Cocrystals of Isoniazid with Polyphenols: Mechanochemical Synthesis and Molecular Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Liquid-Assisted Grinding and Crystallization
2.2. Infrared Spectroscopy
2.3. X-ray Diffraction
3. Results and Discussion
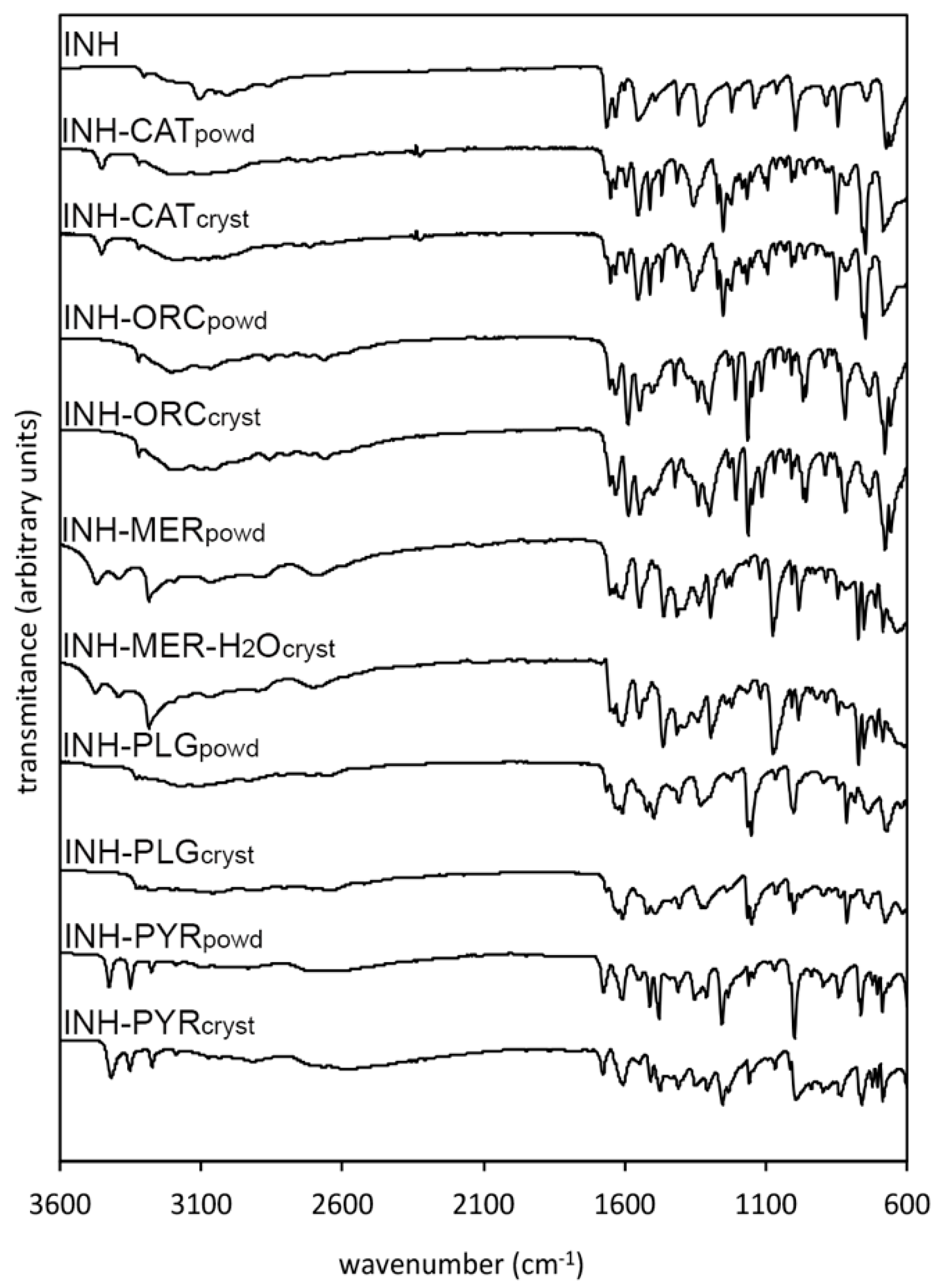
3.1. IR Spectroscopy
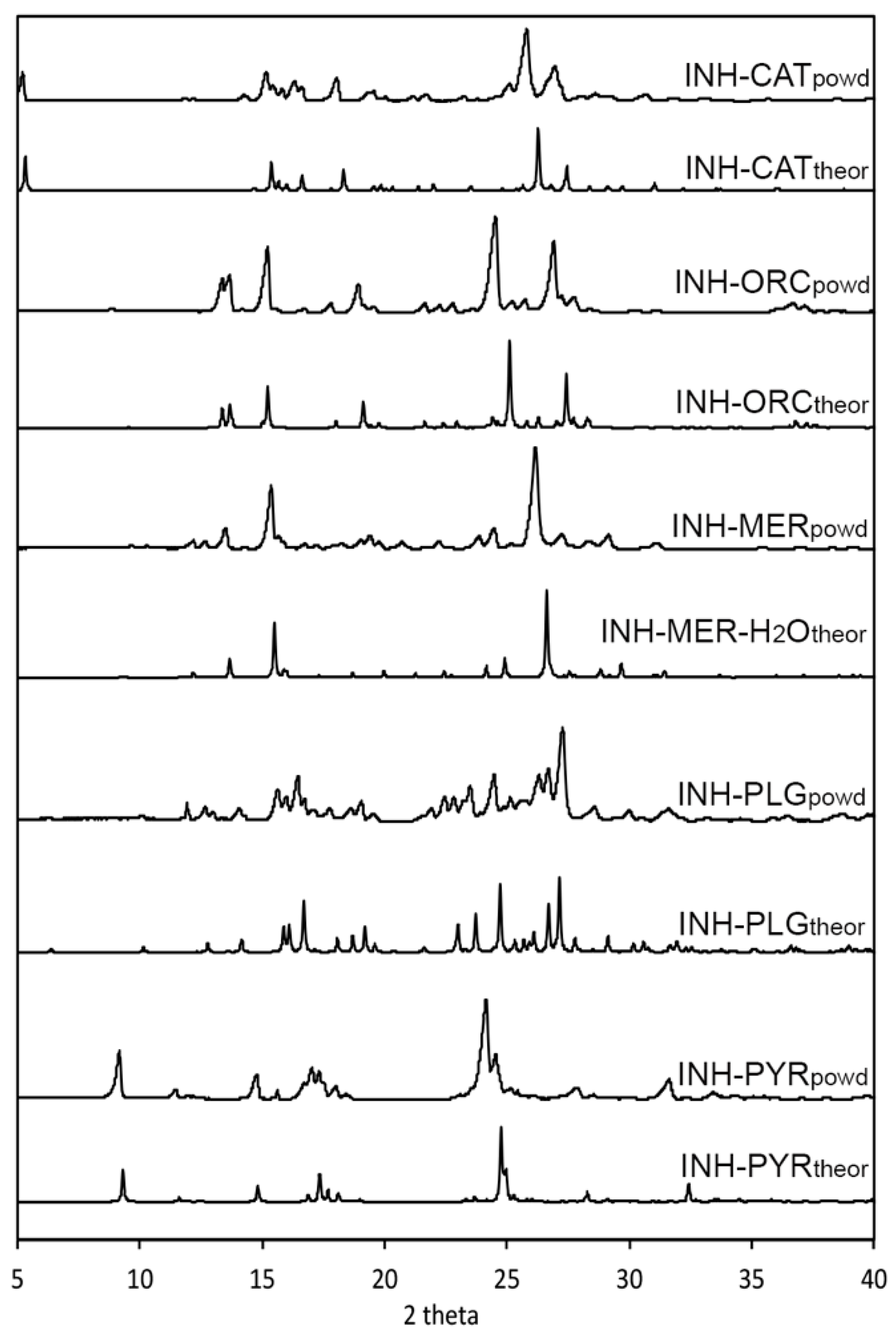
3.2. Powder X-ray Diffraction
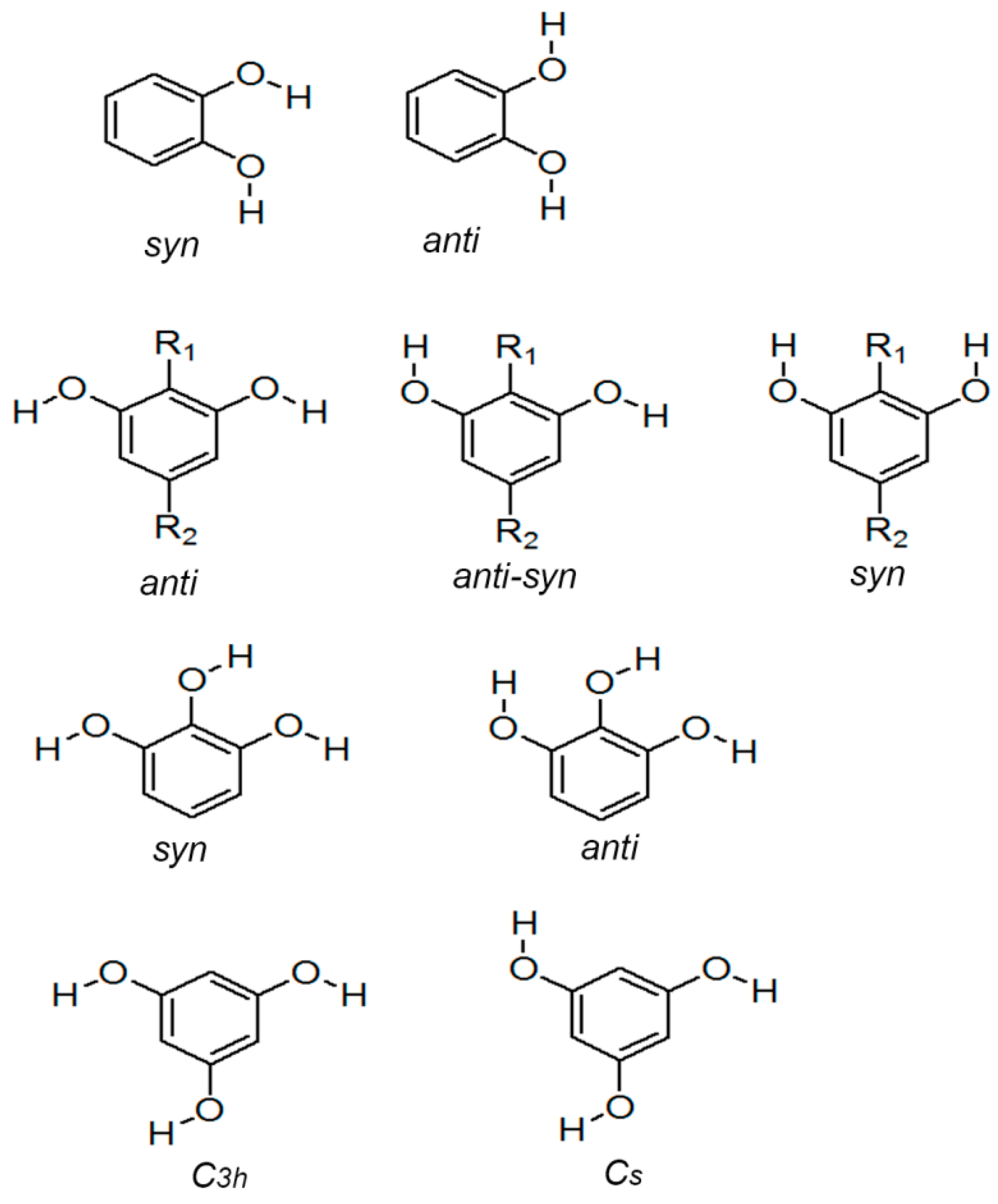
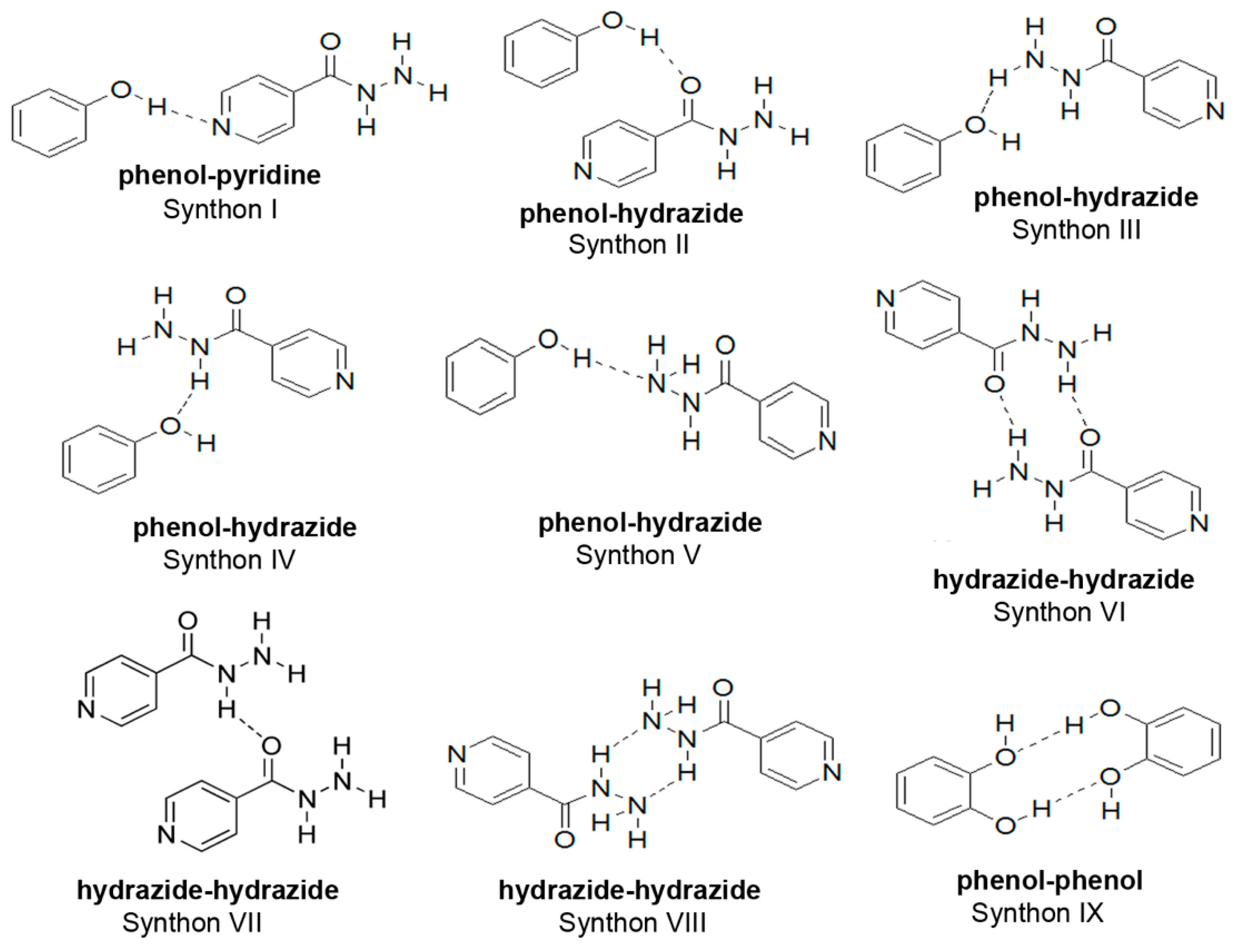
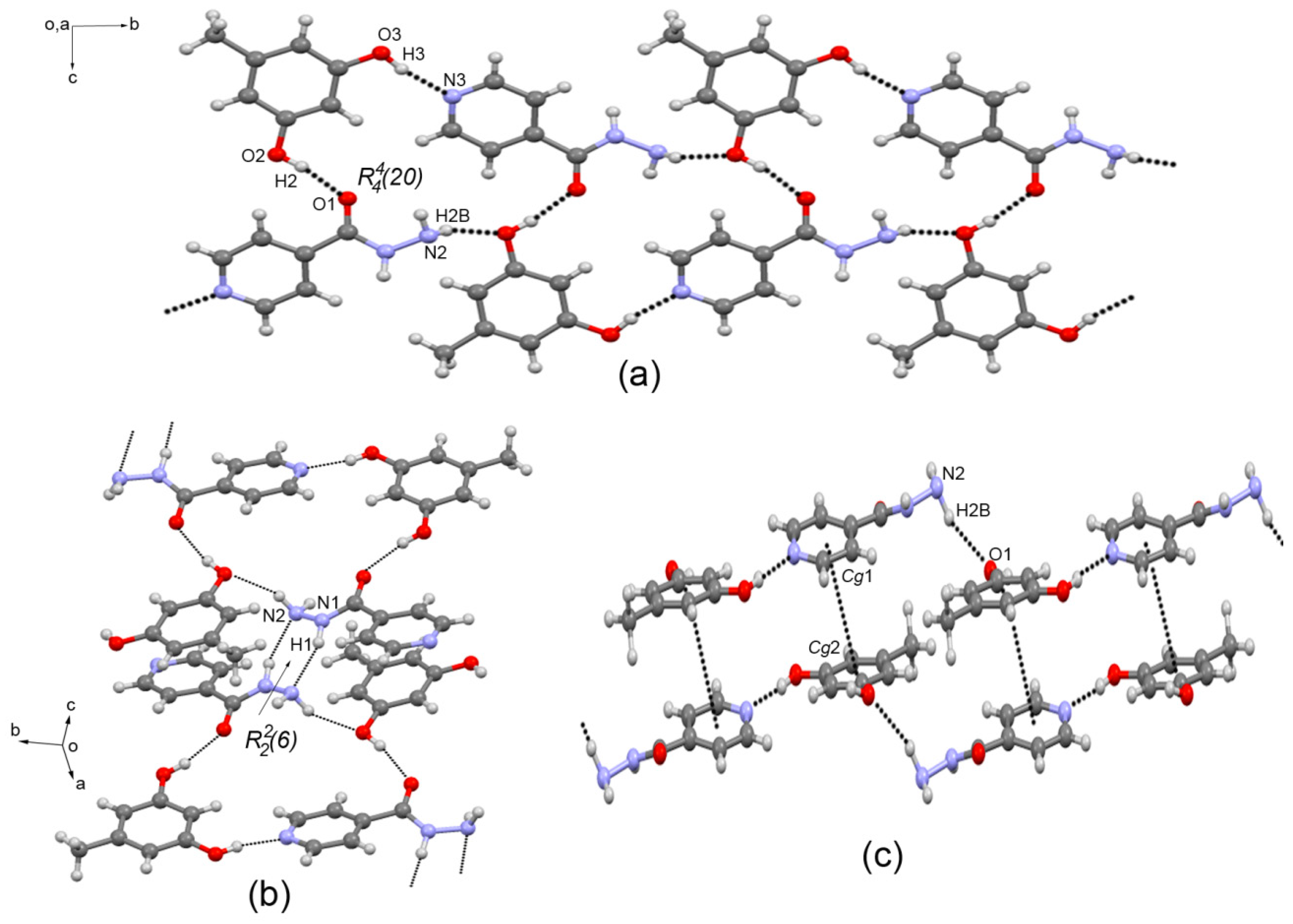
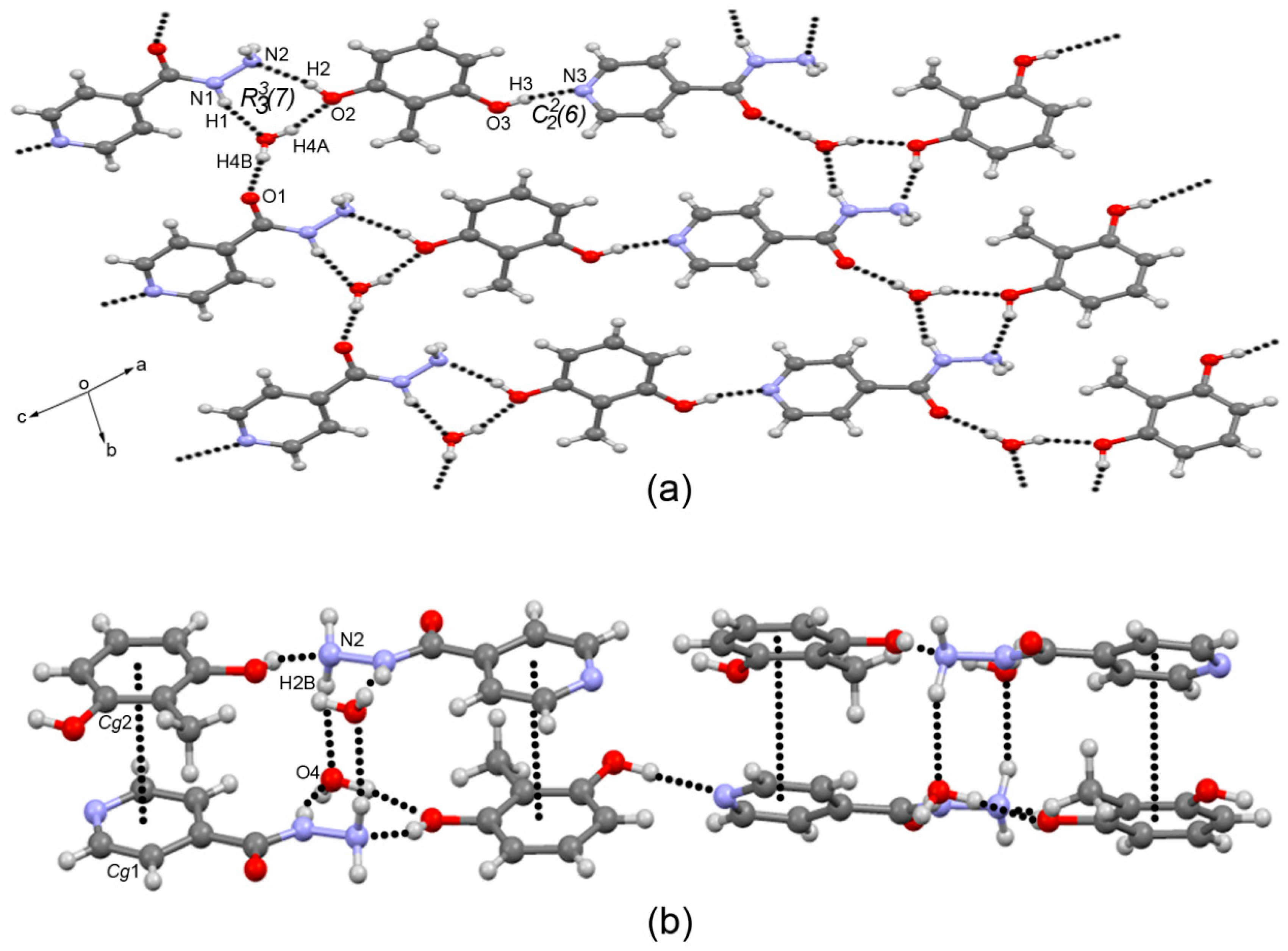
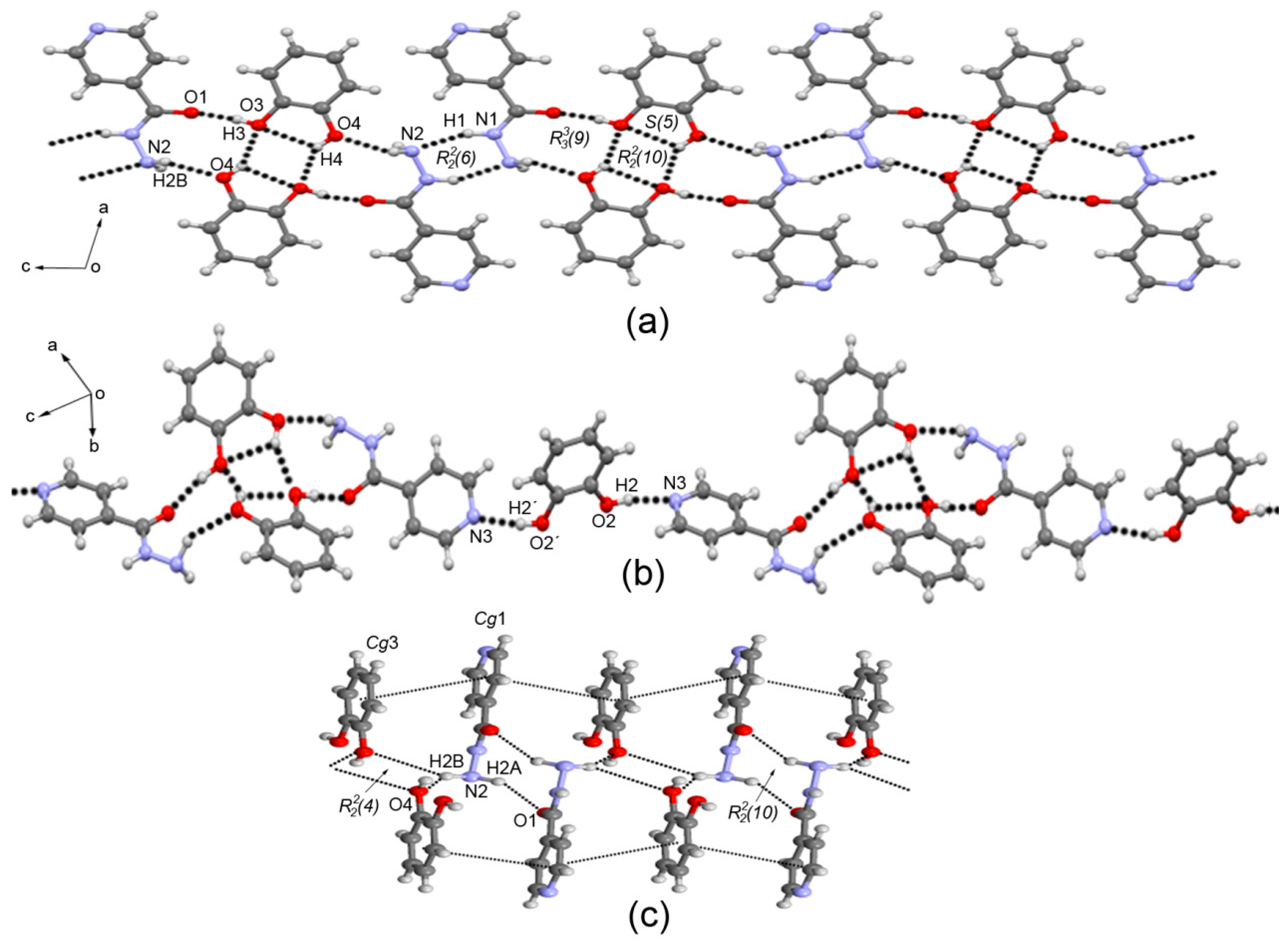
