New Mononuclear Complex of Europium(III) and Benzoic Acid: From Synthesis and Crystal Structure Solution to Luminescence Emission
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis
2.2.1. Synthesis of Complex 2: [Eu(OOCC6H5)3·(H2O)3]
2.2.2. Synthesis of Complex 3: [Eu(OOCC6H5)3·(HOOCC6H5)2]
3. Results and Discussion
3.1. Infrared Spectroscopy
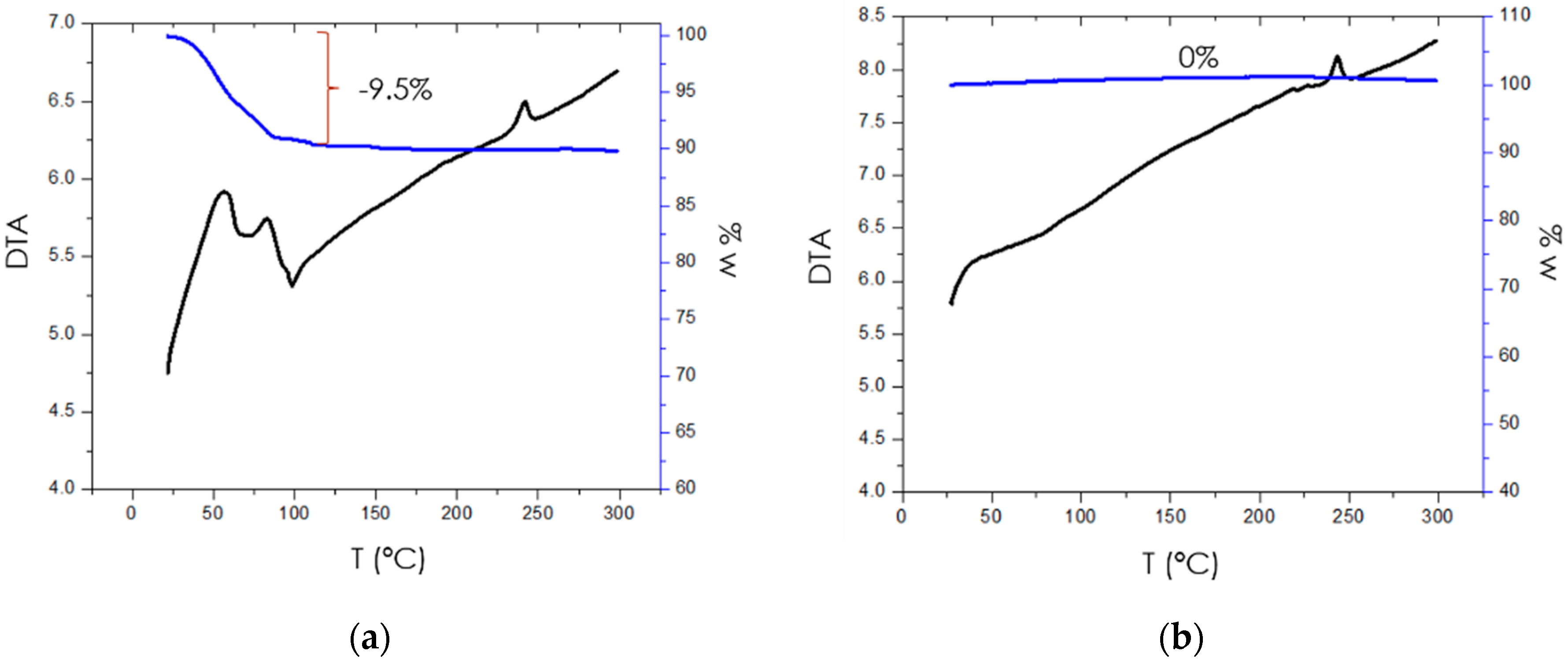
3.2. Thermogravimetric Analysis
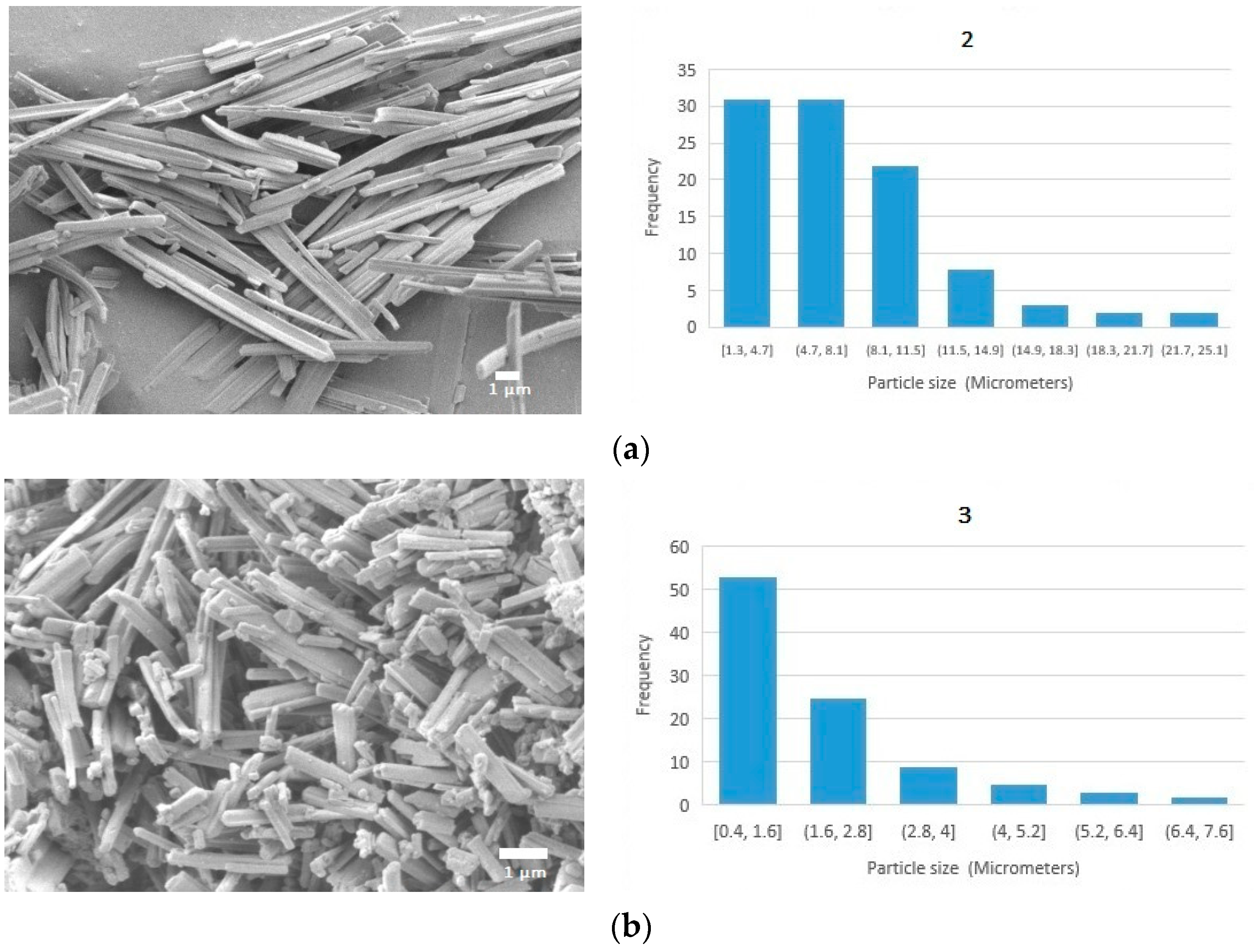
3.3. Morphologic Studies
3.3.1. Scanning Electron Microscopy
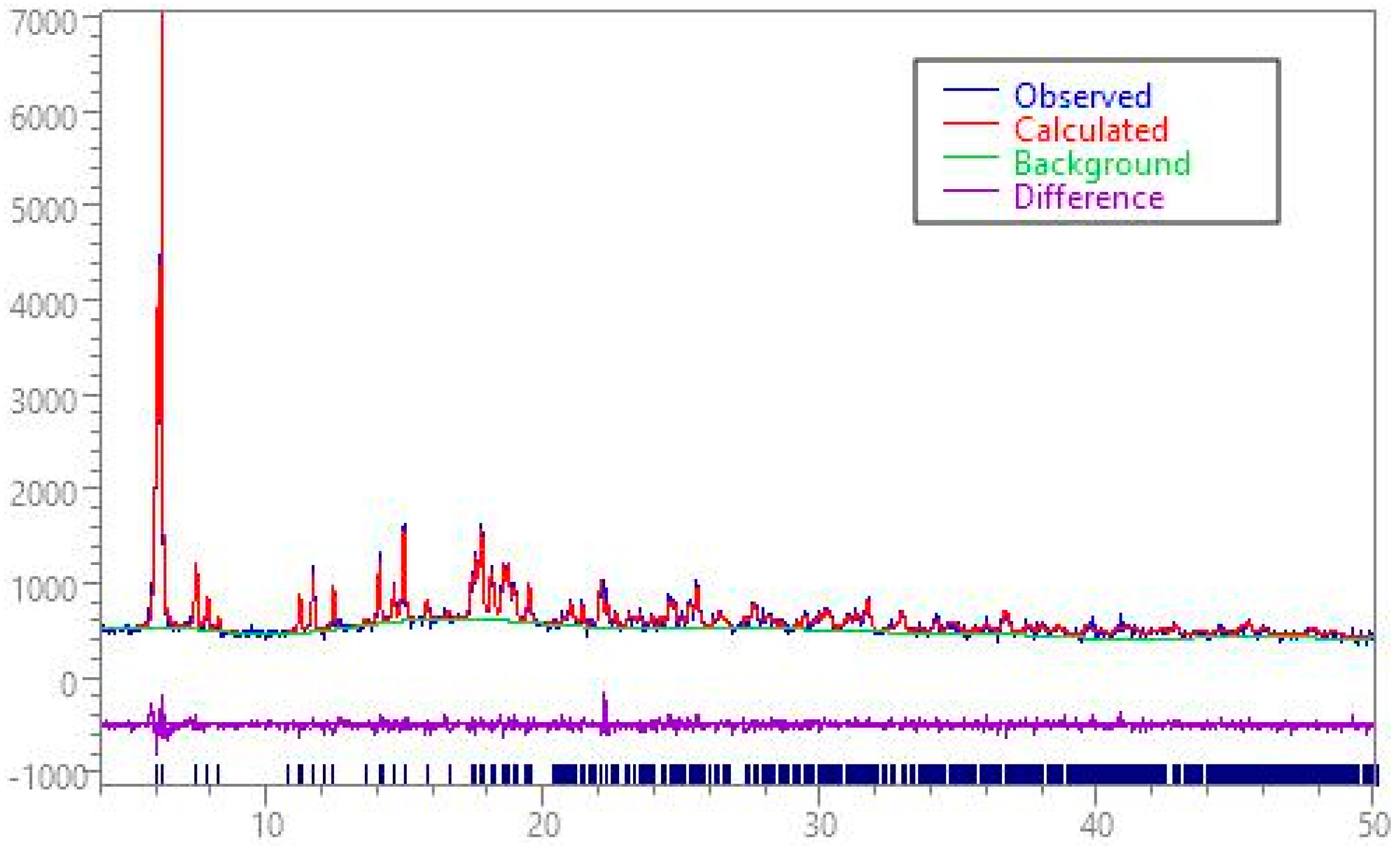
3.3.2. X-ray Powder Diffraction Data
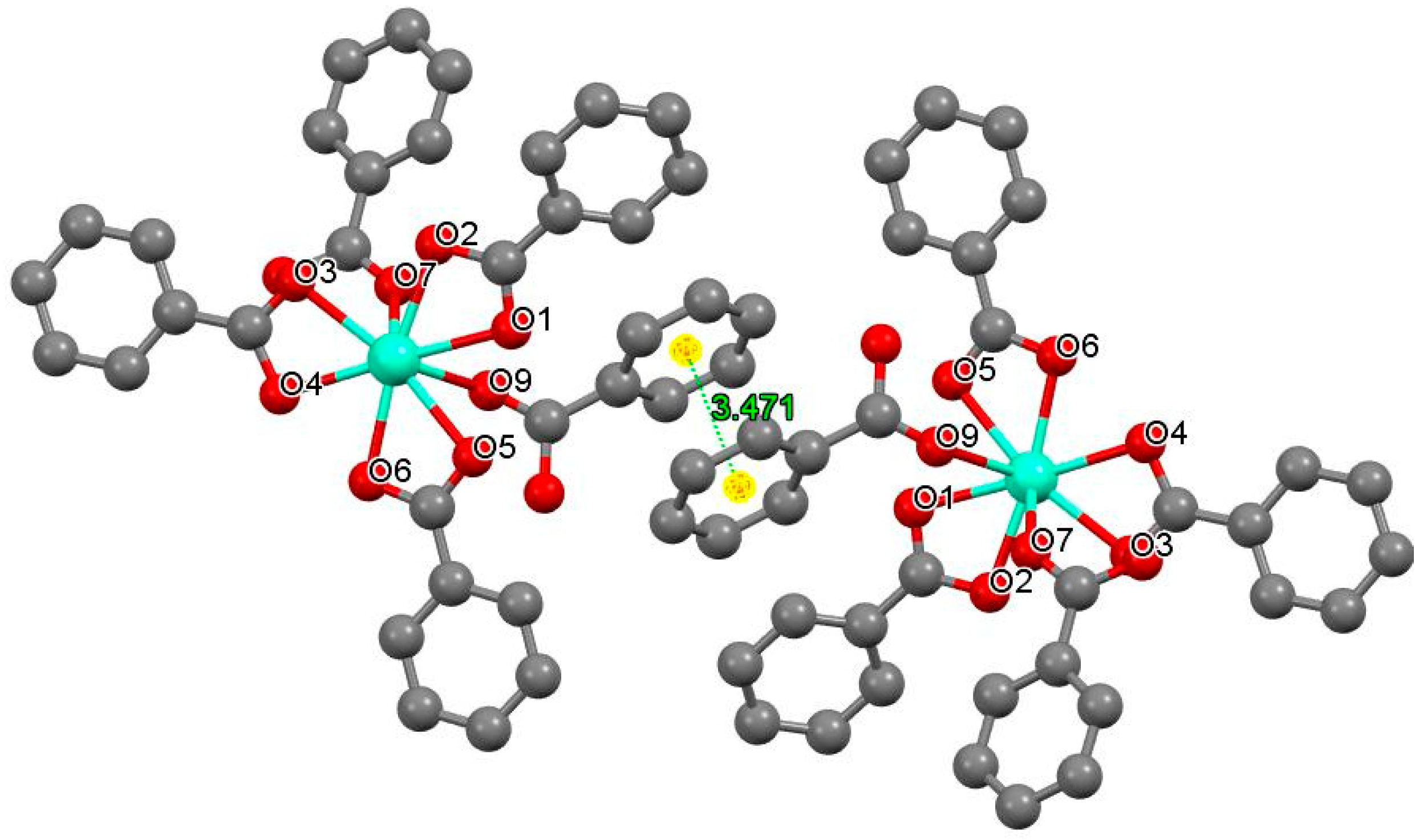
3.4. Crystal Structure Solution
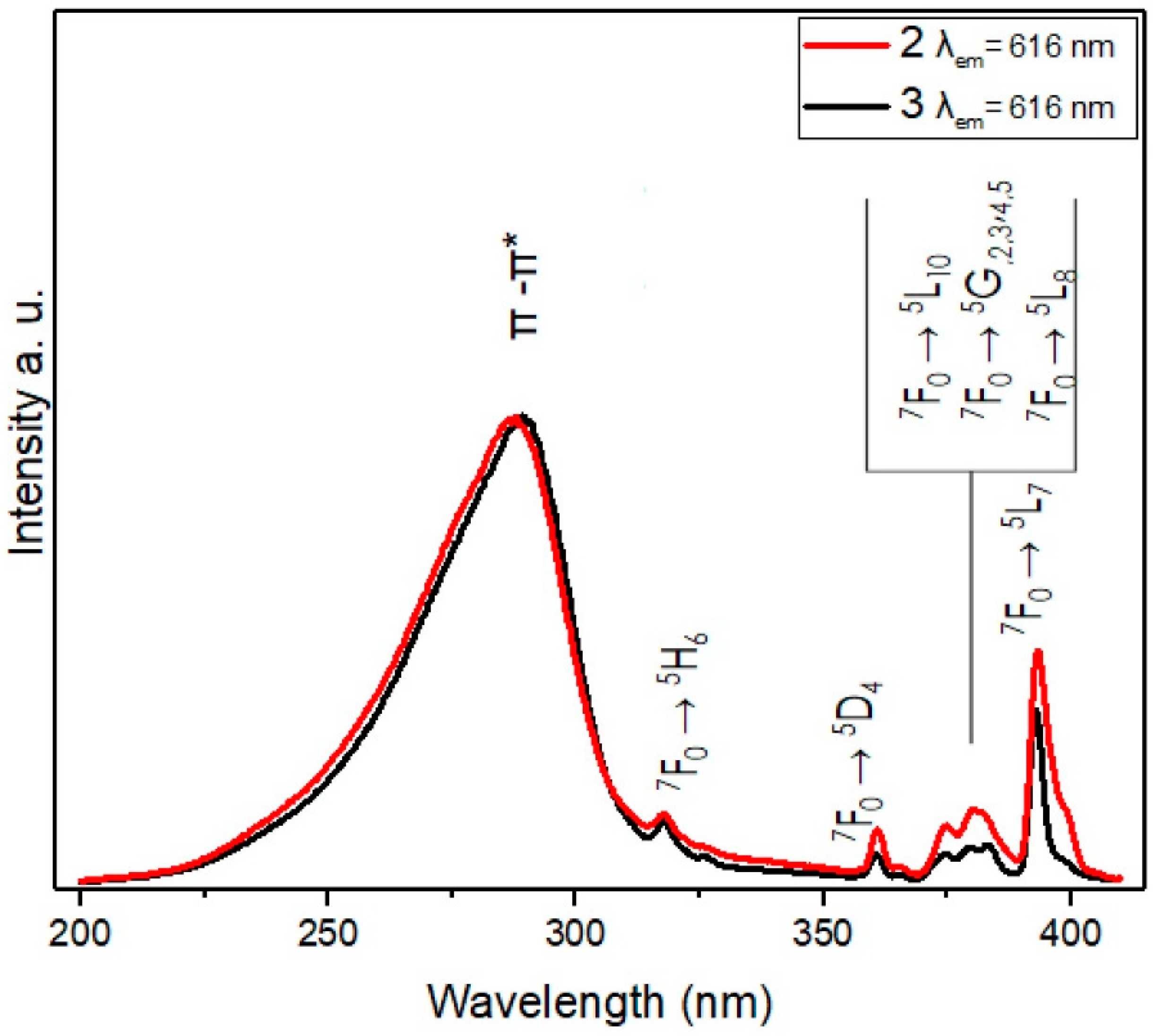
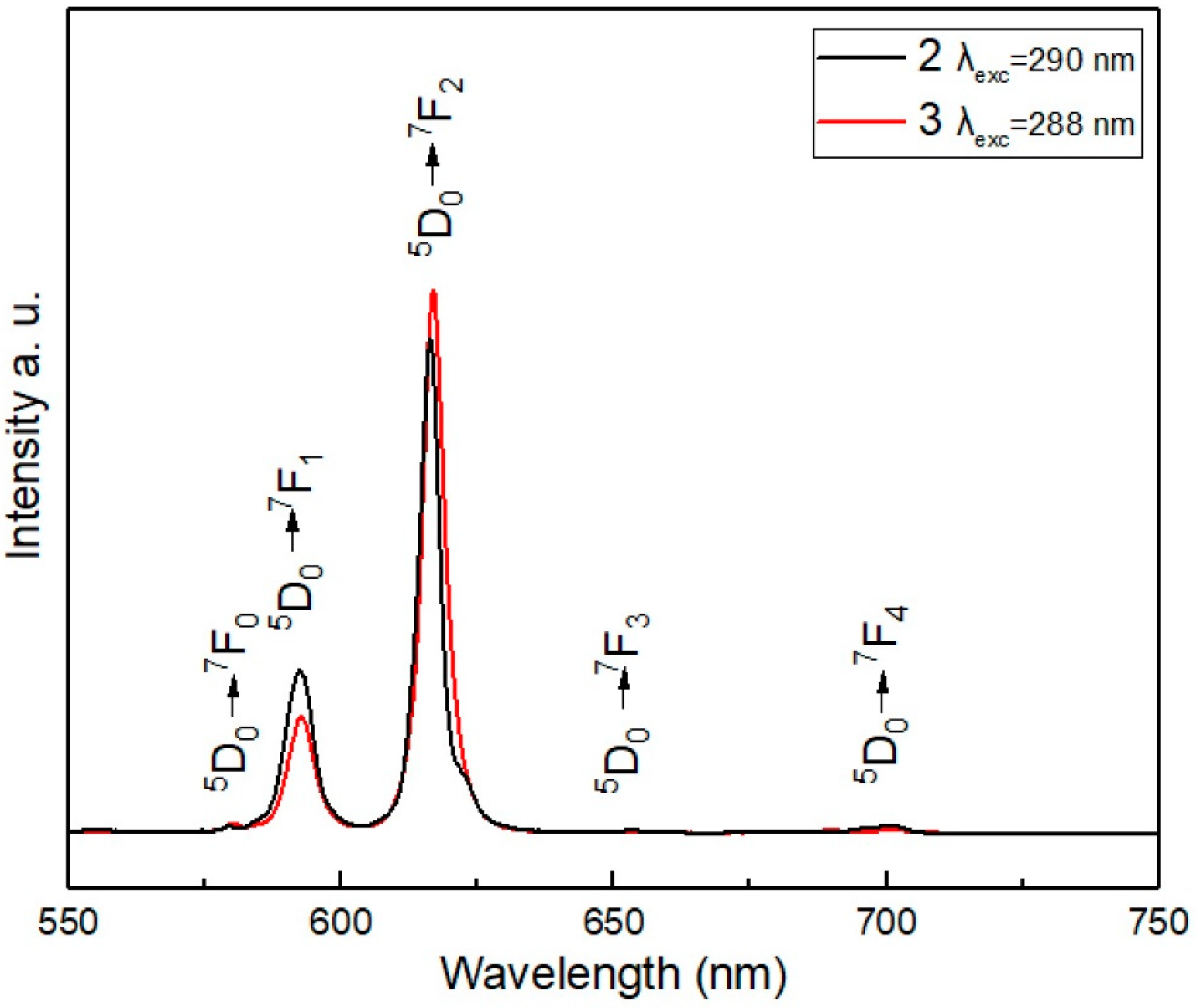
3.5. Luminescence Studies: Excitation and Emission Spectra
3.5.1. Excitation and Emission Spectra
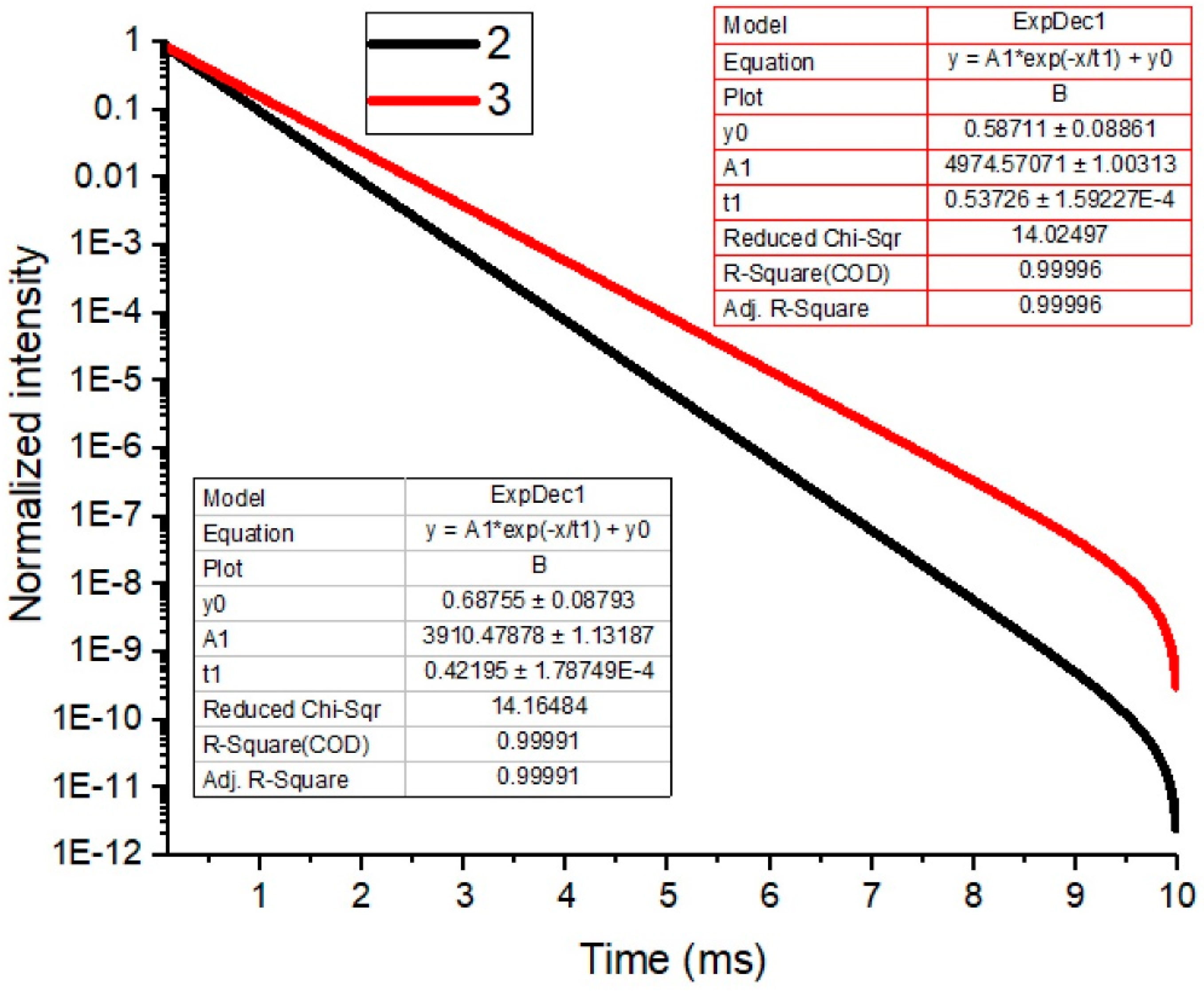
3.5.2. Luminescence Decay Profiles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Doat, A.; Fanjul, M.; Pellé, F.; Hollande, E.; Lebugle, A. Europium-doped bioapatite: A new photostable biological probe, internalizable by human cells. Biomaterials 2003, 24, 3365–3371. [Google Scholar] [CrossRef]
- Lai, J.; Wang, T.; Zhang, H.; Ye, L.; Yan, C.; Gu, W. Modulating the photoluminescence of europium oxide nanoparticles by controlling thermal decomposition conditions. J. Lumin. 2019, 214, 116534. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, F.; Liu, C.; Yu, C.; Zhang, M.; He, Q.; Xiong, Y.; Xu, Z.; Yang, S.; Liao, G. A novel nanotheranostic agent for dual-mode imaging-guided cancer therapy based on europium complexes-grafted-oxidative dopamine. Chem. Eng. J. 2019, 357, 237–247. [Google Scholar] [CrossRef]
- Xuan, Y.; Song, X.L.; Yang, X.Q.; Zhang, R.Y.; Song, Z.Y.; Zhao, D.H.; Hou, X.L.; An, J.; Zhang, X.S.; Zhao, Y. Di Bismuth particles imbedded degradable nanohydrogel prepared by one-step method for tumor dual-mode imaging and chemo-photothermal combined therapy. Chem. Eng. J. 2019, 375, 122000. [Google Scholar] [CrossRef]
- Decadt, R.; Van Hecke, K.; Depla, D.; Leus, K.; Weinberger, D.; Van Driessche, I.; Van Der Voort, P.; Van Deun, R. Synthesis, Crystal Structures, and Luminescence Properties of Carboxylate Based Rare-Earth Coordination Polymers. Inorg. Chem. 2012, 51, 11623–11634. [Google Scholar] [CrossRef]
- Singh, R.; King, A.; Nayak, B.B. Reddish emission of europium doped zinc oxide nanophosphor prepared through precipitation route using sodium borohydride. J. Alloys Compd. 2019, 792, 1191–1199. [Google Scholar] [CrossRef]
- Xu, H.; Sun, Q.; An, Z.; Wei, Y.; Liu, X. Electroluminescence from europium(III) complexes. Coord. Chem. Rev. 2015, 293–294, 228–249. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Mech, A.; Karbowiak, M.; Görller-Walrand, C.; Van Deun, R. The luminescence properties of three tetrakis dibenzoylmethane europium(III) complexes with different counter ions. J. Alloys Compd. 2008, 451, 215–219. [Google Scholar] [CrossRef]
