The Role of Substitution in the Apex Position of the Bent-Core on Mesomorphic Properties of New Series of Liquid Crystalline Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Protected Central Cores
2.2. Synthesis of the Target Materials
2.3. Synthesis of the Central Cores
2.4. Synthesis of Intermediates and Target Compounds
2.5. Synthesis of Target Compounds
2.6. Characterization
2.7. Equipment and Set-up for Studies of Mesomorphic Property
3. Results
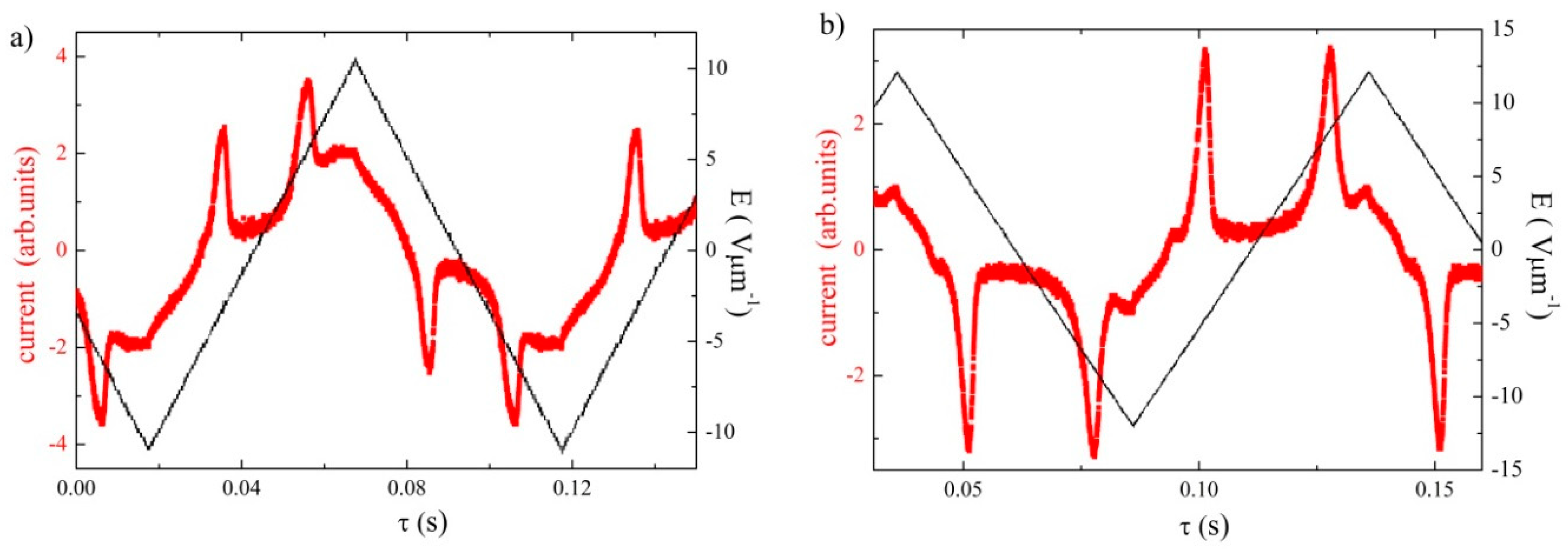
3.1. Mesomorphic Properties and Electro-Optical Behaviour
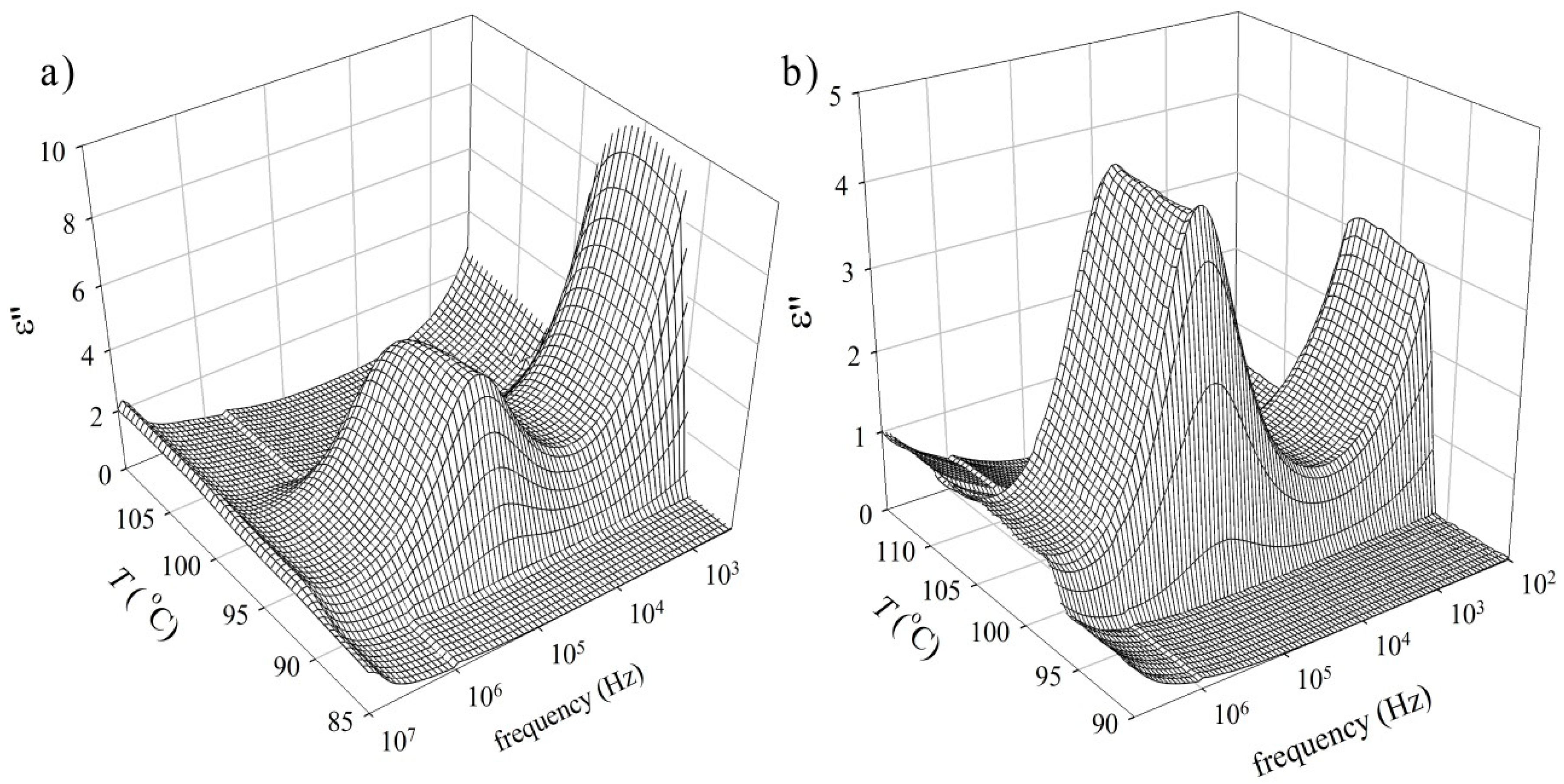
3.2. Dielectric Spectroscopy
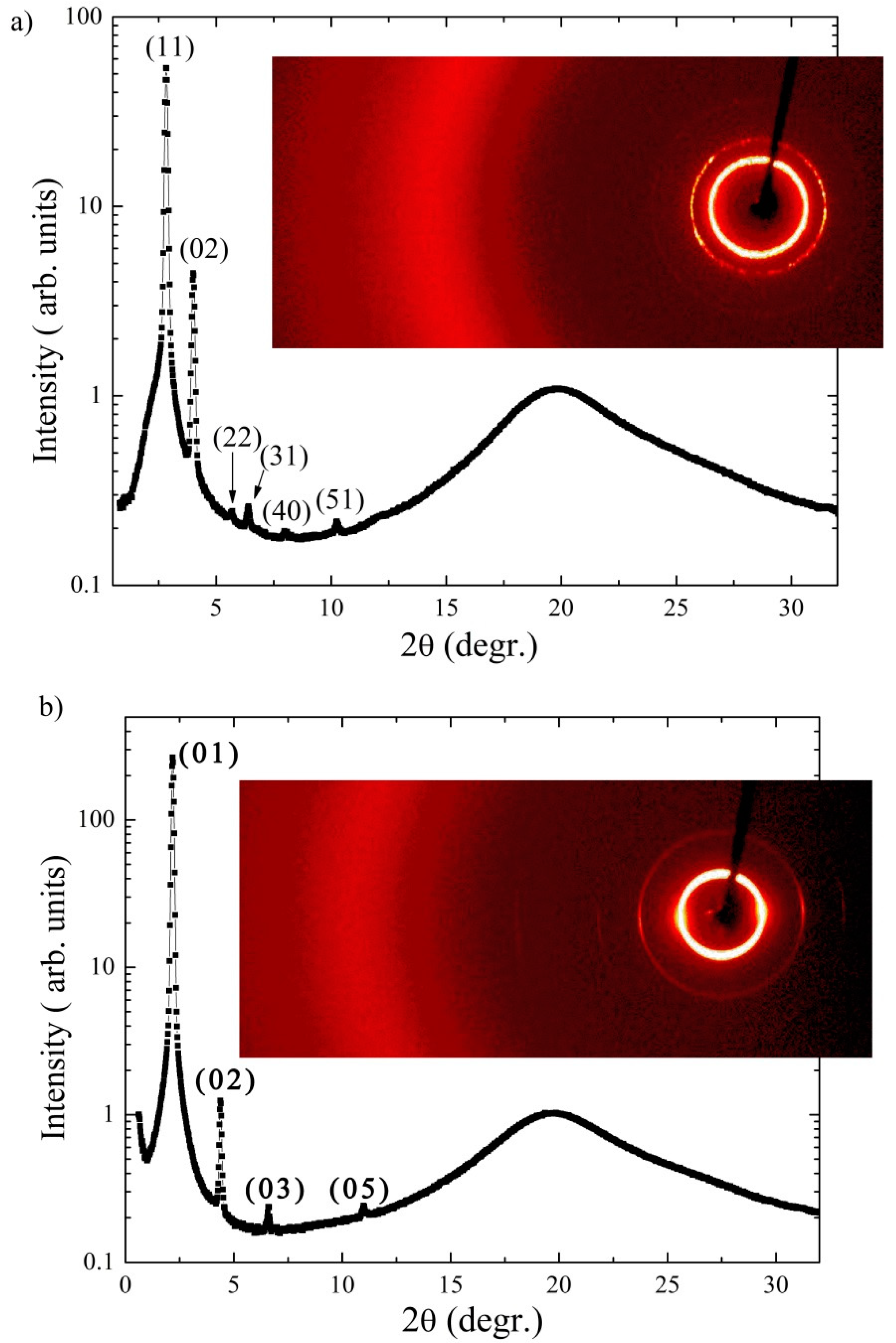
3.3. X-ray Measurements
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vorländer, D. Die Richtung der Kohlenstoff-Valenzen in Benzol-Abkömmlingen. Ber. Dtsch. Chem. Ges. 1929, 62, 2831–2835. [Google Scholar] [CrossRef]
- Link, D.R.; Natale, G.; Shao, R.; Maclennan, J.E.; Clark, N.A.; Körblova, E.; Walba, D.M. Spontaneous Formation of Macroscopic Chiral Domains in a Fluid Smectic Phase of Achiral Molecules. Science 1997, 278, 1924–1927. [Google Scholar] [CrossRef] [PubMed]
- Niori, T.; Sekine, T.; Watanabe, J.; Furukawa, T.; Takezoe, H. Distinct ferroelectric smectic liquid crystals consisting of banana shaped achiral molecules. J. Mater. Chem. 1996, 6, 1231–1233. [Google Scholar] [CrossRef]
- Hideo, T.; Yoichi, T. Bent-Core Liquid Crystals: Their Mysterious and Attractive World. Jpn. J. Appl. Phys. 2006, 45, 597–625. [Google Scholar] [CrossRef]