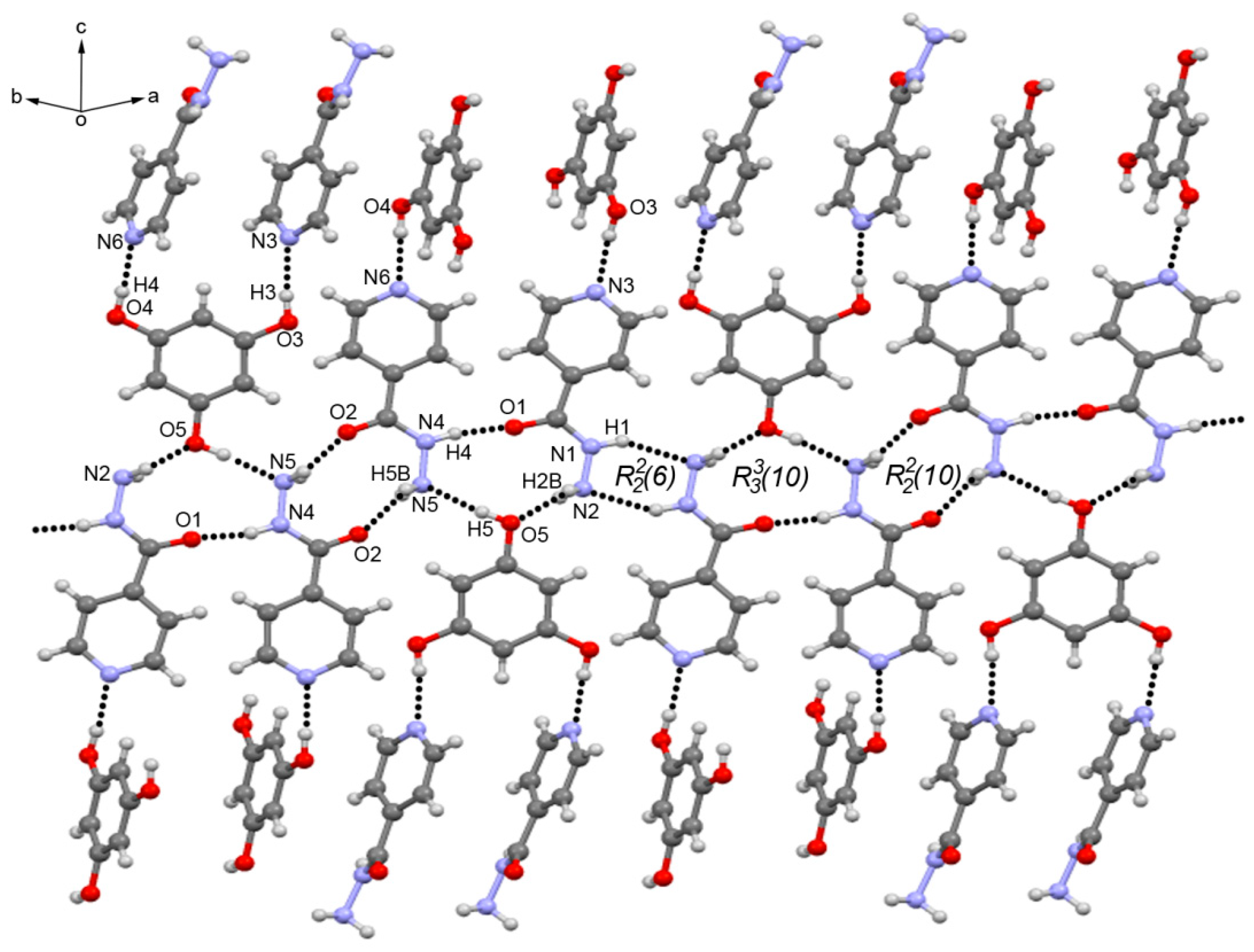
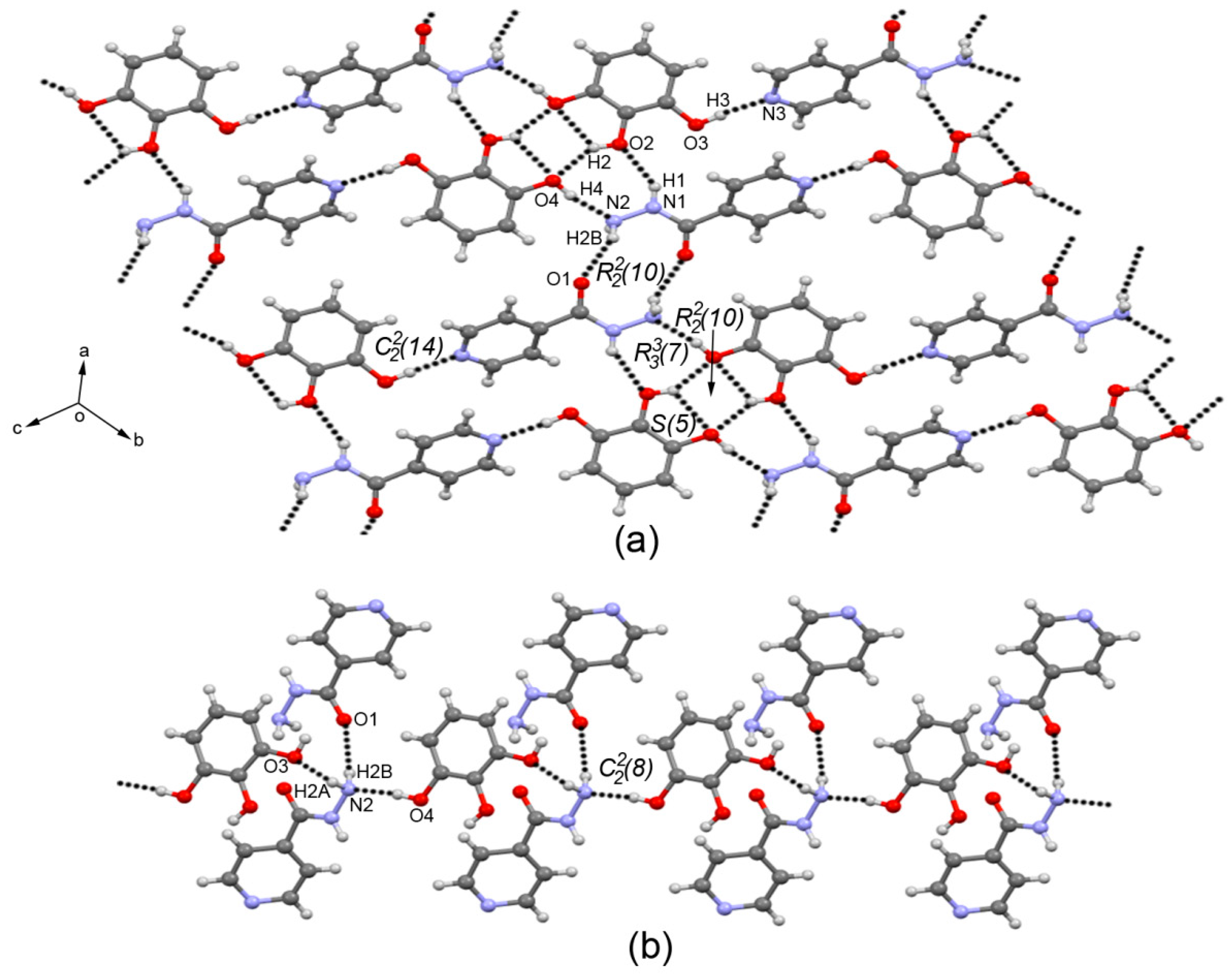
3.3. Crystal Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical cocrystals: From serendipity to design to application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Lewinsohn, D.M.; Leonard, M.K.; LoBue, P.A.; Cohn, D.L.; Daley, C.L.; Desmond, E.; Keane, J.; Lewinsohn, D.A.; Loeffler, A.M.; Mazurek, G.H. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin. Infect. Dis. 2017, 64, e1–e33. [Google Scholar] [CrossRef] [PubMed]
