Novel Pb(II) Complexes: X-Ray Structures, Hirshfeld Surface Analysis and DFT Calculations
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Crystallography
2.3. Other Physical Measurements
2.4. Synthesis
2.4.1. [Pb2L2(NCS)4)] (1)
2.4.2. [Pb2L2(NO3)4)]·2MeOH (2)
2.5. Hirshfeld Surface Analysis
2.6. Theoretical Methods
3. Results and Discussion
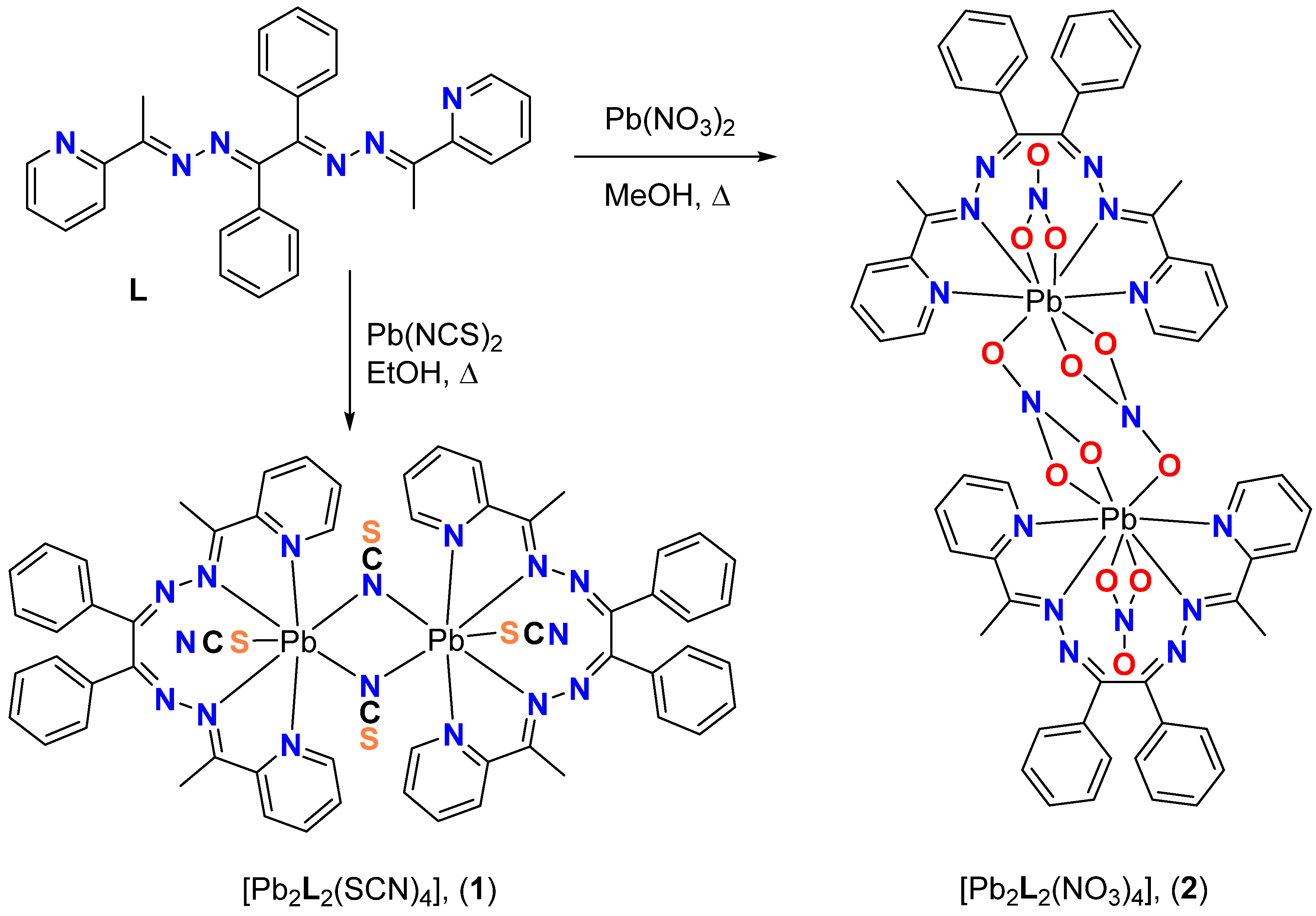
3.1. Synthetic Considerations
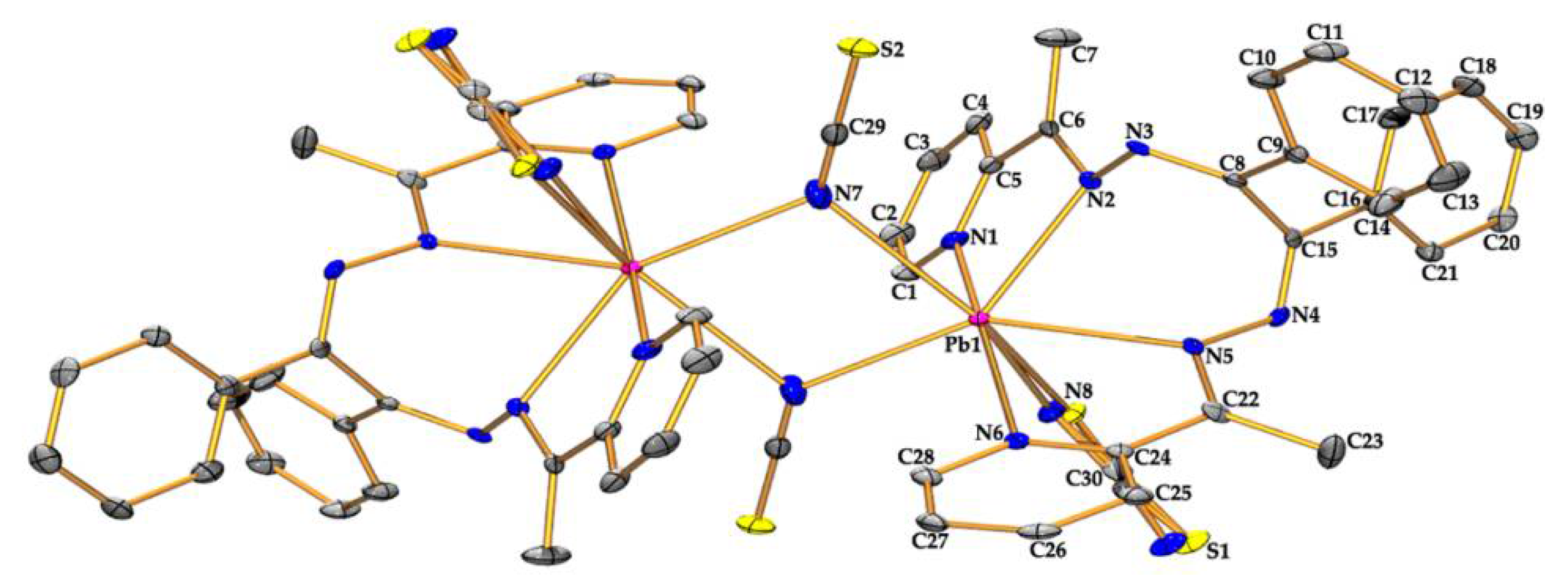
3.2. Structural Description
3.3. Hirshfeld Surface
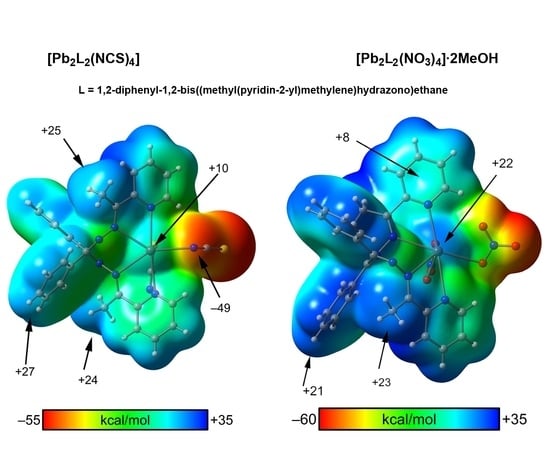
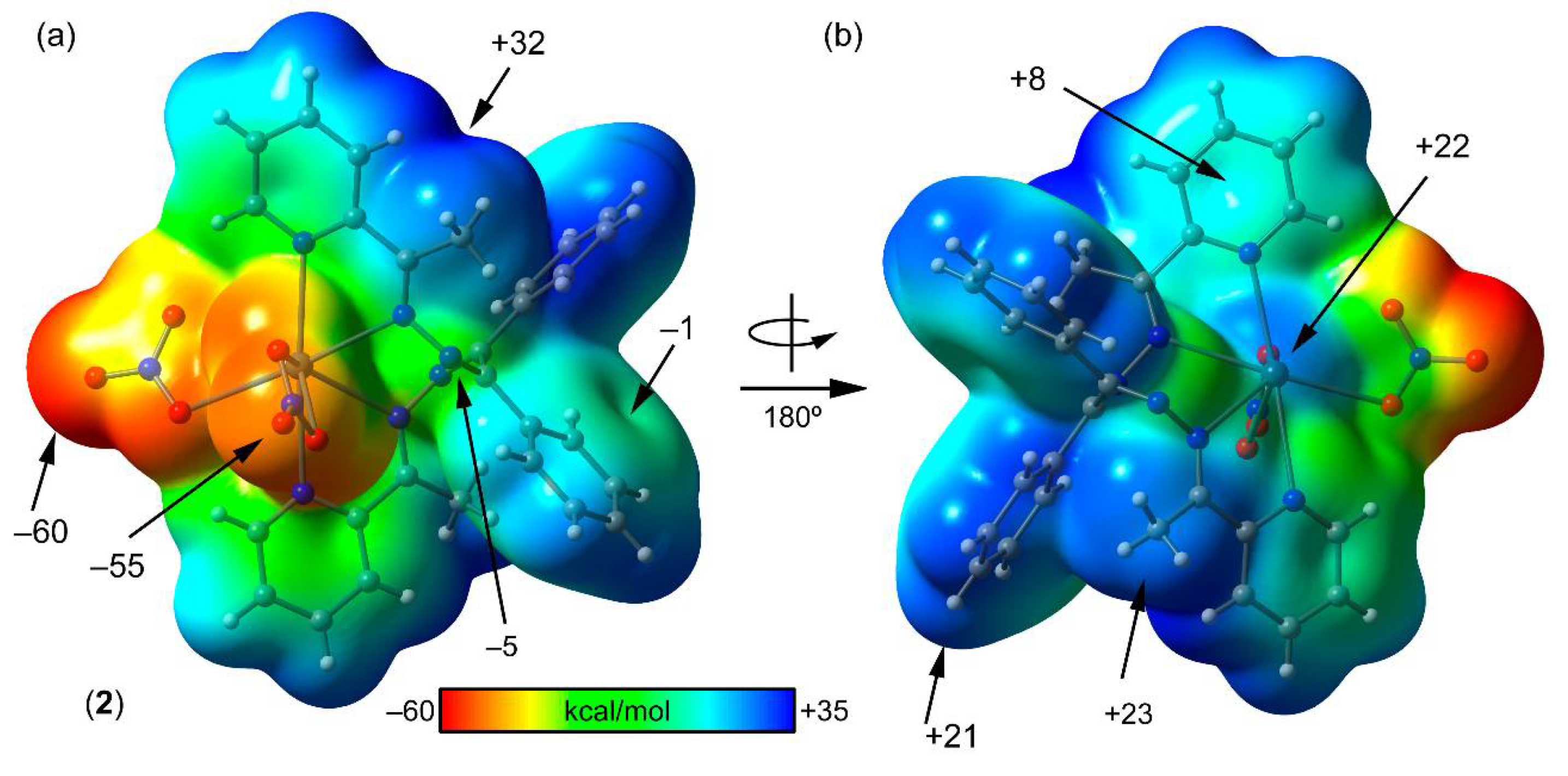
3.4. Mep Surface Analysis
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casas, J.S.; Sordo, J. Lead: Chemistry, Analytical Aspects, Environmental Impact and Health Effects; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Bauzá, A.; Seth, S.K.; Frontera, A. Tetrel bonding interactions at work: Impact on tin and lead coordination compounds. Coord. Chem. Rev. 2019, 384, 107–125. [Google Scholar] [CrossRef]
- Reger, D.L.; Wright, T.D.; Little, C.A.; Lamba, J.J.S.; Smith, M.D. Control of the Stereochemical Impact of the Lone Pair in Lead(II) Tris(pyrazolyl)methane Complexes. Improved Preparation of Na{B[3,5-(CF3)2C6H3]4}. Inorg. Chem. 2001, 40, 3810–3814. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, H.; Schollmeyer, D. Synthesis of and Structural Studies on Lead(II) Cysteamin Complexes. Inorg. Chem. 2004, 43, 5529–5536. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Gurbanov, A.V.; Guseinov, F.I.; Guedes da Silva, M.F.C. Noncovalent interactions in metal complex catalysis. Coord. Chem. Rev. 2019, 387, 32–46. [Google Scholar] [CrossRef]
