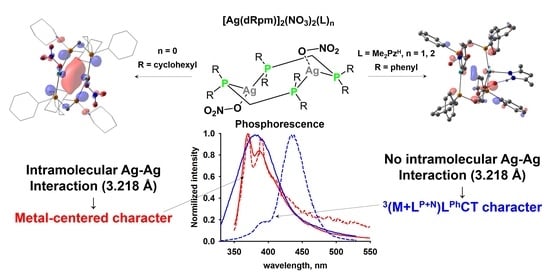
Dinuclear Silver(I) Nitrate Complexes with Bridging Bisphosphinomethanes: Argentophilicity and Luminescence
Abstract
1. Introduction
2. Materials and Methods
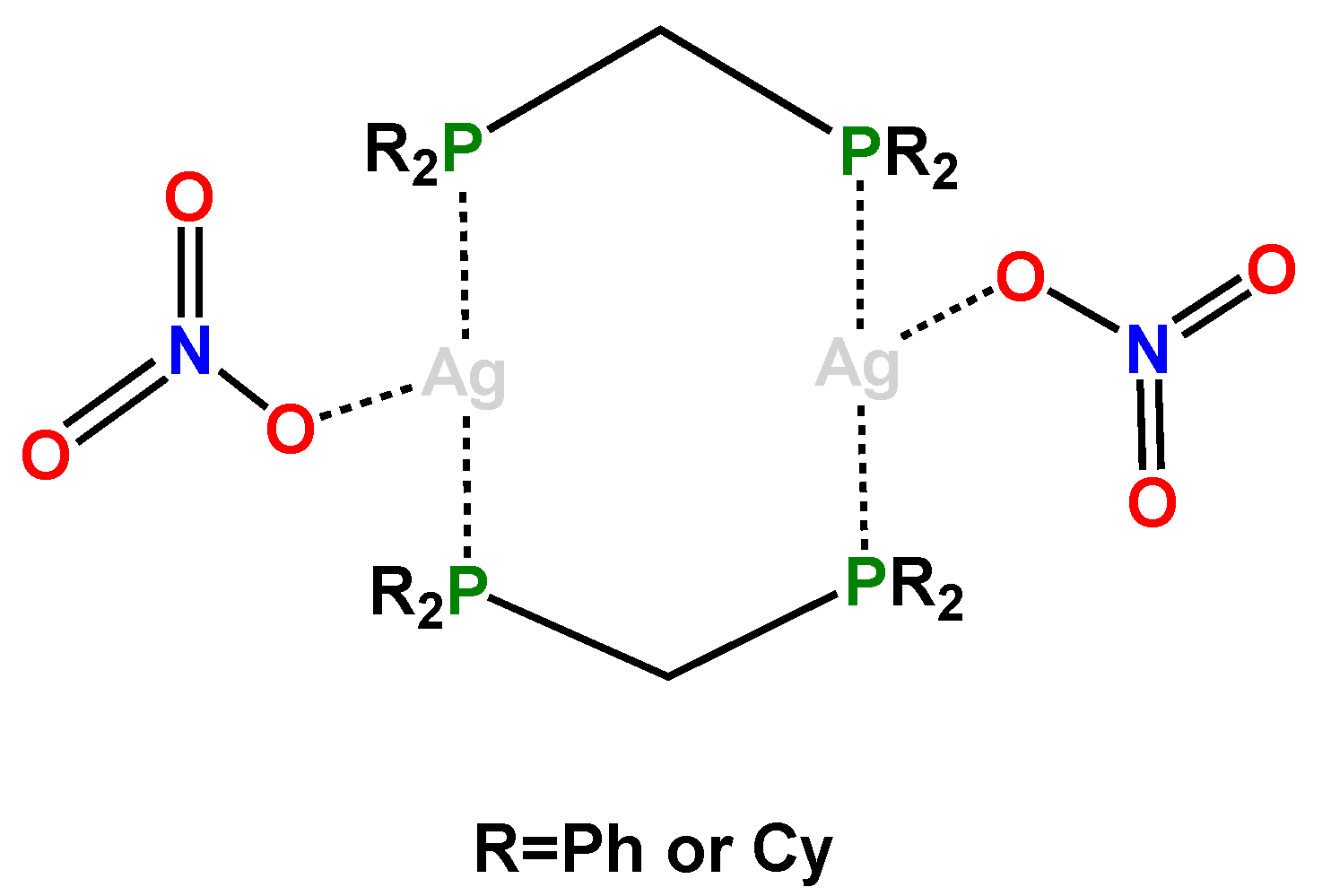
3. Results and Discussion
3.1. Synthetic Procedures
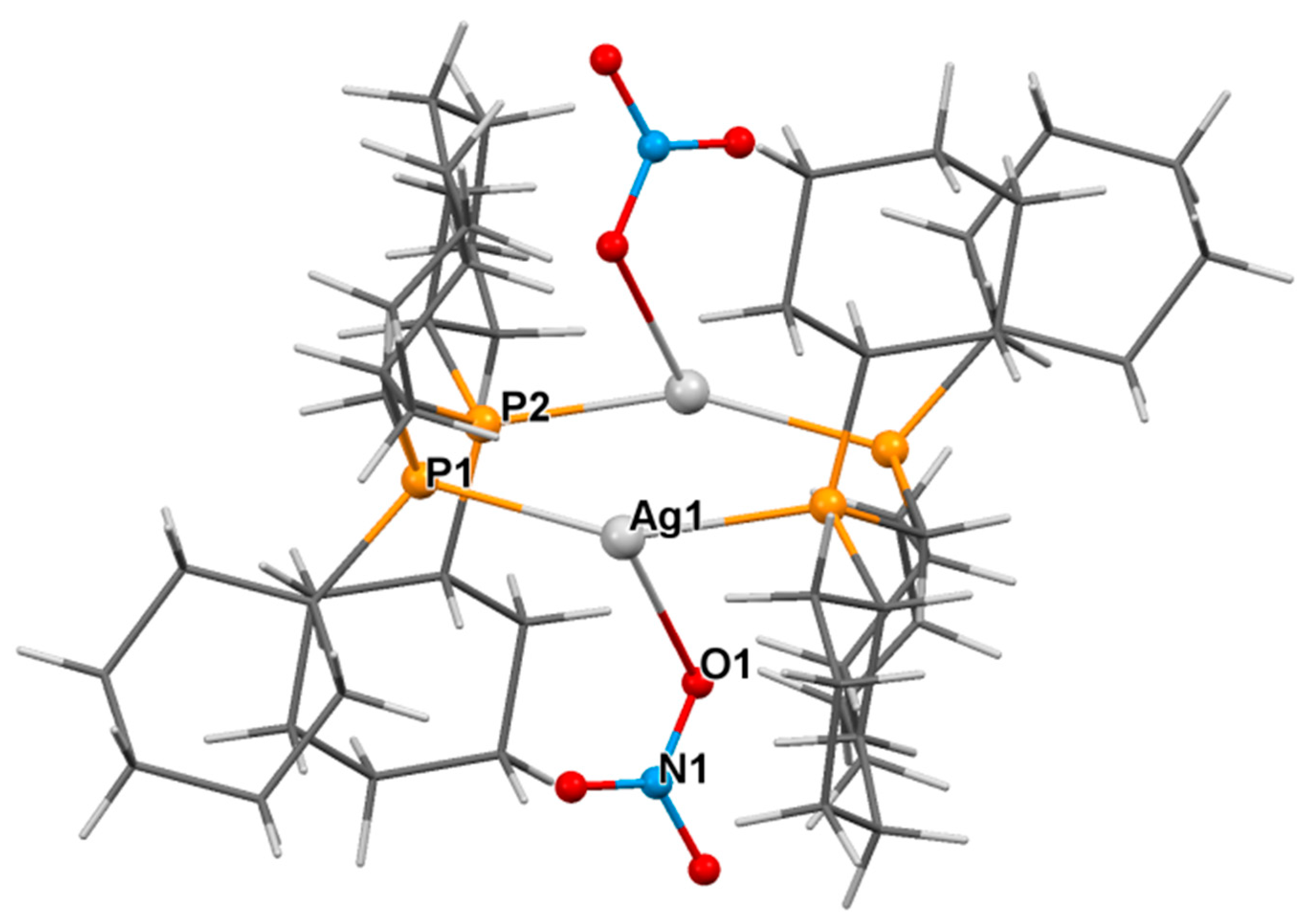
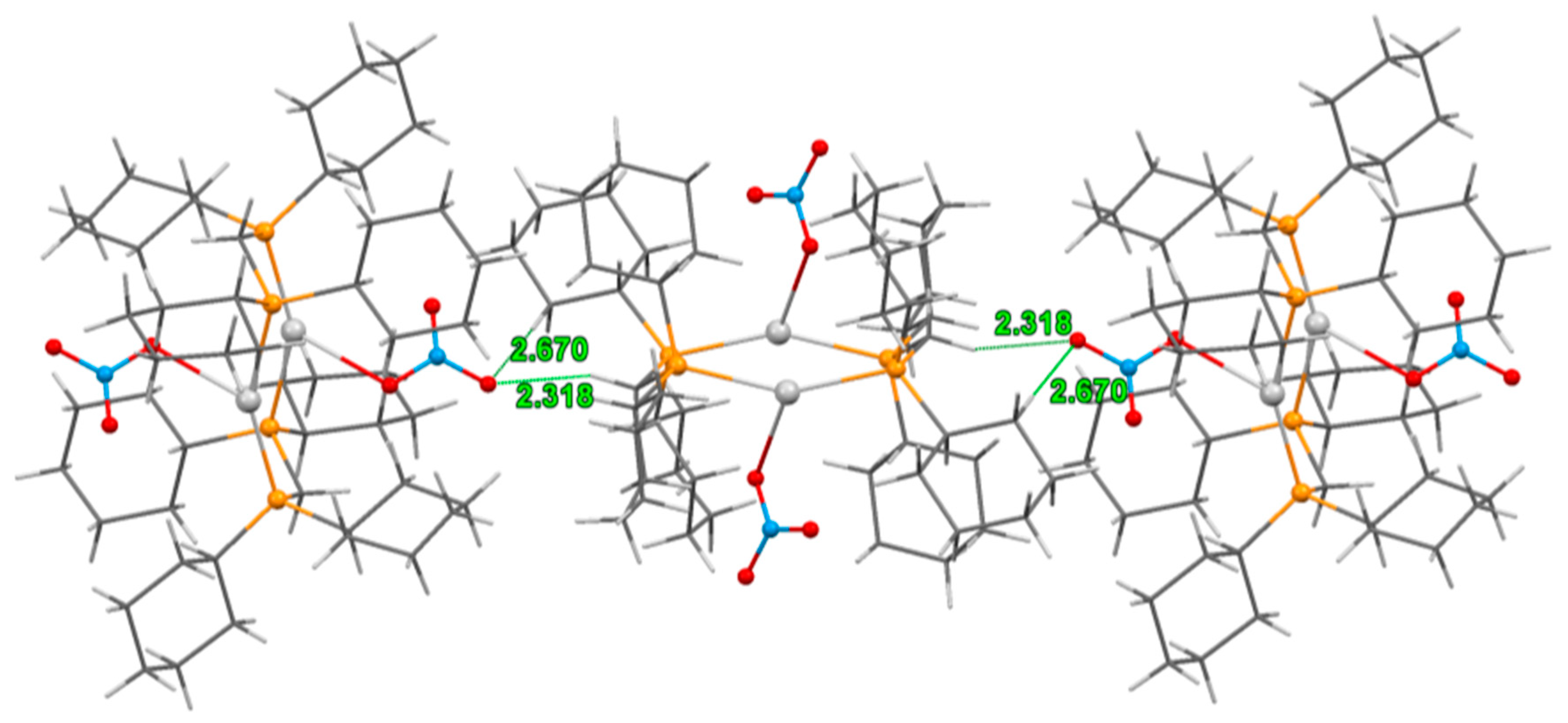
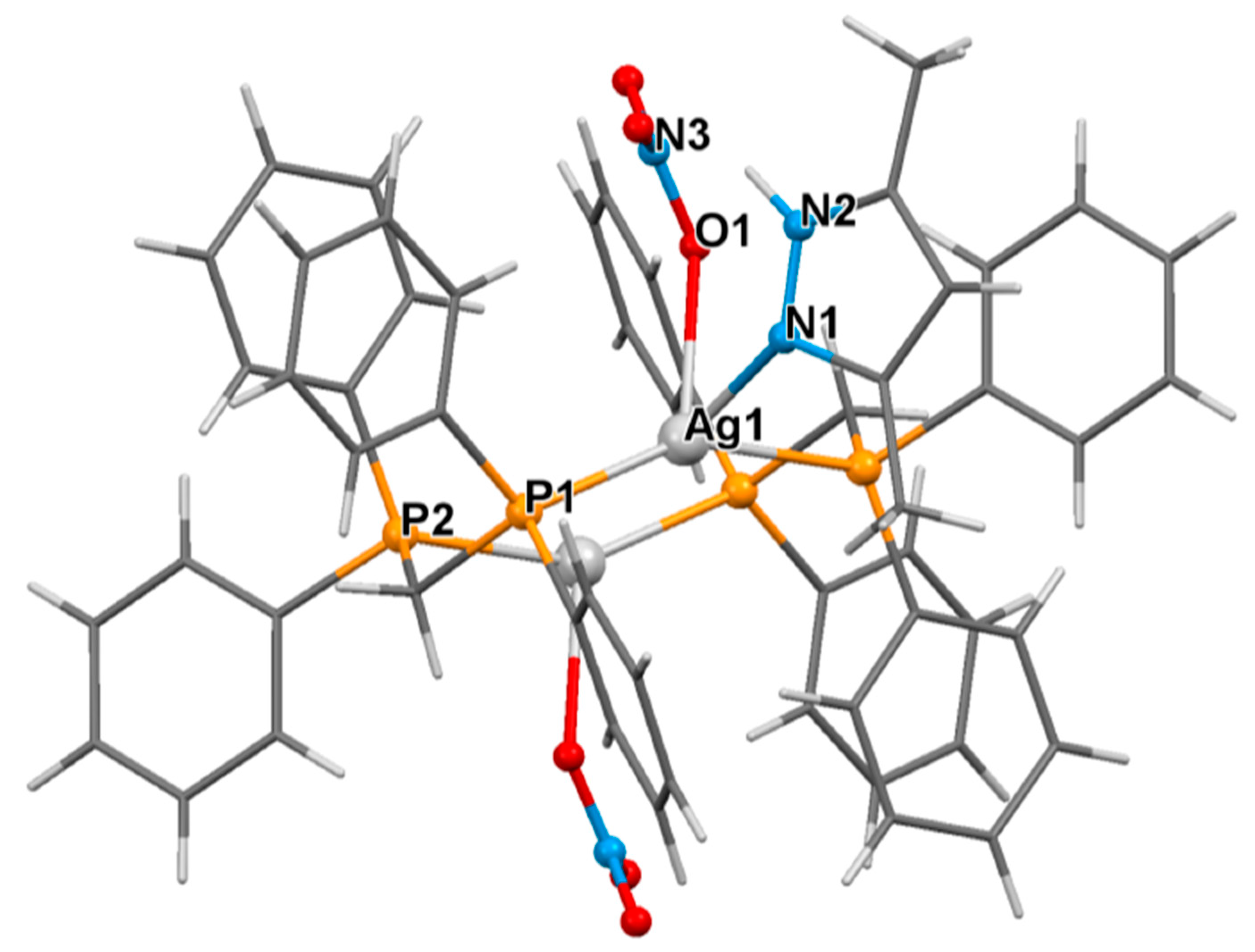
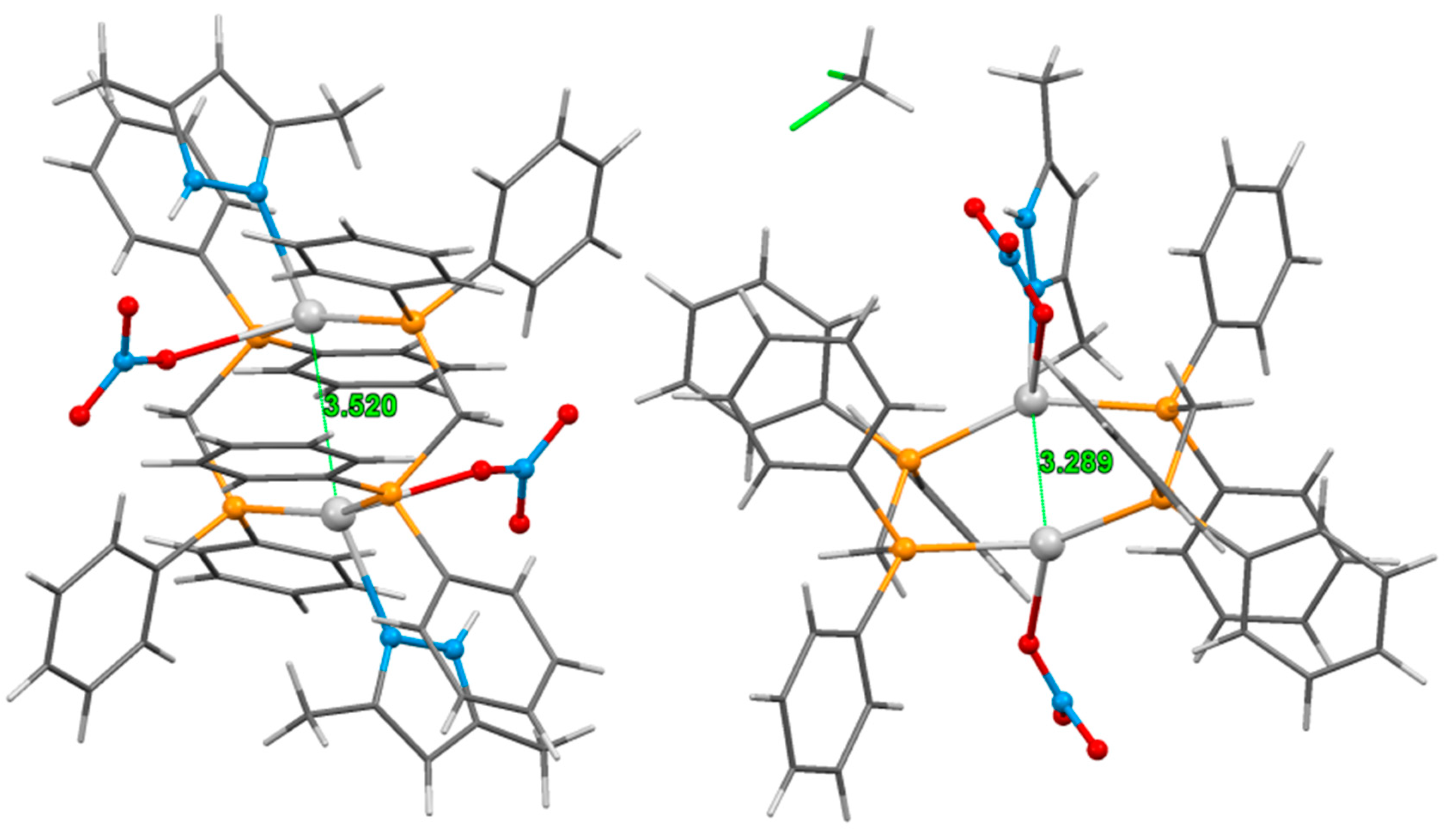
3.2. Crystal Structure of Complexes
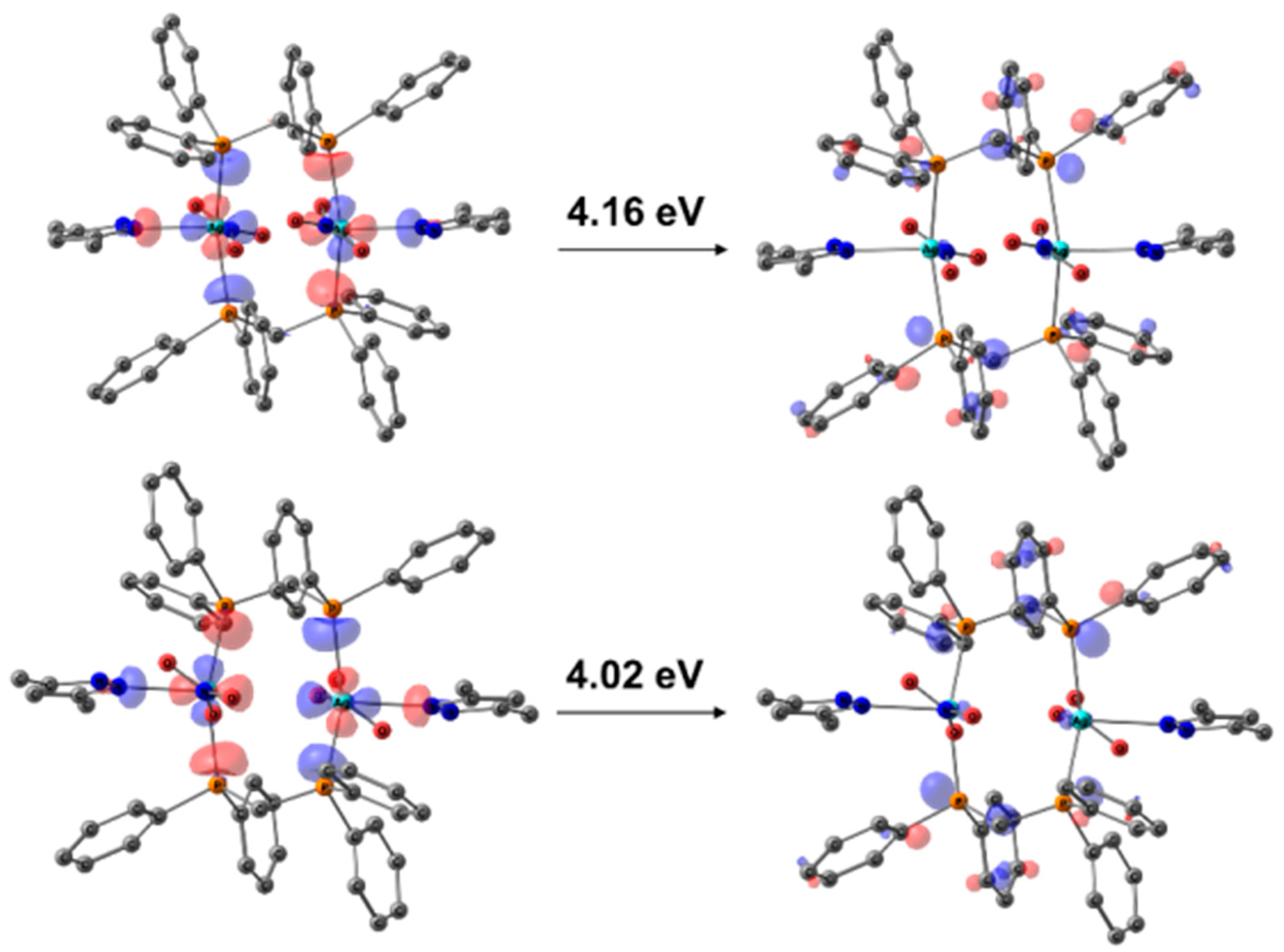
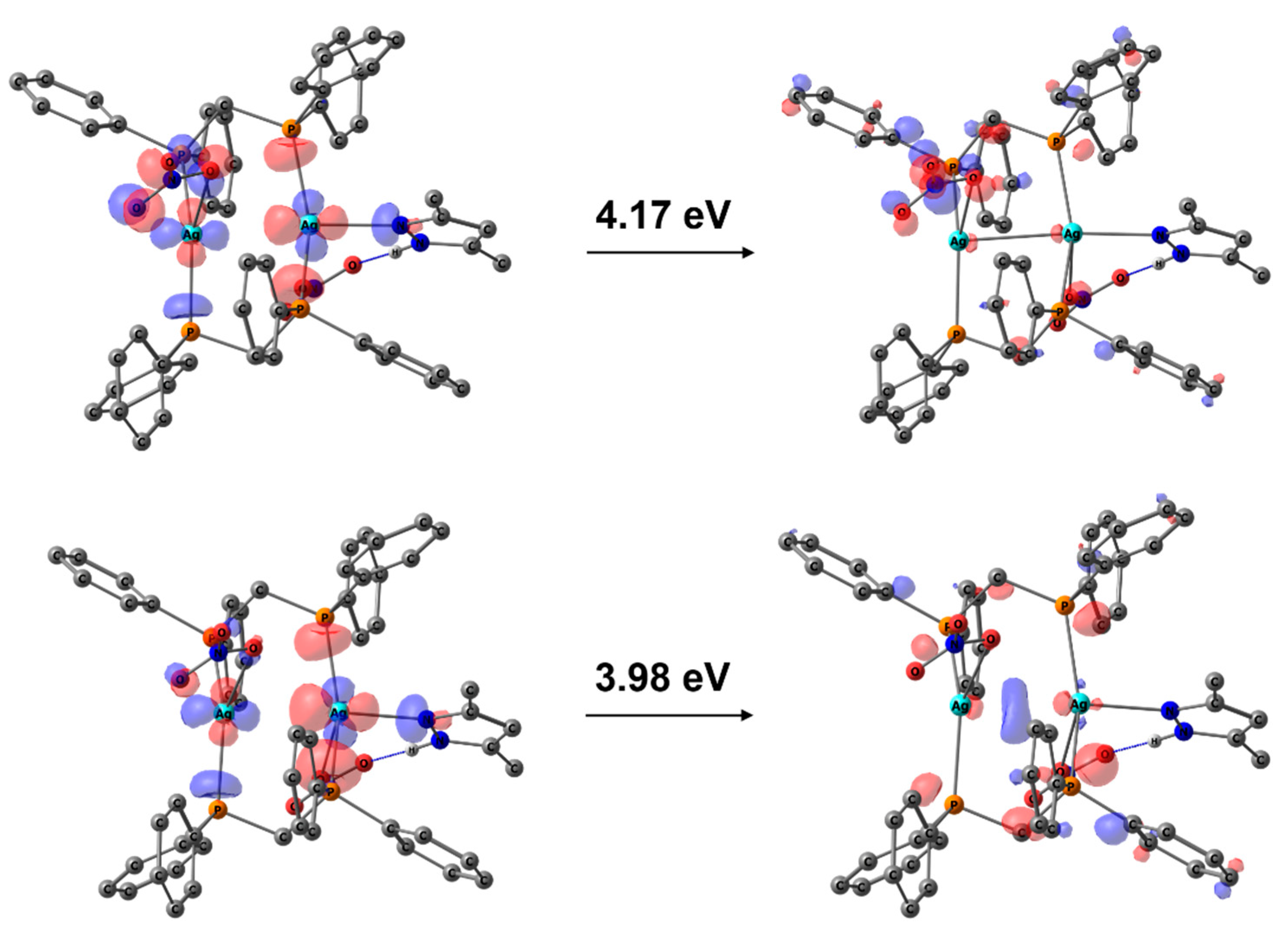
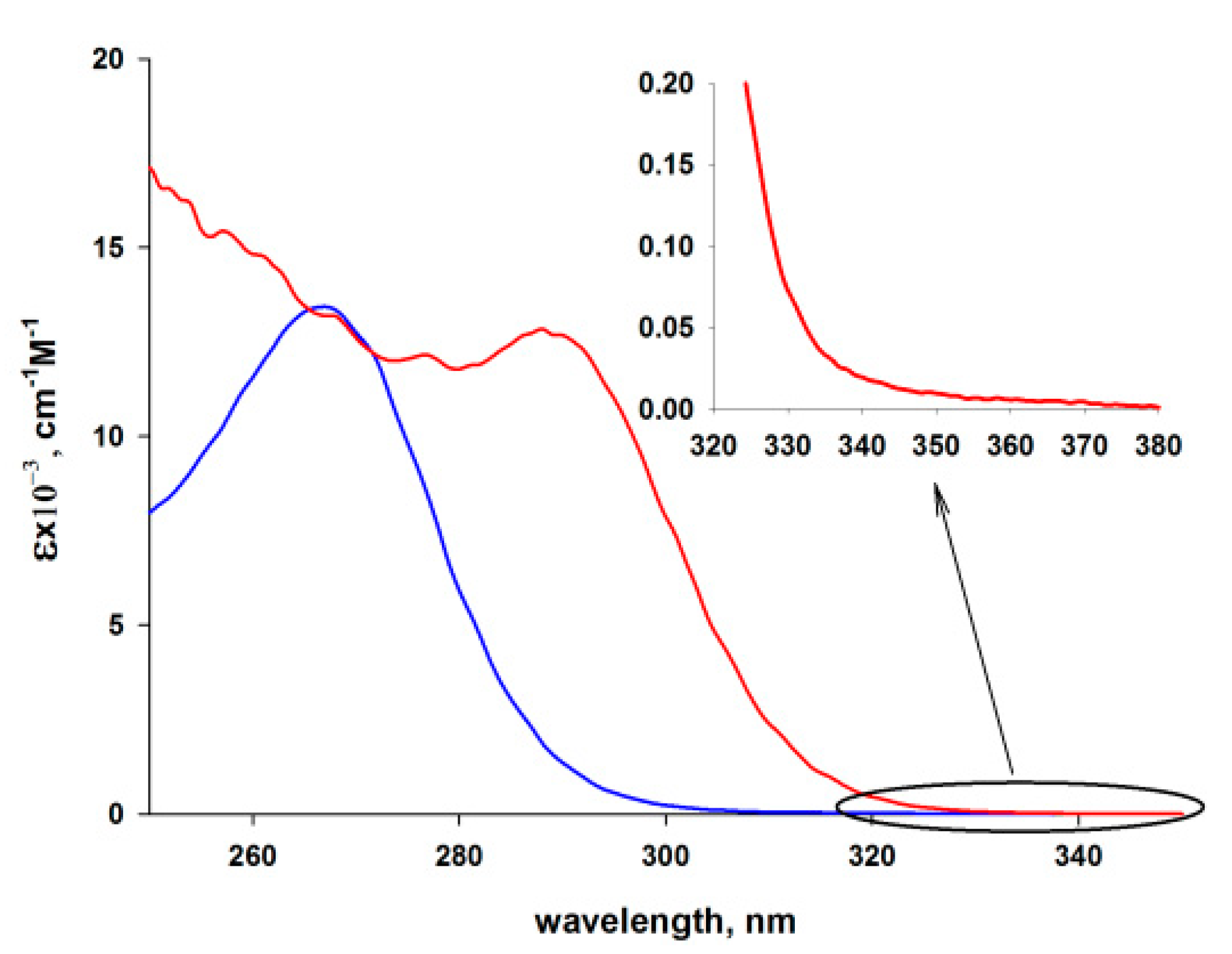
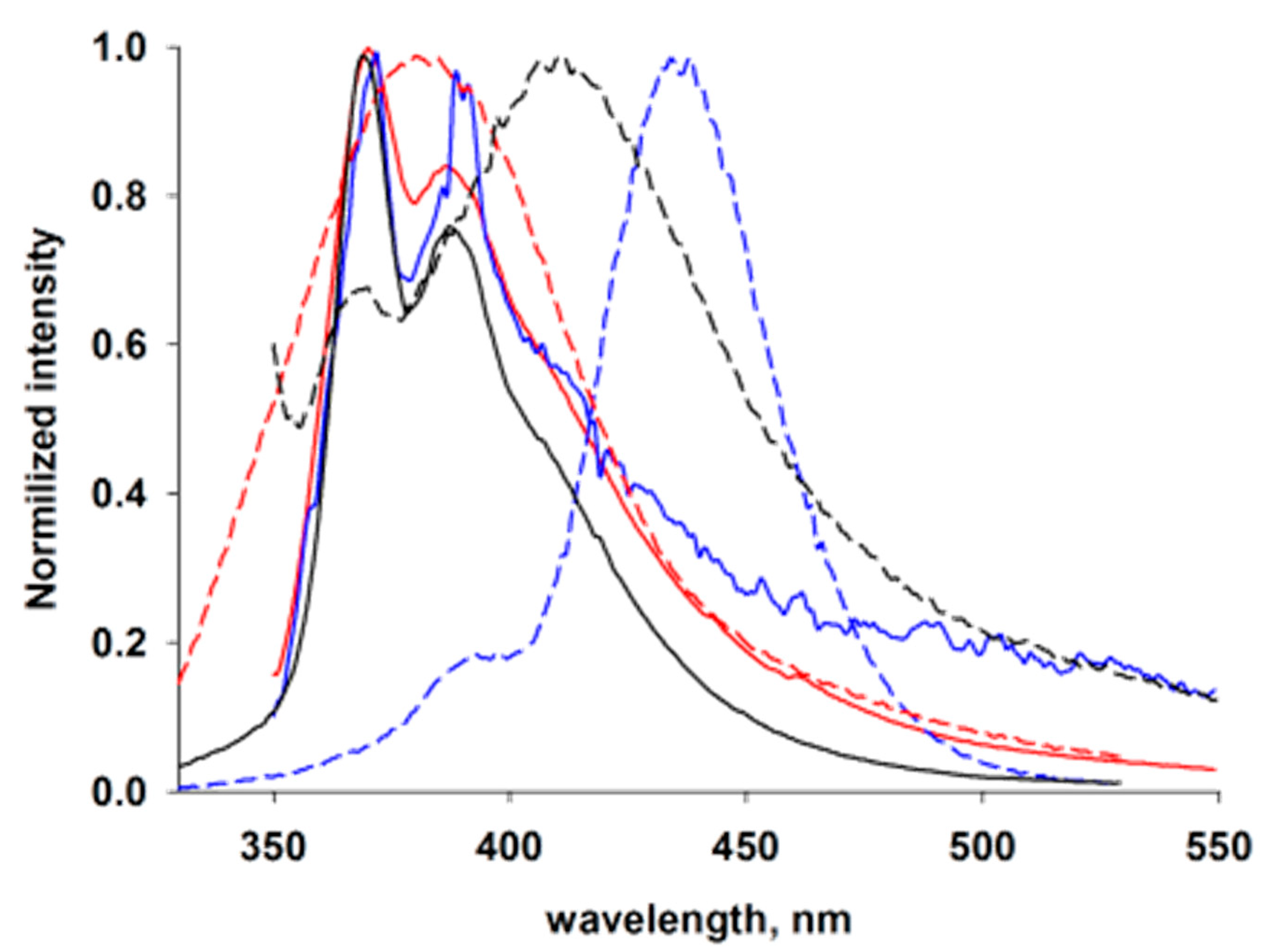
3.3. Photophysical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yam, V.W.; Au, V.K.; Leung, S.Y. Light-Emitting Self-Assembled Materials Based on d(8) and d(10) Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Argentophilic interactions. Angew. Chem. Int. Ed. 2015, 54, 746–784. [Google Scholar] [CrossRef]
- Dolan, N.S.; Scamp, R.J.; Yang, T.; Berry, J.F.; Schomaker, J.M. Catalyst-Controlled and Tunable, Chemoselective Silver-Catalyzed Intermolecular Nitrene Transfer: Experimental and Computational Studies. J. Am. Chem. Soc. 2016, 138, 14658–14667. [Google Scholar] [CrossRef]
- Alderson, J.M.; Corbin, J.R.; Schomaker, J.M. Tunable, Chemo- and Site-Selective Nitrene Transfer Reactions through the Rational Design of Silver(I) Catalysts. Acc. Chem. Res. 2017, 50, 2147–2158. [Google Scholar] [CrossRef]
