Abstract
Layered perovskite compounds of SrBi2Nb2−xVxO9 (x = 0.0, 0.02, 0.04, 0.06, and 0.08) were synthesized via a solid-state reaction method. Their optical properties, electronic band structures, and photocatalytic activities under visible light irradiation were investigated for the first time. The incorporation of vanadium into the SrBi2Nb2O9 lattice reduced the bandgap energies, allowing the compounds to effectively absorb visible light. Electronic band structure calculations revealed that the V 3d orbitals caused bandgap narrowing by forming an additional band below the conduction band, while the O 2p and Bi 6p orbitals dominantly contributed to the formation of the valence band. The photocatalytic activity tested under visible light irradiation (>420 nm) revealed that it increased linearly with the V content in the SrBi2Nb2−xVxO9 compounds until saturation at x = 0.06, which was attributed to improved visible light absorption originating from the reduced bandgap energies.
1. Introduction
Semiconductor-based photocatalysis has attracted great interest in the last decade because of its application to degrade organic pollutants into environmentally benign chemicals at a low cost [1,2,3]; in particular, TiO2 has been widely studied due to its high chemical stability, photocatalytic activity, and non-toxicity [4,5]. However, given its large bandgap energy (~3.2 eV), TiO2 is active only under ultraviolet (UV) light (~4% of the sunlight), which diminishes the energy efficiency. Hence, for efficient use of sunlight and application purposes, development of novel photocatalysts having high activity under visible light (VIS) (~45% of the sunlight) is strongly required [1]. To date, searching novel materials [6,7,8,9] and/or modifying the band structures of existing materials are usually employed to obtain VIS-active photocatalysts [10,11]. For developing a new material, one feasible way is the synthesis of complex oxides that leads to the formation of a new valence- or conduction-band state above the O 2p orbital or metal d orbitals through orbital hybridization [6,12,13,14].
SrBi2Nb2O9 (SBN) compounds with layered perovskite structures are of great interest in the fields of dielectrics, ferroelectrics, and photocatalysts [15,16,17,18]; for instance, Shu et al. investigated their electronic band structures, relaxation energies, and bonding mechanisms by using the first-principle calculation, showing that the ferroelectric properties of SBN compounds mainly originated from the covalent effect between B-site cations and oxygen ions, which was enhanced by Bi–O hybridization [19]. Li et al. prepared ABi2Nb2O9 (A = Ca, Sr, and Ba) compounds and analyzed their electronic band structure, optical properties, and photocatalytic water-splitting activity (under UV light); in this case, SBN exhibited much higher activity than the other two compounds (i.e., CaBi2Nb2O9 and BaBi2Nb2O9) because its lattice was more distorted, and the angle between their corner-linked NbO6 octahedra was closer to 180° than the others [20]. On the other hand, vanadium substitution/incorporation at niobium sites has been reported to influence the dielectric and ferroelectric properties of these compounds [21,22]. Wu et al. [23] observed comparable direct current (DC) conductivity among V-incorporated SBN compounds regardless of the V content and, more interestingly, demonstrated that their ferroelectric properties were significantly enhanced by incorporating up to 10 at% of vanadium, while the layered perovskite structure was preserved.
In the present study, we synthesized a series of SrBi2Nb2−xVxO9 (x = 0.0, 0.02, 0.04, 0.06, and 0.08, hereafter denoted as SBNV0, SBNV02, SBNV04, SBNV06, and SBNV08, respectively) compounds via a solid-state reaction route to investigate the influence of V incorporation on their light absorptions, band structures, and photocatalytic activities under VIS. In particular, we focused on the relationship between the changes in the crystal (e.g., in the lattice parameters) and band structures.