- Olvera, A.; Shi, G.; Djieutedjeu, H.; Page, A.; Uher, C.; Kioupakis, E.; Poudeu, P.F.P. Pb7Bi4Se13: A Lillianite Homologue with Promising Thermoelectric Properties. Inorg. Chem. 2015, 54, 746–755. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Bauzá, A.; Frontera, A. Concurrent agostic and tetrel bonding interactions in lead(ii) complexes with an isonicotinohydrazide based ligand and several anions. Dalton Trans. 2016, 45, 4965–4969. [Google Scholar] [CrossRef]
- Roy, S.; Drew, M.G.B.; Bauzá, A.; Frontera, A.; Chattopadhyay, S. Non-covalent tetrel bonding interactions in hemidirectional lead(II) complexes with nickel(II)-salen type metallo-ligands. New J. Chem. 2018, 42, 6062–6076. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Seth, S.K.; Zubkov, F.I.; López-Torres, E.; Bacchi, A.; Stilinović, V.; Frontera, A. Supramolecular Assemblies in Pb(II) Complexes with Hydrazido-Based Ligands. Crystals 2019, 9, 323. [Google Scholar] [CrossRef]
- Servati, G.M.; Stilinović, V.; Bauzá, A.; Frontera, A.; McArdle, P.; Van Derveer, D.; Ng, S.W.; Mahmoudi, G. Design of Lead(II) Metal-Organic Frameworks Based on Covalent and Tetrel Bonding. Chem. Eur. J. 2015, 21, 17951–17958. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Park, M.; Moon, D.; Lee, K.; Hong, S.; Zou, Y.; Hong, C.S.; Lah, M.S.A. A chiral pentadecanuclear metallamacrocycle with a sextuple twisted möbius topology. J. Am. Chem. Soc. 2007, 129, 14142–14143. [Google Scholar] [CrossRef]
- De, S.; Drew, M.G.B.; Rzepa, H.S.; Datta, D. Linking number analysis of a self-assembled lemniscular Möbius-metallamacrocycle. New J. Chem. 2008, 32, 1831–1834. [Google Scholar] [CrossRef]
- Rzepa, H.S. Linking number analysis of a pentadecanuclear metallamacrocycle: A möbius-craig system revealed. Inorg. Chem. 2008, 47, 8932–8934. [Google Scholar] [CrossRef] [PubMed]
