Abstract
The use of bio-based material for repairing concrete is a relatively new method. Therefore, more results from simulated real-condition experiments are needed before being applied on a practical scale. In the recent past, several studies have been conducted on the improvement of bio-based repair materials. In this study, the bio-based material involving yeast, glucose, and calcium acetate mixed in a Tris buffer solution showed the potential to develop a microbial process leading to the precipitation of calcium carbonate. We investigated the factors affecting the precipitation rate of the calcium carbonate of bio-based materials for repairing leakage in the concrete specimens. Based on a series of experiments involving temperature, the type of dry yeast, and the concentration of the Tris buffer solution, the composition of bio-based materials with the highest precipitation rate of calcium carbonate was selected. The selected mixture could be applied to repair leakage of concrete until the cracks are sealed entirely.
1. Introduction
Current infrastructure development generally uses concrete structures to increase the sturdiness. Easy and inexpensive maintenance are the reasons for the high demand for the use of concrete in infrastructure. Structural constraints and poor work quality are some of the causes of structural defects, so concrete quality control is required. One example of a construction defect of a bridge is a leak in the gaps or joints of structural members used in bridge structures. When it rains, the bridge serviceability will decrease.
Per the Standard specifications for concrete structures 2007 “Design” no. 15 by JSCE [1] and British Standard Institution BS 8110-1:1997 [2], cracks smaller than 0.3 mm are acceptable for aggressive environmental conditions. Invisible and undetectable, the location of some cracks may be inaccessible [3]. The location of the cracking as another factor to be considered when repairing damaged structures with conventional repair materials, as it may considerably complicate the repair. Repairing concrete cracks using conventional techniques to cover the crack gap is less effective, especially for deep cracks. The use of cement-based grout with higher viscosity in conventional methods is less effective for treating cracks in concrete interiors over large areas. The alkaline material that comes out of the concrete will cause environmental pollution. Therefore, the same material as the concrete material must be used for repairs.
Bio-based material for the improvement of durability and properties of concrete is a relatively recent advance technology. In a self-healing approach, isolated micro-organisms can be applied to effective to cracks in fractured concrete [4]. The precipitated calcium carbonate is achieved by the effects of metabolic bacterial activity [5]. Bacteria culture can be injected into the concrete surface to trigger self-healing [6]. Self-healing considerably reduced the water permeability of cracked concrete on the surface in a parking garage sprayed by cultured bacteria [7]. Many researchers have been studying the bacteria-based approach to increase the strength and improve the characteristics of concrete using methods such as concrete embedded with bacteria exposed to a harsh environment [8].
In other studies, a better effect was produced by directly adding bacteria to the concrete mix compared to the injection and spraying method [9,10,11,12]. Due to the ureolytic activity of the bacteria, microbial precipitation plugged the cracks. The lifespan of the bacteria tends to decrease due to the severe environment inside the concrete matrix [9], which results in a reduction in the efficiency of crack sealing over time. The encapsulation of bacteria provides the necessary protection to increase their lifespan [13]. Wang et al. [14] used a bacterial spore suspension with hydrogel sheets to produce hydrogel encapsulating with the spores before mixing them into mortar specimens. The calcium carbonate precipitation filled a crack width of 0.5 mm and decreased the water permeability to 68%. In another study, the immobilization of encapsulated bacteria enhanced their performance by filling a crack width of 0.97 mm [15]. In the most recent study, bacteria encapsulation techniques, through various carrier compounds using graphite nanoplates, produced better results for a crack width of 0.81 mm [16]. The pre-cracked specimens were sealed at three and seven days. In a recent parallel trend, Achal [17] and Mostavi [18] evaluated self-healing materials’ performance and durability based on the depth of the crack plugged. They healed the crack depths of 27.2, 19, and 35 mm. Achal [17] remediated cracks with biocement induced by Bacillus sp. CT-5, whereas Mostavi [18] used double-walled sodium silicate microcapsules with an in-situ polymerization method. The encapsulation technique has been proven to be more effective among the self-healing approaches due to the prolonged performance of the bacteria. The yeast-based mixtures filled are able to fill larger sized or deeper cracks.
Self-healing with microbial-induced calcium carbonate precipitation (MICP) as a liquid-based repair technique has been intensively studied [19,20,21,22,23,24]. The bio-based material mixtures typically consist of a micro-organism, a calcium source, and an organic carbon source. Commercially available dry yeast was used as a micro-organism due to its effectiveness and untreated nature before use. When dry yeast is used as the micro-organism, carbon dioxide produced through the microbial metabolic processes consumes an organic carbon source, such as glucose, providing carbonate ions. The carbonate ions lead to a reaction with the calcium ions present in the mixture, leading to the precipitation of calcium carbonate depending on the pH level of the mixture. CaCO3 precipitates according to the following reactions:
C6H12O6 → 2 CO2 + 2 C2H5OH,
CO2 + H2O → CO32− + 2H+,
Ca2+ + CO32−→ CaCO3 ↓.
