Effect of Equal Channel Angular Extrusion on the Thermal Conductivity of an AX52 Magnesium Alloy
Abstract
1. Introduction
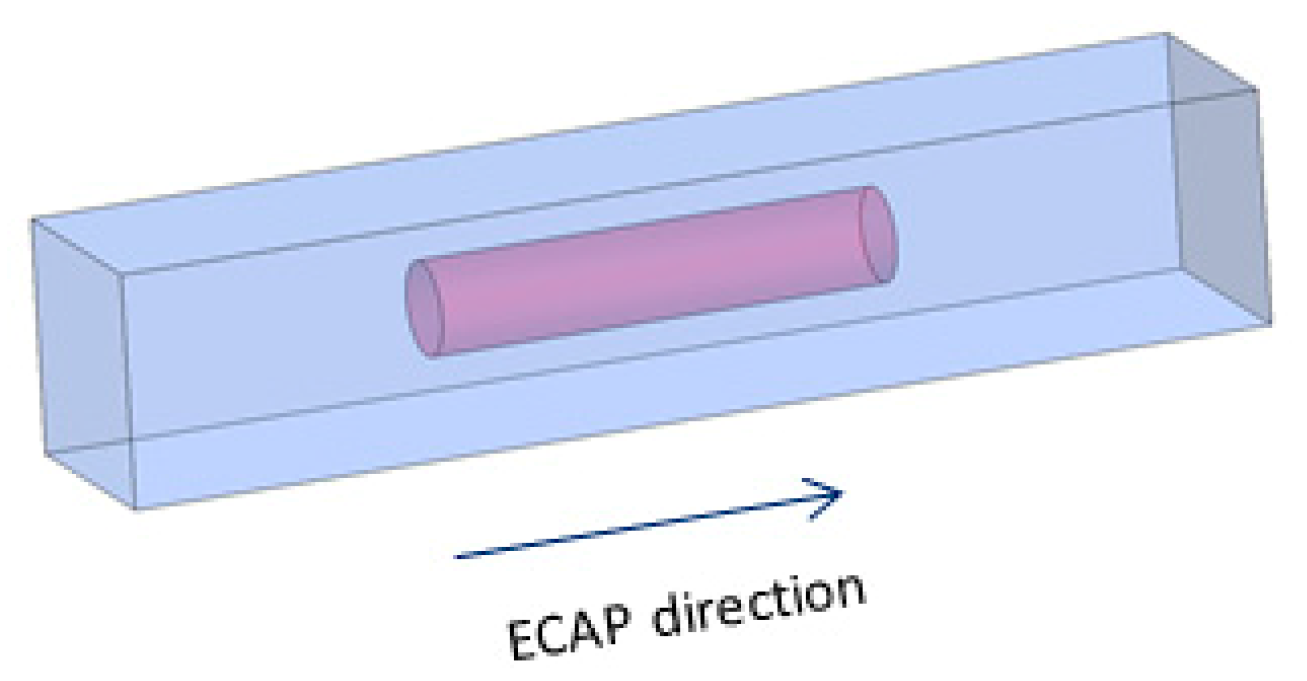
2. Materials and Methods
3. Results
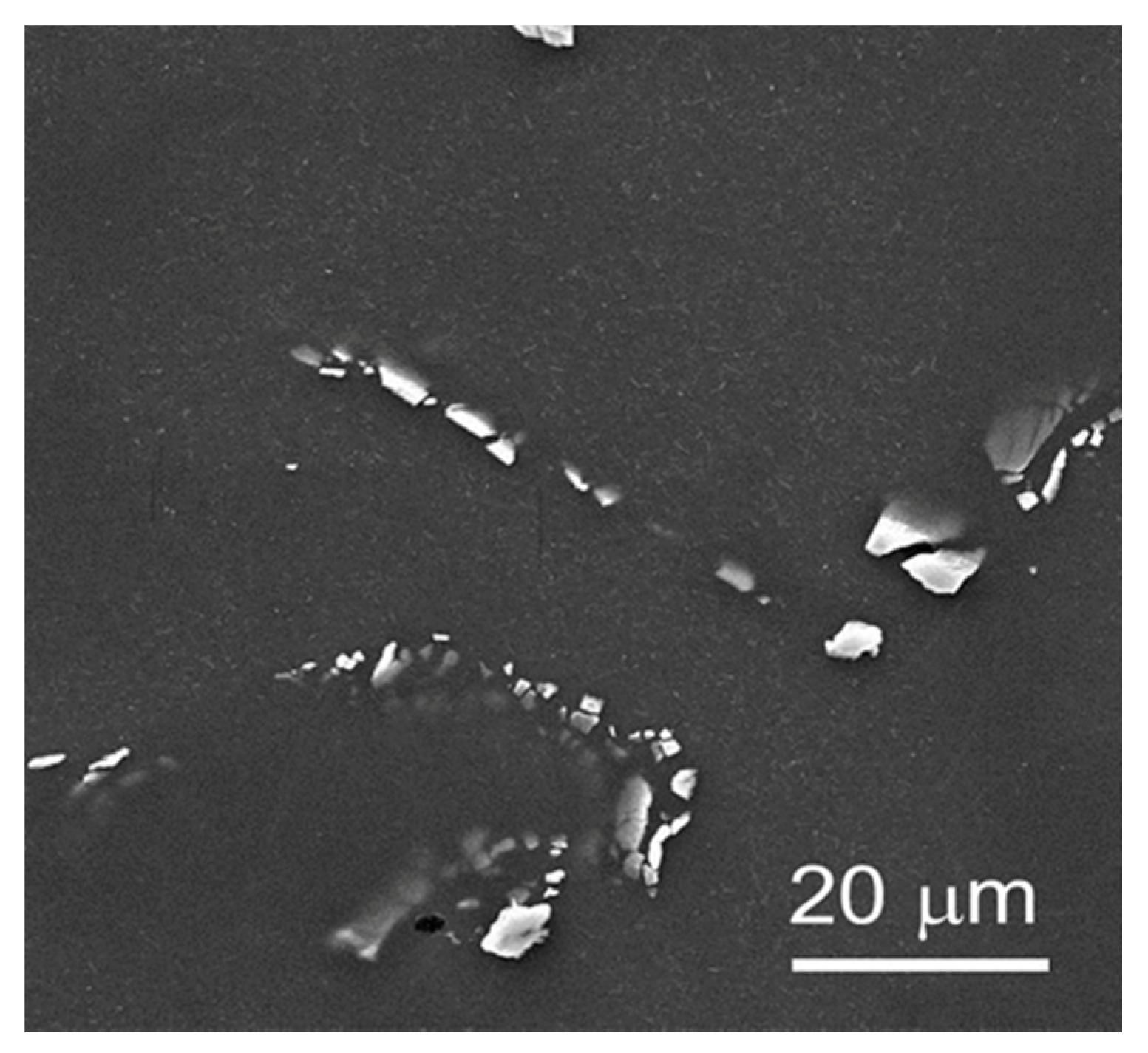