- Sankar, J.; Mori, S.; Saito, S.; Rath, H.; Suzuki, M.; Inokuma, Y.; Shinokubo, H.; Kim, K.S.; Yoon, Z.S.; Shin, J.Y.; et al. Unambiguous identification of Möbius aromaticity for meso-aryl-substituted [28]hexaphyrins(1.1.1.1.1.1). J. Am. Chem. Soc. 2008, 130, 13568–13579. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.M.; Yoon, M.C.; Lim, J.M.; Rath, H.; Naoda, K.; Osuka, A.; Kim, D. Reversal of Hückel (anti)aromaticity in the lowest triplet states of hexaphyrins and spectroscopic evidence for Baird’s rule. Nat. Chem. 2015, 7, 418–422. [Google Scholar] [CrossRef]
- Masoumi, A.; Gargari, M.S.; Mahmoudi, G.; Machura, B.; Lynch, V.; Giester, G.; Abedi, M.; Hazendonk, P. Structural diversity in manganese(II) complexes with multidentate N-donor imino pyridyl ligands. Z. Anorg. Allg. Chem. 2015, 641, 1176–1181. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Stilinović, V.; Gargari, M.S.; Bauzá, A.; Zaragoza, G.; Kaminsky, W.; Lynch, V.; Choquesillo-Lazarte, D.; Sivakumar, K.; Khandari, A.A.; et al. From monomers to polymers: Steric and supramolecular effects on dimensionality of coordination architectures of heteroleptic mercury(ii) halogenide-tetradentate Schiff base complexes. CrystEngComm 2015, 17, 3493–3502. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Zaręba, J.K.; Gurbanov, A.V.; Bauzá, A.; Zubkov, F.I.; Kubicki, M.; Stilinović, V.; Kinzhybalo, V.; Frontera, A. Benzyl Dihydrazone versus Thiosemicarbazone Schiff Base: Effects on the Supramolecular Arrangement of Cobalt Thiocyanate Complexes and the Generation of CoN6 and CoN4S2 Coordination Spheres. Eur. J. Inorg Chem. 2017, 4763–4772. [Google Scholar] [CrossRef]
- Afkhami, F.A.; Mahmoudi, G.; Gurbanov, A.V.; Zubkov, F.I.; Qu, F.; Gupta, A.; Safin, D.A. Solvent-driven azide-induced mononuclear discrete versus one-dimensional polymeric aromatic Möbius cadmium(ii) complexes of an N6 tetradentate helical ligand. Dalton Trans. 2017, 46, 14888–14896. [Google Scholar] [CrossRef]
