Orientation Control of Helical Nanofilament Phase and Its Chiroptical Applications
Abstract
1. Introduction
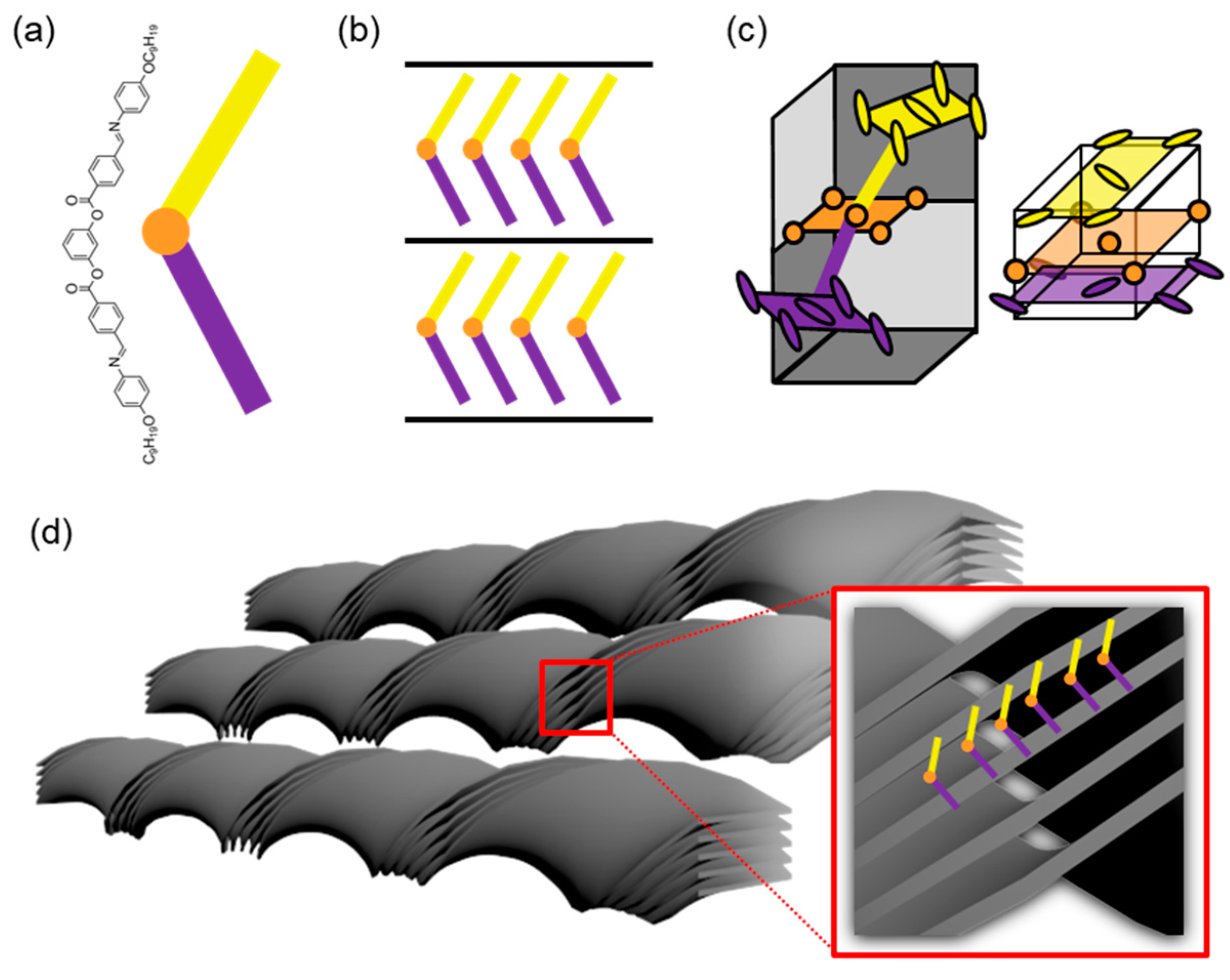
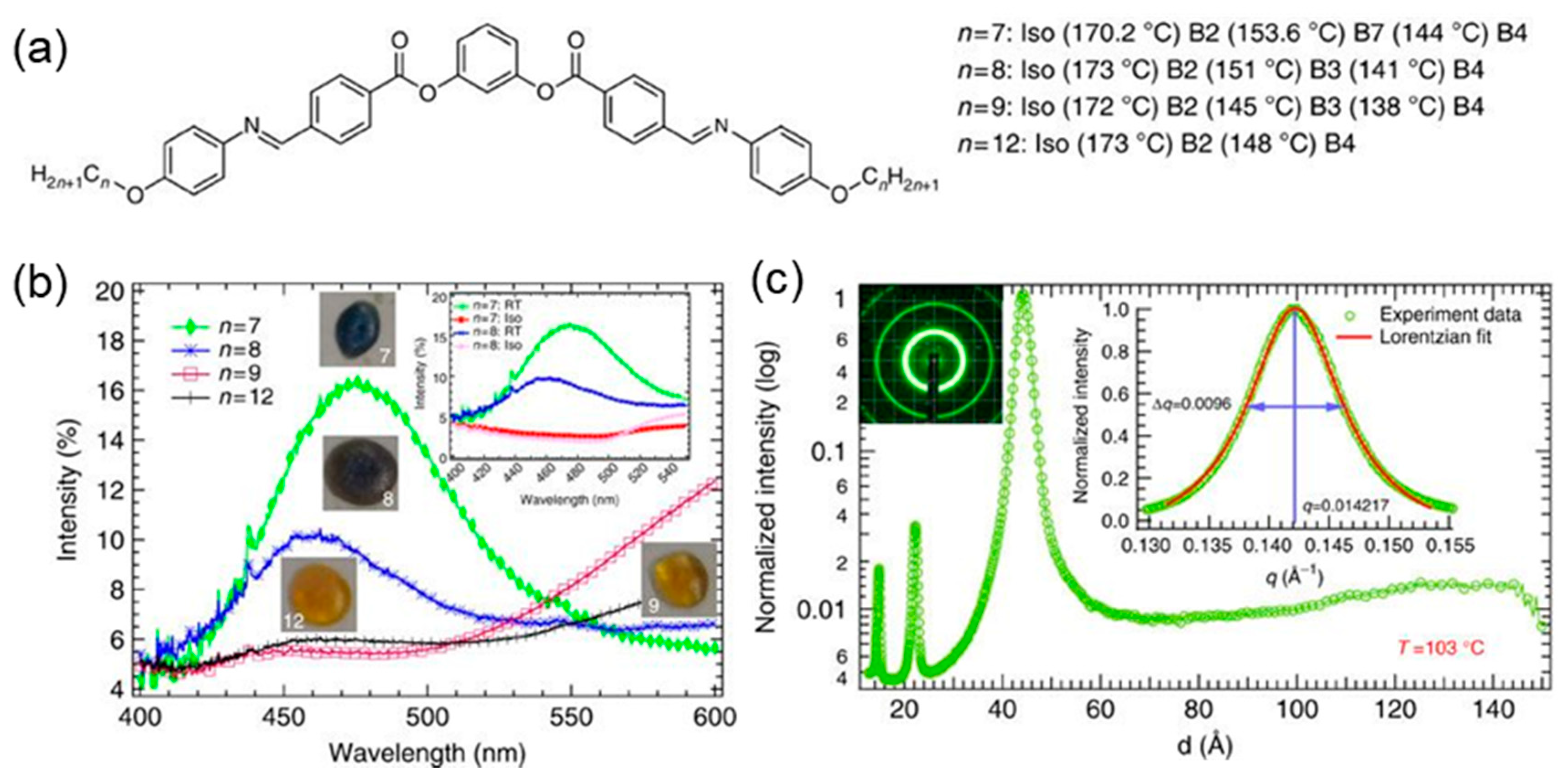
2. Helical Nanofilament Phase
3. Orientation Control of HNFs
3.1. Conventional Alignment Methods to Orient HNFs
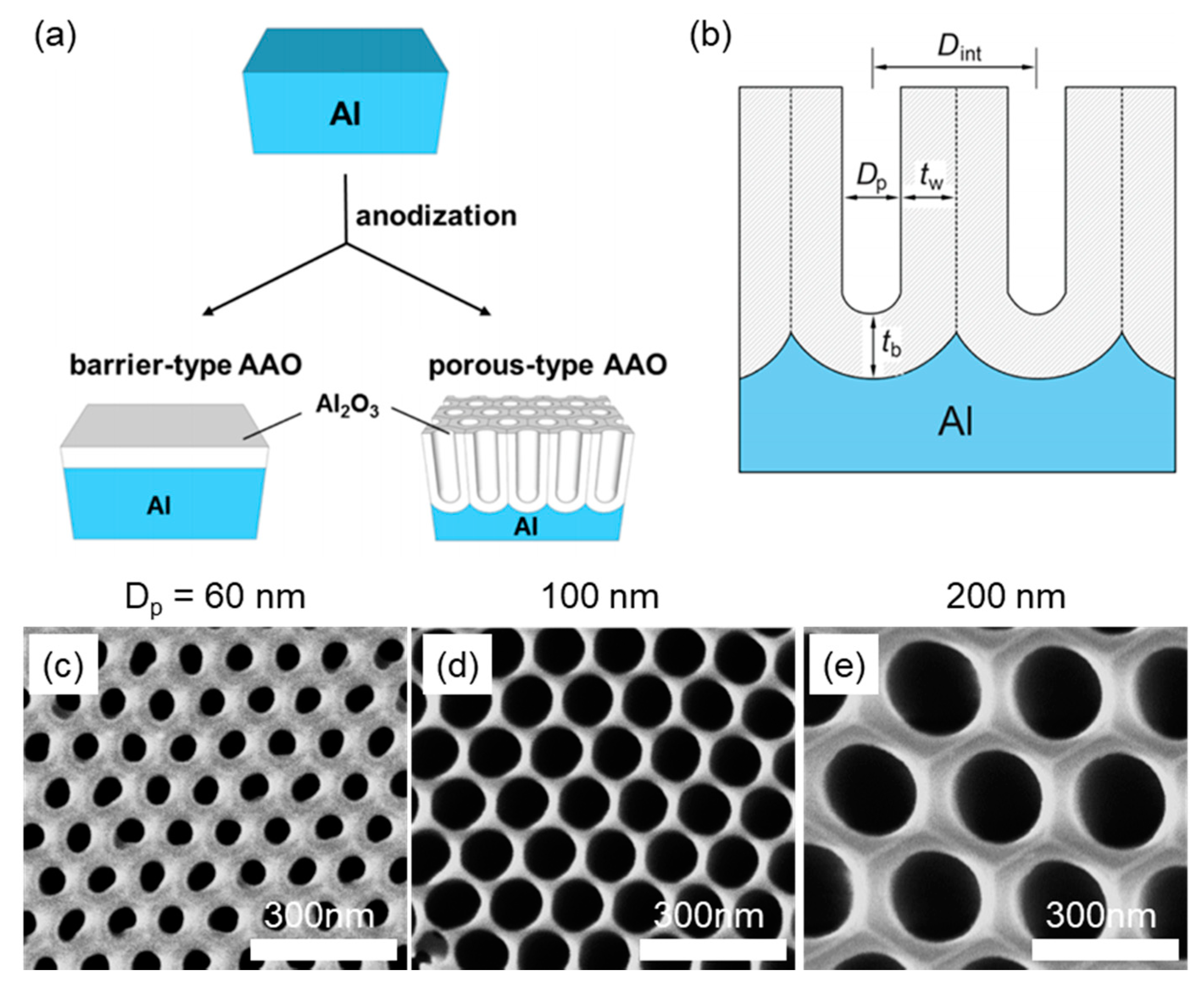
3.2. Cylindrical Nanoconfinement
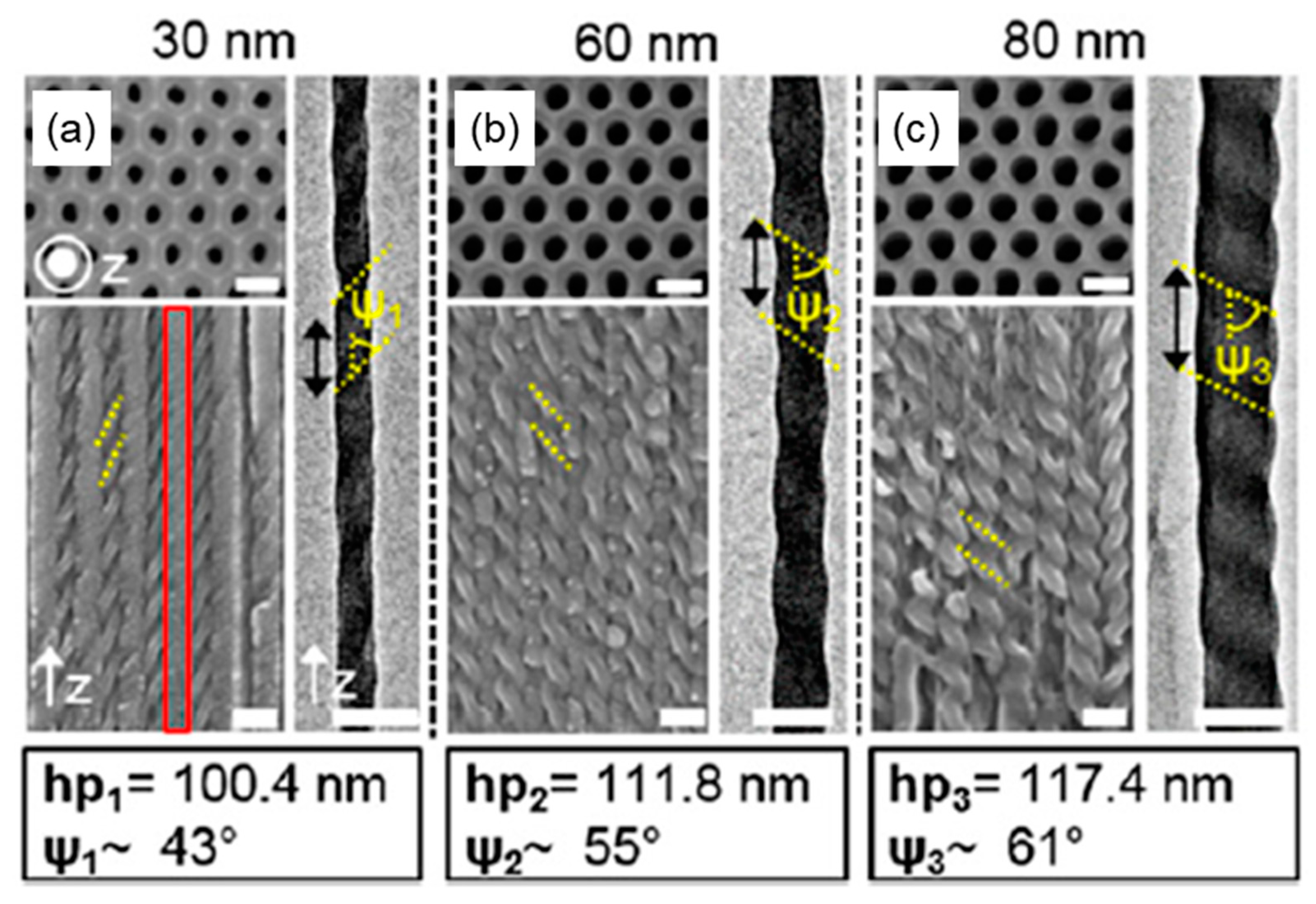
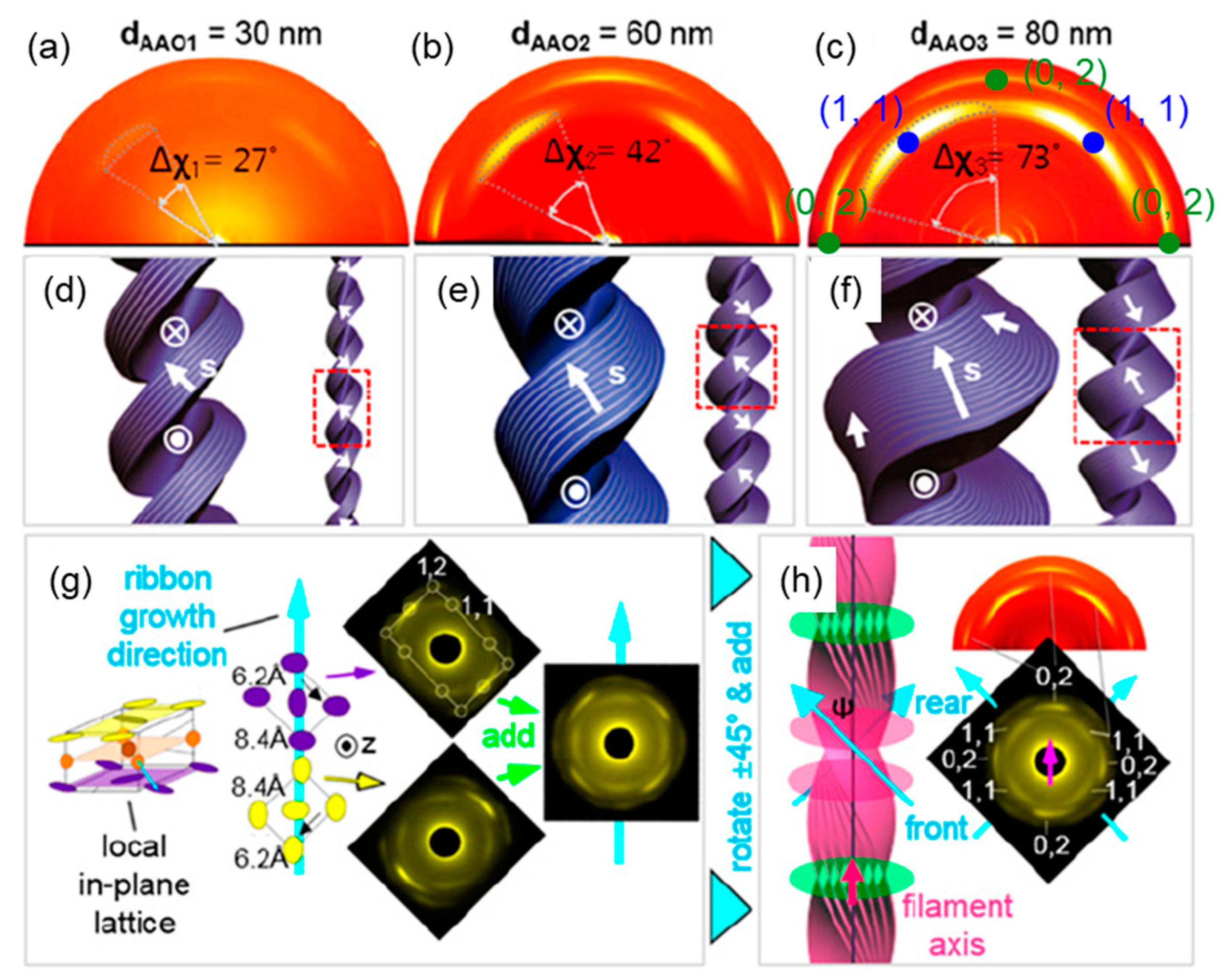
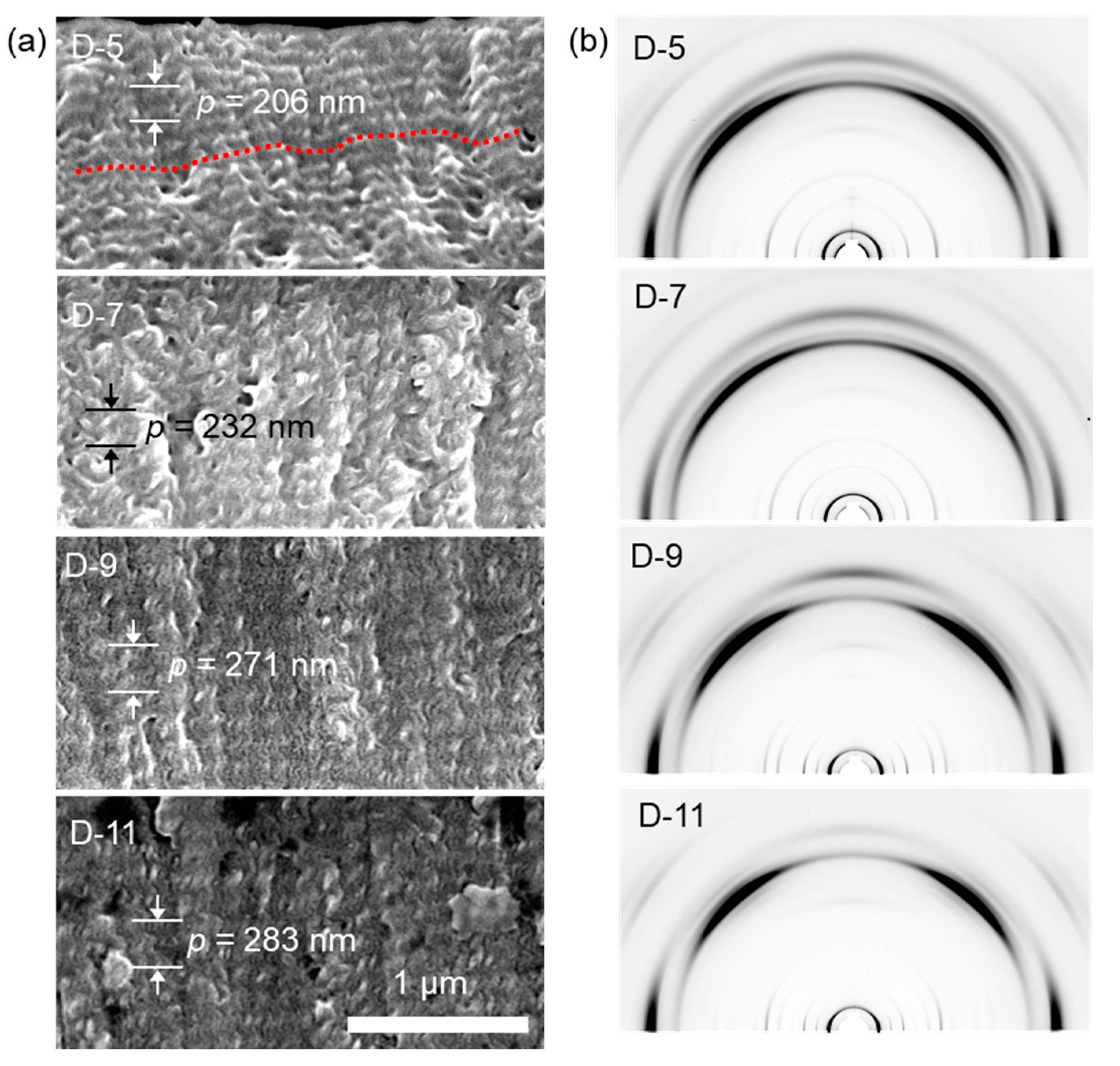
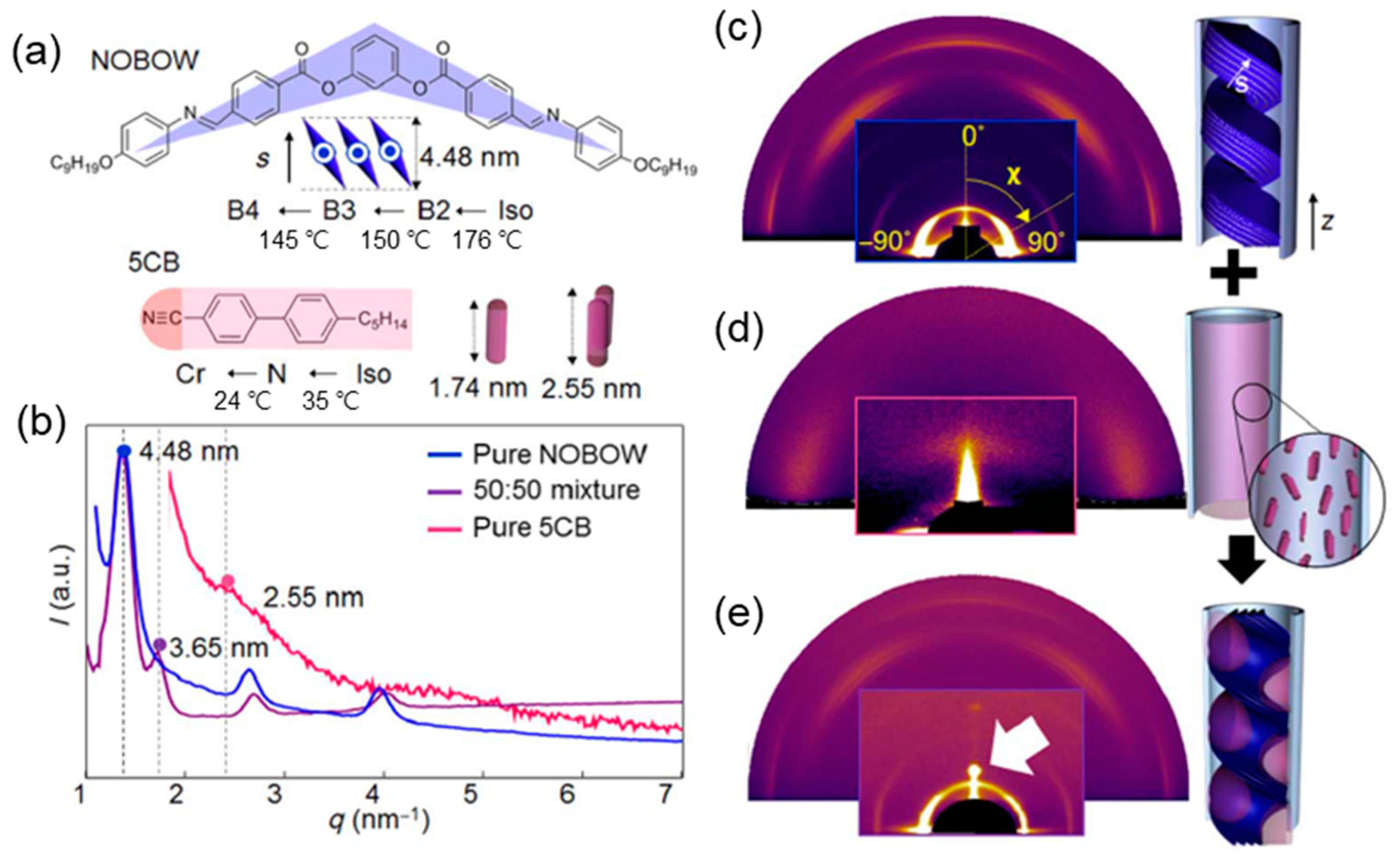
3.2.1. Nanoconfinement of HNFs
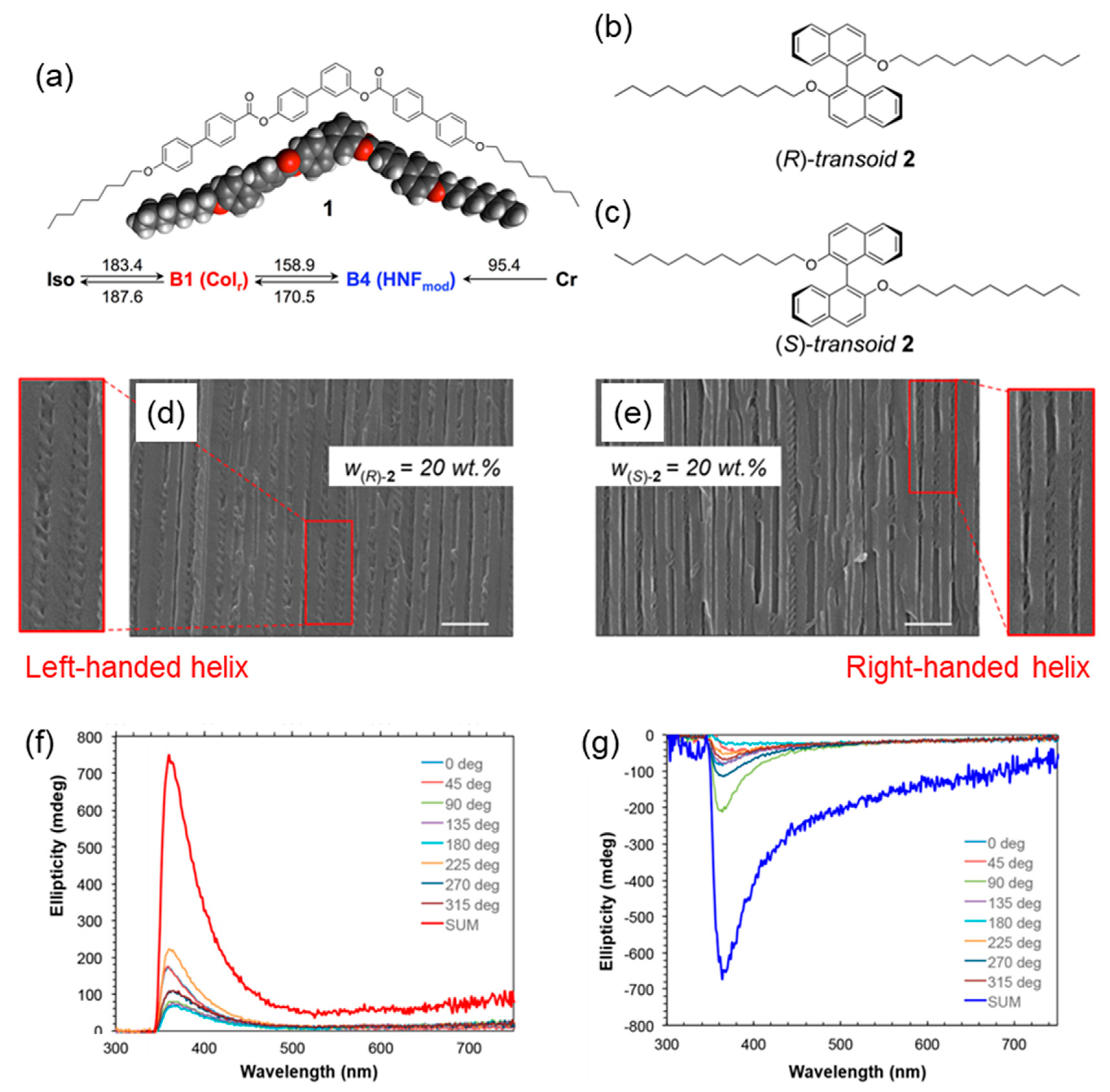
3.2.2. Chirality Control of HNFs with Cylindrical Nanoconfinement
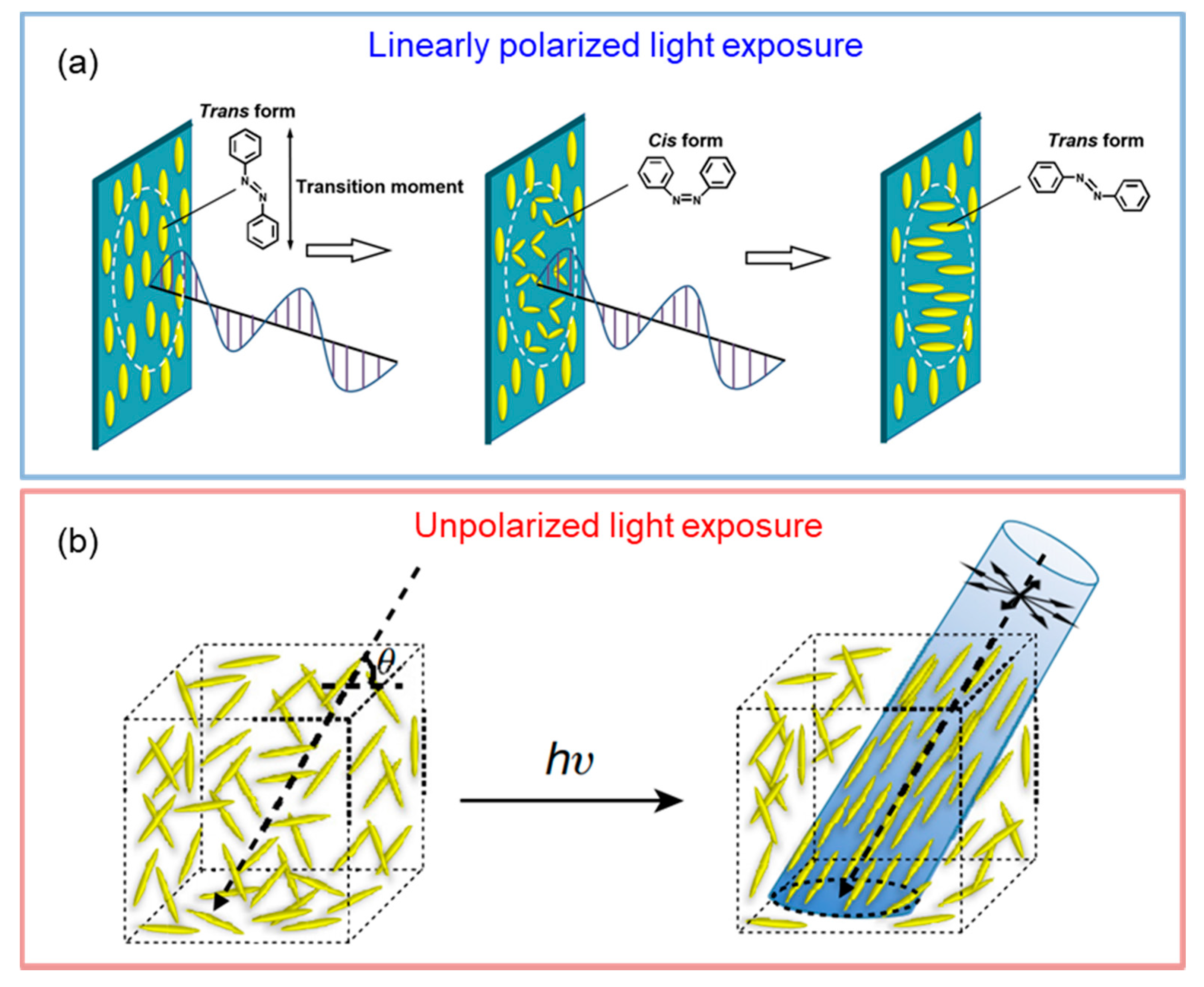
3.3. Photoalignment of Azobenzene
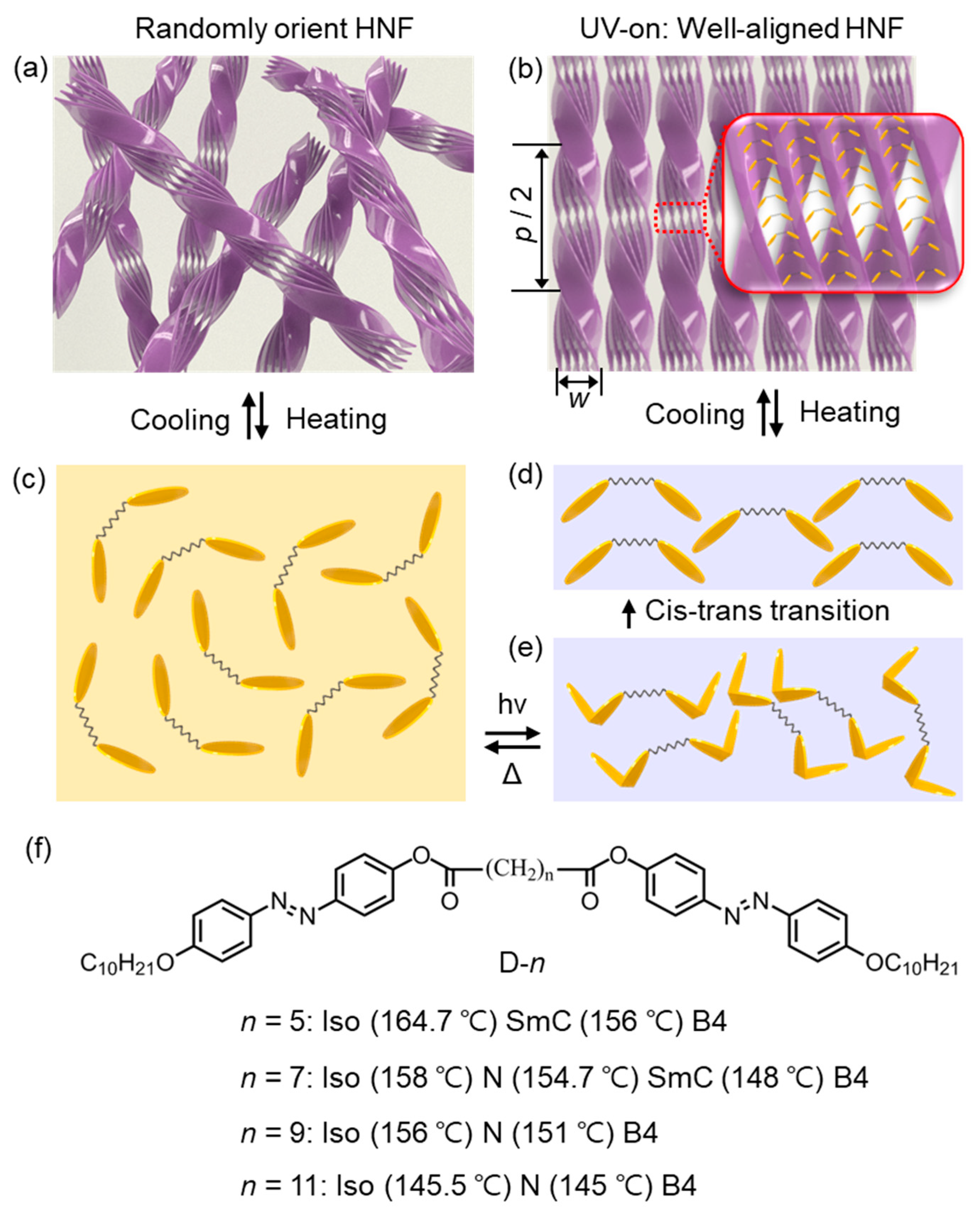
3.3.1. Photoalignment of HNFs
4. Optical Applications of HNFs
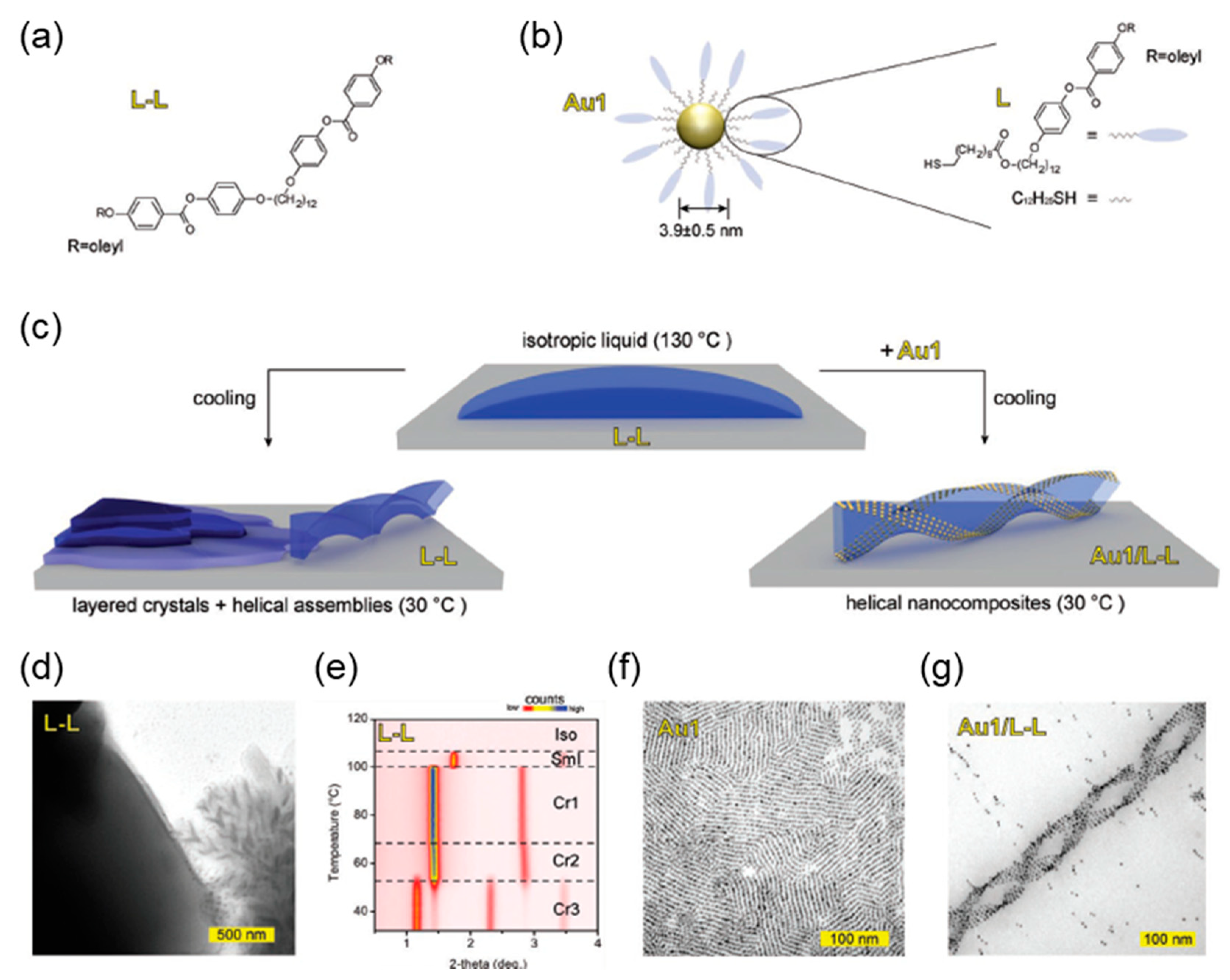
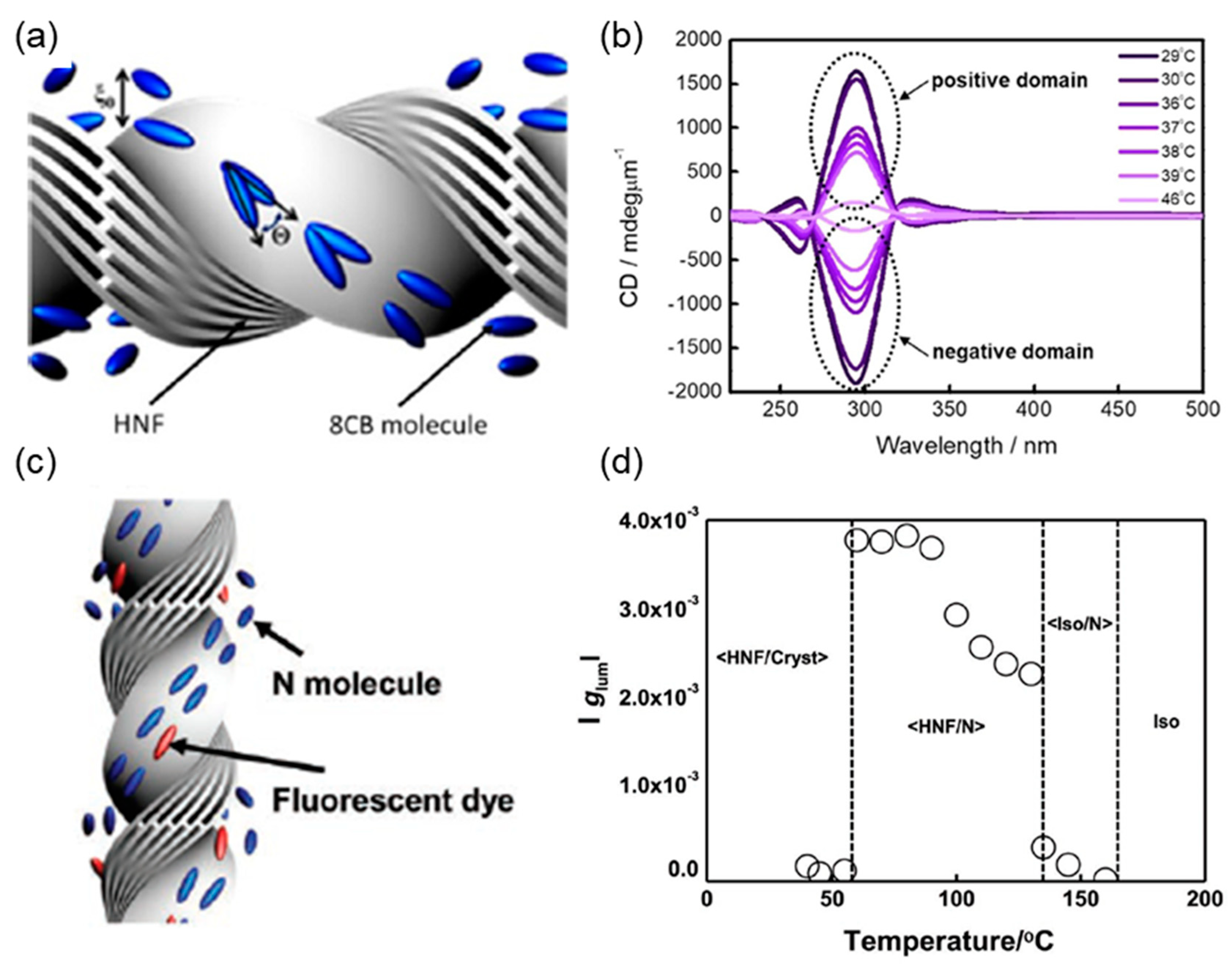
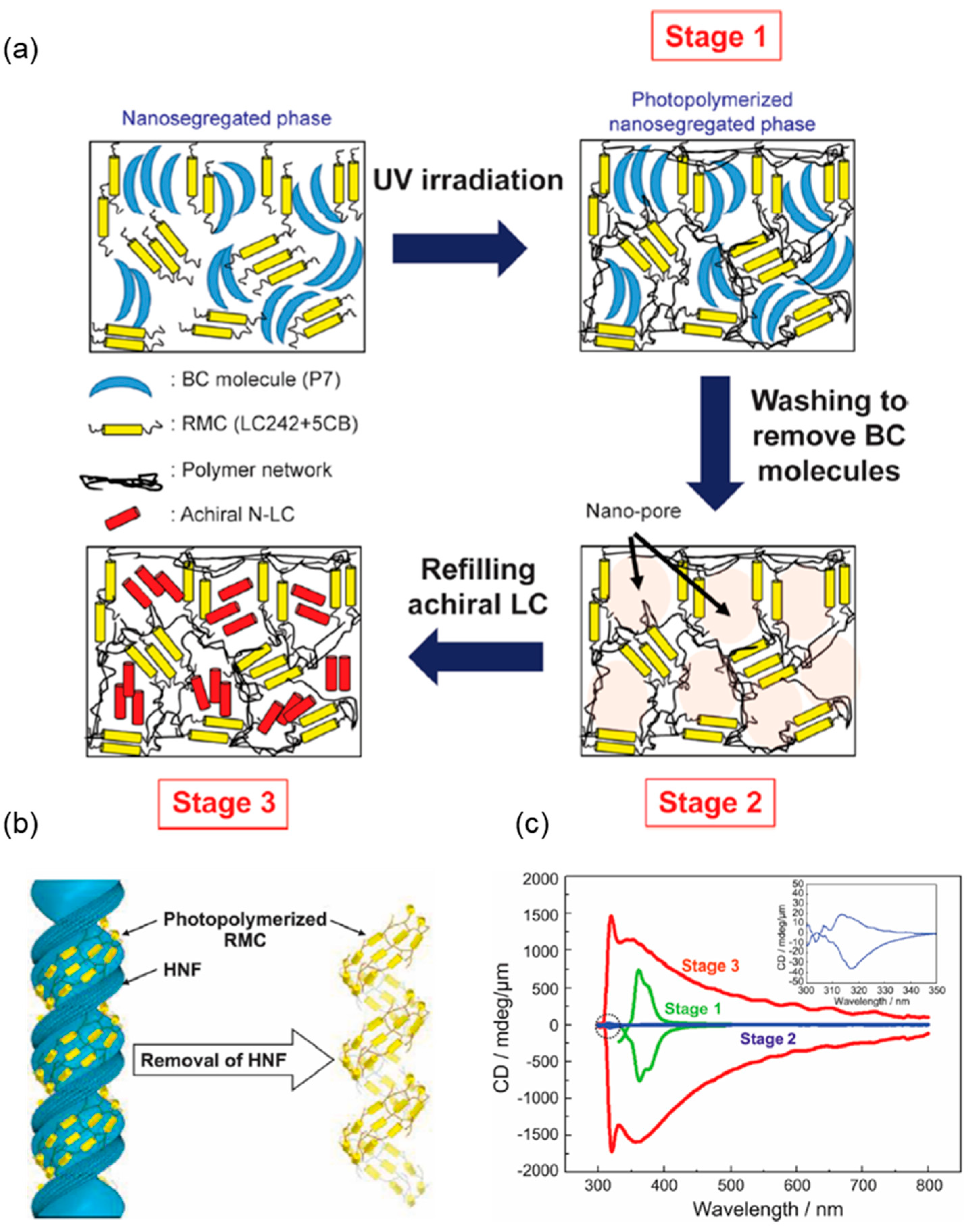
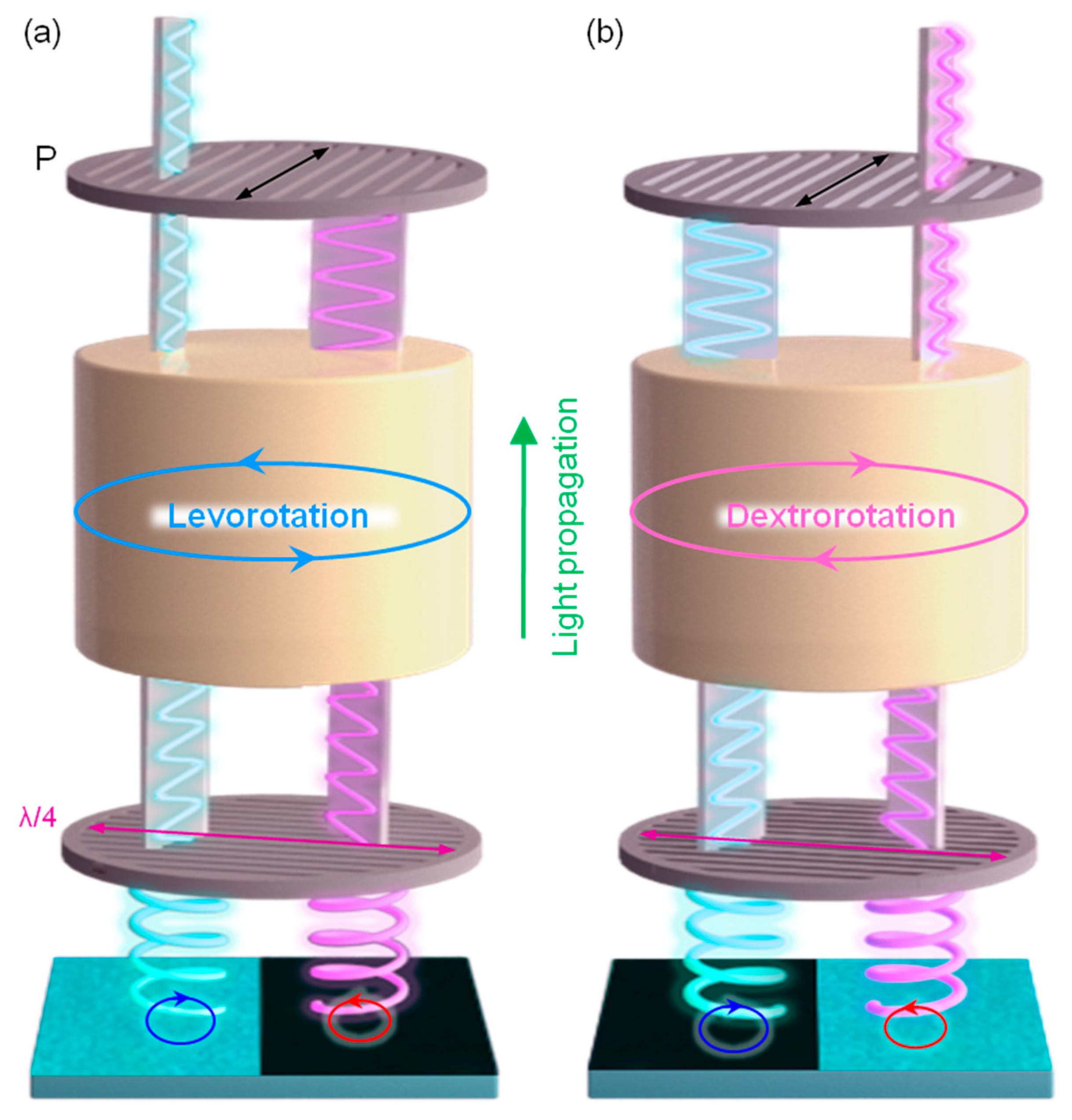
4.1. Template for Chiral Assembly of the Guest Material
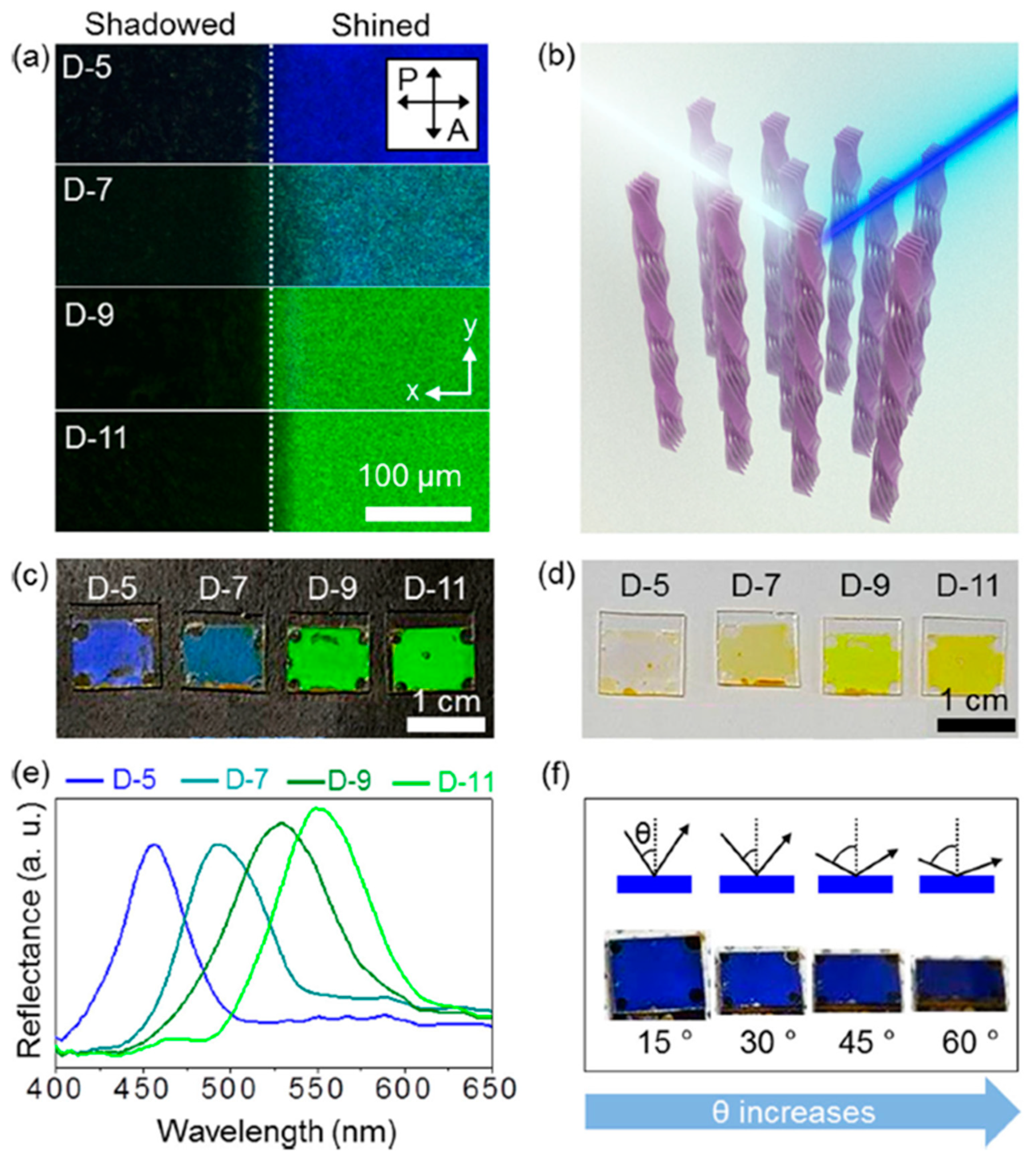
4.2. Photonic Crystal Made of Aligned HNFs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kragt, A.J.J.; Hoekstra, D.C.; Stallinga, S.; Broer, D.J.; Schenning, A.P.H.J. 3D helix engineering in chiral photonic materials. Adv. Mater. 2019, 31, 1903120. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Lee, K.M.; White, T.J.; Jeong, K.-U. Cholesteric liquid crystal paints: In situ photopolymerization of helicoidally stacked multilayer nanostructures for flexible broadband mirrors. NPG Asia Mater. 2018, 10, 1061–1068. [Google Scholar] [CrossRef]
- Lim, C.; Bae, S.; Jeong, S.M.; Ha, N.Y. Manipulation of structural colors in liquid-crystal helical structures deformed by surface controls. ACS Appl. Mater. Interfaces 2018, 10, 12060–12065. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Yokota, M.; Hisakado, Y.; Yang, H.; Kajiyama, T. Polymer-stabilized liquid crystal blue phases. Nat. Mater. 2002, 1, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-G.; Yuan, C.-L.; Hu, W.; Bisoyi, H.K.; Tang, M.J.; Liu, Z.; Sun, P.-Z.; Yang, W.-Q.; Wang, X.-Q.; Shen, D.; et al. Light-patterned crystallographic direction of a self-organized 3D soft photonic crystal. Adv. Mater. 2017, 29, 1703165. [Google Scholar] [CrossRef]
- Hough, L.E.; Jung, H.T.; Kruerke, D.; Heberling, M.S.; Nakata, M.; Jones, C.D.; Chen, D.; Link, D.R.; Zasadzinski, J.; Heppke, G.; et al. Helical nanofilament phases. Science 2009, 325, 456–460. [Google Scholar] [CrossRef]
- Park, W.; Ha, T.; Kim, T.-T.; Zep, A.; Ahn, H.; Shin, T.J.; Sim, K.I.; Jung, T.S.; Kim, J.H.; Pociecha, D.; et al. Directed self-assembly of a helical nanofilament liquid crystal phase for use as structural color reflectors. NPG Asia Mater. 2019, 11, 45. [Google Scholar] [CrossRef]
- Guo, D.-Y.; Chen, C.-W.; Li, C.-C.; Jau, H.-C.; Lin, K.-H.; Feng, T.-M.; Wang, C.-T.; Bunning, T.J.; Khoo, I.C.; Lin, T.-H. Reconfiguration of three-dimensional liquid-crystalline photonic crystals by electrostriction. Nat. Mater. 2020, 19, 94–101. [Google Scholar] [CrossRef]
- Zheng, Z.-G.; Li, Y.; Bisoyi, H.K.; Wang, L.; Bunning, T.J.; Li, Q. Three-dimensional control of the helical axis of a chiral nematic liquid crystal by light. Nature 2016, 531, 352–356. [Google Scholar] [CrossRef]
