Salt-Templated Platinum-Copper Porous Macrobeams for Ethanol Oxidation
Abstract
1. Introduction
2. Results and Discussion
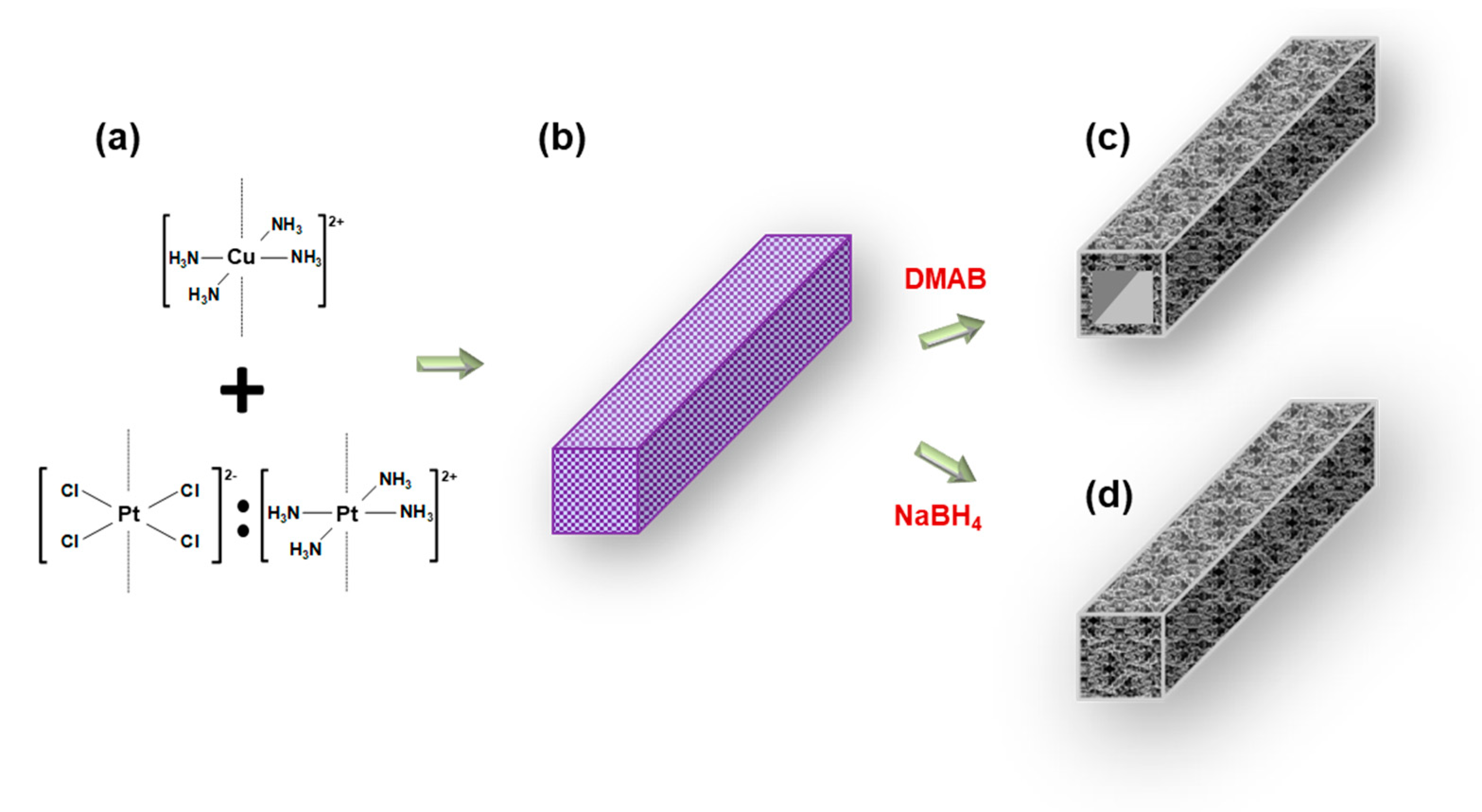
2.1. Platinum-Copper Macrobeam Synthesis Scheme
2.2. Macrobeam Structure
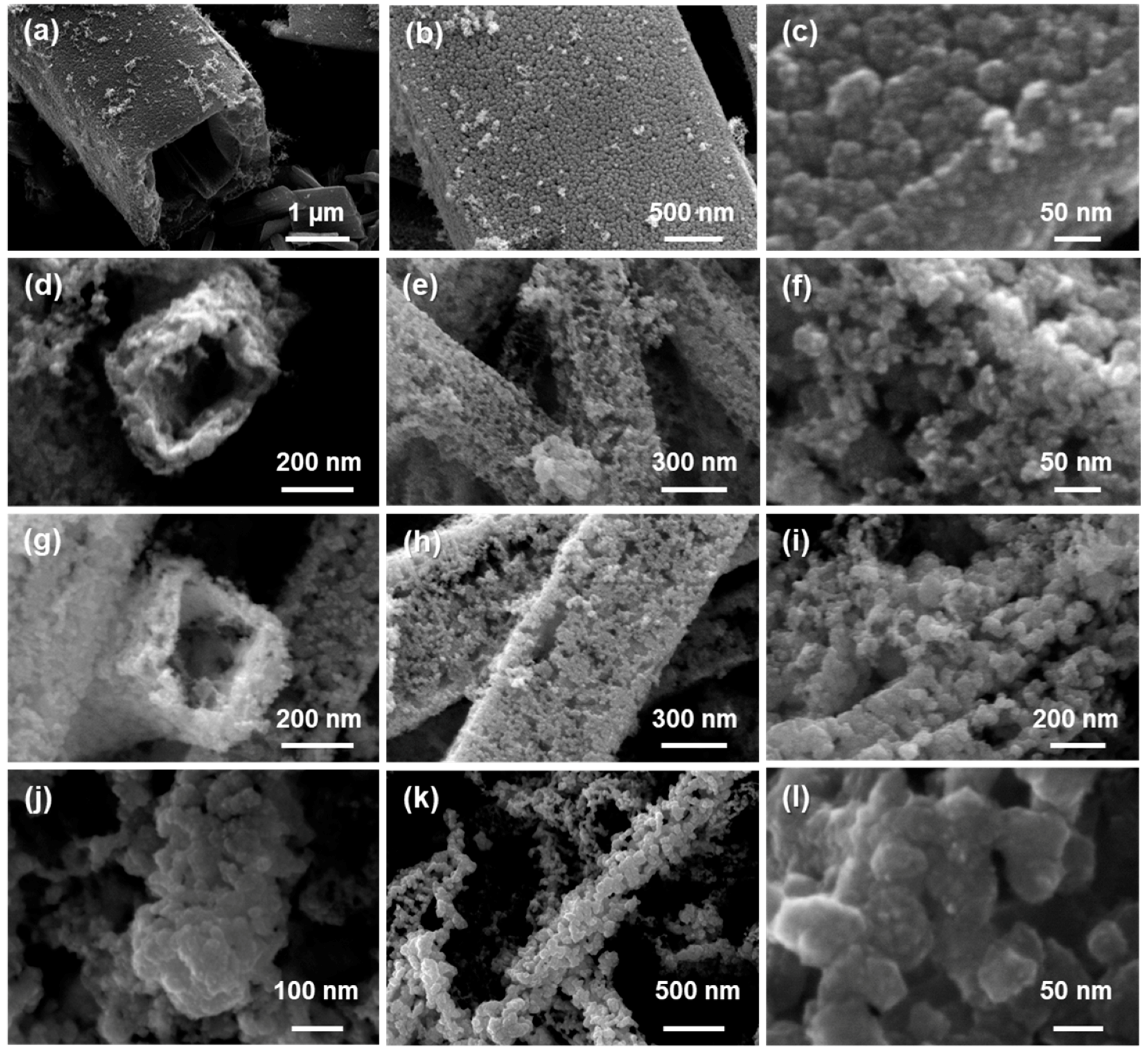
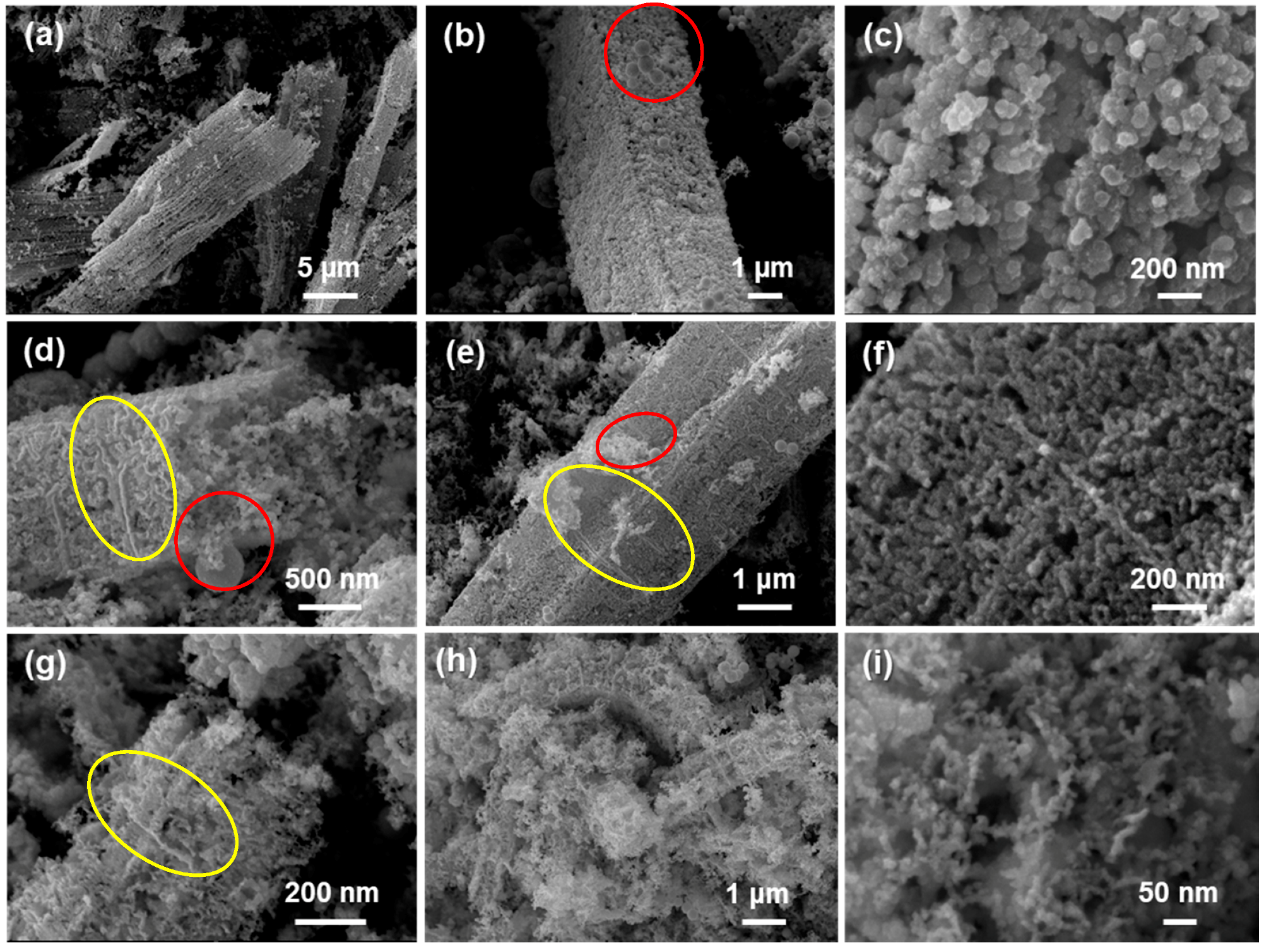
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Proposed Reduction-Dissolution Mechanism
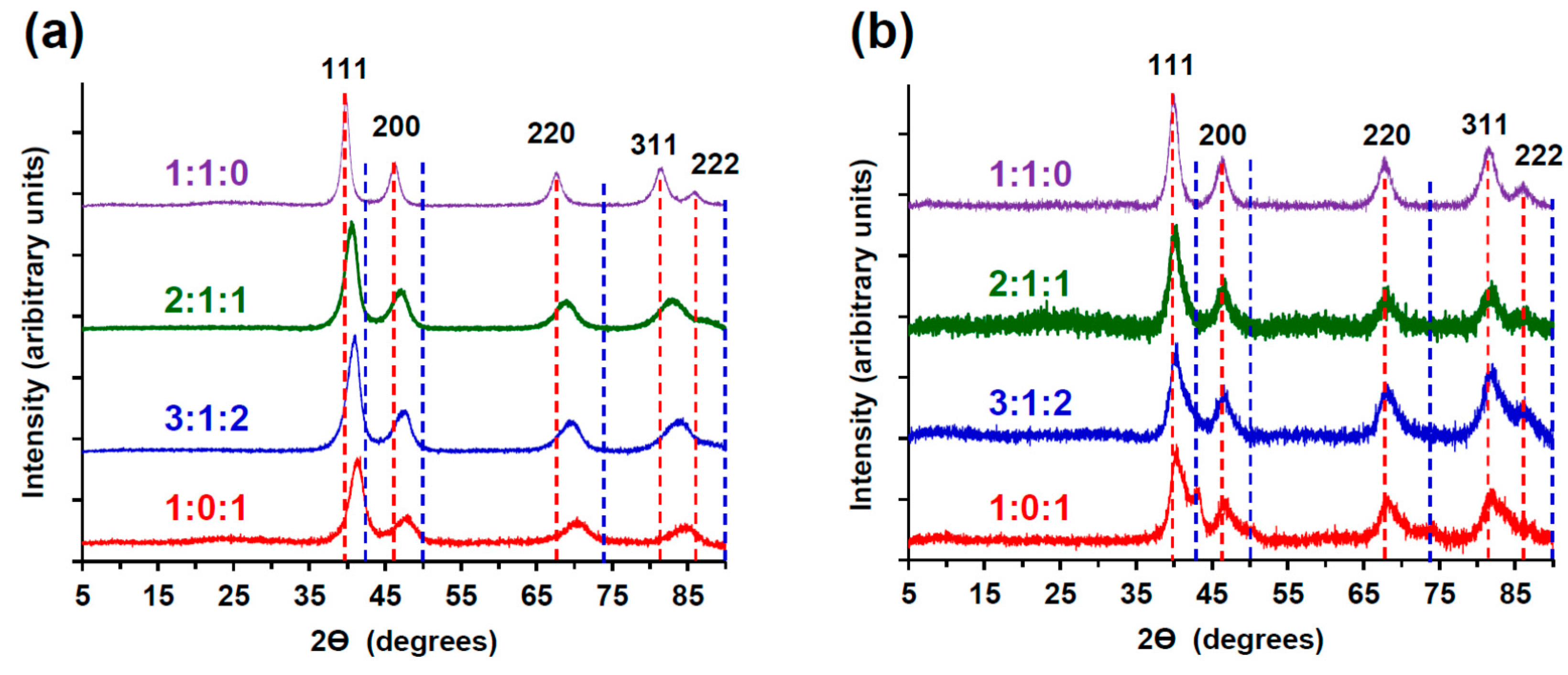
2.2.3. X-ray Diffractometry
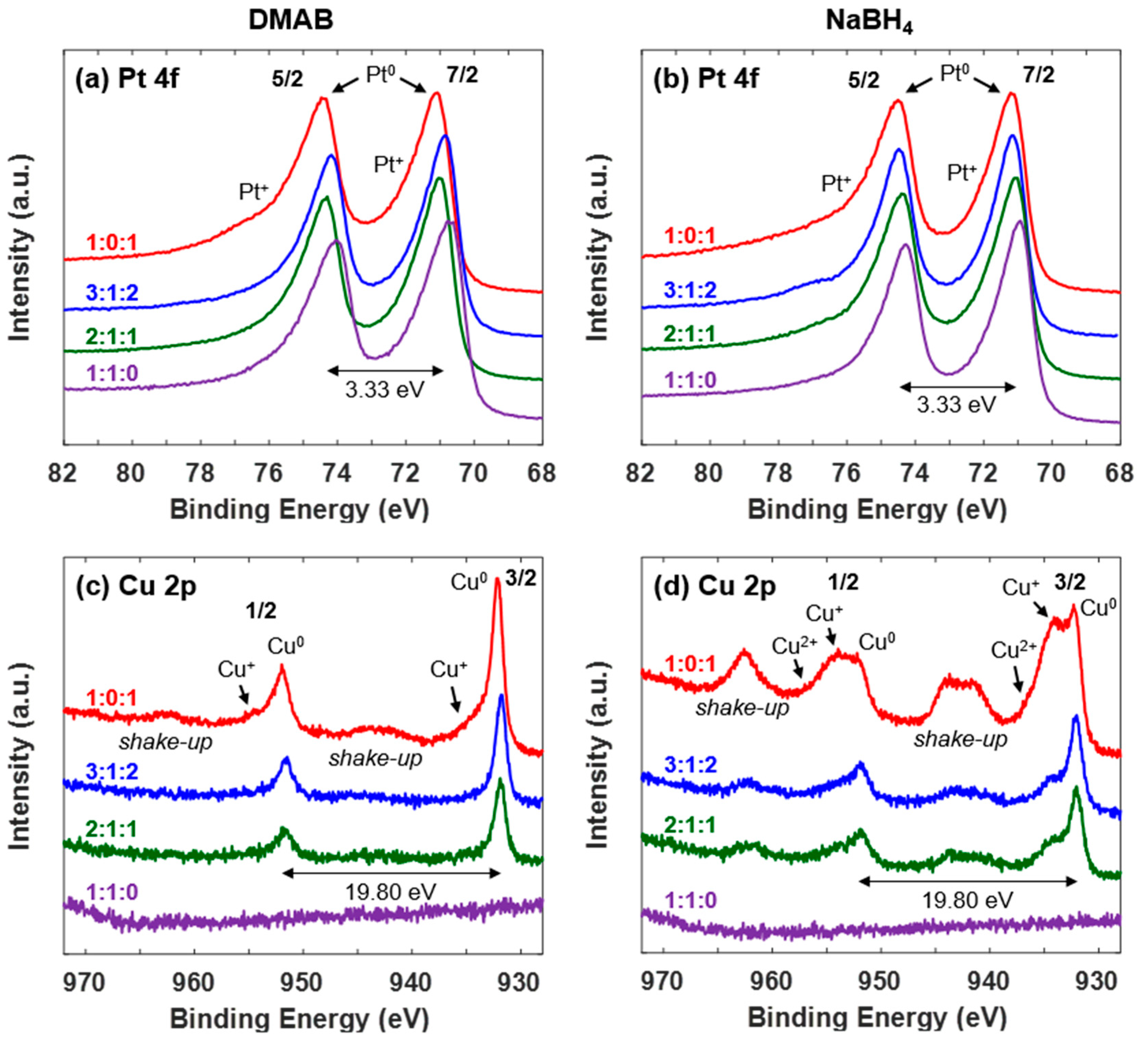
2.2.4. X-ray Photoelectron Spectroscopy (XPS)
2.3. Pt–Cu Macrobeam Electrochemical Characterization
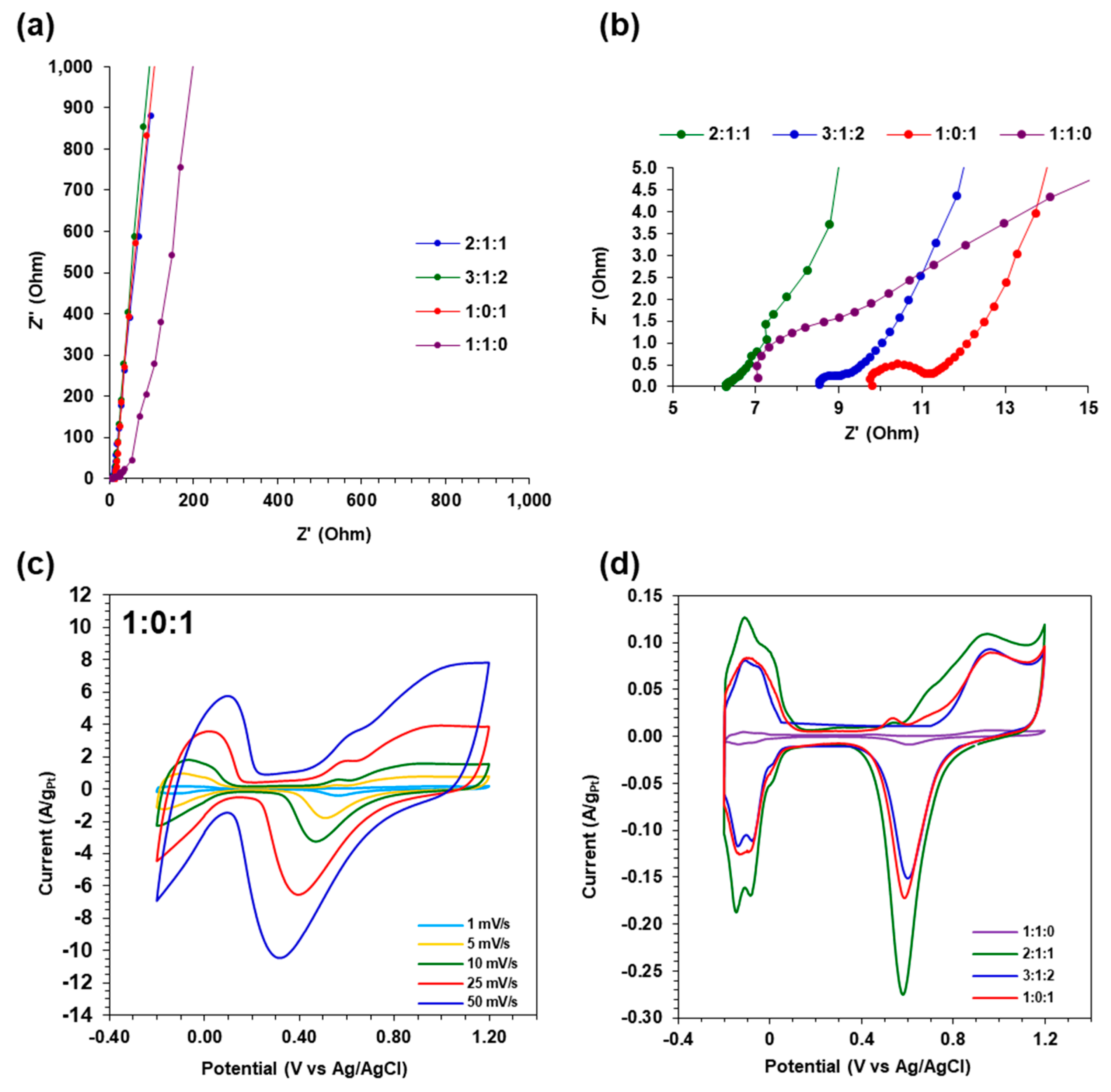
2.3.1. Electrochemical Impedance Spectroscopy (EIS) in 0.5 M H2SO4
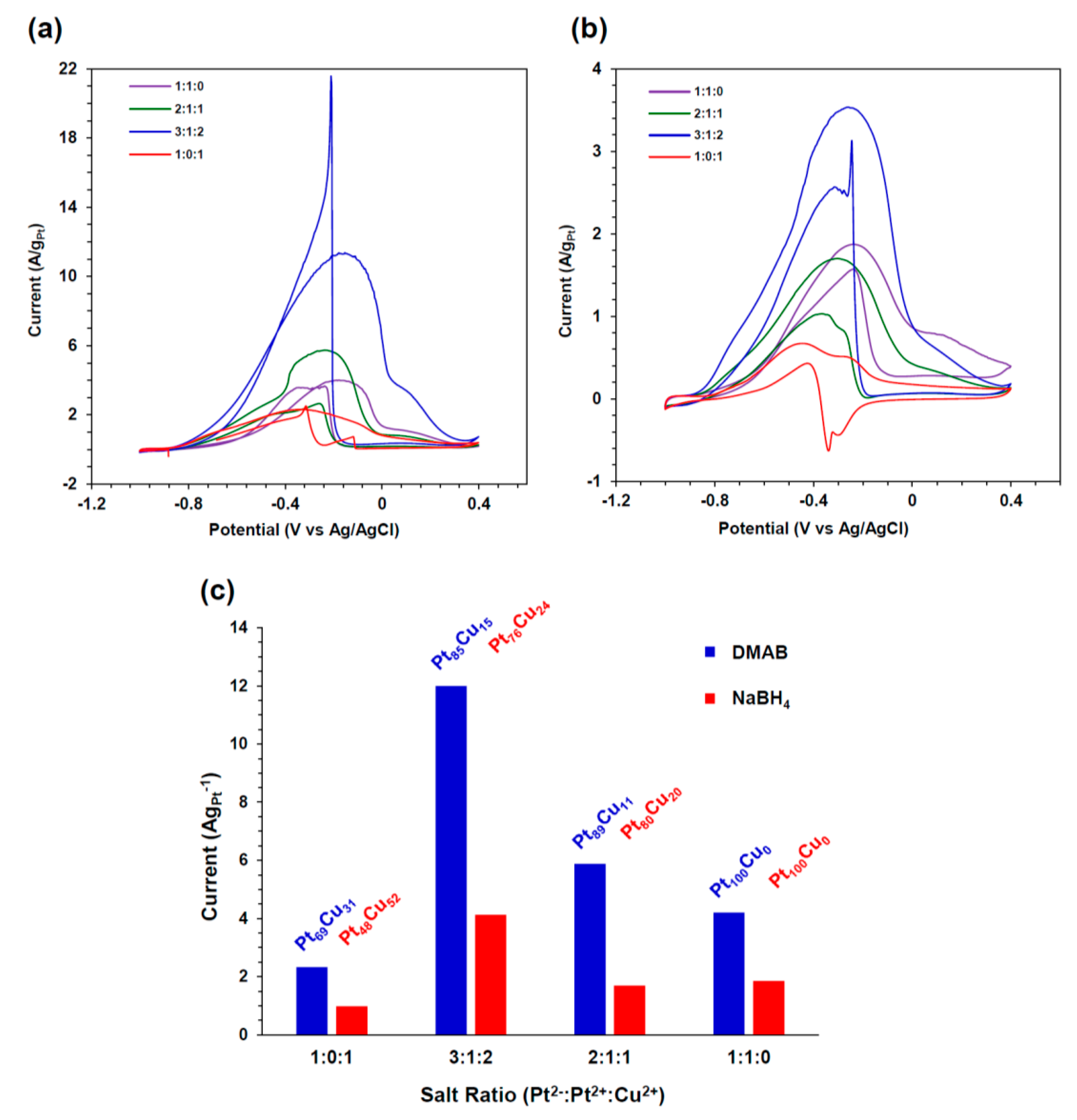
2.3.2. Ethanol Oxidation in 1 M KOH/1 M Ethanol
3. Materials and Methods
3.1. Copper-Platinum Macrobeam Synthesis
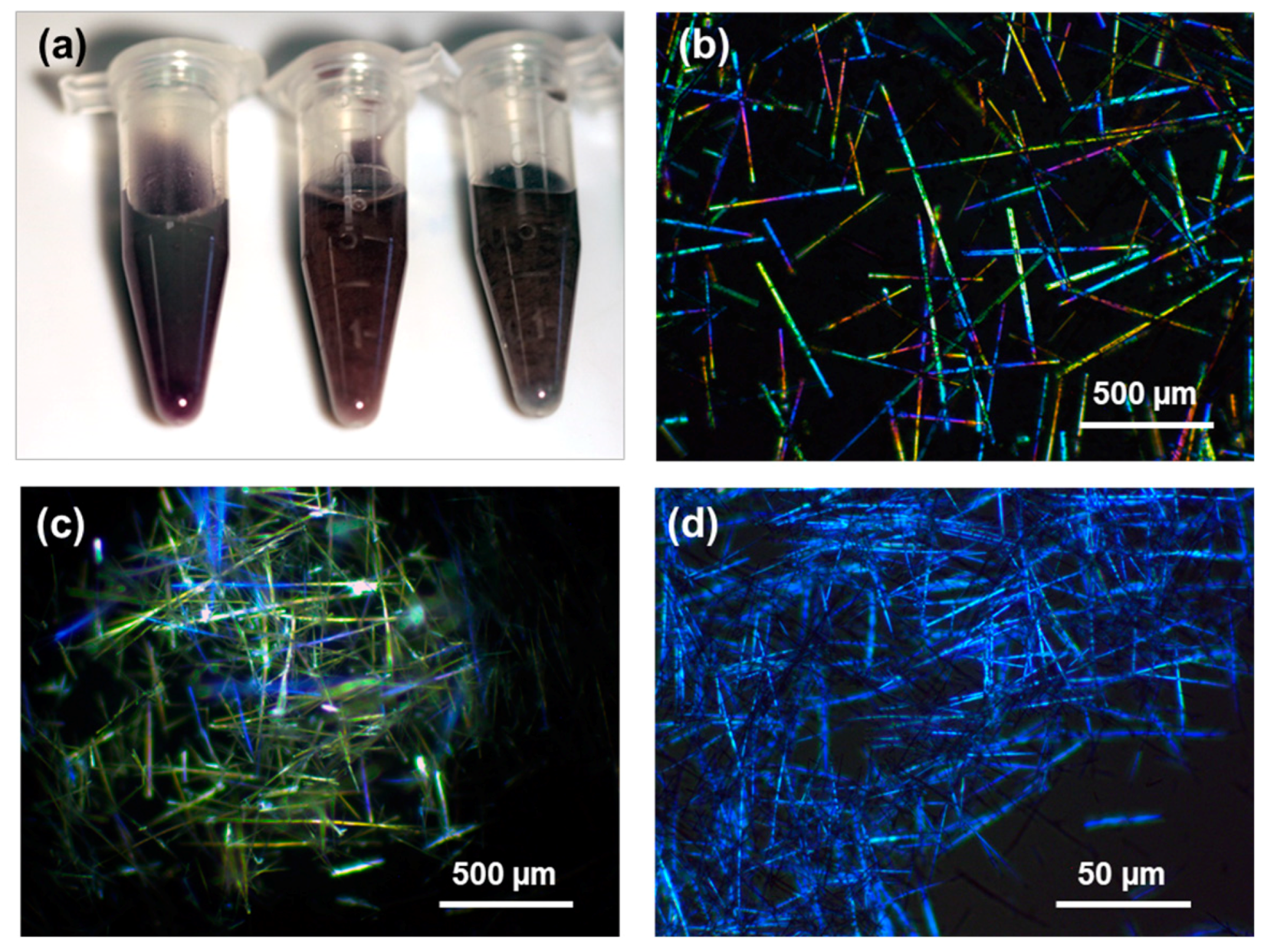
3.2. Polarized Optical Microscopy
3.3. Scanning Electron Microscopy
3.4. X-ray Diffractometry
3.5. X-ray Photoelectron Spectroscopy
3.6. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, A.; Holt-Hindle, P. Platinum-Based Nanostructured Materials: Synthesis, Properties, and Applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yang, H. Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 2009, 4, 143–164. [Google Scholar] [CrossRef]
- Qiu, X.; Dai, Y.; Zhu, X.; Zhang, H.; Wu, P.; Tang, Y.; Wei, S. Template-engaged synthesis of hollow porous platinum–palladium alloy nanospheres for efficient methanol electro-oxidation. J. Power Sources 2016, 302, 195–201. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Tonegawa, A.; Komatsu, M.; Wang, H.; Wang, L.; Nemoto, Y.; Suzuki, N.; Kuroda, K. Electrochemical Synthesis of Mesoporous Pt–Au Binary Alloys with Tunable Compositions for Enhancement of Electrochemical Performance. J. Am. Chem. Soc. 2012, 134, 5100–5109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, S.; Cai, M.; Zhang, Y.; Li, R.; Sun, X. Porous dendritic platinum nanotubes with extremely high activity and stability for oxygen reduction reaction. Sci. Rep. 2013, 3, 1526. [Google Scholar] [CrossRef]
- Chen, J.; Lim, B.; Lee, E.P.; Xia, Y. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95. [Google Scholar] [CrossRef]
- Liu, L.; Pippel, E.; Scholz, R.; Gösele, U. Nanoporous Pt−Co Alloy Nanowires: Fabrication, Characterization, and Electrocatalytic Properties. Nano Lett. 2009, 9, 4352–4358. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Marković, N.M. Improved Oxygen Reduction Activity on Pt3Ni(111) via Increased Surface Site Availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef]
- Wei, L.; Paramaconi, R.; Lars, B.; Annette, F.; Jipei, Y.; Anne-Kristin, H.; Dorin, G.; Zhikun, Z.; Stefan, K.; Nikolai, G.; et al. Bimetallic Aerogels: High-Performance Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 9849–9852. [Google Scholar]
- Lim, B.; Wang, J.; Camargo, P.H.C.; Cobley, C.M.; Kim, M.J.; Xia, Y. Twin-Induced Growth of Palladium–Platinum Alloy Nanocrystals. Angew. Chem. Int. Ed. 2009, 48, 6304–6308. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt Bimetallic Nanodendrites with High Activity for Oxygen Reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-N.; He, L.-L.; Chen, F.-Y.; Wang, A.-J.; Xue, M.-W.; Feng, J.-J. Simple one-pot synthesis of platinum-palladium nanoflowers with enhanced catalytic activity and methanol-tolerance for oxygen reduction in acid media. Electrochim. Acta 2014, 137, 431–438. [Google Scholar] [CrossRef]