- Jákli, A. Liquid crystals of the twenty-first century–nematic phase of bent-core molecules. Liq. Cryst. Rev. 2013, 1, 65–82. [Google Scholar] [CrossRef]
- Madsen, L.A.; Dingemans, T.J.; Nakata, M.; Samulski, E.T. Thermotropic Biaxial Nematic Liquid Crystals. Phys. Rev. Lett. 2004, 92, 145505. [Google Scholar] [CrossRef]
- Tschierske, C.; Photinos, D.J. Biaxial nematic phases. J. Mater. Chem. 2010, 20, 4263–4294. [Google Scholar] [CrossRef]
- Pelzl, G.; Eremin, A.; Diele, S.; Kresse, H.; Weissflog, W. Spontaneous chiral ordering in the nematic phase of an achiral banana-shaped compound. J. Mater. Chem. 2002, 12, 2591–2593. [Google Scholar] [CrossRef]
- Vita, F.; Adamo, F.C.; Francescangeli, O. Polar order in bent-core nematics: An overview. J. Mol. Liq. 2018. [Google Scholar] [CrossRef]
- Connor, P.L.M.; Mandle, R.J. Chemically induced splay nematic phase with micron scale periodicity. Soft Matter 2020, 16, 324–329. [Google Scholar] [CrossRef]
- Jing, H.; Xu, M.; Xiang, Y.; Wang, E.; Liu, D.; Poryvai, A.; Kohout, M.; Éber, N.; Buka, Á. Light Tunable Gratings Based on Flexoelectric Effect in Photoresponsive Bent-Core Nematics. Adv. Opt. Mater. 2019, 7, 1801790. [Google Scholar] [CrossRef]
- Reddy, R.A.; Tschierske, C. Bent-core liquid crystals: Polar order, superstructural chirality and spontaneous desymmetrisation in soft matter systems. J. Mater. Chem. 2006, 16, 907–961. [Google Scholar] [CrossRef]
- Tschierske, C. Development of Structural Complexity by Liquid-Crystal Self-assembly. Angew. Chem. Int. Ed. 2013, 52, 8828–8878. [Google Scholar] [CrossRef] [PubMed]