Notably, calcium carbonate is the main reaction product of the process of microbial metabolism formed by the carbonation of hydration products, and its properties are not harmful to concrete. Besides the material properties, the viscosity of the mixture is lower than conventional materials used for crack repair, such as an epoxy resin. This may overcome the shortcomings related to conventional repair materials. The water tightness of defective concrete is effectively improved due to mixtures that can penetrate to the deeper zones and fill the gaps formed between concrete members, which is of interest for the repair of concrete in wet areas given the difficulty of repairing by injection.
The microbial metabolic process of the bio-material mixture promotes calcium carbonate precipitation, as shown in Figure 1. It is important to manage the pH levels of the mixtures to facilitate the calcium carbonate precipitation to support the reaction.
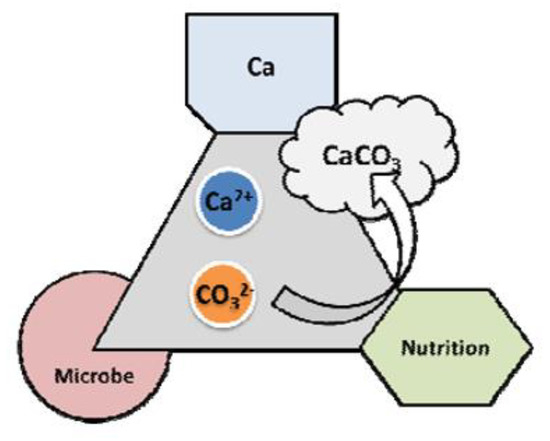
Figure 1.
Basic constituents of bio-based repair materials.
In this study, we used Tris hydroxymethyl aminomethane buffer solution, which has an alkali buffering function. Hydrochloric acid was used to adjust the initial pH of the Tris buffer solution to 9.0 and/or 8.0.
The source of calcium ions used for the mixtures should have higher solubility and lack detrimental effects on concrete materials. The material should also be available on the commercial market and be cost-effective. Based on the factors mentioned above, calcium acetate was chosen for this study. Commercially available dry yeast was used as the microorganism. The dry yeast anaerobically ferments in oxygen-free environments. During metabolism, this microorganism requires an organic carbon source as a food source. In the presence of glucose (C6H12O6), yeast converts carbohydrates to carbon dioxide and alcohol through anaerobic fermentation.
Calcium carbonate is one of the many bio-mineral compounds produced by organisms. Crystallographically, calcium carbonate occurs in three anhydrous polymorphs forms: Calcite, aragonite, and vaterite. In the thermodynamic stability, calcite is more stable than aragonite, and vaterite is the least. It was caused by vaterite to easily transforms into calcite [25]. Vaterite plays an essential role in biological life and health, although its amount is far less than calcite and aragonite [26].
Based on the abovementioned context, the main aim of this research was to examine the factors affecting the precipitation rate of calcium carbonate through the microbial metabolic process of yeast-based mixtures and the applicability of bio-based material for repairing water leakages in concrete.
2. Materials and Methods
2.1. Basic Constituents of Bio-Based Repair Materials
Figure 1 shows the microbial metabolic process of the bio-material mixtures, which promotes calcium carbonate precipitation. To facilitate the precipitation of calcium carbonate, the pH levels in the mixtures must be controlled. The Tris buffer solution (Tris-hydroxymethyl aminomethane) with an alkali buffering function was used in this study. Hydrochloric acid was used to adjust the initial pH of the Tris buffer solution to 8.0. The use of hydrochloric acid should be minimized when adjusting pH levels to ensure that there is no adverse effect on the integrity of the hardened concrete.
The source of calcium ions used for mixtures was chosen with the following characteristics: no harmful effect on the concrete material and soluble. Calcium acetate was chosen for this study based as it is cost-effective and commercially available. Commercially available dry yeast was used as a microorganism; it is anaerobic and active in an oxygen-free environment. Organic carbon sources are necessary for the metabolism of the microorganism. In the presence of glucose (C6H12O6) as an organic carbon source, the yeast converts carbohydrates into carbon dioxide and alcohol through anaerobic fermentation.
The highly alkaline environments exhibited by the pore solution in concrete create harsh conditions for the yeast. The micro-organism must exist in mixtures with a lower pH, which was thus initially controlled to be as low as possible. According to the previous research, less calcium carbonate precipitates when the pH is less than 7.5 [22]. To maintain appropriate pH levels in the mixtures examined, the pH was initially adjusted to 8.0 at room temperature controlled at 20 °C. The specifications of the basic constituent materials and alkaline buffer solution are described in Table 1 and Table 2, respectively.

Table 1.
Specifications of the basic constituent material.

Table 2.
Specifications of alkaline (Tris) buffer solution.
According to Yamamoto [27], the appropriate composition of the basic constituents is mixed. The basic composition of the yeast-based mixtures at room temperature controlled to 20 °C is shown in Table 3.

Table 3.
The basic composition of yeast-based mixtures.