- Scior, T.; Meneses-Morales, I.; Garcés-Eisele, S.J.; Domeyer, D.; Laufer, S. Antitubercular isoniazid and drug resistance of Mycobacterium tuberculosis—A review. Arch. Pharm. Pharm. Med. Chem. 2002, 335, 511–525. [Google Scholar] [CrossRef]
- Sarcevica, I.; Orola, L.; Veidis, M.V.; Podjava, A.; Belyakov, S. Crystal and Molecular Structure and Stability of Isoniazid Cocrystals with Selected Carboxylic Acids. Cryst. Growth Des. 2013, 13, 1082–1090. [Google Scholar] [CrossRef]
- Swapna, B.; Maddileti, D.; Nangia, A. Cocrystals of the Tuberculosis Drug Isoniazid: Polymorphism, Isostructurality and Stability. Cryst. Growth Des. 2014, 14, 5991–6005. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Novel solid forms of the anti-tuberculosis drug, Isoniazid: Ternary and polymorphic cocrystals. CrystEngComm 2013, 15, 5877–5887. [Google Scholar] [CrossRef]
- Mashhadi, S.M.A.; Yunus, U.; Bhatti, M.H.; Tahir, M.N. Isoniazid cocrystals with anti-oxidant hydroxy benzoic acids. J. Mol. Struct. 2014, 1076, 446–452. [Google Scholar] [CrossRef]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Naturforsch. C 2006, 61, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, B.; Dubey, A.; Verma, A.; Tiwari, S. Antibacterial