Abstract
In wastewater treatment, an alternative to the widely used aerobic nitrification with subsequent anoxic denitrification method is the combination of nitration and anammox (AMX) in one system. This study focuses on the co-immobilization of AMX and ammonia-oxidizing bacteria into a polyvinyl alcohol (PVA) hydrogel, and its effective use in nitrogen removal (NR). The NR process was performed in nine consecutive, repeated batches. By optimizing the conditions of the biotransformations, there was equal utilization of nitrogen in both sources, N–NH4+ and N–NO2−, at 100% NR during the sixth repetition. A significant increase in the immobilized co-culture activity was also detected per cycle. The maximum value of the NR rate was 3.46 mg N (L h)−1, and 100% NR efficiency was achieved with an initial concentration of 100.3 mg N L−1 for N–NH4+ and 60.1 mg N L−1 for N–NO2−, during the eighth batch biotransformation.
1. Introduction
Organic nitrogen-containing compounds are among the most important pollutants of wastewater, and the removal of nitrogen is one of the crucial steps in wastewater treatment. These compounds represent a main factor in eutrophication, and they have an effect on the oxygen content of receiving waters as well as the toxicity to aquatic organisms and human beings [1,2]. For decades, a removal process based on aerobic nitrification with subsequent anoxic denitrification was used for the removal of such nitrogen-based compounds. The discovery of anaerobic oxidation of ammonium (anammox; AMX), with essential advantages of high nitrogen removal rate (NRR), environmental friendliness, low operational costs and low occupied areas, has been recognized as an attractive alternative for the treatment of nitrogen-rich wastewater streams [3,4,5]. The AMX process operates by oxidizing ammonia to nitrogen gas with nitrite as an electron acceptor under anoxic conditions. Meanwhile, the growth of AMX bacteria is supported by carbon dioxide fixation (Equation (1)), which is an advantage for the treatment of highly nitrogen-loaded wastewaters containing low biodegradable organic carbons [6,7,8].
NH4+ + 1.3NO2− + 0.066HCO3− + 0.13H+ → 1.02 N2 + 0.26NO3− + 0.066CH2O2.5N0.15 + 2.03 H2O
As nitrite is needed for the AMX process at a molar ratio of 1:1.32 with ammonium [9], part of the ammonium in wastewater must be oxidized to nitrite with a pre-treatment system, such as the nitrosation process, realized by ammonia oxidizing bacteria (AOB), as shown in Equation (2) [6,7,10].
2NH4+ + 3O2 → 2NO2− + 2 H2O + 4H+
Nevertheless, compared to conventional nitrification and denitrification, AMX consumes 100% less organic carbon and saves 90% of the operational costs associated with sludge disposal [11]. An alternative pre-treatment system for wastewater is the combination of nitrosation and AMX in one system. So far, it has mainly been applied for wastewaters with high concentrations of ammonia and low concentrations of biodegradable organic substances [12]. Functional AMX bacteria are very sensitive and are easily inhibited by many factors, such as low temperature (optimal range is 20–43 °C), extreme pH value (optimum pH 6.5–8.8), high salinity as well as the presence of organic matter, phosphates, sulfides and other inhibitors [4,7].
For this reason, immobilization of microbial cells has received increasing interest in wastewater treatment in order to minimize the risk of biomass wash-out from the reactors and to provide a stabilized treatment [13]. The repeated use of immobilized biomass could handle the long start-up of AMX bacteria because of the very slow growth rate (0.072/day at 32 °C) and low yield coefficient (0.13 g dry weight/g NH4–N oxidized) [11]. Immobilization of AMX bacteria through the entrapment method has been reported in several previous papers, using polyethylene glycol gel carriers [14,15] and polyvinyl alcohol (PVA) cryogel, prepared by physical cross-linking through the freezing/thawing method [13].
The aim of this research was to evaluate the effectiveness of co-immobilization of AMX bacteria and AOB by the entrapment method, immobilizing both bacteria into a PVA hydrogel, and its application in nitrogen removal (NR) during the water treatment process, using a real medium. The key issue in this application, however, is the harmonization of activities of both cultures. Therefore, we have focused on different process aspects to achieve this in co-immobilized form. Compared to other gel systems, immobilization using this method offers several advantages, such as a low matrix cost, inexpensive and simple gel preparation, uncomplicated separation from the reaction mixture and low diffusion limits. In addition, this matrix has excellent mechanical stability and is almost non-degradable [16].