2.2. Tube Precipitation Test Method
The measurement of calcium ion concentration and pH in the test tube mixture was started after the reaction began. The mixtures of each material were prepared according to Table 3. The Tris buffer solution was then added to the mixture in the glass beaker size 500 mL. It was subsequently stirred at 500 rpm for 5 min until the material was homogenized and dissolved into the solution. Tris buffer solution was added to the mixture to create a 120 mL volume solution. After separating the samples into 4 test tubes (30 mL each), the test tubes were placed in a temperature-controlled room for the measurements.
After time had elapsed, filtration was carried out to measure the precipitation rate of the calcium carbonate (weight method). A commercial pH meter (Handy type Digital PH Meter SK-620PHII with standard probe PHP-31, SATO, Tokyo, Japan) and calcium ion electrode meter (Handheld Ion/pH Meter IM-32P, DKK-TOA, Tokyo, Japan) were used to measure the pH and the concentration of calcium ions of mixtures. Each test was carried out using two test tubes to confirm the reliability of the precipitation results. Figure 2 shows the scheme and measurement of mixtures.
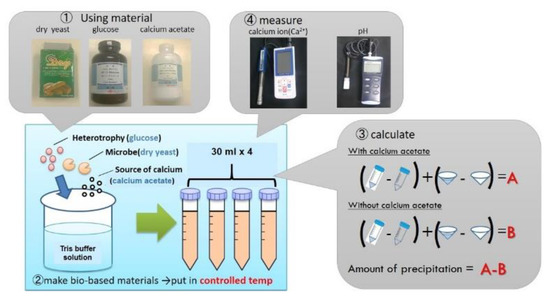
Figure 2.
Scheme of mixtures and measurement.
The decreasing rate of calcium ions and precipitated calcium carbonate were calculated based on the results of the calcium ions measured using the following:
Based on the concentration of calcium ions and pH measured, the concentration of carbonate ions was estimated using the following equations:
where CaCO3 is the amount of precipitates (g), m is the amount of solution (L), M is the molar mass of calcium carbonate (100.09 g/mol), pKa is the acid dissociation constant at 20 °C (8.2), [C0] is the initial concentration of calcium ions (g/L), [Ca] is the measured concentration of calcium ions (g/L), [Q] is the concentration of calcium acetate (mol/L), [B] is the concentration of base (mol/L), [BH+] is the concentration of acid (mol/L), [H+] is the concentration of hydrogen ions (mol/L), and [CO32−] is the estimated concentration of carbonate ion (mol/L).
2.3. XRD and FT-IR Test
X-ray diffraction (XRD) was used to observe the precipitated calcium carbonate as the result of the involvement of bacteria in mineral production and to verify the functionally of the bacteria-based concrete repair system. The sample used for the XRD analysis was the residue that had been left in the test tube for 24, 48, and 72 h under room conditions. The crushed and pulverized sample was found to have a size of less than 10 microns was determined using X-ray diffraction. The crushed and pulverized sample with a size of fewer than 10 microns was prepared, and the XRD test was carried out at the diffraction angle (2θ) between 5° and 50°. The components of the sample obtained from the XRD measurements were identified by comparing them with the existing Powder Diffraction File PDF-4/Mineral 2015 XRD database established by the International Centre for Diffraction Data [28].
Fourier transform infrared (FT-IR) spectroscopy is a tool used to analyze chemical compound based on the interaction between a functional group in compounds using infrared radiation. The functional groups that are exposed to infrared radiation vibrate and produce a specific infrared spectrum for a particular functional group of molecules. The functional group absorbs energy at a certain wavelength, and a transition occurs from the ground state to the specific state position. The absorption of energy at a certain wavelength by molecules in a compound produces a peak in the infrared spectrum band diagram [29]. FT-IR spectroscopy is versatile and non-destructive because it does not ruin the structure of the compounds present in the sample after analysis. FT-IR does not required a sample preparation step or derivatization.
3. Results
3.1. Effect of Temperature
The effect of temperature was examined with respect to the precipitation rate up to 72 h after mixing. Figure 3a shows the precipitates remaining in the tubes after up to 24 h post-filtering.
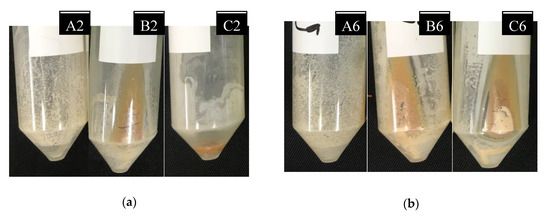
Figure 3.
(a) Precipitates after 24 h for mixtures A2, B2, and C2; (b) precipitates after 72 h for mixtures A6, B6, and C6.
Visually, calcium carbonate precipitation adhering to the test tube increased with the increase in temperature. The A mixture was test tube sampled at 30 °C, and B and C were sampled at 20 and 10 °C, respectively. The precipitation of CaCO3 in the case of the A mixtures was observed to be greater than the precipitation of both the B and C mixtures. Figure 3b shows the precipitates left in the tubes 72 h after filtering. The precipitation of CaCO3 after 72 h was observed to be greater than those at 24 and 48 h.