- Etxebarria, J.; Blanca Ros, M. Bent-core liquid crystals in the route to functional materials. J. Mater. Chem. 2008, 18, 2919–2926. [Google Scholar] [CrossRef]
- Pelzl, G.; Diele, S.; Weissflog, W. Banana-Shaped Compounds—A New Field of Liquid Crystals. Adv. Mater. 1999, 11, 707–724. [Google Scholar] [CrossRef]
- Shen, D.; Pegenau, A.; Diele, S.; Wirth, I.; Tschierske, C. Molecular Design of Nonchiral Bent-Core Liquid Crystals with Antiferroelectric Properties. J. Am. Chem. Soc. 2000, 122, 1593–1601. [Google Scholar] [CrossRef]
- Weissflog, W.; Naumann, G.; Kosata, B.; Schroder, M.W.; Eremin, A.; Diele, S.; Vakhovskaya, Z.; Kresse, H.; Friedemann, R.; Krishnan, S.A.R.; et al. Ten isomeric five-ring bent-core mesogens: The influence of the direction of the carboxyl connecting groups on the mesophase behaviour. J. Mater. Chem. 2005, 15, 4328–4337. [Google Scholar] [CrossRef]
- Kohout, M.; Svoboda, J.; Novotná, V.; Glogarová, M.; Pociecha, D. Non-symmetrical bent-shaped liquid crystals with five ester groups. Liq. Cryst. 2010, 37, 987–996. [Google Scholar] [CrossRef]
- Kohout, M.; Svoboda, J.; Novotná, V.; Pociecha, D. Non-symmetrical bent-shaped liquid crystals based on a laterally substituted naphthalene central core with four ester groups. Liq. Cryst. 2011, 38, 1099–1110. [Google Scholar] [CrossRef]
- Kohout, M.; Svoboda, J.; Novotná, V.; Pociecha, D.; Glogarová, M.; Gorecka, E. A nematic-polar columnar phase sequence in new bent-shaped liquid crystals based on a 7-hydroxynaphthalene-2-carboxylic acid core. J. Mater. Chem. 2009, 19, 3153–3160. [Google Scholar] [CrossRef]