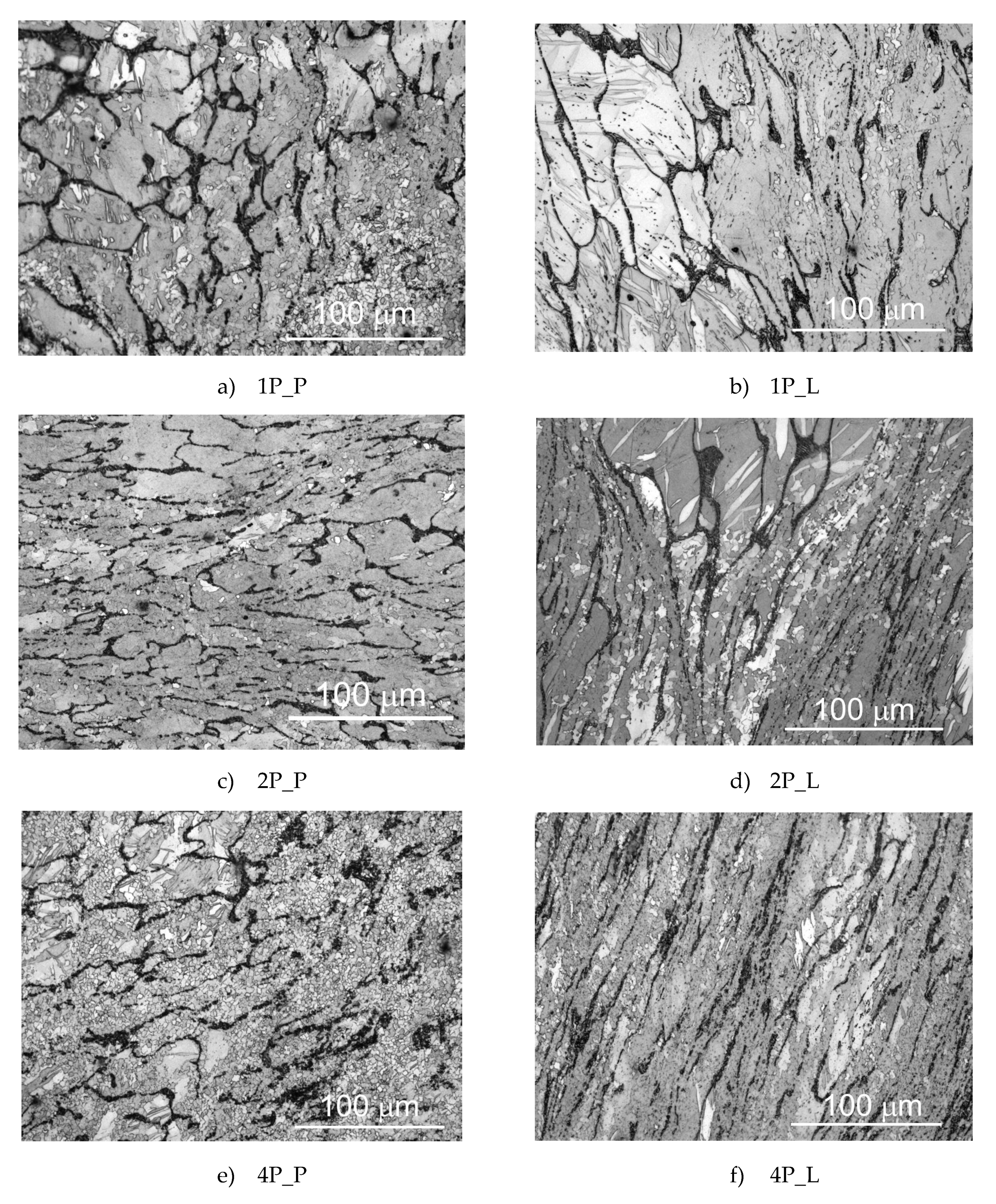
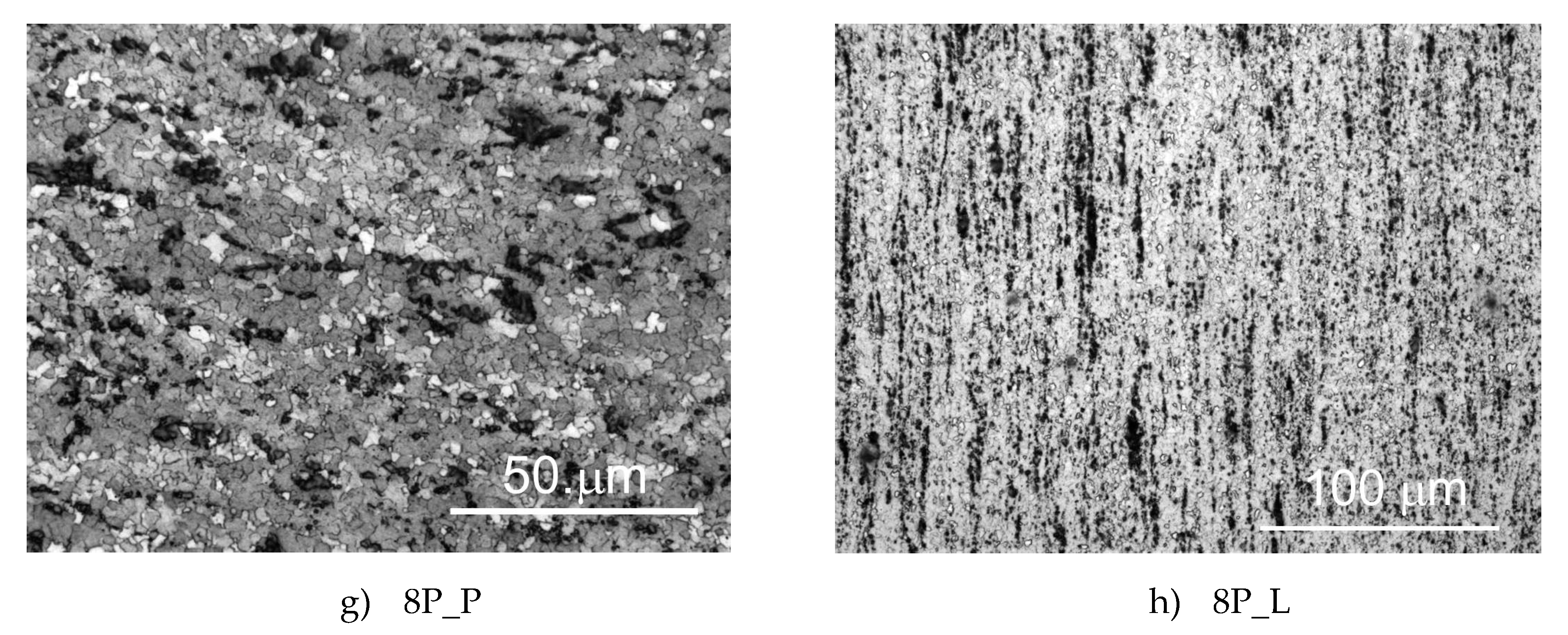
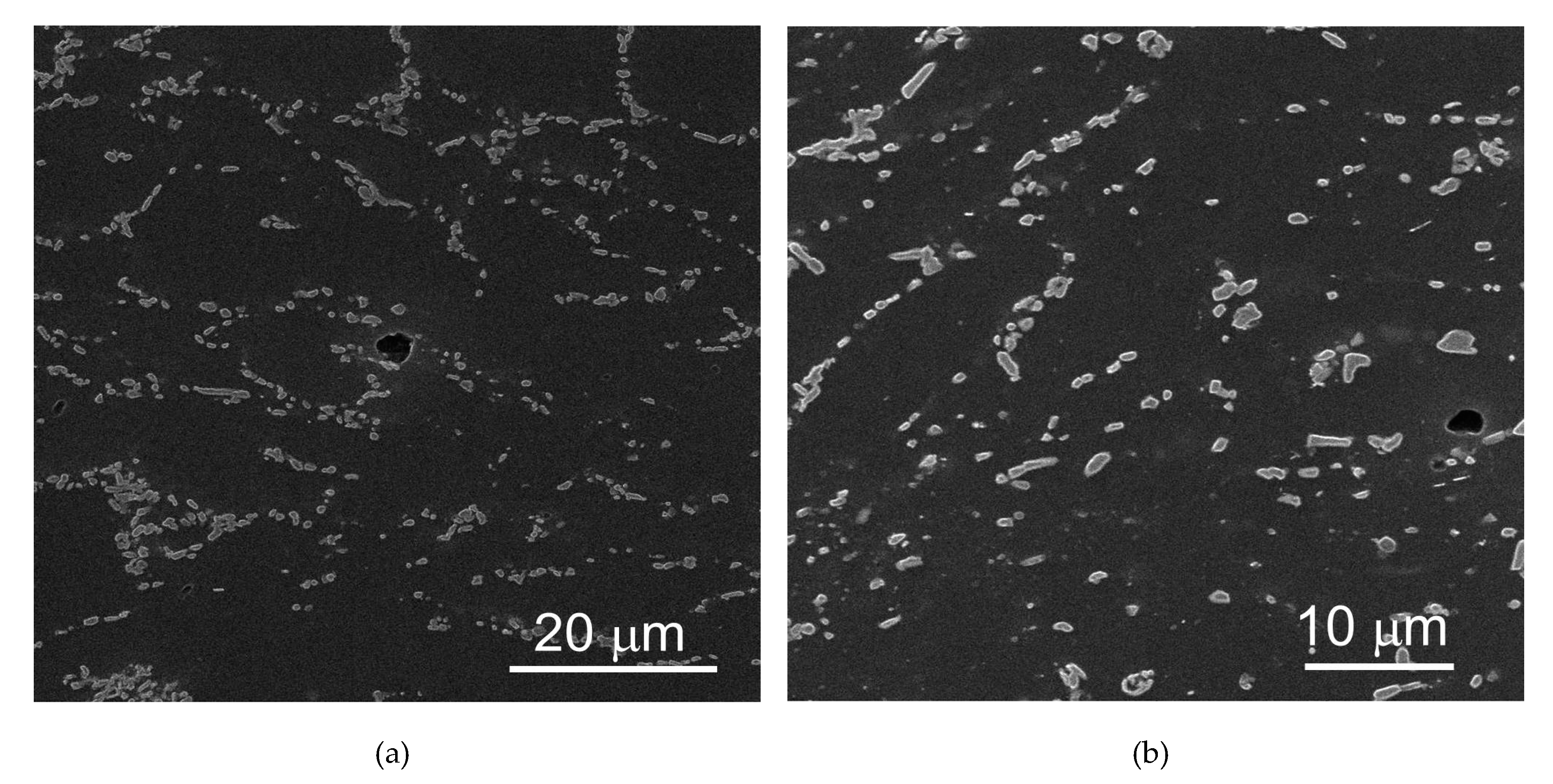
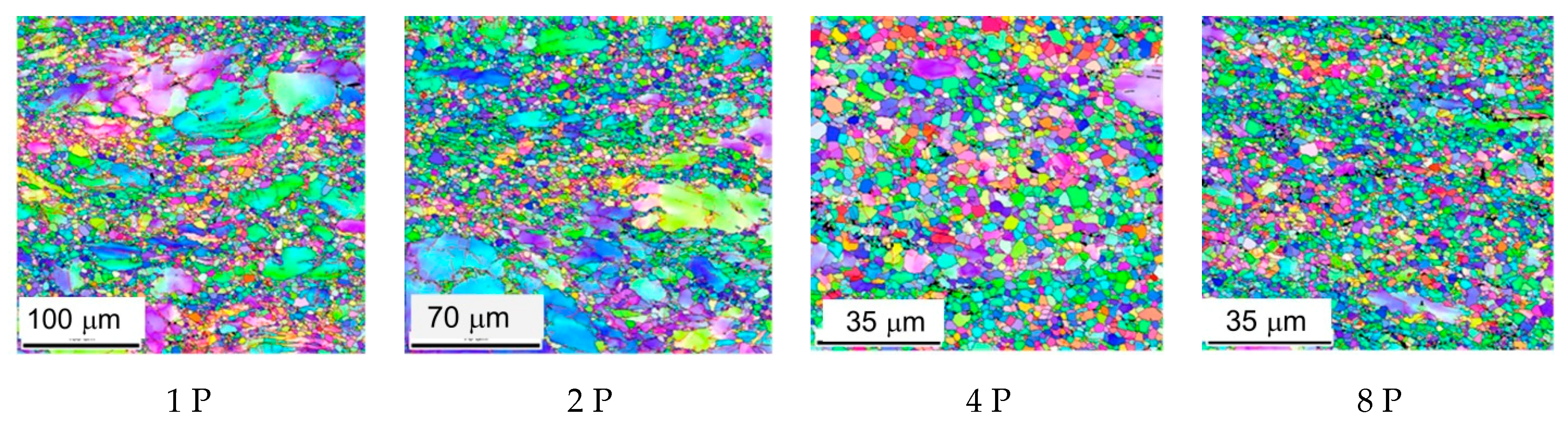
3.1. Microstructure of the Samples
