Low Temperature Infrared Study of Carbon Monoxide Adsorption on Rh/CeO2
Abstract
1. Introduction
2. Results and Discussion
2.1. CO Adsorption on CeO2
2.1.1. Irreversible Adsorption
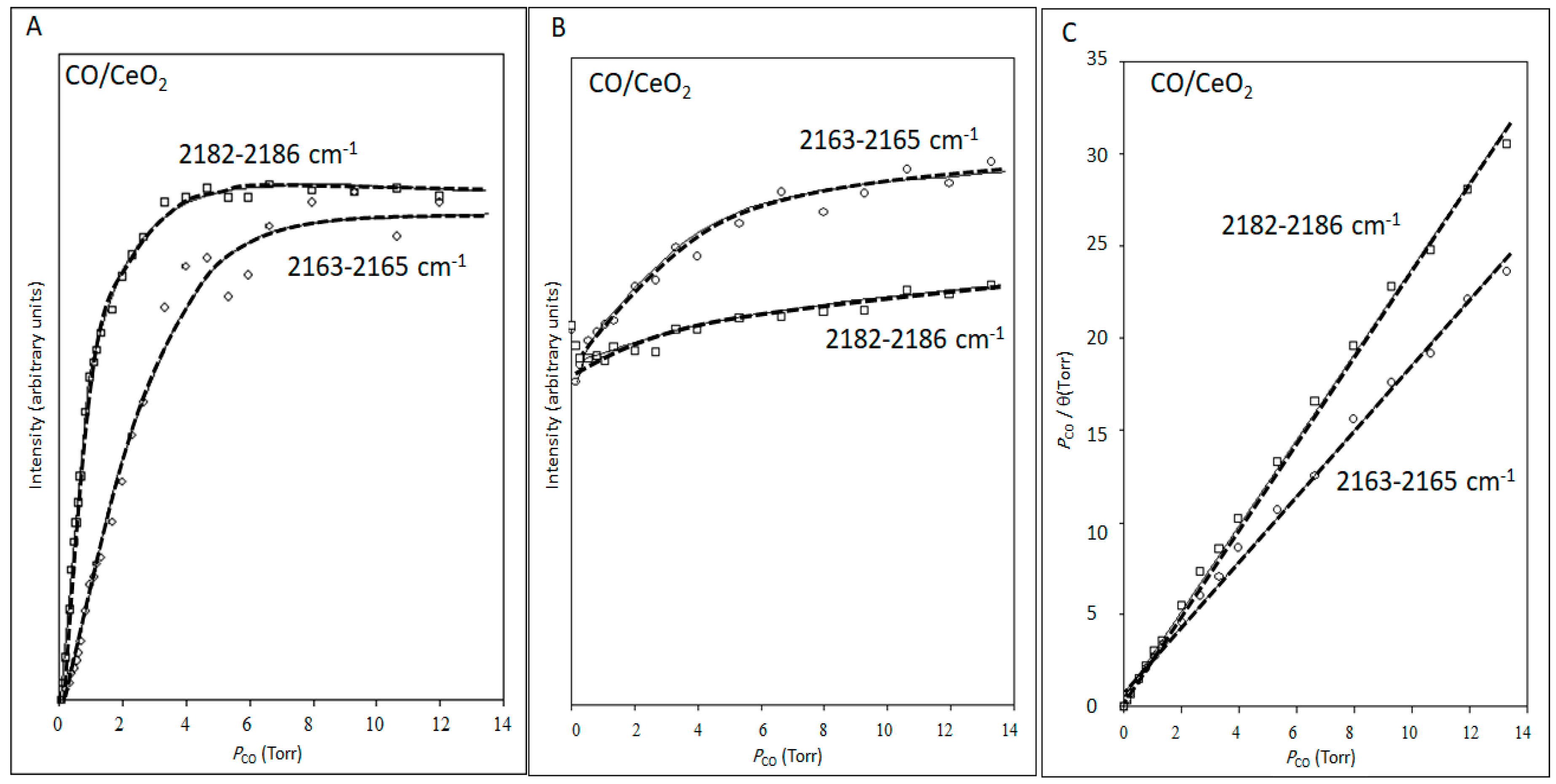
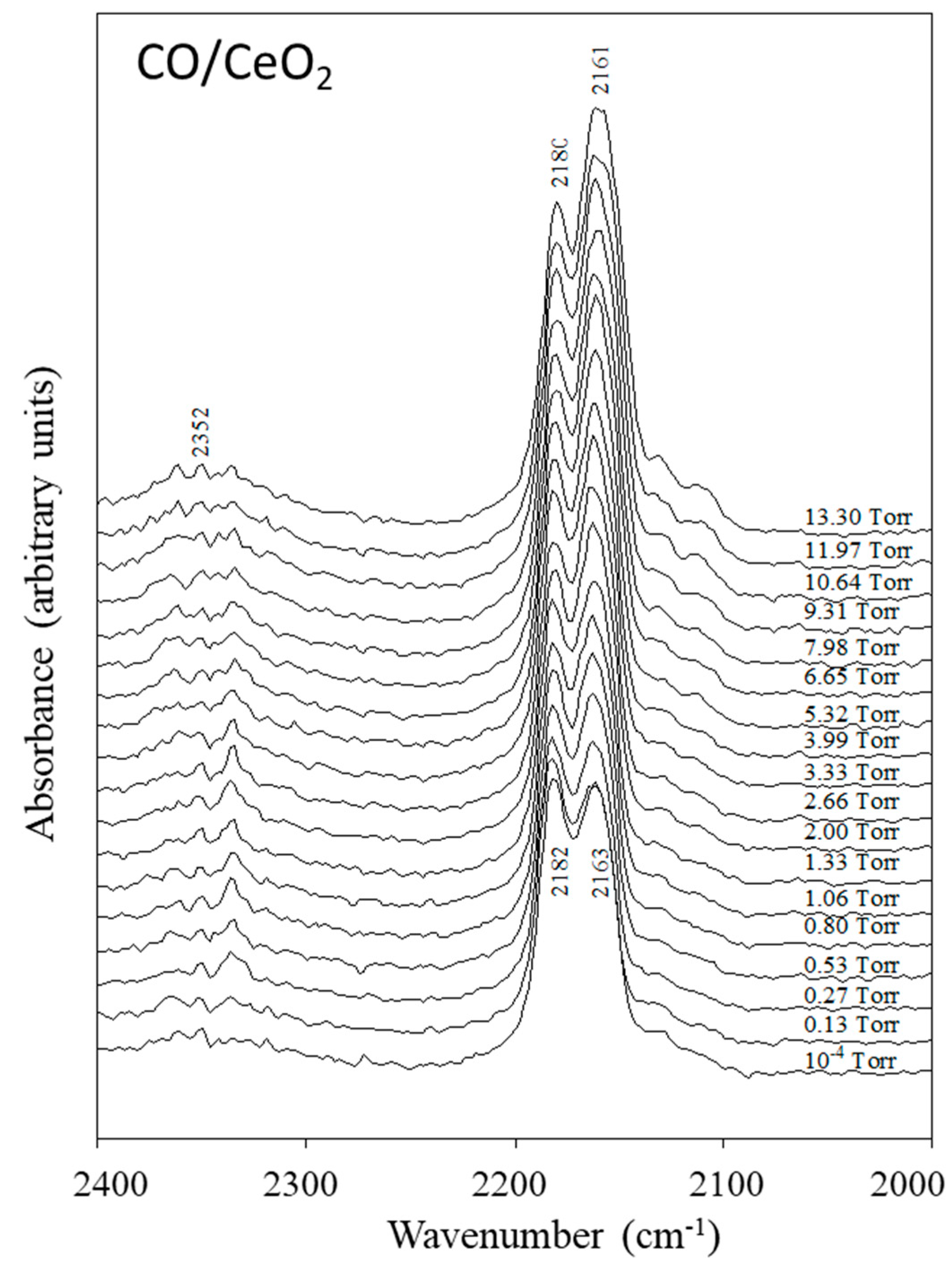
2.1.2. Reversible Adsorption
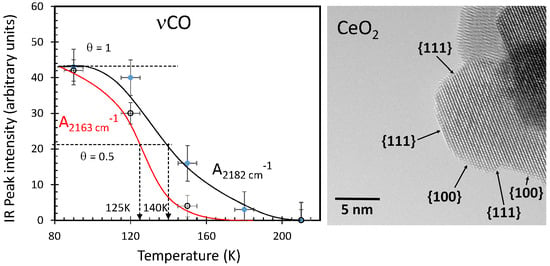
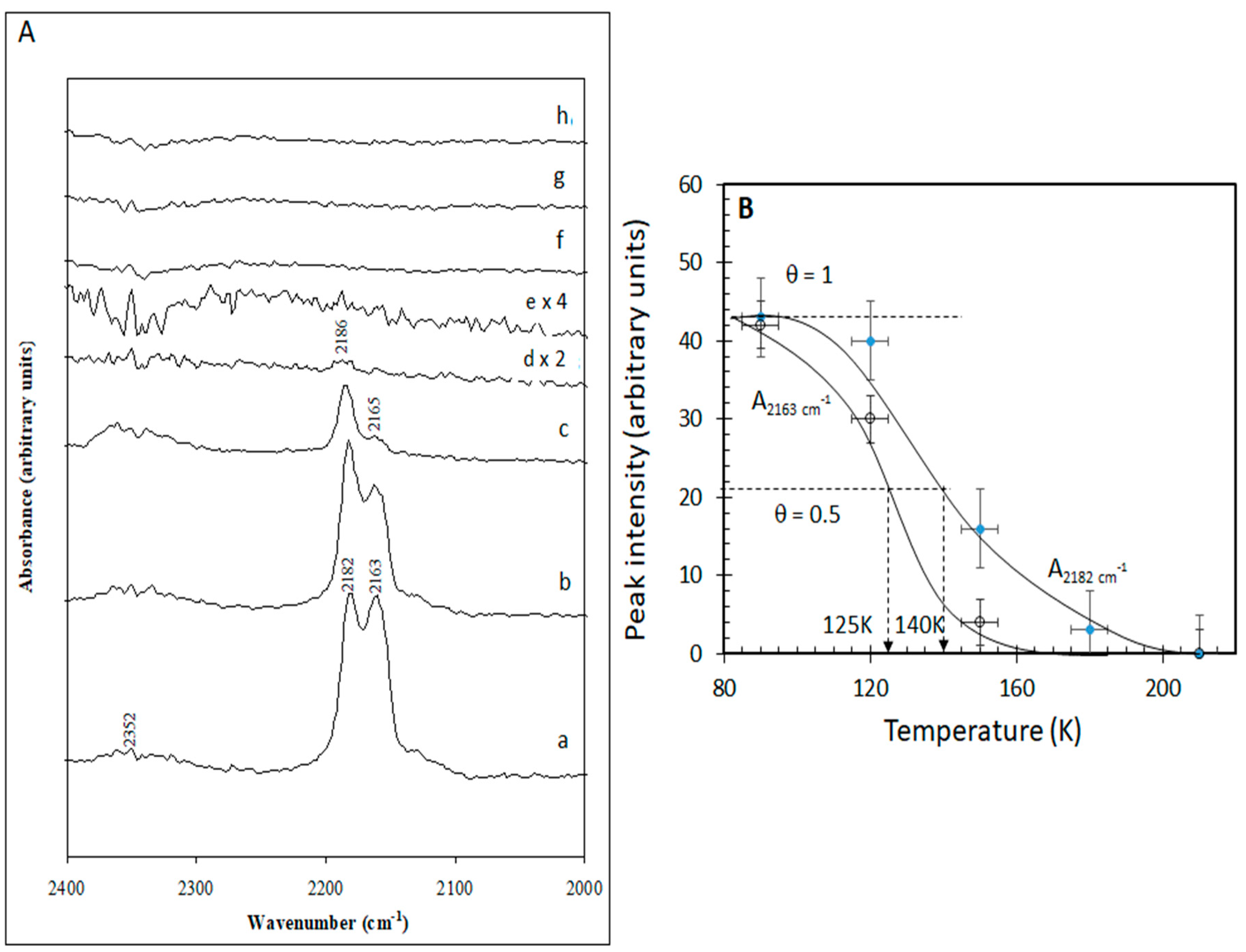
2.1.3. Temperature Effect
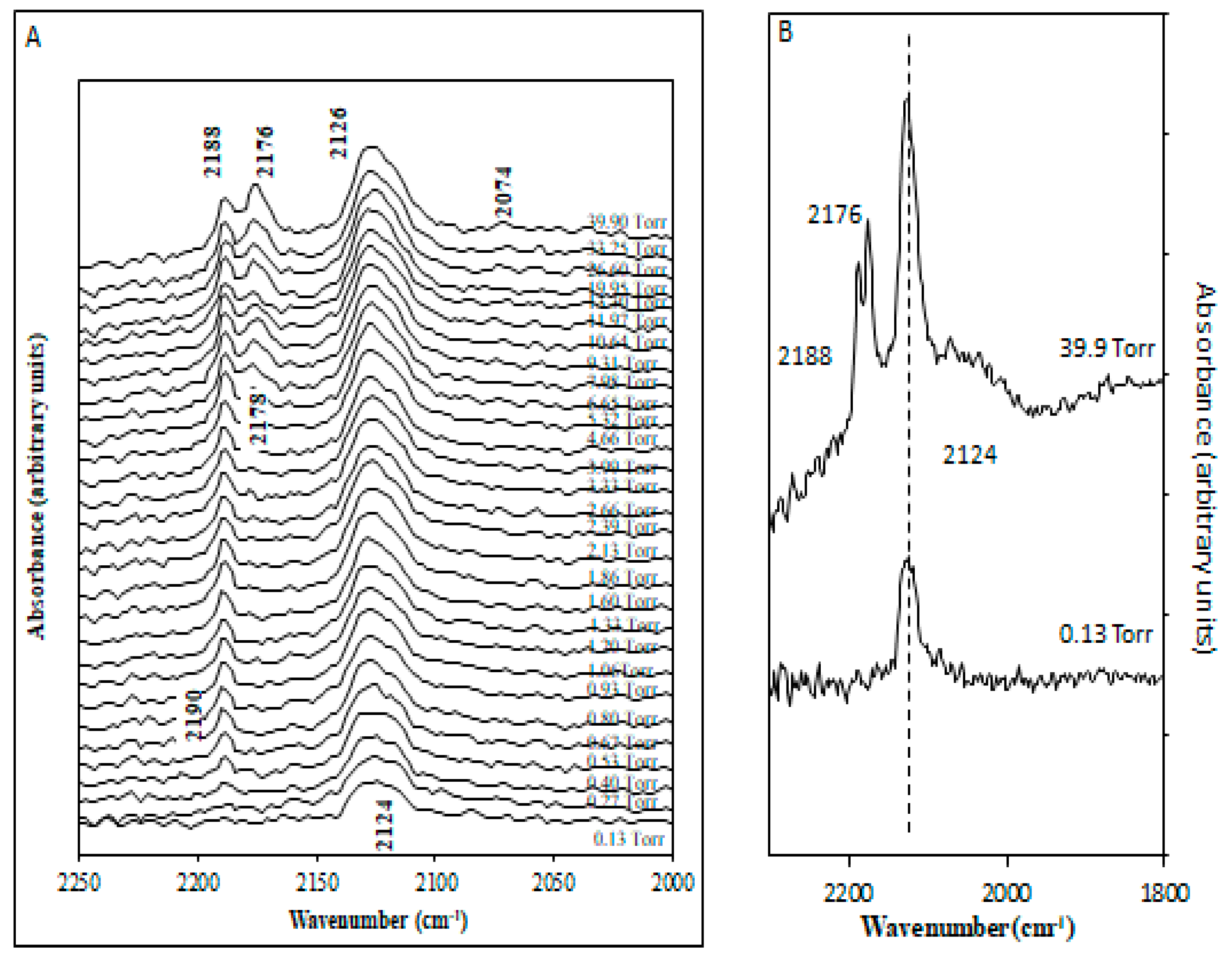
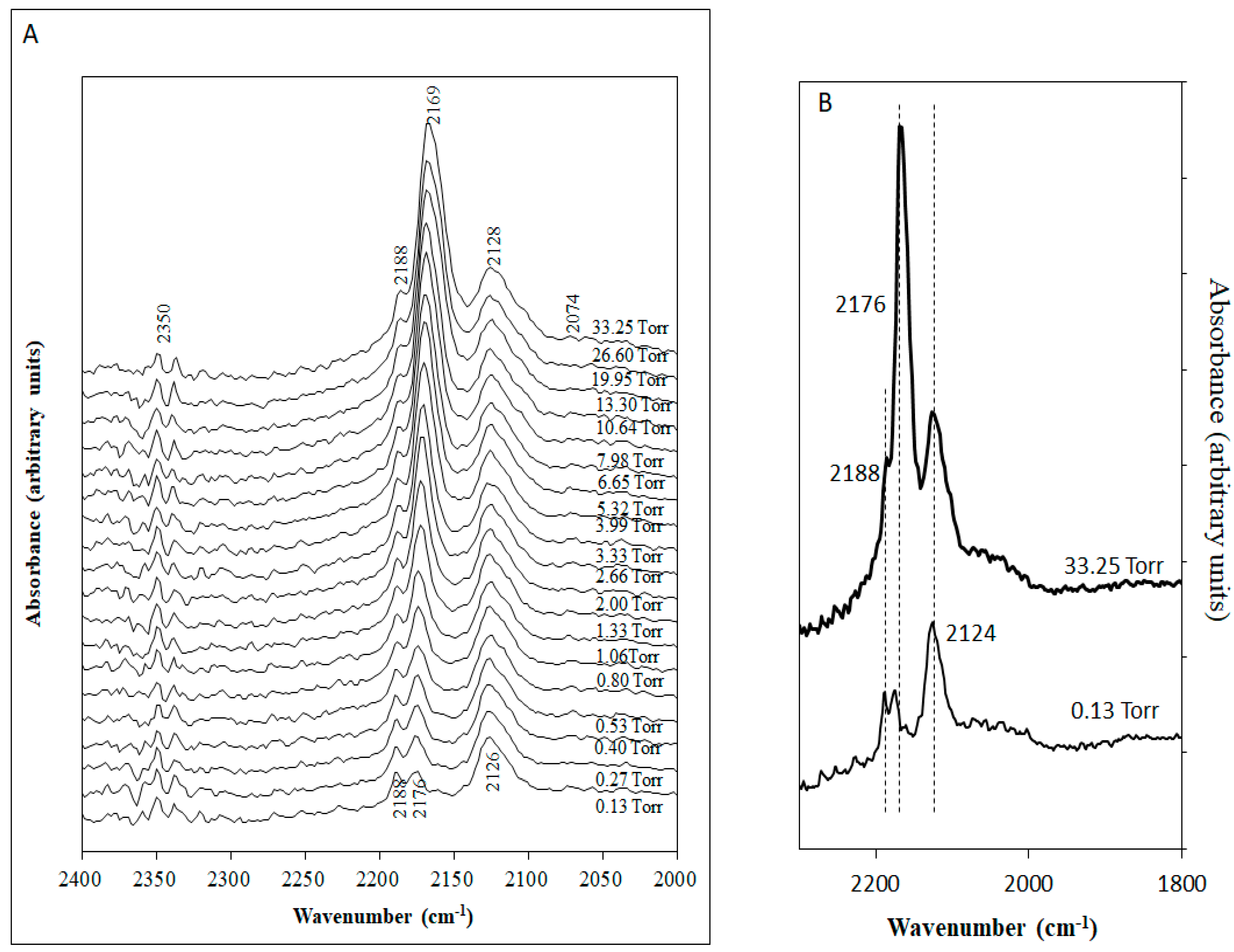
2.2. CO Adsorption on Rh/CeO2
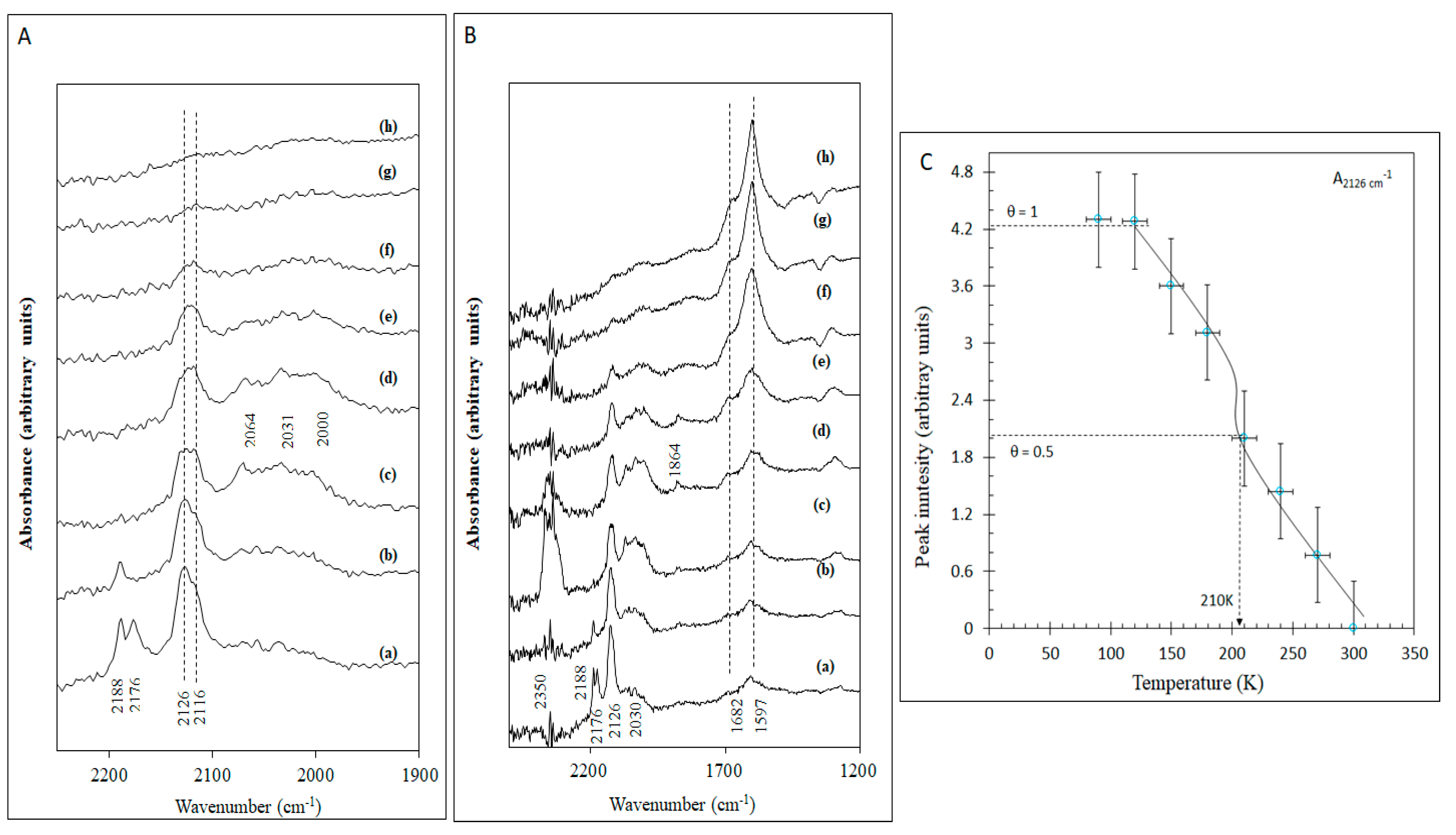
2.2.1. Irreversible Adsorption
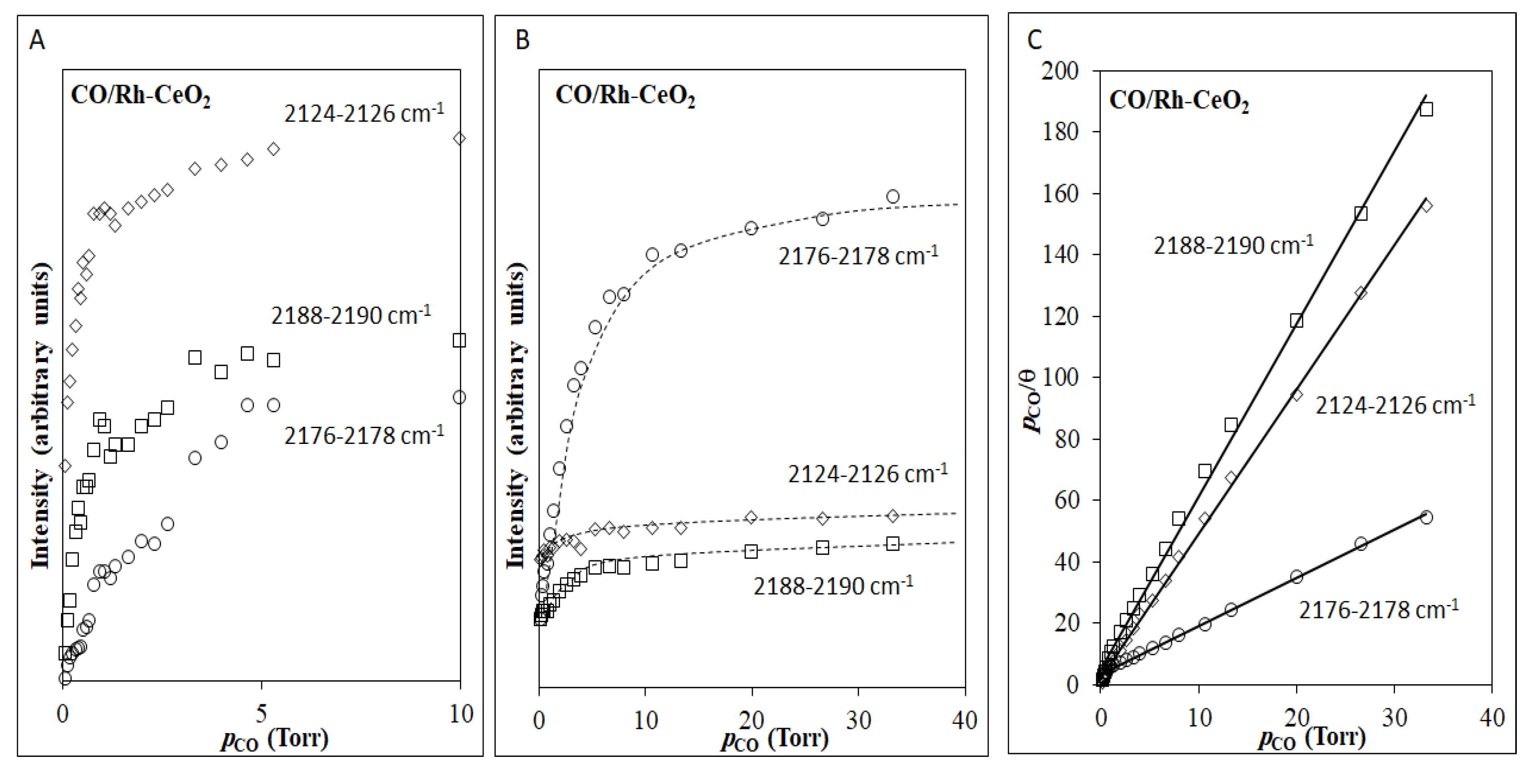
2.2.2. Reversible Adsorption
2.2.3. Temperature Effect
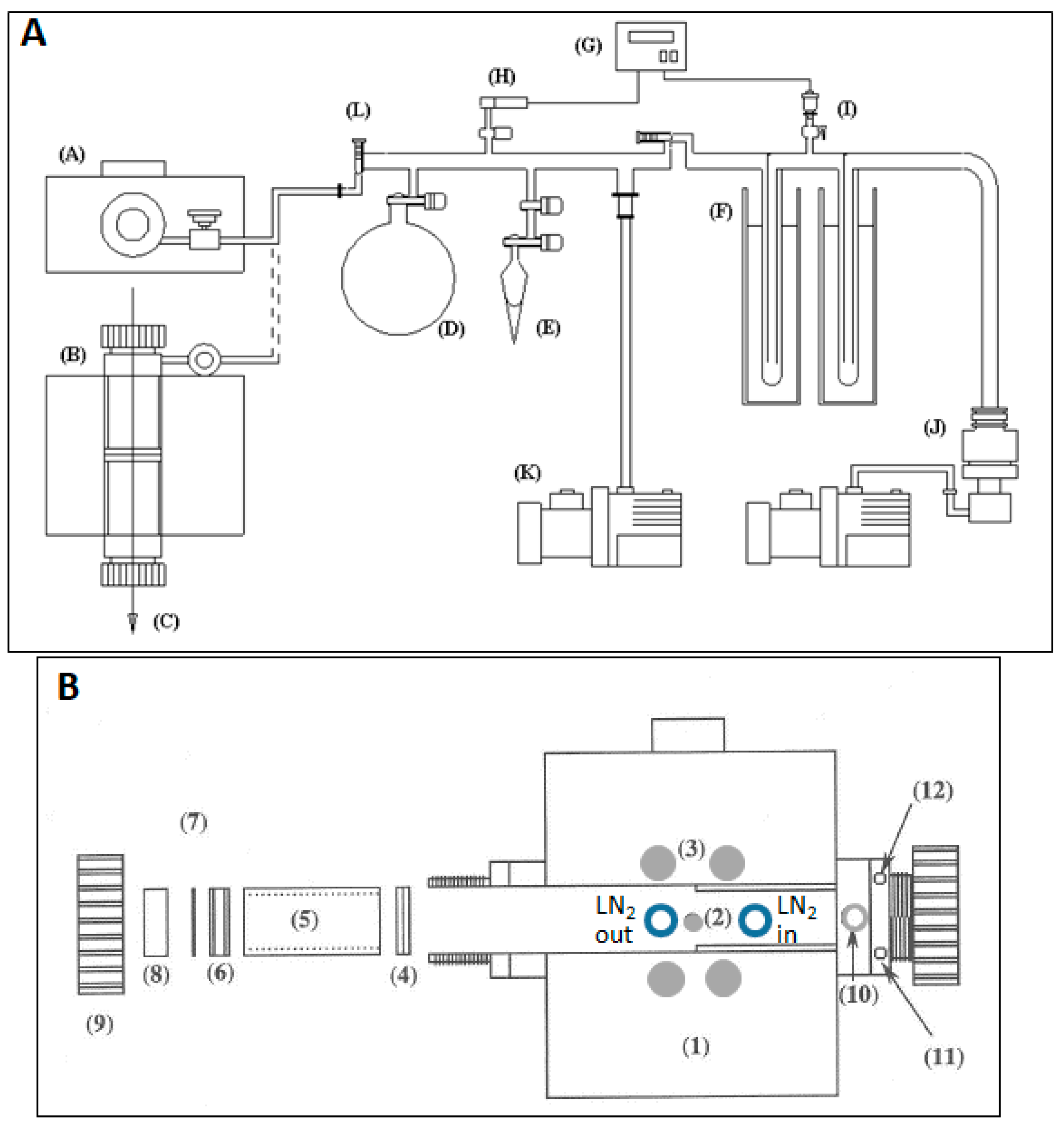
3. Experimental
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, M.; Noei, H.; Fink, K.; Muhler, M.; Wang, Y.; Wöll, C. The Surface Science Approach for Understanding Reactions on Oxide Powders: The Importance of IR Spectroscopy. Angew. Chem. Int. Ed. 2012, 51, 4731–4734. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.E.; Grinberg, I.; Rappe, A.M. Orbital-Specific Analysis of CO Chemisorption on Transition-Metal Surfaces. J. Phys. Chem. C 2008, 112, 1963–1966. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Cui, X.; Huang, T.; Wang, B.; Luo, Y.; Yang, J.; Hou, J. What Are the Adsorption Sites for CO on the Reduced TiO2(110)-1 × 1 Surface? J. Am. Chem. Soc. 2009, 131, 7958–7959. [Google Scholar] [CrossRef]
