Mineral Vesicles and Chemical Gardens from Carbonate-Rich Alkaline Brines of Lake Magadi, Kenya
Abstract
1. Introduction
2. Materials and Methods
2.1. Geological Setting, Sampling and Hydrochemical Analysis
2.2. Magadi Gardens and Vesicles Synthesis
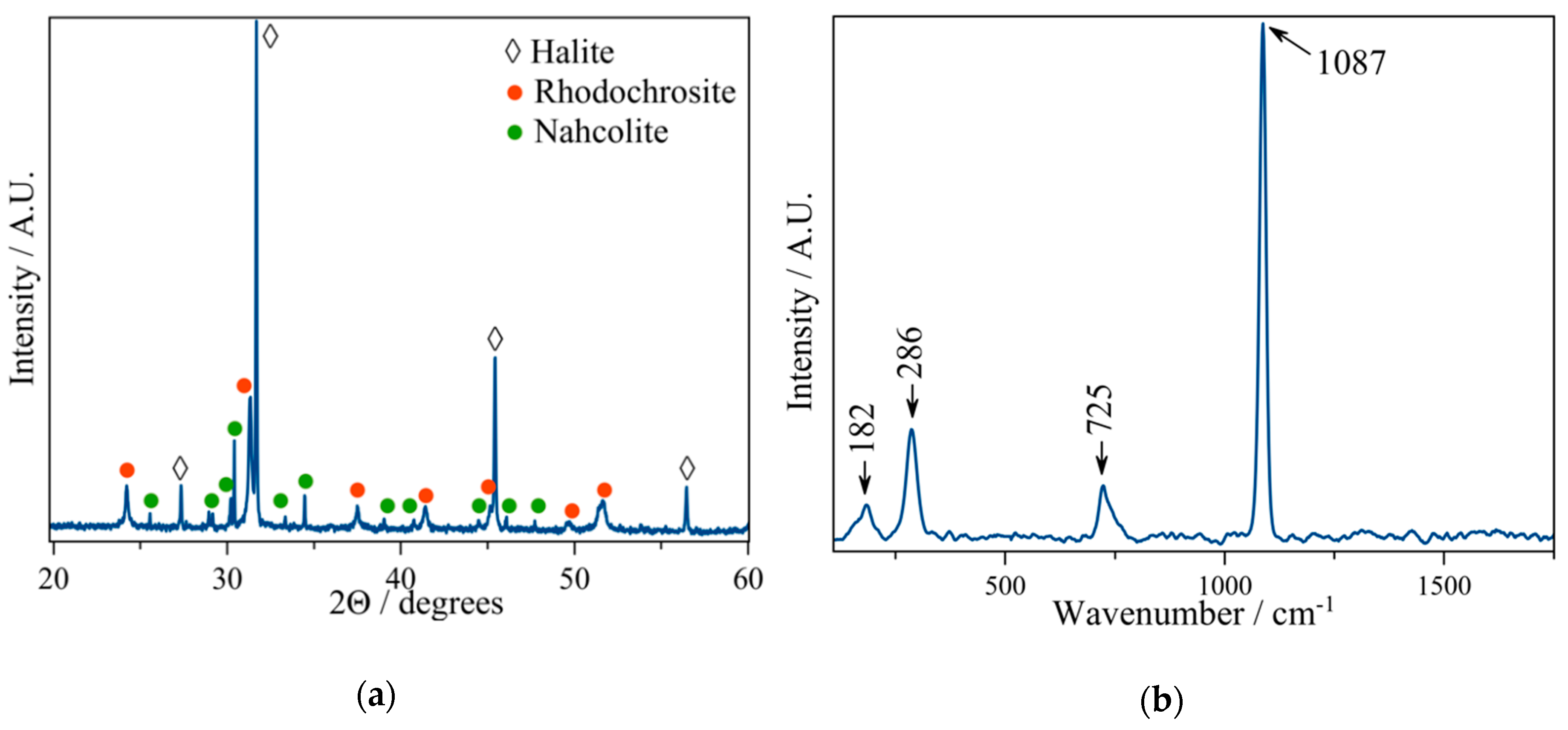
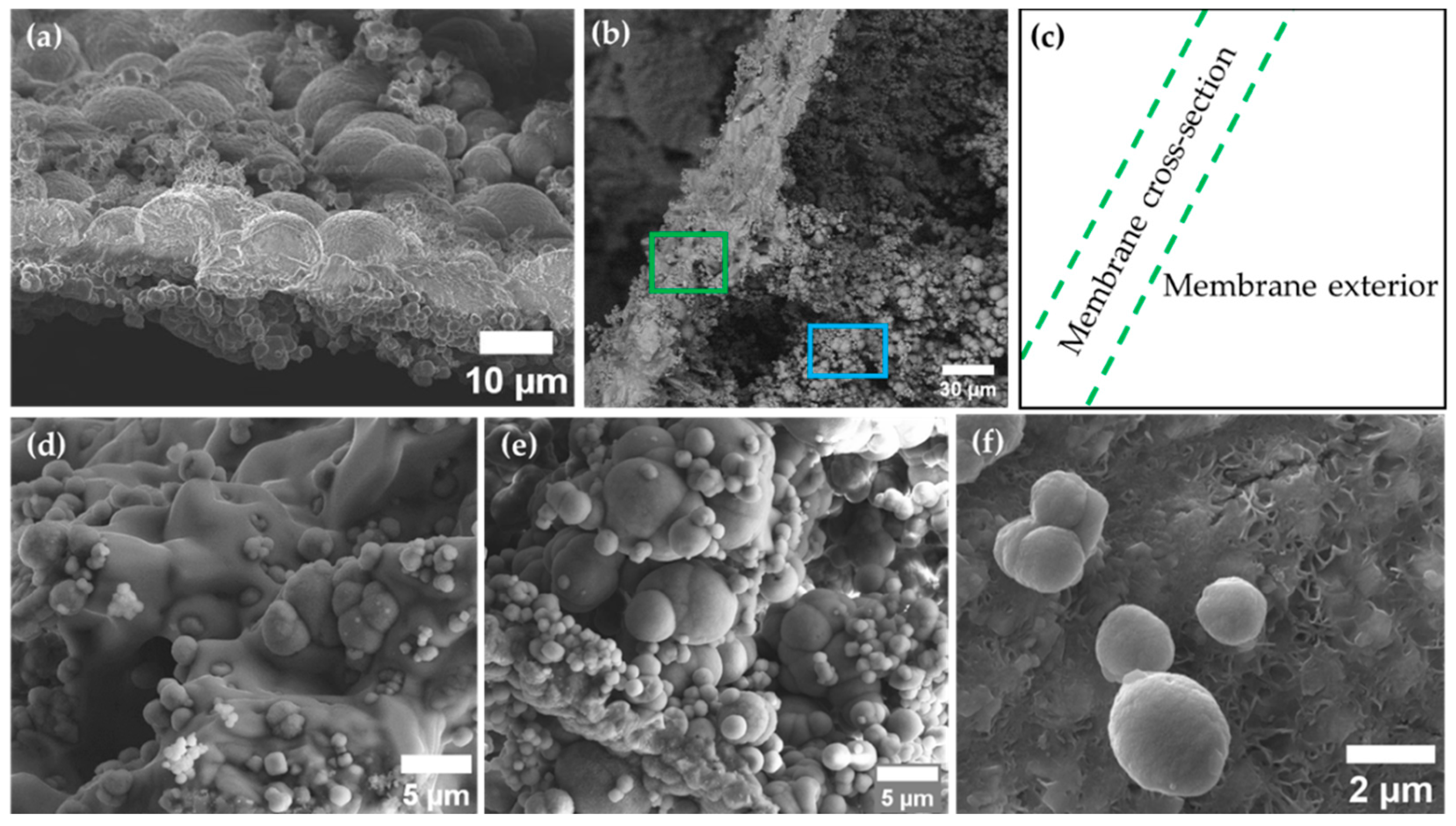