- Schwartz, M.; Lenzini, G.; Geng, Y.; Ronne, P.B.; Ryan, P.Y.A.; Lagerwall, J.P.F. Cholesteric liquid crystal shells as enabling material for information-rich design and architecture. Adv. Mater. 2018, 30, 1707382. [Google Scholar] [CrossRef]
- Xiong, R.; Yu, S.; Kang, S.; Adstedt, K.M.; Nepal, D.; Bunning, T.J.; Tsukruk, V.V. Integration of optical surface structures with chiral nanocellulose for enhanced chiroptical properties. Adv. Mater. 2020, 32, 1905600. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, A.; Bus, T.; Broer, D.J.; Schenning, A.P.H.J. Patterned full-color reflective coating based on photonic cholesteric liquid-crystalline particles. ACS Appl. Mater. Interfaces 2019, 11, 14376–14382. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ma, L.-L.; Hu, W.; Shen, Z.-X.; Bisoyi, H.K.; Wu, S.-B.; Ge, S.-J.; Li, Q.; Lu, Y.-Q. Chirality invertible superstructure mediated active planar optics. Nat. Commun. 2019, 10, 2518. [Google Scholar] [CrossRef]
- Tsai, E.; Richardson, J.M.; Korblova, E.; Nakata, M.; Chen, D.; Shen, Y.; Shao, R.; Clark, N.A.; Walba, D.M. A modulated helical nanofilament phase. Angew. Chem. Int. Ed. 2013, 52, 5254–5257. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yi, Y.; Chen, D.; Korblova, E.; Walba, D.M.; Clark, N.A.; Yoon, D.K. Self-assembled hydrophobic surface generated from a helical nanofilament (B4) liquid crystal phase. Soft Matter 2013, 9, 2793–2797. [Google Scholar] [CrossRef]
- Callahan, R.A.; Coffey, D.C.; Chen, D.; Clark, N.A.; Rumbles, G.; Walba, D.M. Charge generation measured for fullerene-helical nanofilament liquid crystal heterojunctions. ACS Appl. Mater. Interfaces 2014, 6, 4823–4830. [Google Scholar] [CrossRef] [PubMed]
- Matraszek, J.; Topnani, N.; Vaupotic, N.; Takezoe, H.; Mieczkowski, J.; Pociecha, D.; Gorecka, D. Monolayer filaments versus multilayer stacking of bent-core molecules. Angew. Chem. Int. Ed. 2016, 55, 3468–3472. [Google Scholar] [CrossRef]
- Li, L.; Salamonczyk, M.; Jakli, A.; Hegmann, T. A dual modulated homochiral helical nanofilament phase with local columnar ordering formed by bent core liquid crystals: Effects of molecular chirality. Small 2016, 12, 3944–3955. [Google Scholar] [CrossRef]
- Li, L.; Salamonczyk, M.; Shadpour, S.; Zhu, C.; Jakli, A.; Hegmann, T. An unusual type of polymorphism in a liquid crystal. Nat. Commun. 2018, 9, 714. [Google Scholar] [CrossRef]
- Park, W.; Wolska, J.M.; Pociecha, D.; Gorecka, E.; Yoon, D.K. Direct visualization of optical activity in chiral substances using a helical nanofilament (B4) liquid crystal phase. Adv. Opt. Mater. 2019, 7, 1901399. [Google Scholar] [CrossRef]
- Oh, J.-W.; Chung, W.-J.; Heo, K.; Jin, H.-E.; Lee, B.Y.; Wang, E.; Zueger, C.; Wong, W.; Meyer, J.; Kim, C.; et al. Biomimetic virus-based colourimetric sensors. Nat. Commun. 2013, 5, 3043. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Stathopulos, H.; Lieberzeit, P.A.; Dickert, F.L. Solvent vapour detection with cholesteric liquid crystals-optical and mass-sensitive evaluation of the sensor mechanism. Sensor 2010, 10, 4887–4897. [Google Scholar] [CrossRef] [PubMed]
- Winterbottom, D.A.; Narayanaswamy, R.; Raimundo, I.M., Jr. Cholesteric liquid crystals for detection of organic vapours. Sens. Actuator B Chem. 2003, 90, 52–57. [Google Scholar] [CrossRef]
