Numerical Simulation and Experimental Study on Commercial Diesel Reforming Over an Advanced Pt/Rh Three-Way Catalyst
Abstract
:1. Introduction
2. Theory and Experiment
2.1. Catalytic Reforming Kinetics of Representative Hydrocarbons
2.2. Catalyst Preparation
2.3. Catalyst Characterizations
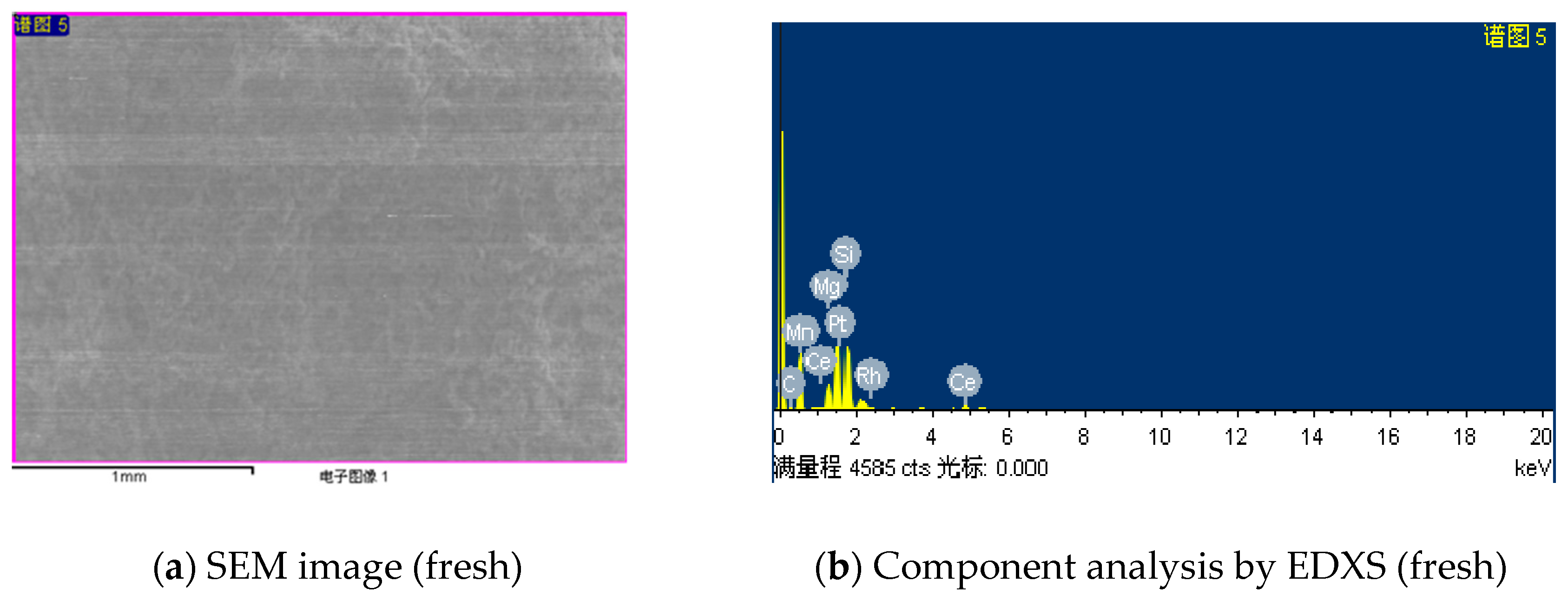
- Scanning Electronic Microscopy (SEM) is adopted using a JSM-5610LV (JEOL Ltd., Tokyo, Japan) and energy-dispersive X-ray spectroscopy (EDXS) with a Phoenix detector (EDAX, Mahwah, NJ, USA). The fresh catalyst samples are deposited on carbon double-face tape for SEM and EDXS analysis, simultaneously they are substituted with silicon supports for used catalysts to analyze the carbon deposition.
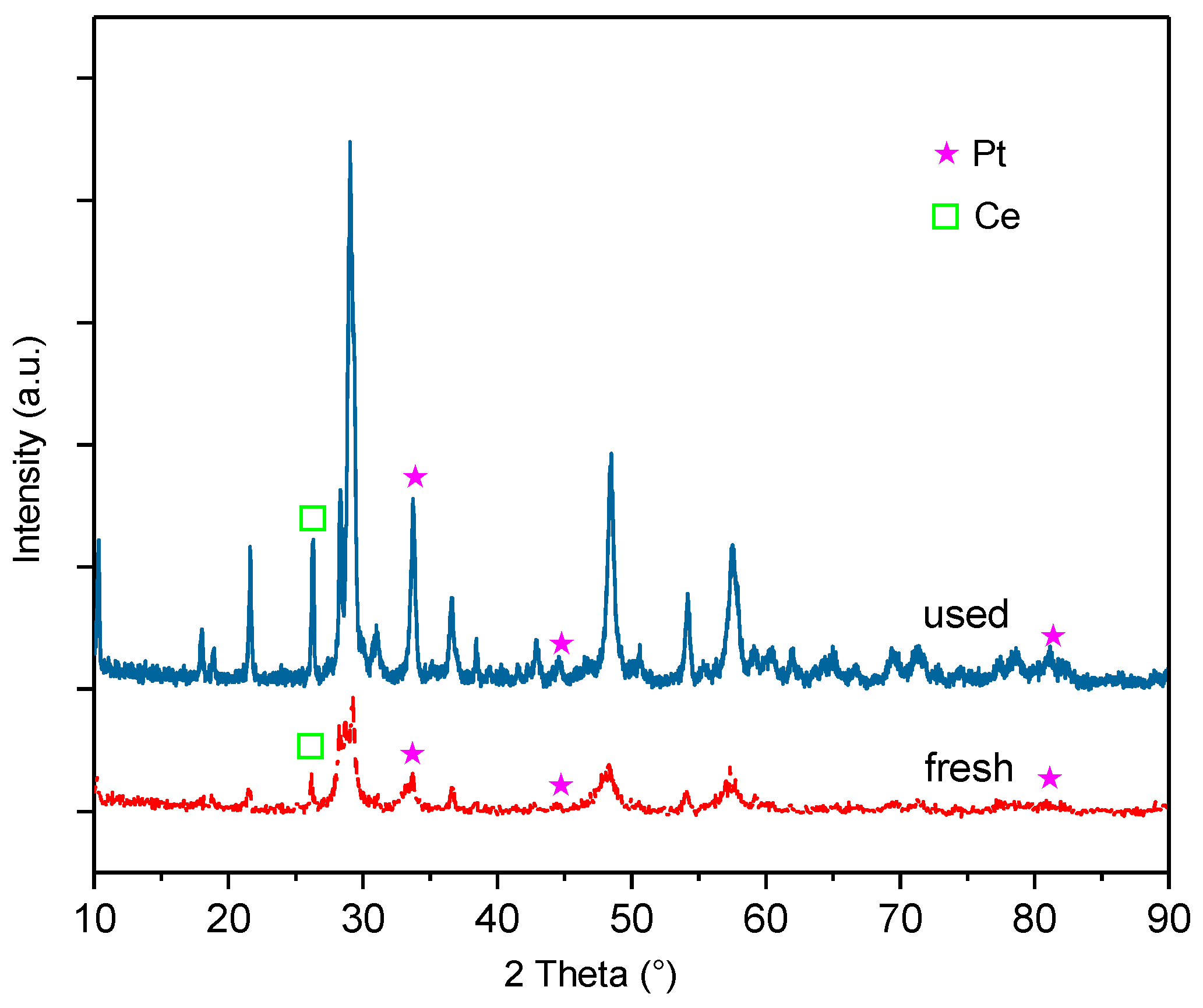
- X-ray diffraction (XRD) is adopted to determine the crystal phase of the powder samples. A Bruker D8 Advance scanning 2θ from 10° to 90° in the scan mode (0.02°, 1s), using Ni filtered Cu Kα radiation is employed. The samples are pre-reduced offline under an H2 flow at 500 °C for 2 h. The crystal phase is identified by comparing the diffractograms with powder diffraction files.