Figure 4 shows the mass of precipitates left in the tubes after filtering each mixture after 72 h.
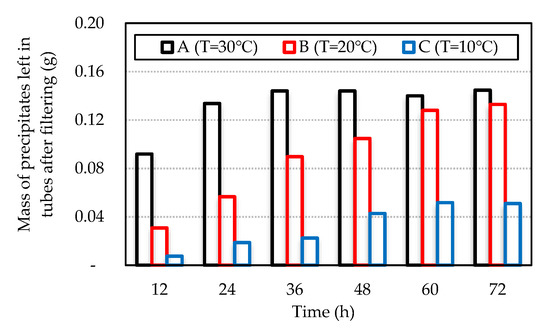
Figure 4.
Mass of the precipitates left in tubes after filtering.
The results were confirmed by the calcium ion measurement in the tested mixtures. The relationship between the decreasing rate of calcium ion and the precipitation rate through filter paper at constant temperature is shown in Figure 5a. The decreasing rate of the calcium ions was measured using the initial concentration of calcium ions (C0), and the concentration of calcium ions was measured (Ca) based on Equation (4). The average difference between the decreasing rate of calcium ions measured and the precipitation rate calculated by the filtered paper was less than 30%. The results confirmed that the decreasing rate of calcium ions was similar to the precipitation rate recorded by measuring the filter paper. The maximum precipitation of calcium carbonate was reached at 30 °C. At 30 °C, after 24 h of elapsed time, the concentration of calcium ions decreased by more than 90%, while the pH gradually lowered below 7.0.
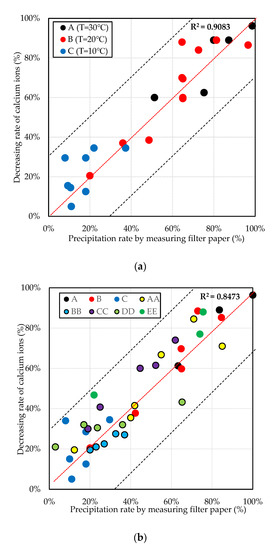
Figure 5.
The relation between the decreasing rate of calcium ions and the precipitation rate by filtered paper at (a) constant temperature and (b) with temperature change.
Figure 5b shows the decreasing rate of calcium ions and pH of the yeast-based mixtures at different temperatures for up to 72 h. AA indicates the temperature change from 20 to 30 °C, BB indicates the temperature change from 20 to 10 °C, CC indicates the temperature change from 10 to 30 °C, DD indicates the temperature change from 10 to 20 °C, and EE indicates the uncontrolled room temperature conditions. As a result, the pH decreased due to the metabolic process in the mixtures. Otherwise, the precipitation rate of the calcium carbonate was slower when the temperature was lower. According to Putri [30], the 20–30 °C range is the optimum temperature range for increasing the precipitation of calcium carbonate.
The results confirmed that the decreasing rate of calcium ions had a strong association with precipitation rate by weighing filter paper. The decreasing rate of calcium ions was nearly equal to the amount of precipitation of the calcium carbonate. A larger decreasing rate of the calcium ions in the early stages of the tests means that the precipitation rate of the calcium carbonate is higher. Therefore, the decreasing rate of the calcium ions was used as an indicator of the precipitation rate of calcium carbonate under the test tube conditions.
3.2. Effect of the Type of Dry Yeast
In previous research, Kubo [21], Yamamoto [27], and Putri [30] used one type of dry yeast as the microorganism. Since there are many varieties of commercial dry yeast on the market, the effects of the dry yeast types were examined to enhance the precipitation rate of the calcium carbonate in bio-based repair materials composed of yeast, calcium acetate, and glucose. Three types of commercial dry yeast were used in this study, as shown in Table 4. To assess the influence of the different types of dry yeast, FT-IR was used.

Table 4.
Types of dry yeast for each group.
A, B, and C were used to represent dry yeast types A, B, and C (Table 4), respectively. For each group, triplicate sets of 60 mL of liquid media were prepared. The concentration of the Tris buffer solution was set to 0.5 mol/L and the initial pH was set to 8.0. For each group, the mix proportion was set in terms of the basic composition mixture (series 1), duplicate (series 2), and triplicate of basic composition materials (series 3), separately. The dry temperature of 20 °C was controlled to test mixtures for up to 48 h.
Figure 6 shows the relationship between the carbonate ions estimated by Equation (6) projected for all types of dry yeast and the changes in the pH measured in the mixture tested. Based on the graph above, two groups were confirmed. When the concentration of carbonate ion was lower than 0.06 mol/L, the pH remained higher than 7.5. When the concentration of the carbonate ion was 0.06 mol/L or more, the pH declined to a maximum of 6.50; the calcium carbonate concentration increased with the decreasing pH. The precipitation of calcium carbonate in the threefold basic composition mixture using dry yeast type B was the largest. However, regardless of the type of dry yeast, the relationship between pH and the concentration of carbonate ions was linear. From this research, adjusting the concentration of the Tris buffer solution in the mixtures using dry yeast type A or C suggested the precipitation of calcium carbonate will increase.