- Pham, Y.H.; Trush, V.A.; Amirkhanov, V.M.; Gawryszewska, P. Structural and spectroscopic study of the europium complex with N-(diphenylphosphoryl)pyrazine-2-carboxamide. Opt. Mater. 2017, 74, 197–200. [Google Scholar] [CrossRef]
- Akerboom, S.; Meijer, M.S.; Siegler, M.A.; Fu, W.T.; Bouwman, E. Structure, Photo-and triboluminescence of the lanthanoid dibenzoylmethanates: HNEt3[Ln(dbm)4]. J. Lumin. 2014, 145, 278–282. [Google Scholar] [CrossRef]
- Hilder, M.; Lezhnina, M.; Junk, P.C.; Kynast, U.H. Spectroscopic properties of lanthanoid benzene carboxylates in the solid state: Part 3. N-heteroaromatic benzoates and 2-furanates. Polyhedron 2013, 52, 804–809. [Google Scholar] [CrossRef]
- Lee, B.H.; Chung, K.H.; Shin, H.S.; Park, Y.J.; Moon, H. Europium(III) complexes of polyfunctional carboxylic acids: Luminescence probes of possible binding sites in fulvic acid. J. Colloid Interface Sci. 1997, 188, 439–443. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Poteat, J.L.; Zhang, L. Complexation of europium using polyethylene carboxylic acid. React. Polym. 1993, 20, 99–109. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.L.P.; Sivakumar, S. Lanthanide benzoates: A versatile building block for the construction of efficient light emitting materials. Dalt. Trans. 2013, 42, 2663–2678. [Google Scholar] [CrossRef]