- Soon, A.; Söhnel, T.; Idriss, H. Plane-wave pseudopotential density functional theory periodic slab calculations of CO adsorption on Cu2O(111) surface. Surf. Sci. 2005, 579, 131–140. [Google Scholar] [CrossRef]
- Blomqvist, J.; Lehmany, L.; Salo, P. CO adsorption on metal-oxide surfaces doped with transition-metal adatoms. Phys. Status Solidi B 2012, 249, 1046–1057. [Google Scholar] [CrossRef]
- Huang, M.; Fabris, S. CO Adsorption and Oxidation on Ceria Surfaces from DFT+U Calculations. J. Phys. Chem. C 2008, 112, 8643–8648. [Google Scholar] [CrossRef]
- Li, C.; Sakata, Y.; Arai, T.; Domen, K.; Maruya, K.I.; Onishi, T.J. Adsorption of Carbon Monoxide and Carbon Dioxide on Cerium Oxide studied by Fourier- transform Infrared Spectroscopy. Part 2.-Formation of Formate Species on Partially Reduced CeO, at Room Temperature. J. Chem. Soc. Faraday Trans. I Phys. Chem. Condens. Phases 1989, 85, 1451–1461. [Google Scholar] [CrossRef]
- Yang, C.; Yu, X.; Heißler, S.; Nefedov, A.; Colussi, S.; Llorca, J.; Trovarelli, A.; Wang, Y.; Christof Wöll, C. Surface Faceting and Reconstruction of Ceria Nanoparticles. Angew. Chem. Int. Ed. 2017, 56, 375–379. [Google Scholar] [CrossRef]