- Mak, C.L.; Bostick, B.C.; Yassin, N.M.; Campbell, M.G. Argentophilic Interactions in Solution: An EXAFS Study of Silver(I) Nitrene Transfer Catalysts. Inorg. Chem. 2018, 57, 5720–5722. [Google Scholar] [CrossRef]
- Grachova, E.V. Design of Supramolecular Cluster Compounds of Copper Subgroup Metals Based on Polydentate Phosphine Ligands. Russ. J. Gen. Chem. 2019, 89, 1102–1114. [Google Scholar] [CrossRef]
- Ho, D.M.; Bau, R. Preparation and structural characterization of [Ag2(dpm)2(NO3)2] and [Ag4(dpm)4(NO3)2]2+[PF6]2−: Conformational flexibility in the M2P4 core structure of bis(diphenylphosphino)methane complexes. Inorg. Chem. 1983, 22, 4073–4079. [Google Scholar] [CrossRef]
- Che, C.-M.; Kwong, H.-L.; Yam, V.W.-W.; Cho, K.-C. Spectroscopic properties and redox chemistry of the phosphorescent excited state of [Au2(dppm)2]2+[dppm = bis(diphenylphosphino)methane]. J. Chem. Soc. Chem. Commun. 1989, 885–886. [Google Scholar] [CrossRef]
- Che, C.-M.; Tse, M.-C.; Chan, M.C.W.; Cheung, K.-K.; Phillips, D.L.; Leung, K.-H. Spectroscopic Evidence for Argentophilicity in Structurally Characterized Luminescent Binuclear Silver(I) Complexes. J. Am. Chem. Soc. 2000, 122, 2464–2468. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Lai, S.W.; Che, C.M.; Fu, W.F.; Zhou, Z.Y.; Zhu, N. Structural variations and spectroscopic properties of luminescent mono- and multinuclear silver(I) and copper(I) complexes bearing phosphine and cyanide ligands. Inorg. Chem. 2005, 44, 1511–1524. [Google Scholar] [CrossRef]