2.3. Membrane Characterization
3. Results
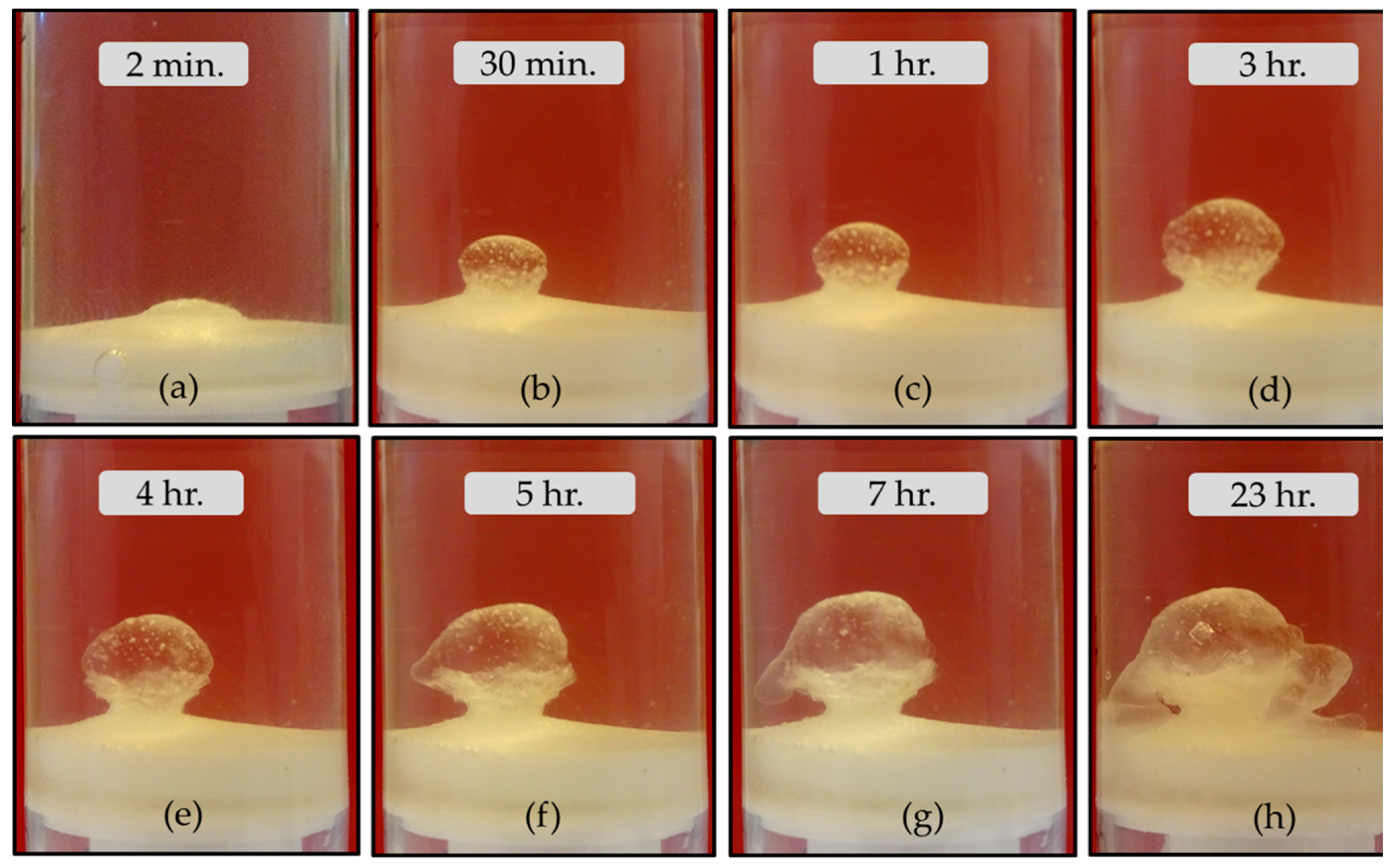
3.1. The Magadi Gardens Growth Process
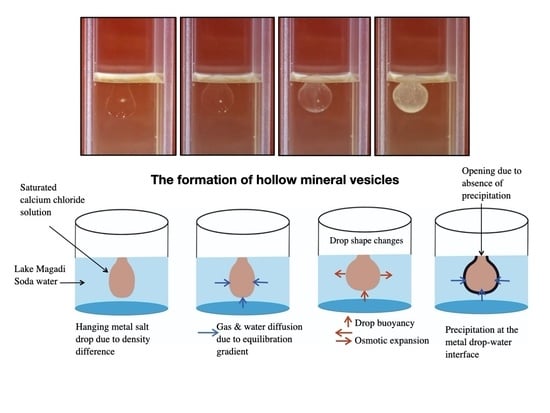
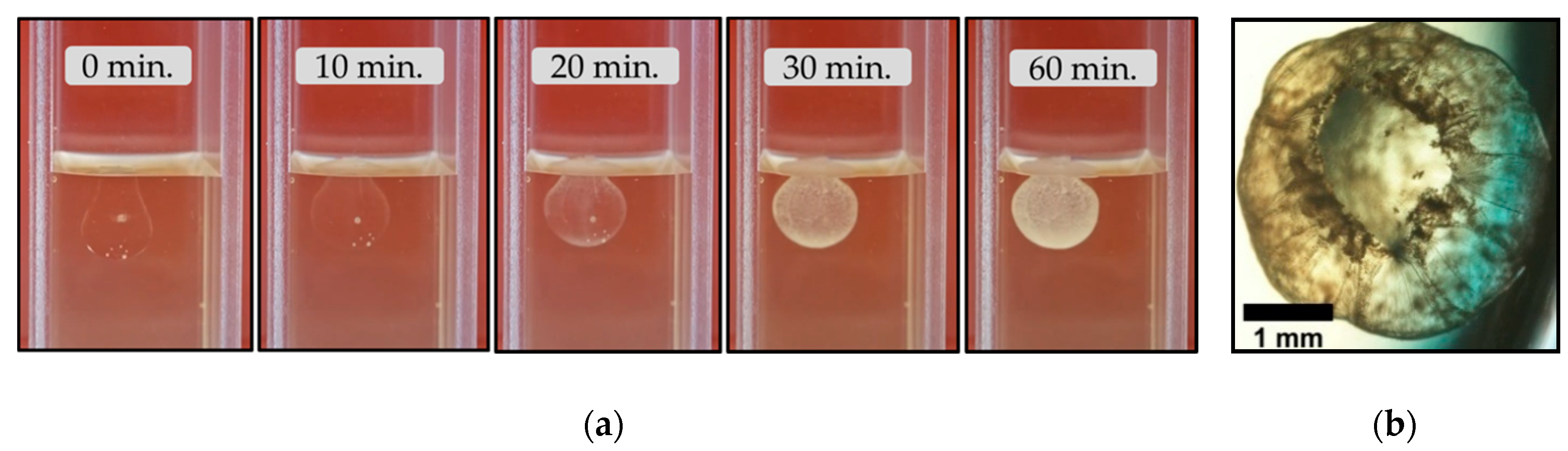
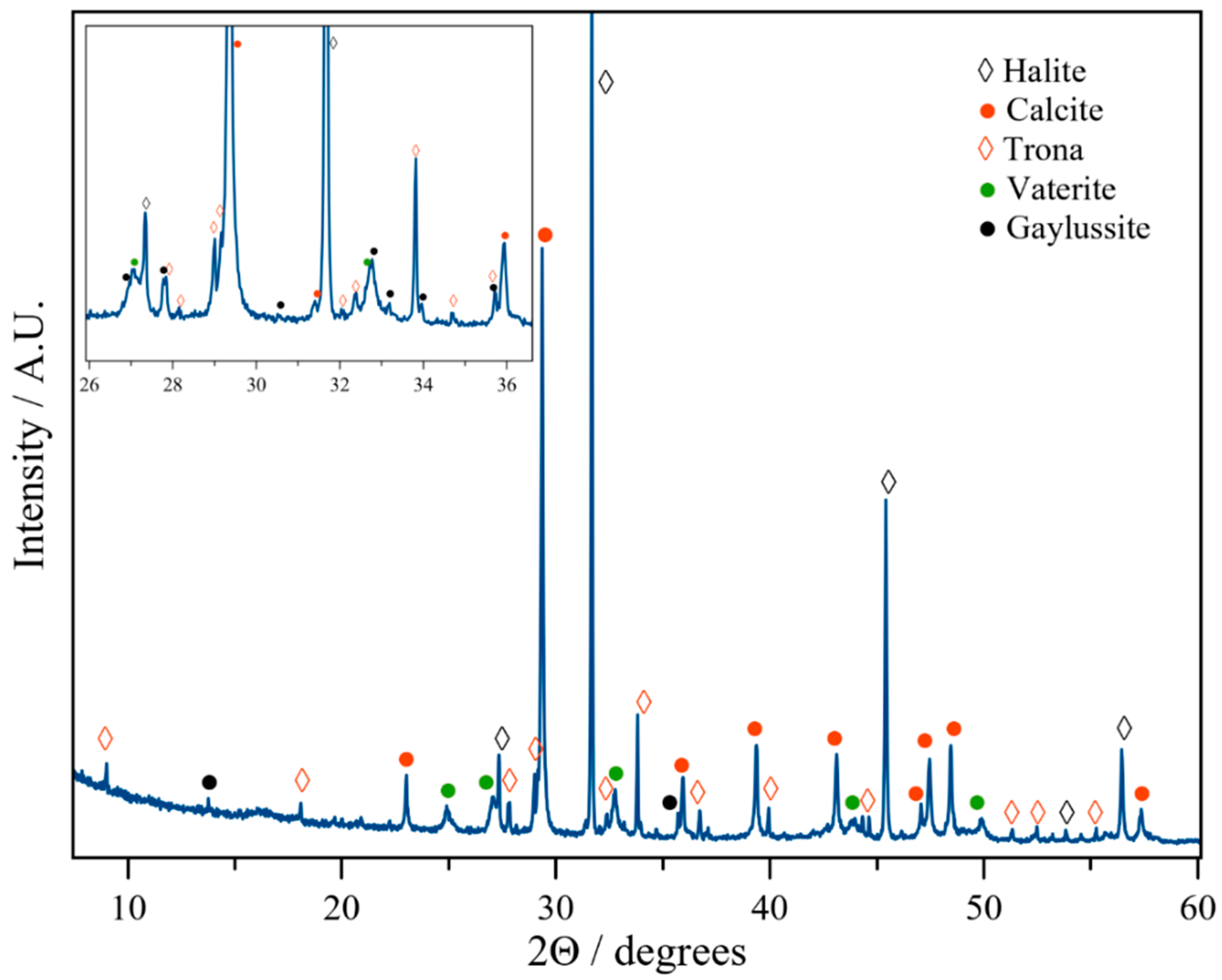
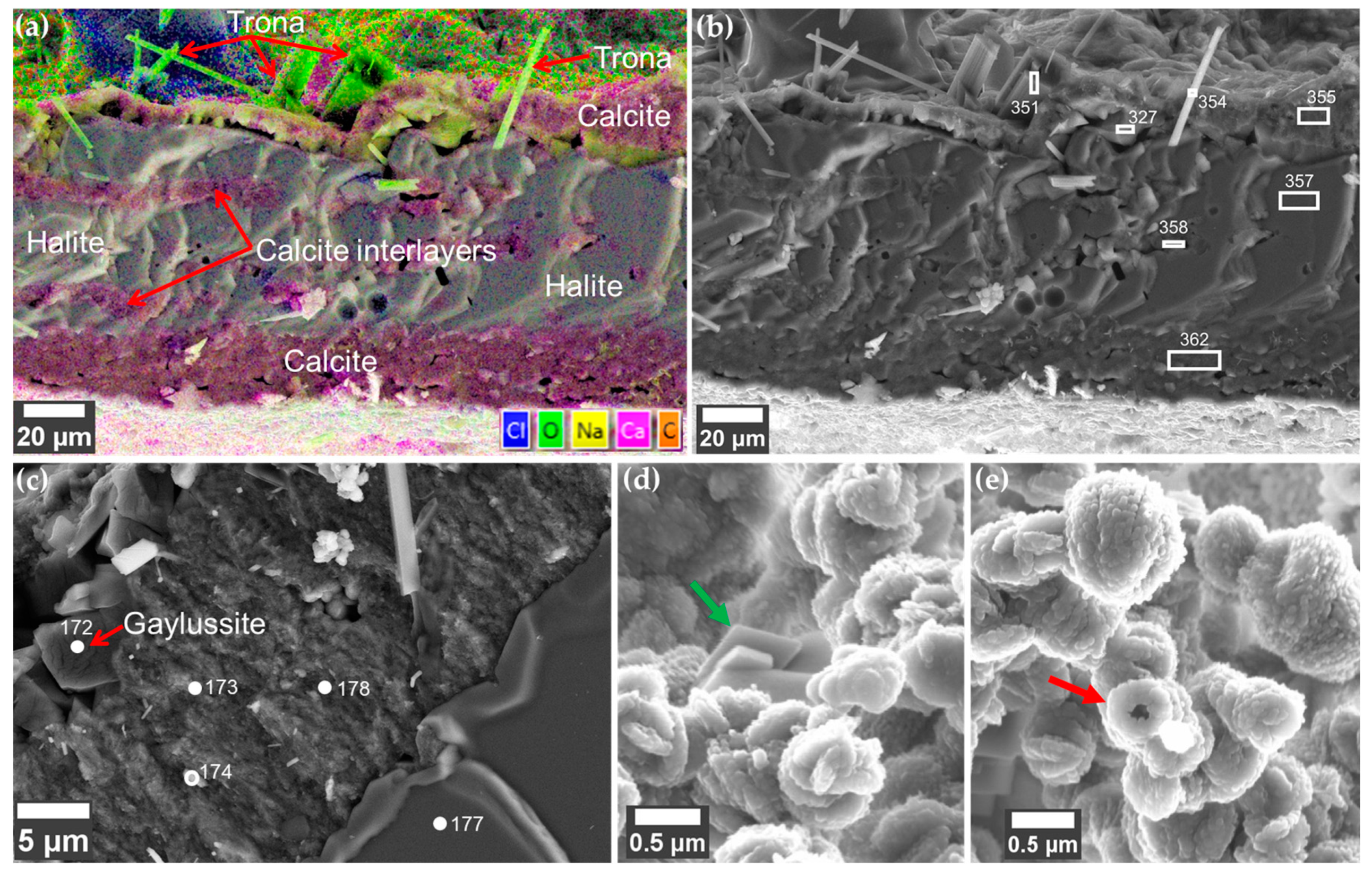
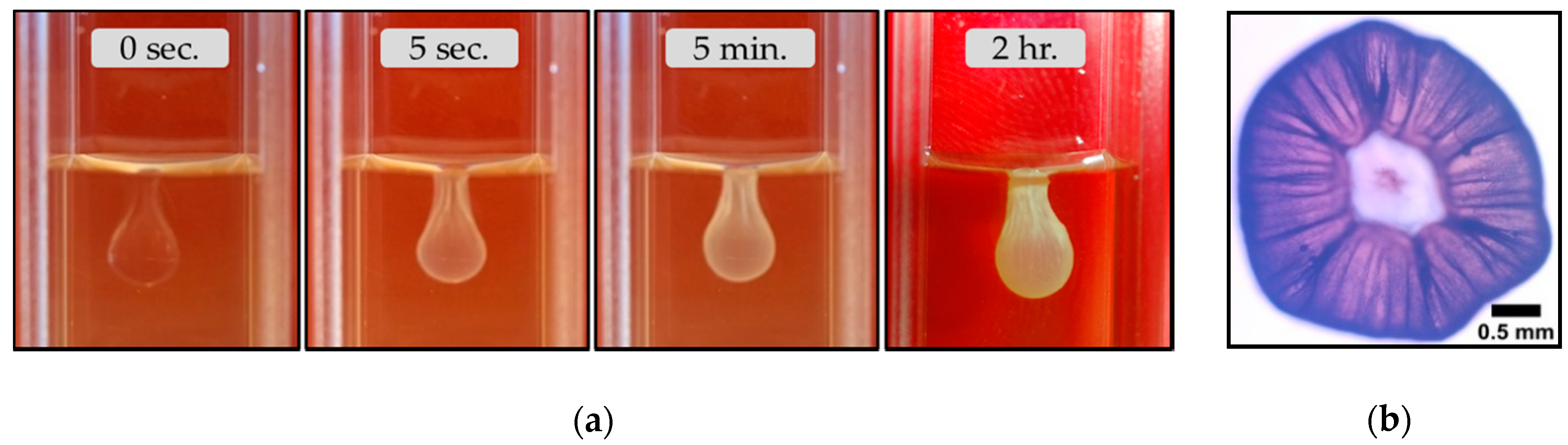
3.2. The Magadi Vesicles Growth Process and Characterization
