Hierarchical PtIn/Mg(Al)O Derived from Reconstructed PtIn-hydrotalcite-like Compounds for Highly Efficient Propane Dehydrogenation
Abstract
:1. Introduction
2. Results and Discussion
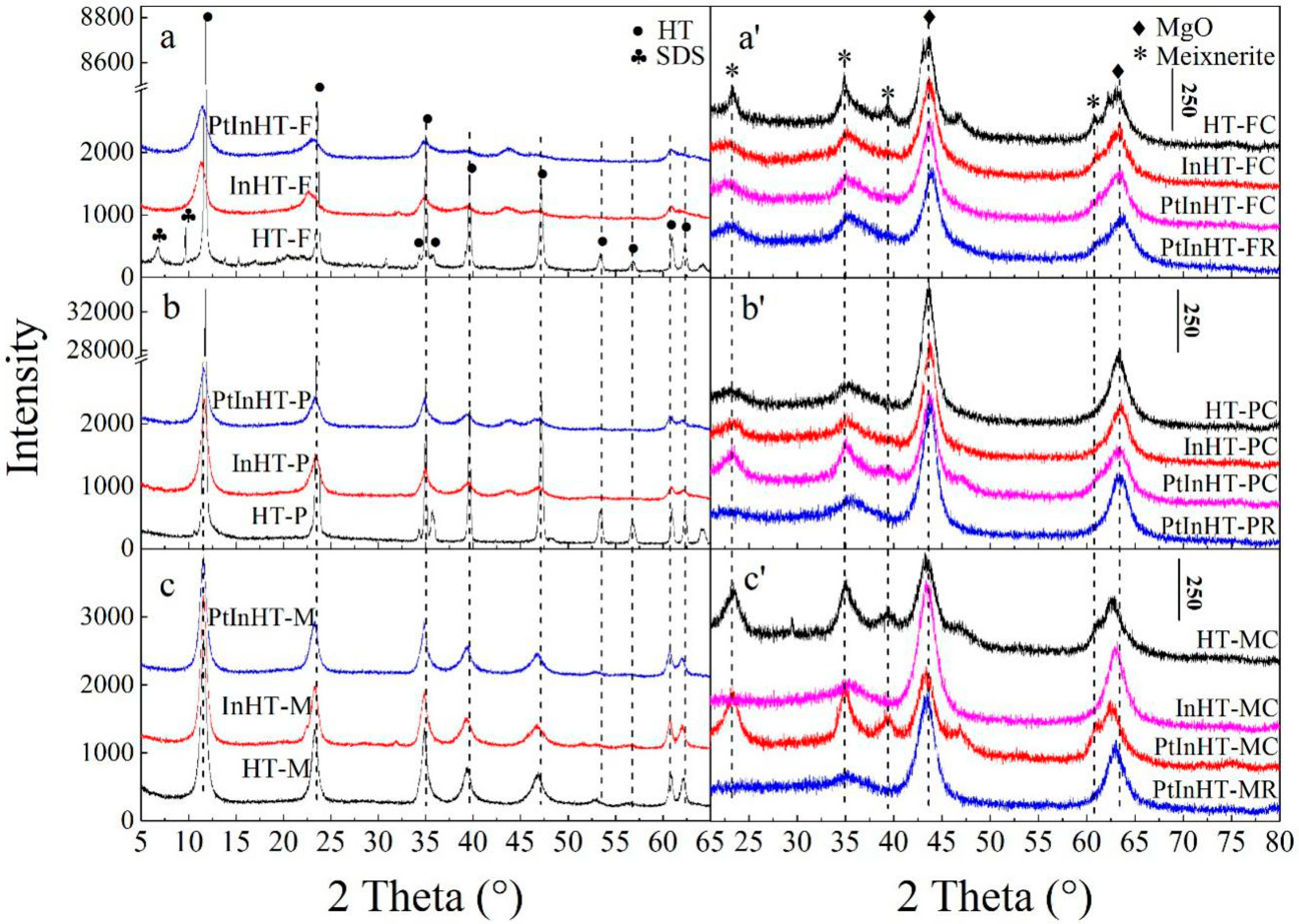
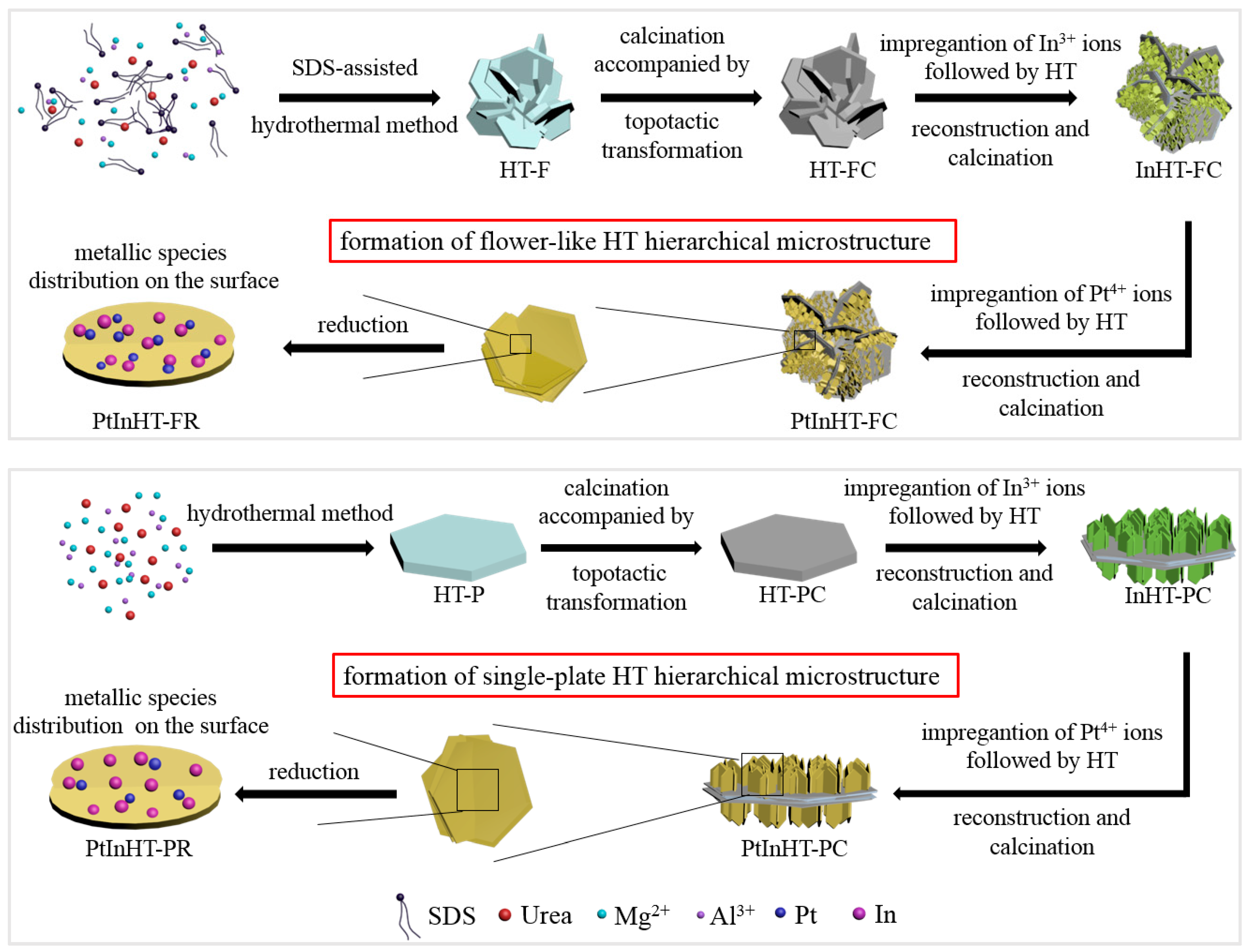
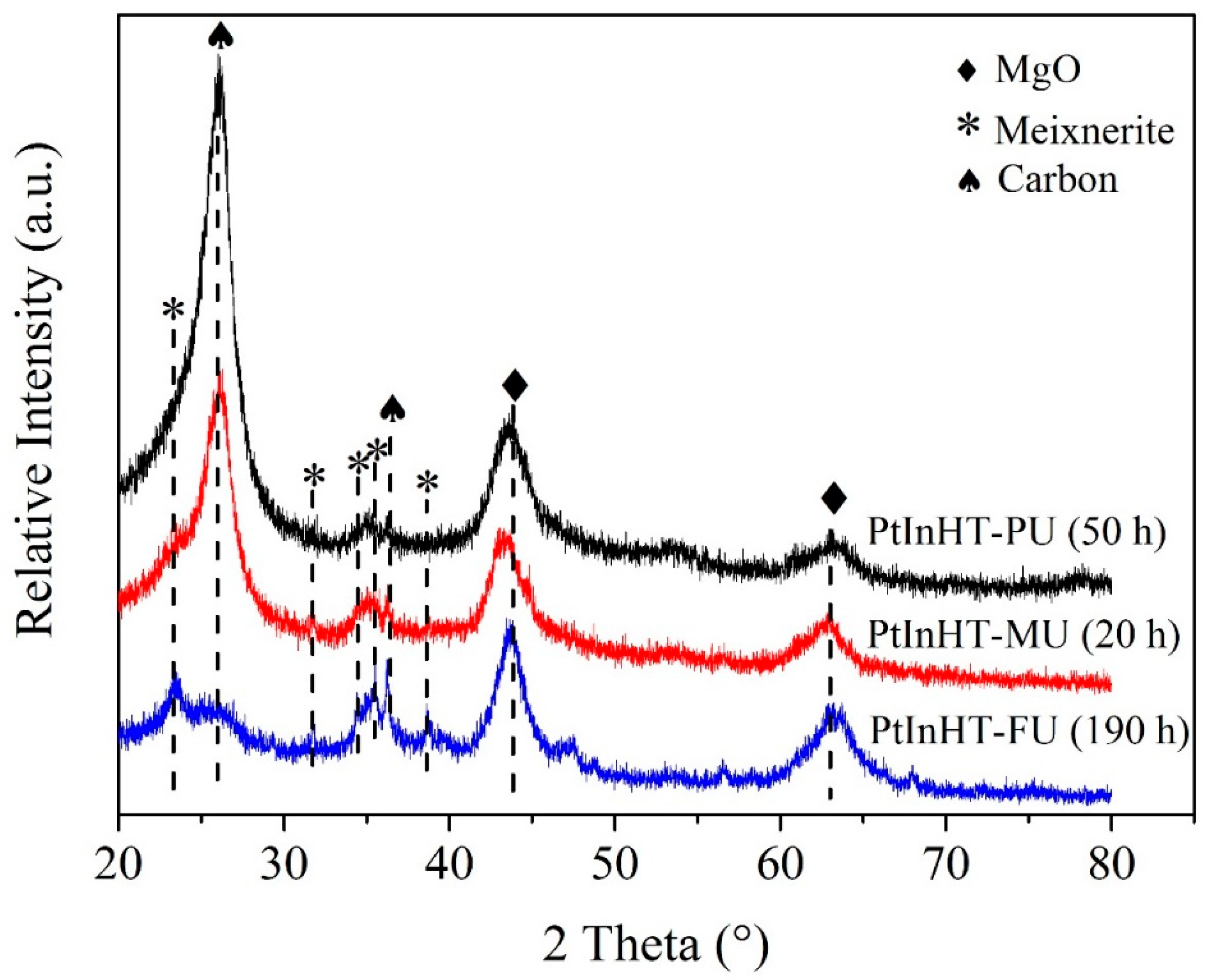
2.1. Phase Structure and Reconstruction Study
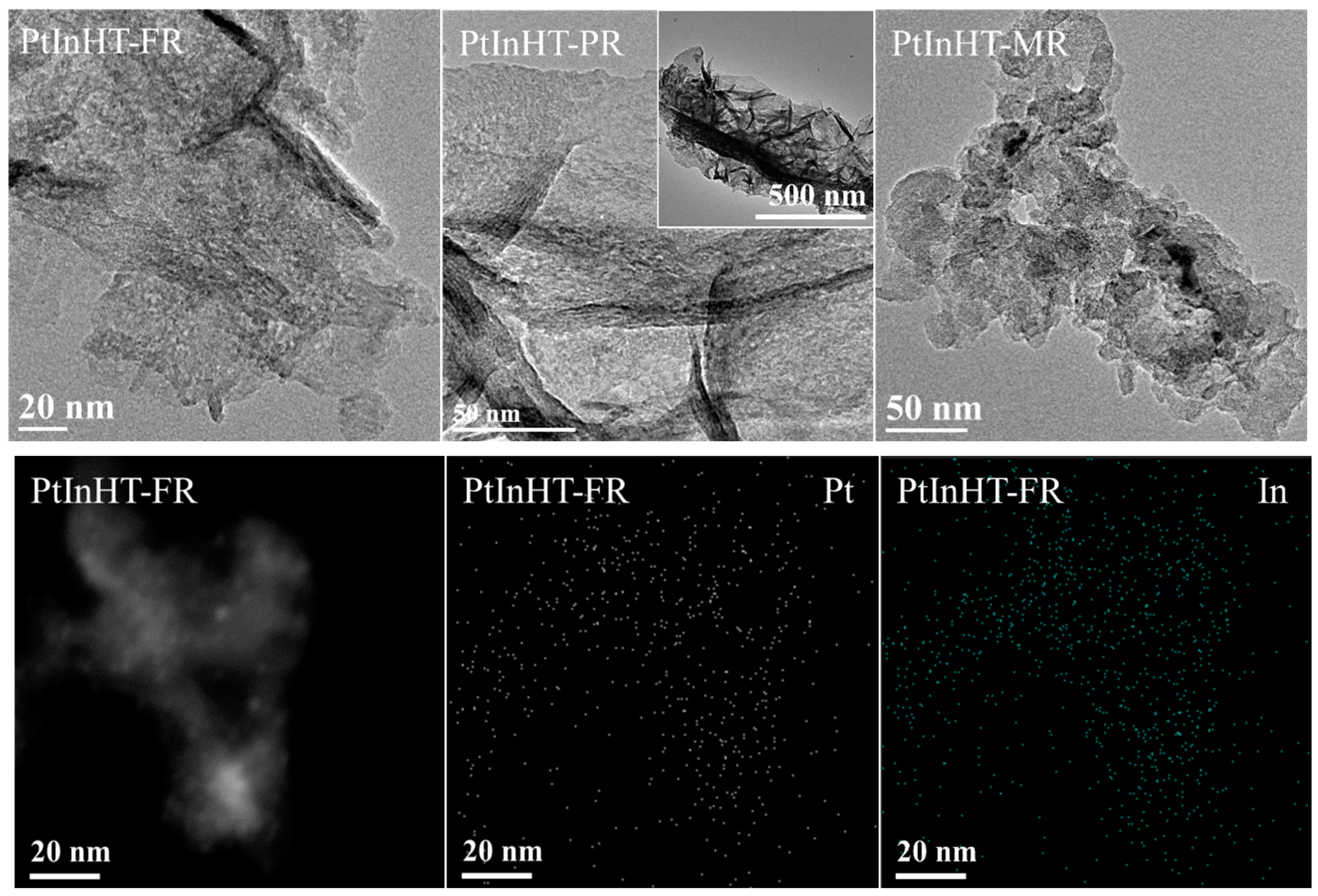
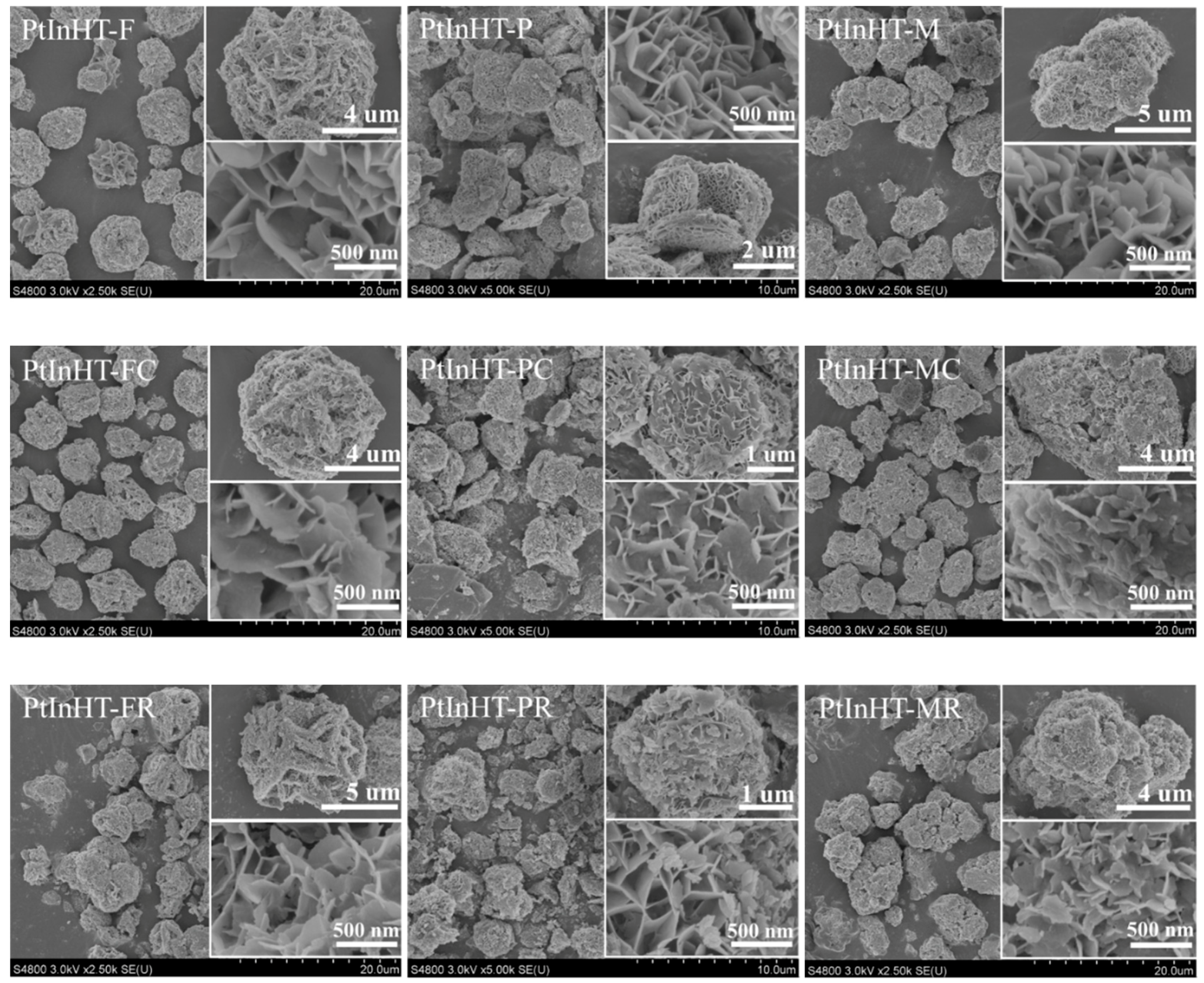
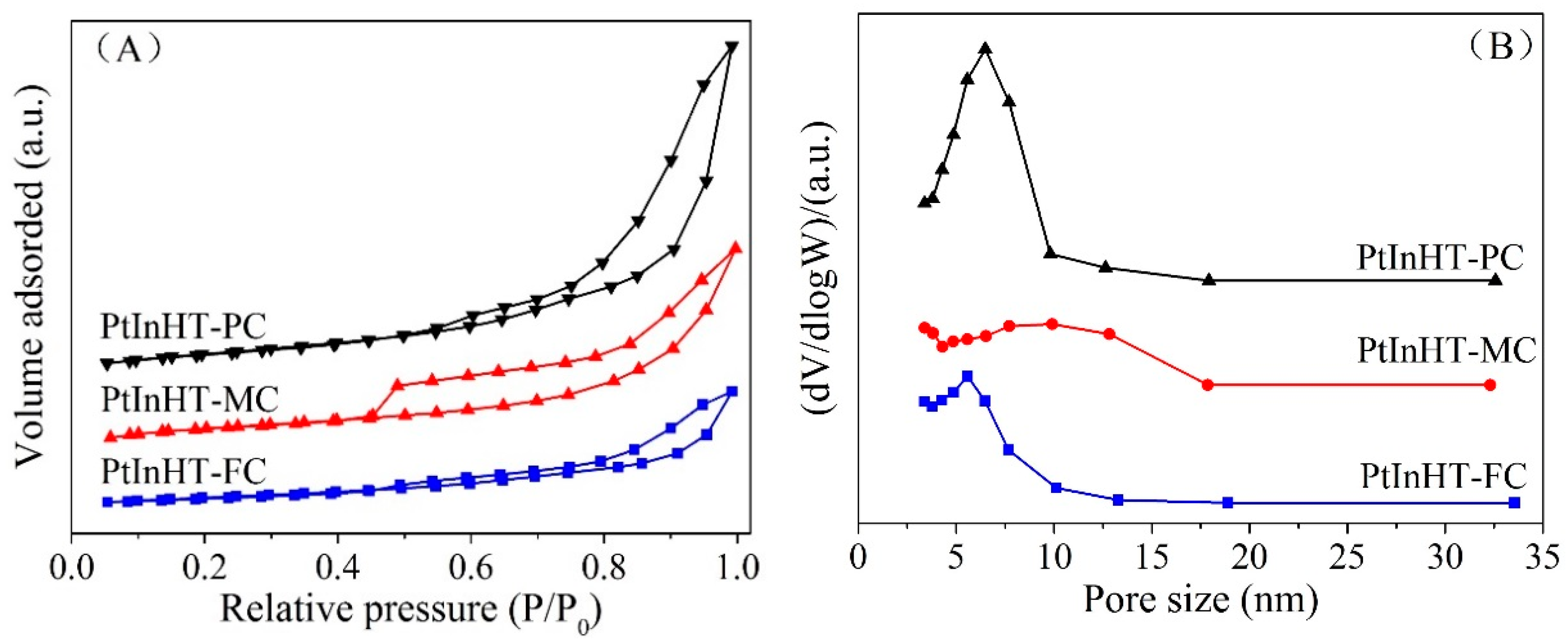
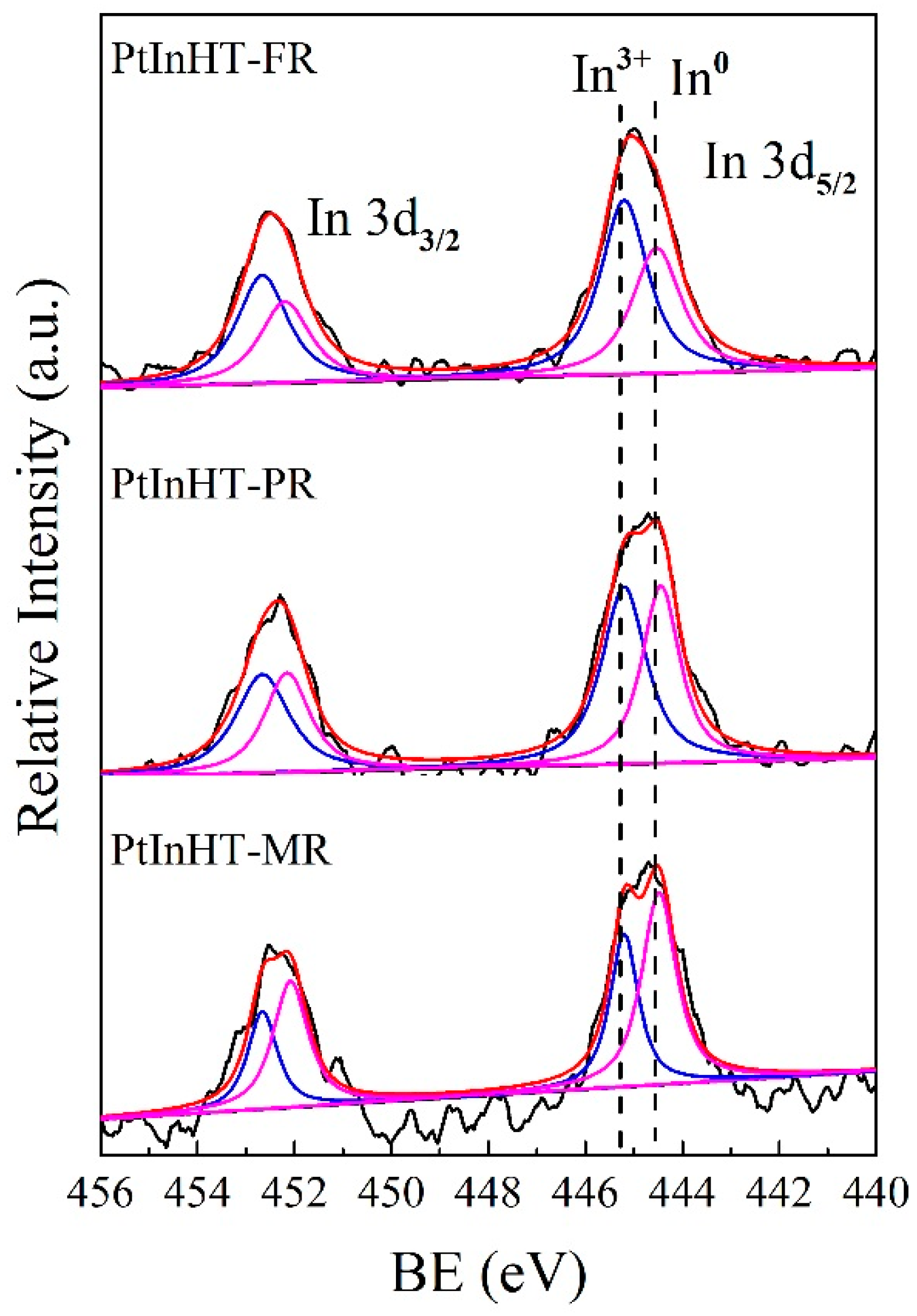
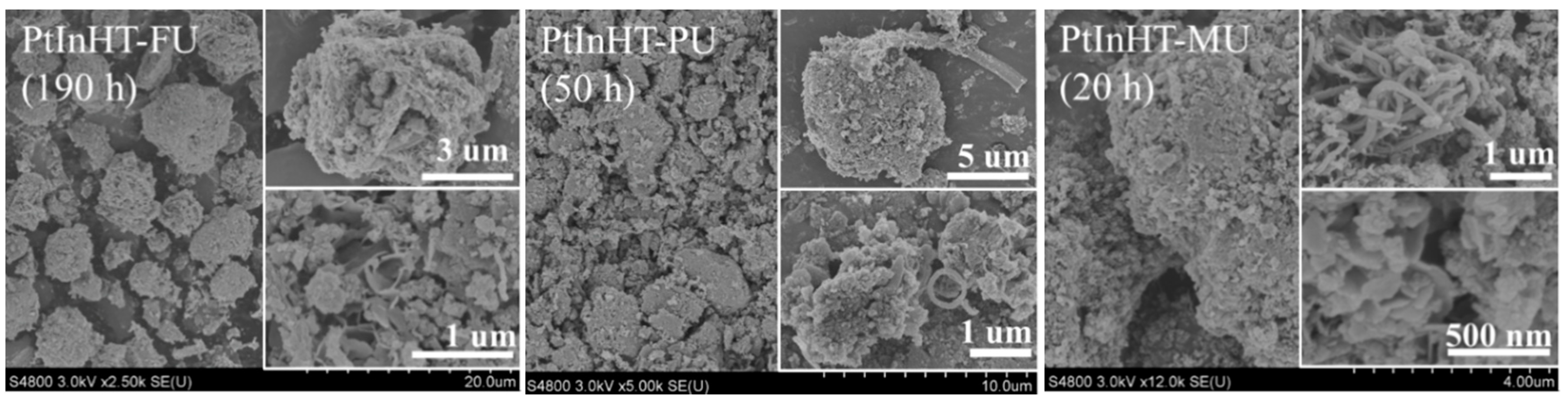
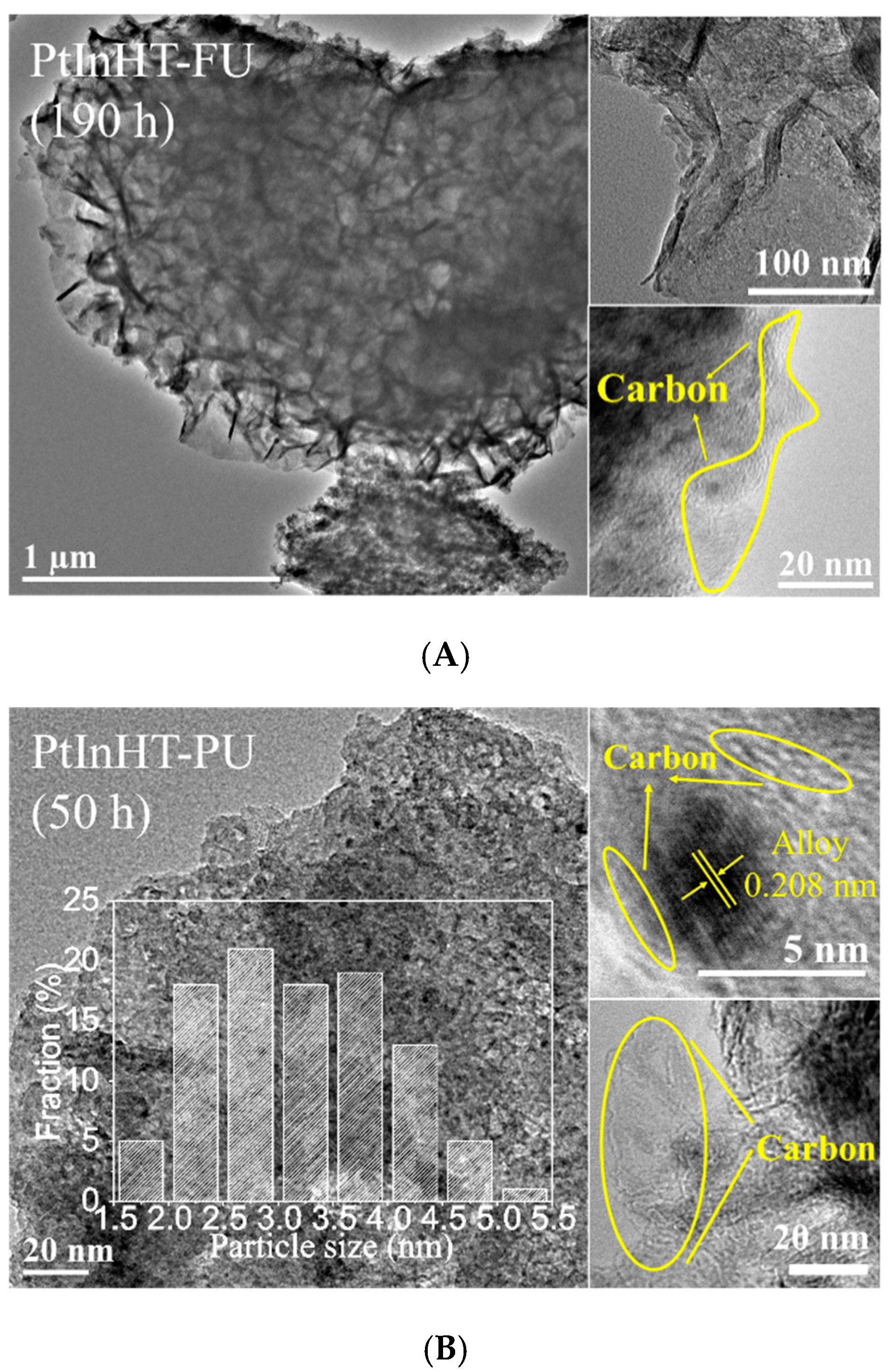
2.2. Morphology, Texture and Pt–In Interaction Analysis
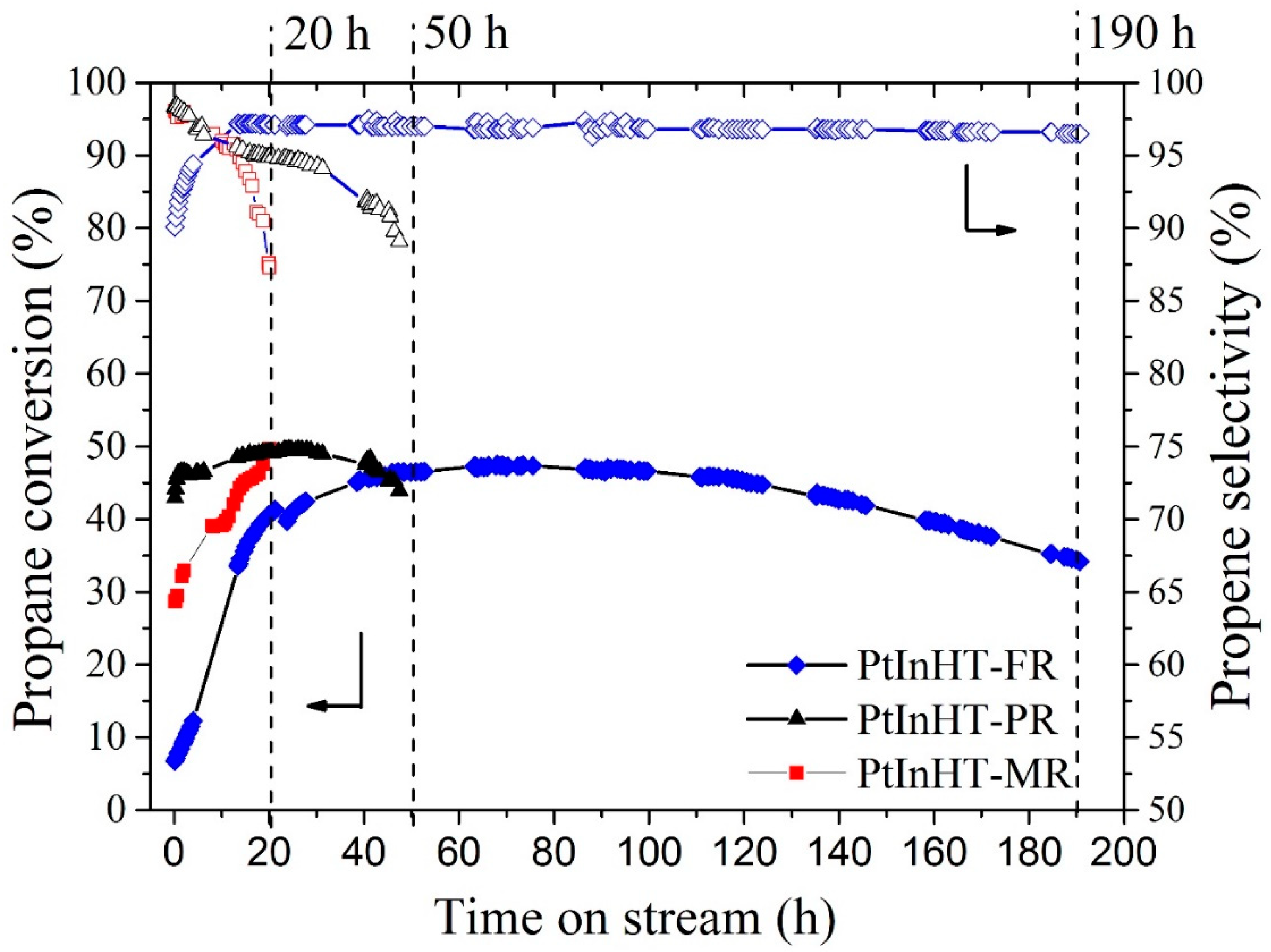
2.3. Catalytic Performance and Structure–Activity Relationship Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation
3.3. Characterizations
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Yang, M.L.; Yu, Y.; Zhu, Y.A.; Sui, Z.J.; Zhou, X.G.; Holmen, A.; Chen, D. Size-Dependent Reaction Mechanism and Kinetics for Propane Dehydrogenation over Pt Catalysts. ACS Catal. 2015, 5, 6310–6319. [Google Scholar] [CrossRef]
- Xiong, H.; Lin, S.; Goetze, J.; Pletcher, P.; Guo, H.; Kovarik, L.; Artyushkova, K.; Weckhuysen, B.M.; Datye, A.K. Thermally Stable and Regenerable Platinum–Tin Clusters for Propane Dehydrogenation Prepared by Atom Trapping on Ceria. Angew. Chem. Int. Ed. 2017, 56, 8986–8991. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Lang, W.Z.; Li, P.P.; Yan, X.; Guo, Y.J. The properties and catalytic performance of PtIn/Mg(Al)O catalysts for the propane dehydrogenation reaction: Effects of pH value in preparing Mg(Al)O supports by the co-precipitation method. J. Catal. 2016, 338, 104–114. [Google Scholar] [CrossRef]
- Cai, W.; Mu, R.; Zha, S.; Sun, G.; Chen, S.; Zhao, Z.J.; Li, H.; Tian, H.; Tang, Y.; Tao, F.; et al. Subsurface catalysis-mediated selectivity of dehydrogenation reaction. Sci. Adv. 2018, 4, eaar5418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Ge, Q.; Xu, H.; Li, W. Effects of Ce addition on the Pt-Sn/gamma-Al2O3 catalyst for propane dehydrogenation to propylene. Appl. Catal. A 2006, 315, 58–67. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, W.I.; Kim, J.R.; Oh, K.; Koh, H.L. Effect of Direct Reduction Treatment on Pt–Sn/Al2O3 Catalyst for Propane Dehydrogenation. Catalysts 2019, 9, 446. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.Z.; Li, P.P.; Yan, X.; Guo, Y.J. Analysis of the catalytic activity induction and deactivation of PtIn/Mg(Al)O catalysts for propane dehydrogenation reaction. RSC Adv. 2015, 5, 64689–64695. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.Z.; Li, P.P.; Long, L.L.; Yan, X.; Guo, Y.J. The influences of Mg/Al molar ratio on the properties of PtIn/Mg(Al)O-x catalysts for propane dehydrogenation reaction. Chem. Eng. J. 2016, 284, 1068–1079. [Google Scholar] [CrossRef]