- Piché, D.; Harvey, P.D. The lowest energy excited states of the binuclear silver(I) halide complexes, Ag2(dmb)2X2. Metal-centered or charge transfer states? Canad. J. Chem. 1994, 72, 705–713. [Google Scholar] [CrossRef]
- Chan, J. Studies of metal binding reactions in metallothioneins by spectroscopic, molecular biology, and molecular modeling techniques. Coord. Chem. Rev. 2002, 233-234, 319–339. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, T.; Pan, H.; Yuan, Y.; Chen, L.; Liu, M.; Zhang, K.; Zhang, S.; Wu, P.; Xu, J. Photoemission mechanism of water-soluble silver nanoclusters: Ligand-to-metal-metal charge transfer vs strong coupling between surface plasmon and emitters. J. Am. Chem. Soc. 2014, 136, 1686–1689. [Google Scholar] [CrossRef]
- Yang, T.; Dai, S.; Tan, H.; Zong, Y.; Liu, Y.; Chen, J.; Zhang, K.; Wu, P.; Zhang, S.; Xu, J.; et al. Mechanism of Photoluminescence in Ag Nanoclusters: Metal-Centered Emission versus Synergistic Effect in Ligand-Centered Emission. J. Phys. Chem. C 2019, 123, 18638–18645. [Google Scholar] [CrossRef]
- Blake, A.J.; Donamaria, R.; Lippolis, V.; Lopez-de-Luzuriaga, J.M.; Manso, E.; Monge, M.; Olmos, M.E. Influence of crown thioether ligands in the structures and of perhalophenyl groups in the optical properties of complexes with argentoaurophilic interactions. Inorg. Chem. 2014, 53, 10471–10484. [Google Scholar] [CrossRef]
- Donamaria, R.; Lippolis, V.; Lopez-de-Luzuriaga, J.M.; Monge, M.; Nieddu, M.; Olmos, M.E. Influence of the Number of Metallophilic Interactions and Structures on the Optical Properties of Heterometallic Au/Ag Complexes with Mixed-Donor Macrocyclic Ligands. Inorg. Chem. 2018, 57, 11099–11112. [Google Scholar] [CrossRef]
