Abstract
A concurrent bienzymatic cascade for the synthesis of optically pure (S)-4-methoxymandelonitrile benzoate ((S)-3) starting from 4-anisaldehyde (1) has been developed. The cascade involves an enantioselective Manihot esculenta hydroxynitrile lyase-catalyzed hydrocyanation of 1, and the subsequent benzoylation of the resulting cyanohydrin (S)-2 catalyzed by Candida antarctica lipase A in organic solvent. To accomplish this new direct synthesis of the protected enantiopure cyanohydrin, both enzymes were immobilized and each biocatalytic step was studied separately in search for a window of compatibility. In addition, potential cross-interactions between the two reactions were identified. Optimization of the cascade resulted in 81% conversion of the aldehyde to the corresponding benzoyl cyanohydrin with 98% enantiomeric excess.
1. Introduction
In the past decades, the field of biocatalysis has grown exponentially, becoming an industrially attractive technology for the production of chiral pharmaceutical intermediates and precursors in the synthesis of fine chemicals [1,2]. Recently, considerable progress has been made in developing enzymatic cascades for the production of valuable chemical products [3,4,5,6]. These are synthetic processes that combine two or more enzymes in one pot and can be performed in a concurrent (simultaneous) or sequential mode [7]. The combination of individual reaction steps in a one-pot process has several advantages in terms of process efficiency and sustainability, since the solvent consumption and waste generation is generally decreased due to the lower number of work-up steps [5]. Furthermore, the coupling of reactions has the potential to drive equilibria towards the desired products, hence reducing the required excess of reagents [8]. There are, however, some challenges associated with the design of enzymatic cascades, mainly due to enzymes often having different optimum conditions. Furthermore, the presence of side products of certain reactions may lead to enzyme inhibition, which could be avoided by running the reactions in a sequential mode [9,10].
A current challenge in the field of enzymatic cascades is the coupling of a hydroxynitrile lyase (HNL)-catalyzed cyanohydrin synthesis with an acylation catalyzed by a lipase. The immediate acylation of the formed cyanohydrin could prevent the back reaction from taking place and yield a chemically more stable product. In addition, if a lipase with appropriate enantioselectivity is chosen, the enantiopurity of the final product can be enhanced.
However, the one-pot combination of the two enzymes is not trivial, for they have very different requirements. It is well known that water acts as a competing nucleophile in lipase-catalyzed transesterification reactions [11], whereas HNLs require relatively high water content when working in organic solvents [12,13,14]. Due to these different water content requirements, the cascade synthesis of acylated cyanohydrins catalyzed by an HNL and a lipase is a challenging task. Hanefeld et al. attempted to couple the hydrocyanation of benzaldehyde with the acetylation of the formed mandelonitrile, in order to shift the equilibrium of the first reaction. The reactions were catalyzed by Hevea brasiliensis hydroxynitrile lyase (HbHNL) and Candida antarctica lipase B (CALB), respectively. However, they encountered problems due to hydrolysis of the acyl donor and subsequent deactivation of the HbHNL [10]. As an alternative to the bienzymatic synthesis of acylated cyanohydrins, researchers have studied kinetic resolution of acylated cyanohydrins and dynamic kinetic resolution approaches combining an unselective hydrocyanation with an enantioselective transesterification [15,16,17,18,19,20]. Nevertheless, it has been shown that under certain conditions HNLs can work at low water content [21], opening the possibility for combining the two mentioned enantioselective enzymatic steps.
In the present study, we have selected the hydrocyanation of 4-anisaldehyde (1) combined with the benzoylation of the formed cyanohydrin (2) to yield 4-methoxymandelonitrile benzoate (3) as a model reaction (Scheme 1). The hydrocyanation of 4-anisaldehyde requires a particularly high excess of HCN to drive the equilibrium towards the cyanohydrin, compared to other benzaldehydes [22,23,24], which is attributed to the positive mesomeric effect of the para-methoxy substituent. The direct benzoylation of the cyanohydrin was envisioned as a possible solution to avoid isolating this instable product. The obtained cascade product 3 is interesting, as it can be transformed in a single hydrogenation step into (S)-tembamide [25], a naturally occurring N-(β-hydroxy) amide for which antiviral (HIV) and hypoglycemic activity have been reported [26,27].
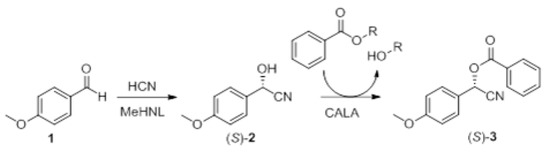
Scheme 1.
Proposed cascade synthesis of (S)-4-methoxymandelonitrile benzoate.
Herein, Manihot esculenta hydroxynitrile lyase (MeHNL)—an S-selective enzyme [28] that can catalyze the hydrocyanation of 4-anisaldehyde—was studied. For the benzoylation of the resulting cyanohydrin, Candida antarctica lipase A (CALA) was chosen, which is known to accept bulky substrates and displayed preference for (S)-4-methoxymandelonitrile benzoate in a prior hydrolase screening (see supporting information Table S1 and Figure S1). Moreover, CALA is a robust industrial enzyme, and was shown to exhibit acyltransferase activity even in aqueous media [29], which we deemed an attractive feature in our envisioned cascade.
2. Results and Discussion
A crucial step in the development of the intended cascade was the identification of a suitable benzoyl donor that would facilitate the CALA-catalyzed transesterification. Several donors were screened in the CALA-catalyzed benzoylation of 4-methoxymandelonitrile (see Figure S2). Of the screened esters, phenyl benzoate afforded the highest reaction rate.
For a successful combination of MeHNL and CALA in the cascade synthesis of (S)-4-methoxymandelonitrile benzoate, the enzymatic hydrocyanation and transesterification reactions were subjected to a systematic study of the relevant reaction variables. The effects of temperature and water activity on both reactions were evaluated and a suitable carrier was selected for the immobilization of each enzyme. In a preliminary solvent screening, isopropyl ether was selected as reaction solvent due to its compatibility with both enzymes and the relatively high solubility of the substrates.
2.1. Influence of Reaction Temperature
In general, temperature affects enzymatic stability and has a great effect on the reaction rate of enzyme-catalyzed reactions and their enantioselectivity. The influence of temperature on the enantioselectivity of enzymatic reactions can be explained by a simple theoretical model [30]. Depending on whether the reaction takes place below or above the so-called racemic temperature, a decrease in temperature will increase or decrease the enantioselectivity, respectively.
According to literature, MeHNL shows optimal hydrocyanation activity at ~40 °C [31]. A reduction in reaction temperature below 40 °C would decrease enzymatic activity, but is expected to slightly increase its enantioselectivity, since MeHNL displays an elevated racemic temperature of 580 °C in the hydrocyanation of phenylpropionaldehyde in dibutylether [14]. Furthermore, decreasing the reaction temperature should reduce the rate of the nonenzymatic hydrocyanation of 4-anisaldehyde to a higher extent than the enzymatic reaction, which would also contribute to increase the enantiomeric excess (e.e.) of the cyanohydrin [32,33,34]. Finally, the reaction must be performed at a rather low temperature, since the boiling point of HCN is 25.6 °C [35]. Based on this information, hydrocyanation reactions were performed at 5, 10, and 20 °C.
Within the studied temperature range, the hydrocyanation rate increased moderately with the reaction temperature (see Figure 1). On the other hand, the enantioselectivity of the enzymatic reaction slightly increased at lower temperatures, as expected. These results suggest that the optimal reaction temperature, which would afford excellent enantioselectivity with a satisfactory reaction rate, would be 10 °C.