2. Results and Discussion
2.1. Crystal Structure and Morphology
The SBN ceramic (family of the Aurivillius phases) has an orthorhombic crystal structure (space group A21am) and a layered perovskite structure [24]. Figure 1a shows a schematic crystal structure of SBN, in which a pseudo-perovskite block layer of (SrNb2O7)2− is sandwiched between (Bi2O2)2+ layers along the c-axis [19]. The XRD patterns of the V-substituted SBN powders, synthesized at 850 °C, are shown in Figure 1b; all the XRD peaks were indexed to the orthorhombic structure of SrBi2Nb2O9 (JCPDS No. 86-1190). In addition, all the powders exhibited high crystallinity, and no other impurity phases were observed, regardless of the V content. Notably, it was known that the layered perovskite structure of the SBN retained up to the V content of 15 at% [21]. Figure 1c shows the variation in lattice parameters with increasing V content, which was calculated via a least-square method by using MAUD software [25]. When the V content was increased from 0 to 0.08, the a value slightly increased until x = 0.04 and then rapidly decreased; similarly, the b value decreased linearly until x = 0.06 and then saturated, while the c value decreased abruptly at x = 0.02 and then increased linearly. As a result, the unit-cell volume was reduced by the V incorporation, probably because of the smaller radius of the V5+ ion (0.54 Å) compared to the Nb5+ ion (0.64 Å) [21]. This change in the lattice parameters confirmed that the V5+ ions were successfully incorporated in the SBN structure.
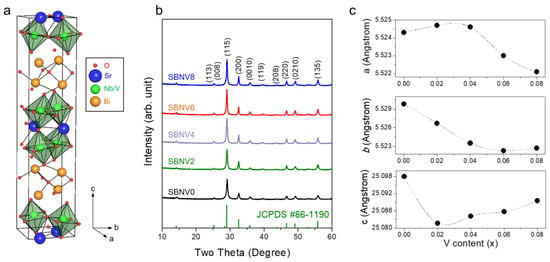
Figure 1.
(a) Crystal structure of a layered perovskite compound of SrBi2Nb2O9. (b) X-ray diffraction patterns and (c) lattice parameter variation of SrBi2Nb2−xVxO9 compounds (x = 0, 0.02, 0.04, 0.06, and 0.08, denoted as SBNV0, SBNV02, SBNV04, SBNV06, and SBNV08, respectively).
Figure 2 and Figure S2 show the SEM images and the corresponding particle size distribution of the SBNV0 and SBNV06 powders; the former exhibited a spherical and rounded morphology, with an average particle size of ~1 μm (Figure 2a,b), while the latter showed large and irregular morphology with fragments (0.3–2 μm in size), which was commonly observed in powders prepared via a solid-state reaction route [26,27]. The specific surface area of SBNV06 was 2.9 m2/g, three times larger than that of SBNV0 (0.9 m2/g) (See Table S1).
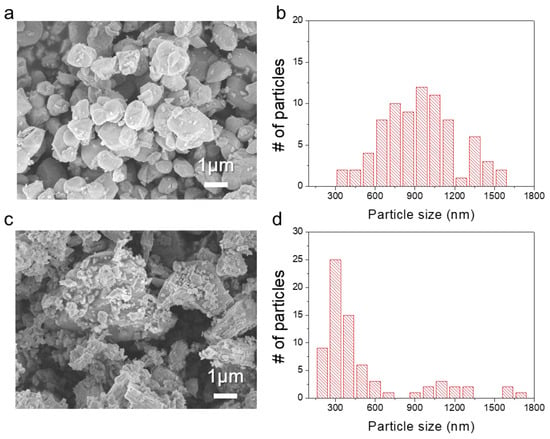
Figure 2.
Field emission scanning electron microscopy images and corresponding particle size distributions of SrBi2Nb2−xVxO9 powders. (a,b) x = 0 (SBNV0) and (c,d) x = 0.06 (SBNV06).
2.2. Optical Properties and Electronic Band Structure
The light absorption characteristics (i.e., bandgap energy and absorption coefficient) of a photocatalyst are generally one of the most critical factors affecting its photocatalytic activity [2]. Hence, the light absorption properties of the SBNV0, SBNV02, SBNV04, SBNV06, and SBNV08 powders were investigated via UV–VIS diffuse reflectance spectroscopy (Figure 3a). The SBNV0 (without V incorporation) had an absorption edge at ~386 nm, comparable to previous results [20]. When increasing the V content, the absorption edge was shifted toward longer wavelengths, i.e., absorption tails/shoulders appeared in the visible-light (VIS) region; in particular, SBNV06 and SBNV08 exhibited higher light absorption than SBNV02 and SNBV04 in the 400–700 nm range. Since SrBi2Nb2O9 had a direct bandgap [20], the optical bandgap values could be estimated by extrapolating the tangents at the linear portion of the (αhν)2 versus hν plots (Tauc plots, here α is the absorption coefficient obtained from the Kubelka-Munk function (α/S) = (1−R)2/(2R), S is scattering coefficient, R is diffuse reflectance, h is Plank constant, and ν is a frequency of light). As shown in Figure 3b,c, the estimated bandgaps of SBNV0, SBNV02, SBNV04, SBNV06, and SBNV08 were 3.21, 3.13, 2.95, 2.75, and 2.71 eV, respectively, that is, the bandgap values decreased with increasing V content (See Table S2). This result indicated that the light absorption properties of SBN were enhanced by the V incorporation, possibly resulting from the change in the local crystal structure. As a result, the generation of charge carriers in SBNV06 and SBNV08 under VIS was expected to be higher than in SBNV0, SBNV02, and SBNV04.