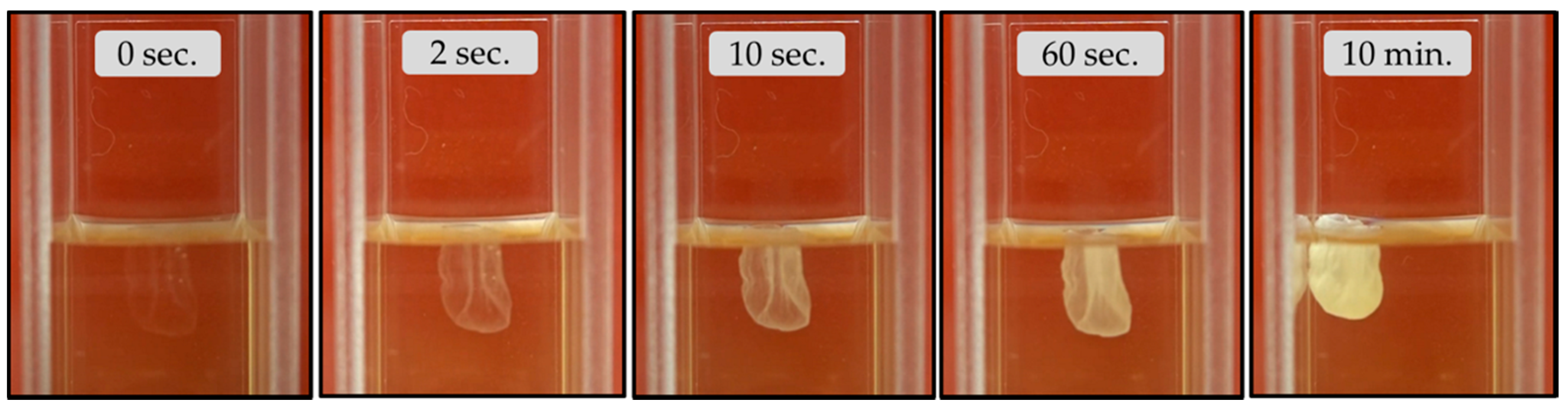
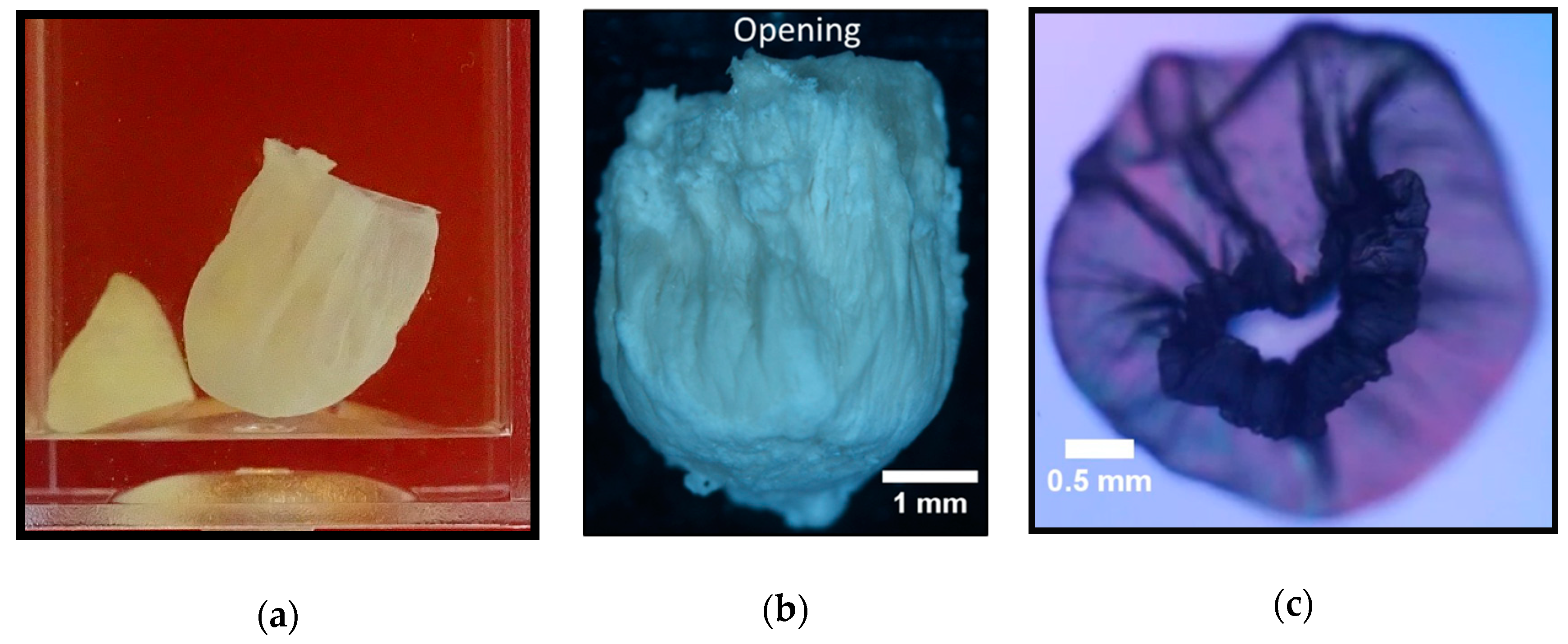
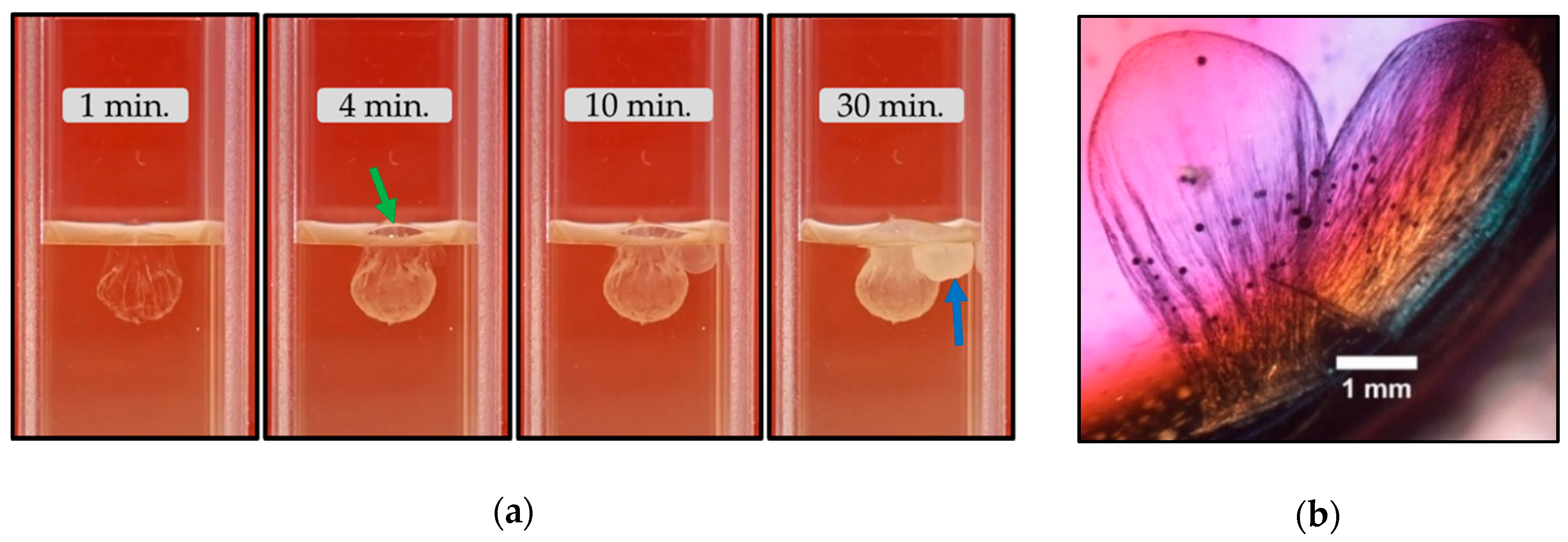
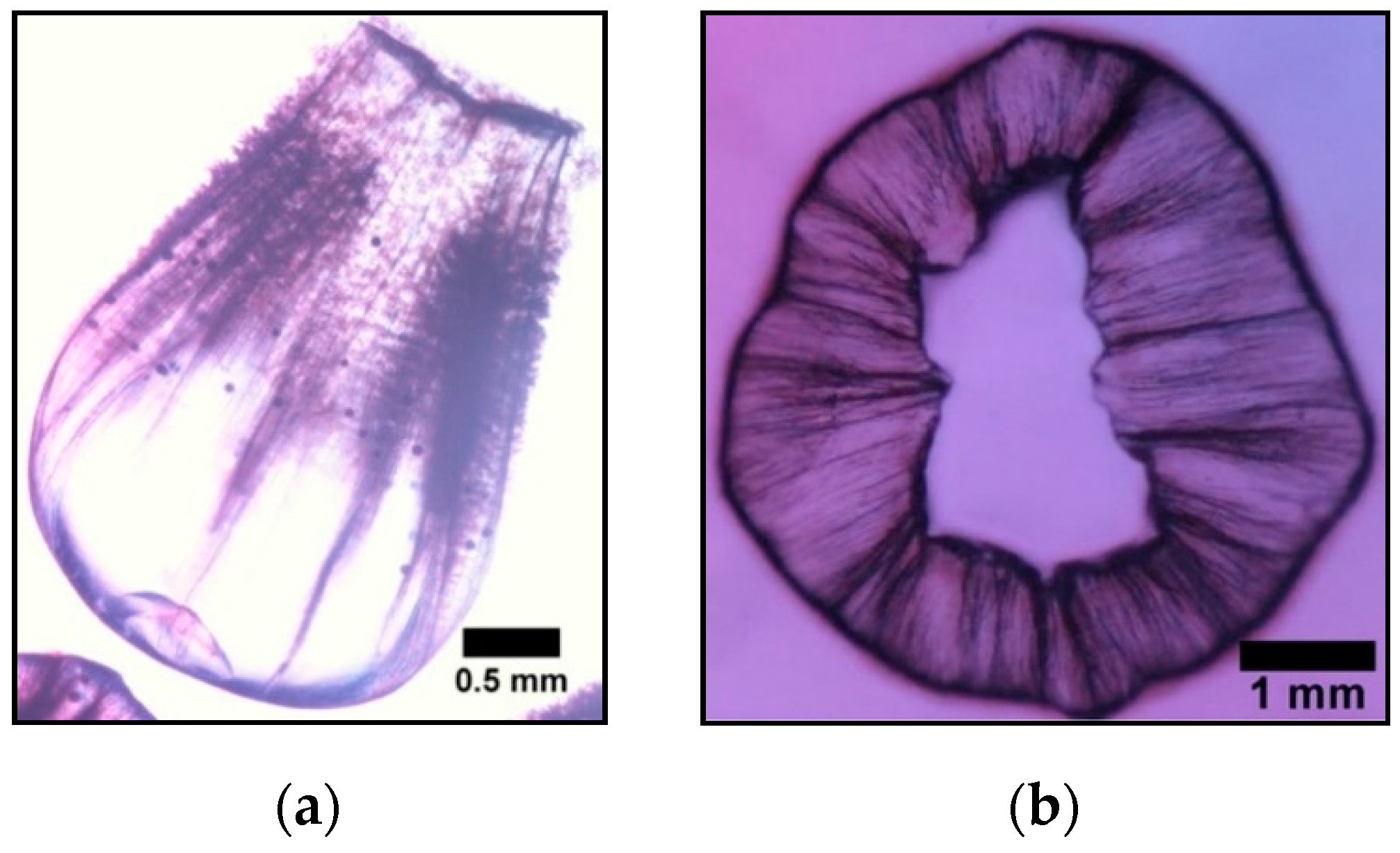
3.2.1. Calcium Chloride Vesicles
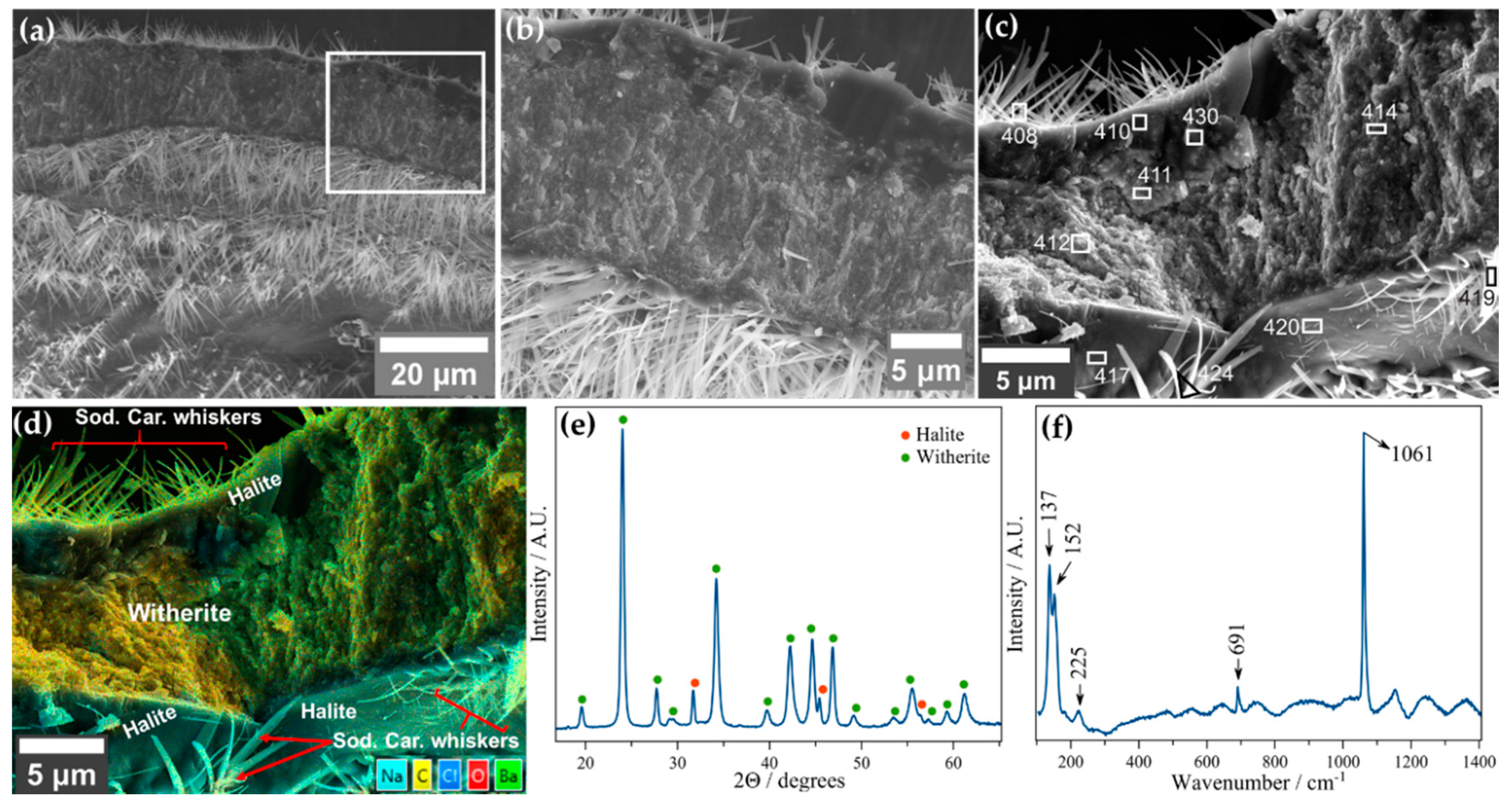
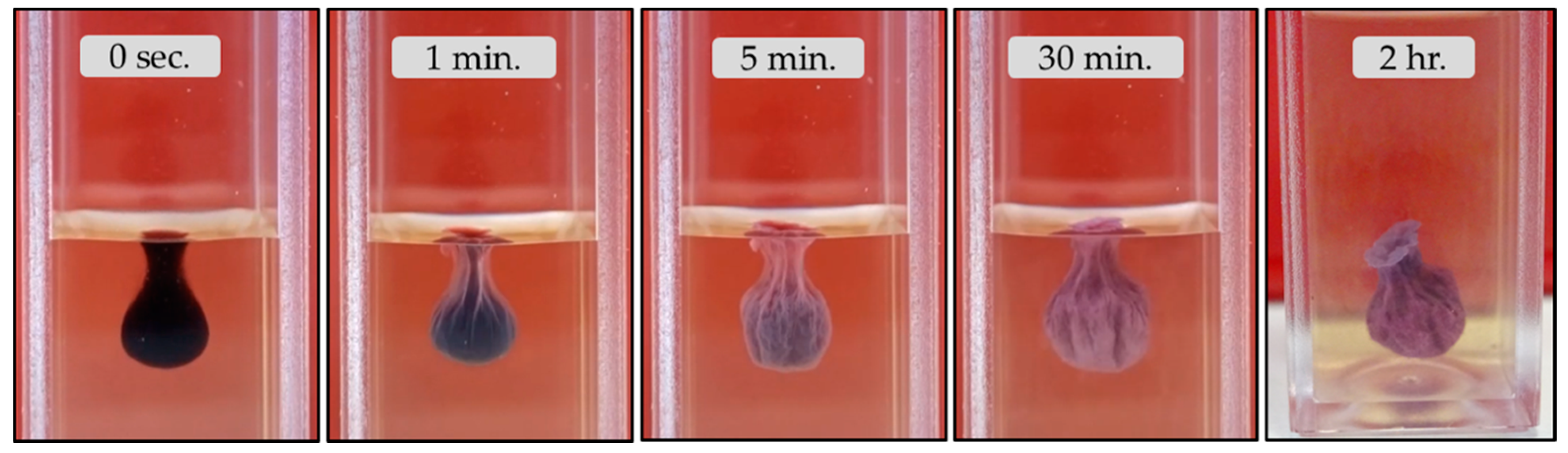
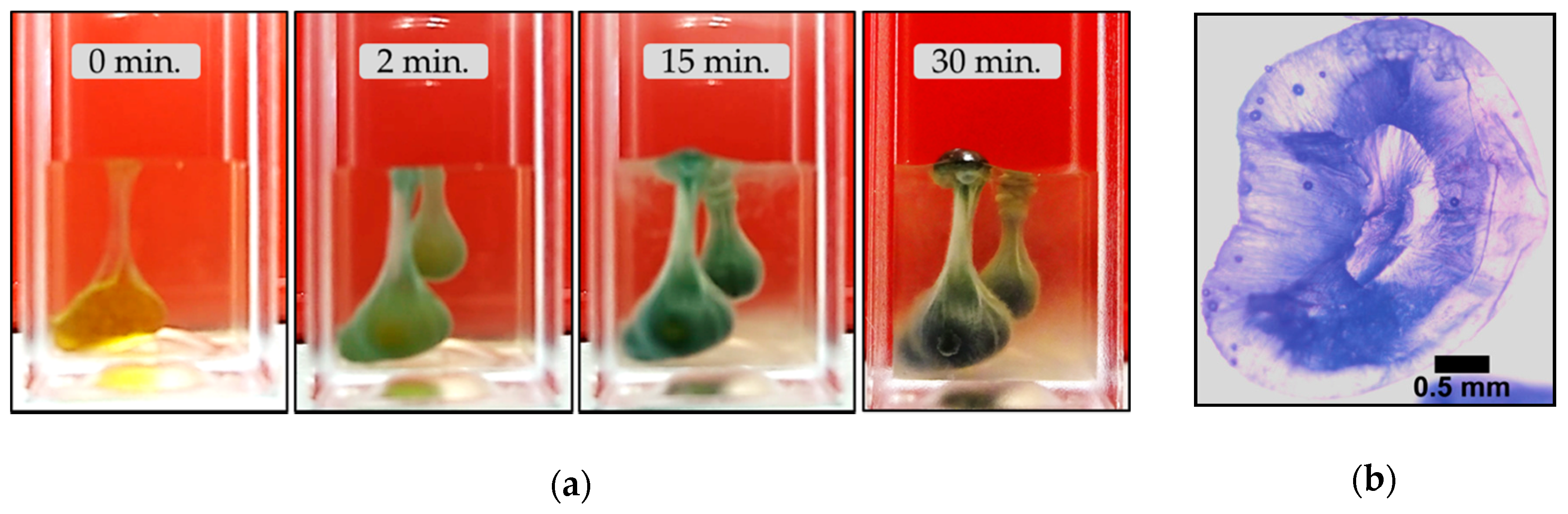