- Amaranatha Reddy, R.; Baumeister, U.; Keith, C.; Hahn, H.; Lang, H.; Tschierske, C. Influence of the core structure on the development of polar order and superstructural chirality in liquid crystalline phases formed by silylated bent-core molecules: Lateral substituents. Soft Matter 2007, 3, 558–570. [Google Scholar] [CrossRef]
- Kovalenko, L.; Weissflog, W.; Grande, S.; Diele, S.; Pelzl, G.; Wirth, I. Dimorphism SmA-B2 in bent-core mesogens with perfluorinated terminal chains. Liq. Cryst. 2000, 27, 683–687. [Google Scholar] [CrossRef]
- Alaasar, M.; Prehm, M.; Tschierske, C. Influence of halogen substituent on the mesomorphic properties of five-ring banana-shaped molecules with azobenzene wings. Liq. Cryst. 2013, 40, 656–668. [Google Scholar] [CrossRef]
- Bedel, J.P.; Rouillon, J.C.; Marcerou, J.P.; Laguerre, M.; Nguyen, H.T.; Achard, M.F. Influence of fluoro substituents on the mesophase behaviour of banana-shaped molecules. J. Mater. Chem. 2002, 12, 2214–2220. [Google Scholar] [CrossRef]
- Dantlgraber, G.; Keith, C.; Baumeister, U.; Tschierske, C. Polar and chiral mesophases formed by m-terphenyl derived bent-core molecules with lateral F-substituents. J. Mater. Chem. 2007, 17, 3419–3426. [Google Scholar] [CrossRef]
- Nadasi, H.; Weissflog, W.; Eremin, A.; Pelzl, G.; Diele, S.; Das, B.; Grande, S. Ferroelectric and antiferroelectric “banana phases” of new fluorinated five-ring bent-core mesogens. J. Mater. Chem. 2002, 12, 1316–1324. [Google Scholar] [CrossRef]