- Seth, S.K.; Bauzá, A.; Mahmoudi, G.; Stilinović, V.; López-Torres, E.; Zaragoza, G.; Keramidas, A.D.; Frontera, A. On the importance of Pb⋯X (X = O, N, S, Br) tetrel bonding interactions in a series of tetra- and hexa-coordinated Pb(ii) compounds. CrystEngComm 2018, 20, 5033–5044. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Afkhami, F.A.; Castiñeiras, A.; García-Santos, I.; Gurbanov, A.; Zubkov, F.I.; Mitoraj, M.P.; Kukułka, M.; Sagan, F.; Szczepanik, D.W.; et al. Quasi-aromatic Möbius Metal Chelates. Inorg. Chem. 2018, 57, 4395–4408. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Mahmoudi, G.; Afkhami, F.A.; Castiñeiras, A.; Giester, G.; Konyaeva, I.A.; Khandar, A.A.; Qu, F.; Gupta, A.; Sagan, F.; et al. Effect of Solvent on the Structural Diversity of Quasi-Aromatic Möbius Cadmium(II) Complexes Fabricated from the Bulky N 6 Tetradentate Helical Ligand. Cryst. Growth Des. 2019, 19, 1649–1659. [Google Scholar] [CrossRef]
- Afkhami, F.A.; Mahmoudi, G.; White, J.M.; Lipkowski, J.; Konyaeva, I.A.; Safin, D.A. Möbius-like metal chelates constructed from CdHal2 (Hal = Cl, Br, I) and benzilbis(pyridin-2-yl)methylidenehydrazone. Inorg. Chim. Acta 2019, 484, 481–490. [Google Scholar] [CrossRef]
- Afkhami, F.A.; Mahmoudi, G.; Khandar, A.A.; White, J.M.; Konyaeva, I.A.; Safin, D.A. Metal chelates constructed from CdHal2 (Hal = Cl, Br, I) and 1,2-diphenyl-1,2-bis((phenyl(pyridin-2-yl)methylene)hydrazono)ethane. J. Molec. Struct. 2019, 1176, 743–750. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Afkhami, F.A.; Mahmoudi, G.; Khandar, A.A.; Gurbanov, A.V.; Zubkov, F.I.; Waterman, R.; Babashkina, M.G.; Szczepanik, D.W.; Jena, H.S.; et al. Structural versatility of the quasi-aromatic Möbius type zinc(ii)-pseudohalide complexes-experimental and theoretical investigations. RSC Adv. 2019, 9, 23764–23773. [Google Scholar] [CrossRef]