- Ai, P.; Mauro, M.; Gourlaouen, C.; Carrara, S.; De Cola, L.; Tobon, Y.; Giovanella, U.; Botta, C.; Danopoulos, A.A.; Braunstein, P. Bonding, Luminescence, Metallophilicity in Linear Au3 and Au2Ag Chains Stabilized by Rigid Diphosphanyl NHC Ligands. Inorg. Chem. 2016, 55, 8527–8542. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Chen, R.; Tang, Y.; Wan, Y.; Chen, T.; Zheng, C.; Qi, Y.; Cheng, Y.; Huang, W. Promoting Singlet/triplet Exciton Transformation in Organic Optoelectronic Molecules: Role of Excited State Transition Configuration. Sci. Rep. 2017, 7, 6225. [Google Scholar] [CrossRef]
- Das, S.; Sharma, S.; Singh, H.B.; Butcher, R.J. Metallophilic Mercuraazamacrocycles Derived from Bis{6-formyl-(2,3,4-trimethoxy)phenyl}mercury: Reactivity with d10 and d8 Metal Ions. Eur. J. Inorg. Chem. 2018, 2018, 4093–4105. [Google Scholar] [CrossRef]
- Titov, A.A.; Filippov, O.A.; Smol’yakov, A.F.; Averin, A.A.; Shubina, E.S. Synthesis, structures and luminescence of multinuclear silver(I) pyrazolate adducts with 1,10-phenanthroline derivatives. Dalton Trans. 2019, 48, 8410–8417. [Google Scholar] [CrossRef]
- Emashova, S.K.; Titov, A.A.; Filippov, O.A.; Smol’yakov, A.F.; Titova, E.M.; Epstein, L.M.; Shubina, E.S. Luminescent AgI Complexes with 2,2′-Bipyridine Derivatives Featuring [Ag-(CF3)2Pyrazolate]4 Units. Eur. J. Inorg. Chem. 2019, 2019, 4855–4861. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydrogen bridges in crystal engineering: Interactions without borders. Acc. Chem. Res. 2002, 35, 565–573. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Bis-μ-[methylenebis(diphenylphosphine)]-dinitratodisilver(I) dichloroform solvate. Acta Crystallogr. Sect. C. 1990, 46, 235–238. [Google Scholar] [CrossRef]
- Cui, Y.-Z.; Yuan, Y.; Li, Z.-F.; Liu, M.; Jin, Q.-H.; Jiang, N.; Cui, L.-N.; Gao, S. From ring, chain to network: Synthesis, characterization, luminescent properties of silver(I) complexes constructed by diphosphine ligands and various N-donor ligands. Polyhedron 2016, 112, 118–129. [Google Scholar] [CrossRef]
- Qiu, Q.-M.; Huang, X.; Zhao, Y.-H.; Liu, M.; Jin, Q.-H.; Li, Z.-F.; Zhang, Z.-W.; Zhang, C.-L.; Meng, Q.-X. Synthesis, structure and terahertz spectra of six Ag(I) complexes of bis(diphenylphosphino)methane with 4,4′-bipyridine and its derivations. Polyhedron 2014, 83, 16–23. [Google Scholar] [CrossRef]
- Titov, A.A.; Filippov, O.A.; Smol’yakov, A.F.; Baranova, K.F.; Titova, E.M.; Averin, A.A.; Shubina, E.S. Dinuclear Cu(I) and Ag(I) Pyrazolates Supported with Tertiary Phosphines: Synthesis, Structures, and Photophysical Properties. Eur. J. Inorg. Chem. 2019, 2019, 821–827. [Google Scholar] [CrossRef]
- Titov, A.A.; Filippov, O.A.; Smol’yakov, A.F.; Averin, A.A.; Shubina, E.S. Copper(I) complex with BINAP and 3,5-dimethylpyrazole: Synthesis and photoluminescent properties. Mendeleev Commun. 2019, 29, 570–572. [Google Scholar] [CrossRef]
