Oxygen Evolution Reaction of Co-Mn-O Electrocatalyst Prepared by Solution Combustion Synthesis
Abstract
1. Introduction
(CoxMn3−x)O4 + 8CO2 + 13H2O + 3N2
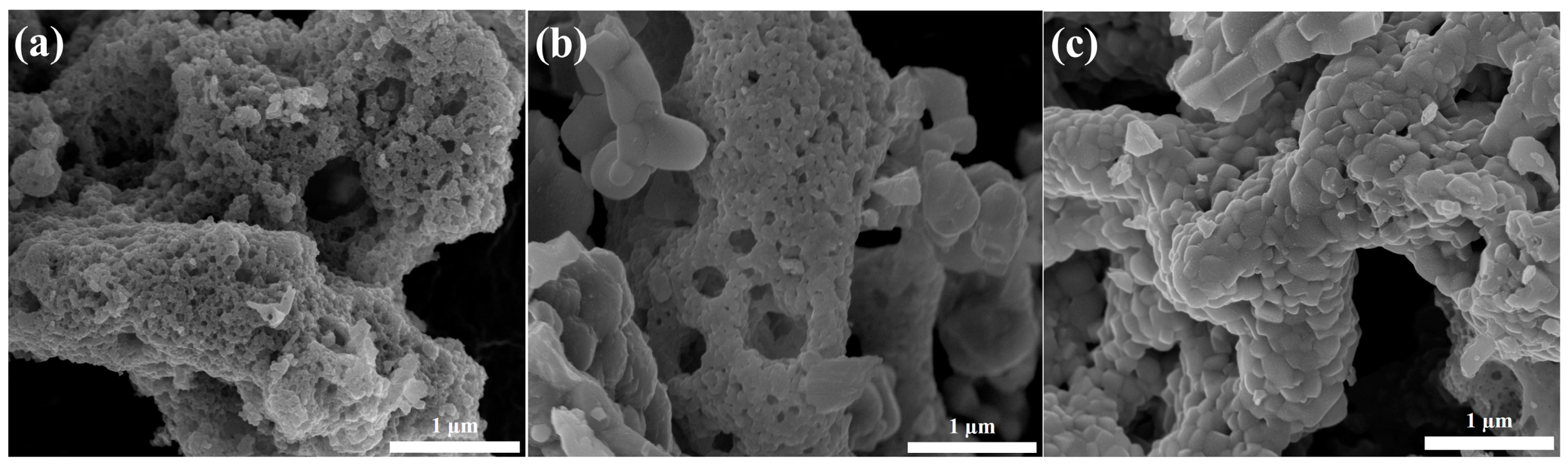
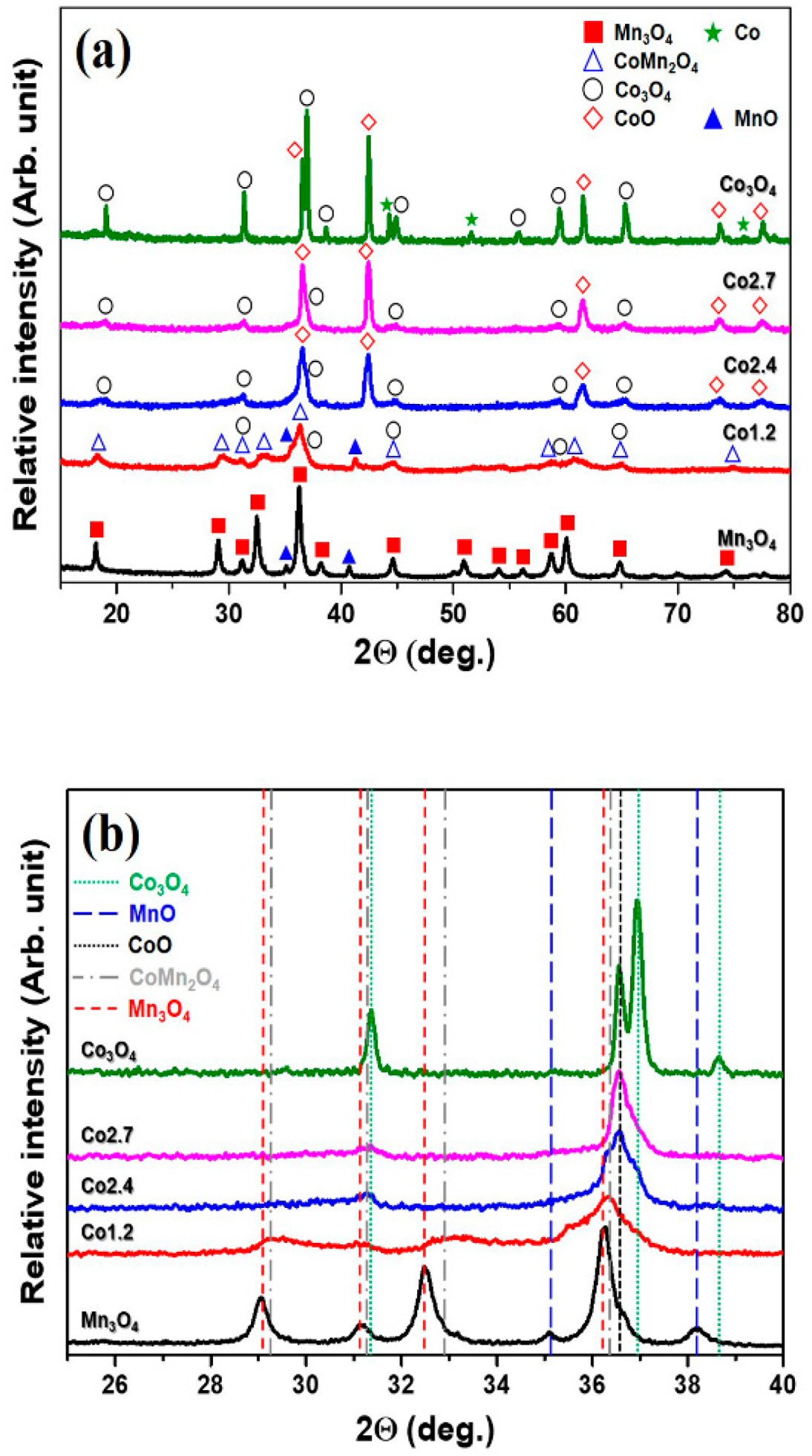
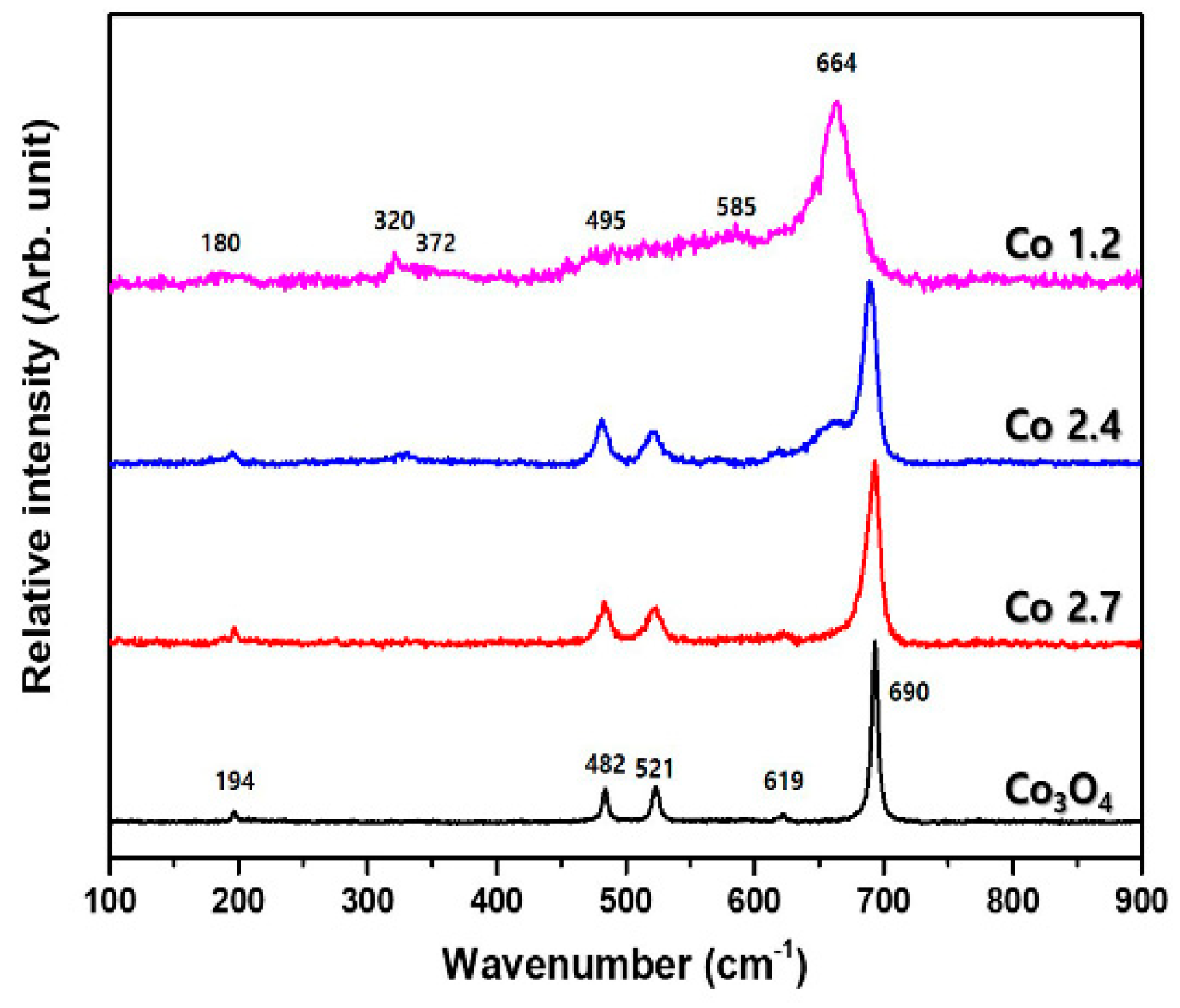
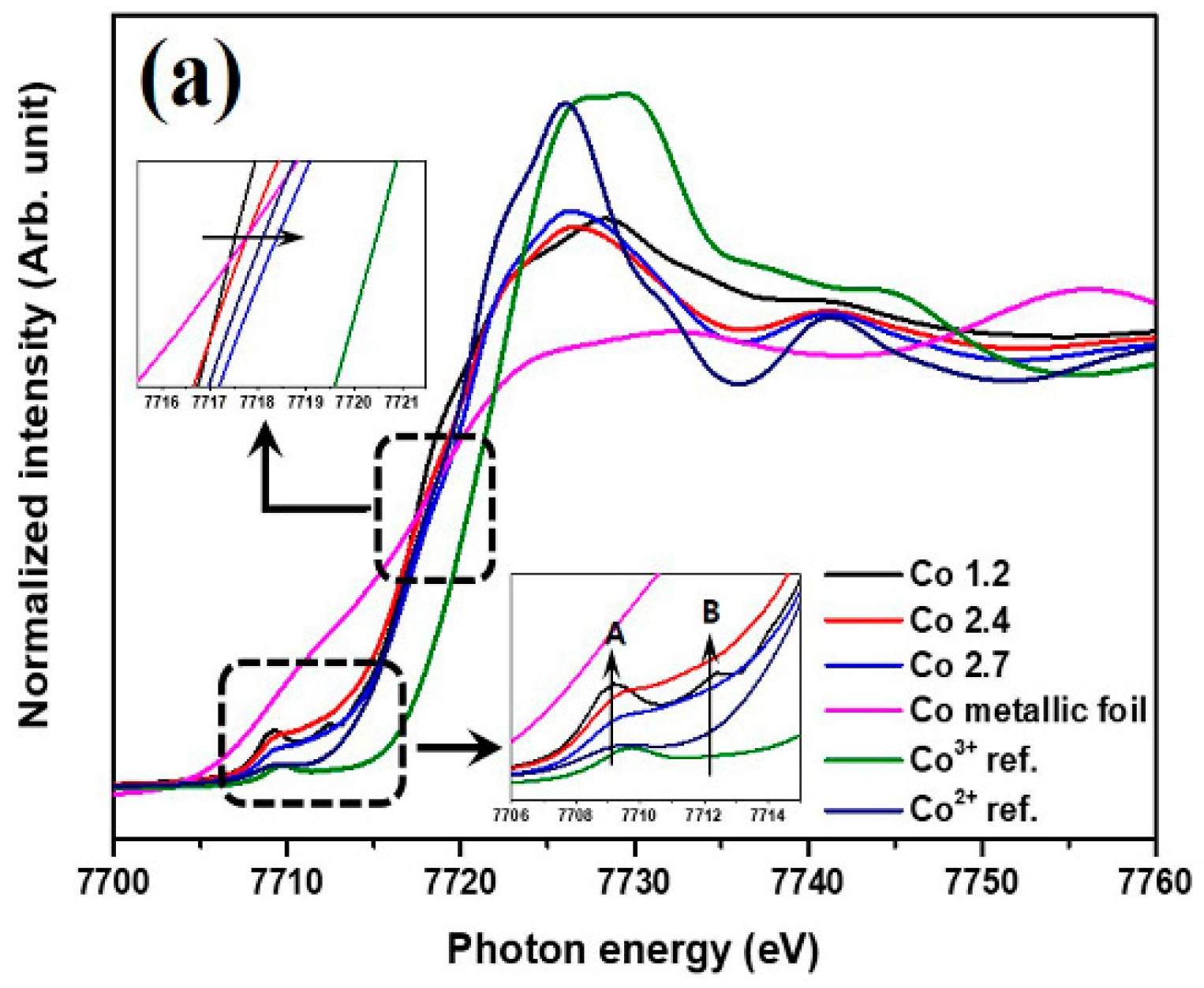
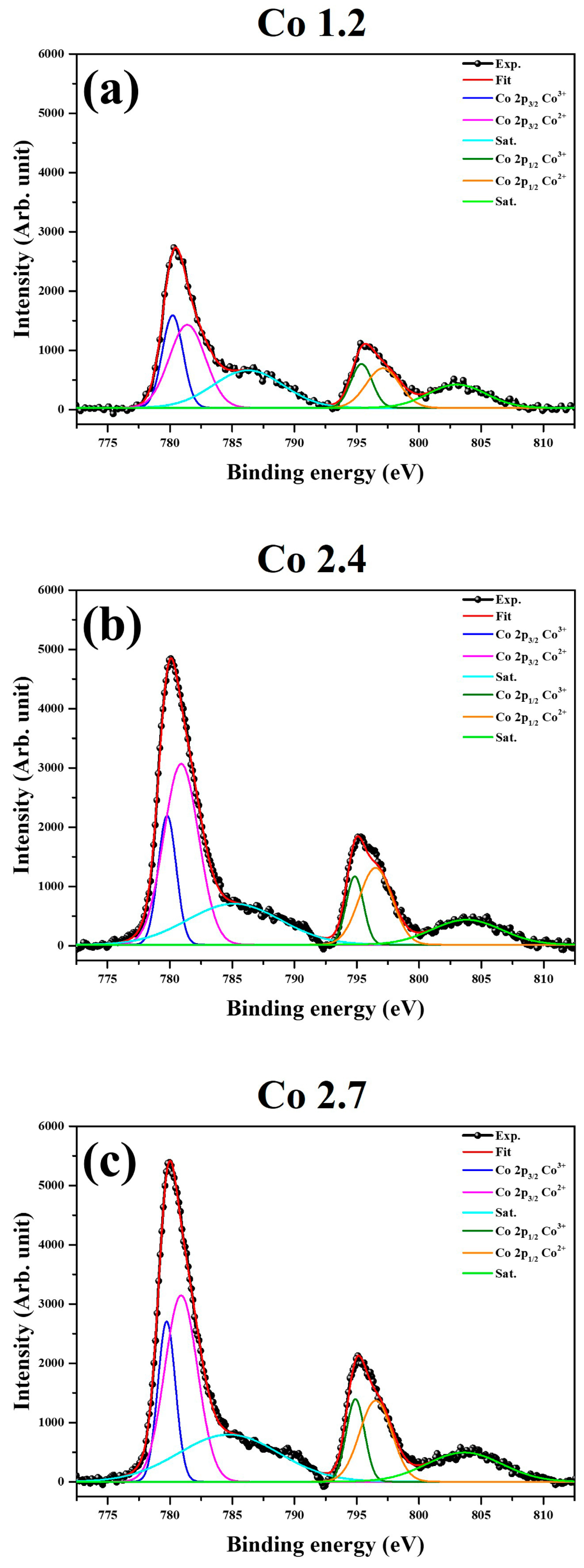
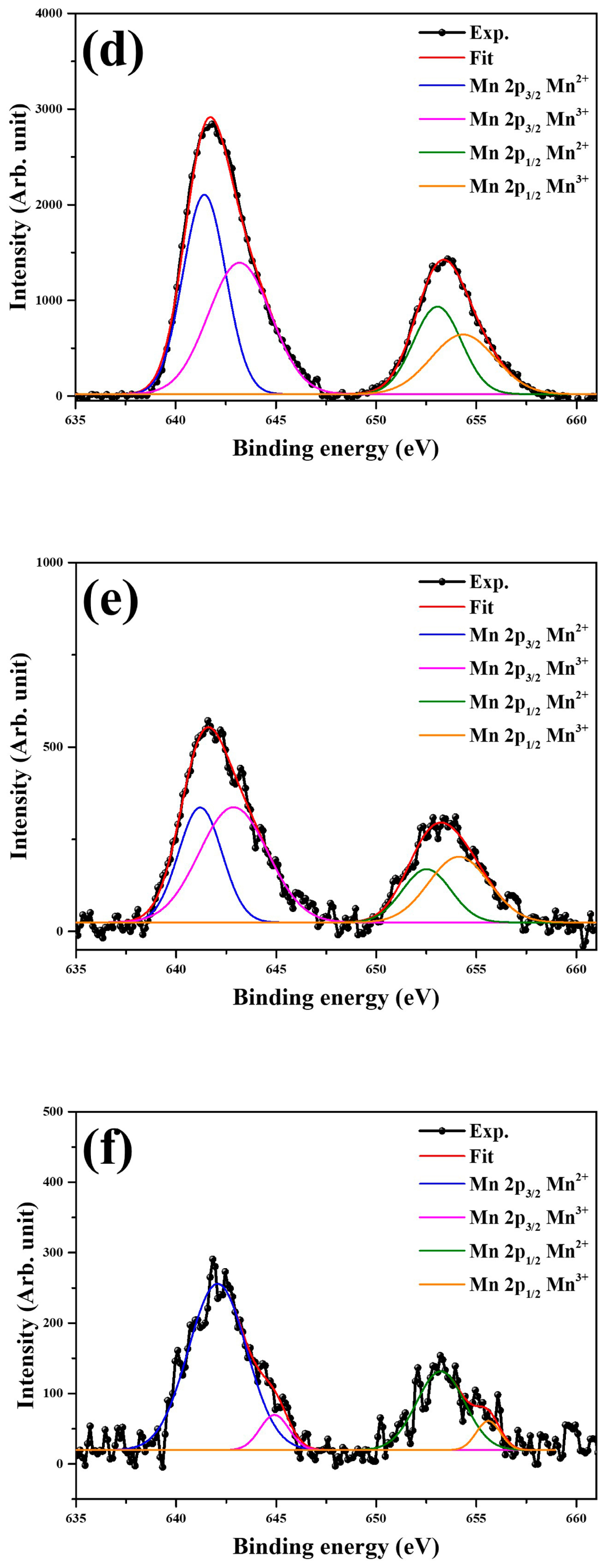
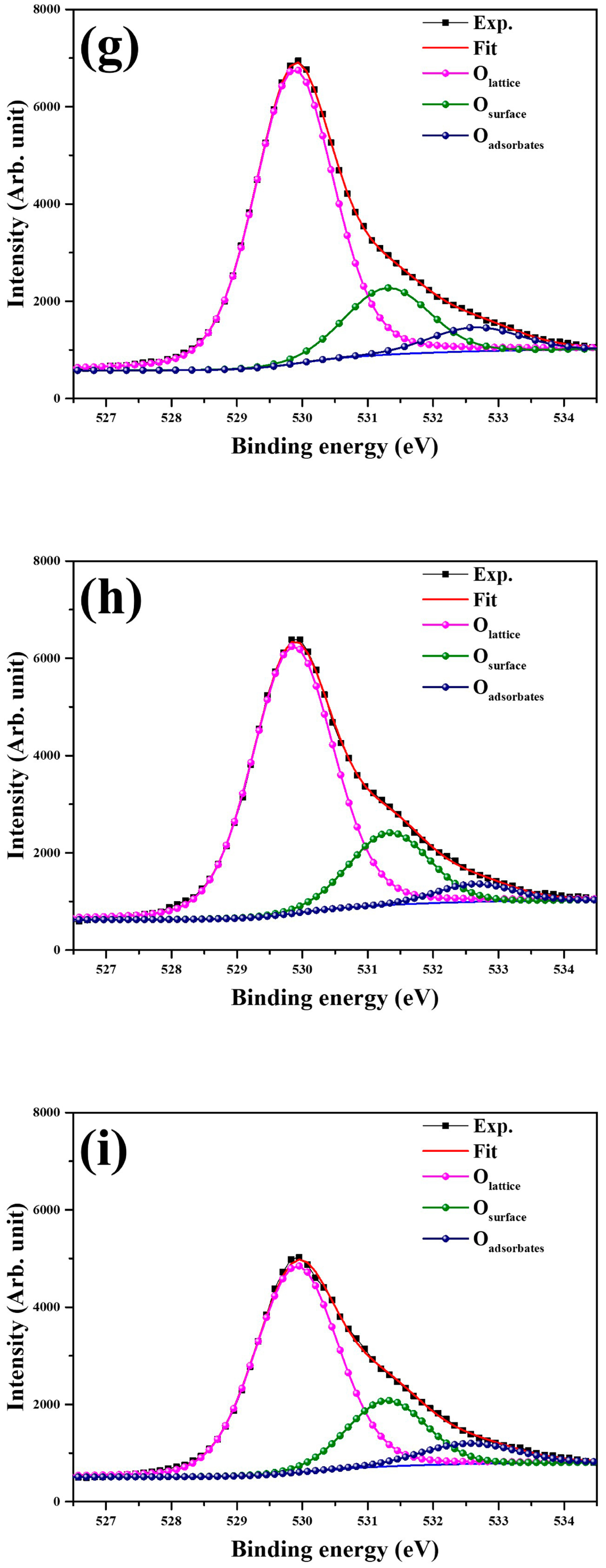
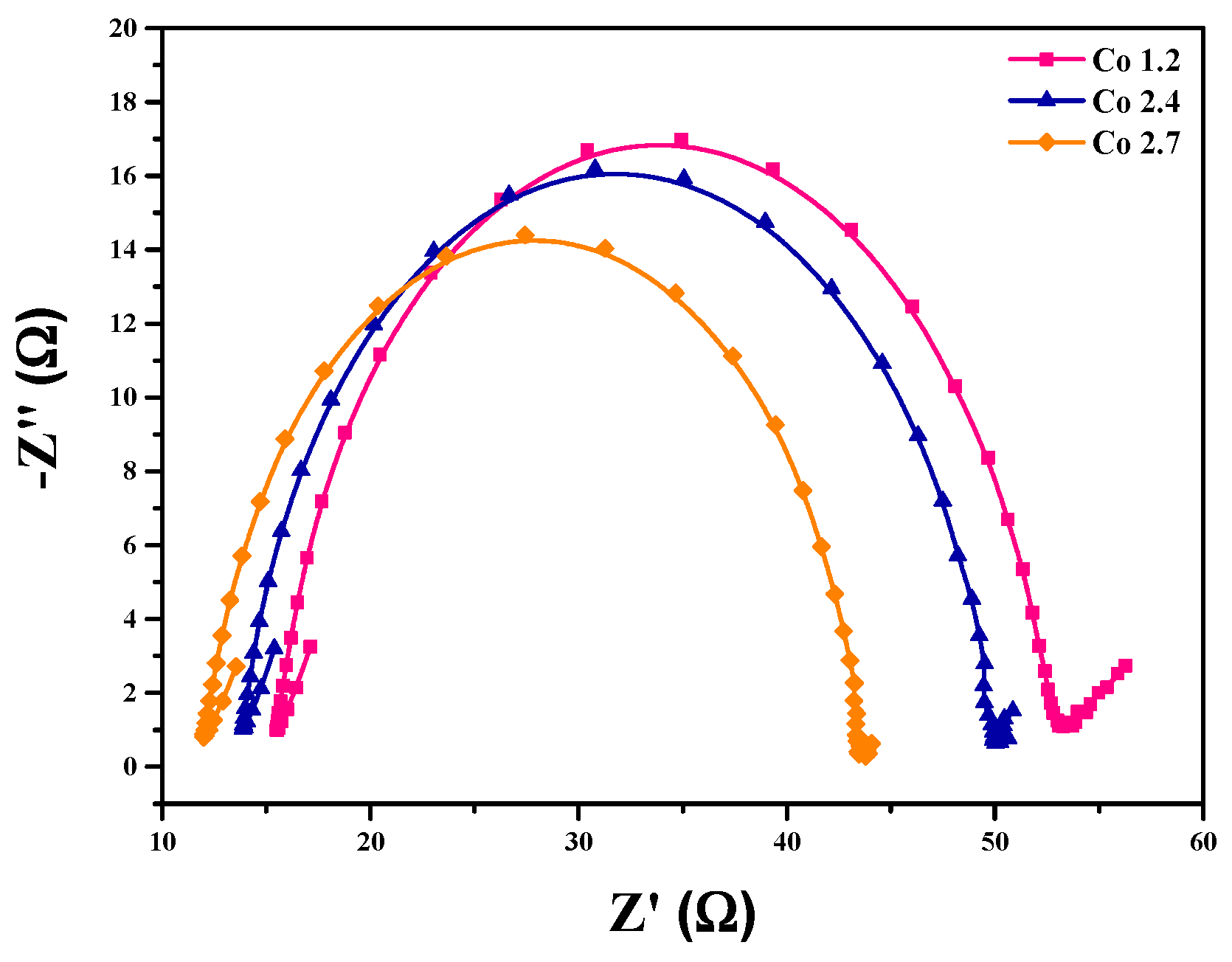
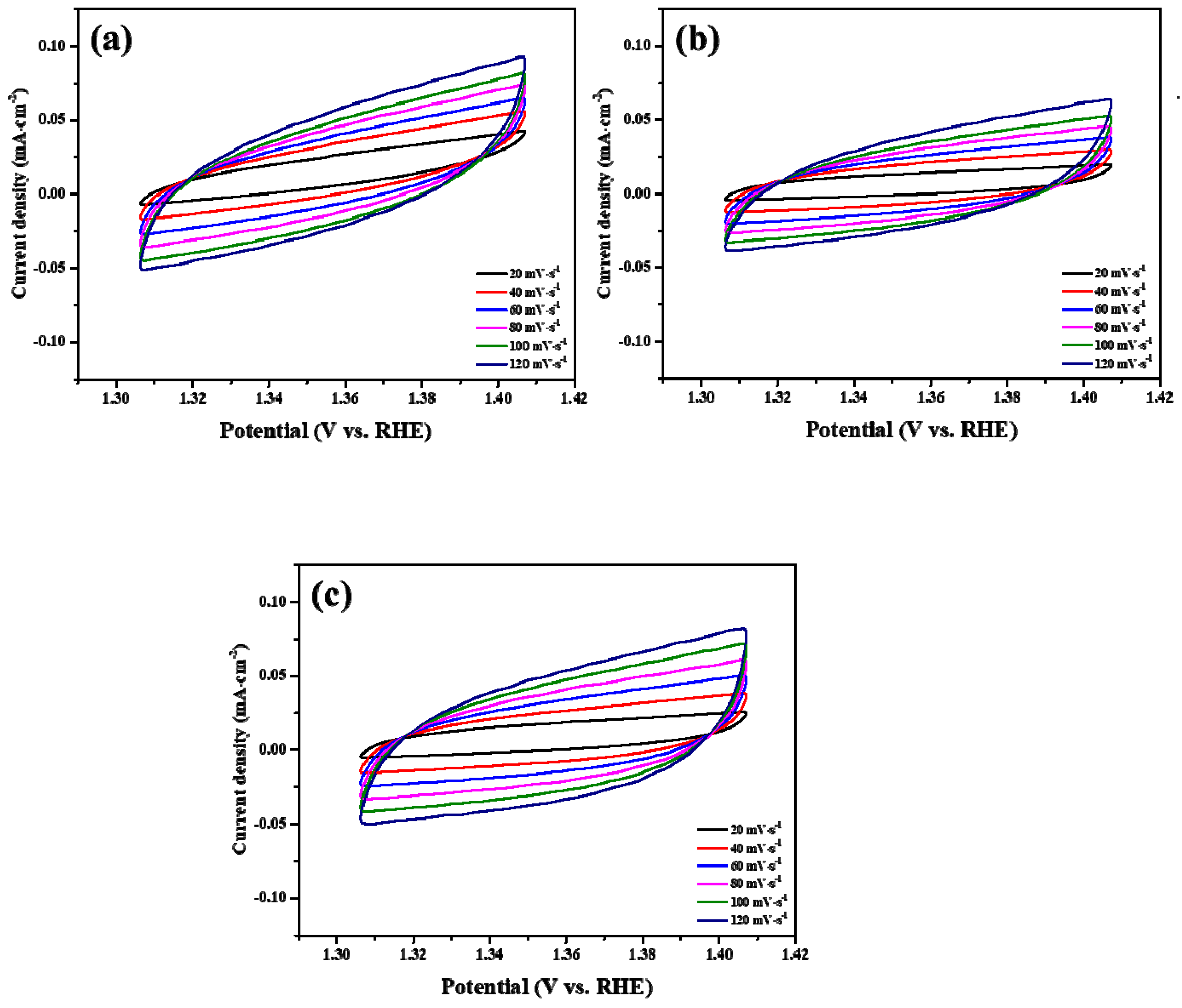
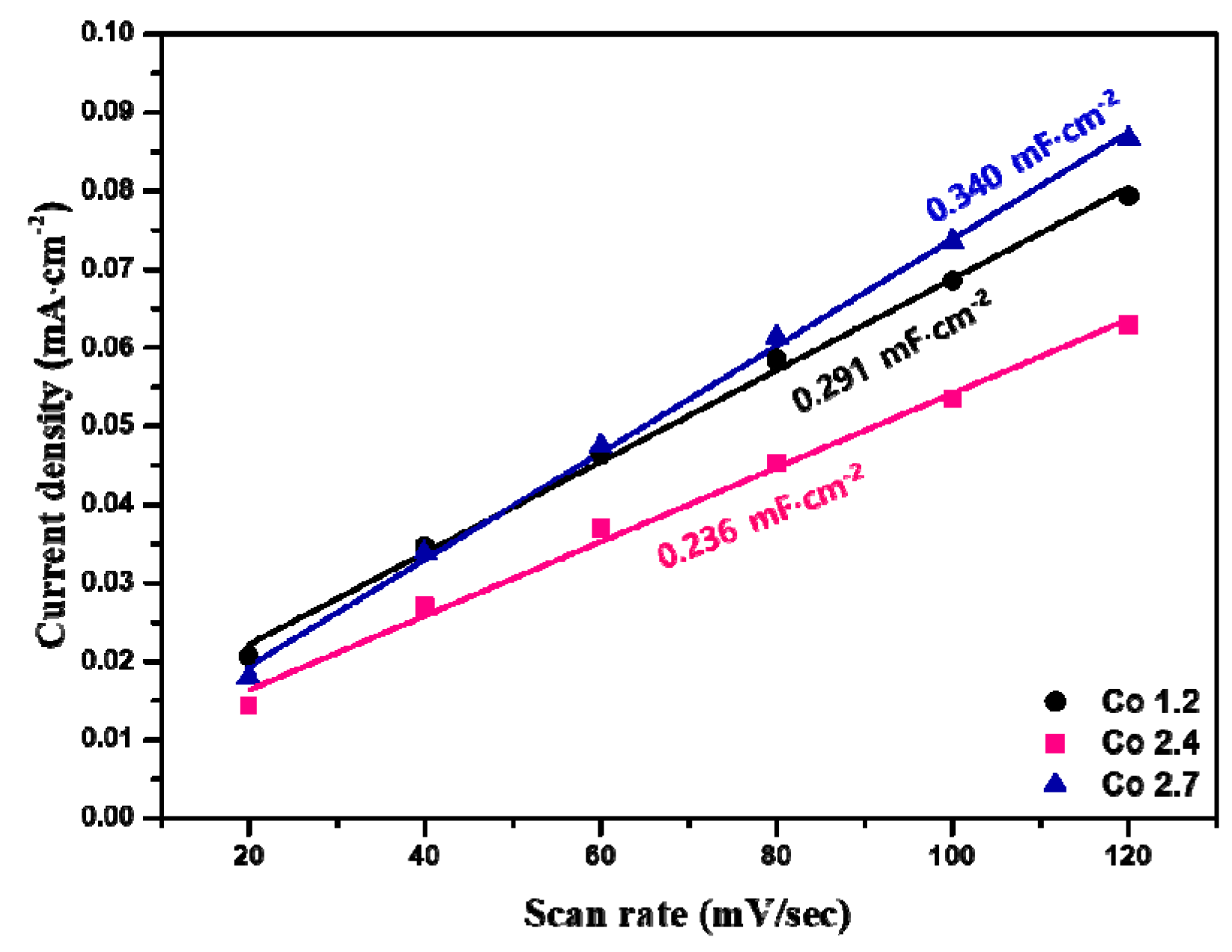
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of CMO Powders
3.2. Structural Characterization
3.3. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amarilla, J.M.; Petrov, K.; Picó, F.; Avdeev, G.; Rojo, J.M.; Rojas, R.M. Sucrose-aided combustion synthesis of nanosized LiMn1.99−yLiyM0.01O4 (M=Al3+, Ni2+, Cr3+, Co3+, y = 0.01 and 0.06) spinels characterization and electrochemical behavior at 25 and at 55 °C in rechargeable lithium cells. J. Power Sources 2009, 191, 591–600. [Google Scholar] [CrossRef]
- Manikandan, P.; Ananth, M.V.; PremKumar, T.; Raju, M.; Periasamy, P.; Manimaran, K. Solution combustion synthesis of layered LiNi0.5Mn0.5O2 and its characterization as cathode material for lithium-ion cells. J. Power Sources 2011, 196, 10148–10155. