Abstract
Hormone sensitive lipase is a central enzyme in triacylglycerol hydrolysis, lipid modification, and transformation of various lipids. Microbial hormone-sensitive lipases, which are highly similar to a catalytic domain of mammalian equivalents, have attracted strong attention due to their application potentials. Here, characterization and a preliminary X-ray crystallographic analysis of a novel bacterial homologue of hormone-sensitive lipase (HaLip1) from Halocynthiibacter arcticus is reported. Sequence analysis shows that HaLip1 has a conserved serine residue within the GDSAG motif. In addition, a characteristic HGGG motif for oxyanion formation was identified. The HaLip1 protein was overexpressed in E. coli. SDS-PAGE, overlay assay, and mass analysis were performed to confirm purity and activity of HaLip1 protein. Furthermore, HaLip1 was crystallized in a condtion consisting of 25% (w/v) PEG 3350, 0.1 M Hepes-KOH, pH 7.5, 0.2 M sodium chloride. Diffraction data were processed to 1.30 Å with an Rmerge of 7.3%. The crystals of HaLip1 belong to the P212121, with unit cell parameters of a = 54.6 Å, b = 59.5 Å, and c = 82.9 Å.
1. Introduction
Hormone-sensitive lipases (HSLs, E.C. 3.1.1.79), a subfamily of lipases/esterase, have functional roles in hydrolysis of triacylglycerol, lipid modification, and energy homeostasis [1,2,3]. In addition, these enzymes are involved in the chemical transformation of a wide range of chemical compounds including carbohydrates, fatty acids, and steroids. In mammals, HSLs consist of an N-terminal protein–protein interaction domain and a C-terminal catalytic functional domain [4,5]. In contrast, bacterial HSLs, which are highly homologous to mammalian C-terminal domains, have been identified [6]. These bacterial HSLs have a highly conserved catalytic triad of Ser–His–Asp, with the functional Ser located in a characteristic GD(T)SAG motif. These HSLs are classified into two subfamilies of GDSAG and GTSAG based on their sequence motif. In addition, a characteristic HGGG motif was suggested to be responsible for the formation of oxyanion hole [7,8,9].
Although several bacterial HSLs were identified from metagenomic DNA libraries and several bacteria [10,11,12,13,14,15], there is limited information available on HSLs from extremophiles. Here, we describe crystallization, and preliminary X-ray diffraction analysis of a novel hormone-sensitive lipase (HaLip1) from Halocynthiibacter arcticus, which was isolated from a marine sediment in the Arctic region [16]. Although the genome of H. arcticus is supposed to have 4675 protein-coding genes, information about its gene products is still largely unknown [17]. Specifically, structural information on HSLs from the genus Halocynthiibacter are largely unavailable. Therefore, structural studies of HaLip1 will provide molecular understanding on the catalytic mechanism of bacterial HSLs at molecular level. Furthermore, considering the fact that bacterial HSLs have attracted great interest due to their industrial potential [18,19], HaLip1 could be an invaluable biocatalyst with unique properties for biotechnological applications.
2. Materials and Methods
2.1. Materials
DNA modifying enzymes and restriction enzymes were obtained from New England BioLabs (Ipswich, MA, USA) or Takara Biomedical Korea (Seoul, Korea). DNA purification kits and other molecular biology kits were purchased from Qiagen Korea (Daejon, Korea). Protein columns and other reagents were obtained from GE Healthcare Korea (Seoul, Korea).
2.2. Cloning and Purification
Psychrophilic bacteria of H. arcticus (KCTC 42129) were grown and genomic DNA was purified as described previously [13]. The HaLip1 gene was amplified by polymerase chain reaction and final product was subcloned into pET-21a. No signal sequence was found in HaLip1. The following primers using NheI and XhoI were used (forward primer: 5’-ATGCTA GCTAGC ATGGCACAAGTCACC-3’, and reverse primer: 5’-GTACCG CTCGAG GGCAAGAAATGCCCG-3’). E. coli BL21(λDE3) cells transformed with a recombinant plasmid (pET21a-HaLip1) were cultured in LB medium. After 1 mM isopropyl-β-D-1-thiogalactoside (IPTG) induction for 18 h at 27 °C, bacterial cells were harvested and then suspended in cell lysis buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 50 mM imidazole). After sonication and centrifugation at 4 °C, the final supernatant fractions were loaded onto a His-tag column, followed by an imidazole gradient elution (from 50 to 200 mM). Finally, the pooled fractions were desalted using a PD-10 column and stored in a storage buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl).