- Svoboda, J.; Novotna, V.; Kozmik, V.; Glogarova, M.; Weissflog, W.; Diele, S.; Pelzl, G. A novel type of banana liquid crystals based on 1-substituted naphthalene-2,7-diol cores. J. Mater. Chem. 2003, 13, 2104–2110. [Google Scholar] [CrossRef]
- Weissflog, W.; Nadasi, H.; Dunemann, U.; Pelzl, G.; Diele, S.; Eremin, A.; Kresse, H. Influence of lateral substituents on the mesophase behaviour of banana-shaped mesogens. J. Mater. Chem. 2001, 11, 2748–2758. [Google Scholar] [CrossRef]
- Weissflog, W.; Shreenivasa Murthy, H.N.; Diele, S.; Pelzl, G. Relationships between molecular structure and physical properties in bent-core mesogens. Philos. Trans. R. Soc. A 2006, 364, 2657–2679. [Google Scholar] [CrossRef]
- Umadevi, S.; Radhika, S.; Sadashiva, B.K. The SmCPA phase in five-ring bent-core compounds derived from 5-methoxyisophthalic acid. Liq. Cryst. 2006, 33, 139–147. [Google Scholar] [CrossRef]
- Kozmík, V.; Polášek, P.; Seidler, A.; Kohout, M.; Svoboda, J.; Novotná, V.; Glogarová, M.; Pociecha, D. The effect of a thiophene ring in the outer position on mesomorphic properties of the bent-shaped liquid crystals. J. Mater. Chem. 2010, 20, 7430–7435. [Google Scholar] [CrossRef]
- Kohout, M.; Kozmík, V.; Slabochová, M.; Tůma, J.; Svoboda, J.; Novotná, V.; Pociecha, D. Bent-shaped liquid crystals based on 4-substituted 3-hydroxybenzoic acid central core. Liq. Cryst. 2015, 42, 87–103. [Google Scholar] [CrossRef]
- Tůma, J.; Kohout, M.; Svoboda, J.; Novotná, V.; Pociecha, D. Bent-shaped liquid crystals based on 4-substituted 3-hydroxybenzoic acid central core–Part II. Liq. Cryst. 2016, 43, 547–563. [Google Scholar] [CrossRef]