- Nakajima, A.; Nakanishi, T.; Kitagawa, Y.; Seki, T.; Ito, H.; Fushimi, K.; Hasegawa, Y. Hyper-stable organo-Eu III luminophore under high temperature for photo-industrial application. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Ramya, A.R.; Reddy, M.L.P.; Cowley, A.H.; Vasudevan, K.V. Synthesis, crystal structure, and photoluminescence of homodinuclear lanthanide 4-(dibenzylamino)benzoate complexes. Inorg. Chem. 2010, 49, 2407–2415. [Google Scholar] [CrossRef]
- Carter, K.P.; Thomas, K.E.; Pope, S.J.A.; Holmberg, R.J.; Butcher, R.; Murugesu, M.; Cahill, C.L. Supramolecular Assembly of Molecular Rare-Earth-3,5-Dichlorobenzoic Acid-2,2′:6′,2″-Terpyridine Materials: Structural Systematics, Luminescence Properties, and Magnetic Behavior. Inorg. Chem. 2016, 55, 6902–6915. [Google Scholar] [CrossRef]
- Carter, K.P.; Pope, S.J.A.; Cahill, C.L. A series of Ln-p-chlorobenzoic acid-terpyridine complexes: Lanthanide contraction effects, supramolecular interactions and luminescent behavior. CrystEngComm 2014, 16, 1873–1884. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Zhang, J.J.; Wang, Z.Q.; Lin, H. Photon Conversion and Radiation Synergism in Eu/Tb Complexes Incorporated Poly Methyl Methacrylate. Adv. Mater. Sci. Eng. 2016, 2016, 2618253. [Google Scholar] [CrossRef]
- Wang, Z.M.; Van De Burgt, L.J.; Choppin, G.R. Spectroscopic study of lanthanide(III) complexes with carboxylic acids. Inorg. Chim. Acta 1999, 293, 167–177. [Google Scholar] [CrossRef]
- Champness, N.R. The future of metal-organic frameworks. Dalt. Trans. 2011, 40, 10311–10315. [Google Scholar] [CrossRef] [PubMed]