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Chen, Y.; Ma, Y. Solution-combustion synthesis of ε-MnO2 for supercapacitors. Mater. Lett. 2010, 64, 61–64. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Balasubramanian, K. Solution combustion synthesis of Fe2O3/C, Fe2O3-SnO2/C, Fe2O3-ZnO/C composites and their electrochemical characterization in non-aqueous electrolyte for supercapacitor application. Int. J. Electrochem. Sci. 2009, 4, 878–886. [Google Scholar]
- Gupta, A.; Chemelewski, W.D.; Mullins, C.B.; Goodenough, J.B. High-rate oxygen evolution reaction on Al-doped LiNiO2. Adv. Mater. 2015, 27, 6063–6067. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Gim, J.; Anh, L.T.; Kim, J. Partially reduced Co3O4/graphene nanocomposite as an anode material for secondary lithium ion battery. Electrochim. Acta 2013, 100, 63–71. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution combustion synthesis of nanoscale materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Erri, P.; Pranda, P.; Varma, A. Oxidizer-fuel interactions in aqueous combustion synthesis. 1. Iron(III) nitrate-model fuels. Ind. Eng. Chem. Res. 2004, 43, 3092–3096. [Google Scholar] [CrossRef]
- Wen, W.; Wu, J.-M. Nanomaterials via solution combustion synthesis: A step nearer to controllability. RSC Adv. 2014, 4, 58090–58100. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Rogachev, A.S. Structural macrokinetics of SHS processes. Pure Appl. Chem. 1992, 64, 941–953. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, C.; Yin, X.; Li, J.; Cao, S.; Zhang, C.; Voyles, P.M.; Wang, X. Metastable intermediates in amorphous titanium oxide: A hidden role leading to ultra-stable photoanode protection. Nano Lett. 2018, 18, 5335–5342. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Li, Q.; Wei, Q.; Zhang, P.; Wang, Q.; Lv, F.; An, Q.; Chen, W.; Mai, L. Metastable amorphous chromium–vanadium oxide nanoparticles with superior performance as a new lithium battery cathode. Nano Res. 2014, 7, 1604–1612. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Y.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Xiao, P.; Zhang, Z.; Lim, S.H.; Li, B.; Wang, X.; et al. Dual-phase spinel MnCo2O4 and spinel MnCo2O4/nanocarbon hybrids for electrocatalytic oxygen reduction and evolution. ACS Appl. Mater. Interfaces 2014, 6, 12684–12691. [Google Scholar] [CrossRef]
- Han, H.; Lee, J.S.; Ryu, J.H.; Kim, K.M.; Jones, J.L.; Lim, J.; Guillemet-Fritsch, S.; Lee, H.C.; Mhin, S. Effect of high cobalt concentration on hopping motion in cobalt manganese spinel oxide (CoxMn3−xO4, x ≥ 2.3). J. Phys. Chem. C 2016, 120, 13667–13674. [Google Scholar] [CrossRef]
- Han, H.; Lee, J.S.; Lim, J.; Park, K.R.; Kim, K.M.; Ryu, J.H.; Yeo, S.; Forrester, J.; Mhin, S. Synthesis of CoxMn1−xO4 (0.9 ≤ x ≤ 2.7) nanopowders with controlled phase and composition via a gel-combustion method. Ceram. Int. 2016, 42, 17168–17173. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345–7352. [Google Scholar] [CrossRef]