3.2.2. Barium Chloride Vesicles
3.2.3. Manganese Chloride Vesicles
3.2.4. Cobalt, Magnesium, Zinc, and Iron (II) Mineral Vesicles
4. Discussion
4.1. The Growth Process of Magadi Mineral Gardens
- 1)
- The pellets of the less soluble and less acidic salts of Ba2+, Mn2+ and Fe2+ (Table 2) do not react with the Magadi water.
- 2)
- In the case of Fe3+ (FeCl3 and Fe2(SO4)3·9H2O), Cu2+ (CuCl2·6H2O and CuSO4·5H2O) and Zn2+ (ZnCl2 and ZnSO4·7H2O), the experiment resulted in the bursting of the initial membrane. The bursting of the membrane was accompanied by the bubbling of gases, and subsequent breaking of the forming mineral membranes in multiple pieces that later sediment (Videos S1–S3).
- 3)
- Unlike the aforementioned metals, the salts of Ca2+ (CaCl2·2H2O) and Co2+ (CoCl2·6H2O) and Co(NO3)2·6H2O) form tubular membranes, as shown in Figure 1 and Figure 2 respectively. Likewise, Mg2+ (MgCl2·6H2O) pellets immersed in Magadi water form mineral gardens (not presented here) that are morphologically similar to Co (Co(NO3)2·6H2O) gardens.