- Utochnikova, V.V.; Solodukhin, N.N.; Aslandukov, A.N.; Marciniak, L.; Bushmarinov, I.S.; Vashchenko, A.A.; Kuzmina, N.P. Lanthanide tetrafluorobenzoates as emitters for OLEDs: New approach for host selection. Org. Electron. Phys. Mater. Appl. 2017, 44, 85–93. [Google Scholar] [CrossRef]
- Fiedler, T.; Hilder, M.; Junk, P.C.; Kynast, U.H.; Lezhnina, M.M.; Warzala, M. Synthesis, structural and spectroscopic studies on the lanthanoid p-aminobenzoates and derived optically functional polyurethane composites. Eur. J. Inorg. Chem. 2007, 2007, 291–301. [Google Scholar] [CrossRef]
- Chen, X.-F.; Duan, C.-Y.; Zhu, X.-H.; You, X.-Z.; Raj, S.S.S.; Fun, H.-K.; Wu, J. Triboluminescence and crystal structures of europium(III) complexes. Mater. Chem. Phys. 2001, 72, 11–15. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.X.; Zhang, L.; Chen, Y.; Zhang, Q.T. Co-luminescence properties of terbium ions-benzoic acid-phen complexes doped with europium ions. Rare Met. 2013, 32, 599–604. [Google Scholar] [CrossRef]
- Borges, A.S.; Dutra, J.D.L.; Freire, R.O.; Moura, R.T.; Da Silva, J.G.; Malta, O.L.; Araujo, M.H.; Brito, H.F. Synthesis and characterization of the europium(III) pentakis(picrate) complexes with imidazolium countercations: Structural and photoluminescence study. Inorg. Chem. 2012, 51, 12867–12878. [Google Scholar] [CrossRef]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Hilder, M.; Junk, P.C.; Kynast, U.H.; Lezhnina, M.M. Spectroscopic properties of lanthanoid benzene carboxylates in the solid state: Part 1. J. Photochem. Photobiol. A Chem. 2009, 202, 10–20. [Google Scholar] [CrossRef]