- Tůma, J.; Kohout, M.; Svoboda, J.; Novotná, V.; Pociecha, D. Bent-core liquid crystals based on 6-substituted 3-hydroxybenzoic acid: The role of substitution and linkage group orientation on mesomorphic properties. Liq. Cryst. 2016, 43, 1889–1900. [Google Scholar] [CrossRef]
- Pallová, L.; Kozmík, V.; Kohout, M.; Svoboda, J.; Novotná, V.; Pociecha, D. Bent-core liquid crystals with a 2-substituted 3-hydroxybenzoic acid central core. Liq. Cryst. 2017, 44, 1306–1315. [Google Scholar] [CrossRef]
- Skopalová, H.; Kozmík, V.; Šmahel, M.; Svoboda, J.; Pacherová, O.; Kohout, M.; Novotná, V. Mesomorphic properties of non-symmetric bent-core liquid crystals with a lateral substituent in the apex position. Liq. Cryst. 2020. submitted. [Google Scholar]
- Chamberland, S.; Grüschow, S.; Sherman, D.H.; Williams, R.M. Synthesis of Potential Early-Stage Intermediates in the Biosynthesis of FR900482 and Mitomycin C. Org. Lett. 2009, 11, 791–794. [Google Scholar] [CrossRef]
- Richardson, S.K.; Jeganathan, A.; Mani, R.S.; Haley, B.E.; Watt, D.S.; Trusal, L.R. Synthesis and biological activity of C-4 and C-15 Aryl azide derivatives of anguidine. Tetrahedron 1987, 43, 2925–2934. [Google Scholar] [CrossRef]
- Kohout, M.; Tuma, J.; Svoboda, J.; Novotna, V.; Gorecka, E.; Pociecha, D. 3-Hydroxycinnamic acid-a new central core for the design of bent-shaped liquid crystals. J. Mater. Chem. C 2013, 1, 4962–4969. [Google Scholar] [CrossRef]
- Monte, M.J.S.; Almeida, A.R.R.P.; Matos, M.A.R. Thermodynamic Study on the Sublimation of Five Aminomethoxybenzoic Acids. J. Chem. Eng. Data 2010, 55, 419–423. [Google Scholar] [CrossRef]