- Rohmann, C.; Metson, J.B.; Idriss, H. DFT study of carbon monoxide adsorption on α-Al2O3(0001). Surf. Sci. 2011, 605, 1694–1703. [Google Scholar] [CrossRef]
- Khriachtchev, L. Physics and Chemistry at Low Temperatures; Pan Stanford Publishing: Singapore, 2011. [Google Scholar]
- Wang, Y.; Wöll, C. IR spectroscopic investigations of chemical and photochemical reactions on metal oxides: bridging the materials gap. Chem. Soc. Rev. 2017, 46, 1875–1932. [Google Scholar] [CrossRef]
- Song, Y.L.; Yin, L.L.; Zhang, J.; Hu, P.; Gong, X.Q.; Lu, G. A DFT + U study of CO oxidation at CeO2(110) and (111) surfaces with oxygen vacancies. Surf. Sci. 2013, 618, 140–147. [Google Scholar] [CrossRef]
- Spezzati, G.; Benavidez, A.D.; DeLaRiva, A.T.; Su, Y.; Hofmann, J.P.; Asahina, S.; Olivier, E.J.; Neethling, J.H.; Millere, J.T.; Datye, A.K.; et al. CO oxidation by Pd supported on CeO2(100) and CeO2(111) facets. Appl. Catal. B Environ. 2019, 243, 36–46. [Google Scholar] [CrossRef]
- Song, W.; Chen, L.; Deng, J.; Jing, M.; Zheng, H.; Liu, J.; Zhao, Z. Combination of Density Functional Theory and Microkinetic Study to the Mn-Doped CeO2 Catalysts for CO Oxidation: A Case Study to Understand the Doping Metal Content. J. Phys. Chem. C 2018, 122, 25290−25300. [Google Scholar] [CrossRef]