- Pham, H.N.; Sattler, J.J.H.B.; Weckhuysen, B.M.; Datye, A.K. Role of Sn in the Regeneration of Pt/γ-Al2O3 Light Alkane Dehydrogenation Catalysts. ACS Catal. 2016, 6, 2257–2264. [Google Scholar] [CrossRef]
- Zhu, Y.; An, Z.; Song, H.; Xiang, X.; Yan, W.; He, J. Lattice-Confined Sn (IV/II) Stabilizing Raft-Like Pt Clusters: High Selectivity and Durability in Propane Dehydrogenation. ACS Catal. 2017, 7, 6973–6978. [Google Scholar] [CrossRef]
- Sun, P.; Siddiqi, G.; Vining, W.C.; Chi, M.; Bell, A.T. Novel Pt/Mg(In)(Al)O catalysts for ethane and propane dehydrogenation. J. Catal. 2011, 282, 165–174. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Cao, Y.; He, H.Y.; Fan, K.N.; Zhuang, J.H. Dehydrogenation of propane over In2O3-Al2O3 mixed oxide in the presence of carbon dioxide. J. Catal. 2010, 272, 101–108. [Google Scholar] [CrossRef]
- Redekop, E.A.; Galvita, V.V.; Poelman, H.; Bliznuk, V.; Detavernier, C.; Marin, G.B. Delivering a Modifying Element to Metal Nanoparticles via Support: Pt–Ga Alloying during the Reduction of Pt/Mg(Al,Ga)Ox Catalysts and Its Effects on Propane Dehydrogenation. ACS Catal. 2014, 4, 1812–1824. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, K.; Wang, C.; Ma, L.; Zhang, Q.; Liu, Q.; Chen, L.; Chen, L.; Zhang, Q.; Tian, Z. The properties and catalytic performance of PtSn/Mg(x-Ga)AlO catalysts for ethane dehydrogenation. RSC Adv. 2017, 7, 22836–22844. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Zhao, Z.J.; Mu, R.; Zha, S.; Li, L.; Chen, S.; Zang, K.; Luo, J.; Li, Z.; Purdy, S.C.; et al. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. Nat. Commun. 2018, 9, 4454. [Google Scholar] [CrossRef] [PubMed]
- Wegener, E.C.; Wu, Z.; Tseng, H.T.; Gallagher, J.R.; Ren, Y.; Diaz, R.E.; Ribeiro, F.H.; Miller, J.T. Structure and reactivity of Pt–In intermetallic alloy nanoparticles: Highly selective catalysts for ethane dehydrogenation. Catal. Today. 2018, 299, 146–153. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, F.; Liu, G.; Zeng, L.; Zhao, Z.J.; Gong, J.L. Effects of Ga doping on Pt/CeO2-Al2O3 catalysts for propane dehydrogenation. AIChE J. 2016, 62, 4365–4376. [Google Scholar] [CrossRef]
- Siddiqi, G.; Sun, P.; Galvita, V.; Bell, A.T. Catalyst performance of novel Pt/Mg(Ga)(Al)O catalysts for alkane dehydrogenation. J. Catal. 2010, 274, 200–206. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, L.; Zhao, Z.J.; Tian, H.; Wu, T.; Gong, J.L. Platinum-Modified ZnO/Al2O3 for Propane Dehydrogenation: Minimized Platinum Usage and Improved Catalytic Stability. ACS Catal. 2016, 6, 2158–2162. [Google Scholar] [CrossRef]
- Del Angel, G.; Bonilla, A.; Peña, Y.; Navarrete, J.; Fierro, J.L.G.; Acosta, D.R. Effect of lanthanum on the catalytic properties of PtSn/γ-Al2O3 bimetallic catalysts prepared by successive impregnation and controlled surface reaction. J. Catal. 2003, 219, 63–73. [Google Scholar] [CrossRef]