- Shibaev, P.V.; Sanford, R.L.; Chiappetta, D.; Rivera, P. Novel color changing pH sensors based on cholesteric polymers. Mol. Cryst. Liq. Cryst. 2007, 479, 161–167. [Google Scholar] [CrossRef]
- Noh, K.-G.; Park, S.-Y. Biosensor array of interpenetrating polymer network with photonic film templated from reactive cholesteric liquid crystal and enzyme-immobilized hydrogel polymer. Adv. Funct. Mater. 2018, 28, 1707562. [Google Scholar] [CrossRef]
- Yoshida, J.; Tamura, S.; Yuge, H.; Watanabe, G. Left- and right-circularly polarized light-sensing based on colored and mechano-responsive chiral nematic liquid crystals. Soft Matter 2018, 14, 27–30. [Google Scholar] [CrossRef]
- Shadpour, S.; Nemati, A.; Salamonczyk, M.; Prevot, M.E.; Liu, J.; Boyd, N.J.; Wilson, M.R.; Zhu, C.; Hegmann, E.; Jakli, A.I.; et al. Missing link between helical nano- and microfilaments in B4 phase bent-core liquid crystals, and deciphering which chiral center controls the filament handedness. Small 2020, 16, 1905591. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.; Jo, S.-Y.; Araoka, F.; Yoon, D.K.; Choi, S.-W. Photomodulated supramolecular chirality in achiral photoresponsive rodlike compounds nansegregated from the helical nanofilaments of achiral bent-core molecules. ACS Appl. Mater. Interfaces 2015, 7, 22686–22691. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, B.; Kim, S.K.; Won, J.C.; Kim, Y.H.; Kim, S.-H. Robust microfluidic encapsulation of cholesteric liquid crystals toward photonic ink capsules. Adv. Mater. 2015, 27, 627–633. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, S.K.; Won, J.C.; Kim, Y.H.; Kim, S.H. Reconfigurable photonic capsules containing cholesteric liquid crystals with planar alignment. Angew. Chem. Int. Ed. 2015, 54, 15266–15270. [Google Scholar] [CrossRef]
- Lin, P.; Wei, Z.; Yan, Q.; Chen, Y.; Wu, M.; Xie, J.; Zeng, M.; Wang, W.; Xu, J.; Cheng, Z. Blue phase liquid crystal microcapsules: Confined 3D structure inducing fascinating properties. J. Mater. Chem. C 2019, 7, 4822–4827. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Qi, H.; Hamad, W.Y.; MacLachlan, M.J. Free-standing mesoporous silica films with tunable chiral nematic structures. Nature 2010, 468, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Ha, T.; Jung, T.S.; Sim, K.I.; Kim, J.H.; Wolska, J.M.; Pociecha, D.; Gorecka, E.; Kim, T.-T.; Yoon, D.K. Security use of the chiral photonic film made of helical liquid crystal structures. Nanoscale 2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.H.; Lee, S.; Walba, D.M.; Clark, N.A.; Lee, S.B.; Yoon, D.K. Orientation control over bent-core smectic liquid crystal phases. Liq. Cryst. 2014, 41, 328–341. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Shin, T.J.; Korblova, E.; Walba, D.M.; Clark, N.A.; Lee, S.B.; Yoon, D.K. Multistep hierarchical self-assembly of chiral nanopore arrays. Proc. Natl. Acad. Sci. USA 2014, 111, 14342–14347. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Shin, T.J.; Tsai, E.; Richardson, J.M.; Korblova, E.; Walba, D.M.; Clark, N.A.; Lee, S.B.; Yoon, D.K. Physico-chemical confinement of helical nanofilaments. Soft Matter 2015, 11, 3653–3659. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Tsai, E.; Richardson, J.M.; Korblova, E.; Walba, D.M.; Clark, N.A.; Lee, S.B.; Yoon, D.K. Multidimensional helical nanostructures in multiscale nanochannels. Langmuir 2015, 31, 8156–8161. [Google Scholar] [CrossRef]