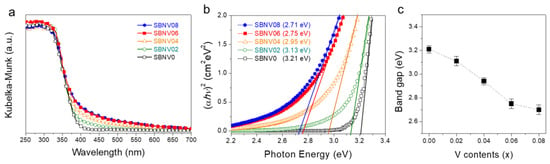
Figure 3.
Optical properties. (a) Kubelka-Munk function. (b) (αhν)2 versus photon energy. (c) Bandgap values of SrBi2Nb2−xVxO9 powders, with x = 0, 0.02, 0.04, 0.06, and 0.08 (SBNV0, SBNV02, SBNV04, SBNV06, and SBNV08, respectively).
Next, the influence of V incorporation on VIS absorption of SBN compounds was further investigated via calculating the electronic band structure and density of state (DOS) of the synthesized powders. DFT calculations were conducted using the atomic positions reported in the literature [24] and the lattice parameters derived from the XRD analysis. Figure 4 shows the results of band structure and partial DOS calculations of SBNV0 and SBNV06. SBNV0 exhibited a direct bandgap (consistent with a previous study), the calculated bandgap value was 2.4 eV (~25% underestimated compared with the experimentally obtained value, Figure 4a) [28], and the valence band consisted of O 2p, Bi 6s, and Bi 6p orbitals, while the conduction band mainly consisted of Nb 4d orbitals (Figure 4b). On the other hand, SBNV06 (i.e., after V incorporation) retained a direct bandgap, but the bandgap value decreased to 2.1 eV, enabling VIS absorption by red-shifting the absorption edge. This bandgap narrowing was attributed to the formation of V 3d orbitals at a lower energy level than that of the Nb 4d orbitals, where the conduction band was widened by the hybridization of both orbitals.
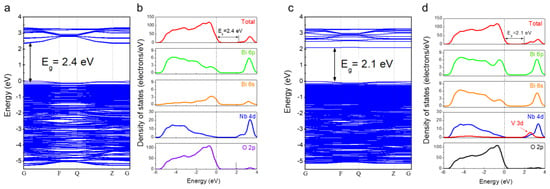
Figure 4.
Electronic band structure and partial density of states (PDOSs) of SrBi2Nb2−xVxO9 powders. (a,b) x = 0 (SBNV0) and (c,d) x = 0.6 (SBNV06).
Then, the conduction- and valence-band positions of SBNV0 and SBNV06 were calculated using the following empirical equation [29]:
where EVB is the valence-band edge potential, EN is the electronegativity of the semiconductor, given by the geometric mean of the electronegativities of the constituent atoms, Ee is the energy of the free electrons on the hydrogen scale (~4.5 eV), and Eg is the bandgap value (experimentally obtained) of the semiconductor. It should be noted that the conduction band position obtained from this equation may largely deviate from the values obtained from other experimental techniques such as Mott–Schottky measurements. Figure 5 shows the schematic band structure of the conduction and valence bands for the SBN powders with and without V incorporation, derived from the experimentally obtained bandgap values, the partial DOS, and the results of the above equation. The conduction-band edge of SBNV0 was located at −0.24 V versus reversible hydrogen electrode (RHE), i.e., higher than the reduction potential of water (H+/H2, 0 V versus RHE), while its valence-band edge was positioned at +2.96 V versus RHE, i.e., lower than the oxidation potential of water (H2O/O2, +1.23 V versus RHE). Therefore, SBNV0 could potentially split water into H2 and O2 gases under UV light illumination [20]. In the case of SBNV06, due to hybridization of the V 3d and Nb 4d orbitals, the conduction-band edge was located at a lower energy level compared to SBNV0 (i.e., −0.02 V versus RHE), while the valence-band edge was located at +2.73 V versus RHE. This result indicated that the SBNV06 was VIS-active, and it could split water thermodynamically.