- Afkhami, F.A.; Mahmoudi, G.; Qu, F.; Gupta, A.; Zangrando, E.; Frontera, A.; Safin, D.A. Complexes of BiCl3 with hydrazone derived ligands: Möbius-like discrete metal chelate versus salt-like porous polymeric structure. Inorg. Chim. Acta 2020, 502, 119350. [Google Scholar] [CrossRef]
- Bruker. SAINT, version 6.36a; Bruker-AXS Inc.: Madison, WI, USA, 2002. [Google Scholar]
- Bruker. SMART, Version 5.625 and SADABS, Version 2.03a; Bruker AXS Inc.: Madison, WI, USA, 2001. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. ActaCryst. 2008, A64, 112–122. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. ActaCryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX suit for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Seth, S.K.; Saha, I.; Estarellas, C.; Frontera, A.; Kar, T.; Mukhopadhyay, S. Supramolecular Self-Assembly of M-IDA Complexes Involving Lone-Pair⋯π Interactions: Crystal Structures, Hirshfeld Surface Analysis, and DFT Calculations [H2IDA = iminodiacetic acid, M = Cu(II), Ni(II)]. Cryst. Growth Des. 2011, 11, 3250–3265. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Cryst. 2004, B60, 627–668. [Google Scholar] [CrossRef]
- Rohl, A.L.; Moret, M.; Kaminsky, W.; Claborn, K.; Mckinnon, J.J.; Kahr, B. Hirshfeld Surfaces Identify Inadequacies in Computations of Intermolecular Interactions in Crystals: Pentamorphic 1,8-Dihydroxyanthraquinone. Cryst. Growth Des. 2008, 8, 4517–4525. [Google Scholar] [CrossRef]
- Seth, S.K. Tuning the formation of MOFs by pH influence: X-ray structural variations and Hirshfeld surface analyses of 2-amino-5-nitropyridine with cadmium chloride. Acta Cryst. 2013, 15, 1772–1781. [Google Scholar] [CrossRef]
- Seth, S.K.; Bauzá, A.; Frontera, A. Screening polymorphism in a Ni(II) metal–organic framework: Experimental observations, Hirshfeld surface analyses and DFT studies. CrystEngComm 2018, 20, 746–754. [Google Scholar] [CrossRef]