- Anniyev, T.; Kaya, S.; Rajasekaran, S.; Ogasawara, H.; Nordlund, D.; Nilsson, A. Tuning the Metal–Adsorbate Chemical Bond through the Ligand Effect on Platinum Subsurface Alloys. Angew. Chem. Int. Ed. 2012, 51, 7724–7728. [Google Scholar] [CrossRef]
- Cui, C.; Gan, L.; Li, H.-H.; Yu, S.-H.; Heggen, M.; Strasser, P. Octahedral PtNi Nanoparticle Catalysts: Exceptional Oxygen Reduction Activity by Tuning the Alloy Particle Surface Composition. Nano Lett. 2012, 12, 5885–5889. [Google Scholar] [CrossRef]
- Zhou, W.-P.; Yang, X.; Vukmirovic, M.B.; Koel, B.E.; Jiao, J.; Peng, G.; Mavrikakis, M.; Adzic, R.R. Improving Electrocatalysts for O2 Reduction by Fine-Tuning the Pt−Support Interaction: Pt Monolayer on the Surfaces of a Pd3Fe(111) Single-Crystal Alloy. J. Am. Chem. Soc. 2009, 131, 12755–12762. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Strasser, P. Electrocatalysis on Bimetallic Surfaces: Modifying Catalytic Reactivity for Oxygen Reduction by Voltammetric Surface Dealloying. J. Am. Chem. Soc. 2007, 129, 12624–12625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, Q.; Lu, H.; Hu, B.; Xie, Y.; Yu, G. Glucose-directed synthesis of Pt-Cu alloy nanowires networks and their electro-catalytic performance for ethylene glycol oxidation. J. Alloys Compd. 2017, 727, 475–483. [Google Scholar] [CrossRef]
- Baumgärtner, M.E.; Raub, J. The Electrodeposition of Platinum and Platinum Alloys. Platinum Met. Rev. 1988, 32, 188–197. [Google Scholar]
- Kloke, A.; Köhler, C.; Gerwig, R.; Zengerle, R.; Kerzenmacher, S. Cyclic Electrodeposition of PtCu Alloy: Facile Fabrication of Highly Porous Platinum Electrodes. Adv. Mater. 2012, 24, 2916–2921. [Google Scholar] [CrossRef]
- Wei, Z.D.; Feng, Y.C.; Li, L.; Liao, M.J.; Fu, Y.; Sun, C.X.; Shao, Z.G.; Shen, P.K. Electrochemically synthesized Cu/Pt core-shell catalysts on a porous carbon electrode for polymer electrolyte membrane fuel cells. J. Power Sources 2008, 180, 84–91. [Google Scholar] [CrossRef]