- de Oliveira, T.C.; Santos, H.P.; Lahoud, M.G.; Franco, D.F.; Freire, R.O.; Dutra, J.D.L.; Cuin, A.; de Lima, J.F.; Marques, L.F. Elucidating the energy transfer process in mononuclear and binuclear lanthanide complexes of the anti-inflammatory drug ibuprofen: From synthesis to high luminescence emission. J. Lumin. 2017, 181, 196–210. [Google Scholar] [CrossRef]
- Albuquerque, R.Q.; Freire, R.O.; Malta, O.L. On the use of ligand field parameters in the study of coordinated water molecules in Eu3+ complexes. J. Phys. Chem. A 2005, 109, 4607–4610. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.S.A.; Galal, H.R.; Dessouky, A.F.M.; El-Naggar, M.; Mekkawi, D.; Ali, S.S.; Attya, G.M. Fluorescence and photostability studies of anthracene-9-carboxylic acid in different media. Int. J. Photoenergy 2000, 2, 47–53. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, J.; Li, D.; Lin, H. Multiligand Europium Complexes Incorporated Polyvinylpyrrolidone for Enhanced Solar Cell. Adv. Mater. Sci. Eng. 2019, 2019, 6180656. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Strelcov, E.; Kolmakov, A. Chemical sensors from lead metallophthalocyanine whiskers. Proc. IEEE Sensors 2007, 2, 636–639. [Google Scholar] [CrossRef]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Stewart Computational Chemistry—MOPAC Home Page. Available online: http://www.openmopac.net/index.html (accessed on 20 May 2020).