- Mei, D.; Ge, Q. A DFT + U study of structure and reducibility of CenO2n-x (n ≤ 4, 0 ≤ x ≤ n) nanoclusters. Comput. Theor. Chem. 2012, 987, 25–31. [Google Scholar] [CrossRef]
- Graciani, J.; Márquez, A.M.; Plata, J.J.; Ortega, Y.; Hernández, N.C.; Meyer, A.; Zicovich-Wilson, C.M.; Sanz, J.F. Comparative Study on the Performance of Hybrid DFT Functionals in Highly Correlated Oxides: The Case of CeO2 and Ce2O3. J. Chem. Theory Comput. 2011, 7, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mullins, D.R.; Overbury, S.H. CO desorption and oxidation on CeO2-supported Rh: Evidence for two types of Rh sites. J. Catal. 2006, 243, 158–164. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Soria, J.; Conesa, J.C. Spectroscopic Study of Active Phase-Support Interactions on a RhOx /CeO2 Catalyst: Evidence for Electronic Interactions. J. Catal. 1997, 168, 364–373. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Sun, T.; Wang, S. The Promoting Effect of Fe Doping on Rh/CeO2 for the Ethanol Synthesis. Catal. Lett. 2015, 145, 1741–1749. [Google Scholar] [CrossRef]
- Soria, J.; Martinez-Arias, A.; Fierro, J.L.G.; Conesa, J.C. Effect of outgassing treatments on the surface reactivity of Rh/CeO2, catalysts: CO adsorption. Vacuum 1995, 461, 1201–1204. [Google Scholar] [CrossRef]