2.3. Biochemical Characterization
Overlay assay of HaLip1with 4-methylumbelliferyl acetate was carried out as described previously [20,21]. The hydrolytic activity of HaLip1 was confirmed by observing the fluorescence of 4-methylumbelliferone. Determination of molecular mass was performed using a Voyager Bio-Spectrometry system in positive ion mode (NICEM, Seoul, Korea). Sequences of HaLip1 and its related hydrolases were obtained from SWISS = PROT. Multiple sequence alignments were performed with Clustal Omega and ESPript.
2.4. Crystallization
The crystallization screening trials were carried out by sitting-drop vapor-diffusion method using an automated crystallization robot (SPT Labtech, Boston, MA, USA) with commercial screening kits of MCSG 1T~4T (Anatrace, Maumee, OH, USA), JCSG-plus (Molecular Dimensions, Maumee, OH, USA), and PGA Screen (Molecular Dimensions, Maumee, OH, USA) [22]. The crystallization trials contained 300 nL of protein solution with an equal volume of reservoir solution in a 96-well plate. After 3 days, crystals of HaLip1 appeared in several well reservoirs. The single crystal of HaLip1 for X-ray analysis was obtained under the MCSG1T #95 condition of 25% (w/v) PEG 3350, 0.1 M Hepes-NaOH, pH 7.5, and 0.2 M sodium chloride.
2.5. X-ray Diffraction Data Collection and Data Processing
The diffraction-quality crystals of HaLip1 were transferred to a paratone oil containing cryo-protectant solution (Hampton Research, Aliso Viejo, CA, USA). After brief and gentle soaking, these crystals were effectively mounted on a synchrotron facility. X-ray diffraction data were collected using an Eiger X 9M detector (Dectris, Baden, Switzerland) at beamline 5C of the Pohang Light Source (PAL, Pohang, Korea). The final crystals were rotated with 1.0° oscillation range per frame. Diffraction data were collected, processed, and finally indexed using HKL2000 (Table 1). The HaLip1 crystal belongs to a primitive orthorhombic space group. Further analysis of the integrated intensities showed that the space group of HaLip1 was P212121 (see also Table S1).

Table 1.
X-ray data collection statistics of HaLip1.
3. Results and Discussion
Multiple sequence alignments of HaLip1 with three homologs in protein data bank (PDB) showed that all of them share common sequence motifs, which are necessary for their function and regulation (Figure 1). Specifically, HaLip1 showed significant sequence identity with a slightly acidophilic carboxylesterase (EstFa_R) from Ferroplasma acidiphilum (3WJ2, 28.2%) [23], a chloramphenicol-metabolizing enzyme (EstDL136) from a metagenome (6AAE, 31.5%) [24], and an alkaline esterase (Est8) from a metagenomic source (4YPV, 25.5%) [25].
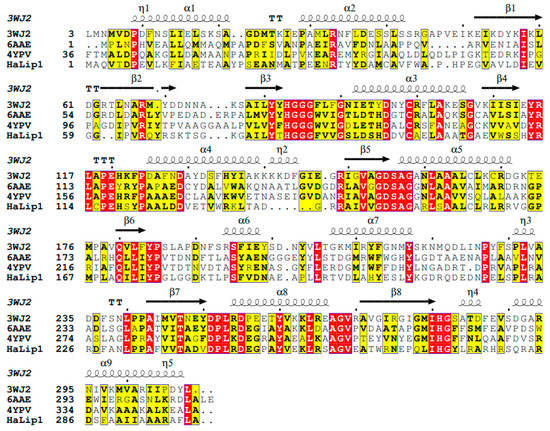
Figure 1.
Multiple sequence alignments including HaLip1 and three related enzymes. Identical and highly conserved amino acids are shown in red and yellow, respectively.
Three highly conserved amino acids of Ser147, Asp242, and His272 could form a catalytic triad, with Ser147 which is located in a characteristic GDSAG motif. In HaLip1 and related homologs, Asp, not Glu, is observed, although Glu is often used [13,26,27]. Furthermore, a highly conserved HGGG motif, which is suggested to be involved in oxyanion hole formation [6,28], was identified. In primary sequence analysis, HaLip1 has high percentages of small amino acids such as Gly (7.3%) and Ala (14.7%). Furthermore, the total number of acidic amino acids (Asp + Glu) was 41, while that of basic amino acids (Lys + Arg) was 31. Interestingly, this property is also frequently observed in psychrophilic enzymes [29].