- Jiang, F.; Zeng, L.; Li, S.; Liu, G.; Wang, S.; Gong, J.L. Propane Dehydrogenation over Pt/TiO2–Al2O3 Catalysts. ACS Catal. 2015, 5, 438–447. [Google Scholar] [CrossRef]
- De Miguel, S.R.; Jablonski, E.L.; Castro, A.A.; Scelza, O.A. Highly selective and stable multimetallic catalysts for propane dehydrogenation. J. Chem. Technol. Biotechnol. 2000, 75, 596–600. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Qiu, A.; Wang, Y.; Xu, Y.; Wu, P. Propane dehydrogenation on PtSn/ZSM-5 catalyst: Effect of tin as a promoter. Catal. Commun. 2006, 7, 860–866. [Google Scholar] [CrossRef]
- Shen, L.L.; Xia, K.; Lang, W.Z.; Chu, L.F.; Yan, X.; Guo, Y.J. The effects of calcination temperature of support on PtIn/Mg(Al)O catalysts for propane dehydrogenation reaction. Chem. Eng. J. 2017, 324, 336–346. [Google Scholar] [CrossRef]
- Nawaz, Z.; Tang, X.; Wang, Y.; Wei, F. Parametric Characterization and Influence of Tin on the Performance of Pt−Sn/SAPO-34 Catalyst for Selective Propane Dehydrogenation to Propylene. Ind. Eng. Chem. Res. 2010, 49, 1274–1280. [Google Scholar] [CrossRef]
- Lin, L.; Yang, W.; Jia, J.; Xu, Z.; Zhang, T.; Fan, Y.; Kou, Y.; Shen, J. Surface structure and reaction performances of highly dispersed and supported bimetallic catalysts. Sci. China Ser. B Chem. 1999, 42, 571. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T. Preparation of supported metal catalysts starting from hydrotalcites as the precursors and their improvements by adopting “memory effect”. Catal. Surv. Asia. 2007, 11, 1–30. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Ifebajo, A.O.; Gazi, M. Magnetic LDH-based CoO–NiFe2O4 catalyst with enhanced performance and recyclability for efficient decolorization of azo dye via Fenton-like reactions. Appl. Catal. B 2019, 243, 243–252. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Stepanova, L.N.; Gulyaeva, T.I.; Erenburg, S.B.; Trubina, S.V.; Kvashnina, K.; Nizovskii, A.I.; Kalinkin, A.V.; Zaikovskii, V.I.; Bukhtiyarov, V.I.; et al. Zinc influence on the formation and properties of Pt/Mg(Zn)AlOx catalysts synthesized from layered hydroxides. J. Catal. 2016, 341, 13–23. [Google Scholar] [CrossRef]
- Hibino, T.; Tsunashima, A. Characterization of Repeatedly Reconstructed Mg−Al Hydrotalcite-like Compounds: Gradual Segregation of Aluminum from the Structure. Chem. Mater. 1998, 10, 4055–4061. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, J.; Peng, Y.; Zhou, J.; Ruan, X.; Liu, J.; Liu, Q.; Xi, Y.; Frost, R.; Qian, G. An investigation into mechanism of cation adsorption by reconstruction of calcined layered double hydroxide. Microporous Mesoporous Mater. 