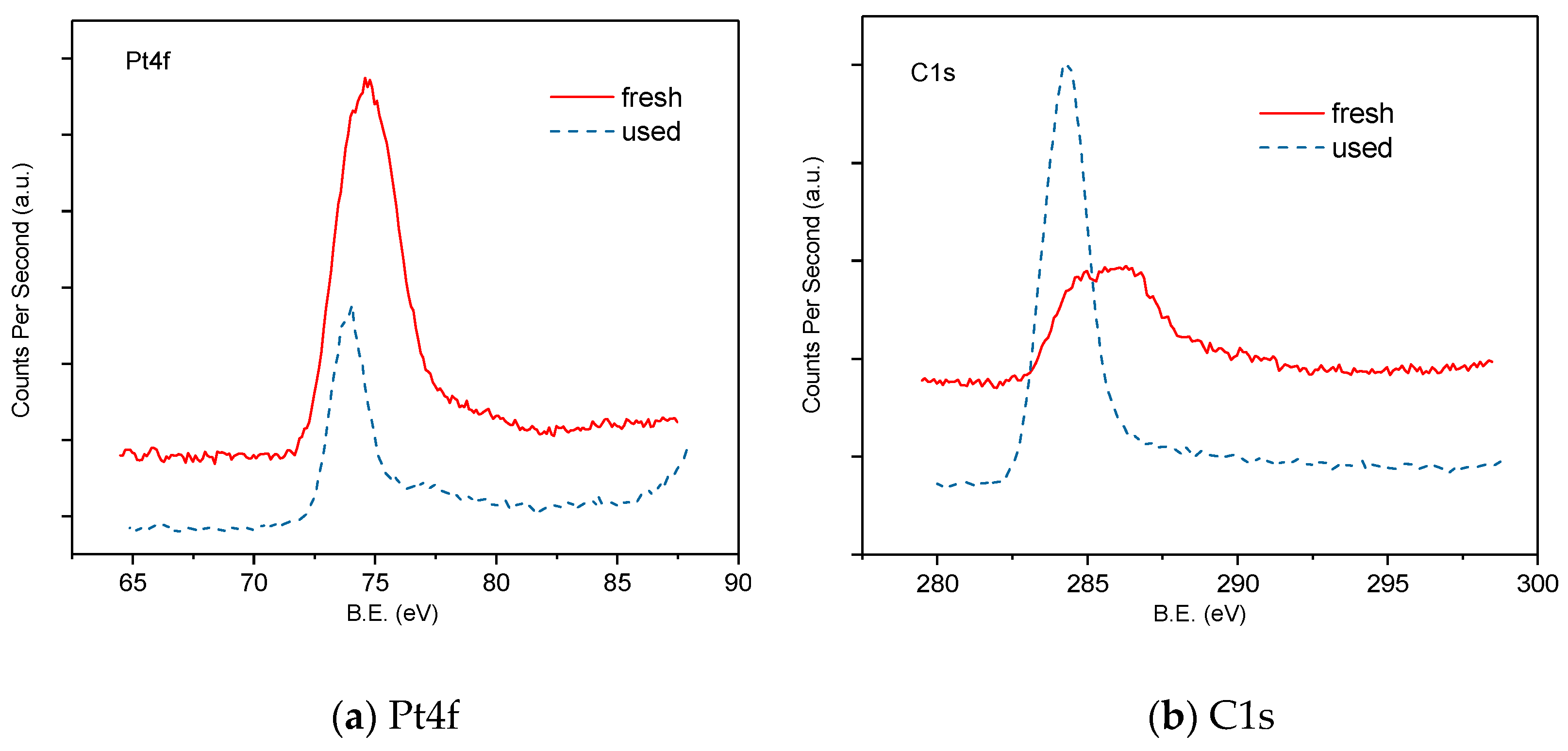
- X-ray photoelectron spectroscopy (XPS) is conducted using a Thermo Fisher Scientific ESCALAB 250xi scanning ESCA microprobe (Waltham, MA, USA) with double anode Al/Mg radiation and a multichannel detector. Prior to the measurement, the samples are reduced in H2 at 400 °C for 2 h.