The recombinant HaLip1 was overexpressed and purified to an electrophoretic homogeneity using an Ni2+-affinity His-tag column (Figure 2A). The hydrolytic activity of HaLip1 was examined by an overlay assay with 4-methylumbelliferyl acetate [10,13]. As shown in Figure 2B, high fluorescence was observed at the same position where HaLip1 was located in native-page. The molecular mass of HaLip1 was determined using MALDI-TOF mass analysis, which indicated a main peak (m/z) at 33.3 kDa. This value is highly consistent with the calculated mass of HaLip1.
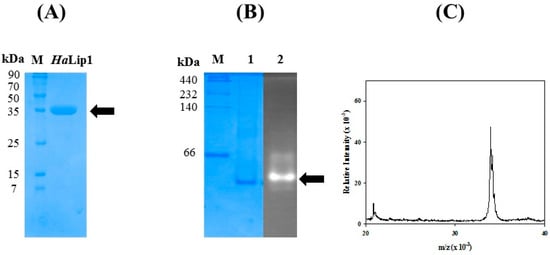
Figure 2.
Biochemical characterization of HaLip1. (A) SDS-PAGE analysis of purified HaLip1. (B) Overlay hydrolytic analysis of HaLip1. 1: Molecular markers, 2: coomassie brilliant Blue staining in native-page, 3: fluorescence due to the formation of cleave product was shown. (C) Mass analysis of HaLip1.
For crystallization studies, purified HaLip1 (14.2 mg/mL) was screened with commercially available crystallization kits [22]. A diffraction-quality crystal of HaLip1 was obtained under the MCSG1T #95 condition of 25% (w/v) PEG 3350, 0.1 M Hepes-NaOH, pH 7.5, 0.2 M sodium chloride. The diffraction-quality crystal grew to final dimensions of 0.4 × 0.2 × 0.3 mm3 (Figure 3). The X-ray radiation maintains isotropic diffraction throughout 360° rotation with 1° per each frame. The crystals of HaLip1 belonged to a P212121 space group with cell parameter of a = 54.6 Å, b = 59.5 Å, and c = 82.9 Å. The diffraction data were collected and processed to 1.3 Å resolution with an Rmerge value of 7.3% (Table 1). The data collection and processing statistics are summarized in Table 1. Considering one HaLip1 molecule per asymmetric unit, the Matthews coefficient (VM) is 2.06 Å3/Da. This value corresponds to 40.4% solvent content [30]. The structural determination of HaLip1 is in progress, and further structural studies will elucidate the catalytic mechanism of HaLip1 at molecular level.
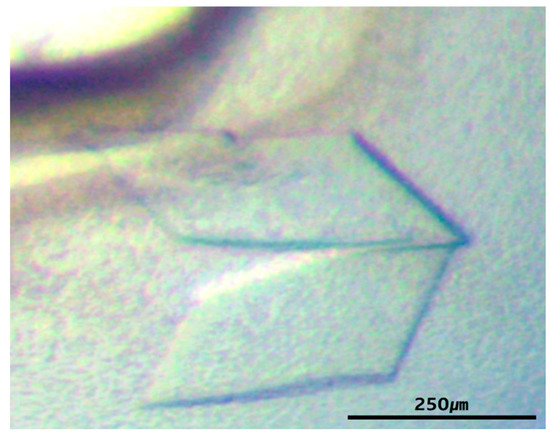
Figure 3.
Crystals images of HaLip1. These crystals grew to final dimensions of 0.4 × 0.2 × 0.3 mm3. A scale bar is also shown in the image.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/10/11/963/s1, Figure S1: Statistics of X-ray diffraction data processing HaLip1 crystal
Author Contributions
S.J., W.Y., and T.D.K. identified, expressed, and purified HaLip1. J.H., H.D., H.-W.K., and J.H.L. crystallized and obtained x-ray diffraction data. K.K.K., J.H.L., and T.D.K. coordinated the whole project. J.H.L., and T.D.K. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Polar Research Institute (KOPRI grant number PM20030).
Conflicts of Interest
The authors declare no conflicts of financial interests.