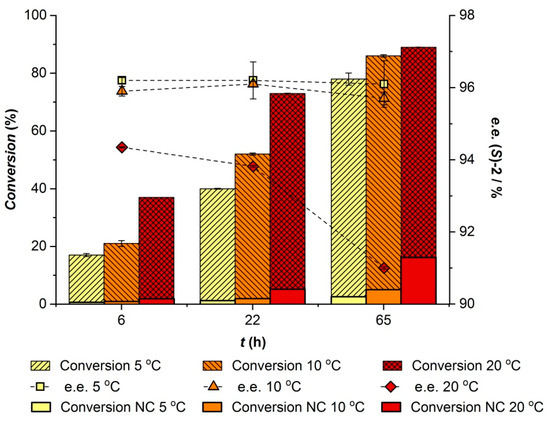
Figure 1.
Effect of reaction temperature on the MeHNL-catalyzed hydrocyanation of 100 mM 4-anisaldehyde in a biphasic system using 6.5 equivalents of HCN. Negative control (NC) shows the rate of the unselective background reaction.
In the case of CALA, based on the data from kinetic resolution studies, the lipase was expected to show increased enantioselectivity with lower temperatures [36,37]. However, immobilized CALA has been reported to display a temperature optimum of 90 °C [38], thus the conversion will be limited by low reaction rates at low temperatures.
The effect of reaction temperature on the CALA-catalyzed benzoylation of racemic 4-methoxymandelonitrile was studied in isopropyl ether without added water in the range of 5 to 25 °C. The results showed that, when reducing the temperature, the enantioselectivity of CALA was slightly enhanced (E value of 7 at 5 and 10 °C and 6 at 25 °C), although this was at the expense of a pronounced reduction of reaction rate, as shown in Figure 2. Within the studied temperature range, the CALA-catalyzed benzoylation of 4-methoxymandelonitrile can be achieved in relatively high yields with optimal performance at 25 °C. Although CALA shows preference for conversion of the (S)-enantiomer, it is not selective enough to afford an enantiopure product. However, when preceded by an enantioselective hydrocyanation, the transesterification reaction can enhance the enantiopurity of the final product. Therefore, the observed minor effect of reaction temperature on the enantiomeric ratio can be neglected.
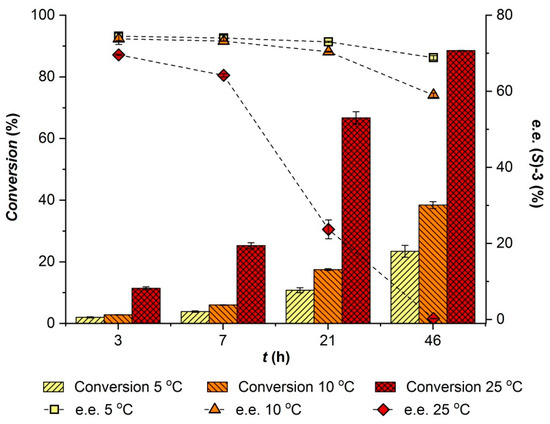
Figure 2.
Effect of temperature on CALA-catalyzed benzoylation of 65 mM (±)-4-methoxymandelonitrile using 200 mM phenyl benzoate.
2.2. Selection of Enzyme Carriers
In our designed cascade, we envisioned a low-water organic solvent system for which the immobilization of both enzymes would be required. In organic solvents, free lipases generally express low catalytic activity and they tend to form aggregates which can lead to mass transfer limitations [39]. Adsorption is believed to enhance the catalytic activity of lipases due to hydrophobic interactions between the support and part of the enzyme yielding the active enzyme conformation by opening the lid [40]. Similarly, immobilization of hydroxynitrile lyases can improve their activity and stability towards organic solvents [41], and may lead to higher enantioselectivity [42]. In this study, CALA was adsorbed on Relizyme EXE309, a macroporous bead based on a methyl methacrylate/styrene copolymer that was recommended by the manufacturer for effective lipase adsorption. For MeHNL, adsorption on Celite R-633 was identified as the best immobilization method (see supporting information Table S2).
2.3. Influence of Water Activity
When working with enzymes in organic solvents, their activity is greatly affected by the water content in the reaction medium. This variable is best quantified in terms of thermodynamic water activity (aw), which is equal in all phases in equilibrium [43]. Water is believed to act as a molecular lubricant, increasing the conformational flexibility of enzymes and, thus, their catalytic activity [44]. The water content also affects enzyme selectivity in a complex way, which involves interactions with the reaction medium and the substrates [14].
In the case of HNLs it has been shown that when working at low water activities, the enzymes are insufficiently hydrated, resulting in activity and selectivity loss [12,13,14]. Lipases, on the other hand, can generally withstand lower water content [45], and some studies have shown that aw does not significantly influence the enantioselectivity of lipases [14,45,46,47]. Furthermore, water competes with the substrate alcohol (in this case, cyanohydrin) for the nucleophilic attack of the acyl-enzyme intermediate, leading to ester hydrolysis [48].
A prerequisite for the combination of both enzymes in a concurrent mode is to find a suitable aw range that allows for sufficient MeHNL activity and high selectivity, while minimizing ester hydrolysis catalyzed by CALA. In order to evaluate the aw effect on both enzymatic reactions, a salt hydrate system that could cover a wide range of aw values was selected: Na2HPO4/Na2HPO4·2H2O/Na2HPO4·7H2O/Na2HPO4·12H2O. These salt pairs afford water activity values of 0.15, 0.57 and 0.74, respectively, at 20 °C [43]. The hydrocyanation and benzoylation reactions were studied independently using immobilized MeHNL and CALA and the three salt pairs.
Before evaluating the influence of aw on MeHNL, the effect of the phosphate salt hydrate pairs on the background hydrocyanation reaction was studied. The chemical hydrocyanation of 100 mM 4-anisaldehyde in isopropyl ether was performed using 6.5 equivalents of HCN and 0.5 mmol salt pair per mL of reaction. This revealed that the Na2HPO4/Na2HPO4·2H2O pair could convert 32% of the aldehyde in 24 h, whereas the other two salt pairs afforded a much lower chemical background reaction rate with only 4% conversion after 24 h. The negative control, where no salt was added, showed 1% conversion.
The effect of the three water activity values provided by the phosphate salts on the MeHNL-catalyzed hydrocyanation of 4-anisaldehyde was then evaluated (see Figure 3). Results at aw = 0.15 are difficult to interpret due to the above-mentioned catalytic effect of the salt. It seems, however, that the enzyme is hardly active at this low water activity. Comparison of the results at the two highest water activity values, where the background reaction is similarly low, shows that the activity and selectivity of MeHNL increased with increasing aw. Hence, to ensure a high e.e. of the resulting cyanohydrin, it is necessary to work at a high aw for the combination of MeHNL and CALA.
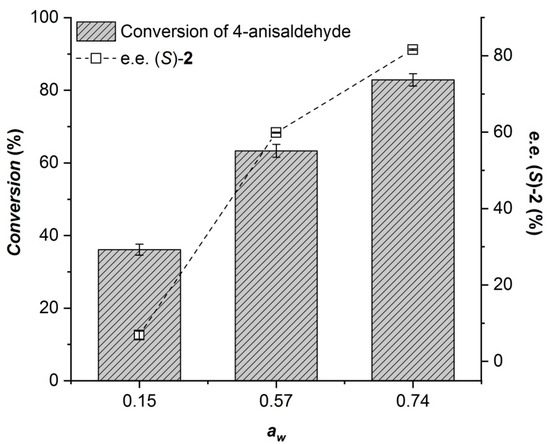
Figure 3.
Effect of water activity on the MeHNL-catalyzed hydrocyanation of 90 mM 4-anisaldehyde using 6.5 equivalents of HCN. Conversion and enantiomeric excess (e.e.) values taken after 23 h of reaction.