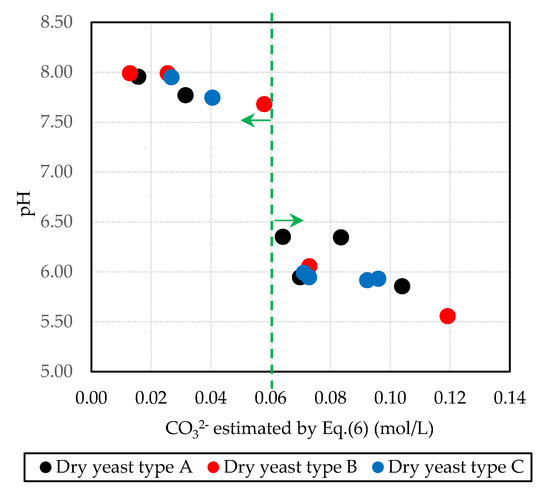
Figure 6.
Estimated carbonate ions and pH for up to 48 h (pH 8.0, 0.5.0 mol/L) for all mixtures.
The results showed the effects of the type of dry yeast on the precipitation rate of calcium carbonate. Based on the concentration of calcium ions and carbonate ions, there was no significant difference in the results obtained using all types of dry yeast. Therefore, all types of dry yeast can be used as the basic constituents of yeast-based mixtures where the concentration of the Tris buffer solution is 0.50 mol/L and the initial pH is 8.0.
3.3. Effect of Different Concentrations of Yeast-Based Mixtures on Calcium Carbonate Precipitation
According to Kawaai [19] and Putri [30,31,32], the initial pH levels and concentration of the mixture influence the precipitation rate, particularly at the beginning of the reaction. Correspondingly, the most effective compositions in the early phases contribute to increasing the amount of precipitation, as well as speeding up the reaction rate due to the effectiveness of sealing the leakage in concrete. Therefore, finding the optimal proportions of yeast, glucose, and calcium acetate is necessary to increase the deposition rate after 72 h. This composition can be used to repair cracks in concrete. The concentration and pH of the mixture strongly influence the deposition rate at the beginning of the reaction. The composition of the mixture must be determine to produce a large amount of precipitation of the calcium carbonate crystals and a fast reaction rate at the beginning of the reaction so that gaps in the concrete can be filled immediately.
In this study, calcium carbonate precipitation was produced from the process of microbial metabolism of the bio-material. The microbes require suitable environmental conditions to produce a large amount of calcium carbonate precipitate from microbial metabolism. A highly alkaline environment disturbs the microbial metabolism of yeast. These microorganisms need to exist in mixtures with a relatively lower pH. According to past research, calcium carbonate did not develop when the pH was less than 7.5 [22]. The initial pH has to be adjusted to the range of 8.0 and 9.0 using a Tris buffer solution at ambient conditions controlled to 20 °C.
Table 5 shows the composition of the liquid medium for each group. The initial pH values of the liquid were 8.0 and 9.0, which are represented by letters A and B in the group label, respectively. The concentration of the Tris buffer solution was controlled to 1.0, 0.75, 0.5, and 0.25 mol/L for each group. The mix proportion was set to the basic mixture (series 1), duplicate (series 2), triplicate (series 3), and quadruple the basic composition (series 4) for each concentration of Tris solution. The samples were examined at 10, 20, and 30 °C and the temperature was changed from 10 to 30 °C for up to 72 h. The A1 mixture at 20 °C was used as the benchmark for the other compositions.

Table 5.
The mix proportion of the basic constituents for each group.
The results showed a smaller amount of calcium carbonate was precipitated in the early phase. The calcium carbonate precipitation was over 20% after 48 h. In the concentration range of 0.5 to 0.75 mol/L, the basic mixture (A1) mixed in with the Tris buffer solution seemed to be sufficient as indicated by the higher decreasing rate measured after 72 h.
To determine the suitable concentration of Tris buffer solution given the initial pH, the A2, A3, A4, and B series were examined in comparison to the A1 case. They were involved higher groupings of the yeast mixed in the four concentrations of Tris buffer solution with an initial pH of 9.0 or 8.0. Figure 7 shows that estimated calcium ions production decreased following 24 h in each Tris buffer solution concentration.
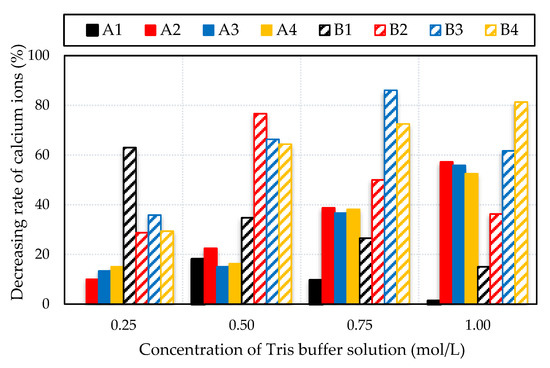
Figure 7.