- Ševccíková, K.; Nehasil, V.; Vorokhta, M.; Stanislav Haviar, S.; Matolín, V.; Matolínová, I.; Mašek, K.; Igor Píš, I.; Kobayashi, K.; Kobata, M.; et al. Altering properties of cerium oxide thin films by Rh doping. Mater. Res. Bull. 2015, 67, 5–13. [Google Scholar]
- Gayen, A.; Priolkar, K.R.; Sarode, P.R.; Jayaram, V.; Hedge, M.S.; Subbanna, G.N.; Emura, S. Ce1-xRhxO2-δ solid solution formation in combustion-synthesized Rh/CeO2 catalyst studied by XRD, TEM XPS, and EXAFS. Chem. Mater. 2004, 16, 2317–2328. [Google Scholar] [CrossRef]
- Sheng, P.Y.; Chiu, W.W.; Yee, A.; Morrison, S.J.; Idriss, H. Hydrogen production from ethanol over bimetallic Rh-M/CeO2 (M = Pd or Pt). Catal. Today 2007, 129, 313–321. [Google Scholar] [CrossRef]
- Frank, M.; Kuhnemuth, R.; Baumer, M.; Freund, H.J. Oxide-supported Rh particle structure probed with carbon monoxide. Surf. Sci. 1999, 427, 288–293. [Google Scholar] [CrossRef]
- Platero, E.E.; Coluccia, S.; Zecchina, A. Dipole coupling and chemical shifts in CO overlayers adsorved on NiO. Surf. Sci. 1986, 171, 465–482. [Google Scholar] [CrossRef]
- Zecchina, A.; Scarano, D.; Bordiga, S.; Ricchiardi, G.; Spoto, G.; Geobaldo, F. IR studies of CO and NO adsorbed on well characterized oxide single microcrystals. Catal. Today 1996, 27, 403–435. [Google Scholar] [CrossRef]