activity of phenolics compounds against pathogenic bacteria. Int. J. Pharm. Sci. Rev. Res. 2015, 35, 16–18. [Google Scholar]
- Sarma, B.; Saikia, B. Hydrogen bond synthon competition in the stabilization of theophylline cocrystals. CrystEngComm 2014, 16, 4753–4765. [Google Scholar] [CrossRef]
- Magaña-Vergara, N.E.; de la Cruz-Cruz, P.; Peraza-Campos, A.L.; Martínez-Martínez, F.J.; González-González, J.S. Mechanochemical Synthesis and Crystal Structure of the Lidocaine-Phloroglucinol Hydrate 1:1:1 Complex. Crystals 2018, 8, 130. [Google Scholar] [CrossRef]
- Bolla, G.; Sanphui, P.; Nangia, A. Solubility Advantage of Tenoxicam Phenolic Cocrystals Compared to Salts. Cryst. Growth Des. 2013, 13, 1988–2003. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Nangia, A. Fast Dissolving Curcumin Cocrystals. Cryst. Growth Des. 2011, 11, 4135–4145. [Google Scholar] [CrossRef]
- Karki, S.; Friscic, T.; Fabian, L.; Jones, W. New solid forms of artemisinin obtained through cocrystallisation. CrystEngComm 2010, 12, 4038–4041. [Google Scholar] [CrossRef]
- CrysAlisPro, version 1.171.36.32; Oxford Diffraction Ltd.: Abingdon, UK, 2013.