Decreasing rate of calcium ions after 24 h.
The precipitation tended to be higher than 62% of the decreasing rate after 24 h when the B1 mixture was mixed with a concentration of 0.25 mol/L Tris buffer solution with an initial pH of 9.0. After 24 h, the pH measured for the B1 to B4 mixtures changed to 8.43, 6.16, 6.04, and 6.03, respectively.
The results suggested that the faster generation of carbonate ions was due to the use of a low concentration of the mixture, which increased the precipitation of CaCO3. Otherwise, the decreasing rate of calcium ions seemed to be larger when the solution was controlled to a concentration of 0.5 mol/L, in B2, B3, and B4 mixtures. The decrease rates of the calcium ions for the B2, B3, and B4 mixtures were above 60% and their pH after 24 h were 8.09, 6.30, and 6.14, respectively. A similar tendency of the decreasing rate of calcium ions with the precipitation process was found for B3 and B4 mixed in the Tris buffer solution concentrations of 0.75 and 1.0 mol/L, respectively. The results suggested that the use of a higher concentration of basic constituent mixtures produced more calcium carbonate precipitation.
The results showed that calcium carbonate precipitation depend on the composition of the yeast-based mixtures within 72 h after mixing. Tris buffer solution was observed to be sufficient at the concentrations of 0.5 to 0.75 mol/L and 0.75 to 1.0 mol/L and at an initial pH of 9.0 and 8.0, respectively. At an initial pH of 9.0, the reaction was observed to be faster initially, potentially due to a lower concentration of Tris buffer solution being reacted with a higher-pH mixture. The highest precipitation was produced by B3 at an initial pH adjusted to 9.0 where the Tris buffer solution was controlled to 0.75 mol/L. The dry yeast concentration was 27 g/L, mixed with 0.15 mol/L of calcium acetate and 0.3 mol/L of glucose.
The FT-IR evaluation proved the presence of calcium carbonate in the crystal, which is related to calcium carbonate precipitation produced by bio-based materials. Figure 8 depicts the FT-IR spectra for the precipitate in the test tube. For sample 1, the peaks at 1392.35 cm−1, 1031.73 cm−1, and 871.667 cm−1 demonstrated the existence of calcium carbonate. For precipitates of samples 2 and 3, the signals at 1403.924 cm−1, 1033.658 cm−1, and 871.67 cm−1 showed the presence of calcium carbonate. A comparable trend was observed in the pattern for sample 4, which showed peaks at 1392.35 cm−1, 1031.73 cm−1, and 871.667 cm−1. The precipitate for sample 5 was CaCO3, as demonstrated by the peaks at 1394.281 cm−1, 1029.801 cm−1, and 871.67 cm−1. These spectra were analyzed and aligned with the standard spectrum of calcium carbonate from the National Institute of Standards and Technology (NIST) Standard Reference Database [33]. Therefore, this spectrum showed that the majority of precipitate was composed of calcium carbonate.
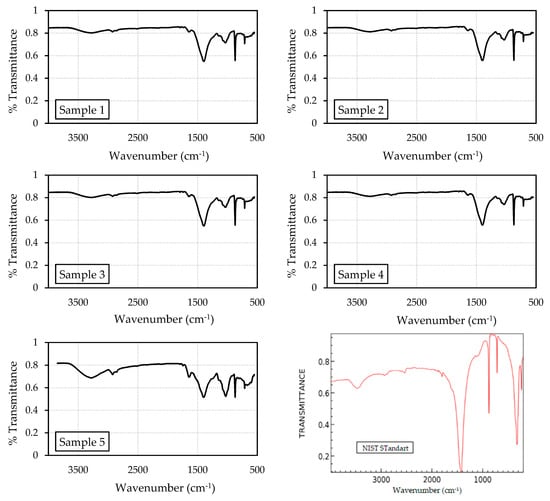
Figure 8.
Spectra of FT-IR analysis of five different sample and standard spectra from NIST [33].
The same precipitates were examined by XRD to confirm the polymorph as crystals. The sample 1 precipitate XRD pattern is presented in Figure 9.
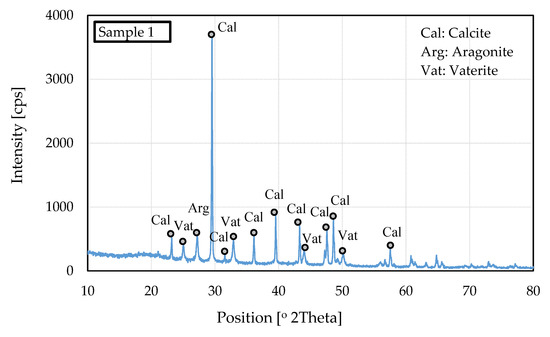
Figure 9.
XRD pattern for sample 1 specimen.