2017, 242, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Fan, G.; Yang, Y.; Li, F. Multi-level three-dimensional Mg–Al layered double hydroxide hierarchical microstructures with enhanced basic catalytic property. J. Colloid Interface Sci. 2014, 432, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ma, X.; An, Z.; Zhu, Y.; Wang, W.; He, J. Pseudo-single-atom Platinum Induced by the Promoter Confined in Brucite-like Lattice for Catalytic Reforming. ChemCatChem 2016, 8, 1773–1777. [Google Scholar] [CrossRef]
- Gao, Z.; Sasaki, K.; Qiu, X. Structural Memory Effect of Mg–Al and Zn–Al layered Double Hydroxides in the Presence of Different Natural Humic Acids: Process and Mechanism. Langmuir 2018, 34, 5386–5395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.; Wan, L.; Xue, M.; Duan, Y.; Liu, X. Synergistic effect between Sn and K promoters on supported platinum catalyst for isobutane dehydrogenation. J. Nat. Gas Chem. 2011, 20, 639–646. [Google Scholar] [CrossRef]
- Im, J.; Choi, M. Physicochemical Stabilization of Pt against Sintering for a Dehydrogenation Catalyst with High Activity, Selectivity, and Durability. ACS Catal. 2016, 6, 2819–2826. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S.I. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]




| Sample | Lattice Parameter a (nm) a | Lattice Parameter c (nm) b | Lc (nm) c | |
|---|---|---|---|---|
| HT | -F | 0.3037 | 2.2551 | 233.5 |
| -P | 0.3039 | 2.2588 | 235.7 | |
| -M | 0.3045 | 2.2847 | 64.1 | |
| InHT | -F | 0.3051 | 2.3246 | 34.2 |
| -P | 0.3041 | 2.2673 | 48.4 | |
| -M | 0.3049 | 2.2858 | 60.1 | |