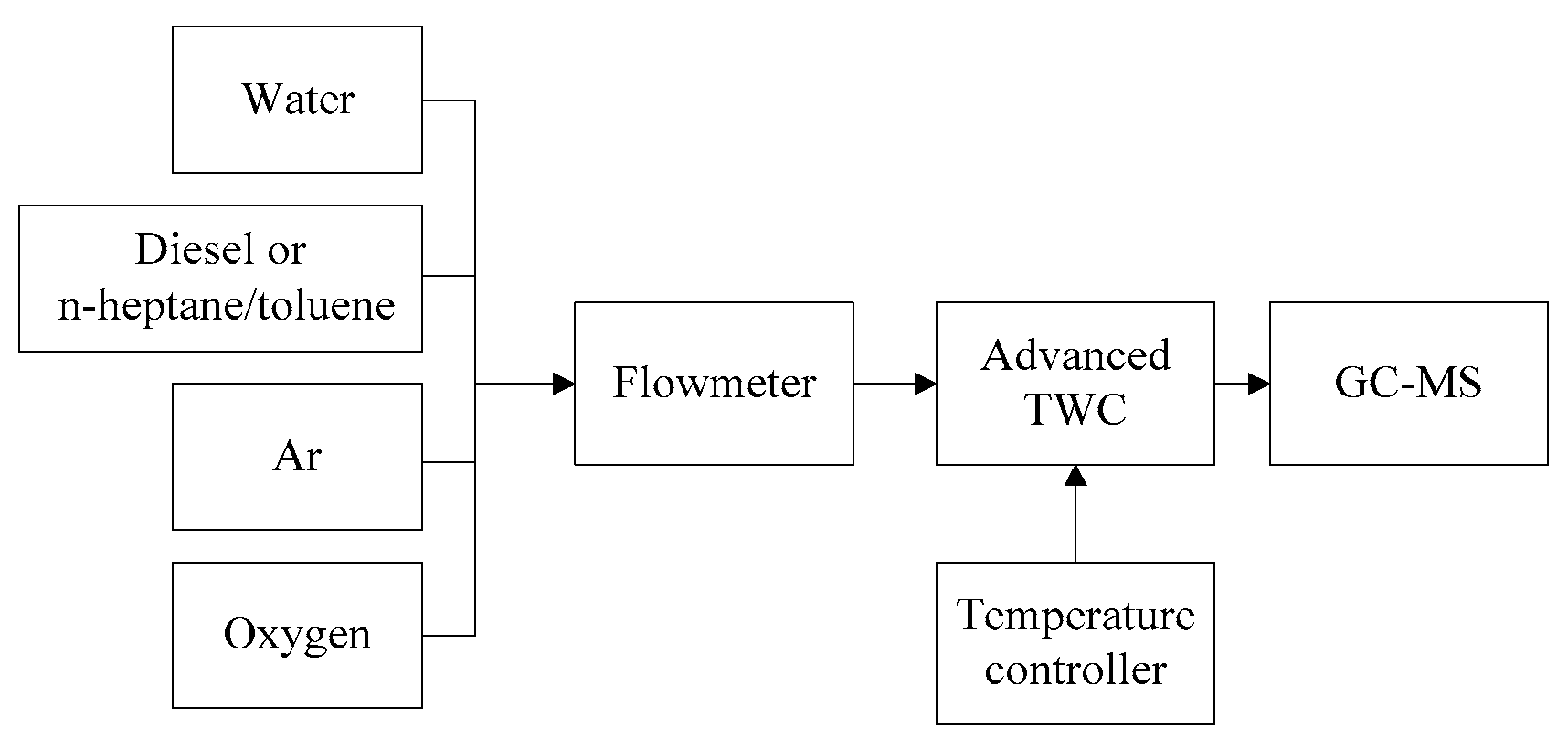
2.4. Experimental Procedure
3. Results and Discussion
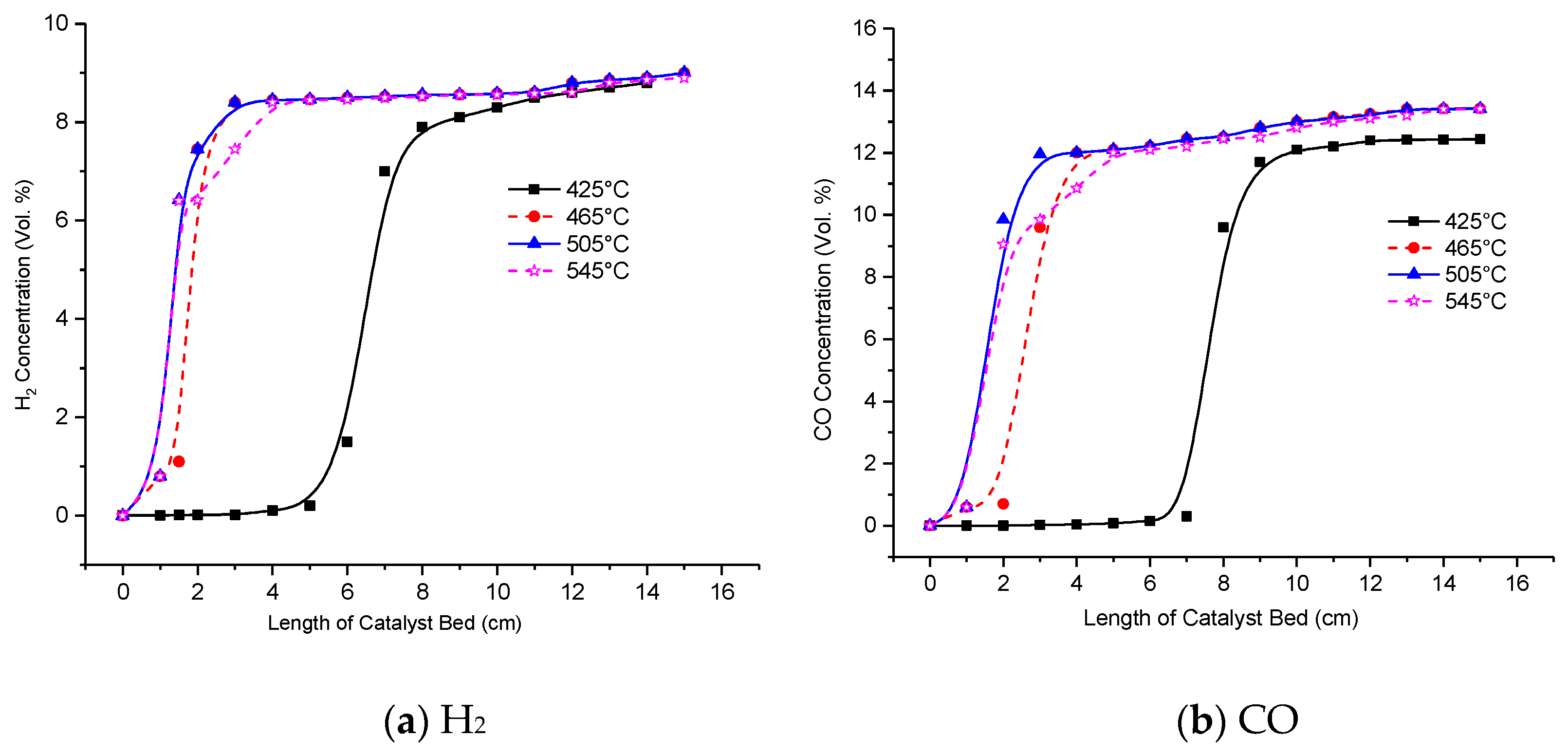
3.1. Effect of Reaction Temperature on Reformer Product
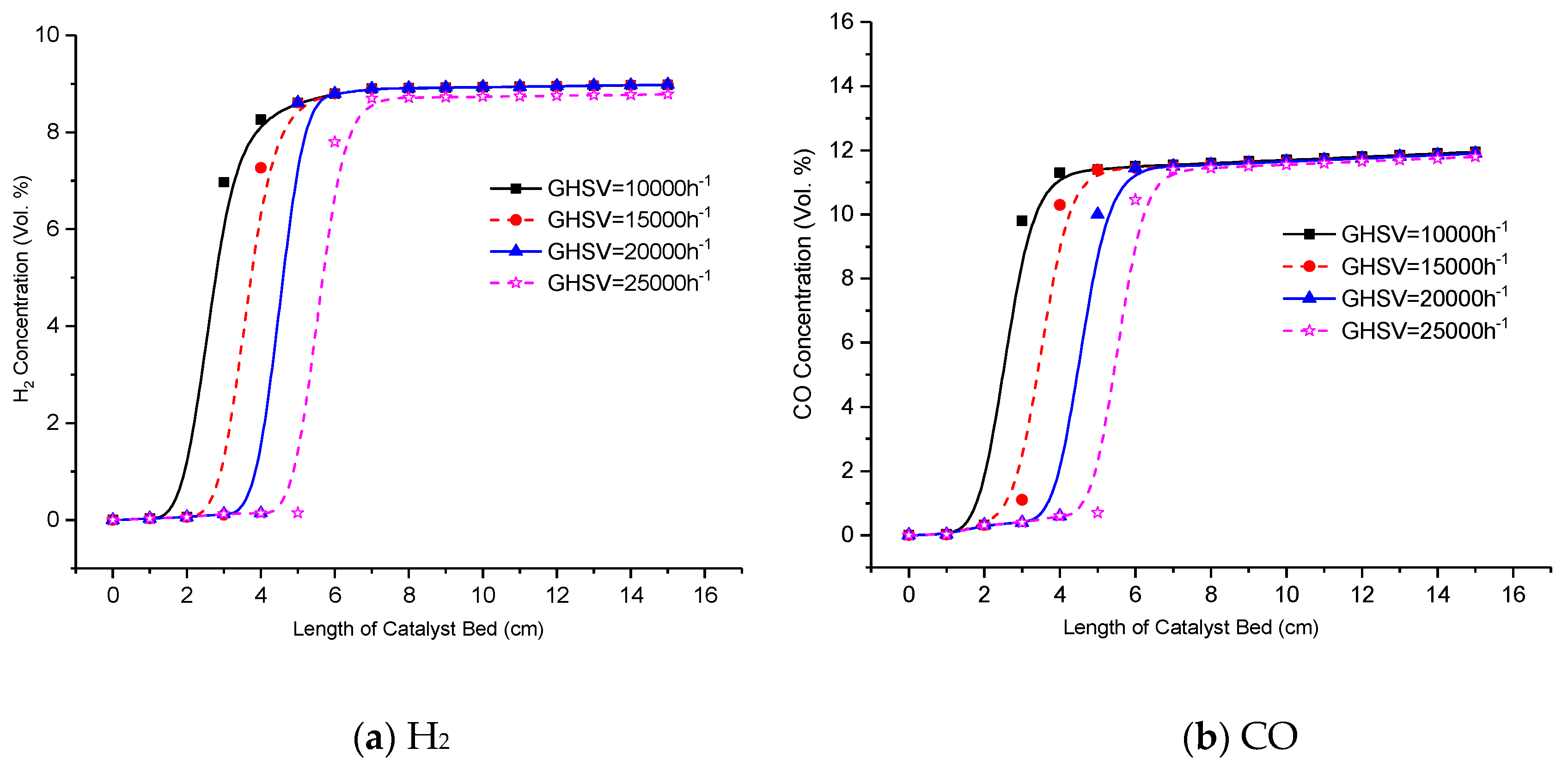
3.2. Effect of GHSV on the Reformer Product
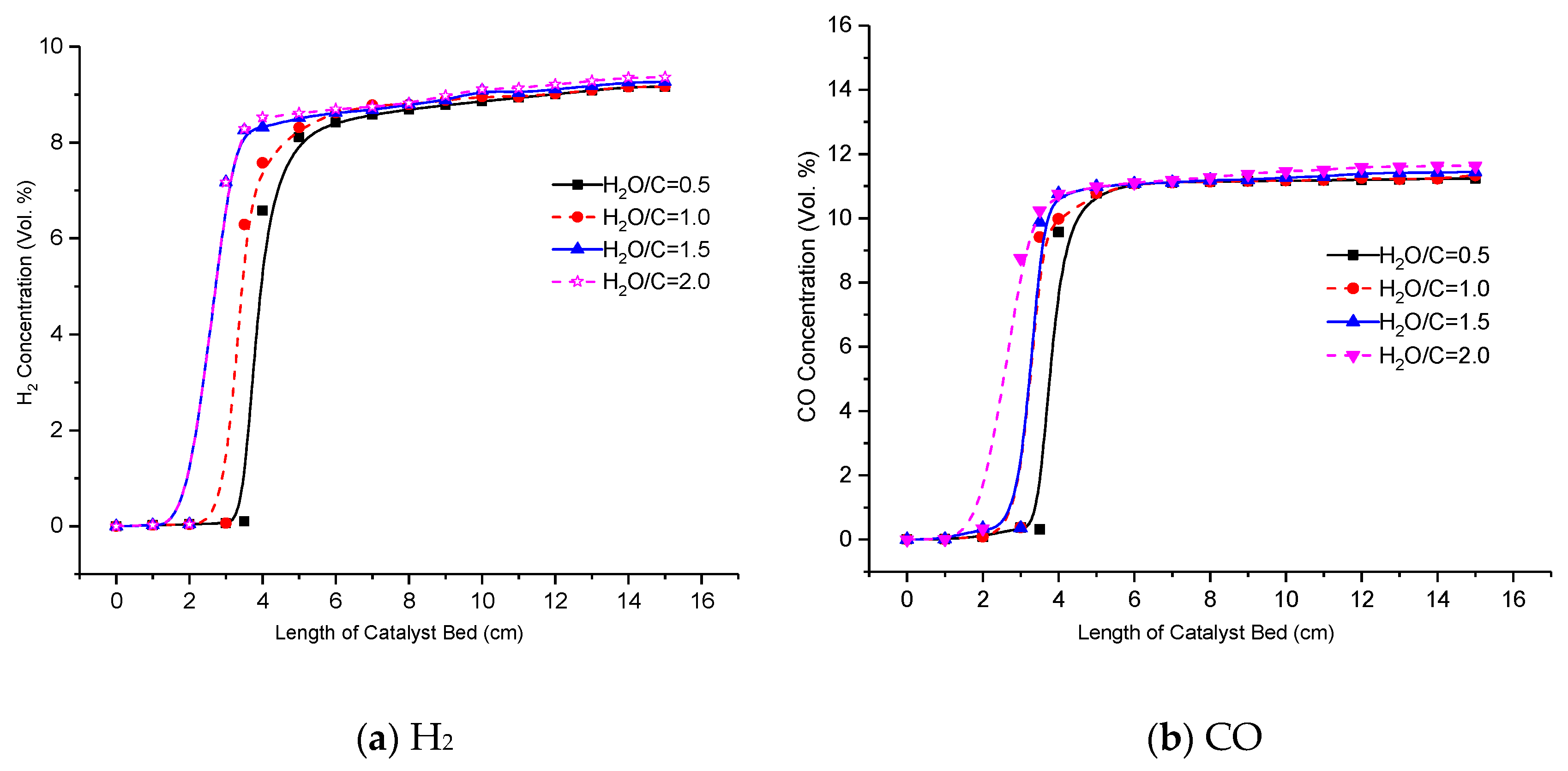