- Han, L.; Cui, P.; He, H.; Liu, H.; Peng, Z.; Yang, J. A seed-mediated approach to the morphology-controlled synthesis of bimetallic copper–platinum alloy nanoparticles with enhanced electrocatalytic performance for the methanol oxidation reaction. J. Power Sources 2015, 286, 488–494. [Google Scholar] [CrossRef]
- Xu, D.; Liu, Z.; Yang, H.; Liu, Q.; Zhang, J.; Fang, J.; Zou, S.; Sun, K. Solution-Based Evolution and Enhanced Methanol Oxidation Activity of Monodisperse Platinum–Copper Nanocubes. Angew. Chem. Int. Ed. 2009, 48, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Cheng, H.; Zhao, Y.; Hu, Y.; Liu, Y.; Dai, L.; Qu, L. Newly-Designed Complex Ternary Pt/PdCu Nanoboxes Anchored on Three-Dimensional Graphene Framework for Highly Efficient Ethanol Oxidation. Adv. Mater. 2012, 24, 5493–5498. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Fu, G.; Chen, Y.; Tang, Y.; Lu, T. Autocatalysis and Selective Oxidative Etching Induced Synthesis of Platinum–Copper Bimetallic Alloy Nanodendrites Electrocatalysts. ACS Appl. Mater. Interfaces 2014, 6, 7301–7308. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Liu, H.; You, N.; Wu, J.; Sun, D.; Xu, L.; Tang, Y.; Chen, Y. Dendritic platinum–copper bimetallic nanoassemblies with tunable composition and structure: Arginine-driven self-assembly and enhanced electrocatalytic activity. Nano Res. 2016, 9, 755–765. [Google Scholar] [CrossRef]
- Qiu, H.J.; Shen, X.; Wang, J.Q.; Hirata, A.; Fujita, T.; Wang, Y.; Chen, M.W. Aligned Nanoporous Pt–Cu Bimetallic Microwires with High Catalytic Activity toward Methanol Electrooxidation. ACS Catal. 2015, 5, 3779–3785. [Google Scholar] [CrossRef]