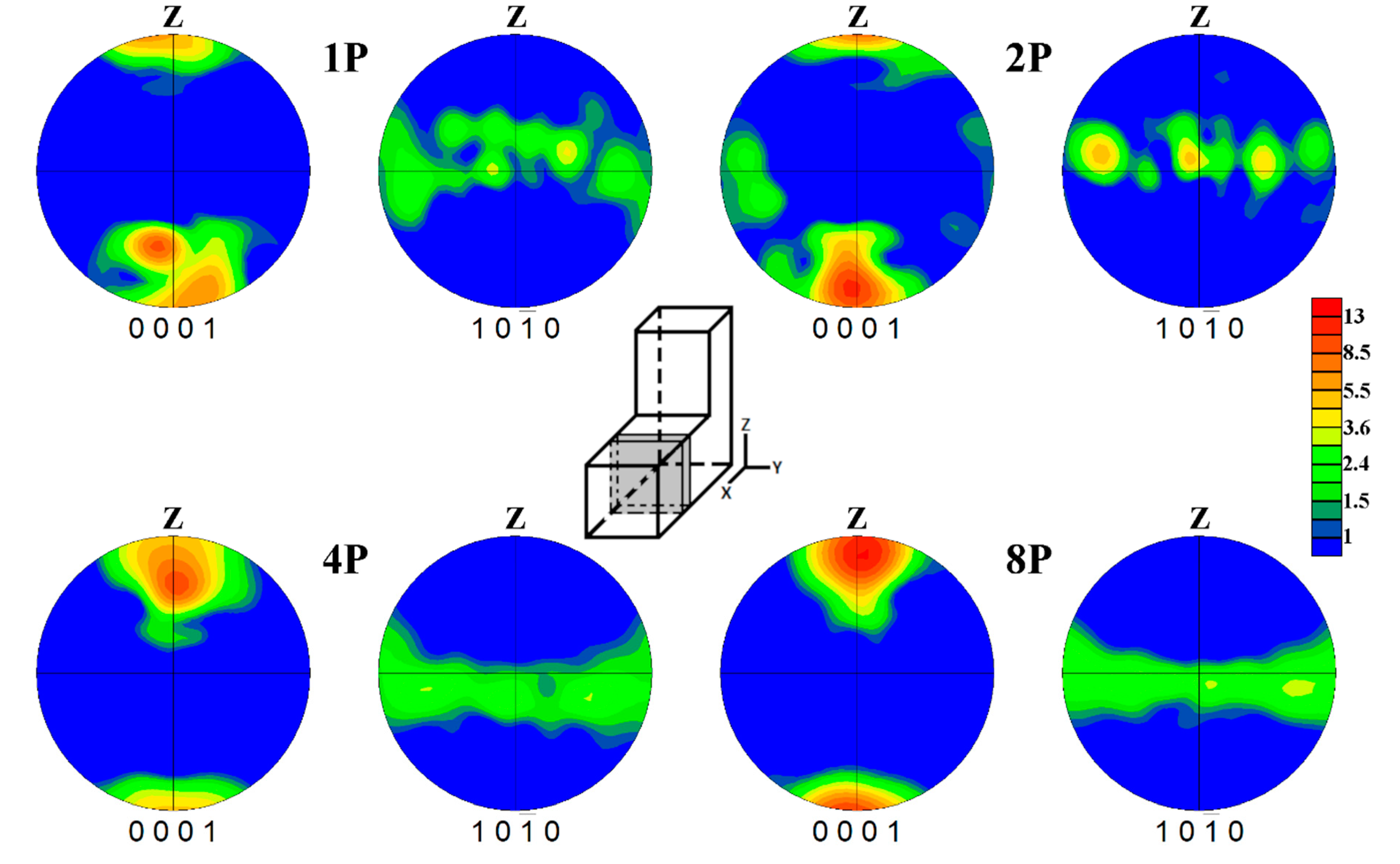
3.2. Texture of Samples
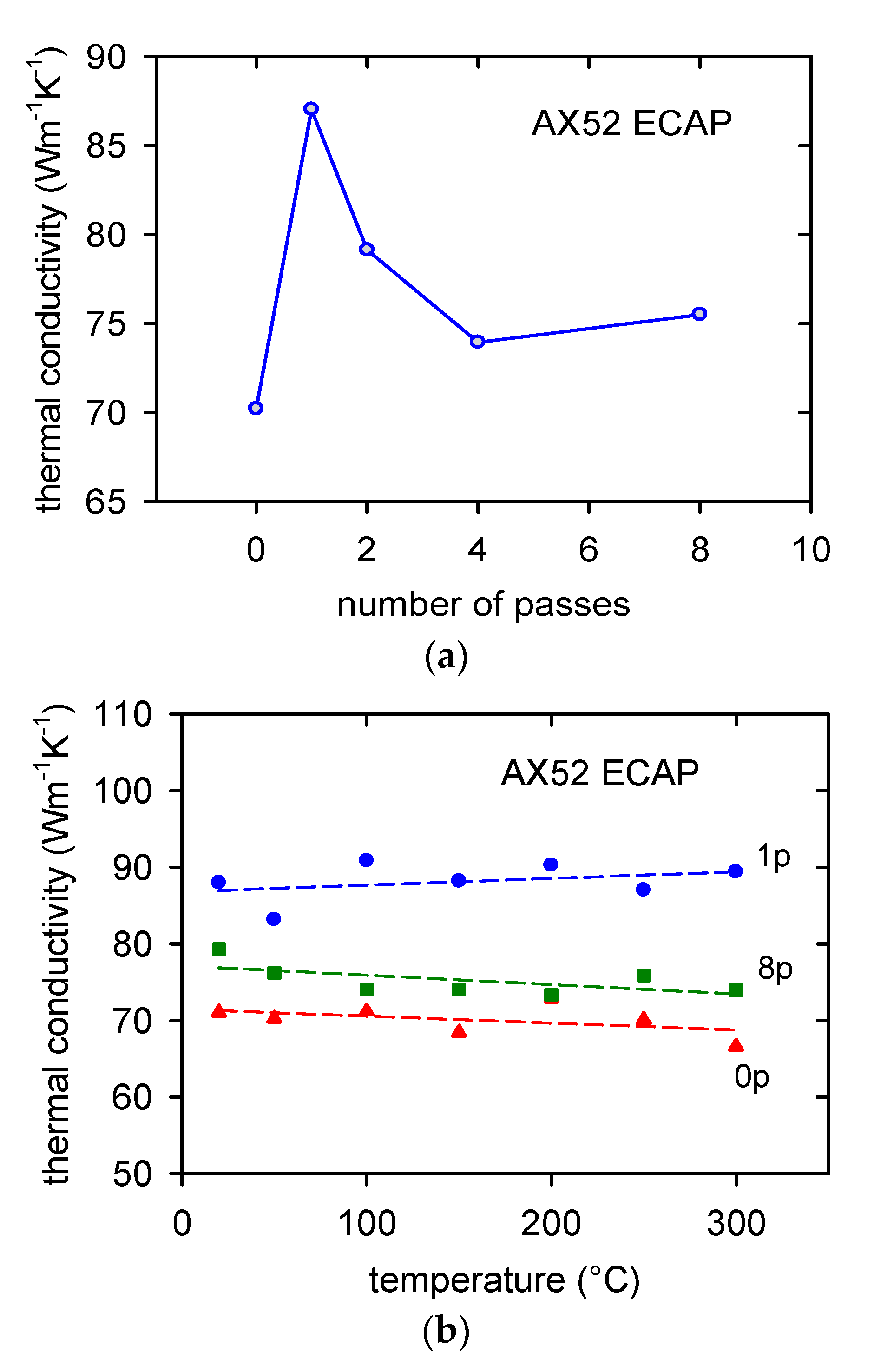
3.3. Thermal Properties
4. Discussion
5. Conclusions
- The microstructure was substantially refined by equal channel angular (ECA) pressing performed via route A. The grain size decreased after eight passes from 63 to 3.4 µm.
- The pronounced texture was formed during the ECAP processing, where the basal planes were primarily oriented parallel to the processing direction.
- The thermal conductivity was perceptibly influenced by severe plastic deformation. The thermal conductivity, estimated at room temperature, increased after the first ECAP pass and then decreased after the subsequent passes.