2. Results and Discussion
The NR process using immobilized microbial co-cultures of AMX bacteria and AOB occurred in repeated batch-mode biotransformations. This experimental setup was made mainly because of the separate influences on each batch process (such as pH maintenance, nutrient limitation) which were evaluated after each separate batch. Another inspiration was to reach high NR efficiency. There were nine successive repetitions realized, as illustrated in Figure 1. The operational parameters were improved throughout the entire process and resulted in an increase of the nitrogen removal rate (NRR) and achieved a 100%-successful transformation of substrate nitrogen compounds contained in the medium.
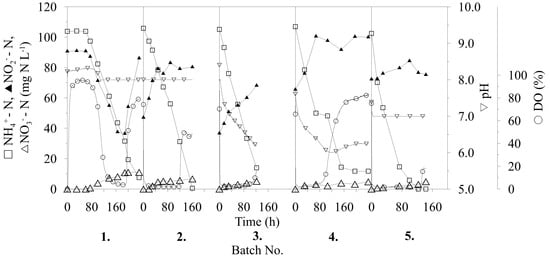
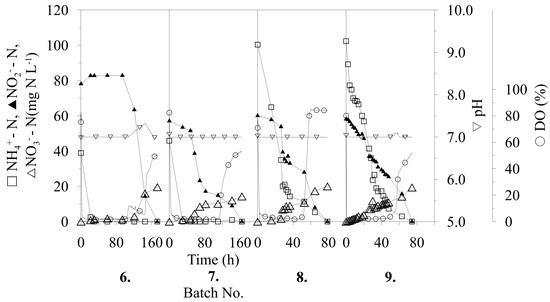
Figure 1.
Repeated batch biotransformations with immobilized co-culture of anammox (AMX) bacteria and ammonia oxidizing bacteria AOB.
Figure 1 illustrates the process of each cycle of repeated biotransformation, and, as shown, low activity of the immobilized cells was observed during the first 60 h of the first biotransformation. This was most likely caused by the highly stressful aerobic conditions of the immobilization process to which the biomass was exposed for a short time beforehand. After this time, the oxygen started to decrease in the medium, as observed by a decrease in the dissolved oxygen (DO) content to 10%, presumably caused by production of nitrogen gas (a final compound of nitrogen conversion). The higher activity of the immobilized bacteria also presented simultaneous utilization of N–NH4+ and N–NO2−, probably caused by the suppression of the metabolic activity of one or more representatives of the immobilized AMX consortium also presented by formation of ions N–NO3−. Utilization of N–NO2− slowed around the middle of the first repeated batch and, instead of decreasing, it started to increase, according to the fermentation time. An increase of N–NO2− concentration and continuous utilization of N–NH4+ was more likely caused by the activity of AOB, which became dominant in the co-immobilized culture. As reported [17], AOB forms a thick layer around anammox cells which, after AOB domination, may eliminate the substrate (ammonia) access to AMX consortium (Equations (1,2)). During this first batch biotransformation, 52% of the total nitrogen was removed from compounds. The NRR, calculated from 0 to 30 h for each batch, was constant at 0 mg N (L h)−1 because of the long lag phase. After 256 h of the first biotransformation, the whole volume of the medium was separated through a sieve, subsequently fed with fresh medium and the next biotransformation was started.
As shown in Figure 1, the trend of nitrite production continued during the second batch, this time from the beginning of biotransformation. On the other hand, the low value of DO provides a prerequisite, indicating that the AMX consortium was only temporarily suppressed and not inhibited, presented by production of gaseous nitrogen and formation of ions N–NO3−. The NR and NRRs achieved were similar to the results of the first batch, owing to increasing nitrite production.