- Jiang, W.; Hong, C.; Wei, H.; Wu, Z.; Ye, S.; Huang, C.-H. A green-emitting iridium complex used for sensitizing europium ion with high quantum yield. Inorg. Chim. Acta 2017, 459, 124–130. [Google Scholar] [CrossRef]
- Wang, A.-L.; Zhou, D.; Chen, Y.-N.; Li, J.J.; Zhang, H.-X.; Zhao, Y.-L.; Chu, H. Crystal structure and photoluminescence of europium, terbium and samarium compounds with halogen-benzoate and 2,4,6-tri(2-pyridyl)-s-triazine. J. Lumin. 2016, 177, 22–30. [Google Scholar] [CrossRef]
- Hreniak, D.; Speghini, A.; Bettinelli, M.; Strek, W. Spectroscopic investigations of nanostructured LiNbO3 doped with Eu3+. J. Lumin. 2006, 119–120, 219–223. [Google Scholar] [CrossRef]
- Medina, D.Y.; Orozco, S.; Hernandez, I.; Hernandez, R.T.; Falcony, C. Characterization of europium doped lanthanum oxide films prepared by spray pyrolysis. J. Non-Cryst. Solids 2011, 357, 3740–3743. [Google Scholar] [CrossRef]
- García, R.N.; Desirena, H.; López-Luke, T.; Guerrero-Contreras, J.; Jayasankar, C.; Quintero-Torres, R.; De La Rosa, E. Spectroscopic properties of Eu3+/Nd3+ co-doped phosphate glasses and opaque glass-ceramics. Opt. Mater. 2015, 46, 34–39. [Google Scholar] [CrossRef]
- Crystal, P.H.; Fujihara, S.; Tokumo, K. Multiband Orange-Red Luminescence of Eu3+ Ions Based on the Pyrochlore-Structured Host Crystal. Chem. Mater. 2005, 17, 5587–5593. [Google Scholar]
- Yang, C.; Fu, L.-M.; Wang, Y.; Zhang, J.-P.; Wong, W.-T.; Ai, X.-C.; Qiao, Y.-F.; Zou, B.-S.; Gui, L.-L. A Highly Luminescent Europium Complex Showing Visible-Light-Sensitized Red Emission: Direct Observation of the Singlet Pathway. Angew. Chem. 2004, 116, 5120–5123. [Google Scholar] [CrossRef]
- Yan, B.; Song, Y.S. Spectroscopic study on the photophysical properties of lanthanide complexes with 2,2′-bipyridine-N, N′-dioxide. J. Fluoresc. 2004, 14, 289–294. [Google Scholar] [CrossRef]
- Wu, M.; You, J.; Song, Z. Luminescence Study of the Interaction of Carboxymethyl Chitosan with Metal Ions. J. Appl. Spectrosc. 2014, 81, 602–606. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Eliseeva, S.V.; Scopelliti, R.; Bünzli, J.-C.G. Influence of Symmetry on the Luminescence and Radiative Lifetime of Nine-Coordinate Europium Complexes. Inorg. Chem. 2015, 54, 9166–9173. [Google Scholar] [CrossRef]
- Moreau, P.; Colette-Maatouk, S.; Vitorge, P.; Gareil, P.; Reiller, P.E. Complexation of europium(III) by hydroxybenzoic acids: A time-resolved luminescence spectroscopy study. Inorganica Chim. Acta 2015, 432, 81–88. [Google Scholar] [CrossRef]