- Kim, H.; Zep, A.; Ryu, S.H.; Ahn, H.; Shin, T.J.; Lee, S.B.; Pociecha, D.; Gorecka, E.; Yoon, D.K. Linkage-length dependent structuring behavior of bent-core molecules in helical nanostructures. Soft Matter 2016, 12, 3326–3330. [Google Scholar] [CrossRef]
- Kim, H.; Ryu, S.H.; Tuchband, M.; Shin, T.J.; Korblova, E.; Walba, D.M.; Clark, N.A.; Yoon, D.K. Structural transitions and guest/host complexing of liquid crystal helical nanofilaments induced by nanoconfinement. Sci. Adv. 2017, 3, e1602102. [Google Scholar] [CrossRef]
- Zep, A.; Sitkowska, K.; Pociecha, D.; Gorecka, E. Photoresponsive helical nanofilaments of B4 phase. J. Mater. Chem. C 2014, 2, 2323–2327. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, X.; Prehm, M.; Liu, F.; Grimm, S.; Geuss, M.; Steinhart, M.; Tschierske, C.; Ungar, G. Honeycombs in honeycombs: Complex liquid crystal alumina composite mesostructures. ACS Nano 2014, 8, 4500–4509. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Izumi, T.; Hoshino, Y.; Takanishi, Y.; Ishikawa, K.; Watanabe, J.; Takezoe, H. Circular-polarization-induced enantiomeric excess in liquid crystals on an achiral, bent-shaped mesogen. Angew. Chem. Int. Ed. 2006, 45, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Alaasar, M. Azobenzene-containing bent-core liquid crystals: An overview. Liq. Cryst. 2016, 43, 2208–2243. [Google Scholar] [CrossRef]
- Park, W.; Feringan, B.; Yang, M.; Ryu, S.H.; Ahn, H.; Shin, T.J.; Sierra, T.; Gimenez, T.; Yoon, D.K. Manipulation of supramolecular columnar structures of H-bonded donor-acceptor units through geometrical nanoconfinement. ChemPhysChem 2019, 20, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Baginski, M.; Tupikowska, M.; Gonzalez-Rubio, G.; Wojcik, M.; Lewandowski, W. Shaping liquid crystals with gold nanoparticles: Helical assemblies with tunable and hierarchical structures via thin-film cooperative interaction. Adv. Mater. 2019, 32, 1904581. [Google Scholar] [CrossRef]
- Jeon, S.-W.; Kim, D.-Y.; Araoka, F.; Jeong, K.-U.; Choi, S.-W. Nanosegregated chiral materials with self-assembled hierarchical mesophases: Effect of thermotropic and photoinduced polymorphism in rodlike molecules. Chem. Eur. J. 2017, 23, 17794–17799. [Google Scholar] [CrossRef]
- Kim, B.-C.; Choi, H.-J.; Lee, J.-J.; Araoka, F.; Choi, S.-W. Circularly polarized luminescence induced by chiral super nanospaces. Adv. Funct. Mater. 2019, 29, 1903246. [Google Scholar] [CrossRef]
- Sekine, T.; Niori, T.; Sone, M.; Watanabe, J.; Choi, S.-W.; Takanishi, Y.; Takezoe, H. Origin of helix in achiral banana-shaped molecular system. Jpn. J. Appl. Phys. 1997, 36, 6455–6463. [Google Scholar] [CrossRef]
- Zhang, C.; Diorio, N.; Lavrentovich, O.D.; Jakli, A. Helical nanofilaments of bent-core liquid crystals with a second twist. Nat. Commun. 2014, 5, 3302. [Google Scholar] [CrossRef]
- Zhang, P.; Kragt, A.J.J.; Schenning, A.P.H.J.; de Haan, L.T.; Zhou, G. An easily coatable temperature responsive cholesteric liquid crystal oligomer for making structural colour pattern. J. Mater. Chem. C 2018, 6, 7184–7187. [Google Scholar] [CrossRef]