- Legutko, P.; Jakubek, T.; Kaspera, W.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Strong enhancement of deSoot activity of transition metal oxides by alkali doping: Additive effects of potassium and nitric oxide. Top. Catal. 2017, 60, 162–170. [Google Scholar] [CrossRef]
- Widjaja, E.; Sampanthar, J.T.; Han, X.D.; Goh, E. Use of Raman microscopy and band-target entropy minimization technique to differentiate physical mixture from chemical mixture in mixed metal oxides. Catal. Today 2008, 131, 21–27. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C 1988, 21, L199–L201. [Google Scholar] [CrossRef]
- Gallant, D.; Pezolet, M.; Simard, S. Optical and physical properties of cobalt oxide films electrogenerated in bicarbonate aqueous media. J. Phys. Chem. B 2006, 100, 6871–6880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, L. Synthesis of sphere-like Co3O4 nanocrystals via a simple polyol route. Mater. Lett. 2007, 61, 4894–4896. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Wu, H.B.; Zhang, G.; Lou, X.W. Controlled synthesis of hierarchical CoxMn3−xO4 array micro-/nanostructures with tunable morphology and composition as integrated electrodes for lithium-ion batteries. Energy Environ. Sci. 2013, 6, 2664–2671. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, L.; Liu, J.; Wang, S.; Sun, G. Effect of surface manganese valence of manganese oxides on the activity of the oxygen reduction reaction in alkaline media. ACS Catal. 2014, 4, 457–463. [Google Scholar] [CrossRef]
- Boosting electrochemical water oxidation through replacement of Oh Co sites in cobalt oxide spinel with manganese. Chem. Commun. 2017, 53, 8018–8021. [CrossRef] [PubMed]
- Wang, Y.; Zhou, T.; Jiang, K.; Da, P.; Peng, Z.; Tang, J.; Kong, B.; Cai, W.; Yang, Z.; Zheng, G. Reduced mesoporous Co3O4 nanowires as efficient water oxidation electrocatalysts and supercapacitor electrodes. Adv. Energy Mater. 2014, 4, 1400696. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hung, S.F.; Chen, H.Y.; Chan, T.S.; Chen, H.M.; Liu, B. In operando identification of geometrical-site-dependent water oxidation activity of spinel Co3O4. J. Am. Chem. Soc. 2016, 138, 36–39. [Google Scholar] [CrossRef]
- Sa, Y.J.; Kwon, K.; Cheon, J.Y.; Kleitz, F.; Joo, S.H. Ordered mesoporous Co3O4 spinels as stable, bifunctional, noble metal-free oxygen electrocatalysts. J. Mater. Chem. A 2013, 1, 9992–10001. [Google Scholar] [CrossRef]
- Wu, J.; Xue, Y.; Yan, X.; Yan, W.; Cheng, Q.; Xie, Y. Co3O4 Nanocrystals on single-walled carbon nanotubes as a highly efficient oxygen-evolving catalyst. Nano Res. 2012, 5, 521–530. [Google Scholar] [CrossRef]