- Titov, A.A.; Filippov, O.A.; Smol’yakov, A.F.; Godovikov, I.A.; Shakirova, J.R.; Tunik, S.P.; Podkorytov, I.S.; Shubina, E.S. Luminescent Complexes of the Trinuclear Silver(I) and Copper(I) Pyrazolates Supported with Bis(diphenylphosphino)methane. Inorg. Chem. 2019, 58, 8645–8656. [Google Scholar] [CrossRef]

| 1 | 2a | 2a/2b | |
|---|---|---|---|
| Empirical Formula | C₂₅H₄₆AgNO₃P₂ | C₅₅H₅₃Ag₂N₄O₆P₄ | C117H116Ag4Cl₂N₅O₆P₄ |
| Formula weight | 578.44 | 1205.63 | 2675.24 |
| Diffractometer | Bruker SMART APEX CCD | Bruker SMART APEX CCD | Bruker SMART APEX CCD |
| Scan mode | ϕ and ω scans | ϕ and ω scans | ω and ϕ scans |
| Anode [Wavelength, Å] | MoKα [0.71073] sealed tube | MoKα [0.71073] sealed tube | MoKα [0.71073] sealed tube |
| Crystal Dimensions, mm | 0.1 × 0.15 × 0.36 | 0.08 × 0.11 × 0.15 | 0.08 × 0.12 × 0.44 |
| Crystal color | colorless | colorless | colorless |
| Crystal system | tetragonal | monoclinic | triclinic |
| a, Å | 29.8637(11) | 10.940(2) | 10.9934(13) |
| b, Å | 29.8637(11) | 19.805(4) | 11.9540(14) |
| c, Å | 12.6441(5) | 11.719(2) | 22.858(3) |
| α, deg | 90 | 90 | 77.727(2) |
| β, deg | 90 | 91.087(4) | 82.796(2) |
| γ, deg | 90 | 90 | 85.688(2) |
| Volume, ų | 11276.5(9) | 2538.5(9) | 2908.5(6) |
| Density, g cm⁻³ | 1.363 | 1.577 | 1.527 |
| Temperature, K | 120 | 120 | 120 |
| Tmin/Tmax | 0.7056/0.7461 | 0.6339/0.7460 | 0.6141/0.7459 |
| μ, mm⁻¹ | 0.853 | 0.953 | 0.930 |
| Space group | I4₁/a | P2₁/n | P1 |
| Z | 16 | 2 | 2 |