- Scarano, D.; Zecchina, A. Adsorbate-adsorbent and adsorbate-adsorbate interactions in CO overlayers adsorbed on g-Cr20’ 3 as investigated by infrared spectroscopy. Spectrochim. Acta Part A Mol. Spectrosc. 1987, 43, 1441–1445. [Google Scholar] [CrossRef]
- Zaki, M.I.; Vielhaber, B.; Knozinger, H. Low-temperature carbon monoxide adsorption and state of molybdena supported on alumina, titania, ceria, and zirconia. An infrared spectroscopic investigation. J. Phys. Chem. 1986, 90, 3176–3183. [Google Scholar]
- Binet, C.; Daturi, M.; Lavalley, J.C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Tasker, P.W. The stability of ionic crystal surfaces. J. Phys. C: Solid State Phys. 1979, 12, 4977–4984. [Google Scholar] [CrossRef]
- Muggelberg, C.; Castell, M.R.; Briggs, G.A.D.; Goddard, D.T. An STM study of the UO2(100) surface. Appl. Surf. Sci. 1999, 142, 124–128. [Google Scholar] [CrossRef]
- Nolan, M.; Grigoleit, S.; Sayle, D.C.; Parker, S.C. Graeme W. Watson, G.W. Density functional theory studies of the structure and electronic structure of pure and defective low index surfaces of ceria. Surf. Sci. 2005, 576, 217–229. [Google Scholar] [CrossRef]
- Ballinger, T.H.; Yates, J.T. High-Temperature Behavior of Rh/Al2O3 Catalysts. J. Phys. Chem. 1991, 95, 1694–1698. [Google Scholar] [CrossRef]
- Diaz, A.L.; Quigley, W.W.C.; Yamamoto, H.D.; Bussell, M.E. Infrared Spectroscopy and Temperature Programmed Desorption Study of CO on Rh/Al2O3 Catalysts: Probing Overlayer and Support Sites. Langmuir 1994, 10, 1461–1471. [Google Scholar] [CrossRef]