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. 1995, A51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Sailatha, E.; Seshadri, S.; Kumaresan, S. FTIR, FT Raman spectra and molecular structural confirmation of isoniazid. Indian J. Pure Appl. Phys. 2009, 47, 12–18. [Google Scholar]
- Saucedo-Balderas, M.M.; Delgado-Alfaro, R.A.; Martínez-Martínez, F.J.; Ortegón-Reyna, D.; Bernabé-Pineda, M.; Zúñiga-Lemus, O.; González-González, J.S. Synthesis, Molecular Structure of Diethyl Phenylenebis(Methylene)Dicarbamates and FTIR Spectroscopy Molecular Recognition Study with Benzenediols. J. Braz. Chem. Soc. 2015, 26, 396–402. [Google Scholar] [CrossRef]
- Mohammed-Zieglera, I.; Billes, F. Vibrational spectroscopic calculations on pyrogallol and gallic acid. J. Mol. Struct. THEOCHEM 2002, 618, 259–265. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The nature of π–π interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Intl. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Vedernikova, I.; Proynov, E.; Salahub, D.; Haemers, A. Local atomic and orbital reactivity indices from density functional calculations for hydrogen-bonded 1,2-dihydroxybenzene. Int. J. Quantum Chem. 2000, 77, 161–173. [Google Scholar] [CrossRef]
- Khan, M.; Enkelmann, V.; Brunklaus, G. Solid-State NMR and X-ray Analysis of Structural Transformations in O−H···N Heterosynthons Formed by Hydrogen-Bond-Mediated Molecular Recognition. J. Org. Chem. 2009, 74, 2261–2270. [Google Scholar] [CrossRef]
- Veidis, M.V.; Orola, L.; Mutikainen, I.; Sarcevica, I. The conformation of pyrogallol as a result of cocrystallization with N-heterocyclic bases. CrystEngComm 2012, 14, 7253–7257. [Google Scholar] [CrossRef]
- Braun, D.E.; Tocher, D.A.; Price, S.L.; Griesser, U.J. The complexity of hydration of phloroglucinol: A comprehensive structural and thermodynamic characterization. J. Phys. Chem. B 2012, 116, 3961–3972. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Grobelny, P.; Thakur, T.S.; Desiraju, G.R. Polymorphs, Pseudopolymorphs, and Co-Crystals of Orcinol: Exploring the Structural Landscape with High Throughput Crystallography. Cryst. Growth Des. 2011, 11, 2637–2653. [Google Scholar] [CrossRef]
- Thakuria, R.; Cherukuvada, S.; Nangia, A. Crystal Structures of Pyrogallol, Its Hydrate, and Stable Multiple Z′ Cocrystals with N-Heterocycles Containing Metastable Conformers of Pyrogallol. Cryst. Growth Des. 2012, 12, 3944–3953. [Google Scholar] [CrossRef]
- Gangavaram, S.; Raghavender, S.; Sanphui, P.; Pal, S.; Manjunatha, S.G.; Nambiar, S.; Nangia, A. Polymorphs and Cocrystals of Nalidixic Acid. Cryst. Growth Des. 2012, 12, 4963–4971. [Google Scholar] [CrossRef]
- Kamalakaran, A.S. Molecular Adducts of Isoniazid: Crystal Structure, Electronic Properties, and Hirshfeld Surface Analysis. J. Struct. Chem. 2018, 59, 1518–1533. [Google Scholar] [CrossRef]
- Vishweshwar, P.; Nangia, A.; Lynch, V.M. Supramolecular synthons in phenol–isonicotinamide adducts. CrystEngComm 2003, 5, 164–168. [Google Scholar] [CrossRef]
- Khan, M.; Enkelmann, V.; Brunklaus, G. O-H···N Heterosynthon: A Robust Supramolecular Unit for Crystal Engineering. Cryst. Growth Des. 2009, 9, 2354–2362. [Google Scholar] [CrossRef]
- Corpinot, M.K.; Kresimir Bucar, D.K. A Practical Guide to the Design of Molecular Crystals. Cryst. Growth Des. 2019, 19, 1426–1453. [Google Scholar] [CrossRef]
- Bis, J.A.; Vishweshwar, P.; Weyna, D.; Zaworotko, M.J. Hierarchy of Supramolecular Synthons: Persistent Hydroxyl···Pyridine Hydrogen Bonds in Cocrystals That Contain a Cyano Acceptor. Mol. Pharm. 2007, 4, 401–416. [Google Scholar] [CrossRef]

| Cocrystal | INH-ORC | INH-MER-H2O | INH-CAT | INH-PLG | INH-PYR |
|---|---|---|---|---|---|
| Molecular formula | C6H7N3O·C7H8O2 | C6H7N3O·C7H8O2·H2O | C6H7N3O·C6H6O2 | C6H7N3O·C6H6O3 | C6H7N3O·C6H6O3 |
| Mr Formula weight (g·mol−1) | 261.28 | 279.29 | 302.31 | 400.40 | 263.25 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Triclinic |
| Space group | P21/n | P21/c | P2/c | P21/c | P-1 |
| a, b, c (Å) | 7.3607(9), 13.2387(14), 12.9532(17) | 10.2646(8), 6.8865(5), 19.0382(13) | 17.5391(13), 7.0947(5), 12.1518(9) | 7.1867(5), 9.1733(7), 27.751(2) | 7.7020(6), 8.2347(11), 10.6669(15) |
| α, β, γ (°) | 90, 92.118(11), 90 | 90, 94.671(8), 90 | 90, 108.616(8), 90 | 90, 92.721(7),90 | 107.521(12), 107.527(10), 97.396(9) |
| V (Å3) | 1261.4(3) | 1341.29(17) | 1432.99(19) | 1827.4(2) | 597.02(13) |
| Z | 4 | 4 | 4 | 4 | 2 |
| μ (mm−1) | 0.100 | 0.104 | 0.104 | 0.109 | 0.112 |
| T (K) | 130(2) | 130(2) | 130(2) | 130(2) | 130(2) |
| ρcalcd (g·cm−3) | 1.376 | 1.383 | 1.401 | 1.455 | 1.464 |
| Crystal size (mm) | 0.470 × 0.320 × 0.210 | 0.550 × 0.420 × 0.320 | 0.480 × 0.380 × 0.260 | 0.460 × 0.370 × 0.290 | 0.460 × 0.370 × 0.290 |
| F(000) | 552 | 592 | 636 | 840 | 276 |