- McKendry, I.G.; Thenuwara, A.C.; Shumlas, S.L.; Peng, H.; Aulin, Y.V.; Chinnam, P.R.; Borguet, E.; Strongin, D.R.; Zdilla, M.J. Systematic Doping of Cobalt into Layered Manganese Oxide Sheets Substantially Enhances Water Oxidation Catalysis. Inorg. Chem. 2018, 57, 557–564. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Evans, D.; Yang, W. Well-dispersed Co3O4/Co2MnO4 nanocomposites as a synergistic bifunctional catalyst for oxygen reduction and oxygen evolution reactions. Nanoscale 2013, 5, 5312–5315. [Google Scholar] [CrossRef]
- Meng, Y.; Song, W.; Huang, H.; Ren, Z.; Chen, S.; Suib, S. Structure−property relationship of bifunctional MnO2 nanostructures: Highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tian, L.; Atkins, S.; Liu, Y.; Murowchick, J.; Chen, X. Converting CoMoO4 into CoO/MoOx for overall water splitting by hydrogenation. ACS Sustain. Chem. Eng. 2016, 4, 3743–3749. [Google Scholar] [CrossRef]
- Masa, J.; Weide, P.; Peeters, D.; Sinev, I.; Xia, W.; Sun, Z.; Somsen, C.; Muhler, M.; Schuhmann, W. Amorphous cobalt boride (Co2B) as a highly efficient nonprecious catalyst for electrochemical water splitting: Oxygen and hydrogen evolution. Adv. Energy Mater. 2016, 6, 1502313. [Google Scholar] [CrossRef]