In the case of CALA, at aw = 0.57 and below, no significant difference in the enantioselectivity of the enzyme (E = 5) or in the selectivity for transesterification over hydrolysis was observed (see Figure 4). When working at aw = 0.74, however, the hydrolysis rate increased significantly at the expense of the transesterification reaction. Although the enantioselectivity also increased under these conditions (E = 9), it is not a sufficient improvement to compensate for the increased hydrolysis rate. Considering the observed effects of water activity on MeHNL and CALA, we concluded that it should be possible to combine both enzymatic reactions at aw = 0.57.
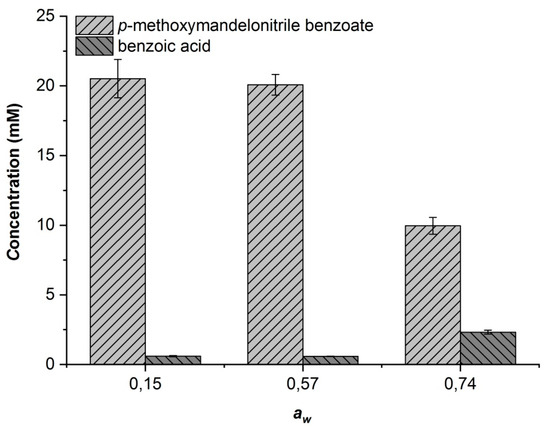
Figure 4.
Effect of the different phosphate hydrate pairs on the CALA-catalyzed benzoylation of 66 mM (±)-4-methoxymandelonitrile using 200 mM vinyl benzoate after 22 h of reaction.
2.4. Cross Interactions
When performing reactions in one-pot, the reagents, products, or byproducts of one reaction may affect the other reactions. Thus, for an optimized cascade, the effect of hydrocyanation reagents on the CALA-catalyzed transesterification reaction as well as the influence of the benzoyl donor and the transesterification byproduct on the MeHNL-catalyzed hydrocyanation reaction was evaluated. Further, the influence of the side product benzoic acid on both transesterification and hydrocyanation reactions was studied.
The influence of 4-anisaldehyde and HCN on the reaction rate and selectivity of the CALA-catalyzed benzoylation of 4-methoxymandelonitrile was evaluated. As shown in Figure 5A, both 4-anisaldehyde and HCN exert a moderate negative effect on the benzoylation rate. Moreover, both compounds reduce the selectivity of CALA towards transesterification (see Figure 5B). Regarding the enantioselectivity of the benzoylation reaction, it was not significantly affected by the presence of either of the hydrocyanation substrates, with the E values varying between 5 and 6. Similarly, the effect of benzoic acid on CALA was studied in a benzoylation reaction of 70 mM (±)-4-methoxymandelonitirile containing one equivalent of benzoic acid. The reaction rate was 20% slower than that of the control.
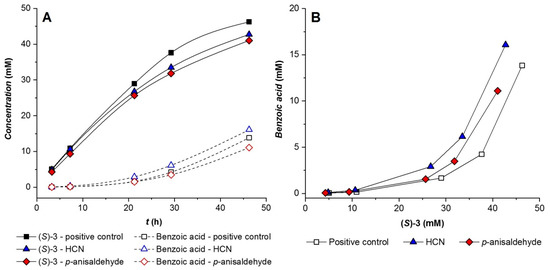
Figure 5.
Effect of 60 mM 4-anisaldehyde and 209 mM HCN on the transesterification and hydrolysis rate of CALA (A) as well as its selectivity towards transesterification over hydrolysis (B) using 65 mM (±)-4-methoxymandelonitrile and 200 mM phenyl benzoate.
The influence of phenyl benzoate and its leaving group, phenol, on the MeHNL-catalyzed hydrocyanation of 4-methoxymandelonitrile was studied. Addition of either three equivalents of phenyl benzoate or phenol to the hydrocyanation reaction afforded (S)-4-methoxymandelonitrile with the same yield and enantiopurity as the control reaction (data not shown). To evaluate the effect of the side product benzoic acid on the hydrocyanation reaction, an experiment was performed with addition of different amounts of the acid to the reaction mixture. As shown in Figure 6, benzoic acid exerts a strong negative effect on the hydrocyanation rate of MeHNL. Thus, in order to achieve a cascade synthesis of 3 with high yield and selectivity, the CALA-catalyzed hydrolysis of phenyl benzoate and 3 should be minimized as much as possible. This observation is not surprising, since benzoic acid has previously been reported as a competitive inhibitor for other hydroxynitrile lyases [49,50,51]. Based on these results, the observed negative effect of benzoic acid on CALA is minor when compared to its effect on MeHNL.
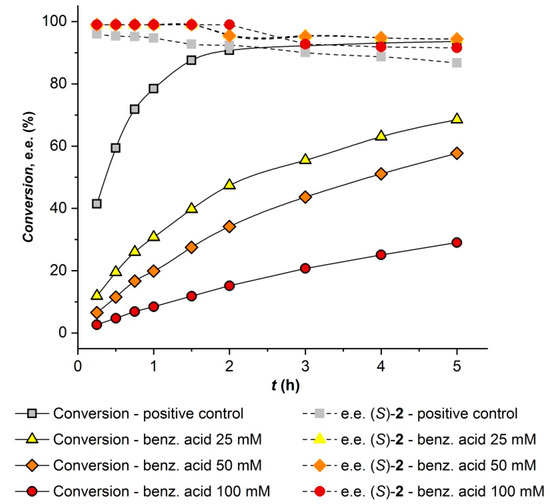
Figure 6.
Effect of 25 mM, 50 mM, or 100 mM benzoic acid on MeHNL-catalyzed hydrocyanation of 100 mM 4-anisaldehyde using 6.5 equivalents of HCN. With exception of the positive control, the enantiomeric excess values overlap at low reaction times.
2.5. Cascade Synthesis of (S)-4-Methoxymandelonitrile Benzoate
For the first cascade synthesis test (see Table 1, entry 1), the used reaction conditions in terms of enzyme amount, temperature, water buffering salts and the time point of CALA addition were selected based on the results obtained before. The reaction temperature was set at 10 °C, Na2HPO4·2H2O/Na2HPO4·7H2O was added to set the water activity to aw = 0.57, and the lipase was added after 16 h of hydrocyanation reaction with the idea to minimize the exposure of MeHNL to benzoic acid. In this first reaction, a low reaction rate of the overall process was observed, resulting in only 10.5% conversion to the corresponding ester with a fair e.e. of 89% after 47 h reaction time. The results, however, showed that both enzymes were simultaneously active after adding the lipase to the reaction mixture, since the 4-anisaldehyde was further converted and (S)-4-methoxymandelonitrile benzoate was also formed. The same process was repeated at 25 °C (Table 1, entry 2) resulting in a significantly improved reaction rate, while maintaining the enantiomeric excess of the ester. Based on these observations, all further reactions were performed at room temperature (20 °C).

Table 1.
Cascade synthesis of (S)-4-methoxymandelonitrile benzoate catalyzed by immobilized MeHNL and CALA starting from 100 mM 4-anisaldehyde and 650 mM HCN using 200–400 mM phenyl benzoate. Reactions were performed in duplicate and relative standard deviations were generally below 2%.
For comparison, in experiment 3 (Table 1, entry 3) a concurrent approach, where both enzymes were added from the beginning, was tested. Due to the inhibitory effect of 4-anisaldehyde and HCN on the acyltransferase activity of CALA, a higher amount of lipase was added. This approach significantly improved the enantiopurity of the final ester (97% e.e.), while maintaining a good reaction rate. After 30.5 h of reaction, 10 mM cyanohydrin was still present in the reaction mixture. This suggested that the transesterification rate was not too high in comparison to the MeHNL-catalyzed hydrocyanation. Therefore, it was decided to maintain the CALA/MeHNL ratio for further reactions. Strikingly, the results could be further improved by omitting the water buffering salts from the system (Table 1, entry 4). Elimination of these salts provided overall higher yield and selectivity, which may be explained by the previously identified negative effect of low water activity on the enzymatic hydrocyanation reaction. Moreover, comparison of the benzoic acid concentration in experiments 3 and 4 indicated that in the former cascade (including the salts) the hydrolysis reaction was not suppressed to a higher extent than in the latter (without salts). Thus, the use of the salt hydrate pair did not further reduce the hydrolysis reaction in the studied system.