- Wey, J.P.; Neely, W.C.; Worley, S.D. Infrared Spectroscopy at High Pressure: Interaction of CO with Oxidized Rh/Al2O3. J. Catal. 1992, 134, 378–381. [Google Scholar] [CrossRef]
- Risse, T.; Carlsson, A.; Bäumer, M.; Klüner, T.; Freund, H.J. Using IR intensities as a probe for studyingthe surface chemical bond. Surf. Sci. 2003, 546, L829–L835. [Google Scholar] [CrossRef]
- Greenler, R.G.; Dudek, J.A.; Beck, D.E. Field enhancement at crystal steps: An intensity effect in vibrational spectra of adsorbed molecules. Surf. Sci. 1984, 145, L453–L458. [Google Scholar] [CrossRef]
- Hayden, B.E.; Kretzschmar, K.; Bradshaw, A.M.; Greenler, R.G. An infrared study of the adsorption of CO on a stepped platinum surface. Surf. Sci. 1985, 149, 394–406. [Google Scholar] [CrossRef]
- Mullins, D.R. The surface chemistry of cerium oxide. Surf. Sci. Rep. 2015, 70, 42–85. [Google Scholar] [CrossRef]
- Bashir, S.; Idriss, H. Mechanistic study of the role of Au, Pd and Au–Pd in the surface reactions of ethanol over TiO2 in the dark and under photo-excitation. Catal. Sci. Technol. 2017, 7, 5301–5320. [Google Scholar] [CrossRef]