Based on the XRD analysis, the three polymorphs of CaCO3 (calcite, vaterite, and aragonite) coexisted. Calcite was the main polymorph, with nine peaks. There was only one peak of the aragonite polymorph in the precipitate, showing that the calcium carbonate precipitate was stable because calcite is the stable polymorph of the crystal. In addition, the XRD method showed the effect of the changes in temperature [30]. When temperature increased, the intensities of the calcite reflections increased and became the major phase. The XRD analysis emphasized that calcite should be the dominant polymorph of calcium carbonate.
4. Conclusions
A series of experiments were conducted to examine the factors affecting the precipitation rate of calcium carbonate through the microbial metabolic process of yeast-based mixtures for concrete repair. Initially, the test tube method was used to investigate the pH and precipitation rate of calcium carbonate by weighing the filter paper. The calcium ions in the mixture were measured to obtain the decreasing rate of the calcium ions. We found that the correlation between the decreasing rate of calcium ions and the precipitation rate could be determined by weighing the filter paper. The result almost equal when the decreasing rate of calcium ions is large and the calcium carbonate precipitation rate is high. The decreasing rate of calcium was used as an index of the precipitation rate of calcium carbonate in this study.
Secondly, the investigation showed the effect of the changes in temperature on the precipitation of calcium carbonate. Based on the mass of precipitates left in the tubes after filtering, the maximum precipitation of calcium carbonate was observed at 30 °C, and the lowest at 10 °C. When the temperature increased, the intensity of the calcite reaction increased and became the major phase of calcium carbonate.
Third, a series of experiments was performed to evaluate the effects of dry yeast type on enhancing the precipitation rate of calcium carbonate. The result showed that there was no significant difference in the calcium carbonate precipitation rate in the results obtained from all types of dry yeast. Therefore, all types of dry yeast can be used as the basic constituent of yeast-based mixtures with a Tris buffer solution concentration of 0.5 mol/L and an initial pH of 8.0. The FT-IR spectra for all types of dry yeast were indicative of the existence of calcium carbonate.
Fourth, the results showed that calcium carbonate precipitates depended on the concentration of the yeast-based mixtures 72 h after mixing. The most precipitate was produced by the B3 mixture with the initial pH adjusted to 9.0 and the Tris buffer solution controlled to 0.75 mol/L.
Author Contributions
P.Y.P. performed all the experiments, analyzed the data, and wrote the original draft; I.U. supervised this work and suggested how to proceed with the study; N.S. conducted writing, review, and editing; F.R. completed part of the experiments and analyzed the data; T.A. partly analyzed the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Japan Grant-in-Aid for Scientific Research (B): 15H04025 and a grant from Universitas Negeri Padang (Penelitian Dasar). The authors greatly appreciate the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Japan Society of Civil Engineers. Standard specifications for concrete structures-2007: Design. In JSCE Guidelines for Concrete; JSCE Concrete Committee: Tokyo, Japan, 2007. [Google Scholar]
- British Standart Institution, BS 8110-1:1997. Structural Use of Concrete. Part 1: Code of Practice for Design and Construction; British Standart Institution: London, UK, 1997. [Google Scholar]
- Muhammad, N.Z.; Shafaghat, A.; Keyvanfar, A.; Majid, M.Z.A.; Ghoshal, S.; Mohammadyan-Yasouj, S.; Ganiyu, A.A.; Kouchaksaraei, M.S.; Kamyab, H.; Taheri, M.M.; et al. Tests and methods of evaluating the self-healing efficiency of concrete: A review. Constr. Build. Mater. 2016, 112, 1123–1132. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Keyvanfar, A.; Andalib, R.; Samadi, M.; Shafaghat, A.; Kamyab, H.; Majid, M.Z.A.; Zin, R.M.; Fulazzaky, M.A.; Lee, C.T.; et al. Application ofProteus mirabilisandProteus vulgarismixture to design self-healing concrete. Desalin. Water Treat. 2013, 52, 3623–3630. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Schlangen, E. A Two Component Bacteria-Based Self-Healing Concrete. In Proceedings of the 2nd International Conference on Concrete Repair, Rehabilitation and Retrofitting, Cape Town, South Africa, 24–26 November 2009; pp. 215–220. [Google Scholar]
- Sangadji, S.; Wiktor, V.; Jonkers, H.; Schlangen, E. Injecting a Liquid Bacteria-Based Repair System to Make Porous Network Concrete Healed. In ICSHM 2013: Proceedings of the 4th International Conference on Self-Healing Materials, Ghent, Belgium, 16–20 June 2013; Magnel Laboratory for Concrete Research: Ghent, Belgium, 2013; pp. 118–122. [Google Scholar]