- Seth, S.K. Structural characterization and Hirshfeld surface analysis of a CoII complex with imidazo[1,2-a]-pyridine. Acta Cryst. 2018, E74, 600–606. [Google Scholar] [CrossRef]
- Maity, T.; Mandal, H.; Bauza, A.; Samanta, B.C.; Frontera, A.; Seth, S.K. Quantifying conventional C–H⋯π(aryl) and unconventional C–H⋯π(chelate) interactions in dinuclear Cu(II) complexes: Experimental observations, Hirshfeld surface and theoretical DFT study. New J. Chem. 2018, 42, 10202–10213. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer 3.1; University of Western Australia: Perth, Australia, 2007. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A dispersion correction for density functionals, Hartree-Fock and semi-empirical quantum chemical methods DFT-D3. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]


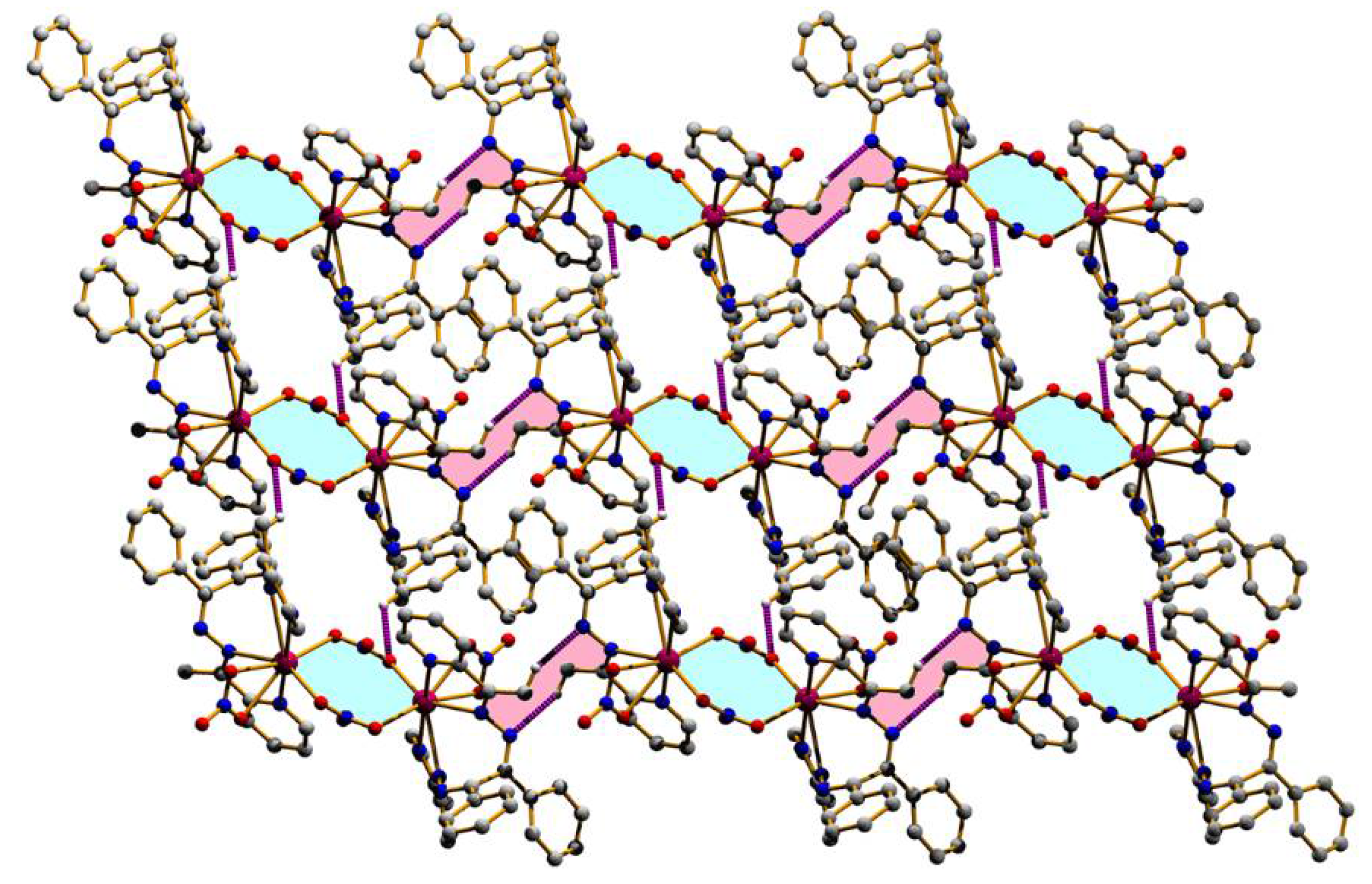
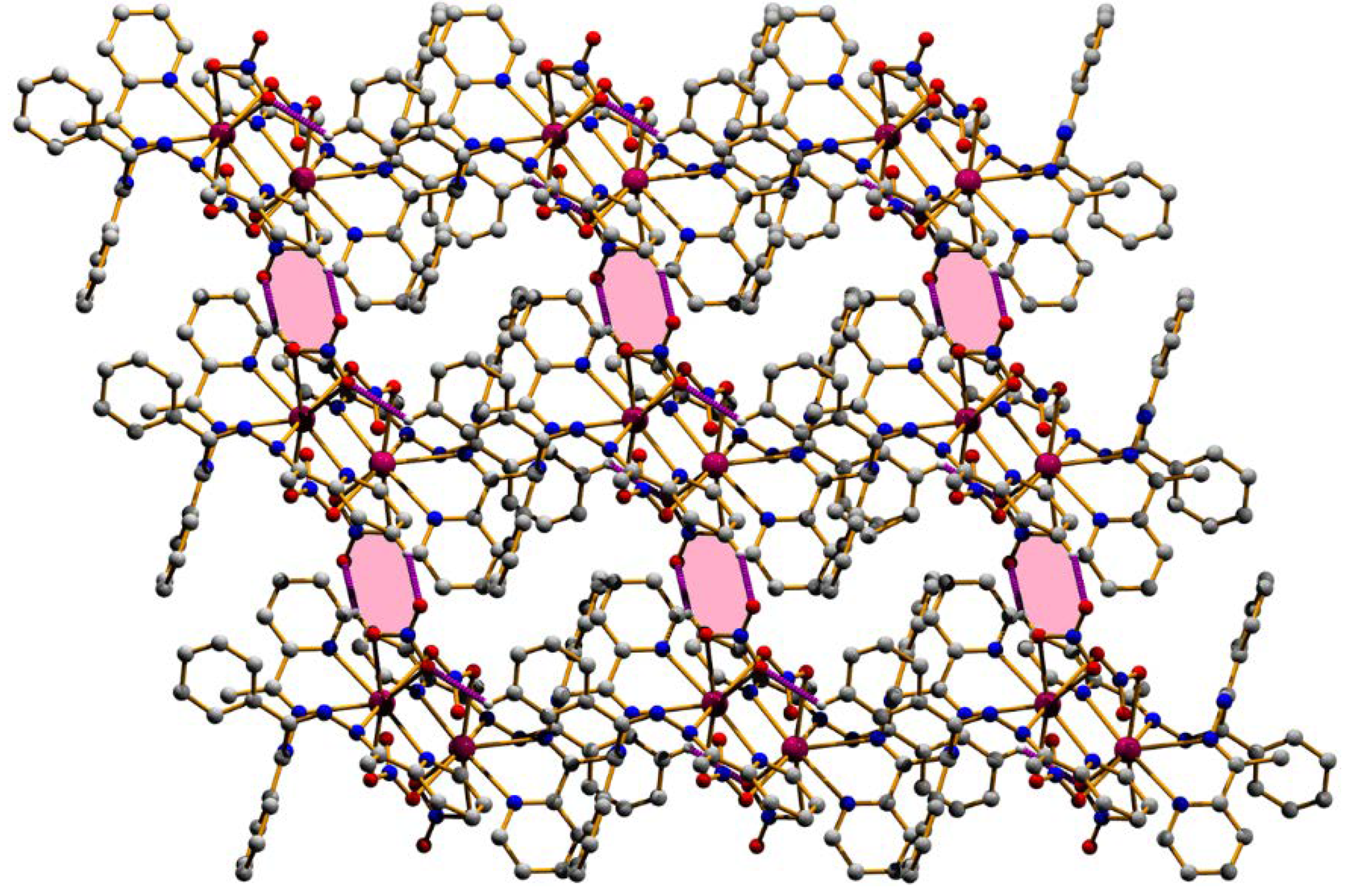
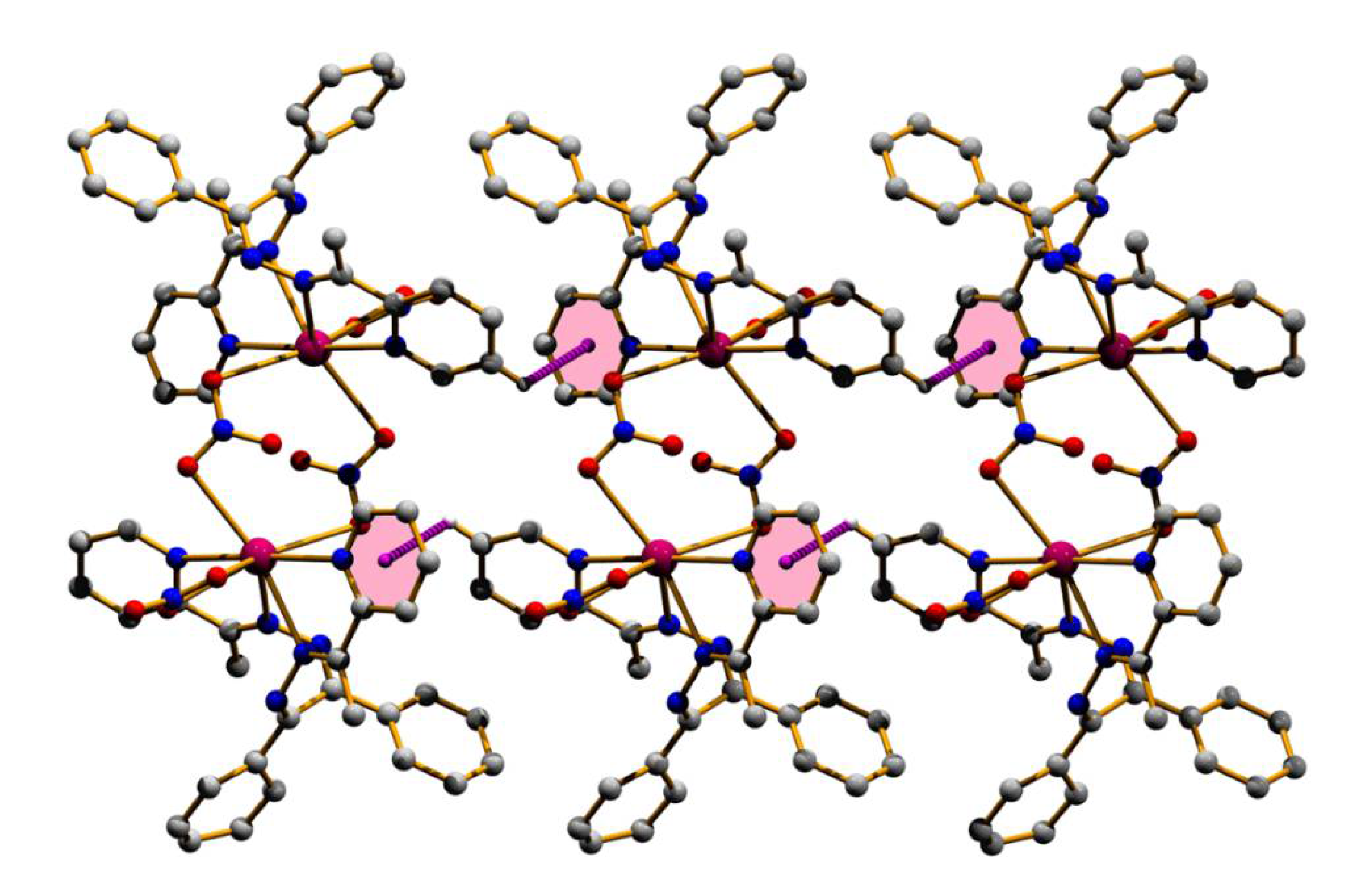


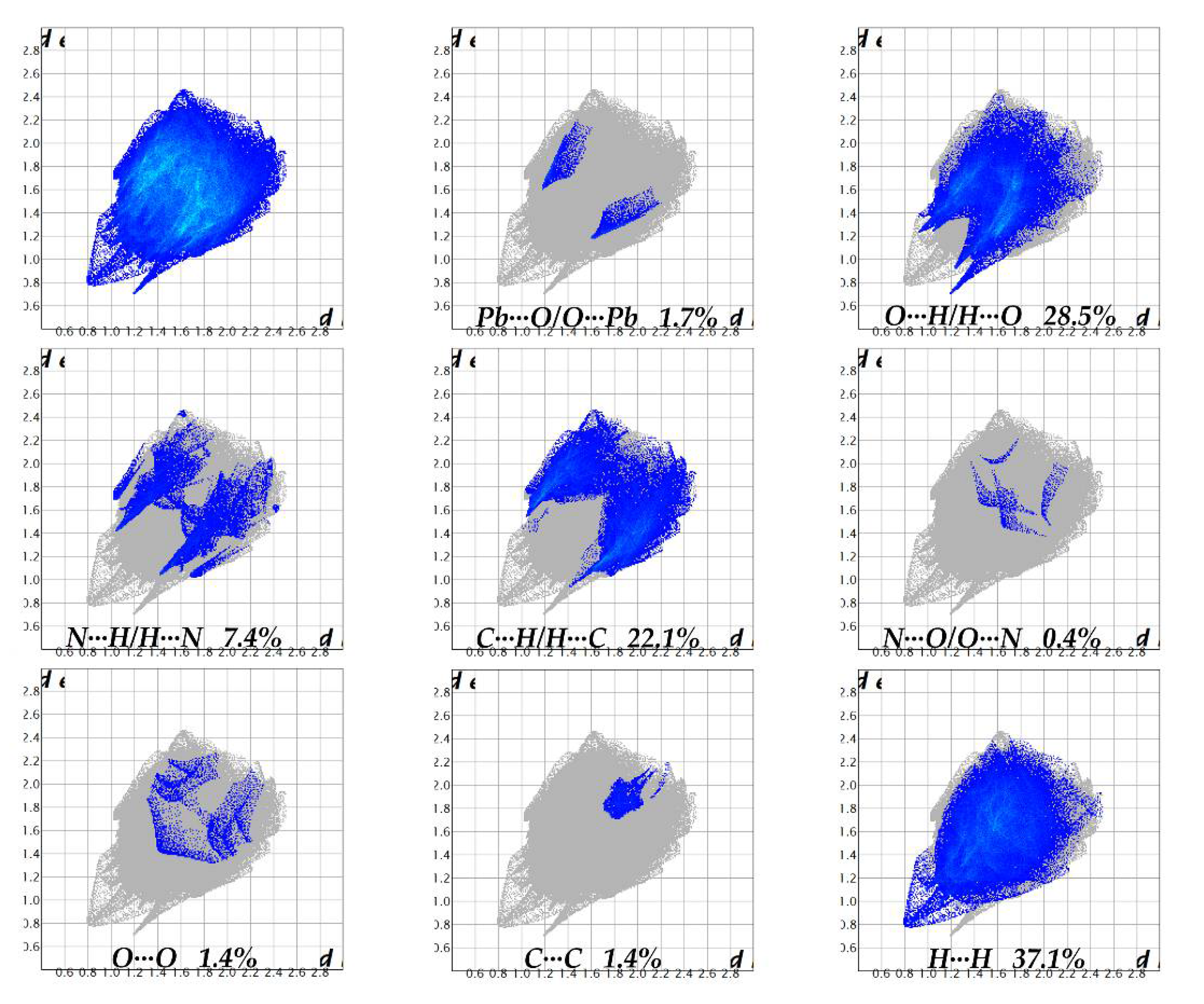

| Structure | (1) | (2) |
|---|---|---|
| Empirical formula | C60H48N16Pb2S4 | C57H52N16O13Pb2 |
| Formula Weight | 1535.76 | 1583.52 |
| Temperature (K) | 100(2) | 100(2) |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Triclinic |
| space group | P‒1 | P‒1 |
| a, b, c (Å) | 9.2815(7), 10.4330(8), 16.3791(11) | 10.0664(6),11.0250(7),14.1786(10) |
| α, β, γ (°) | 81.444(3), 74.809(3), 78.168(4) | 105.444(3), 95.245(3), 104.866(3) |
| Volume (Å3) | 1490.51(19) | 1444.27(16) |
| Z / Density (calc.) (Mg/m3) | 1/1.711 | 1/1.821 |
| Absorption coefficient (mm−1) | 5.834 | 5.901 |
| F(000) | 748 | 774 |
| Crystal size (mm3) | 0.45 × 0.40 × 0.20 | 0.45 × 0.25 × 0.11 |
| θ range for data collection | 2.274 to 25.342 | 1.512 to 26.242 |