- The temperature dependence of the thermal conductivity was only weak.
- The influence of the structural parameters on the thermal conductivity is a complex problem, where the conductivity is a result of the interplay of various electron and phonon scattering processes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krajňák, T.; Minárik, P.; Stráská, J.; Gubicza, J.; Máthis, K.; Janeček, M. Influence of equal channel angular pressing on texture, microstructure and mechanical properties of extruded AX41 magnesium. J. Alloys Compd. 2017, 705, 273–282. [Google Scholar] [CrossRef]
- Trojanová, Z.; Halmešová, K.; Drozd, Z.; Šíma, V.; Lukáč, P.; Džugan, J.; Minárik, P. Thermal conductivity of an AZ31 sheet after accumulative roll bonding. Crystals 2018, 8, 278. [Google Scholar] [CrossRef]
- Drozd, Z.; Trojanová, Z.; Halmešová, K.; Džugan, J.; Lukáč, P.; Minárik, P. Anisotropy of Thermal Expansion in an AZ31 Magnesium Alloy Subjected to the Accumulative Roll Bonding. Acta Phys. Pol. A 2018, 134, 820–823. [Google Scholar] [CrossRef]
- Trojanová, Z.; Drozd, Z.; Lukáč, P.; Minárik, P.; Džugan, J.; Halmešová, K. Amplitude-dependent internal friction in AZ31 alloy sheets submitted to accumulative roll bonding. Low Temp. Phys. Fiz. Nizk. Temp. 2018, 44, 966–972. [Google Scholar] [CrossRef]
- Ying, T.; Zheng, M.Y.; Li, Z.T.; Qiao, X.G.; Xu, S.W. Thermal conductivity of as-cast and as-extruded binary Mg–Zn alloys. J. Alloys Compd. 2015, 621, 250–255. [Google Scholar] [CrossRef]
- Ying, T.; Zheng, M.Y.; Li, Z.T.; Qiao, X.G. Thermal conductivity of as cast and as extruded binary Mg-Al alloys. J. Alloys Compd. 2014, 608, 19–24. [Google Scholar] [CrossRef]
- Liu, Y.F.; Jia, X.J.; Qiao, X.G.; Xu, S.W.; Zheng, M.Y. Effect of La content on Microstructure, thermal conductivity and mechanical properties of Mg-4Al magnesium alloys. J. Alloys Compd. 2019, 806, 71–78. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Z.; Liu, H.; Chen, Y.; Ding, W.; Xiao, S. Electrical and thermal conductivity in Mg-5Sn alloy at different aging status. Mater. Des. 2015, 84, 48–52. [Google Scholar] [CrossRef]
- Pan, H.; Pan, F.; Yang, R.; Peng, J.; Tang, A.; Huang, Q.; Song, K.; Gao, Z. Thermal and electrical conductivity of Mg-Zn-Al alloys. Mater. Sci. Technol. 2014, 30, 988–994. [Google Scholar] [CrossRef]
- Oh, G.Y.; Jung, Y.G.; Yang, W.; Kim, S.K.; Lim, H.K.; Kim, Y.J. Investigation of thermal conductivity and mechanical properties of Mg-4Zn-0.5Ca-xY alloys. Mater. Trans. 2015, 56, 1887–1892. [Google Scholar] [CrossRef]
- Rudajevová, A.; Staněk, M.; Lukáč, P. Determination of thermal diffusivity and thermal conductivity of Mg-Al alloys. Mater. Sci. Eng. A 2003, 341, 152–157. [Google Scholar] [CrossRef]
- Su, C.; Li, D.; Ying, T.; Zhou, L.; Li, L.; Zeng, X. Effect of Nd content and heat treatment on the thermal conductivity of Mg Nd alloys. J. Alloys Compd. 2015, 685, 114–121. [Google Scholar] [CrossRef]
- Yamasaki, M.; Kawamura, Y. Thermal diffusivity and thermal conductivity of Mg-Zn-rare earth element alloys with long-period stacking ordered phase. Scripta Mater. 2009, 60, 264–267. [Google Scholar] [CrossRef]
- Rudajevová, A.; Lukáč, P. Comparison of the thermal properties of AM20 and AS21 magnesium alloys. Mater. Sci. Eng. A 2005, 397, 16–21. [Google Scholar] [CrossRef]
- Lee, S.; Ham, H.J.; Kwon, S.Y.; Kim, S.W.; Suh, C.M. Thermal conductivity of magnesium alloys in the temperature range from −125 °C to 400 °C. Int. J. Thermophys. 2013, 34, 2343–2350. [Google Scholar] [CrossRef]
- Perkguleryuz, M. Magnesium Alloys and Their Applications; Kainer, K.U., Ed.; Willey-VCH: Weinheim, Germany, 2003; pp. 65–85. [Google Scholar]
- Ninomiya, R.; Ojiro, T.; Kubota, K. Improved heat resistance of Mg-Al alloys by the Ca addition. Acta Metall. 1995, 43, 669–674. [Google Scholar] [CrossRef]
- Gjestland, H.; Nussbaum, G.; Regazzoni, G.; Lohne, O.; Bauger, O. Stress-relaxation and creep behaviour of some rapidly solidified magnesium alloys. Mater. Sci. Eng. A 1991, 134, 1197–1200. [Google Scholar] [CrossRef]
- Terada, Y.; Ishimatsu, N.; Sota, R.; Sato, T.; Ohori, K. Creep Characteristics of Ca-Added Die-Cast AM50 Magnesium Alloys. Mater. Sci. Forum 2003, 419, 459–464. [Google Scholar] [CrossRef]