- Hartmann, R.W.; Heindl, A.; Schoenenberger, H. Ring-substituted 1,2-dialkylated 1,2-bis(hydroxyphenyl)ethanes. 2. Synthesis and estrogen receptor binding affinity of 4,4’-, 5,5’-, and 6,6’-disubstituted metahexestrols. J. Med. Chem. 1984, 27, 577–585. [Google Scholar] [CrossRef]


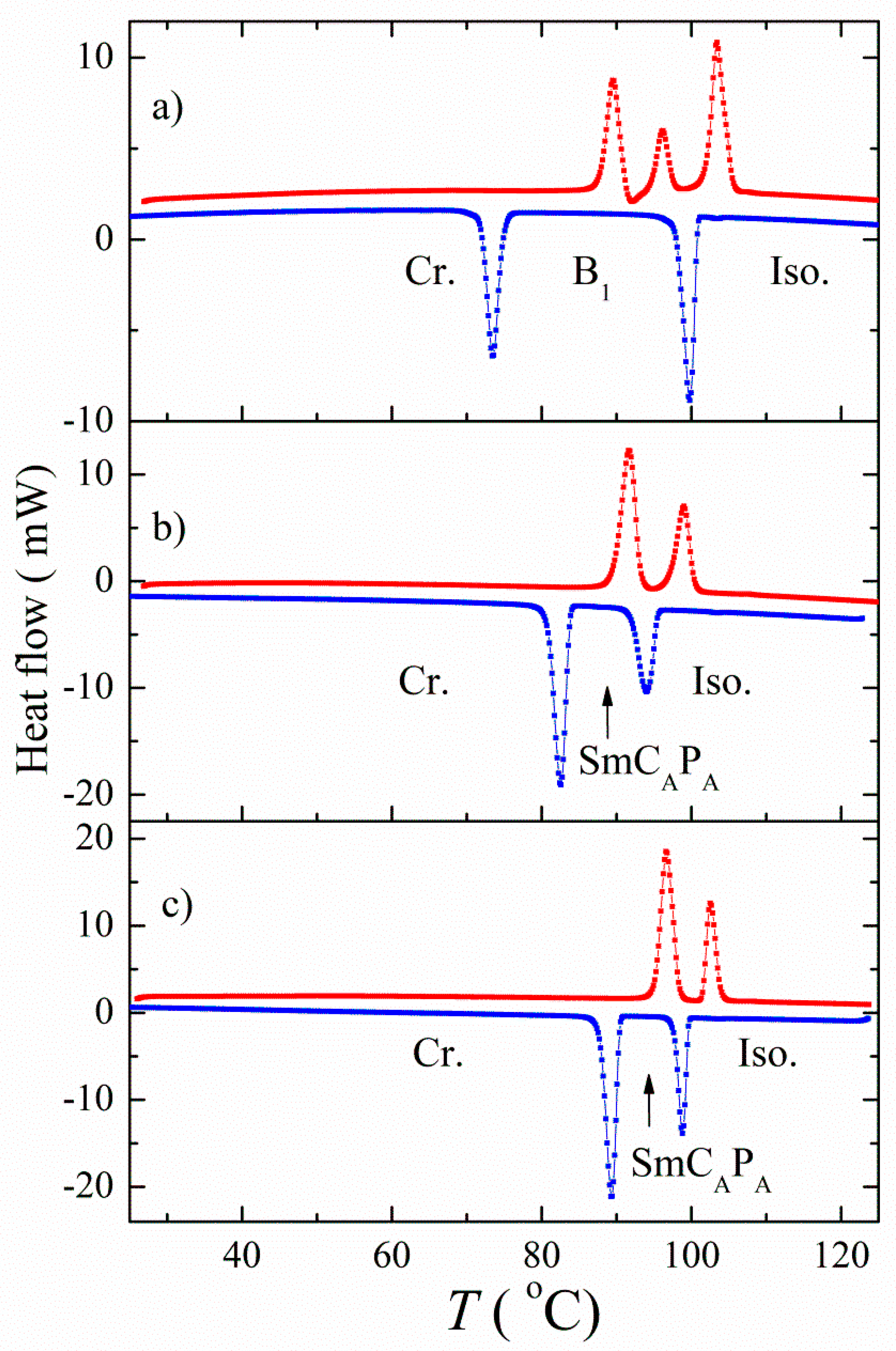
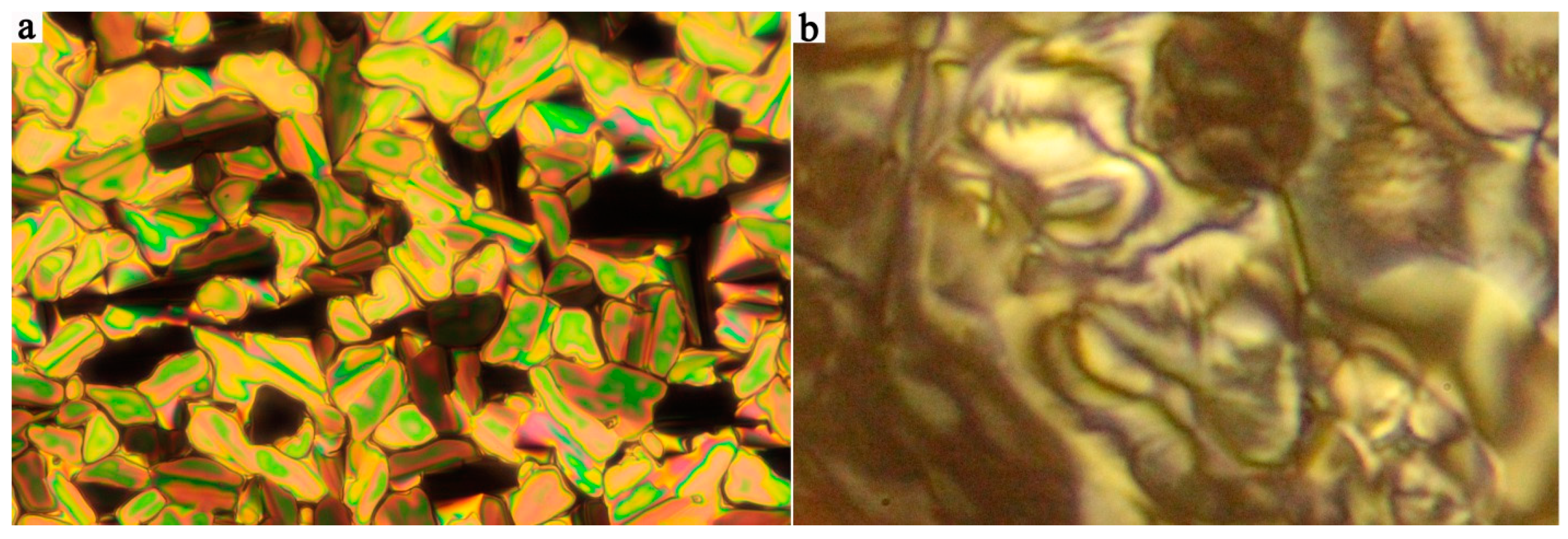
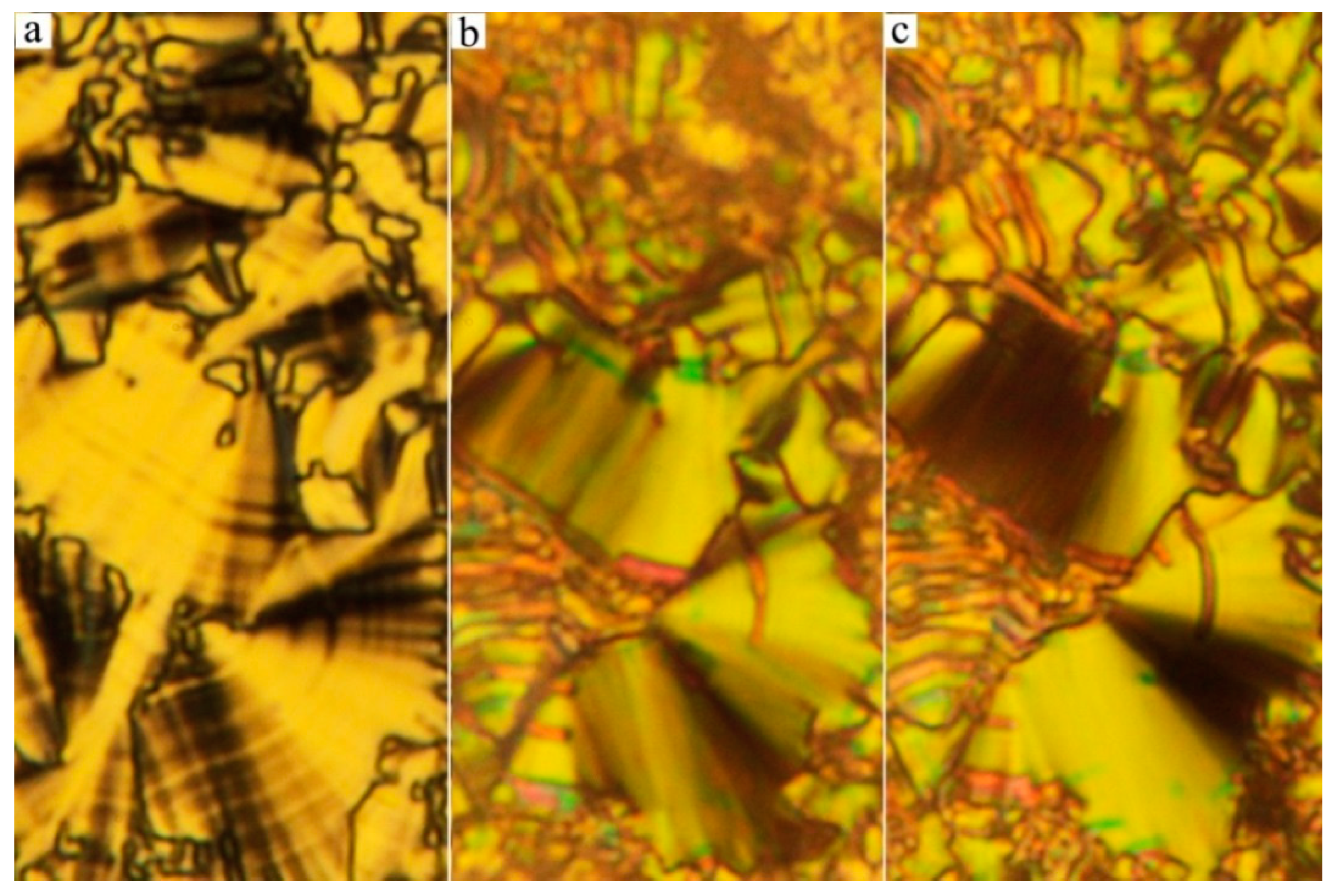
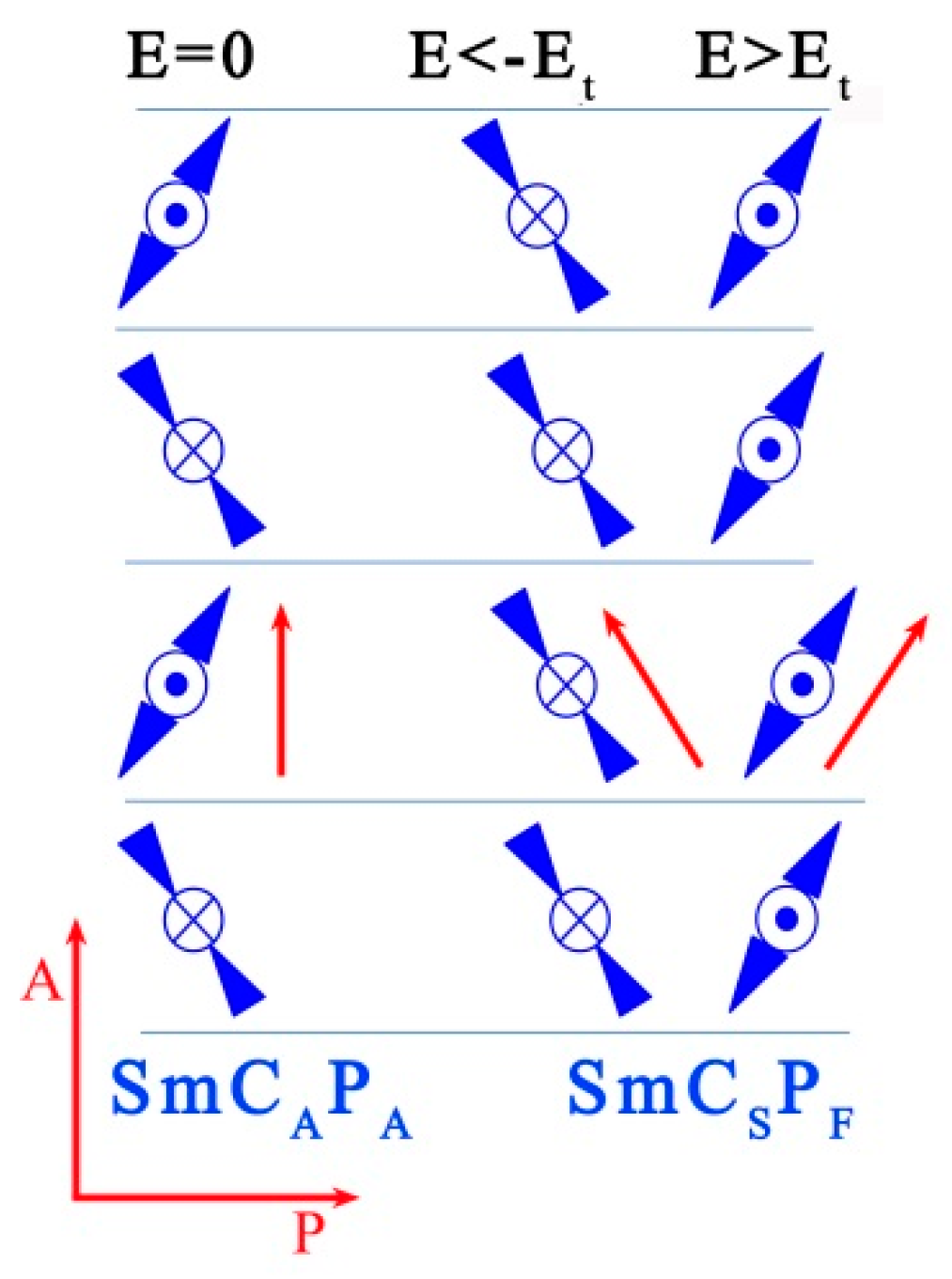

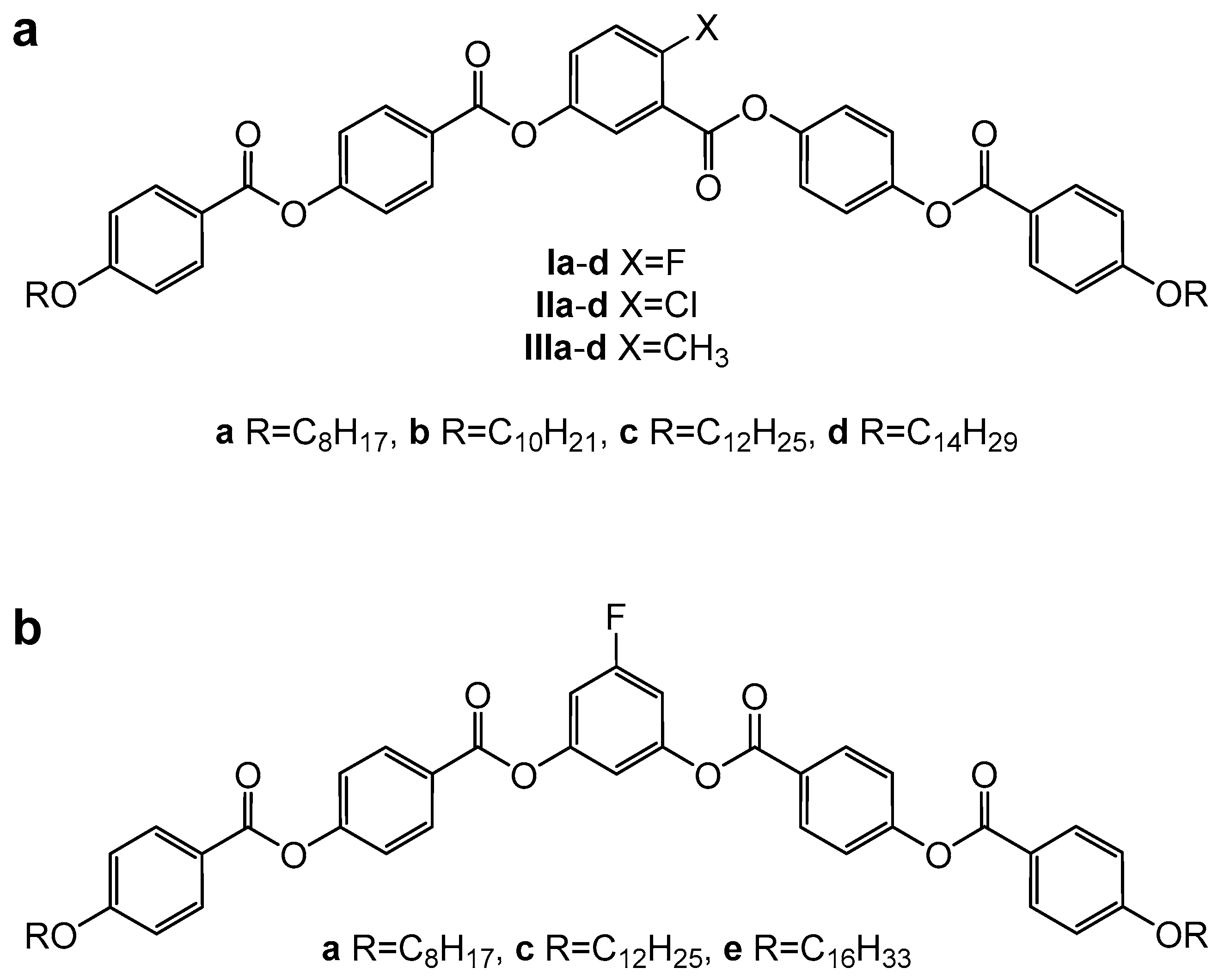
| Comp. | X | R | m.p. ΔH | Tcr ΔH | M | Tiso ΔH | Iso |
|---|---|---|---|---|---|---|---|
| Ia | F | C8H17 | 123 (+50.0) | 104 (−29.6) | B1 | 121 (−50.0) | • |
| Ib | F | C10H21 | 88 (+18.7) | 75 (−16.6) | B1 | 101 (−18.8) | • |
| Ic | F | C12H25 | 95 (+31.9) | 83 (−33.5) | SmCAPA | 96 (−19.1) | • |
| Id | F | C14H29 | 95 (+44.3) | 90 (−44.3) | SmCAPA | 100 (−21.1) | • |
| IIa | Cl | C8H17 | 118 (+45.7) | 101 (−44.0) | − | − | • |
| IIb | Cl | C10H21 | 107 (+40.0) | 100 (−37.6) | − | − | • |
| IIc | Cl | C12H25 | 106 (+41.0) | 101 (−37.4) | − | − | • |
| IId | Cl | C14H29 | 107 (+50.1) | 102 (−42.8) | − | − | • |
| IIIa | CH3 | C8H17 | 125 (+45.3) | 103 (−44.9) | − | − | • |
| IIIb | CH3 | C10H21 | 108 (+66.1) | 100 (−32.6) | − | − | • |
| IIIc | CH3 | C12H25 | 88 (+35.9) | 99 (−25.1) | − | − | • |
| IIId | CH3 | C14H29 | 66 (+11.4) | 65 (−14.2) | CrX | 103 (−25.9) | • |
| IVa | NO2 | C8H17 | 127 (+39.1) | 125 (−37.4) | − | − | • |
| IVb | NO2 | C10H21 | 129 (+42.1) | 126 (−41.9) | − | − | • |
| IVc | NO2 | C12H25 | 132 (+44.2) | 128 (−42.6) | − | − | • |