| F(000) | 4864 | 1226 | 1360 |
| Reflections collected | 54587 | 23611 | 37353 |
| Independent reflections | 5546 | 4995 | 16342 |
| Reflections (I > 2σ (I)) | 4576 | 3545 | 9421 |
| Parameters | 234 | 308 | 740 |
| Rint | 0.0350 | 0.1053 | 0.0860 |
| 2θmin–2θmax | 2.728–51.998 | 4.040–52.000 | 3.670–59.410 |
| wR₂ (all reflections) | 0.2032 | 0.1571 | 0.1221 |
| R₁ (I > σ (I)) | 0.0717 | 0.0866 | 0.0561 |
| GOF | 1.027 | 1.220 | 0.972 |
| ρmin/ρmₐₓ, eÅ⁻³ | −1.042/1.733 | −1.338/0.927 | −1.011/1.385 |
| Bonds Lengths | Angles | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | ||||
| Ag1-P1 | 2.396(2) | Ag1-P1 | 2.403(2) | Ag1-Ag1-P2 | 82.90(4) | Ag1-Ag1-P2 | 81.59(5) |
| Ag1-O1 | 2.557(9) | Ag1-O1 | 2.554(6) | Ag1-P1-C1 | 111.1(2) | Ag1-O1-N3 | 129.8(5) |
| Ag1-Ag1 | 2.9810(7) | Ag1-N1 | 2.443(6) | Ag1-P1-C2 | 115.5(2) | Ag1-N1-N2 | 124.7(5) |
| Ag1-P2 | 2.428(2) | Ag1-Ag1 | 3.218(1) | Ag1-P1-C8 | 113.2(3) | P1-Ag1-O1 | 126.5(1) |
| P1-C1 | 1.838(8) | Ag1-P2 | 2.442(2) | Ag1-O1-N1 | 106.9(5) | P1-Ag1-N1 | 98.6(2) |
| P1-C2 | 1.850(6) | P1-C4 | 1.846(8) | P1-Ag1-O1 | 119.1(2) | P1-Ag1-Ag1 | 88.90(5) |
| P1-C8 | 1.85(1) | P1-C17 | 1.801(8) | P1-Ag1-Ag1 | 94.21(4) | P1-Ag1-P2 | 151.61(8) |
| P2-C1 | 1.827(7) | P1-C23 | 1.828(8) | P1-C1-P2 | 114.4(3) | O1-Ag1-N1 | 84.1(2) |
| P2-C17 | 1.84(1) | P2-C4 | 1.822(8) | P1-Ag1-P2 | 152.22(6) | O1-Ag1-Ag1 | 91.5(1) |
| P2-C23 | 1.844(6) | P2-C5 | 1.827(8) | O1-Ag1-Ag1 | 74.4(2) | O1-Ag1-P2 | 80.7(1) |
| O1-N1 | 1.27(1) | P2-C11 | 1.804(8) | O1-Ag1-P2 | 86.9(2) | N1-Ag1-Ag1 | 172.5(2) |