Salt-free experiments with varying initial amounts of benzoyl donor (Table 1, entries 4–6) showed a detrimental effect of higher donor concentrations on the hydrocyanation reaction, while the amount of hydrolysis side product, benzoic acid, increased substantially. In all cases, however, excellent enantiopurity of (S)-4-methoxymandelonitrile benzoate (99% e.e.) was maintained throughout the process. These results prompted us to test gradual addition of phenyl benzoate to the process, which was found to minimize the hydrolytic side reaction at the expense of some loss in reaction rate (see Table 1, entry 7), leading to 64% yield of ester 3 (98% e.e.) in 49 h, with 50% less benzoic acid produced compared to Table 1, entry 5. Finally, we hypothesized that a reduction of the lipase units could further decrease the amount of formed benzoic acid. Using 0.5 U/ml CALA afforded ester 3 (98% e.e.) with 81% yield in 122 h, with a total conversion of 4-anisaldehyde of 95% (see Table 1, entry 8 and Figure S3). The reaction time required for high conversion values could be reduced by approximately one day when the reaction temperature was increased from 20 to 25 °C (see Table 1, entry 10).
Furthermore, we also tested the possibility of decreasing the amount of HCN used in the cascade. However, when performing the cascade using 5 instead of 6.5 equivalents of HCN (using the same conditions detailed in Table 1, entry 7), after 122 h only 60 mM (S)-3 was produced with a poor enantiomeric excess of 87%. Similarly, a decrease in yield and enantiomeric excess of (S)-2 was observed in the immobilized MeHNL-catalyzed hydrocyanation of 4-anisaldehyde when decreasing the HCN concentration (see Figure S4).
Overall, our results demonstrate that the concurrent combination of a MeHNL-catalyzed hydrocyanation with a CALA-catalyzed transesterification is possible after careful investigation of the individual reactions in search for compatible conditions. However, further optimization is required to improve the cascade, since 5% residual 4-anisaldehyde could not be converted by MeHNL (see Table 1, entry 8 and Figure S4A). Presumably, this is due to an observed increase in benzoic acid concentration as the cascade proceeds. Moreover, roughly 11 mM cyanohydrin 2 remained in the reaction media, which could not be further converted to 3 by CALA. This indicates a rather low affinity of the lipase for 4-methoxymandelonitrile, which, at low concentrations, cannot compete with the water present in the reaction medium for the acyl-enzyme complex. Hence, hydrolysis is the main reaction taking place in the end, further increasing the amount of benzoic acid.
The use of an engineered CALA with enhanced selectivity towards transesterification reactions versus hydrolysis could significantly improve the performance of our designed cascade [29].
3. Materials and Methods
3.1. Enzymes
Hydroxynitrile lyase from Manihot esculenta was heterologously expressed in E. coli K12 Top 10F’ harboring the pSE420-MeHNL plasmid, which was kindly provided by Dr. Kerstin Steiner from the Austrian Centre of Industrial Biotechnology (ACIB). 2 × YT medium (100 mL, containing 100 µg/mL ampicillin) was inoculated from a glycerol stock (10 µL) and the culture was incubated overnight at 37 °C, 200 rpm. A 5 mL aliquot of this preculture (OD600 = 4.4) was used to inoculate 2 × YT medium (500 mL, containing 100 µg/mL ampicillin), and the new culture was grown at 37 °C, 200 rpm. At an optical density at 600 nm (OD600) of 0.8, IPTG was added to a final concentration of 0.1 mM and the culture was further incubated at 18 °C, 160 rpm for 48 h. Cells were harvested by centrifugation at 4000 g, 4 °C for 10 min, washed with 25 mM sodium acetate buffer, pH 5.8, and centrifuged again (4000 g, 4 °C for 30 min). Cell pellets were resuspended in 25 mM sodium acetate, pH 5.8 (4 mL per g of wet cells), and 10 mL aliquots were sonicated at 4 °C using a Fisher Scientific Model 120 Sonic Dismembrator at 60% amplitude, 2 s pulse, 5 s pause for 7 min. The resulting crude extract was centrifuged to remove cell debris (18,500 g, 4 °C for 45 min) and the obtained cell free extract was stored at −20 °C. Concentration of cell free extract was performed in an Amicon Ultra Filter Device with 10 kDa cut-off at 4 °C.
Candida antarctica lipase A (Novozym® CALA L, LDN 00025) was purchased from Novozymes.
3.1.1. Enzyme Activity Measurements
The enzymatic activity of free MeHNL was measured following the cleavage of racemic mandelonitrile into benzaldehyde and HCN, according to a literature procedure [52]. Samples were prepared by diluting the MeHNL cell free extract in 5 mM phosphate buffer, pH 6.5. The enzymatic activity of immobilized MeHNL was determined following the hydrocyanation of 4-anisaldehyde (100 mM) using 6.5 equivalents of HCN in isopropyl ether at 20 °C, 800 rpm. A negative control was performed using the enzyme support instead. Samples were analyzed by chiral HPLC.
The enzymatic activity of CALA was measured according to a modified literature procedure following the hydrolysis of tributyrin in phosphate buffer at pH 7 [53]. The resulting butyric acid was titrated with 0.1 M sodium hydroxide, and the consumption of the latter was recorded as a function of time. The enzymatic activity of immobilized CALA was determined following the hydrolysis of 4-nitrophenyl butyrate (1.67 mM) in acetonitrile/50 mM phosphate pH 7.4 (1/5). A calibration curve of the product 4-nitrophenol was used to calculate the reaction rate based on the absorbance of the reaction mixture at 410 nm.
3.1.2. Enzyme Immobilization
General procedure for MeHNL: In a 5 mL glass vial containing a magnetic stirring bar and the carrier (100 mg), MeHNL-containing cell-free extract (40 µL) was added dropwise while stirring. The mixture was stirred for 30 min and was then dried at room temperature under high vacuum for 48 h.
Immobilization of CALA: CALA (30 g), Relizyme EXE309 (30 g), and demineralized water (30 mL) were mixed in a 100 mL bottle. The pH was adjusted to 6 with a solution of 2 M sodium hydroxide and the mixture was incubated at room temperature in an orbital shaker overnight. After filtering the solution, the activity of the supernatant was measured and compared to the initial activity of the enzyme solution before immobilization, giving an immobilization yield of 84%. The immobilized enzyme was washed several times with demineralized water and dried at room temperature under high vacuum for 48 h.
3.1.3. Evaluation of the MeHNL Immobilisates
To 1.5 mL glass vials containing different MeHNL immobilisates (15 mg) and Na2HPO4·2H2O (30 mg, 0.17 mmol), each a solution of 100 mM 4-anisaldehyde and 650 mM HCN in isopropyl ether (500 µL) was added. Duplicate reactions per immobilisate were incubated at 20 °C in an orbital shaker at 800 rpm. Samples (20 µL) were analyzed by HPLC.
3.2. Chemicals
Unless otherwise indicated, reagents and organic solvents were purchased from Fisher Scientific, Sigma-Aldrich, TCI Chemicals and Acros Organics, and were of the highest available purity.
Celite R633 was a gift from Imerys S.A., Relizyme EXE309 was kindly provided by Resindion S.r.l., and SYLOID silicas were a gift from Grace Davison, Inc. (±)-4-Methoxymandelonitrile was synthetized via chemical hydrocyanation of 4-anisaldehyde following a literature procedure [22].