3.3. Effect of H2O/C Ratio on Reformer Product
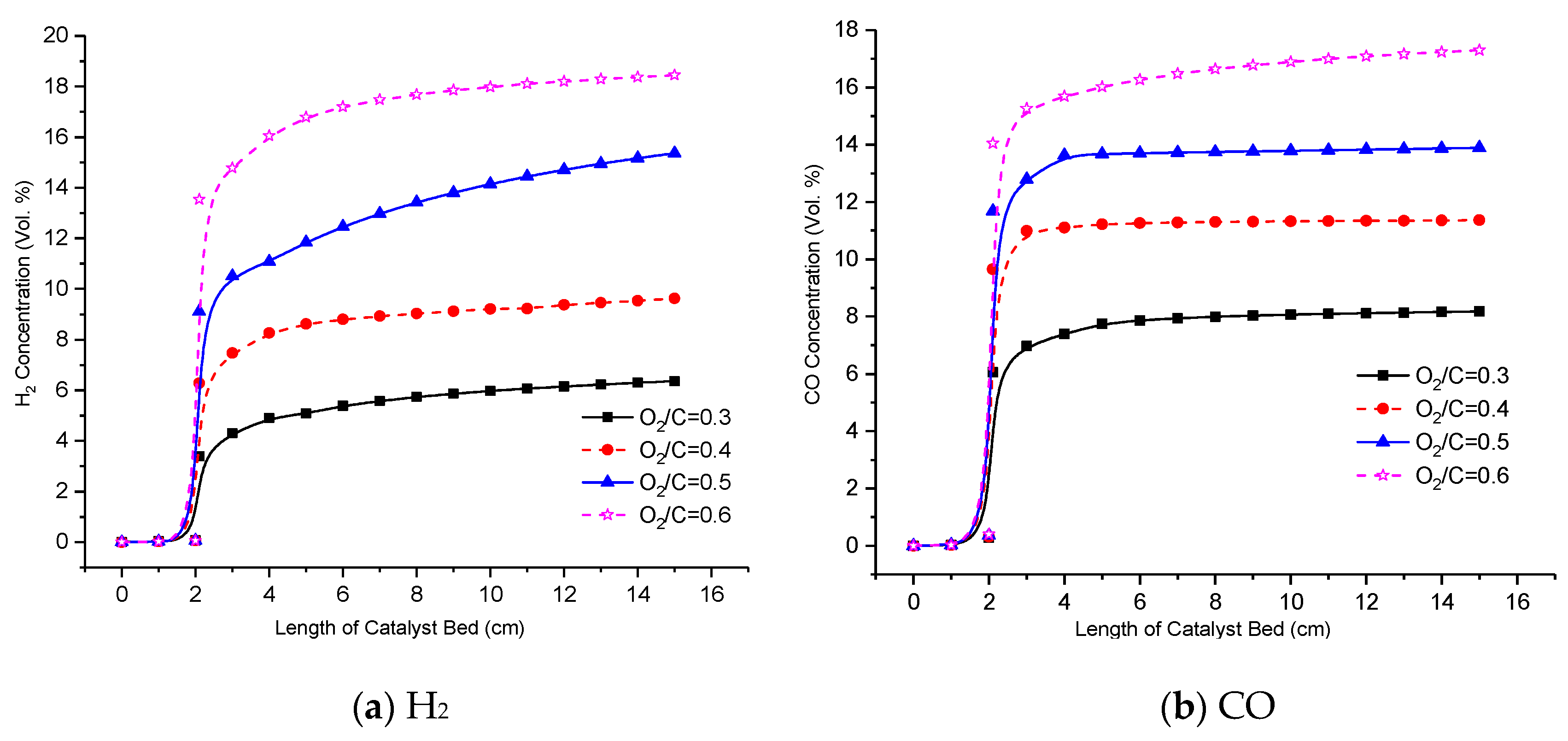
3.4. Effect of the O2/C Ratio on Reformer Product
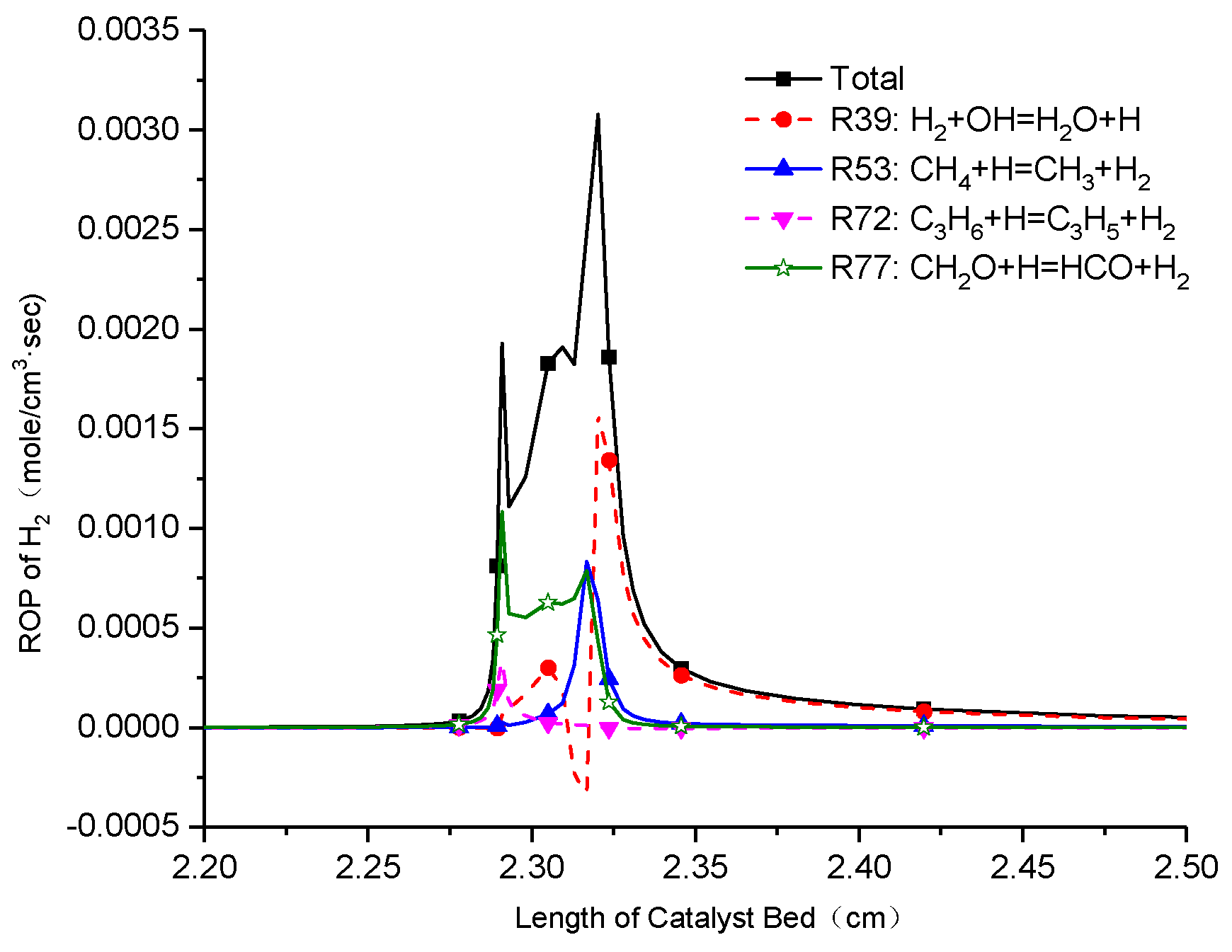
3.5. Sensitivity and Rate-of-Production Analysis of the H2 Concentration
3.6. Texture Analysis of the Catalyst
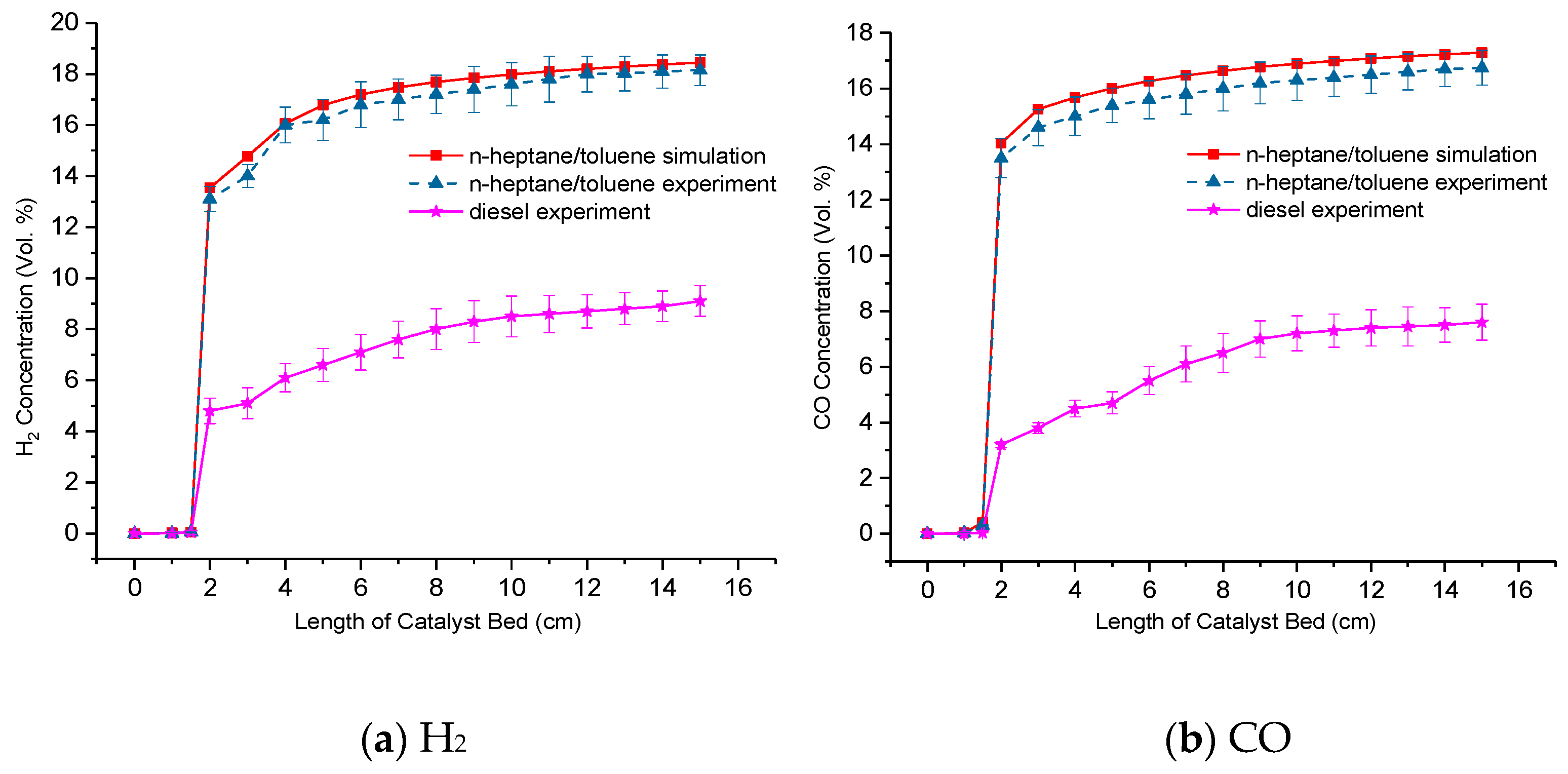
3.7. Experimental Analysis Results of H2 and CO Production
4. Conclusions
- In consideration of the stoichiometric ratio of H2/CO, the POX reaction is most likely to occur during the reaction process owing to the partial oxidation reforming of toluene, and other reactions involved in n-heptane/toluene reforming may be SR or ATR.
