12) | 180 | 180.00(4) |
| γ,° | 89.7(5)–90.0 (7) av. 89.85 | 86.42(13)–89.49(13) av. 88.05 | 89.94(14)–89.04(13) av. 89.49 | 88.3(4)–89.0(4) av. 88.65 |
| S(OC-6) | 2.599 | 2.299 | 3.786 | 3.819 |
| S(ebcT-6) | 13.151 | 13.485 | 11.286 | 11.206 |
| Parameter | [Fe(L)2](BF4)2 |
|---|---|
| T1/2, K | 175 |
| ∆H, kJ/mol | 15.2 |
| ∆S, J/mol | 86.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikova, E.K.; Aleshin, D.Y.; Nikovskiy, I.A.; Denisov, G.L.; Nelyubina, Y.V. Spin State Behavior of A Spin-Crossover Iron(II) Complex with N,N′-Disubstituted 2,6-bis(pyrazol-3-yl)pyridine: A Combined Study by X-ray Diffraction and NMR Spectroscopy. Crystals 2020, 10, 793. https://doi.org/10.3390/cryst10090793
Melnikova EK, Aleshin DY, Nikovskiy IA, Denisov GL, Nelyubina YV. Spin State Behavior of A Spin-Crossover Iron(II) Complex with N,N′-Disubstituted 2,6-bis(pyrazol-3-yl)pyridine: A Combined Study by X-ray Diffraction and NMR Spectroscopy. Crystals. 2020; 10(9):793. https://doi.org/10.3390/cryst10090793
Chicago/Turabian StyleMelnikova, Elizaveta K., Dmitry Yu. Aleshin, Igor A. Nikovskiy, Gleb L. Denisov, and Yulia V. Nelyubina. 2020. "Spin State Behavior of A Spin-Crossover Iron(II) Complex with N,N′-Disubstituted 2,6-bis(pyrazol-3-yl)pyridine: A Combined Study by X-ray Diffraction and NMR Spectroscopy" Crystals 10, no. 9: 793. https://doi.org/10.3390/cryst10090793
APA StyleMelnikova, E. K., Aleshin, D. Y., Nikovskiy, I. A., Denisov, G. L., & Nelyubina, Y. V. (2020). Spin State Behavior of A Spin-Crossover Iron(II) Complex with N,N′-Disubstituted 2,6-bis(pyrazol-3-yl)pyridine: A Combined Study by X-ray Diffraction and NMR Spectroscopy. Crystals, 10(9), 793. https://doi.org/10.3390/cryst10090793