| PtInHT | -F | 0.3052 | 2.3027 | 42.6 |
| -P | 0.3049 | 2.2764 | 44.2 | |
| -M | 0.3050 | 2.2936 | 51.3 | |
| Sample | SBETa (m2·g−1) | Vpb (cm3·g−1) | dpc (nm) | DPtd (%) |
|---|---|---|---|---|
| PtInHT-FC | 57.9 | 0.17 | 5.58 | 33.6 |
| PtInHT-PC | 131.6 | 0.48 | 6.48 | 21.1 |
| PtInHT-MC | 144.4 | 0.31 | 9.91 | 27.3 |
| Catalyst | BE (eV) | In3+/In0a | |||
|---|---|---|---|---|---|
| In3d5/2 | In3d3/2 | ||||
| In3+ | In0 | In3+ | In0 | ||
| PtInHT-FR | 445.2 | 444.5 | 452.7 | 452.2 | 1.34 |
| PtInHT-PR | 445.2 | 444.4 | 452.7 | 452.1 | 1.17 |
| PtInHT-MR | 445.2 | 444.4 | 452.7 | 452.0 | 0.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, M.; Song, Z.; Liu, S.; Wang, J.; Zhang, L. Hierarchical PtIn/Mg(Al)O Derived from Reconstructed PtIn-hydrotalcite-like Compounds for Highly Efficient Propane Dehydrogenation. Catalysts 2019, 9, 767. https://doi.org/10.3390/catal9090767
Li J, Zhang M, Song Z, Liu S, Wang J, Zhang L. Hierarchical PtIn/Mg(Al)O Derived from Reconstructed PtIn-hydrotalcite-like Compounds for Highly Efficient Propane Dehydrogenation. Catalysts. 2019; 9(9):767. https://doi.org/10.3390/catal9090767
Chicago/Turabian StyleLi, Jiaxin, Ming Zhang, Zhen Song, Shuo Liu, Jiameng Wang, and Lihong Zhang. 2019. "Hierarchical PtIn/Mg(Al)O Derived from Reconstructed PtIn-hydrotalcite-like Compounds for Highly Efficient Propane Dehydrogenation" Catalysts 9, no. 9: 767. https://doi.org/10.3390/catal9090767