EVB = EN − Ee + 0.5 × Eg,
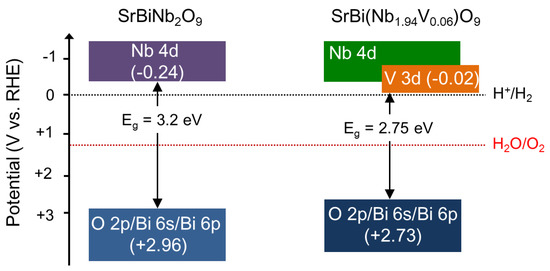
Figure 5.
Schematic band structure and positions (on the electrochemical scale) of SrBi2Nb2O9 and SrBi2Nb2−xVxO9 (x = 0.06).
2.3. Visible-Light-Induced Photocatalytic Performance
The VIS-induced photocatalytic performance of SBNV0, SBNV02, SBNV04, SBNV06, and SBNV08 were tested by checking the decomposition of RhB dye under VIS illumination (λ > 420 nm). As shown in Figure 6a,b, no decomposition of the RhB dye was observed in the blank experiment (i.e., without photocatalyst powder). In contrast, all the powders achieved noticeable degradation of the RhB dye; after 5 h, the decomposition efficiencies of SBNV0, SBNV02, SBNV04, SBNV06, and SBNV08 were, respectively, ~48%, ~56%, ~63%, 78%, and 75%, which meant also that SBNV06 was the most efficient.
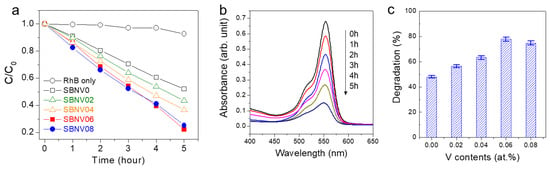
Figure 6.
Visible light (VIS)-induced photocatalytic activity of SrBi2Nb2−xVxO9 powders with x = 0, 0.02, 0.04, 0.06, and 0.08 (SBNV0, SBNV02, SBNV04, SBNV06, and SBNV08, respectively), under irradiation with a halogen lamp having a 420 nm cut-off filter: (a) Concentration change profiles with irradiation time. (b) Variation of the absorbance of the RhB dye solution with time for the SBNV06 (See Figure S3 for the others). (c) Comparison of RhB dye decomposition efficiency after 5 h. The error bar was obtained from three measurements of each sample.
Critical factors influencing the photocatalytic activity of photocatalysts are light absorption (charge generation), charge transport, and number of active sites [30,31]. Regarding light absorption efficiency, smaller bandgap values, i.e., efficient light absorption, facilitates the generation of charge carriers in the photocatalyst. On the other hand, the charge transport efficiency is greatly affected by electron/hole mobilities, defects, and particle size [31]. In the case of the number of active sites, a large surface area is preferred because it provides a greater number of surface active sites for the adsorption of organic molecules, thus increasing their decomposition/oxidation. As shown in Figure 3, SBNV06 and SBNV08 exhibited smaller bandgaps and larger VIS absorption compared to SBNV0, SBNV02, and SBNV04, which allowed improved charge generation under VIS irradiation. Furthermore, the particle sizes of the V-incorporated SBN powders (Figure 2) were smaller than that of SBNV0; therefore, the specific surface area of SBNV06 was larger than that of SBNV0 (Table S1), corresponding to a large number of surface active sites. Regarding charge transport efficiency, since SrBi2Nb2O9 had an extremely low electron mobility (<10−5 cm2/V·s) [32], SBNV0 could be expected to have a poor charge transport efficiency. Besides, excess doping or substitution generates a greater number of defects; hence, SBNV08 may have far more defects that would significantly decrease its charge transport efficiency compared to SBNV06. As a result, the highest photocatalytic activity of SBNV06 could be mainly attributed to the improved visible absorption (via V incorporation) and larger surface area (resulting from a smaller particle size).