- Xiang, J.; Varanytsia, A.; Minkowski, F.; Paterson, D.A.; Storey, J.M.D.; Imrie, C.T.; Lavrentovich, O.D.; Palffy-Muhoray, P. Electrically tunable laser based on oblique heliconical cholesteric liquid crystal. Proc. Natl. Acad. Sci. USA 2016, 113, 12925–12928. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.Y.; Ohtsuka, Y.; Jeong, S.M.; Nishimura, S.; Suzaki, G.; Takanishi, Y.; Ishikawa, K.; Takezoe, H. Fabrication of a simultaneous red-green-blue reflector using single-pitched cholesteric liquid crystals. Nat. Mater. 2008, 17, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Bsoul, A.; Walus, K.; Hamad, W.Y.; MacLachlan, M.J. Photonic patterns printed in chiral nematic mesoporous resins. Angew. Chem. Int. Ed. 2015, 54, 4304–4308. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, D.; Gutierrez-Cuevas, K.G.; Bisoyi, H.K.; Fan, J.; Zola, R.S.; Li, G.; Urbas, A.M.; Bunning, T.J.; Weitz, D.A.; et al. Optically reconfigurable chiral microspheres of self-organized helical superstructures with handedness inversion. Mater. Horiz. 2017, 4, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lewis, L.; Hamad, W.Y.; MacLachlan, M.J. Pressure-responsive hierarchical chiral photonic aerogels. Adv. Mater. 2019, 31, 1808186. [Google Scholar] [CrossRef]
- Qu, D.; Chu, G.; Martin, P.; Vasilyev, G.; Vilensky, R.; Zussman, E. Modulating the structural orientation of nanocellulose composites through mechano-stimuli. ACS Appl. Mater. Interfaces 2019, 11, 40443–40450. [Google Scholar] [CrossRef]
- Chen, R.; Lee, Y.-H.; Zhan, T.; Yin, K.; An, Z.; Wu, S.-T. Multistimuli-responsive self-organized liquid crystal bragg gratings. Adv. Opt. Mater. 2019, 7, 1900101. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, D.; Jia, H.; Lei, K.; Zheng, Z.; Wang, X. Humidity and heat dual response cellulose nanocrystals/poly(N-isopropylacrylamide) composite films with cyclic performance. ACS Appl. Mater. Interfaces 2019, 11, 39192–39200. [Google Scholar] [CrossRef]
- Yuan, C.-I.; Huang, W.; Zheng, Z.-G.; Liu, B.; Bisoyi, H.K.; Li, Y.; Shen, D.; Lu, Y.; Li, Q. Stimulated transformation of soft helix among helicoidal, heliconical, and their inverse helices. Sci. Adv. 2019, 5, eaax9501. [Google Scholar] [CrossRef]
- Araoka, F.; Sugiyama, G.; Ishikawa, K.; Takezoe, H. Highly ordered helical nanofilament assembly aligned by a nematic director field. Adv. Funct. Mater. 2013. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Shin, T.J.; Cha, Y.J.; Korblova, E.; Walba, D.M.; Clark, N.A.; Lee, S.B.; Yoon, D.K. Alignment of helical nanofilaments on the surfaces of various self-assembled monolayers. Soft Matter 2013, 9, 6185–6191. [Google Scholar] [CrossRef]
- Yoon, D.K.; Yi, Y.; Shen, Y.; Korblova, E.D.; Walba, D.M.; Smalyukh, I.I.; Clark, N.A. Orientation of a helical nanofilament (B4) liquid-crystal phase: Topographic control of confinement, shear flow, and temperature gradients. Adv. Mater. 2011, 23, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Park, S.J. Porous anodic aluminum oxide: Anodization and templated synthesis of functional nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Foley, L.; Park, W.; Yang, M.; Carlson, E.; Korblova, E.; Yoon, D.K.; Walba, D.M. Nanoconfinement of the low-temperature dark conglomerate: Structural control from focal conics to helical nanofilaments. Chem. Eur. J. 2019, 25, 7438–7442. [Google Scholar] [CrossRef]
- You, R.; Park, W.; Carlson, E.; Ryu, S.H.; Shin, M.J.; Guzman, E.; Ahn, H.; Shin, T.J.; Walba, D.M.; Clark, N.A.; et al. Nanoconfined heliconical structure of twist-bend nematic liquid crystal phase. Liq. Cryst. 2019, 46, 316–325. [Google Scholar] [CrossRef]