- The optimization of the reforming reaction conditions and the catalyst to acquire further conversion of the carbon monoxide to hydrogen by the WGSR will boost the produced hydrogen levels even further. The preferred reaction conditions (reaction temperature 505 °C, GHSV 10000 1/h, H2O/C ratio 2.0 and O2/C ratio 0.6) are determined.
- The reaction of OH/H2O2 chemistry and the H abstraction reaction of n-heptane by OH are the most sensitive reactions on the H2 yield, as well as the reforming process of n-heptane/toluene moves forward to H2 yield.
- The coke formation and carbon deposition take place on the catalyst surface by SEM/EDXS and XPS analysis. The diffraction peaks ascribed to metallic Pt are observed, and no obvious peaks characteristic of Rh are detected by the XRD pattern analysis.
- The characteristics of concentration trend for n-heptane/toluene reforming can represent the H2 and CO yield features for diesel reforming in a way, while the difference of the average H2 and CO concentration results is remarkable. In order to benefit from representing the diesel reforming as accurately as possible by means of a numerical simulation method, a multi-component wide distillation surrogate fuel ought to be proposed in further studies.
- Future work comprises the investigation of the different impacts of a multi-component wide distillation surrogate fuel reforming on the H2 and CO concentration. Additionally, the plan is to study the physical and chemical properties of the catalyst before and after surrogate diesel reforming tests.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| TWC | three-way catalyst |
| SEM | scanning electron microscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| POX | partial oxidation reforming |
| SR | steam reforming |
| ATR | autothermal reforming |
| PAH | polynuclear aromatic hydrocarbons |
| CFFBR | circulating fast fluidized bed reactors |
| CFFBMR | circulating fast fluidized bed membrane reactors |
| ALD | atomic layer deposition |
| TG | thermal gravimetric |
| GHSV | gas hourly space velocity |
| WGSR | water-gas shift reaction |
| ROP | rate-of-production |
| EDXS | energy-dispersive X-ray spectroscopy |
References
- Granlund, M.Z.; Jansson, K.; Nilsson, M.; Dawody, J.; Pettersson, L.J. Evaluation of Co, La, and Mn promoted Rh catalysts for autothermal reforming of commercial diesel: Aging and characterization. Appl. Catal. B Environ. 2015, 172, 145–153. [Google Scholar] [CrossRef]
- Xu, X.; Li, P.; Shen, Y. Small-scale reforming of diesel and jet fuels to make hydrogen and syngas for fuel cells: A review. Appl. Energy 2013, 108, 202–217. [Google Scholar] [CrossRef]
- Bowers, B.; Zhao, J.; Ruffo, M.; Khan, R.; Dattatraya, D.; Dushman, N.; Beziat, J.; Boudjemaa, F. Onboard fuel processor for PEM fuel cell vehicles. Int. J. Hydrogen Energy 2007, 32, 1437–1442. [Google Scholar] [CrossRef]
- Han, G.; Lee, S.; Bae, J. Diesel autothermal reforming with hydrogen peroxide for low-oxygen environments. Appl. Energy 2015, 156, 99–106. [Google Scholar] [CrossRef]
- Tribalis, A.; Panagiotou, G.D.; Bourikas, K.; Sygellou, L.; Kennou, S.; Ladas, S.; Lycourghiotis, A.; Kordulis, C. Ni Catalysts Supported on Modified Alumina for Diesel Steam Reforming. Catalysts 2016, 6, 11. [Google Scholar] [CrossRef]
- Dolanc, G.; Pregelj, B.; Petrovčič, J.; Pasel, J.; Kolb, G. Control of autothermal reforming reactor of diesel fuel. J. Power Sources 2016, 313, 223–232. [Google Scholar] [CrossRef]
- Koo, K.Y.; Park, M.G.; Jung, U.H.; Kim, S.H.; Yoon, W.L. Diesel pre-reforming over highly dispersed nano-sized Ni catalysts supported on MgO–Al2O3 mixed oxides. Int. J. Hydrogen Energy 2014, 39, 10941–10950. [Google Scholar] [CrossRef]
- Zhou, W.; Ke, Y.; Wang, Q.; Wan, S.; Lin, J.; Zhang, J.; Hui, K. Development of cylindrical laminated methanol steam reforming microreactor with cascading metal foams as catalyst support. Fuel 2017, 191, 46–53. [Google Scholar] [CrossRef]
- Martin, S.; Kraaij, G.; Ascher, T.; Baltzopoulou, P.; Karagiannakis, G.; Wails, D.; Wörner, A. Direct steam reforming of diesel and diesel–biodiesel blends for distributed hydrogen generation. Int. J. Hydrogen Energy 2015, 40, 75–84. [Google Scholar] [CrossRef]
- Pasel, J.; Samsun, R.C.; Tschauder, A.; Peters, R.; Stolten, D. A novel reactor type for autothermal reforming of diesel fuel and kerosene. Appl. Energy 2015, 150, 176–184. [Google Scholar] [CrossRef]
- Achouri, I.E.; Abatzoglou, N.; Fauteux-Lefebvre, C.; Braidy, N. Diesel steam reforming: Comparison of two nickel aluminate catalysts prepared by wet-impregnation and co-precipitation. Catal. Today 2013, 207, 13–20. [Google Scholar] [CrossRef]
- Xu, L.; Mi, W.; Su, Q. Hydrogen production through diesel steam reforming over rare-earth promoted Ni/γ-Al2O3 catalysts. J. Nat. Gas Chem. 2011, 20, 287–293. [Google Scholar] [CrossRef]
- Brown, L.F. A comparative study of fuels for on-board hydrogen production for fuel-cell-powered automobiles. Int. J. Hydrogen Energy 2001, 26, 381–397. [Google Scholar] [CrossRef]
- Tsolakis, A.; Megaritis, A.; Golunski, S.E. Reaction profiles during exhaust-assisted reforming of diesel engine fuels. Energy Fuels 2005, 19, 744–752. [Google Scholar] [CrossRef]
- Thormann, J.; Pfeifer, P.; Schubert, K.; Kunz, U. Reforming of diesel fuel in a micro reactor for APU systems. Chem. Eng. J. 2008, 135, S74–S81. [Google Scholar] [CrossRef]
- Ra, Y.; Reitz, R.D. A reduced chemical kinetic model for IC engine combustion simulations with primary reference fuels. Combust. Flame 2008, 155, 713–738. [Google Scholar] [CrossRef]
- Pitz, W.J.; Mueller, C.J. Recent progress in the development of diesel surrogate fuels. Prog. Energy Combust. Sci. 2011, 37, 330–350. [Google Scholar] [CrossRef]
- Wang, H.; Yao, M.; Yue, Z.; Jia, M.; Reitz, R.D. A reduced toluene reference fuel chemical kinetic mechanism for combustion and polycyclic-aromatic hydrocarbon predictions. Combust. Flame 2015, 162, 2390–2404. [Google Scholar] [CrossRef]
- Ra, Y.; Reitz, R.D. A combustion model for IC engine combustion simulations with multi-component fuels. Combust. Flame 2011, 158, 69–90. [Google Scholar] [CrossRef]
- Abashar, M. Steam reforming of n-heptane for production of hydrogen and syngas. Int. J. Hydrogen Energy 2013, 38, 861–869. [Google Scholar] [CrossRef]
- Hamoule, T.; Peyrovi, M.; Rashidzadeh, M.; Toosi, M. Catalytic reforming of n-heptane over Pt/Al-HMS catalysts. Catal. Commun. 2011, 16, 234–239. [Google Scholar] [CrossRef]