- Masa, J.; Sinev, I.; Mistry, H.; Ventosa, E.; Mata, M.; Arbiol, J.; Muhler, M.; Cuenya, B.; Schuhmann, W. Ultrathin high surface area nickel boride (NixB) nanosheets as highly efficient electrocatalyst for oxygen evolution. Adv. Energy Mater. 2017, 7, 1700381. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, J.; Yanzhang, R.; Du, M.; Wang, Q.; Gao, G.; Wu, J.; Wu, G.; Zhang, M.; Liu, B.; et al. When cubic cobalt sulfide meets layered molybdenum disulfide: A core–shell system toward synergetic electrocatalytic water splitting. Adv. Mater. 2015, 27, 4752–4759. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Cheng, N.; Pu, Z.; Xing, W.; Sun, X. NiSe nanowire film supported on nickel foam: An efficient and stable 3D bifunctional electrode for full water splitting. Angew. Chem. Int. Ed. 2015, 54, 9351–9355. [Google Scholar] [CrossRef]
- Koza, J.A.; Hull, C.M.; Liu, Y.C.; Switzer, J.A. Deposition of β-Co(OH)2 films by electrochemical reduction of Tris(ethylenediamine)cobalt(III) in alkaline solution. Chem. Mater. 2013, 25, 1922–1926. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]


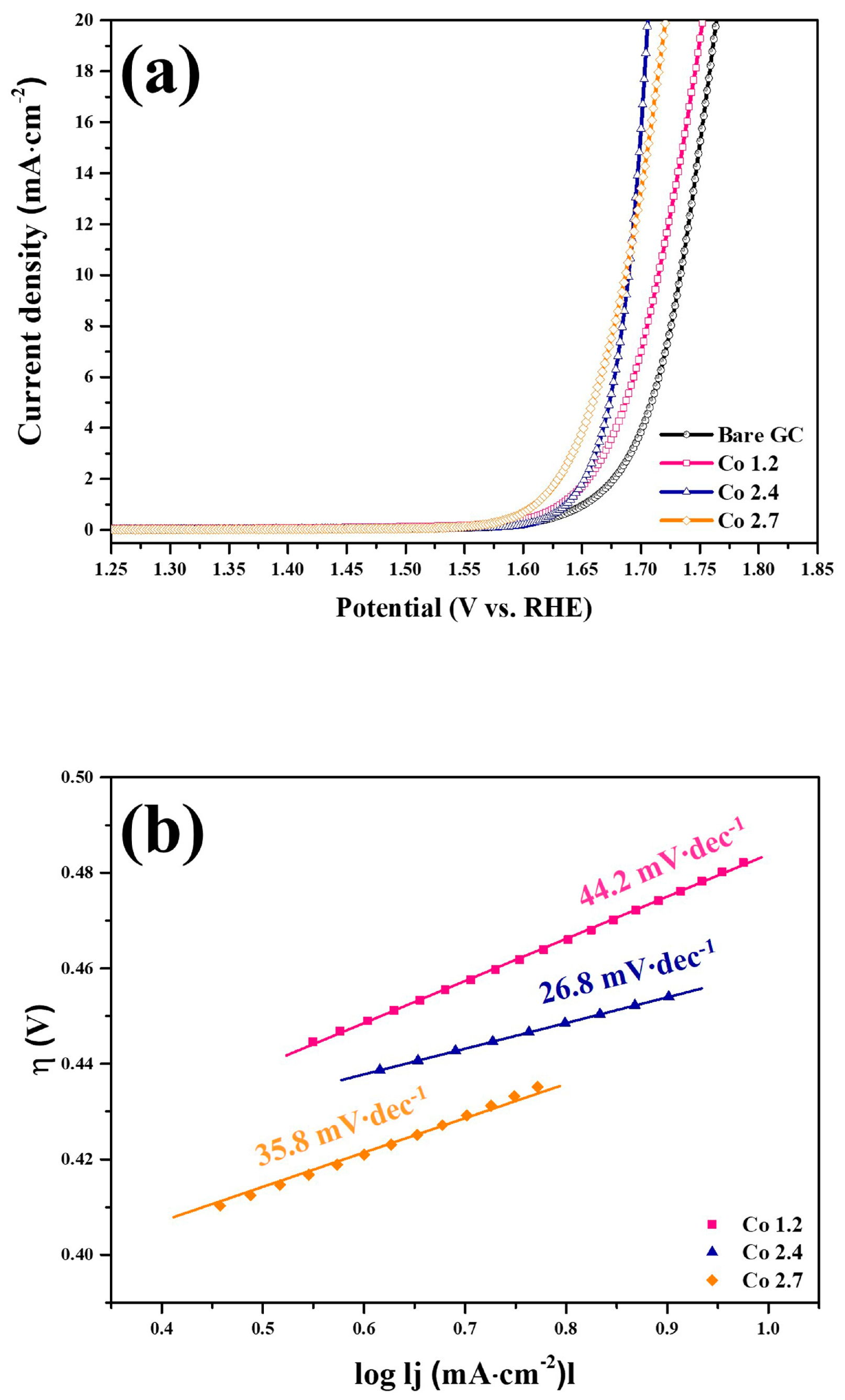

| Catalysts | Electrolyte | Current Density (mA cm−2) | Overpotential (mV) | Tafel Slope (mV dec−1) | References |
|---|---|---|---|---|---|
| Co- and Mn-based oxides | 1 M KOH | 10 | 450 | 35.8 | This work |
| Mesoporous Co3O4 | 0.1 M KOH | 10 | 411 | 80 | [27] |
| CoO/CNT | 1 M KOH | 10 | 550 | 108 | [28] |
| K0.04[Co0.42Mn0.58O2] | 1 M KOH | 10 | 420 | 110 | [29] |