- Zhang, N.; Bu, L.; Guo, S.; Guo, J.; Huang, X. Screw Thread-Like Platinum–Copper Nanowires Bounded with High-Index Facets for Efficient Electrocatalysis. Nano Lett. 2016, 16, 5037–5043. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, S.; Wilson, A.R.; Ha, Y.-C.; Wiley, B.J. Optically transparent hydrogen evolution catalysts made from networks of copper–platinum core–shell nanowires. Energy Environ. Sci. 2014, 7, 1461–1467. [Google Scholar] [CrossRef]
- Gong, M.; Yao, Z.; Lai, F.; Chen, Y.; Tang, Y. Platinum–copper alloy nanocrystals supported on reduced graphene oxide: One-pot synthesis and electrocatalytic applications. Carbon 2015, 91, 338–345. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Nosheen, F.; Wang, P.; Zhang, J.; Zhuang, J.; Wang, X. Fine Tuning of the Structure of Pt–Cu Alloy Nanocrystals by Glycine-Mediated Sequential Reduction Kinetics. Small 2013, 9, 3063–3069. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Shen, P.K. Concave Platinum–Copper Octopod Nanoframes Bounded with Multiple High-Index Facets for Efficient Electrooxidation Catalysis. ACS Nano 2017, 11, 11946–11953. [Google Scholar] [CrossRef] [PubMed]
- Nosheen, F.; Zhang, Z.; Xiang, G.; Xu, B.; Yang, Y.; Saleem, F.; Xu, X.; Zhang, J.; Wang, X. Three-dimensional hierarchical Pt-Cu superstructures. Nano Res. 2015, 8, 832–838. [Google Scholar] [CrossRef]
- Li, F.; Guo, Y.; Chen, M.; Qiu, H.; Sun, X.; Wang, W.; Liu, Y.; Gao, J. Comparison study of electrocatalytic activity of reduced graphene oxide supported Pt–Cu bimetallic or Pt nanoparticles for the electrooxidation of methanol and ethanol. Int. J. Hydrogen Energy 2013, 38, 14242–14249. [Google Scholar] [CrossRef]
- Tseng, C.-J.; Lo, S.-T.; Lo, S.-C.; Chu, P.P. Characterization of Pt-Cu binary catalysts for oxygen reduction for fuel cell applications. Mater. Chem. Phys. 2006, 100, 385–390. [Google Scholar] [CrossRef]