- Barkleit, A.; Kretzschmar, J.; Tsushima, S.; Acker, M. Americium(iii) and europium(iii) complex formation with lactate at elevated temperatures studied by spectroscopy and quantum chemical calculations. Dalt. Trans. 2014, 43, 11221–11232. [Google Scholar] [CrossRef]


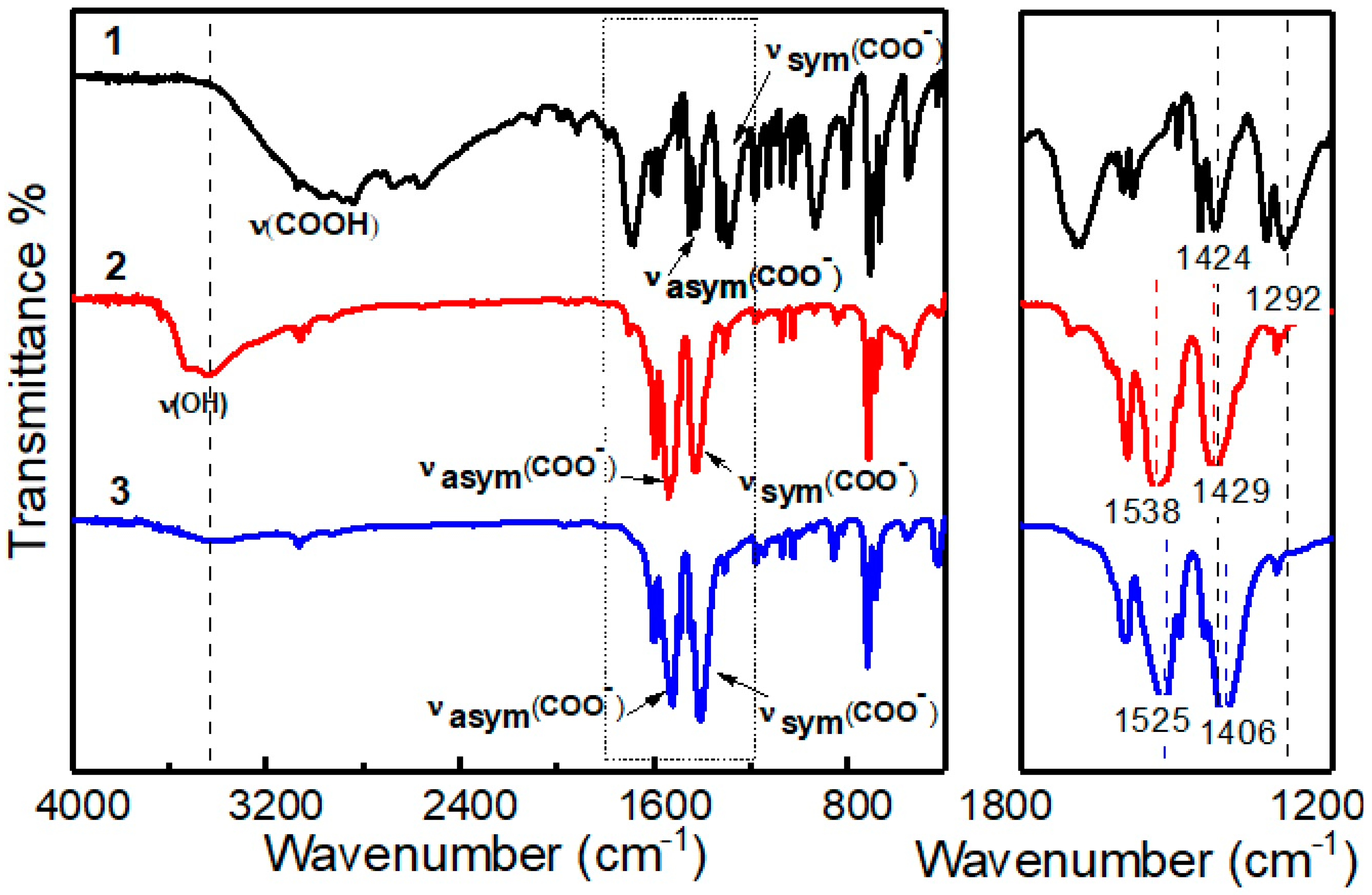


| Complex | Identification | Vibrations (cm−1) | Δv (cm−1) | ||
|---|---|---|---|---|---|
| v (HO–H) | vasym (COO−) | vsym (COO−) | asym-sym | ||
| Benzoic acid a | 1 | – | 1424 | 1292 | 132 |
| [Eu(OOCC6H5)3·(H2O)3] | 2 | 3438 | 1538 | 1429 | 109 |
| Eu(OOCC6H5)3·(HOOCC6H5)2 | 3 | – | 1525 | 1406 | 119 |
| Bond Distances of Europium–Oxygen(Å) | |||
|---|---|---|---|
| Eu–O1 2.3637(3) | Eu–O3 2.3895(2) | Eu–O5 2.3866(3) | Eu–O7 2.3409(2) |
| Eu–O2 2.3001(2) | Eu–O4 2.3324(3) | Eu–O6 2.3236(2) | Eu–O9 2.3347(2) |
| Formula, molecular weight | Eu C35H27O10, 760.56 |
| Temperature (K), (Å) | 293, 1.54056 |
| System, space group | Triclinic, P-1 |
| a, b, c (Å); α, β, γ (°) | 24.496(3), 15.166(2), 5.0934(8), 92.408(1), 91.007(3), 74.814(6), |
| V (Å3), Z | 1824.42, 2 |
| 2θ range, step (°) | 4–40, 0.02 |
| Nr. of data points | 3701 |
| Nr. of Bragg refl., parameters | 884, 112 |
| Rp (%), Rwp (%), X2 | 3.876, 5.032, 1.564 |
| Rexp (%), RBragg (%) | 4.024, 1.397 |
| CCDC depository nr. | CCDC 19771999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Fuentes, C.; Ruiz-Guerrero, R.; Morales-Ramírez, A.d.J.; Molina-Maldonado, P.; Medina-Velazquez, D.Y. New Mononuclear Complex of Europium(III) and Benzoic Acid: From Synthesis and Crystal Structure Solution to Luminescence Emission. Crystals 2020, 10, 674. https://doi.org/10.3390/cryst10080674
Hernández-Fuentes C, Ruiz-Guerrero R, Morales-Ramírez AdJ, Molina-Maldonado P, Medina-Velazquez DY. New Mononuclear Complex of Europium(III) and Benzoic Acid: From Synthesis and Crystal Structure Solution to Luminescence Emission. Crystals. 2020; 10(8):674. https://doi.org/10.3390/cryst10080674
Chicago/Turabian StyleHernández-Fuentes, Carlos, Rosario Ruiz-Guerrero, Angel de Jesús Morales-Ramírez, Paulina Molina-Maldonado, and Dulce Y. Medina-Velazquez. 2020. "New Mononuclear Complex of Europium(III) and Benzoic Acid: From Synthesis and Crystal Structure Solution to Luminescence Emission" Crystals 10, no. 8: 674. https://doi.org/10.3390/cryst10080674
APA StyleHernández-Fuentes, C., Ruiz-Guerrero, R., Morales-Ramírez, A. d. J., Molina-Maldonado, P., & Medina-Velazquez, D. Y. (2020). New Mononuclear Complex of Europium(III) and Benzoic Acid: From Synthesis and Crystal Structure Solution to Luminescence Emission. Crystals, 10(8), 674. https://doi.org/10.3390/cryst10080674