| O2-N1 | 1.244(8) | P2-Ag1 | 2.442(2) | N1-Ag1-P2 | 91.7(2) | ||
| O3-N1 | 1.12 (1) | O1-N3 | 1.25(1) | N2-N1-C1 | 101.8(6) | ||
| O2-N3 | 1.24(1) | ||||||
| O3-N3 | 1.24(1) | ||||||
| N1-N2 | 1.31(1) | ||||||
| N1-C1 | 1.50(1) | ||||||
| N1-C2 | 1.58(1) | ||||||
| N2-C3 | 1.34(1) | ||||||
| N2-C2 | 1.02(1) | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranova, K.F.; Titov, A.A.; Filippov, O.A.; Smol’yakov, A.F.; Averin, A.A.; Shubina, E.S. Dinuclear Silver(I) Nitrate Complexes with Bridging Bisphosphinomethanes: Argentophilicity and Luminescence. Crystals 2020, 10, 881. https://doi.org/10.3390/cryst10100881
Baranova KF, Titov AA, Filippov OA, Smol’yakov AF, Averin AA, Shubina ES. Dinuclear Silver(I) Nitrate Complexes with Bridging Bisphosphinomethanes: Argentophilicity and Luminescence. Crystals. 2020; 10(10):881. https://doi.org/10.3390/cryst10100881
Chicago/Turabian StyleBaranova, Kristina F., Aleksei A. Titov, Oleg A. Filippov, Alexander F. Smol’yakov, Alexey A. Averin, and Elena S. Shubina. 2020. "Dinuclear Silver(I) Nitrate Complexes with Bridging Bisphosphinomethanes: Argentophilicity and Luminescence" Crystals 10, no. 10: 881. https://doi.org/10.3390/cryst10100881
APA StyleBaranova, K. F., Titov, A. A., Filippov, O. A., Smol’yakov, A. F., Averin, A. A., & Shubina, E. S. (2020). Dinuclear Silver(I) Nitrate Complexes with Bridging Bisphosphinomethanes: Argentophilicity and Luminescence. Crystals, 10(10), 881. https://doi.org/10.3390/cryst10100881