| IVd | NO2 | C14H29 | 129 (+47.2) | 126 (−48.6) | − | − | • |
| T/ºC | a/Å | b/Å | d/Å | l/Å | γ/degr. | |
|---|---|---|---|---|---|---|
| Ia | 114 | 32.5 | 41.8 | 45.8 | 24 | |
| Ib | 100 | 43.8 | 44.2 | 50.1 | 28 | |
| Ic | 95 | 40.3 | 54.5 | 42.3 | ||
| Id | 99 | 42.2 | 58.9 | 44.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skopalová, H.; Špaček, P.; Kozmík, V.; Svoboda, J.; Novotná, V.; Pociecha, D.; Kohout, M. The Role of Substitution in the Apex Position of the Bent-Core on Mesomorphic Properties of New Series of Liquid Crystalline Materials. Crystals 2020, 10, 735. https://doi.org/10.3390/cryst10090735
Skopalová H, Špaček P, Kozmík V, Svoboda J, Novotná V, Pociecha D, Kohout M. The Role of Substitution in the Apex Position of the Bent-Core on Mesomorphic Properties of New Series of Liquid Crystalline Materials. Crystals. 2020; 10(9):735. https://doi.org/10.3390/cryst10090735
Chicago/Turabian StyleSkopalová, Helena, Petr Špaček, Václav Kozmík, Jiří Svoboda, Vladimíra Novotná, Damian Pociecha, and Michal Kohout. 2020. "The Role of Substitution in the Apex Position of the Bent-Core on Mesomorphic Properties of New Series of Liquid Crystalline Materials" Crystals 10, no. 9: 735. https://doi.org/10.3390/cryst10090735
APA StyleSkopalová, H., Špaček, P., Kozmík, V., Svoboda, J., Novotná, V., Pociecha, D., & Kohout, M. (2020). The Role of Substitution in the Apex Position of the Bent-Core on Mesomorphic Properties of New Series of Liquid Crystalline Materials. Crystals, 10(9), 735. https://doi.org/10.3390/cryst10090735