- Zhang, R.; Zeng, X.; Kim, B.; Bushby, R.J.; Shin, K.; Baker, P.J.; Percec, V.; Leowanawat, P.; Ungar, G. Columnar liquid crystals in cylindrical nanoconfinement. ACS Nano 2015, 9, 1759–1766. [Google Scholar] [CrossRef]
- Ryu, S.H.; Ahn, H.; Shin, T.J.; Yoon, D.K. Direct observation of liquid crystal phases under nanoconfinement: A grazing incidence X-ray diffraction study. Liq. Cryst. 2016, 44, 713–721. [Google Scholar] [CrossRef]
- Zep, A.; Salamonczyk, M.; Vaupotic, N.; Pociecha, D.; Gorecka, E. Physical gels made of liquid crystalline B4 phase. Chem. Commun. 2013, 49, 3119–3121. [Google Scholar] [CrossRef]
- Chen, D.; Maclennan, J.E.; Shao, R.; Yoon, D.K.; Wang, H.; Korblova, E.; Walba, D.M.; Glaser, M.A.; Clark, N.A. Chirality-preserving growth of helical filaments in the B4 phase of bent-core liquid crystals. J. Am. Chem. Soc. 2011, 133, 12656–12663. [Google Scholar] [CrossRef]
- Shadpour, S.; Nemati, A.; Liu, J.; Hegmann, T. Directing the handedness of helical nanofilaments confined in nanochannels using axially chiral binaphthyl dopants. ACS Appl. Mater. Interfaces 2020, 12, 13456–13463. [Google Scholar] [CrossRef]
- Yu, C.-J.; Kim, D.-W.; Lee, S.-D. Multimode transflective liquid crystal display with a single cell gap using a self-masking process of photoalignment. Appl. Phys. Lett. 2004, 85, 5146–5148. [Google Scholar] [CrossRef]
- Meng, C.; Chen, E.; Wang, L.; Tang, S.; Tsng, M.; Guo, J.; Ye, Y.; Yan, Q.F.; Kwok, H. Color-switchable liquid crystal smart window with multi-layered light guiding structures. Opt. Exp. 2019, 27, 13098–13107. [Google Scholar] [CrossRef]
- Ware, T.H.; McConney, M.E.; Wie, J.J.; Tondiglia, V.P.; White, T.J. Voxelated liquid crystal elastomers. Science 2015, 347, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Lee, K.M.; Evans, D.R.; McConney, M.E.; Kim, D.-Y.; Jeong, K.-U. Giant surfactants for the construction of automatic liquid crystal alignment layers. J. Mater. Chem. C 2019, 7, 8500. [Google Scholar] [CrossRef]
- Lv, J.-A.; Liu, Y.; Wei, J.; Chen, E.; Qin, L.; Yu, Y. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature 2016, 537, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Toshchevikov, V.; Petrova, T.; Saphiannikova, M. Kinetics of light-induced ordering and deformation in LC azobenzene-containing materials. Soft Matter 2017, 13, 2823–2835. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, C.; Wang, H.; Maclennan, J.E.; Glaser, M.A.; Korblova, E.; Walba, D.M.; Rego, J.A.; Soto-Bustamante, E.A.; Clark, N.A. Nanoconfinement of guest materials by helical nanofilament networks of bent-core mesogens. Soft Matter 2013, 9, 462–471. [Google Scholar] [CrossRef]
- Xiong, R.; Yu, S.; Smith, M.J.; Zhou, J.; Krecker, M.; Zhang, L.; Nepal, D.; Bunning, T.J.; Tsukruk, V.V. Self-assembly of emissive nanocellulose/quantum dot nanostructures for chiral fluorescent materials. ACS Nano 2019, 13, 9074–9081. [Google Scholar] [CrossRef]
- Li, J.; Bisoyi, H.K.; Tian, J.; Guo, J.; Li, Q. Optically rewritable transparent liquid crystal displays enabled by light-driven chiral fluorescent molecular switches. Adv. Mater. 2019, 31, 1807751. [Google Scholar] [CrossRef]
- Li, J.; Bisoyi, H.K.; Lin, S.; Guo, J.; Li, Q. 1,2-Dithienyldicyanoethene-based, visible-light-driven, chiral fluorescent molecular switch: Rewritable multimodal photonic devices. Angew. Chem. Int. Ed. 2019, 58, 16052–16056. [Google Scholar] [CrossRef]