- Eid, K.; Wang, H.; He, P.; Wang, K.; Ahamad, T.; Alshehri, S.M.; Yamauchi, Y.; Wang, L. One-step synthesis of porous bimetallic PtCu nanocrystals with high electrocatalytic activity for methanol oxidation reaction. Nanoscale 2015, 7, 16860–16866. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, N.; Jia, S.; Shao, Y. Detection of Glucose Based on Bimetallic PtCu Nanochains Modified Electrodes. Anal. Chem. 2013, 85, 5040–5046. [Google Scholar] [CrossRef] [PubMed]
- Burpo, F.J.; Nagelli, E.A.; Morris, L.A.; McClure, J.P.; Ryu, M.Y.; Palmer, J.L. Direct solution-based reduction synthesis of Au, Pd, and Pt aerogels. J. Mater. Res. 2017, 32, 4153–4165. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Morris, L.A.; McClure, J.P.; Ryu, M.Y.; Palmer, J.L. A Rapid Synthesis Method for Au, Pd, and Pt Aerogels Via Direct Solution-Based Reduction. JoVE 2018, e57875. [Google Scholar] [CrossRef]
- Xiao, X.; Song, H.; Lin, S.; Zhou, Y.; Zhan, X.; Hu, Z.; Zhang, Q.; Sun, J.; Yang, B.; Li, T.; et al. Scalable salt-templated synthesis of two-dimensional transition metal oxides. Nat. Commun. 2016, 7, 11296. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, H.; Jin, H.; Wu, M.; Fang, Y.; Sun, J.; Hu, Z.; Li, T.; Wu, J.; Huang, L.; et al. Salt-Templated Synthesis of 2D Metallic MoN and Other Nitrides. ACS Nano 2017, 11, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
- Burpo, F.J.; Nagelli, E.A.; Winter, S.J.; McClure, J.P.; Bartolucci, S.F.; Burns, A.R.; O’Brien, S.F.; Chu, D.D. Salt-Templated Hierarchically Porous Platinum Macrotube Synthesis. ChemistrySelect 2018, 3, 4542–4546. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Mitropoulos, A.N.; Bartolucci, S.F.; McClure, J.P.; Baker, D.R.; Losch, A.R.; Chu, D.D. Salt-templated platinum–palladium porous macrobeam synthesis. MRS Communications 2019, 9, 280–287. [Google Scholar] [CrossRef]