- González-Marcos, M.; Iñarra, B.; Guil, J.; Gutiérrez-Ortiz, M. Development of an industrial characterisation method for naphtha reforming bimetallic Pt-Sn/Al2O3 catalysts through n-heptane reforming test reactions. Catal. Today 2005, 107, 685–692. [Google Scholar] [CrossRef]
- Susu, A.A. Comparative study of aromatization selectivity during N-heptane reforming on sintered Pt/Al2O3 and Pt-Re/Al2O3 catalysts. J. Chem. Technol. Biotechnol. 2008, 83, 928–942. [Google Scholar] [CrossRef]
- Lakhapatri, S.L.; Abraham, M.A. Deactivation due to sulfur poisoning and carbon deposition on Rh-Ni/Al2O3 catalyst during steam reforming of sulfur-doped n-hexadecane. Appl. Catal. A Gen. 2009, 364, 113–121. [Google Scholar] [CrossRef]
- Villoria, J.; Alvarez-Galvan, M.C.; Al-Zahrani, S.; Palmisano, P.; Specchia, S.; Specchia, V.; Fierro, J.; Navarro, R.; Al-Zahrani, S.; Yerga, R.N. Oxidative reforming of diesel fuel over LaCoO3 perovskite derived catalysts: Influence of perovskite synthesis method on catalyst properties and performance. Appl. Catal. B Environ. 2011, 105, 276–288. [Google Scholar] [CrossRef]
- Craciun, R.; Shereck, B.; Gorte, R. Kinetic studies of methane steam reforming on ceria-supported Pd. Catal. Lett. 1998, 51, 149–153. [Google Scholar] [CrossRef]
- Wang, X.; Gorte, R. A study of steam reforming of hydrocarbon fuels on Pd/ceria. Appl. Catal. A Gen. 2002, 224, 209–218. [Google Scholar] [CrossRef]
- Faure, R.; Rossignol, F.; Chartier, T.; Bonhomme, C.; Maître, A.; Etchegoyen, G.; Del Gallo, P.; Gary, D. Alumina foam catalyst supports for industrial steam reforming processes. J. Eur. Ceram. Soc. 2011, 31, 303–312. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, J.W.; Kim, M.J.; Park, C.J.; Jeong, Y.H.; Choi, H.-Y.; Park, N.-K.; Lee, T.J. Durability tests of Rh/Al-Ce-Zr catalysts coated on NiCrAl metal foam for ATR of dodecane at high temperature. Int. J. Precis. Eng. Manuf. Technol. 2017, 4, 183–189. [Google Scholar] [CrossRef]
- Xu, D.; Wu, B.; Ren, P.; Wang, S.; Huo, C.; Zhang, B.; Guo, W.; Huang, L.; Wen, X.; Qin, Y.; et al. Controllable deposition of Pt nanoparticles into a KL zeolite by atomic layer deposition for highly efficient reforming of n-heptane to aromatics. Catal. Sci. Technol. 2017, 7, 1342–1350. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Yerga, R.N.; Rosa, F.; Briceño, Y.; Alvarez, F.G.; Fierro, J. Performance of La, Ce-modified alumina-supported Pt and Ni catalysts for the oxidative reforming of diesel hydrocarbons. Int. J. Hydrogen Energy 2008, 33, 652–663. [Google Scholar] [CrossRef]
- Twigg, M.V. Catalytic control of emissions from cars. Catal. Today 2011, 163, 33–41. [Google Scholar] [CrossRef]
- Zheng, T.; He, J.; Zhao, Y.; Xia, W.; He, J. Precious metal-support interaction in automotive exhaust catalysts. J. Rare Earths 2014, 32, 97–107. [Google Scholar] [CrossRef]
- Shaula, A.; Karavai, O.; Ivanova, Y.; Mikhalev, S.; Tarelho, L. Strontium ferrite as a three-way catalyst component. Mater. Lett. 2018, 216, 273–276. [Google Scholar] [CrossRef]
- Sugiura, M. Oxygen Storage Materials for Automotive Catalysts: Ceria-Zirconia Solid Solutions. Catal. Surv. Asia 2003, 7, 77–87. [Google Scholar] [CrossRef]
- Karatzas, X.; Jansson, K.; Dawody, J.; Lanza, R.; Pettersson, L.J. Microemulsion and incipient wetness prepared Rh-based catalyst for diesel reforming. Catal. Today 2011, 175, 515–523. [Google Scholar] [CrossRef]
- Han, Z.; Wang, J.; Yan, H.; Shen, M.; Wang, J.; Wang, W.; Yang, M. Performance of dynamic oxygen storage capacity, water–gas shift and steam reforming reactions over Pd-only three-way catalysts. Catal. Today 2010, 158, 481–489. [Google Scholar] [CrossRef]
- Salazar-Villalpando, M.D. Syn-gas generation in the absence of oxygen and isotopic exchange reactions over Rh & Pt/doped-ceria catalysts. Int. J. Hydrogen Energy 2012, 37, 2121–2128. [Google Scholar]
- Nevalainen, P.; Kinnunen, N.M.; Kirveslahti, A.; Kallinen, K.; Maunula, T.; Keenan, M.; Suvanto, M. Formation of NH 3 and N 2 O in a modern natural gas three-way catalyst designed for heavy-duty vehicles: The effects of simulated exhaust gas composition and ageing. Appl. Catal. A Gen. 2018, 552, 30–37. [Google Scholar] [CrossRef]
- Granlund, M.Z.; Görke, O.; Pfeifer, P.; Pettersson, L.J. Comparison between a micro reactor with multiple air inlets and a monolith reactor for oxidative steam reforming of diesel. Int. J. Hydrogen Energy 2014, 39, 18037–18045. [Google Scholar] [CrossRef]
- Mota, N.; Alvarez-Galvan, M.C.; Al-Zahrani, S.; Navarro, R.; Fierro, J.; Al-Zahrani, S. Diesel fuel reforming over catalysts derived from LaCo1−xRuxO3 perovskites with high Ru loading. Int. J. Hydrogen Energy 2012, 37, 7056–7066. [Google Scholar] [CrossRef]
- Grote, M.; Maximini, M.; Yang, Z.; Engelhardt, P.; Köhne, H.; Lucka, K.; Brenner, M. Experimental and computational investigations of a compact steam reformer for fuel oil and diesel fuel. J. Power Sources 2011, 196, 9027–9035. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, H.; Liu, H.; Zhou, X. Self-sustained electrochemical promotion catalysts for partial oxidation reforming of heavy hydrocarbons. Int. J. Hydrogen Energy 2012, 37, 17928–17935. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Zhou, X.; Liu, H.; Wei, Y.; Pramuanjaroenkij, A.; Bordas, A.; Page, M.; Cai, S.; Zhang, X. Development and Study of Self-Sustained Electrochemical Promotion Catalysts for Hydrocarbon Reforming. ECS Trans. 2013, 58, 243–254. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Pan, Z.; Xu, H. Numerical Simulation and Experimental Investigation of Diesel Fuel Reforming over a Pt/CeO2-Al2O3 Catalyst. Energies 2019, 12, 1056. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, Q.; Yao, M.; Yang, B.; Qiu, L.; Reitz, R.D. Development of an n-heptane/toluene/polyaromatic hydrocarbon mechanism and its application for combustion and soot prediction. Int. J. Engine Res. 2013, 14, 434–451. [Google Scholar] [CrossRef]
- Koop, J.; Deutschmann, O. Detailed surface reaction mechanism for Pt-catalyzed abatement of automotive exhaust gases. Appl. Catal. B Environ. 2009, 91, 47–58. [Google Scholar] [CrossRef]
- Chatterjee, D.; Deutschmann, O.; Warnatz, J. Detailed surface reaction mechanism in a three-way catalyst. Faraday Discuss. 2001, 119, 371–384. [Google Scholar] [CrossRef]
- Chen, H.; Shen, H.; Wu, T.; Zuo, C. Numerical simulation and experimental research on combustion characteristics of compression-ignition engine under an O2/CO2 atmosphere. HKIE Trans. 2017, 24, 121–132. [Google Scholar] [CrossRef]
- Internet: Chemkin@reactiondesign.com. Available online: http://www.reactiondesign.com/products/chemkin/chemkin-2/ (accessed on 6 March 2019).