- Xu, M.; Ma, C.; Zhou, J.; Liu, Y.; Wu, X.; Luo, S.; Li, W.; Yu, H.; Wang, Y.; Chen, Z.; et al. Assembling semiconductor quantum dots in hierarchical photonic cellulose nanocrystal films: Circularly polarized luminescent nanomaterials as optical coding labels. J. Mater. Chem. C 2019, 7, 13794–13802. [Google Scholar] [CrossRef]
- Rofouie, P.; Galati, E.; Sun, L.; Helmy, A.S.; Kumacheva, E. Hybrid cholesteric films with tailored polarization rotation. Adv. Funct. Mater. 2019, 29, 1905552. [Google Scholar] [CrossRef]
- Cheng, Z.; Ma, Y.; Yang, L.; Cheng, F.; Hung, Z.; Natan, A.; Li, H.; Chen, Y.; Cao, D.; Huang, Z.; et al. Plasmonic-enhanced cholesteric films: Coassembling anisotropic gold nanorods with cellulose nanocrystals. Adv. Opt. Mater. 2019, 7, 1801816. [Google Scholar] [CrossRef]
- Kuzyk, A.; Schreiber, R.; Fan, Z.; Pardatscher, G.; Roller, E.-M.; Hogele, A.; Simmel, F.C.; Govorov, A.O.; Liedl, T. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 2012, 483, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Gansel, J.K.; Thiel, M.; Rill, M.S.; Decker, M.; Bade, K.; Saile, V.; Freymann, G.V.; Linden, S.; Wegener, M. Gold helix photonic metamaterial as broadband circular polarizer. Science 2009, 325, 1513–1515. [Google Scholar] [CrossRef]
- Kumar, J.; Nakashima, T.; Kawai, T. Circularly polarized luminescence in chiral molecules and supramolecular assemblies. J. Phys. Chem. Lett. 2015, 6, 3445–3452. [Google Scholar] [CrossRef]
- Haryono, A.; Binder, W.H. Controlled arrangement of nanoparticle arrays in block-copolymer domains. Small 2006, 2, 600–611. [Google Scholar] [CrossRef]
- Kim, D.S.; Honglawan, A.; Yang, S.; Yoon, D.K. Arrangement and SERS applications of nanoparticle clusters using liquid crystalline template. ACS Appl. Mater. Interfaces 2017, 9, 7787–7792. [Google Scholar] [CrossRef]
- Cha, Y.J.; Kim, D.S.; Yoon, D.K. Highly aligned plasmonic gold nanorods in a DNA matrix. Adv. Funct. Mater. 2017, 27, 1703790. [Google Scholar] [CrossRef]
- Lee, J.-J.; Kim, B.-C.; Choi, H.-J.; Bae, S.; Araoka, F.; Choi, S.-W. Inverse helical nanofilament networks serving as a chiral nanotemplate. ACS Nano 2020, 14, 5243–5250. [Google Scholar] [CrossRef]
- Jahani, S.; Jacob, Z. All-dielectric metamaterials. Nat. Nanotechnol. 2016, 11, 23–36. [Google Scholar] [PubMed]
- Ginn, J.C.; Brener, I. Realizing optical magnetism from dielectric metamaterials. Phys. Rev. Lett. 2012, 108, 097402. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.K.; Deb, R.; Chen, D.; Korblova, E.; Shao, R.; Ishikawa, K.; Rao, N.V.S.; Walba, D.M.; Smalyukh, I.I.; Clark, N.A. Organization of the polarization splay modulated smectic liquid crystal phase by topographic confinement. Proc. Natl. Acad. Sci. USA 2010, 107, 21311–21315. [Google Scholar] [CrossRef]
- Kim, H.; Ge, J.; Kim, J.; Choi, S.-E.; Lee, H.; Lee, H.; Park, W.; Yin, Y.; Kwon, S. Structural colour printing using a magnetically tunable and lithographically fixable photonic crystal. Nat. Photonics. 2009, 3, 534–540. [Google Scholar] [CrossRef]