| Co3O4/MnCo2O4 | 0.1 M KOH | 10 | 540 | N/A | [30] |
| α-MnO2-SF | 0.1 M KOH | 10 | 490 | 77.5 | [31] |
| CoO/MoOx | 1.0 M KOH | 10 | 490 | 44 | [32] |
| Co2B-500 | 0.1 M KOH | 10 | 380 | 45 | [33] |
| NixB-300 | 1 M KOH | 10 | 380 | 89 | [34] |
| Co9S8@MoS2/CNFs | 1 M KOH | 10 | 430 | 61 | [35] |
| NiSe/NF | 1 M KOH | 10 | 400 | N/A | [36] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.R.; Jeon, J.E.; Ali, G.; Ko, Y.-H.; Lee, J.; Han, H.; Mhin, S. Oxygen Evolution Reaction of Co-Mn-O Electrocatalyst Prepared by Solution Combustion Synthesis. Catalysts 2019, 9, 564. https://doi.org/10.3390/catal9060564
Park KR, Jeon JE, Ali G, Ko Y-H, Lee J, Han H, Mhin S. Oxygen Evolution Reaction of Co-Mn-O Electrocatalyst Prepared by Solution Combustion Synthesis. Catalysts. 2019; 9(6):564. https://doi.org/10.3390/catal9060564
Chicago/Turabian StylePark, Kyoung Ryeol, Jae Eun Jeon, Ghulam Ali, Yong-Ho Ko, Jaewoong Lee, HyukSu Han, and Sungwook Mhin. 2019. "Oxygen Evolution Reaction of Co-Mn-O Electrocatalyst Prepared by Solution Combustion Synthesis" Catalysts 9, no. 6: 564. https://doi.org/10.3390/catal9060564
APA StylePark, K. R., Jeon, J. E., Ali, G., Ko, Y.-H., Lee, J., Han, H., & Mhin, S. (2019). Oxygen Evolution Reaction of Co-Mn-O Electrocatalyst Prepared by Solution Combustion Synthesis. Catalysts, 9(6), 564. https://doi.org/10.3390/catal9060564