For the preparation of hydrogen cyanide solution, potassium cyanide (26 g, 0.4 mol) was dissolved in a stirred mixture of distilled water (200 mL) and diisopropyl ether (iPr2O) (100 mL). The solution was cooled in an ice-water bath and 1.33 M citric acid (100 mL, 0.13 mol, 1 equivalent) was slowly added. The aqueous layer was extracted twice with 50 mL of iPr2O. The combined organic phases were stored in a dark bottle containing 1 M citrate buffer pH 5.5 (10 mL). The HCN concentration was determined by measuring the absorption of the [Ni(CN)4]2− complex ion at 267 nm. HCN solution (20 µL) was added to 3.5 mM NiSO4·6H2O in 1 M NH3 (4 mL) [54]. After vigorous mixing, the solution was further diluted 50 times with 1 M NH3 in a cuvette. The absorption was measured and the resulting HCN concentration was calculated based on a calibration curve.
Synthesis of (±)-4-Methoxymandelonitrile (2)
In a 100 mL round-bottom flask, NaCN (4.4 g, 90 mmol) was dissolved in water (30 mL) and NaHSO3 (9.4 g, 90 mmol) was slowly added. 4-Anisaldehyde (2.4 g, 18 mmol) was dissolved in ethyl acetate (20 mL) and added to the reaction mixture. After 1 h of vigorous stirring, the flask was introduced in an ice-water bath and stirred for another 6 h. The reaction was followed by HPLC until it reached plateau after 90% conversion. Distilled water (30 mL) was added to dissolve the salts and the aqueous phase was extracted ethyl acetate (2 × 10 mL). The combined organic phase was washed with brine, dried over anhydrous MgSO4, and concentrated on a rotary evaporator using a 10 °C bath, whereupon crystallization occurred. The crystals were washed with cold heptane/ethyl acetate (2:1) and stored at 5 °C. HPLC analysis showed that the (±)-4-methoxymandelonitrile crystals contained 3% 4-anisaldehyde impurity.
3.3. Bioconversions
All bioconversions were performed in 1.5 mL glass vials in an orbital shaker at 800 rpm. Unless otherwise stated, the reaction temperature was set at 20 °C.
3.3.1. Benzoyl Donor Screening
To a vial containing immobilized CALA (4.2 mg, 0.46 U), racemic 4-methoxymandelonitrile (200 mM) in isopropyl ether (250 µL) and benzoyl donor (300 mM) in isopropyl ether (500 µL) was added. Reactions were performed in duplicate and were incubated at 25 °C. Samples (20 µL) were taken after 4, 20, and 27.5 h and analyzed by HPLC. The enantioselectivity was calculated according to the method of Chen et al. for conversion values between 5 and 20% [55].
3.3.2. Temperature Effect on MeHNL
4-Anisaldehyde (530 mM) in isopropyl ether (188 µL), HCN (800 mM) in isopropyl ether (812 µL), citrate buffer pH 5 (500 mM, 125 µL), and MeHNL-containing cell-free extract (33.2 µL, 5 U) were incubated at 5, 10, and 20 °C. Reactions were performed in duplicate and a negative control reaction was performed for each temperature value, where no enzyme was added. Samples (20 µL) were taken from the upper organic phase after 6, 22, and 75 h and analyzed by HPLC.
3.3.3. Temperature Effect on CALA
To a vial containing CALA (2 mg, 0.22 U), (±)-4-methoxymandelonitrile (195 mM) in isopropyl ether (125 µL) and phenyl benzoate (300 mM) in isopropyl ether (250 µL) was added. The mixture was incubated at 5, 10, and 25 °C. Reactions were performed in duplicate. Samples (20 µL) were taken after 3, 7, 21, and 46 h and analyzed by HPLC.
3.3.4. Effect of Na2HPO4 Salt Hydrates on Chemical Hydrocyanation
To a solution of 4-anisaldehyde (100 mM) and HCN (650 mM) in isopropyl ether (1 mL), anhydrous Na2HPO4 (70 mg, 0.5 mmol), Na2HPO4·2H2O (85 mg, 0.5 mmol), or Na2HPO4·7H2O (134 mg, 0.5 mmol) was added. Reactions were performed in duplicate and a control reaction without salt was run in parallel. The amount of salt added is based on the maximum solubility of water in isopropyl ether (0.55 wt%) [56] and the water capacity of the Na2HPO4/Na2HPO4·2H2O salt pair (11.2 mmol/g) [57]. Reactions were stopped after 24 h and analyzed by HPLC.
3.3.5. Effect of aw on MeHNL
To a vial containing MeHNL immobilized on Celite R633 (12 mg, 0.36 U), a solution of 4-anisaldehyde (90 mM) and HCN (590 mM) in isopropyl ether (550 µL) was added, together with either anhydrous Na2HPO4 (35 mg, 0.25 mmol), Na2HPO4·2H2O (42 mg, 0.25 mmol) or Na2HPO4·7H2O (67 mg, 0.25 mmol). Reactions were performed in duplicate. Samples (20 µL) were taken after 5 and 24 h and analyzed by HPLC.
3.3.6. Effect of aw on CALA
To a vial containing CALA immobilized on Relizyme EXE309 (4 mg, 0.44 U), a solution of (±)-4-methoxymandelonitrile (100 mM) and vinyl benzoate (300 mM) in isopropyl ether (500 µL) was added, together with either anhydrous Na2HPO4 (35 mg, 0.25 mmol), Na2HPO4·2H2O (42 mg, 0.25 mmol), or Na2HPO4·7H2O (67 mg, 0.25 mmol). Samples (20 µL) were taken after 6, 22, and 30 h and analyzed by HPLC.
3.3.7. Effect of Phenol and Phenyl Benzoate on MeHNL
To a solution of 4-anisaldehyde (100 mM) and HCN (600 mM) in isopropyl ether (670 µL), MeHNL on Celite R633 (8 mg, 0.24 U) and either phenol (18.8 mg, 200 µmol) or phenyl benzoate (39.6 mg, 200 µmol) were added. A control was performed without addition of phenol or phenyl benzoate. Samples (20 µL) were taken after 17 and 22 h and analyzed by HPLC.
3.3.8. Effect of 4-Anisaldehyde and HCN on CALA
To a solution of (±)-4-methoxymandelonitrile (65 mM) and phenyl benzoate (200 mM) in isopropyl ether (375 µL), CALA on Relizyme EXE309 (2 mg, 0.22 U) and either 4-anisaldehyde (200 mM) in isopropyl ether (160 µL) or HCN (700 mM) in isopropyl ether (160 µL) were added. Reactions were performed in duplicate and a control was performed adding isopropyl ether (160 µL) instead of the hydrocyanation reagents. The reactions were incubated at 25 °C. Samples (20 µL) were taken after 3, 7, 21, 29, and 46 h and analyzed by HPLC.
3.3.9. Effect of Benzoic Acid on MeHNL
To a vial containing MeHNL on Celite R633 (15 mg, 0.45 U) and benzoic acid (1.5 mg, 12 µmol; 3 mg, 25 µmol; or 6 mg, 50 µmol), a solution of 4-anisaldehyde (100 mM), HCN (650 mM), and mesitylene (100 mM) (internal standard) in isopropyl ether (500 µL) was added. A control was performed without addition of benzoic acid. Samples (20 µL) were analyzed by HPLC.
3.3.10. Effect of Benzoic Acid on CALA
To a solution of (±)-4-methoxymandelonitrile (100 mM), phenyl benzoate (300 mM) and mesitylene (100 mM) (internal standard) in isopropyl ether (350 µL), CALA on Relizyme EXE309 (2 mg, 0.22 U), and Na2HPO4·2H2O (30 mg, 0.17 mmol), benzoic acid (70 mM) in isopropyl ether (150 µL, 1 equivalent) were added. A control was performed adding isopropyl ether (150 µL) instead of benzoic acid solution. Samples (20 µL) were analyzed by HPLC.