- Burpo, F.; Nagelli, E.; Morris, L.; Woronowicz, K.; Mitropoulos, A. Salt-Mediated Au-Cu Nanofoam and Au-Cu-Pd Porous Macrobeam Synthesis. Molecules 2018, 23, 1701. [Google Scholar] [CrossRef] [PubMed]
- Magnus, G. Ueber einige Verbindungen des Platinchlorürs. Ann. Phys. 1828, 90, 239–242. [Google Scholar] [CrossRef]
- Vauquelin, N.L. Memoire sur le Palladium et le Rhodium. Ann. Chim. 1813, 88, 167–198. [Google Scholar]
- Wagner, C.D. NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000. [Google Scholar]
- de Levie, R. On porous electrodes in electrolyte solutions—IV. Electrochim. Acta 1964, 9, 1231–1245. [Google Scholar] [CrossRef]
- Keiser, H.; Beccu, K.D.; Gutjahr, M.A. Abschätzung der porenstruktur poröser elektroden aus impedanzmessungen. Electrochim. Acta 1976, 21, 539–543. [Google Scholar] [CrossRef]
- Zhan, D.; Velmurugan, J.; Mirkin, M.V. Adsorption/desorption of hydrogen on Pt nanoelectrodes: evidence of surface diffusion and spillover. J. Am. Chem. Soc. 2009, 131, 14756–14760. [Google Scholar] [CrossRef]
- Biegler, T.; Rand, D.A.J.; Woods, R. Limiting oxygen coverage on platinized platinum; Relevance to determination of real platinum area by hydrogen adsorption. J. Electroanal. Chem. Interfacial Electrochem. 1971, 29, 269–277. [Google Scholar] [CrossRef]
- Lai, S.C.S.; Koper, M.T.M. Ethanol electro-oxidation on platinum in alkaline media. PCCP 2009, 11, 10446–10456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, S.; Cai, W.-B. Recent Advances on Electro-Oxidation of Ethanol on Pt- and Pd-Based Catalysts: From Reaction Mechanisms to Catalytic Materials. Catalysts 2015, 5, 1507–1534. [Google Scholar] [CrossRef]