- Qin, L.; Gu, W.; Wei, J.; Yu, Y. Piecewise phototuning of self-organized helical superstructures. Adv. Mater. 2018, 30, 1704941. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Yu, S.; Heo, S.-J.; Shim, J.W.; Yang, S.-M.; Han, M.G.; Lee, H.-S.; Jin, Y.; Lee, S.Y.; Park, N.; et al. Flexible, Angle-independent, structural color reflectors Inspired by morpho butterfly wings. Adv. Mater. 2012, 24, 2375–2379. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Lyu, Q.; Xie, Z.; Li, M.; Wang, K.; Wang, K.; Xiong, B.; Zhang, L.; Zhu, J. Metallosupramolecular photonic elastomers with self-healing capability and angle-independent color. Adv. Mater. 2019, 31, 1805496. [Google Scholar] [CrossRef]
- Chan, C.L.C.; Bay, M.M.; Jacucci, G.; Vadrucci, R.; Williams, C.A.; Kerkhof, G.T.V.D.; Parker, R.M.; Vynck, K.; Frka-Petesic, B.; Vignolini, S. Visual appearance of chiral nematic cellulose-based photonic films: Angular and polarization independent color response with a twist. Adv. Mater. 2019, 31, 1905151. [Google Scholar] [CrossRef]
- Chen, D.; Tuchband, M.R.; Horanyi, B.; Korblova, E.; Walba, D.M.; Claser, M.A.; Maclennan, J.E.; Clark, N.A. Diastereomeric liquid crystal domains at the mesoscale. Nat. Commun. 2015, 6, 7763. [Google Scholar] [CrossRef]
- Tuchband, M.R.; Chen, D.; Horanyi, B.; Shuai, M.; Shen, Y.; Korblova, E.; Walba, D.M.; Kapernaum, N.; Giesselmann, F.; Glaser, M.A.; et al. Manipulating the twist sense of helical nanofilaments of bent-core liquid crystals using rod-shaped, chiral mesogenic dopants. Liq. Cryst. 2016, 43, 1083–1091. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, Z.; Chen, R.; Zhu, C.; Shoemaker, R.K.; Tsai, E.; Walba, D.M.; Glaser, M.A.; Maclennan, J.E.; Chen, D.; et al. Effect of conformational chirality on optical activity observed in a smectic of achiral, bent-core molecules. J. Phys. Chem. B 2017, 121, 6944–6950. [Google Scholar] [CrossRef] [PubMed]
- Amabilino, D.B. How deformation can land a hand to molecular ordering. Science 2009, 325, 402–403. [Google Scholar] [CrossRef]
- Sharma, V.; Crne, M.; Park, J.O.; Srinivasarao, M. Structural origin of circularly polarized iridescence in jeweled beetles. Science 2009, 325, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, W.; Li, W.; Wang, X.; Tang, Z.; Zhang, S.X.-A.; Xu, Y. Uncovering the circular polarization potential of chiral photonic cellulose films for photonic applications. Adv. Mater. 2018, 30, 1705948. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Ding, D.; Yang, X.; Hou, K.; Miao, X.; Wang, D.; Kou, B.; Huang, L.; Tang, Z. Biomimetic chiral photonic crystals. Angew Chem. Int. Ed. 2019, 58, 7783–7787. [Google Scholar] [CrossRef]
- Wang, H.; Bisoyi, H.K.; Urbas, A.M.; Bunning, T.J.; Li, Q. Reversible circularly polarized reflection in a self-organized helical superstructure enabled by a visible-light-driven axially chiral molecular switch. J. Am. Chem. Soc. 2019, 141, 8078–8082. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W.; Yoon, D.K. Orientation Control of Helical Nanofilament Phase and Its Chiroptical Applications. Crystals 2020, 10, 675. https://doi.org/10.3390/cryst10080675
Park W, Yoon DK. Orientation Control of Helical Nanofilament Phase and Its Chiroptical Applications. Crystals. 2020; 10(8):675. https://doi.org/10.3390/cryst10080675
Chicago/Turabian StylePark, Wongi, and Dong Ki Yoon. 2020. "Orientation Control of Helical Nanofilament Phase and Its Chiroptical Applications" Crystals 10, no. 8: 675. https://doi.org/10.3390/cryst10080675
APA StylePark, W., & Yoon, D. K. (2020). Orientation Control of Helical Nanofilament Phase and Its Chiroptical Applications. Crystals, 10(8), 675. https://doi.org/10.3390/cryst10080675