- Wenwen, D.; Yangshan, S.; Xuegang, M.; Feng, X.; Min, Z.; Dengyun, W. Microstructure and mechanical properties of Mg–Al based alloy with calcium and rare earth additions. Mater. Sci. Eng. A 2003, 356, 1–7. [Google Scholar] [CrossRef]
- Luo, A.A. Magnesium casting technology for structural applications. J. Magnes. Alloys. 2013, 1, 2–22. [Google Scholar] [CrossRef]
- Pekguleryuz, M.; Celikin, M. Creep resistance in magnesium alloys. Int. Mater. Rev. 2010, 55, 197–217. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Q.; Liu, Z.; Wang, G.; Wu, G.; Zhu, Y.; Ding, W. Behavior of Mg-Al-Ca alloy during solution heat treatment at 415 °C. J. Mater. Sci. Lett. 2002, 21, 1281–1283. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, W.; Qiu, M.; Zhang, X.; Pan, F. Grain refinement of Ca addition in a twin-roll-cast Mg–3Al–1Zn alloy. Mater. Chem. Phys. 2012, 133, 611–616. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Ye, R.; Liu, G. Effects of the addition of Ca and Sb on the microstructure and mechanical properties of AZ91 magnesium. Mater. Sci. Eng. A 2013, 587, 262–267. [Google Scholar] [CrossRef]
- Luo, A.A. Recent magnesium alloy development for elevated temperature applications. Int. Mater. Rev. 2004, 49, 13–30. [Google Scholar] [CrossRef]
- Pekguleryuz, M.O.; Kaya, A.A. Creep Resistant Magnesium Alloys for Powertrain Applications. Adv. Eng. Mater. 2004, 5, 866–878. [Google Scholar] [CrossRef]
- Luo, A.A.; Powell, B.R.; Sachdev, A.K. Computational phase equilibria and experimental investigation of magnesium–aluminum–calcium alloys. Intermetallics 2012, 24, 22–29. [Google Scholar] [CrossRef]
- Elamami, H.A.; Incesu, A.; Korgiopoulus, K.; Pekguleryuz, M.; Gungor, A. Phase selection and mechanical properties of permanent-mold cast Mg-Al-Ca-Mn alloys and the role of Ca/Al ratio. J. Alloys Compd. 2018, 764, 216–225. [Google Scholar] [CrossRef]
- Krajňák, T.; Minárik, P.; Gubicza, J.; Máthis, K.; Kužel, R.; Janeček, M. Influence of equal channel angular pressing routes on texture, microstructure and mechanical properties of extruded AX41 magnesium alloy. Mater. Charact. 2017, 123, 282–293. [Google Scholar] [CrossRef]
- Trojanová, Z.; Halmešová, K.; Džugan, J.; Palček, P.; Minárik, P.; Lukáč, P. Influence of strain rate on deformation behaviour of an AX52 alloy processed by equal channel angular pressing (ECAP). Lett. Mater. 2018, 8, 517–523. [Google Scholar] [CrossRef]
- Krajňák, T.; Minárik, P.; Stráský, J.; Máthis, K.; Janeček, M. Mechanical properties of ultrafine-grained AX41 magnesium alloy at room temperature and elevated temperatures. Mater. Sci. Eng. A 2018, 731, 438–445. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.G. High strength Mg-Al-Ca alloy with ultrafine grain size sensitive to strain rate. Mater. Sci. Eng. A 2011, 528, 2062–2066. [Google Scholar] [CrossRef]
- Janeček, M.; Krajňák, T.; Minárik, P.; Čížek, J. Structural stability of ultra-fine grained magnesium alloys processed by equal channel angular pressing. Mater. Sci. Eng. 2017, 194, 012022. [Google Scholar] [CrossRef]
- Wang, C.; Ma, A.; Sun, J.; Liu, H.; Huang, H.; Yang, Z. Effect of ECAP process on as-cast and as-homogenized Mg-Al-Ca-Mn alloys with different Mg2Ca morphologies. J. Alloys Compd. 2019, 793, 259–270. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, A.; Liu, H.; Sun, J.; Song, D.; Wang, C.; Yuan, Y.; Jiang, J. Multimodal microstructure and mechanical properties of AZ91 Mg alloy prepared by equal channel angular pressing plus aging. Metals 2018, 8, 763. [Google Scholar] [CrossRef]
- Masoudpanah, S.M.; Mahmudi, R. Effects of rare-earth elements and Ca additions on the microstructure and mechanical properties of AZ31 magnesium alloy processed by ECAP. Mater. Sci. Eng. A 2009, 526, 22–30. [Google Scholar] [CrossRef]
- Hakamada, M.; Watazu, A.; Saito, N.; Iwasaki, H. Dynamic recrystallization during hot compression of as-cast and homogenized noncombustible Mg-9Al-1Zn-1Ca (in mass%) alloys. Mater. Sci. Eng. A 2010, 527, 7143–7146. [Google Scholar] [CrossRef]
- Kaibyshev, R. Dynamic recrystallization in magnesium alloys. In Advances in Wrought Magnesium Alloys; Bettels, C., Barnett, M., Eds.; Woodhead Publishing Limited: Oxford, UK, 2012; pp. 186–225. [Google Scholar] [CrossRef]