- Rezaei, M.; Tabaian, S.H.; Haghshenas, D.F. The Role of Electrodeposited Pd Catalyst Loading on the Mechanisms of Formic Acid Electro-Oxidation. Electrocatalysis 2014, 5, 193–203. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
| Pt2−:Pt2+:Cu2+ | Stoic. Pt:Cu | EDS Pt:Cu | XPS Pt:Cu | |
|---|---|---|---|---|
| NaBH4 | 1:0:1 | 1:1 | 0.5:1 | 0.92:1 |
| 3:1:2 | 2:1 | 1.3:1 | 3.1:1 | |
| 2:1:1 | 3:1 | 2.5:1 | 4.0:1 | |
| 1:1:0 | 1:0 | 1:0 | 1.0:0 | |
| DMAB | 1:0:1 | 1:1 | 0.7:1 | 2.2:1 |
| 3:1:2 | 2:1 | 1.5:1 | 5.8:1 | |
| 2:1:1 | 3:1 | 2.1:1 | 7.9:1 | |
| 1:1:0 | 1:0 | 1:0 | 1.0:0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burpo, F.J.; Nagelli, E.A.; Losch, A.R.; Bui, J.K.; Forcherio, G.T.; Baker, D.R.; McClure, J.P.; Bartolucci, S.F.; Chu, D.D. Salt-Templated Platinum-Copper Porous Macrobeams for Ethanol Oxidation. Catalysts 2019, 9, 662. https://doi.org/10.3390/catal9080662
Burpo FJ, Nagelli EA, Losch AR, Bui JK, Forcherio GT, Baker DR, McClure JP, Bartolucci SF, Chu DD. Salt-Templated Platinum-Copper Porous Macrobeams for Ethanol Oxidation. Catalysts. 2019; 9(8):662. https://doi.org/10.3390/catal9080662
Chicago/Turabian StyleBurpo, F. John, Enoch A. Nagelli, Anchor R. Losch, Jack K. Bui, Gregory T. Forcherio, David R. Baker, Joshua P. McClure, Stephen F. Bartolucci, and Deryn D. Chu. 2019. "Salt-Templated Platinum-Copper Porous Macrobeams for Ethanol Oxidation" Catalysts 9, no. 8: 662. https://doi.org/10.3390/catal9080662
APA StyleBurpo, F. J., Nagelli, E. A., Losch, A. R., Bui, J. K., Forcherio, G. T., Baker, D. R., McClure, J. P., Bartolucci, S. F., & Chu, D. D. (2019). Salt-Templated Platinum-Copper Porous Macrobeams for Ethanol Oxidation. Catalysts, 9(8), 662. https://doi.org/10.3390/catal9080662