| θ range (°) | 3.457–29.354 | 3.566–29.548 | 3.538–29.358 | 3.604–29.502 | 3.884–29.424 |
| Reflections collected | 5891 | 5919 | 6477 | 9139 | 4443 |
| Independent reflections | 2934 | 3143 | 3368 | 4305 | 2768 |
| Data/restraints/parameters | 2934/5/188 | 3143/7/204 | 3368/6/217 | 4305/9/289 | 2768/6/190 |
| Goof | 1.004 | 1.022 | 1.015 | 0.962 | 1.039 |
| R (int) | 0.0269 | 0.0235 | 0.0250 | 0.0323 | 0.0235 |
| Final R indices [I > 2σ(I)], R1/wR2 | 0.0492/0.1184 | 0.0452/0.0966 | 0.0472/0.1013 | 0.0548/0.1327 | 0.0455/0.1036 |
| Largest diff. peak/hole (e·Å−3) | 0.354/0.312 | 0.266/−0.288 | 0.407/−0.283 | 0.292/−0.393 | 0.310/−0.332 |
| Cocrystal | Interaction | D-H | H···A | D···A | D-H···A | Symmetry Code |
|---|---|---|---|---|---|---|
| INH-ORC | N1-H1···N2 | 0.916(16) | 2.127(17) | 2.982(2) | 155.0(18) | −x, −y, 1 − z |
| N2-H2A···O3 | 0.903(17) | 2.352(17) | 3.104(2) | 140.6(16) | ½ − x, −1/2 + y, ½ − z | |
| N2-H2B···O2 | 0.921(16) | 2.165(17) | 3.031(2) | 156.3(16) | 1 − x, −y, 1 − z | |
| O2-H2···O1 | 0.850(2) | 1.900(2) | 2.737(17) | 166.0(2) | ½ + x, ½ − y, ½ + z | |
| O3-H3···N3 | 0.870(2) | 1.880(2) | 2.7459(19) | 170.0(19) | 1 − x, 1 – y, 1 − z | |
| INH-MER-H2O | O3-H3···N3 | 0.900(18) | 1.849(18) | 2.7371(18) | 168.8(19) | 1 + x, y, z |
| O2-H2···N2 | 0.858(15) | 1.926(17) | 2.7540(19) | 161.7(19) | −1 + x, ½ − y, ½ + z | |
| O4-H4A···O2 | 0.870(2) | 1.930(2) | 2.7585(18) | 159.0(3) | −x, −1/2 + y, ½ − z | |
| O4-H4B···O1 | 0.860(2) | 1.920(2) | 2.7723(17) | 174.0(2) | 1 − x, 1 − y, −z | |
| N2-H2B···O4 | 0.914(19) | 2.170(18) | 3.0207(19) | 154.4(17) | 1 + x, y, z | |
| N1-H1···O4 | 0.902(14) | 2.017(14) | 2.8908(19) | 163.0(17) | 1 − x, −y, −z | |
| INH-CAT | O4-H4···O3 | 0.840(2) | 2.060(2) | 2.8041(18) | 147.0(2) | 1 − x, y, ½ − z |
| O3-H3···O1 | 0.859(18) | 1.808(18) | 2.6570(16) | 169.0(2) | x, 1 − y, −1/2 + z | |
| N2-H2B···O4 | 0.908(16) | 2.231(18) | 3.0241(19) | 145.6(15) | 1 − x, 1 − y, 1 − z | |
| N1-H1···N2 | 0.893(16) | 2.067(17) | 2.896(2) | 154.1(17) | 1 − x, y, ½ − z | |
| O2-H2···N3 | 0.879(17) | 1.882(17) | 2.7419(18) | 165.6(19) | x, 1 − y, ½ + z | |
| N2-H2A···O1 | 0.914(16) | 2.231(18) | 3.0637(19) | 151.3(18) | 1 − x, -y, 1 − z | |
| N2-H2B···O4 | 0.908(16) | 2.231(18) | 3.0241(19) | 145.6(15) | 1 − x, 1 − y, 1 − z | |
| INH-PLG | O4-H4···N6 | 0.859(17) | 1.880(17) | 2.738(2) | 179.0(3) | 1 − x, ½ + y, ½ − z |
| O3-H3···N3 | 0.868(17) | 1.931(17) | 2.794(2) | 173.0(3) | 1 − x, -1/2 + y, ½ − z | |
| O5-H5···N5 | 0.870(2) | 1.920(2) | 2.780(2) | 174.0(3) | 1 − x, −y, −z | |
| N4-H4···O1 | 0.930(2) | 2.000(2) | 2.900(2) | 165.0(2) | 1 + x, −1 + y, z | |
| N5-H5B···O2 | 0.918(15) | 2.150(2) | 2.903(3) | 139.0(2) | 1 − x, −y, −z | |
| N1-H1···N2 | 0.910(2) | 2.160(2) | 2.976(3) | 148.4(19) | 1−x,1−y, −z | |
| N2-H2A···O2 | 0.917(19) | 2.170(2) | 3.058(2) | 164.0(2) | 1 − x, 1 − y, −z | |
| N2-H2B···O5 | 0.923(15) | 2.284(17) | 3.075(3) | 143.0(2) | −x, 1 − y, −z | |
| INH-PYR | O2-H2···O4 | 0.860(2) | 1.980(2) | 2.7160(18) | 143.0(2) | 2 − x, 1 − y, −z |
| N1-H1···O2 | 0.882(17) | 2.147(17) | 2.913(2) | 144.9(16) | ||
| O4-H4···N2 | 0.871(19) | 1.844(19) | 2.707(2) | 170.8(19) | 1 − x, 2 − y, −z | |
| O3-H3···N3 | 0.890(2) | 1.860(2) | 2.742(2) | 175.0(2) | 1 − x, 1 − y, 1 − z | |
| N2-H2B···O1 | 0.900(17) | 2.190(18) | 2.886(2) | 133.7(16) | 2 − x, 1 − y, −z | |
| N2-H2A···O3 | 0.908(17) | 2.245(17) | 3.146(2) | 172.0(2) | 1 − x, 1 − y, −z |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, J.S.; Martínez-Santiago, A.M.M.; Martínez-Martínez, F.J.; Emparán-Legaspi, M.J.; Pineda-Contreras, A.; Flores-Alamo, M.; García-Ortega, H. Cocrystals of Isoniazid with Polyphenols: Mechanochemical Synthesis and Molecular Structure. Crystals 2020, 10, 569. https://doi.org/10.3390/cryst10070569
González-González JS, Martínez-Santiago AMM, Martínez-Martínez FJ, Emparán-Legaspi MJ, Pineda-Contreras A, Flores-Alamo M, García-Ortega H. Cocrystals of Isoniazid with Polyphenols: Mechanochemical Synthesis and Molecular Structure. Crystals. 2020; 10(7):569. https://doi.org/10.3390/cryst10070569
Chicago/Turabian StyleGonzález-González, Juan Saulo, Ana María Monserrat Martínez-Santiago, Francisco Javier Martínez-Martínez, María José Emparán-Legaspi, Armando Pineda-Contreras, Marcos Flores-Alamo, and Héctor García-Ortega. 2020. "Cocrystals of Isoniazid with Polyphenols: Mechanochemical Synthesis and Molecular Structure" Crystals 10, no. 7: 569. https://doi.org/10.3390/cryst10070569
APA StyleGonzález-González, J. S., Martínez-Santiago, A. M. M., Martínez-Martínez, F. J., Emparán-Legaspi, M. J., Pineda-Contreras, A., Flores-Alamo, M., & García-Ortega, H. (2020). Cocrystals of Isoniazid with Polyphenols: Mechanochemical Synthesis and Molecular Structure. Crystals, 10(7), 569. https://doi.org/10.3390/cryst10070569