3.3.11. General Procedure for the Cascade Synthesis of (S)-4-Methoxymandelonitrile Benzoate
To a vial containing immobilized MeHNL and CALA, Na2HPO4·2H2O (30 mg, for experiments 1-3, Table 1) was added. A solution of 4-anisaldehyde (360 mM) and mesitylene (360 mM) (internal standard) in isopropyl ether (140 µL) and HCN (900 mM) in isopropyl ether (360 µL) was subsequently added. To this mixture, phenyl benzoate (2-4 equivalents) was added (in case of experiments 7 and 8 from Table 1, 1 equivalent was added each time at t = 0, after 24 h and after 46 h) and the reactions were incubated at 10, 20, or 25 °C. Negative control reactions without enzyme addition were performed in parallel. Reactions were performed in duplicate and 20 µL samples were analyzed by HPLC.
3.4. HPLC Analysis
The quantification of 4-anisaldehyde, (S)- and (R)-4-methoxymandelonitrile and (S)- and (R)-4-methoxymandelonitrile benzoate was achieved using chiral normal phase HPLC. Samples from reaction mixtures were diluted 50× with n-heptane/isopropanol 8:2 v/v and dried over anhydrous magnesium sulfate. Analysis was performed using a Hitachi Elite LaChrom HPLC system, consisting of a VWR Hitachi L-2130 pump, L-2200 auto-sampler, L-2350 column oven, and L-2400 UV detector using a D2 lamp coupled to a Hitachi organizer module. Separation was achieved on a Chiralpak AD-H column (250 mm × 4.6 mm × 5 μm; Daicel, Japan), using n-heptane/isopropanol (82/18) at a flow rate of 0.65 mL/min. The column oven temperature was set at 35 °C and a detection wavelength of 225 nm was chosen. Under these conditions, the compounds were separated with the following retention times; mesitylene (internal standard) 5.0 min, benzoic acid 6.7 min, phenol 7.0 min, phenyl benzoate 7.3 min, 4-anisaldehyde 8.3 min, (S)-4-methoxymandelonitrile 9.6 min, (R)-4-methoxymandelonitrile 10.5 min, (S)-4-methoxymandelonitrile benzoate 13.1 min, and (R)-4-methoxymandelonitrile benzoate 13.9 min.
Reverse-phase HPLC was used for the quantification of benzoic acid. Samples from reaction mixtures were diluted 50 × with acetonitrile/water 1:1 v/v. Analysis was performed using a Shimadzu Nexera XR HPLC system equipped with a DGU-20A5R degassing unit, coupled to two LC-20AD solvent delivery units, a SIL-20AC Autosampler, a CTO-20A column oven, an SPD-20A UV/VIS detector using a D2 lamp, and a CBM-20A system controller. Separation was achieved on a Nucleoshell RP 18 column (150 mm × 3 mm × 2.7 µm; Macherey-Nagel, Germany) at 30 °C, 0.7 mL/min flow rate using MilliQ® water containing 0.1% trifluoroacetic acid (solvent A) and acetonitrile (solvent B) as mobile phase and UV detection at 225 nm. The following eluent program was used; 10% solvent B for 0.5 min, followed by a linear gradient to 45% solvent B in 5.5 min, 45% solvent B for 10 min, another linear gradient to 100% solvent B in 5 min, 100% solvent B for 3 min, and finally a linear gradient to 10% solvent B in 3 min followed by 10% solvent B for 3 min. Under these conditions, the compounds were separated with the following retention times: phenol 4.3 min, benzoic acid 4.9 min, (±)-4-methoxymandelonitrile 5.2 min, 4-anisaldehyde 5.9 min, phenyl benzoate 14.8 min, (±)-4-methoxymandelonitrile benzoate 16.1 min, and mesitylene (internal standard) 19.3 min.
Calibration curves were prepared using commercial 4-anisaldehyde (>99.0%), (R)-4-methoxymandelonitrile (98.0%) and benzoic acid (>99.5%) as well as chemically synthesized (±)-4-methoxymandelonitrile benzoate.
4. Conclusions
In the present work, the successful concurrent cascade synthesis of (S)-4-methoxymandelonitrile benzoate starting from 4-anisaldehyde and catalyzed by MeHNL and CALA was accomplished. Systematically studying each enzymatic reaction first was key for their effective combination. When developing the one-pot cascade, however, further fine-tuning of reaction conditions was required to achieve optimal performance. This way, 95% conversion of 1 with 81% yield of the final ester (S)-3 and excellent enantiopurity of 98% e.e. was achieved. Compared to the enzymatic hydrocyanation performed under the same conditions, which yielded 2 with 85% conversion and 91% e.e. (see Table 1, entry 9, and Figure S4B), this result proves that the CALA-catalyzed benzoylation of (S)-2 coupled to the MeHNL-catalyzed hydrocyanation of 1 can shift the equilibrium of the latter by removal of the unstable intermediate. Nevertheless, there is still room for further improvement, since the low affinity of CALA for cyanohydrin 2 has been identified as the main bottleneck that prevents the cascade from reaching full conversion.
In principle, our approach reported herein can be transferred to the synthesis of other acylated cyanohydrins. In any case, a key requirement for success will be the application of a lipase with high selectivity for transesterification over hydrolysis in microaqueous media.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/6/522/s1, Figure S1: Conversion and enantiomeric excess (e.e.) of the remaining 4-methoxymandelonitrile benzoate after 18 h of hydrolysis reaction, Figure S2: Conversion and enantiomeric excess (e.e.) values obtained for the CALA-catalyzed benzoylation of 67 mM (±)-4-methoxymandelonitrile using 200 mM vinyl benzoate (VB), acetoxime benzoate (AB) or phenyl benzoate (PB), Figure S3: Comparison of the cascade synthesis of (S)- 4-methoxymandelonitrile benzoate catalyzed by MeHNL and CALA starting from 100 mM 1 (A, Table S2, entry 8) and the hydrocyanation of 100 mM 1 catalyzed by MeHNL under the same conditions (B, Table S2, entry 9), Figure S4: Hydrocyanation of 100 mM 4-anisaldehyde catalyzed by immobilized MeHNL using 3-6.5 equivalents of HCN at 20 °C. Table S1: Assigned number and specifications of the lipases screened in the hydrolysis of (±)-4-methoxymandelonitrile benzoate, Table S2: Results of hydrocyanation of 100 mM 4-anisaldehyde with 6.5 equivalents of HCN catalyzed by immobilized MeHNL in isopropyl ether with 0.34 mmol Na2HPO4·2H2O/Na2HPO4·7H2O per mL (water activity of 0.57). Detailed information and additional data on the screening of hydrolases for the selective hydrolysis of (±)-4-methoxymandelonitrile benzoate, the screening of benzoyl donors, the immobilization of MeHNL, the effect of HCN concentration on MeHNL-catalyzed hydrocyanation, the optimized cascade, and the chemical synthesis of (±)-4-methoxymandelonitrile benzoate.
Author Contributions
Conceptualization, L.v.L. and F.H.; methodology, A.S.; validation, L.L., A.S., and L.v.L.; formal analysis, L.L.; investigation, L.L.; resources, A.S., L.v.L. and F.H.; data curation, A.S. and L.v.L.; writing—original draft preparation, L.L. and L.v.L.; writing—review and editing, A.S. and F.H.; visualization, L.L.; supervision, A.S., L.v.L., and F.H..; project administration, A.S. and L.v.L.; funding acquisition, L.v.L., F.H., and A.S.
Funding
This project was funded by the European Union’s Horizon 2020 MSCA ITN-EID program under grant agreement No 634200 (BIOCASCADES). This communication reflects only the beneficiary´s view and the European Commission is not responsible for any use that may be made of the information it contains.