- Yang, Z.; Woo, T.K.; Hermansson, K. Strong and weak adsorption of CO on CeO2 surfaces from first principles calculations. Chem. Phys. Lett. 2004, 396, 384–392. [Google Scholar] [CrossRef]
- Chen, H.T.; Chang, J.G. Computational Investigation of CO Adsorption and Oxidation on Iron-Modified Cerium Oxide. J. Phys. Chem. C 2011, 115, 14745–14753. [Google Scholar] [CrossRef]
- Idriss, H.; Scott, M.; Llorca, J.; Chan, S.C.; Chiu, W.; Sheng, P.Y.; Yee, A.; Blackford, M.A.; Pas, S.J.; Hill, A.J.; et al. A phenomenological study of the metal/oxide interface: The role of catalysis in hydrogen production from renewable sources. ChemSusChem: Chem. Sustain. Energy Mater. 2008, 1, 905–910. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idriss, H.; Llorca, J. Low Temperature Infrared Study of Carbon Monoxide Adsorption on Rh/CeO2. Catalysts 2019, 9, 598. https://doi.org/10.3390/catal9070598
Idriss H, Llorca J. Low Temperature Infrared Study of Carbon Monoxide Adsorption on Rh/CeO2. Catalysts. 2019; 9(7):598. https://doi.org/10.3390/catal9070598
Chicago/Turabian StyleIdriss, Hicham, and Jordi Llorca. 2019. "Low Temperature Infrared Study of Carbon Monoxide Adsorption on Rh/CeO2" Catalysts 9, no. 7: 598. https://doi.org/10.3390/catal9070598
APA StyleIdriss, H., & Llorca, J. (2019). Low Temperature Infrared Study of Carbon Monoxide Adsorption on Rh/CeO2. Catalysts, 9(7), 598. https://doi.org/10.3390/catal9070598