2.1. Influence of pH on the Process
After the second biotransformation, the operation conditions were changed, which were intended to provide an advantage to the AMX part of the immobilized biomass. The first parameter modified was the pH of the process, with reference to published data of pH optimum of 6.5–8.8 for AMX cultivation [7]. According to this fact, a spontaneous decrease of the pH value during the process was performed instead of a pH stat at 8.0, as previously used. Following this change, a decrease in pH from 8.0 to 6.18 (after 122 h) and from pH 7.6 to 6.0 (after 110 h) was observed in the third and fourth biotransformations, respectively. Although there was no change in nitrite production during these processes, an increase in the utilization of N–NH4+ was observed, according to time of biotransformation. For the third batch, an NRR of 0.50 mg N (L h)−1 and 42% NR was achieved, whereas for the fourth batch, the NRR was 0.59 mg N (L h)−1 and 43% NR was achieved. Compared with previously achieved values, and as illustrated in Figure 2, there is a clear increase in the NRR despite a decrease in NR, which represents the activity of the whole process. During the fifth biotransformation, the pH stat was reset to pH 7.0 and, as shown in Figure 1, the increase in N–NO2− concentration was significantly reduced for only 4.4 mg N L−1 in the period 0–120 h, as compared with previous (second to fourth) conversions with an increase of about 31.9 ± 0.3 mg N L−1.
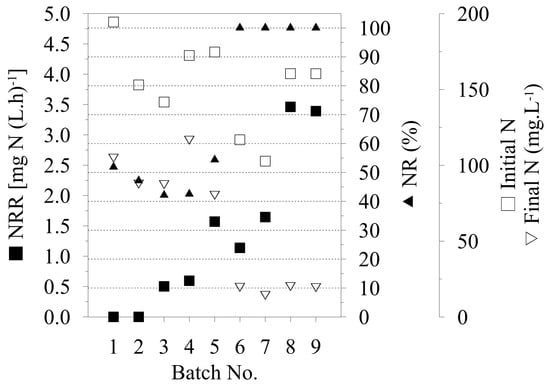
Figure 2.
Nitrogen removal rate (NRR) calculated for the period 0–30 h of each biotransformation cycle, nitrogen removal (NR) calculated for entire duration of biotransformation and total, initial and final nitrogen.
2.2. AMX Bacteria Stimulation
Compared to AOB, AMX bacteria has extremely slow biomass growth (doubling time between 2.1 and 3.9 days) [18]. Therefore, AMX biomass suffers to be overgrown by AOB in the previous repeated batches. To increase the AMX bacterium, the nutritional limitation (decrease of N–NH4+) of AOB was applied. In the following two biotransformations (sixth and seventh), the initial concentration of N–NH4+ was decreased two-fold in an attempt to stimulate AMX bacteria at the expense of the other co-immobilized bacteria. At first, total utilization of N–NH4+ was observed after 25 h of biotransformation (sixth batch). Then, after 100 h, relatively high-speed utilization of N–NO2− was observed, which decreased a concentration to 14.2 mg N L−1 from 78.1 mg N L−1 after 45 h (145th hour of biotransformation) by simultaneous formation of 19.52 mg N L−1 (N–NO3−). The N–NO3− confirms the trend of AMX bacteria activation at the end of this batch. Finally, a total NR of 100% was observed (calculated on initial N–NH4+, N–NO2−) despite it being at the expense of an NRR decrease (Figure 2). During the seventh batch, synchronic utilization of both sources of nitrogen was observed from the beginning of the biotransformation. An NRR of 1.65 mg N (L h)−1 was achieved, as calculated for the period of 0–30 h, which is similar to the value of 1.57 mg N (L h)−1 that was achieved in the fifth biotransformation, with the difference of 100% NR observed during seventh biotransformation (Figure 2). This confirmed the correctness of the chosen procedure in the sixth batch, with a decrease in the initial concentrations of N–NH4+ for AMX stimulation, and led to improved total nitrogen utilization and activity of the whole process with immobilized co-cultures. Furthermore, the effectivity of the process was also apparent from the initial and final content of total N between sixth and ninth batch (Figure 2). The difference between initial and final N content confirms the efficient process strategy in batch numbers eight and nine.