Acknowledgments
We thank Antje Spieß (Institute of Biochemical Engineering, Technische Universität Braunschweig, Germany) for fruitful discussion and for providing an HPLC system for chiral analysis. L. Leemans also thanks Yvonne Goecke (Institute of Biochemical Engineering, Technische Universität Braunschweig, Germany) for technical support with the HPLC equipment. Further, Kerstin Steiner (ACIB, Graz, Austria) is acknowledged for the generous gift of E. coli K12 Top 10F’ clone harboring the pSE420-MeHNL plasmid. Imerys S.A. and Resindion S.r.l. are acknowledged for providing Celite R633 and supplying Relizyme EXE309, respectively.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Choi, J.M.; Han, S.S.; Kim, H.S. Industrial Applications of Enzyme Biocatalysis: Current Status and Future Aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Gröger, H.; Asano, Y. Introduction-Principles and Historical Landmarks of Enzyme Catalysis in Organic Synthesis. In Enzyme Catalysis in Organic Synthesis; Drauz, K., Gröger, H., May, O., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 1–42. [Google Scholar]
- Sperl, J.M.; Sieber, V. Multienzyme Cascade Reactions—Status and Recent Advances. ACS Catal. 2018, 8, 2385–2396. [Google Scholar] [CrossRef]
- France, S.P.; Hepworth, L.J.; Turner, N.J.; Flitsch, S.L. Constructing Biocatalytic Cascades: In Vitro and in Vivo Approaches to de Novo Multi-Enzyme Pathways. ACS Catal. 2017, 7, 710–724. [Google Scholar] [CrossRef]
- Gröger, H.; Hummel, W. Combining the “two Worlds” of Chemocatalysis and Biocatalysis towards Multi-Step One-Pot Processes in Aqueous Media. Curr. Opin. Chem. Biol. 2014, 19, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Gröger, H.; Hummel, W. Chemoenzymatic Multistep One-Pot Processes. Cascade Biocatal. 2014, 427–456. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.; Kroutil, W. Artificial Biocatalytic Linear Cascades for Preparation of Organic Molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Process Integration and Cascade Catalysis. In Green Chemistry and Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 389–408. [Google Scholar]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-Enzymatic Cascade Reactions: Overview and Perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Hanefeld, U.; Straathof, A.J.J.; Heijnen, J.J. Enzymatic Formation and Esterification of (S)-Mandelonitrile. J. Mol. Catal. B Enzym. 2001, 11, 213–218. [Google Scholar] [CrossRef]
- Valivety, R.H.; Halling, P.J.; Macrae, A.R. Water as a Competitive Inhibitor of Lipase-Catalysed Esterification in Organic Media. Biotechnol. Lett. 1993, 15, 1133–1138. [Google Scholar] [CrossRef]
- Paravidino, M.; Sorgedrager, M.; Orru, R.A.A.; Hanefeld, U. Activity and Enantioselectivity of the Hydroxynitrile Lyase MeHNL in Dry Organic Solvents. Chem. Eur. J. 2010, 16, 7596–7604. [Google Scholar] [CrossRef]
- Costes, D.; Wehtje, E.; Adlercreutz, P. Hydroxynitrile Lyase-Catalyzed Synthesis of Cyanohydrins in Organic Solvents Parameters Influencing Activity and Enantiospecificity. Enzyme Microb. Technol. 1999, 25, 384–391. [Google Scholar] [CrossRef]
- Persson, M.; Costes, D.; Wehtje, E.; Adlercreutz, P. Effects of Solvent, Water Activity and Temperature on Lipase and Hydroxynitrile Lyase Enantioselectivity. Enzyme Microb. Technol. 2002, 30, 916–923. [Google Scholar] [CrossRef]
- Li, Y.-X.; Straathof, A.J.J.; Hanefeld, U. Enantioselective Formation of Mandelonitrile Acetate—investigation of a Dynamic Kinetic Resolution. Tetrahedron Asymmetry 2002, 13, 739–743. [Google Scholar] [CrossRef]
- Veum, L.; Kuster, M.; Telalovic, S.; Hanefeld, U.; Maschmeyer, T. Enantioselective Synthesis of Protected Cyanohydrins. Eur. J. Org. Chem. 2002, 2002, 1516–1522. [Google Scholar] [CrossRef]
- Veum, L.; Hanefeld, U. Enantioselective Formation of Mandelonitrile Acetate: Investigation of a Dynamic Kinetic Resolution II. Tetrahedron Asymmetry 2004, 15, 3707–3709. [Google Scholar] [CrossRef]
- Veum, L.; Hanefeld, U. Enantioselective Synthesis of Aliphatic Cyanohydrin Acetates. Synlett 2005, 15, 2382–2384. [Google Scholar] [CrossRef]
- Hietanen, A.; Ekholm, F.S.; Leino, R.; Kanerva, L.T. Applying Biocatalysis to the Synthesis of Diastereomerically Enriched Cyanohydrin Mannosides. Eur. J. Org. Chem. 2010, 2010, 6974–6980. [Google Scholar] [CrossRef]
- Hietanen, A.; Kanerva, L.T. One-Pot Oxidation-Hydrocyanation Sequence Coupled to Lipase-Catalyzed Diastereoresolution in the Chemoenzymatic Synthesis of Sugar Cyanohydrin Esters. Eur. J. Org. Chem. 2012, 2012, 2729–2737. [Google Scholar] [CrossRef]
- Okrob, D.; Paravidino, M.; Orru, R.V.A.; Wiechert, W.; Hanefeld, U.; Pohl, M. Hydroxynitrile Lyase from Arabidopsis thaliana: Identification of Reaction Parameters for Enantiopure Cyanohydrin Synthesis by Pure and Immobilized Catalyst. Adv. Synth. Catal. 2011, 353, 2399–2408. [Google Scholar] [CrossRef]
- Malona, J.A.; Cariou, K.; Spencer, W.T.; Frontier, A.J. Total Synthesis of (±)-Rocaglamide via Oxidation-Initiated Nazarov Cyclization. J. Org. Chem. 2012, 77, 1891–1908. [Google Scholar] [CrossRef]
- Yildirim, D.; Tükel, S.S.; Alagöz, D. Crosslinked Enzyme Aggregates of Hydroxynitrile Lyase Partially Purified from Prunus dulcis Seeds and Its Application for the Synthesis of Enantiopure Cyanohydrins. Biotechnol. Prog. 2014, 30, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Van Langen, L.M.; Van Rantwijk, F.; Sheldon, R.A. Enzymatic Hydrocyanation of a Sterically Hindered Aldehyde. Optimization of a Chemoenzymatic Procedure for (R)-2-Chloromandelic Acid. Org. Process Res. Dev. 2003, 7, 828–831. [Google Scholar] [CrossRef]
- Veum, L.; Pereira, S.R.M.; Van Der Waal, J.C.; Hanefeld, U. Catalytic Hydrogenation of Cyanohydrin Esters as a Novel Approach to N-Acylated β-Amino Alcohols-Reaction Optimisation by a Design of Experiment Approach. Eur. J. Org. Chem. 2006, 2006, 1664–1671. [Google Scholar] [CrossRef]
- Cheng, M.J.; Lee, K.H.; Tsai, I.L.; Chen, I.S. Two New Sesquiterpenoids and Anti-HIV Principles from the Root Bark of Zanthoxylum ailanthoides. Bioorg. Med. Chem. 2005, 13, 5915–5920. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, A.; Kapil, R.S.; Popli, S.P. Coumarins and Alkaloids of Aegle marmelos. Phytochemistry 1973, 12, 2071–2072. [Google Scholar] [CrossRef]