- Ion, S.E.; Humphreys, F.J.; White, H.S. Dynamic recrystallization and the development of microstructure during the high temperature deformation of magnesium. Acta Metall. 1982, 30, 1909–1919. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Hou, J.; Du, W. A review on thermal conductivity of magnesium and its alloys. J. Magnes. Alloys 2020, 8, 78–90. [Google Scholar] [CrossRef]
- Uher, C. Thermal conductivity of metals. In Thermal Conductivity; Tritt, M., Ed.; Kuwen Academic/Plenum Publishers: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- Lumley, R.N.; Polmear, I.J.; Groot, H.; Ferrier, J. Thermal characteristics of heat-treated aluminum high-pressure die-castings. Scr. Mater. 2008, 58, 1006–1009. [Google Scholar] [CrossRef]
- Tong, X.; You, G.; Ding, Y.; Xue, H.; Wang, Y.; Guo, W. Effect of grain size on low-temperature electrical resistivity and thermal conductivity of pure magnesium. Mater. Lett. 2018, 229, 261–264. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, Y.; Luo, H.; Luo, C.; Peng, J. Evolution of the microstructure, texture and thermal conductivity of as-extruded ZM60 magnesium alloy in pre-compression. J. Alloys Compd. 2019, 775, 707–713. [Google Scholar] [CrossRef]
- Bass, J.; Fisher, K.H. Landolt-Börnstein database, New Series III/15a; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Osip’yan, Y.A. Interaction of electrons with dislocations in crystals. Her. Russ. Acad. Sci. 2006, 76, 437–445. [Google Scholar] [CrossRef]
- Fiks, V.B. Interaction of conduction electrons with single dislocation in metals. Sov. Phys. JETP 1981, 53, 1209–1211. [Google Scholar]

| Number of passes | 0 | 1 | 2 | 4 | 8 |
|---|---|---|---|---|---|
| Grain size (μm) | 63 | 28 | 21 | 4 | 3.4 |
| No. of Passes | 0 P | 1 P | 2 P | 4 P | 8 P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T | a | cp | a | cp | a | cp | a | cp | a | cp |
| °C | cm2 s−1 | J kg−1 K−1 | cm2 s−1 | J kg-1 K−1 | cm2 s−1 | J kg−1 K−1 | cm2 s−1 | J kg−1 K−1 | cm2 s−1 | J kg−1 K−1 |
| 20 | 0.42 | 950 | 0.44 | 1122 | 0.40 | 1153 | 0.41 | 1060 | 0.45 | 990 |
| 50 | 0.41 | 963 | 0.42 | 1112 | 0.38 | 1215 | 0.39 | 1140 | 0.44 | 973 |
| 100 | 0.39 | 1027 | 0.40 | 1277 | 0.35 | 1205 | 0.36 | 1137 | 0.41 | 1015 |
| 150 | 0.37 | 1042 | 0.39 | 1273 | 0.33 | 1254 | 0.34 | 1136 | 0.39 | 1067 |
| 200 | 0.37 | 1111 | 0.38 | 1339 | 0.32 | 1282 | 0.33 | 1185 | 0.39 | 1056 |
| 250 | 0.35 | 1131 | 0.37 | 1327 | 0.31 | 1308 | 0.31 | 1185 | 0.38 | 1122 |
| 300 | 0.34 | 1108 | 0.36 | 1403 | 0.30 | 1302 | 0.31 | 1214 | 0.36 | 1154 |
| No. of Passes | 0 P | 1 P | 2 P | 4 P | 8 P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T | α × 10−6 | κ | α × 10−6 | κ | α × 10−6 | κ | α × 10−6 | κ | α × 10−6 | κ |
| °C | K−1 | W m−1 K−1 | K−1 | W m−1 K−1 | K−1 | W m−1 K−1 | K−1 | W m-1 K-1 | K−1 | W m−1 K−1 |
| 20 | 24.96 | 70.2 | 25.42 | 87.0 | 24.81 | 79.1 | 25.66 | 73.9 | 25.08 | 75.5 |
| 50 | 25.36 | 69.4 | 25.89 | 82.3 | 25.30 | 79.0 | 26.07 | 75.5 | 25.42 | 72.5 |
| 100 | 26.02 | 70.3 | 26.67 | 89.9 | 26.11 | 71.9 | 26.75 | 69.2 | 25.98 | 70.4 |
| 150 | 26.68 | 67.6 | 27.45 | 87.2 | 26.91 | 70.3 | 27.44 | 65.0 | 26.53 | 70.3 |
| 200 | 27.33 | 72.0 | 28.23 | 89.3 | 27.72 | 69.4 | 28.12 | 65.6 | 27.09 | 69.5 |
| 250 | 27.99 | 69.3 | 29.02 | 86.0 | 28.53 | 68.3 | 28.80 | 61.3 | 27.65 | 71.8 |
| 300 | 28.65 | 65.8 | 29.80 | 88.4 | 29.34 | 65.5 | 29.48 | 62.6 | 28.21 | 69.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trojanová, Z.; Halmešová, K.; Džugan, J.; Drozd, Z.; Minárik, P.; Lukáč, P. Effect of Equal Channel Angular Extrusion on the Thermal Conductivity of an AX52 Magnesium Alloy. Crystals 2020, 10, 497. https://doi.org/10.3390/cryst10060497
Trojanová Z, Halmešová K, Džugan J, Drozd Z, Minárik P, Lukáč P. Effect of Equal Channel Angular Extrusion on the Thermal Conductivity of an AX52 Magnesium Alloy. Crystals. 2020; 10(6):497. https://doi.org/10.3390/cryst10060497
Chicago/Turabian StyleTrojanová, Zuzanka, Kristýna Halmešová, Ján Džugan, Zdeněk Drozd, Peter Minárik, and Pavel Lukáč. 2020. "Effect of Equal Channel Angular Extrusion on the Thermal Conductivity of an AX52 Magnesium Alloy" Crystals 10, no. 6: 497. https://doi.org/10.3390/cryst10060497
APA StyleTrojanová, Z., Halmešová, K., Džugan, J., Drozd, Z., Minárik, P., & Lukáč, P. (2020). Effect of Equal Channel Angular Extrusion on the Thermal Conductivity of an AX52 Magnesium Alloy. Crystals, 10(6), 497. https://doi.org/10.3390/cryst10060497