| Completeness to θ (%) | 99.8 | 98.9 |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 0.362 and 0.154 | 0.552 and 0.165 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/parameters | 5442/382 | 5905/416 |
| Goodness-of - fit on F2 | 1.028 | 1.051 |
| Final R indices [I > 2σ(I)] | R1 = 0.0283, wR2 = 0.0581 | R1 = 0.0265, wR2 = 0.0529 |
| R indices (all data) | R1 = 0.0337, wR2 = 0.0600 | R1 = 0.0323, wR2 = 0.0548 |
| Largest diff. peak and hole (e.Å−3) | 1.279 and −1.814 | 1.127 and −1.132 |
| Complex-(1) | |||
| Pb(1)-N(1) | 2.629(4) | Pb(1)-N(6) | 2.657(3) |
| Pb(1)-N(2) | 2.645(3) | Pb(1)-N(7) | 2.615(4) |
| Pb(1)-N(5) | 2.745(3) | Pb(1)-S(1) | 2.756(2) |
| O(1)-Pb(1)-N(3) | 60.39(9) | O(1)-Pb(1)-O(7) | 142.78(7) |
| O(1)-Pb(1)-N(4) | 115.35(9) | N(3)-Pb(1)-O(7) | 117.30(7) |
| N(3)-Pb(1)-N(4) | 57.86(9) | N(4)-Pb(1)-O(7) | 84.17(6) |
| Complex-(2) | |||
| Pb(1)-O(1) | 2.614(3) | Pb(1)-N(1) | 2.720(3) |
| Pb(1)-O(3) | 2.621(3) | Pb(1)-N(5) | 2.763(3) |
| Pb(1)-N(2) | 2.670(3) | ||
| O(1)-Pb(1)-O(3) | 48.61(8) | N(2)-Pb(1)-N(1) | 59.79(9) |
| O(1)-Pb(1)-N(2) | 75.78(9) | O(1)-Pb(1)-N(5) | 130.88(9) |
| O(3)-Pb(1)-N(2) | 81.63(9) | O(3)-Pb(1)-N(5) | 90.89(9) |
| O(1)-Pb(1)-N(1) | 70.93(9) | N(2)-Pb(1)-N(5) | 71.13(8) |
| O(3)-Pb(1)-N(1) | 114.86(9) | N(1)-Pb(1)-N(5) | 118.16(8) |
| D–H···A | D–H | H···A | D···A | D–H···A | Symmetry |
|---|---|---|---|---|---|
| Compound (1) | |||||
| C27–H27···S2 | 0.95 | 2.94 | 3.716(5) | 139 | 1 − x, 2 − y, −z |
| C7–H7C···N8 | 0.98 | 2.64 | 3.56(2) | 158 | x, 1 + y, z |
| Compound (2) | |||||
| O7–H71···O3 | 0.84 | 2.04 | 2.849(8) | 162 | ---- |
| C1–H1···O5 | 0.95 | 2.26 | 3.041(5) | 139 | −x, 1 − y, −z |
| C3–H3···O2 | 0.95 | 2.57 | 3.451(5) | 153 | −1 + x, y, z |
| C4–H4···O7 | 0.95 | 2.40 | 3.218(9) | 144 | −1 + x, y, z |