- Tsolakis, A.; Golunski, S. Sensitivity of process efficiency to reaction routes in exhaust-gas reforming of diesel fuel. Chem. Eng. J. 2006, 117, 131–136. [Google Scholar] [CrossRef]
- Tsolakis, A. Catalytic exhaust gas fuel reforming for diesel engines?effects of water addition on hydrogen production and fuel conversion efficiency. Int. J. Hydrogen Energy 2004, 29, 1409–1419. [Google Scholar] [CrossRef]
- Mariagiovanna, M. On-board fuel processor modelling for hydrogen-enriched gasoline fuelled engine. Int. J. Hydrogen Energy 2005, 30, 1483–1490. [Google Scholar]
- Moon, D.J.; Sreekumar, K.; Lee, S.D.; Lee, B.G.; Kim, H.S. Studies on gasoline fuel processor system for fuel-cell powered vehicles application. Appl. Catal. A Gen. 2001, 215, 1–9. [Google Scholar] [CrossRef]
- Karatzas, X.; Dawody, J.; Grant, A.; Svensson, E.E.; Pettersson, L.J. Zone-coated Rh-based monolithic catalyst for autothermal reforming of diesel. Appl. Catal. B Environ. 2011, 101, 226–238. [Google Scholar] [CrossRef]
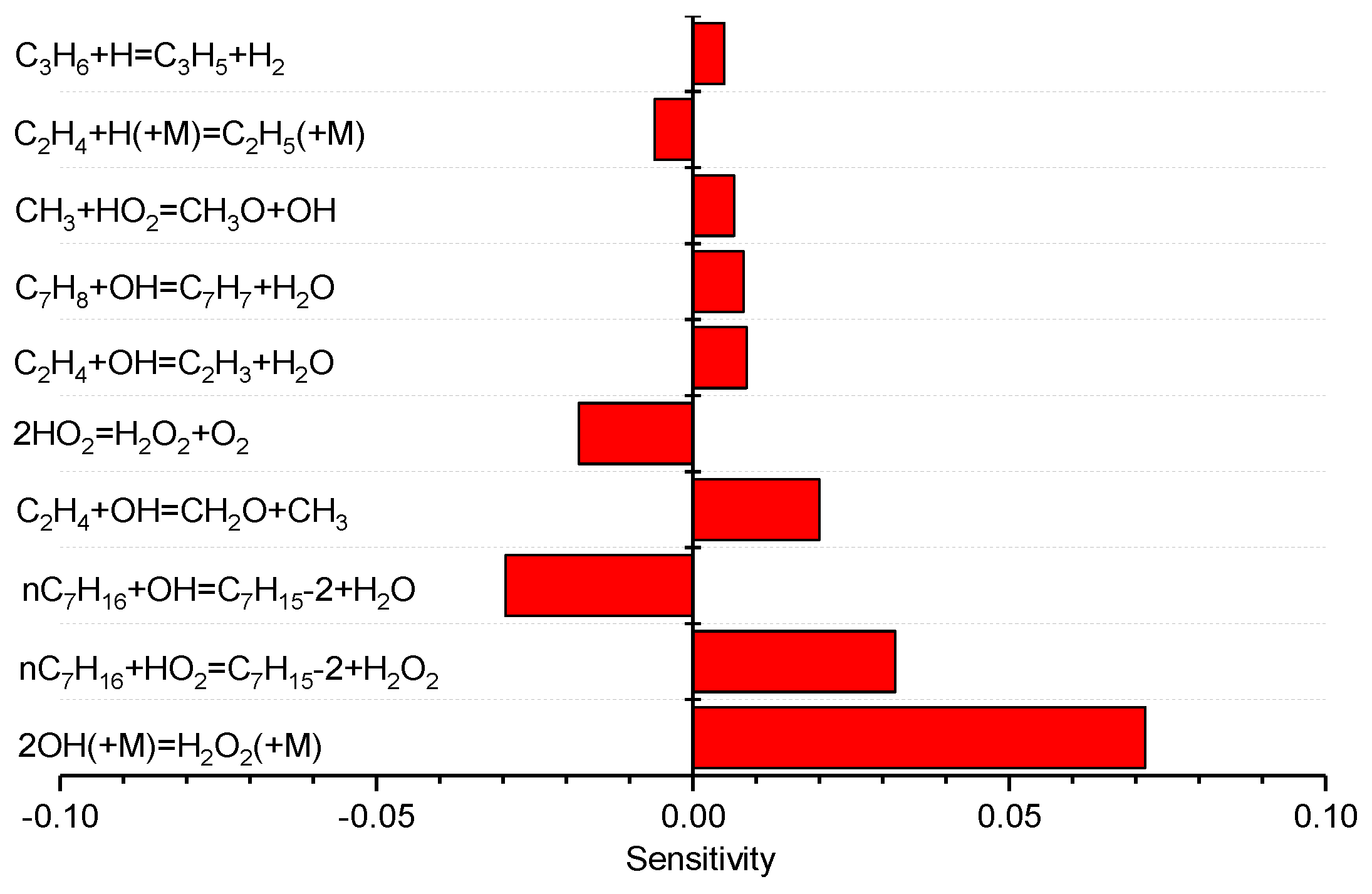

| Reaction Step | Elementary-Step Reaction | A | n | E |
|---|---|---|---|---|
| R.208 | C7H15−2 + C7H8 = C7H7 + nC7h16 | 1.00 × 1011 | 0.0 | 12,000.0 |
| R.209 | A1− + nC7H16 = C7H15−2 + A1 | 2.00 × 1011 | 0.0 | 12,500.0 |
| Catalysts | Average Composition (wt %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | Mn | Al | Si | Pt | Rh | Ce | Zr | |
| Fresh | 1.99 | 47.5 | 1.03 | 15.66 | 23.02 | 1.12 | 0.08 | 4.83 | 4.77 |
| Used | 8.09 | 40.35 | 1.18 | 13.52 | 25.39 | 2.05 | 0.06 | 4.29 | 5.07 |
| Catalysts | Average Surface Composition (atoms %) | |||||
|---|---|---|---|---|---|---|
| Pt | Rh | O | Ce | C | Zr | |
| Fresh | 0.74 | 0.32 | 65.46 | 3.28 | 21.87 | 3.23 |
| Used | 0.41 | 0.2 | 33.88 | 1.43 | 51.9 | 7.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, X.; Pan, Z.; Xu, H. Numerical Simulation and Experimental Study on Commercial Diesel Reforming Over an Advanced Pt/Rh Three-Way Catalyst. Catalysts 2019, 9, 590. https://doi.org/10.3390/catal9070590
Chen H, Wang X, Pan Z, Xu H. Numerical Simulation and Experimental Study on Commercial Diesel Reforming Over an Advanced Pt/Rh Three-Way Catalyst. Catalysts. 2019; 9(7):590. https://doi.org/10.3390/catal9070590
Chicago/Turabian StyleChen, Hanyu, Xi Wang, Zhixiang Pan, and Hongming Xu. 2019. "Numerical Simulation and Experimental Study on Commercial Diesel Reforming Over an Advanced Pt/Rh Three-Way Catalyst" Catalysts 9, no. 7: 590. https://doi.org/10.3390/catal9070590
APA StyleChen, H., Wang, X., Pan, Z., & Xu, H. (2019). Numerical Simulation and Experimental Study on Commercial Diesel Reforming Over an Advanced Pt/Rh Three-Way Catalyst. Catalysts, 9(7), 590. https://doi.org/10.3390/catal9070590