- Wiktor, V.; Jonkers, H.M. Field performance of bacteria-based repair system: Pilot study in a parking garage. Case Stud. Constr. Mater. 2015, 2, 11–17. [Google Scholar] [CrossRef]
- Nosouhian, F.; Mostofinejad, D.; Hasheminejad, H. Concrete Durability Improvement in a Sulfate Environment Using Bacteria. J. Mater. Civ. Eng. 2016, 28, 04015064. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Çopuroğlu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Wang, J.; Van Tittelboom, K.; De Belie, N.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540. [Google Scholar] [CrossRef]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzym. Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Irwan, J.M.; Othman, N. An Overview of Bioconcrete for Structural Repair. Appl. Mech. Mater. 2013, 389, 36–39. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; De Belie, N.; De Muynck, W.; Verstraete, W. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]
- Wang, J.; Snoeck, D.; Van Vlierberghe, S.; Verstraete, W.; De Belie, N. Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr. Build. Mater. 2014, 68, 110–119. [Google Scholar] [CrossRef]
- Wang, J.; Soens, H.; Verstraete, W.; De Belie, N. Self-healing concrete by use of microencapsulated bacterial spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357. [Google Scholar] [CrossRef]
- Achal, V.; Mukerjee, A.; Reddy, M.S. Biogenic treatment improves the durability and remediates the cracks of concrete structures. Constr. Build. Mater. 2013, 48, 1–5. [Google Scholar] [CrossRef]
- Mostavi, E.; Asadi, S.; Hassan, M.; Alansari, M. Evaluation of Self-Healing Mechanisms in Concrete with Double-Walled Sodium Silicate Microcapsules. J. Mater. Civ. Eng. 2015, 27, 04015035. [Google Scholar] [CrossRef]
- Kawaai, K.; Ujike, I.; Yamamoto, S.; Putri, P.Y. Some Considerations on Precipitation Rate of Calcium Carbonate in Bio- Based Materials Used for Concrete Repair; CRC Press: Thessaloniki, Greece, 2016. [Google Scholar]
- SKawasaki, S.; Murao, A.; Hiroyoshi, N.; Tsunekawa, M.; Kaneko, K. Fundamental Study on Novel Grout Cementing Due to Microbial Metabolism. J. Jpn. Soc. Eng. Geol. 2006, 47, 2–12. [Google Scholar] [CrossRef]
- Kubo, F.; Okazaki, S.; Ujike, I. Development of Microbial Metabolic Processes to Repair Concrete Joint Leakage. Adv. Mater. Res. 2013, 845, 158–162. [Google Scholar] [CrossRef]
- Ujike, I.; Kubo, F.; Kawaai, K.; Okazaki, S. Influencing Factors Affecting Microbial Metabolic Processes of Bio Materials Used for Leakage Parts in Concrete. In Proceedings of the 5th International Conference on Concrete Repair, Belfast, Northern Ireland, 1–3 September 2014; Volume 3, pp. 127–133. [Google Scholar]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H.M. Assessment of the functionality of bacteria-based repair system for concrete through ESEM analysis. In EMABM 2015: Proceedings of the 15th Euroseminar on Microscopy Applied to Building Materials, Delft, The Netherlands, 17–19 June 2015; Delft University of Technology: Delft, The Netherlands, 2015; Volume 3, pp. 165–169. [Google Scholar]
- Nan, Z.; Chen, X.; Yang, Q.; Wang, X.; Shi, Z.; Hou, W. Structure transition from aragonite to vaterite and calcite by the assistance of SDBS. J. Colloid Interface Sci. 2008, 325, 331–336. [Google Scholar] [CrossRef]
- Chen, S.-F.; Yu, S.-H.; Jiang, J.; Li, F.; Liu, Y. Polymorph Discrimination of CaCO3Mineral in an Ethanol/Water Solution: Formation of Complex Vaterite Superstructures and Aragonite Rods. Chem. Mater. 2006, 18, 115–122. [Google Scholar] [CrossRef]
- Yamamoto, S. Effect of Temperature on Calcium Carbonate Precipitation of Concrete Repair Materials Using Microbial Metabolism; Ehime University: Ehime, Japan, 2017. (In Japanese) [Google Scholar]
- International Center for Diffraction Data. The Powder Diffraction File (PDF)-4/Minerals; International Center for Diffraction Data: Newtown Square, PA, USA, 2015. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Putri, P.Y.; Kawaai, K.; Ujike, I.; Yamamoto, S. Effect of Temperature on Precipitation Rate of Calcium Carbonate Produced through Microbial Metabolic Process of Bio Materials. Civ. Eng. Dimens. 2016, 18, 103–108. [Google Scholar] [CrossRef][Green Version]
- Putri, P.Y.; Ujike, I.; Kawaai, K. Influence of the Type of Dry Yeast on Precipitation Rate of Calcium Carbonate in Bio-Based Repair Materials. In EcoGRAFI: Proceeding of 2nd International Conference on Bio-based Building Materials, France, 21–23 June 2017; RILEM Publications: Clermont-Ferrand, France, 2017; pp. 133–139. [Google Scholar]
- Putri, P.Y.; Kawaai, K.; Ujike, I.; Okuno, H. Influence of Different Concentration of Tris Buffer Solution on Calcium Carbonate Precipitation in Bio-based Repair Materials. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 1879. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. NIST Standard Reference Database Number 69. 2016. Available online: http://webbook.nist.gov/chemistry/ (accessed on 23 September 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).