The photocatalytic activity of SBNV06 was further compared to that of the frequently studied tungsten oxide (WO3) photocatalyst (synthesized via a precipitation method), which had a comparable bandgap value (~2.7 eV) but much smaller particle size (~200 nm) (Figure 7). Although the initial degradation rate (after 1 h) exhibited by WO3 (29%) was slightly faster than that of SBNV06 (16%), the final degradation rates (after 5 h) were comparable (84% and 80%, respectively). Also, as shown in Figure S4, the reaction rate constant (k, assuming pseudo-first-order reaction kinetics) of WO3 (2.58 × 10−3 min−1) was higher than that of SBNV06 in the time range of 0–150 min. However, at 200–300 min, the SBNV06 showed a higher k value than WO3. These results indicated that the initial photocatalytic reaction of SBNV06 was slow, implying the adsorption of RhB dye molecules on the surface of SBNV06 was a rate-limiting factor (possibly because of surface property and/or smaller surface area than the WO3) at the initial stage of photocatalytic reaction. Therefore, when considering the small bandgap value of 2.75 eV and favorable band positions, the photocatalytic activity of SBNV06 could be further enhanced by controlling its particle size and morphology, via a solution synthesis method [33].
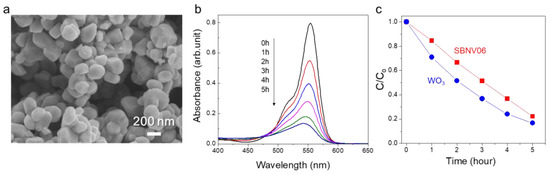
Figure 7.
Comparison of VIS-induced photocatalytic activity between WO3 and SBNV06, under VIS irradiation (λ > 420 nm). (a) SEM image of the WO3 powder. (b) Variation of the absorbance of RhB dye solution with time for the WO3. (c) Concentration change profiles with irradiation time.
3. Materials and Methods
3.1. Preparation of SrBi2Nb2−xVxO9 and WO3 Powders
The SrBi2Nb2−xVxO9 powders were synthesized via a solid-state method [34]. Stoichiometric mixtures of the starting materials (SrCO3, Bi2O3, Nb2O5, and V2O5, all 99.9%) were ball-milled with ZrO2 balls and ethanol for 48 h. Then, the resulting mixtures were dried and successively calcined at 850 °C for 6 h (the samples calcined below 850 °C exhibited impurity phases, see Figure S1). The WO3 powder was synthesized by a precipitation method. Briefly, 0.02 mol of sodium tungstate dihydrate (Na2WO4-2H2O, >99%, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in deionized water (150 mL) and the pH value was adjusted to 10 by using 0.1 M NaOH (98%, Alfa Aesar, Ward Hill, MA, USA) aqueous solution. The resultant mixture was heated to 50 °C for 6 h. After washing and drying, the yellowish powder was annealed at 350 °C for 2 h.
3.2. Characterizations and Density Functional Theory Calculation
The crystal structures and morphologies of the powders were determined by using an X-ray powder diffractometer (XRD) (D8 Advance, Bruker AXS, Karlsruhe, Germany) and a scanning electron microscope (SEM) (JSM-6330F, Jeol, Tokyo, Japan), respectively. The diffuse reflectance spectra were collected with a UV–VIS near-infrared (NIR) spectrophotometer (U-4001, Hitachi, Tokyo, Japan). The specific surface area measurements were conducted using a Brunauer–Emmett–Teller (BET) surface area analyzer (ASAP 2010, Micromeitics, Norcross, USA). The band structure calculation was based on the plane-wave density functional theory (DFT) and performed using the CASTEP code [35]; the applied convergence criterion was 10−6 eV for the total energy change per atom. Generalized gradient approximation (GGA) using Perdew–Burke–Ernzerhof (PBE) functionals and ultrasoft pseudopotentials were calculated and, then, basis set corrections were carried out [36]. Energy calculations were performed using the PBE0 hybrid functional and the norm-conserving pseudopotentials, with a cut-off energy of 830 eV and a 4 × 4 × 2 k-point sampling [37].
3.3. Photocatalytic Performance Test
The photocatalytic activities of the SrBi2Nb2−xVxO9 powders were tested via the decomposition of a rhodamine B (RhB) dye solution (20 mg/L) under VIS illumination by using 100 W tungsten–halogen lamps (OSRAM) with a cut-off filter (λ > 420 nm). A reactor made by Pyrex glass was positioned at the center of a black acrylic box (200 mm × 150 mm × 200 mm) and one of the lamps was positioned at 10 cm from it. Reaction suspensions were prepared by adding the photocatalyst powder (0.3 g) to the RhB dye solution (100 mL) with stirring and sonication. Before the photocatalytic test, the suspensions were successively stirred in the dark (for 60 min) to ensure the adsorption/desorption equilibrium. Aliquots (2 mL) of the suspension were removed at given time intervals, centrifuged, and then the RhB concentration in them was determined by using a Ultraviolet-visible (UV–VIS) spectrophotometer (Lambda 35, Perkin-Elmer, Walthan, MA, USA).