References
- Arner, P.; Langin, D. The role of neutral lipases in human adipose tissue lipolysis. Curr. Opin. Lipidol. 2007, 18, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Trites, M.J.; Clugston, R.D. The role of adipose triglyceride lipase in lipid and glucose homeostasis: Lessons from transgenic mice. Lipids Health Dis. 2019, 18, 204. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysis—A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011, 50, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Krintel, C.; Klint, C.; Lindvall, H.; Mörgelin, M.; Holm, C. Quarternary structure and enzymological properties of the different hormone-sensitive lipase (HSL) isoforms. PLoS ONE 2010, 5, e11193. [Google Scholar] [CrossRef]
- Lampidonis, A.D.; Rogdakis, E.; Voutsinas, G.E.; Stravopodis, D.J. The resurgence of Hormone-Sensitive Lipase (HSL) in mammalian lipolysis. Gene 2011, 477, 1–11. [Google Scholar] [CrossRef]
- Kim, T.D. Bacterial Hormone-Sensitive Lipases (bHSLs): Emerging Enzymes for Biotechnological Applications. J. Microbiol. Biotechnol. 2017, 27, 1907–1915. [Google Scholar] [CrossRef]
- Osterlund, T.; Contreras, J.A.; Holm, C. Identification of essential aspartic acid and histidine residues of hormone-sensitive lipase: Apparent residues of the catalytic triad. FEBS Lett. 1997, 403, 259–262. [Google Scholar] [CrossRef]
- Ngo, T.D.; Ryu, B.H.; Ju, H.; Jang, E.; Park, K.; Kim, K.K.; Kim, T.D. Structural and functional analyses of a bacterial homologue of hormone-sensitive lipase from a metagenomic library. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Li, P.Y.; Ji, P.; Li, C.Y.; Zhang, Y.; Wang, G.L.; Zhang, X.Y.; Xie, B.B.; Qin, Q.L.; Chen, X.L.; Zhou, B.C.; et al. Structural basis for dimerization and catalysis of a novel esterase from the GTSAG motif subfamily of the bacterial hormone-sensitive lipase family. J. Biol. Chem. 2014, 289, 19031–19041. [Google Scholar] [CrossRef]
- Kim, B.Y.; Yoo, W.; Huong Luu Le, L.T.; Kim, K.K.; Kim, H.W.; Lee, J.H.; Kim, Y.O.; Kim, T.D. Characterization and mutation anaylsis of a cold-active bacterial hormone-sensitive lipase from Salinisphaera sp. P7–4. Arch. Biochem. Biophys. 2019, 663, 132–142. [Google Scholar] [CrossRef]
- Park, J.M.; Kang, C.H.; Won, S.M.; Oh, K.H.; Yoon, J.H. Characterization of a novel moderately thermophilic solvent-tolerant esterase isolated from a compost metagenome library. Front. Microbiol. 2020, 10, 3069. [Google Scholar] [CrossRef]
- Kryukova, M.V.; Petrovskaya, L.E.; Kryukova, E.A.; Lomakina, G.Y.; Yakimov, S.A.; Maksimov, E.G.; Boyko, K.M.; Popov, V.O.; Dolgikh, D.A.; Kirpichnikov, M.P. Thermal inactivation of a cold-active esterase PMGL3 isolated from the permafrost metagenomic library. Biomolecules 2019, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Le, L.T.H.L.; Yoo, W.; Lee, C.; Wang, Y.; Jeon, S.; Kim, K.K.; Lee, J.H.; Kim, T.D. Molecular characterization of a novel cold-active hormone-sensitive lipase (HaHSL) from Halocynthiibacter arcticus. Biomolecules 2019, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huo, Y.-Y.; Ji, R.; Kuang, S.; Ji, C.; Xu, X.-W.; Li, J. Structural insights of a hormone sensitive lipase homologue Est22. Sci. Rep. 2016, 6, 28550. [Google Scholar] [CrossRef]
- Jayanath, G.; Mohandas, S.P.; Kachiprath, B.; Solomon, S.; Sajeevan, T.P.; Bright Singh, I.S.; Philip, R. A novel solvent tolerant esterase of GDSGG motif subfamily from solar saltern through metagenomic approach: Recombinant expression and characterization. Int. J. Biol. Macromol. 2018, 119, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.; Lee, Y.M.; Shin, S.C.; Hwang, K.; Hwang, C.Y.; Hong, S.G.; Lee, H.K. Halocynthiibacter arcticus sp. nov., isolated from Arctic marine sediment. Int. J. Syst. Evol. Microbiol. 2015, 65, 3861–3865. [Google Scholar] [CrossRef]