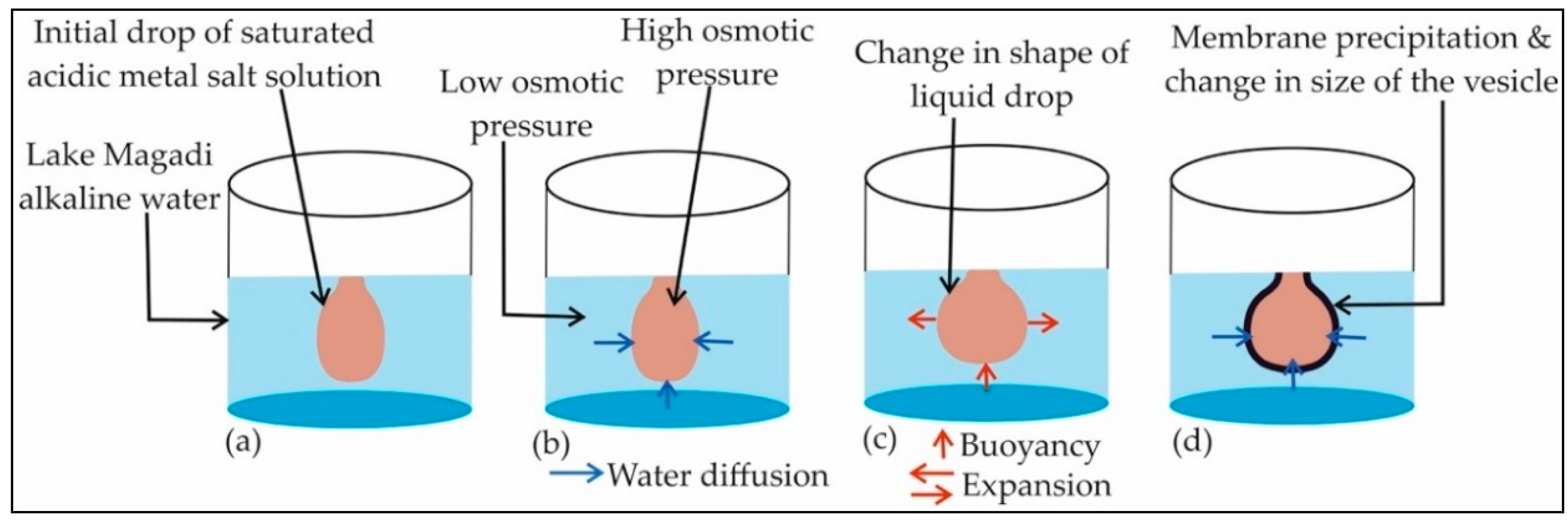
4.2. The Growth Process of Magadi Mineral Vesicles
4.3. Calcium Magadi Mineral Self-Organization and the Origin of Life
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García-Ruiz, J.M.; Otálora, F. Crystal Growth in Geology: Patterns on the Rocks. In Handbook of Crystal Growth; Nishinaga, T., Rudolph, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume II, pp. 1–43. [Google Scholar] [CrossRef]
- Nakouzi, E.; Steinbock, O. Self-organization in precipitation reactions far from the equilibrium. Sci. Adv. 2016, 2, e1601144. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, J.M.; van Zuilen, M.; Bach, W. Mineral self-organization on a lifeless planet. Phys. Life Rev. 2020, (in press). [CrossRef]
- Kellermeier, M.; Glaab, F.; Melero-García, E.; García-Ruiz, J.M. Experimental Techniques for the Growth and Characterization of Silica Biomorphs and Silica Gardens. In Methods in Enzymology; De Yoreo, J.J., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 532, pp. 225–256. [Google Scholar] [CrossRef]
- Barge, L.M.; Cardoso, S.S.S.; Cartwright, J.H.E.; Cooper, G.J.T.; Cronin, L.; De Wit, A.; Doloboff, I.J.; Escribano, B.; Goldstein, R.E.; Haudin, F.; et al. From Chemical Gardens to Chemobrionics. Chem. Rev. 2015, 115, 8652–8703. [Google Scholar] [CrossRef] [PubMed]
- Glaab, F.; Kellermeier, M.; Kunz, W.; Morallon, E.; García-Ruiz, J.M. Formation and Evolution of Chemical Gradients and Potential Differences Across Self-Assembling Inorganic Membranes. Angew. Chemie 2012, 124, 4393–4397. [Google Scholar] [CrossRef]
- Saladino, R.; Di Mauro, E.; García-Ruiz, J.M. A Universal Geochemical Scenario for Formamide Condensation and Prebiotic Chemistry. Chem. A Eur. J. 2019, 25, 3181–3189. [Google Scholar] [CrossRef]
- Satoh, H.; Tsukamoto, K.; Garcia-Ruiz, J.M. Formation of chemical gardens on granitic rock: A new type of alteration for alkaline systems. Eur. J. Mineral. 2014, 26, 415–426. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Nakouzi, E.; Kotopoulou, E.; Tamborrino, L.; Steinbock, O. Biomimetic mineral self-organization from silica-rich spring waters. Sci. Adv. 2017, 3, e1602285. [Google Scholar] [CrossRef]
- García-Ruiz, J.M. Carbonate precipitation into alkaline silica-rich environments. Geology 1998, 26, 843–846. [Google Scholar] [CrossRef]
- Kempe, S.; Degens, E.T. An early soda ocean? Chem. Geol. 1985, 53, 95–108. [Google Scholar] [CrossRef]
- Kempe, S.; Kazmierczak, J.; Degens, E.T. The Soda Ocean Concept and Its Bearing on Biotic Evolution. In Origin, Evolution, and Modern Aspects of Biomineralization in Plants and Animals; Crick, R.E., Ed.; Springer: Boston, MA, USA, 1989; pp. 29–43. [Google Scholar]
- García-Ruiz, J.M. Geochemical Scenarios for the Precipitation of Biomimetic Inorganic Carbonates. In Carbonate Sedimentation and Diagenesis in the Evolving Precambrian World; Grotzinger, J.P., James, N.P., Eds.; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 2000; Volume 67, pp. 75–89. [Google Scholar]
- Kempe, S.; Kazmierczak, J. Soda Ocean Hypothesis. In Encyclopedia of Geobiology; Reitner, J., Thiel, V., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 829–833. [Google Scholar]
- Toner, J.D.; Catling, D.C. A carbonate-rich lake solution to the phosphate problem of the origin of life. Proc. Natl. Acad. Sci. USA 2020, 117, 883–888. [Google Scholar] [CrossRef]
- Eugster, H.P. Chemistry and origin of the brines of Lake Magadi, Kenya. Miner. Soc. Amer. Spec. Pap. 1970, 3, 213–235. [Google Scholar]
- Jones, B.F.; Eugster, H.P.; Rettig, S.L. Hydrochemistry of the Lake Magadi basin, Kenya. Geochim. Cosmochim. Acta 1977, 41, 53–72. [Google Scholar] [CrossRef]
- Baker, B.H. Geology of the Magadi Area; Geological Survey of Kenya: Nairobi, Kenya, 1958; Volume 42, p. 81. [Google Scholar]
- Baker, B.H. Geology of the Area south of Magadi; Geological Survey of Kenya: Nairobi, Kenya, 1963; Volume 61, p. 27. [Google Scholar]
- Deocampo, D.M.; Renaut, R.W. Geochemistry of African Soda Lakes. In Soda Lakes of East Africa; Schagerl, M., Ed.; Springer: Cham, Switzerland, 2016; pp. 77–93. [Google Scholar]
- Garrels, R.M.; Mackenzie, F.T. Origin of the Chemical Compositions of Some Springs and Lakes. In Equilibrium Concepts in Natural Water Systems; Stumm, W., Ed.; ACS: Washington, DC, USA, 1967; pp. 222–242. [Google Scholar] [CrossRef]
- Hardie, L.A.; Eugster, H.P. The Evolution of Closed-Basin Brines. Miner. Soc. Amer. Spec. Pap. 1970, 3, 273–290. [Google Scholar]
- Mattia Bizzarri, B.; Botta, L.; Pérez-Valverde, M.I.; Saladino, R.; Di Mauro, E.; García-Ruiz, J.M. Silica Metal Oxide Vesicles Catalyze Comprehensive Prebiotic Chemistry. Chem. A Eur. J. 2018, 24, 8126–8132. [Google Scholar] [CrossRef]
- Eugster, H.P. Lake Magadi, Kenya, and Its Precursors. In Developments in Sedimentology; Nissenbaum, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1980; Volume 28, pp. 195–232. [Google Scholar] [CrossRef]
- Jones, B.F.; Rettig, S.L.; Eugster, H.P. Silica in Alkaline Brines. Science 1967, 158, 1310–1314. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-5429-3. [Google Scholar]
- Bischoff, W.D.; Sharma, S.K.; MacKenzie, F.T. Carbonate ion disorder in synthetic and biogenic magnesian calcites: A Raman spectral study. Am. Mineral. 1985, 70, 581–589. [Google Scholar]
- Urmos, J.; Sharma, S.K.; Mackenzie, F.T. Characterization of some biogenic carbonates with Raman spectroscopy. Am. Mineral. 1991, 76, 641–646. [Google Scholar]