4. Conclusions
Layered perovskite compounds of SrBi2Nb2−xVxO9 were synthesized at 850 °C via a solid-state reaction method, and their optical properties, electronic band structures, and VIS-induced photocatalytic activities were investigated for the first time. Vanadium incorporation into the SrBi2Nb2O9 lattice reduced the bandgap energies, allowing efficient absorption of VIS. Electronic band structure calculations confirmed that the V 3d orbitals were an additional band just below the Nb 4d orbitals, causing bandgap narrowing by the hybridization of both orbitals. The VIS-induced photocatalytic activity of the synthesized SrBi2Nb2−xVxO9 powders increased linearly with the V content until saturation at x = 0.06, which could be mainly attributed to the improved VIS absorption and larger surface area.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/5/393/s1, Figure S1: XRD patterns of SBNV06 powders calcined at 800, 850 and 900 °C for 6 h, Figure S2: SEM images of (a) SNBV02, (b) SBNV04 and (c) SBNV08 samples, Figure S3: Variation of the absorbance of the RhB dye solution with time for (a) SBNV0, (b) SBNV02, (c) SBNV04, (d) SBNV06, (e) SBNV08, and (f) SBNV10 samples, Figure S4: (a) Pseudo first order reaction kinetics and (b) band-gap values, specific surface areas and reaction rate constants of the SBNV06 and WO3 samples, Table S1: The BET surface area of samples, Table S2: Experimental and calculated bandgap values of samples.
Author Contributions
Conceptualization, S.W.H. and I.S.C.; synthesis and characterizations, T.H.N., S.W.H. and I.S.C.; Data analysis and writing, I.S.C.; funding acquistition, I.S.C.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (grant number NRF- 2019R1A2C2002024).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.A.; O’shea, K. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Cho, I.-S.; Kwak, C.H.; Kim, D.W.; Lee, S.; Hong, K.S. Photophysical, photoelectrochemical, and photocatalytic properties of novel SnWO4 oxide semiconductors with narrow band gaps. J. Phys. Chem. C 2009, 113, 10647–10653. [Google Scholar] [CrossRef]
- Hitoki, G.; Ishikawa, A.; Takata, T.; Kondo, J.N.; Hara, M.; Domen, K. Ta3N5 as a novel visible light-driven photocatalyst (λ < 600 nm). Chem. Lett. 2002, 31, 736–737. [Google Scholar]
- Long, M.; Cai, W.; Cai, J.; Zhou, B.; Chai, X.; Wu, Y. Efficient photocatalytic degradation of phenol over Co3O4/BiVO4 composite under visible light irradiation. J. Phys. Chem. B 2006, 110, 20211–20216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Takanabe, K.; Maeda, K.; Domen, K.; Epping, J.D.; Fu, X.; Antonietti, M.; Wang, X. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem. Int. Ed. 2010, 49, 441–444. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide. Angew. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN: ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Domen, K. New Non-Oxide Photocatalysts Designed for Overall Water Splitting under Visible Light. J. Phys. Chem. C 2007, 111, 7851–7861. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Selective synthesis and visible-light photocatalytic activities of BiVO 4 with different crystalline phases. Mater. Chem. Phys. 2007, 103, 162–167. [Google Scholar] [CrossRef]
- Hosogi, Y.; Tanabe, K.; Kato, H.; Kobayashi, H.; Kudo, A. Energy structure and photocatalytic activity of niobates and tantalates containing Sn (II) with a 5s2 electron configuration. Chem. Lett. 2003, 33, 28–29. [Google Scholar] [CrossRef]