- Semba, H.; Dobashi, Y.; Matsui, T. Expression of Hydroxynitrile Lyase from Manihot esculenta in Yeast and Its Application in (S)-Mandelonitrile Production Using an Immobilized Enzyme Reactor. Biosci. Biotechnol. Biochem. 2008, 72, 1457–1463. [Google Scholar] [CrossRef]
- Müller, J.; Sowa, M.A.; Fredrich, B.; Brundiek, H.; Bornscheuer, U.T. Enhancing the Acyltransferase Activity of Candida antarctica Lipase A by Rational Design. ChemBioChem 2015, 16, 1791–1796. [Google Scholar] [CrossRef]
- Phillips, R.S. Temperature Modulation of the Stereochemistry of Enzymatic Catalysis: Prospects for Exploitation. Trends Biotechnol. 1996, 14, 13–16. [Google Scholar] [CrossRef]
- Dadashipour, M.; Asano, Y. Hydroxynitrile Lyases: Insights into Biochemistry, Discovery and Engineering. ACS Catal. 2011, 1, 1121–1149. [Google Scholar] [CrossRef]
- Willeman, W.F.; Straathof, A.J.J.; Heijnen, J.J. Reaction Temperature Optimization Procedure for the Synthesis of (R)-Mandelonitrile by Prunus amygdalus Hydroxynitrile Lyase Using a Process Model Approach. Enzyme Microb. Technol. 2002, 30, 200–208. [Google Scholar] [CrossRef]
- Ueatrongchit, T.; Tamura, K.; Ohmiya, T.; H-Kittikun, A.; Asano, Y. Hydroxynitrile Lyase from Passiflora edulis: Purification, Characteristics and Application in Asymmetric Synthesis of (R)-Mandelonitrile. Enzyme Microb. Technol. 2010, 46, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Willeman, W.F.; Hanefeld, U.; Straathof, A.J.J.; Heijnen, J.J. Estimation of Kinetic Parameters by Progress Curve Analysis for the Synthesis of (R)-Mandelonitrile by Prunus amygdalus Hydroxynitrile Lyase. Enzyme Microb. Technol. 2000, 27, 423–433. [Google Scholar] [CrossRef]
- O’Neil, M.J.; Patricia, E.; Heckelman, A.S.; Budavari, S. The Merck Index-An Encyclopedia of Chemicals Drugs and Biologicals; Merck and Co.: Whitehouse Station, NJ, USA, 2006; p. 830. [Google Scholar]
- Shakeri, M.; Engström, K.; Sandström, A.G.; Bäckvall, J.E. Highly Enantioselective Resolution of β-Amino Esters by Candida antarctica Lipase A Immobilized in Mesocellular Foam: Application to Dynamic Kinetic Resolution. ChemCatChem 2010, 2, 534–538. [Google Scholar] [CrossRef]
- Ding, W.; Li, M.; Dai, R.; Deng, Y. Lipase-Catalyzed Synthesis of the Chiral Tetrahydroisoquinoline (R)-Salsolinol. Tetrahedron Asymmetry 2012, 23, 1376–1379. [Google Scholar] [CrossRef]
- Zamost, B.L.; Nielsen, H.K.; Starnes, R.L. Thermostable Enzymes for Industrial Applications. J. Ind. Microbiol. 1991, 8, 71–81. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and Application of Lipases in Organic Media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernández-Lafuente, R.; Huguet, J.; Guisán, J.M. A Single Step Purification, Immobilization, and Hyperactivation of Lipases via Interfacial Adsorption on Strongly Hydrophobic Supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]
- Hanefeld, U. Immobilisation of Hydroxynitrile Lyases. Chem. Soc. Rev. 2013, 42, 6308–6321. [Google Scholar] [CrossRef]
- Cabirol, F.L.; Hanefeld, U.; Sheldon, R.A. Immobilized Hydroxynitrile Lyases for Enantioselective Synthesis of Cyanohydrins: Sol-Gels and Cross-Linked Enzyme Aggregates. Adv. Synth. Catal. 2006, 348, 1645–1654. [Google Scholar] [CrossRef]
- Valivety, R.H.J.; Halling, P.; Macrae, A.R. Reaction Rate with Suspended Lipase Catalyst Shows Similar Dependence on Water Activity in Different Organic Solvents. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. 1992, 1118, 218–222. [Google Scholar] [CrossRef]
- Schmitke, J.L.; Wescott, C.R.; Klibanov, A.M. The Mechanistic Dissection of the Plunge in Enzymatic Activity upon Transition from Water to Anhydrous Solvents. J. Am. Chem. Soc. 1996, 118, 3360–3365. [Google Scholar] [CrossRef]
- Wehtje, E.; Costes, D.; Adlercreutz, P. Enantioselectivity of Lipases: Effects of Water Activity. J. Mol. Catal. B Enzym. 1997, 3, 221–230. [Google Scholar] [CrossRef]
- Pepin, P.; Lortie, R. Influence of Water Activity on the Enantioselective Esterification of (R,S)-Ibuprofen by Candida antarctica Lipase B in Solventless Media. Biotechnol. Bioeng. 1999, 63, 502–505. [Google Scholar] [CrossRef]
- Bovara, R.; Carrea, G.; Ottolina, G.; Riva, S. Water Activity Does Not Influence the Enantioselectivity. Biotechnol. Lett. 1993, 15, 169–174. [Google Scholar] [CrossRef]
- Carrea, G.; Riva, S. Properties and Synthetic Applications of Enzymes in Organic Solvents. Angew. Chem. Int. Ed. Engl. 2000, 39, 2226–2254. [Google Scholar] [CrossRef]
- Lauble, H.; Miehlich, B.; Förster, S.; Wajant, H.; Effenberger, F. Crystal Structure of Hydroxynitrile Lyase from Sorghum bicolor in Complex with the Inhibitor Benzoic Acid: A Novel Cyanogenic Enzyme. Biochemistry 2002, 41, 12043–12050. [Google Scholar] [CrossRef]
- Xu, L.L.; Singh, B.K.; Conn, E.E. Purification and Characterization of Mandelonitrile Lyase from Prunus lyonii. Arch. Biochem. Biophys. 1986, 250, 322–328. [Google Scholar] [CrossRef]
- Jaenicke, L.; Preun, J. Chemical Modification of Hydroxynitrile Lyase by Selective Reaction of an Essential Cysteine-SH Group with α,β-unsaturated Propiophenones as Pseudo-substrates. Eur. J. Biochem. 1984, 138, 319–325. [Google Scholar] [CrossRef]
- Hanefeld, U.; Straathof, A.J.J.; Heijnen, J.J. Study of the (S)-Hydroxynitrile Lyase from Hevea brasiliensis: Mechanistic Implications. Biochim. Biophys. Acta—Protein Struct. Mol. Enzymol. 1999, 1432, 185–193. [Google Scholar] [CrossRef]
- Lowe, M.E. Assays for Pancreatic Triglyceride Lipase and Colipase. In Lipase and Phospholipase Protocols. Methods in Molecular Biology; Doolittle, M., Reue, K., Eds.; Humana Press: New York, NY, USA, 1999; Volume 109, pp. 59–70. [Google Scholar]
- Schallmey, M.; Jekel, P.; Tang, L.; Elenkov, M.M.; Höffken, H.W.; Hauer, B.; Janssen, D.B. A single point mutation enhances hydroxynitrile synthesis by halohydrin dehalogenase. Enzyme Microb. Technol. 2015, 70, 50–57. [Google Scholar] [CrossRef]
- Chen, C.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative Analyses of Biochemical Kinetic Resolutions of Enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Sakuth, M.; Mensing, T.; Schuler, J.; Heitmann, W.; Strehlke, G.; Mayer, D. Ethers, Aliphatic. In Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Elvers, B., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; p. 440. [Google Scholar]
- Halling, P.J. Salt Hydrates for Water Activity Control with Biocatalysts in Organic Media. Biotechnol. Tech. 1992, 6, 271–276. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).