| C7–H7B···N3 | 0.98 | 2.55 | 3.373(5) | 141 | −x, 1 − y, 1 − z |
| C7–H7C···N3 | 0.98 | 2.30 | 2.755(5) | 107 | ---- |
| C12–H12···O1 | 0.95 | 2.47 | 3.102(6) | 124 | x, −1 + y, z |
| C15–H15B···O7 | 1.40 | 2.40 | 3.574(9) | 139 | ---- |
| C21–H21···O4 | 0.95 | 2.57 | 3.243(5) | 128 | −1 − x, −y, −z |
| C23–H23C···N4 | 0.98 | 2.39 | 2.789(5) | 104 | ---- |
| C29–H29···O4 | 0.95 | 2.41 | 3.161(5) | 136 | −x, 1 − y, −z |
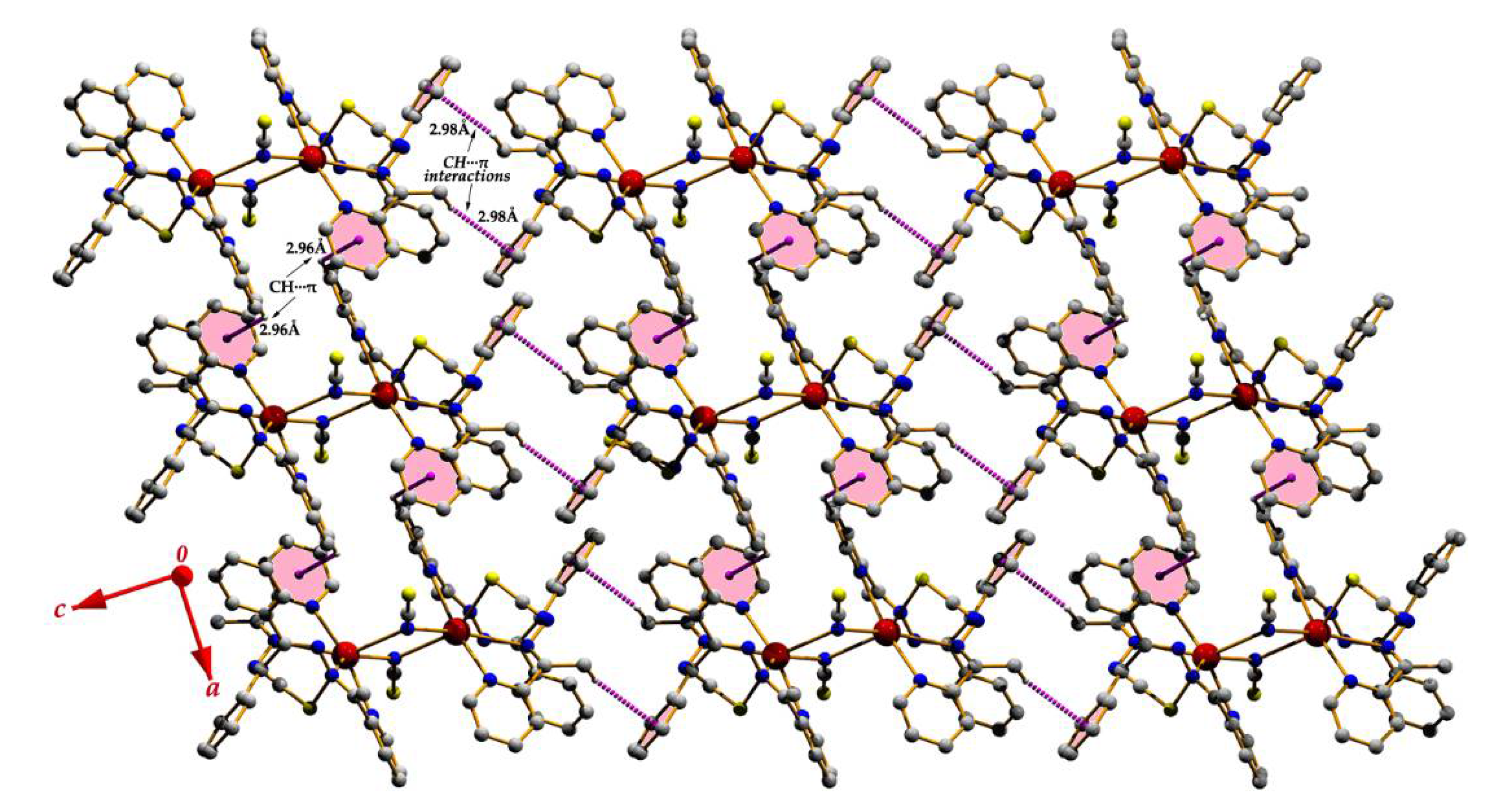
| Y–X∙∙∙Cg(I) | X∙∙∙Cg | Y∙∙∙Cg | Y–X∙∙∙Cg | X-Perp | Symmetry |
|---|---|---|---|---|---|
| Compound (1) | |||||
| C(2)–H(2)∙∙∙Cg(2) | 2.96 | 3.570(5) | 123 | 2.89 | 1 + x, y, z |
| C(23)–H(23C)∙∙∙Cg(4) | 2.98 | 3.560(5) | 119 | 2.73 | −x, 2 − y, 1 − z |
| Compound (2) | |||||
| C(28)–H(28)∙∙∙Cg(18) | 2.96 | 3.413(4) | 110 | 2.80 | 1 + x, y, z |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoudi, G.; Kumar Seth, S.; Bauza Riera, A.; Ivanovich Zubkov, F.; Frontera, A. Novel Pb(II) Complexes: X-Ray Structures, Hirshfeld Surface Analysis and DFT Calculations. Crystals 2020, 10, 568. https://doi.org/10.3390/cryst10070568
Mahmoudi G, Kumar Seth S, Bauza Riera A, Ivanovich Zubkov F, Frontera A. Novel Pb(II) Complexes: X-Ray Structures, Hirshfeld Surface Analysis and DFT Calculations. Crystals. 2020; 10(7):568. https://doi.org/10.3390/cryst10070568
Chicago/Turabian StyleMahmoudi, Ghodrat, Saikat Kumar Seth, Antonio Bauza Riera, Fedor Ivanovich Zubkov, and Antonio Frontera. 2020. "Novel Pb(II) Complexes: X-Ray Structures, Hirshfeld Surface Analysis and DFT Calculations" Crystals 10, no. 7: 568. https://doi.org/10.3390/cryst10070568
APA StyleMahmoudi, G., Kumar Seth, S., Bauza Riera, A., Ivanovich Zubkov, F., & Frontera, A. (2020). Novel Pb(II) Complexes: X-Ray Structures, Hirshfeld Surface Analysis and DFT Calculations. Crystals, 10(7), 568. https://doi.org/10.3390/cryst10070568