2.3. Harmonised Co-Culture Experiments
During the eighth and ninth biotransformations, the initial concentration of N–NH4+ was increased to 100.3 and 102.3 mg N L−1, respectively. The initial concentration of N–NO2− was similar to that used in previous repeated batch biotransformations at 60.1 and 58.1 mg N L−1 for the eighth and ninth biotransformations, respectively. As shown in Figure 1, simultaneous utilization of N–NH4+ and N–NO2− was observed from the beginning of both biotransformations. For both, 100% NR was observed after 75 h (ninth batch) and 78 h (eighth batch).
During the eighth batch, the higher NRR of 3.46 mg N (L h)−1 was achieved in the 0–30 h period (Figure 2), and a similar rate of 3.39 mg N (L h)−1 was observed during the ninth biotransformation. Although the highest achieved value of the NRR was 2.8 times lower than published data on a similar topic [9.8 mg N (L h)−1], the observed NR efficiency in this study achieved 100% compared to reported 85% to 95% by Jenni et al. [12]. However, in this study, only small amount of immobilized biomass was applied (10% w/v particles load; in total 0,6 g of co-immobilized biomass per L of treated waste water). As reported recently [19], further process intensification is possible by the increase of immobilized biomass up to 20 g L−1 of dry cell weight biomass, with no change in immobilized particles structure. Interestingly, this overload of biomass results in linear increase of the specific immobilized biomass activity as well. In the case of AMX and AOB co-immobilization, this may result in a more than 30-fold increase of initial specific activity. The further increase in the NRR can be also obtained by increase of the total amount of used immobilized particles (10% w/v in these experiments) to maximum of 30% (w/v) [20], which results in an increase of total activity of the biotransformation system. Therefore, we believe that this co-immobilized system has a potential for further applications in waste water treatment processes.
3. Materials and Methods
3.1. Medium
The cultivation medium (0.8 L) was composed of real wastewater (received as a product from machine-sludge thickening) obtained from Central WWTP Vrakuňa, Bratislava, Slovakia, and aged tap water (left to sit for 24 h for chlorine evaporation) in a volumetric ratio of 3:5. The characteristics of the wastewater were a pH value of 7.4, a chemical oxygen demand of 370 mg L−1, a five-day biochemical oxygen demand of 138 mg L−1, a suspended solids content of 678 mg L−1, an ammonium nitrogen (N–NH4+) concentration of 275 mg L−1 and a total nitrogen content of 673 mg L−1. The nitrite nitrogen (N–NO2−) and nitrate nitrogen (N–NO3−) contents were not detectable. To increase the N–NO2− concentration, 0.2 g of NaNO2 (Mikrochem, Slovakia) was added into the cultivation medium. The final concentration of N–NH4+ in the cultivation medium was 103 ± 2 mg N L−1, except for the sixth and seventh conversions, which started with concentrations of 38.8 and 45.8 mg N L−1, respectively. The final concentration of N–NO2− was 62 ± 15 mg L−1. The medium was purged with N2 for 15 min to remove the dissolved O2, but this was only performed before the first biotransformation.
3.2. Biomass Immobilisation
Biomass used for immobilization was received from the Institute of Chemical Technology (ICT), Prague, Czech Republic, propagated in lab-scale conditions by use of real wastewater obtained from WWTP Prague. Using fluorescence in situ hybridization (FISH) by ICT, the composition of the received consortium of bacteria was characterized as 20% to 30% AMX bacteria and 70% to 80% of AOB. The biomass (1 ± 0.2 g wet weight) was softly suspended in 10 mL of aged tap water and mixed with 190 mL PVA hydrogel, prepared by using of PVA (20 g) and polyethylene glycol (12 g), which were melted in deionized water (158 mL) at 90 °C until clarification and then cooled to 30 °C. Lens-shaped gel particles with entrapped biomass were prepared by passing the gel mixture through thin nozzles to a hard surface, followed by subsequent drying in an airflow cabinet for 55 min. Particles were dried down to 30% of their initial mass and swollen in a stabilizing solution of 0.1 M sodium sulfate for 30–45 min. Then, the particles were separated through a sieve and washed from the stabilizing solution by deionized water. Using the mentioned LentiKats® method of immobilization, a final amount of 85 g of immobilized biomass was acquired.