- Lee, Y.M.; Baek, K.; Lee, J.; Lee, H.K.; Park, H.; Shin, S.C. Complete genome sequence of Halocynthiibacter arcticus PAMC 20958(T) from an Arctic marine sediment sample. J. Biotechnol. 2016, 224, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Kim, T.D.; Kim, K.K. Carboxylic Ester Hydrolases in Bacteria: Active Site, Structure, Function and Application. Crystals 2019, 9, 597. [Google Scholar] [CrossRef]
- Bae, S.Y.; Ryu, B.H.; Jang, E.; Kim, S.; Kim, T.D. characterization and immobilization of a novel SGNH hydrolase (Est24) from Sinorhizobium meliloti. Appl. Microbiol. Biotechnol. 2013, 97, 1637–1647. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, S.; Yoon, S.; Ryu, Y.; Lee, S.Y.; Kim, T.D. Characterization of a novel oligomeric SGNH-arylestersae from Sinorhizobium meliloti 1021. Int. J. Biol. Macromol. 2010, 46, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Yoo, W.; Park, S.H.; Le, L.T.H.L.; Jeong, C.S.; Ryu, B.H.; Shin, S.C.; Kim, H.W.; Park, H.; Kim, K.K.; et al. Structural and functional characterization of a novel cold-active S-formylglutathione hydrolase (SfSFGH) homolog from Shewanella frigidimarina, a psychrophilic bacterium. Microb. Cell Fact. 2019, 18, 140. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Unno, H.; Oshima, Y.; Hosoya, M.; Fujino, N.; Hirooka, K.; Takahashi, S.; Yamashita, S.; Kusunoki, M.; Nakayama, T. Structural insights into the low pH adaptation of a unique carboxylesterase from Ferroplasma: Altering the pH optima of two carboxylesterases. J. Biol. Chem. 2014, 289, 24499–24510. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, P.A.; Han, K.; Lee, S.W.; Rhee, S. Crystal structure of chloramphenicol-metabolizing enzyme EstDL136 from a metagenome. PLoS ONE 2019, 14, e0210298. [Google Scholar] [CrossRef]
- Pereira, M.R.; Maester, T.C.; Mercaldi, G.F.; de Macedo Lemos, E.G.; Hyvönen, M.; Balan, A. From a metagenomic source to a high-resolution structure of a novel alkaline esterase. Appl. Microbiol. Biotechnol. 2017, 101, 4935–4949. [Google Scholar] [CrossRef]
- Nam, K.H.; Kim, M.Y.; Kim, S.J.; Priyadarshi, A.; Lee, W.H.; Hwang, K.Y. Structural and functional analysis of a novel EstE5 belonging to the subfamily of hormone-sensitive lipase. Biochem. Biophys. Res. Commun. 2009, 379, 553–556. [Google Scholar] [CrossRef]
- Li, P.Y.; Chen, X.L.; Ji, P.; Li, C.Y.; Wang, P.; Zhang, Y.; Xie, B.B.; Qin, Q.L.; Su, H.N.; Zhou, B.C.; et al. Interdomain hydrophobic interactions modulate the thermostability of microbial esterases from the hormone-sensitive lipase family. J. Biol. Chem. 2015, 290, 11188–11198. [Google Scholar] [CrossRef]
- Palm, G.J.; Fernández-Álvaro, E.; Bogdanović, X.; Bartsch, S.; Sczodrok, J.; Singh, R.K.; Böttcher, D.; Atomi, H.; Bornscheuer, U.T.; Hinrichs, W. The crystal structure of an esterase from the hyperthermophilic microorganism Pyrobaculum calidifontis VA1 explains its enantioselectivity. Appl. Microbiol. Biotechnol. 2011, 91, 1061–1072. [Google Scholar] [CrossRef]
- Santiago, M.; Ramírez-Sarmiento, C.A.; Zamora, R.A.; Parra, L.P. Discovery, molecular mechanisms, and industrial applications of cold-active eEnzymes. Front. Microbiol. 2016, 7, 1408. [Google Scholar] [CrossRef]
- Matthews, B.W. Solvent content of protein crystals. J. Mol. Biol. 1968, 33, 491–497. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).