- Burgio, L.; Clark, R.J. Library of FT-Raman spectra of pigments, minerals, pigment media and varnishes, and supplement to existing library of Raman spectra of pigments with visible excitation. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2001, 57, 1491–1521. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochemical Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Glaab, F.; Rieder, J.; García-Ruiz, J.M.; Kunz, W.; Kellermeier, M. Diffusion and precipitation processes in iron-based silica gardens. Phys. Chem. Chem. Phys. 2016, 18, 24850–24858. [Google Scholar] [CrossRef]
- Cartwright, J.H.E.; Escribano, B.; Khokhlov, S.; Sainz-Díaz, C.I. Chemical gardens from silicates and cations of group 2: A comparative study of composition, morphology and microstructure. Phys. Chem. Chem. Phys. 2011, 13, 1030–1036. [Google Scholar] [CrossRef]
- Cardoso, S.S.S.; Cartwright, J.H.E.; Sainz-Díaz, C.I. Carbonate-hydroxide chemical-garden tubes in the soda ocean of Enceladus: Abiotic membranes and microtubular forms of calcium carbonate. Icarus 2019, 319, 337–348. [Google Scholar] [CrossRef]
- Thouvenel-Romans, S.; Steinbock, O. Oscillatory growth of silica tubes in chemical gardens. J. Am. Chem. Soc. 2003, 125, 4338–4341. [Google Scholar] [CrossRef]
- Thouvenel-Romans, S.; Van Saarloos, W.; Steinbock, O. Silica tubes in chemical gardens: Radius selection and its hydrodynamic origin. Europhys. Lett. 2004, 67, 42–48. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; De Gruyter: Berlin, Germany, 2016; pp. 1–29. [Google Scholar]
- Cartwright, J.H.E.; Escribano, B.; Sainz-Díaz, C.I. Chemical-Garden Formation, Morphology, and Composition. I. Effect of the Nature of the Cations. Langmuir 2011, 27, 3286–3293. [Google Scholar] [CrossRef]
- Lowenstein, T.K.; Demicco, R.V. Elevated Eocene Atmospheric CO2 and Its Subsequent Decline. Science 2006, 313, 1928. [Google Scholar] [CrossRef]
- Jagniecki, E.A.; Lowenstein, T.K. Evaporites of the Green River Formation, Bridger and Piceance Creek Basins: Deposition, Diagenesis, Paleobrine Chemistry, and Eocene Atmospheric CO2. In Stratigraphy and Paleolimnology of the Green River Formation, Western USA; Smith, M.E., Carroll, A.R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 277–312. [Google Scholar]
- Lee, H.; Muirhead, J.D.; Fischer, T.P.; Ebinger, C.J.; Kattenhorn, S.A.; Sharp, Z.D.; Kianji, G. Massive and prolonged deep carbon emissions associated with continental rifting. Nat. Geosci. 2016, 9, 145–149. [Google Scholar] [CrossRef]
- Lowenstein, T.K.; Jagniecki, E.A.; Carroll, A.R.; Smith, M.E.; Renaut, R.W.; Owen, R.B. The Green River salt mystery: What was the source of the hyperalkaline lake waters? Earth-Science Rev. 2017, 173, 295–306. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Bots, P.; Benning, L.G.; Rodriguez-Blanco, J.-D.; Roncal-Herrero, T.; Shaw, S. Mechanistic Insights into the Crystallization of Amorphous Calcium Carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. [Google Scholar] [CrossRef]
- Surdam, R.C.; Eugster, H.P. Mineral reactions in the sedimentary deposits of the Lake Magadi region, Kenya. Geol. Soc. Am. Bull. 1976, 87, 1739. [Google Scholar] [CrossRef]
- Eugster, H.P.; Jones, B.F. Gels Composed of Sodium-Aluminium Silicate, Lake Magadi, Kenya. Science 1968, 161, 160–163. [Google Scholar] [CrossRef]
- Reinhardt, M.; Goetz, W.; Duda, J.-P.; Heim, C.; Reitner, J.; Thiel, V. Organic signatures in Pleistocene cherts from Lake Magadi (Kenya) – implications for early Earth hydrothermal deposits. Biogeosciences 2019, 16, 2443–2465. [Google Scholar] [CrossRef]
- Pasek, M.A.; Gull, M.; Herschy, B. Phosphorylation on the early earth. Chem. Geol. 2017, 475, 149–170. [Google Scholar] [CrossRef]
- Plattner, H.; Verkhratsky, A. Inseparable tandem: Evolution chooses ATP and Ca2+ to control life, death and cellular signalling. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150419. [Google Scholar] [CrossRef]

| pH | T (°C) | Conductivity | TDS | Na+ | K+ | SiO2 | Cl− | CO32− | HCO3− | SO42− |
| 10.9 | 37 | 16,200 | 440,000 | 179,460 | 831 | 1080 | 125,000 | 54,900 | 8110 | 2254 |
| F− | PO43− | Mg2+ | Al3+ | Ca2+ | Fetot | B+ | Sr2+ | Ba2+ | Br− | I− |
| 1075 | 418 | 0.023 | <0.005 | <0.5 | <0.05 | 211 | 0.0767 | 0.622 | 725 | 26.6 |
| Salt | BaCl2·2H2O | CaCl2·2H2O | MnCl2·4H2O | CoCl2·6H2O | Co(NO3)2·6H2O |
| pH | 5.2 | 4.6 | 2.3 | 2.6 | 3.5 |
| Solubility | 37 | 81.3 | 77.3 | 56.2 | 103 |
| Salt | CuCl2·6H2O | CuSO4·5H2O | FeCl2·4H2O | FeSO4·7H2O | FeCl3 |
| pH | 0.5 | 3.1 | 1.0 | 2.6 | −1.7 |
| Solubility | 75.7 | 22 | 65 | 29.5 | 91.2 |
| Salt | Fe2(SO4)3·9H2O | MgCl2·6H2O | MgSO4 | ZnCl2 | ZnSO4·7H2O |
| pH | −0.3 | 5.1 | 8.1 | −1.9 | 4.7 |
| Solubility | 440 1 | 56 | 35.5 | 408 | 57.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Getenet, M.; García-Ruiz, J.M.; Verdugo-Escamilla, C.; Guerra-Tschuschke, I. Mineral Vesicles and Chemical Gardens from Carbonate-Rich Alkaline Brines of Lake Magadi, Kenya. Crystals 2020, 10, 467. https://doi.org/10.3390/cryst10060467
Getenet M, García-Ruiz JM, Verdugo-Escamilla C, Guerra-Tschuschke I. Mineral Vesicles and Chemical Gardens from Carbonate-Rich Alkaline Brines of Lake Magadi, Kenya. Crystals. 2020; 10(6):467. https://doi.org/10.3390/cryst10060467
Chicago/Turabian StyleGetenet, Melese, Juan Manuel García-Ruiz, Cristóbal Verdugo-Escamilla, and Isabel Guerra-Tschuschke. 2020. "Mineral Vesicles and Chemical Gardens from Carbonate-Rich Alkaline Brines of Lake Magadi, Kenya" Crystals 10, no. 6: 467. https://doi.org/10.3390/cryst10060467
APA StyleGetenet, M., García-Ruiz, J. M., Verdugo-Escamilla, C., & Guerra-Tschuschke, I. (2020). Mineral Vesicles and Chemical Gardens from Carbonate-Rich Alkaline Brines of Lake Magadi, Kenya. Crystals, 10(6), 467. https://doi.org/10.3390/cryst10060467