3.3. Repeated Batch Biotransformation
Batch biotransformations with immobilized biomass were performed in a 1.3 L BioFlo® 115 fermenter (New Brunswick, USA) in which 0.8 L of the cultivation medium was inoculated with 85 g of LentiKats®. Each biotransformation was carried out at either pH 8.0 (first and second batch) or pH 7.0 (fifth to ninth batch) by automatic addition of 2 M NaOH at 30 °C with 250 rpm stirring. Repeated batch-mode biotransformations occurred when the residual concentrations of N–NH4+ and N–NO2− were reduced to almost 0 mg N L−1 or did not change in the long term. This situation was solved by separating the entire volume of the production medium through a sieve to avoid washing out the LentiKats® with the immobilized biomass. The fermenter was subsequently fed with a fresh cultivation medium. Experiments were duplicated, and datapoints represent mean value of the process (SD was lower than 5%).
3.4. Analytical Assays
The concentrations of N–NH4+, N–NO2− and N–NO3− were determined by methods described in the Standard Methods for the Examination of Water and Wastewater [21], using a BioSpectometer® (Eppendorf, Germany). Dissolved oxygen (DO) was measured using a polarographic DO probe, and the pH value of the medium was measured using a pH probe, both of which are components of the BioFlo® 115 fermenter. NR and NRR were calculated to the depletion of primary sources of nitrogen: N–NH4+ and N–NO2−.
4. Conclusions
The presented results emphasize that the use of a small amount of immobilized biomass (0.6 g of immobilized wet weight per L of treated waste water) can be advantageous in large-scale applications, owing to the low biomass-production rate of AMX bacteria and AOB. The decrease of N–NH4+ leads to starvation of AOB, which stimulates the AMX bacteria and significantly improves the total nitrogen utilization and activity of co-immobilized cultures. This starvation technique is a useful trick for co-culture activities harmonization, which resulted in high overall nitrogen removal activity. At optimal conditions pH = 7 and initial concentrations N–NH4+ 100.3 mg N L−1 and N–NO2− 60.1 mg L−1, at 30 °C with gentle (250 rpm) batch bioreactor stirring, an immobilized consortium was able to remove 160.4 mg N L−1 of initial nitrogen sources from wastewater within 80 h with NR rate 3.46 mg N (L h)−1. This is the first report on the immobilization of AMX bacteria and AOB into a PVA hydrogel to indicate the methods, pH stat and substrate limitation that stimulate the co-immobilized bacteria activity in biotransformations. This co-culture improvement strategy might be beneficial for further co-immobilization studies and applications.
Author Contributions
I.D. investigation, writing—original draft preparation; R.S. methodology; M.R. (Michal Rosenberg) conceptualization, funding acquisition; M.R. (Martin Rebroš) writing—review and editing, methodology, funding acquisition.
Funding
This work was supported by the Ministry of Industry and Trade of the Czech Republic, programme TIP, grant no. FR-TI4/254 and was cofounded by the Slovak Research and Development Agency under contract no. APVV-16-0314. This publication is the result of the project implementation: Comenius University in Bratislava Science Park supported by the Research and Development Operational Programme funded by the ERDF. Grant number: ITMS 26240220086.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ni, B.-J.; Smets, B.F.; Yuan, Z.; Pellicer-Nàcher, C. Model-based evaluation of the role of Anammox on nitric oxide and nitrous oxide productions in membrane aerated biofilm reactor. J. Membr. Sci. 2013, 446, 332–340. [Google Scholar] [CrossRef]
- Paredes, D.; Kuschk, P.; Mbwette, T.S.A.; Stange, F.; Müller, R.A.; Köser, H. New aspects of microbial nitrogen transformations in the context of wastewater treatment—A review. Eng. Life Sci. 2007, 7, 13–25. [Google Scholar] [CrossRef]
- Bi, Z.; Qiao, S.; Zhou, J.; Tang, X.; Cheng, Y. Inhibition and recovery of Anammox biomass subjected to short-term exposure of Cd, Ag, Hg and Pb. Chem. Eng. J. 2014, 244, 89–96. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Ni, J. Effects of Ca2+ on activity restoration of the damaged anammox consortium. Bioresour. Technol. 2013, 143, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Peng, Y.; Zhang, S.; Wang, J.; Gan, Y.; Chang, J.; Wang, S.; Wang, S.; Zhu, G. Performance of anammox UASB reactor treating low strength wastewater under moderate and low temperatures. Bioresour. Technol. 2013, 129, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, H.; Yang, F.; Qiang, H.; Li, H.; Zhang, R. Study of an innovative anaerobic (A)/oxic (O)/anaerobic (A) bioreactor based on denitrification–anammox technology treating low C/N municipal sewage. Chem. Eng. J. 2013, 232, 65–73. [Google Scholar] [CrossRef]
- Magrí, A.; Béline, F.; Dabert, P. Feasibility and interest of the anammox process as treatment alternative for anaerobic digester supernatants in manure processing—An overview. J. Environ. Manag. 2013, 131, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-W.; Wei, Q.-Y.; Urata, K.; Tomoshige, Y.; Zhang, X.-H.; Kawagoshi, Y. Kinetic study on nitrogen removal performance in marine anammox bacterial culture. J. Biosci. Bioeng. 2014, 117, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.-C.; Xing, B.-S.; Yu, J.-J.; Qin, T.-Y.; Chen, S.-X. The importance of the substrate ratio in the operation of the Anammox process in upflow biofilter. Ecol. Eng. 2013, 53, 130–137. [Google Scholar] [CrossRef]
- Isaka, K.; Kimura, Y.; Yamamoto, T.; Osaka, T.; Tsuneda, S. Complete autotrophic denitrification in a single reactor using nitritation and anammox gel carriers. Bioresour. Technol. 2013, 147, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Anjali, G.; Sabumon, P.C. Unprecedented development of anammox in presence of organic carbon using seed biomass from a tannery Common Effluent Treatment Plant (CETP). Bioresour. Technol. 2017, 153, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Jenni, S.; Vlaeminck, S.E.; Morgenroth, E.; Udert, K.M. Successful application of nitritation/anammox to wastewater with elevated organic carbon to ammonia ratios. Water Res. 2014, 49, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Magrí, A.; Vanotti, M.B.; Szögi, A.A. Anammox sludge immobilized in polyvinyl alcohol (PVA) cryogel carriers. Bioresour. Technol. 2012, 114, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Inatomi, Y.; Qiao, S.; Quan, L.; Yamamoto, T.; Isaka, K.; Sumino, T. Innovative treatment system for digester liquor using anammox process. Bioresour. Technol. 2009, 100, 5437–5443. [Google Scholar] [CrossRef] [PubMed]
- Isaka, K.; Date, Y.; Sumino, T.; Tsuneda, S. Ammonium removal performance of anaerobic ammonium-oxidizing bacteria immobilized in polyethylene glycol gel carrier. Appl. Microbiol. Biotechnol. 2007, 76, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Rebroš, M.; Rosenberg, M.; Stloukal, R.; Krištofíková, L. High efficiency ethanol fermentation by entrapment of Zymomonas mobilis into LentiKatsR. Lett. Appl. Microbiol. 2005, 41, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Tian, T.; Duan, X.; Zhou, J.; Cheng, J. Novel single-stage autotrophic nitrogen removal via co-immobilizing partial nitrifying and anammox biomass. Chem. Eng. J. 2013, 230, 19–26. [Google Scholar] [CrossRef]
- Zhang, L.; Narita, Y.; Gao, L.; Ali, M.; Oshiki, M.; Okabe, S. Maximum specific growth rate of anammox bacteria revisited. Water Res. 2017, 116, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Zajkoska, P.; Rosenberg, M.; Heath, R.; Malone, K.J.; Stloukal, R.; Turner, N.J.; Rebros, M. Immobilised whole-cell recombinant monoamine oxidase biocatalysis. Appl. Microbio. Biotechnol. 2015, 99, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Stloukal, R.; Rosenberg, M.; Rebros, M. Method for industrial production of biocatalysts in the form of enzymes or microorganisms immobilized in polyvinyl alcohol gel, their use and devices for their production. WO/2007/104268, 20 December 2007. [Google Scholar]
- APHA, AWWA, WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington DC, WA, USA, 1998. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).