- Jain, S.; Jha, A. Structural and electrical properties of SrBi2VxNb2−xO9 ferroelectric ceramics: Effect of temperature and frequency. J. Electroceram. 2010, 24, 58–63. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.; Zhang, H.; Li, Z.; Sun, J. Electronic structure and photocatalytic properties of ABi2Ta2O9 (A = Ca, Sr, Ba). J. Solid State Chem. 2008, 181, 2653–2659. [Google Scholar] [CrossRef]
- Chung, S.T.; Cho, S.-B. Low-frequency dielectric dispersion and electrical conductivity of pure and La-doped SrBi2Nb2O9 ceramics. J. Korean Phys. Soc. 2008, 52. [Google Scholar]
- Forbess, M.; Seraji, S.; Wu, Y.; Nguyen, C.; Cao, G. Dielectric properties of layered perovskite Sr1−xAxBi2Nb2O9 ferroelectrics (A = La, Ca and x = 0, 0.1). Appl. Phys. Lett. 2000, 76, 2934–2936. [Google Scholar] [CrossRef]
- Shu, H.; Sun, L.; Zhong, X.; Wang, J.; Zhou, Y. Bonding mechanism and relaxation energy of SrBi2B2O9 (B = Ta, Nb): First-principles study. J. Phys. Chem. Solids 2009, 70, 707–712. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.; Zhang, H.; Lv, Z. Band structure and photocatalytic activities for H2 production of ABi2Nb2O9 (A = Ca, Sr, Ba). Int. J. Hydrogen Energy 2010, 35, 2652–2656. [Google Scholar] [CrossRef]
- Wu, Y.; Forbess, M.J.; Seraji, S.; Limmer, S.J.; Chou, T.P.; Nguyen, C.; Cao, G. Doping effect in layer structured SrBi2Nb2O9 ferroelectrics. J. Appl. Phys. 2001, 90, 5296–5302. [Google Scholar] [CrossRef]
- Goel, P.; Yadav, K. Effect of V5+ doping on Structural and Dielectric properties of SrBi2Nb2O9 Synthesized at low Temperature. Phys. B Condens. Matter 2006, 382, 245–251. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, G. Influences of vanadium doping on ferroelectric properties of strontium bismuth niobates. J. Mater. Sci. Lett. 2000, 19, 267–269. [Google Scholar] [CrossRef]
- Kennedy, B.J. Structure ofABi2Nb2O9 (A = Sr, Ba): Refinement of Powder Neutron Diffraction Data. J. Solid State Chem. 1996, 126, 135–141. [Google Scholar]
- Lutterotti, L. Total pattern fitting for the combined size–strain–stress–texture determination in thin film diffraction. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Noh, T.H.; Hwang, S.W.; Kim, J.U.; Yu, H.K.; Seo, H.; Ahn, B.; Kim, D.W.; Cho, I.S. Optical properties and visible light-induced photocatalytic activity of bismuth sillenites (Bi12XO20, X = Si, Ge, Ti). Ceram. Int. 2017, 43, 12102–12108. [Google Scholar] [CrossRef]
- Perdew, J.P. Density functional theory and the band gap problem. Int. J. Quantum Chem. 1985, 28, 497–523. [Google Scholar] [CrossRef]
- Butler, M.; Ginley, D. Prediction of flatband potentials at semiconductor-electrolyte interfaces from atomic electronegativities. J. Electrochem. Soc. 1978, 125, 228–232. [Google Scholar] [CrossRef]
- Nethercot, A.H., Jr. Prediction of Fermi energies and photoelectric thresholds based on electronegativity concepts. Phys. Rev. Lett. 1974, 33, 1088. [Google Scholar] [CrossRef]
- Li, Z.; Feng, J.; Yan, S.; Zou, Z. Solar fuel production: Strategies and new opportunities with nanostructures. Nano Today 2015, 10, 468–486. [Google Scholar] [CrossRef]
- Mills, A.; Davies, R.H.; Worsley, D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Palanduz, A.; Smyth, D. Defect chemistry and charge transport in SrBi2Nb2O9. J. Electroceram. 2003, 11, 191–206. [Google Scholar] [CrossRef]
- Xie, H.; Wang, K.; Jiang, Y.; Zhao, Y.; Wang, X. Hydrothermal Synthesis and Characterization of SrBi2Nb2O9 Nanoplates. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2015, 45, 80–85. [Google Scholar] [CrossRef]
- Cho, I.-S.; Bae, S.T.; Kim, D.H.; Hong, K.S. Effects of crystal and electronic structures of ANb2O6 (A = Ca, Sr, Ba) metaniobate compounds on their photocatalytic H2 evolution from pure water. Int. J. Hydrogen Energy 2010, 35, 12954–12960. [Google Scholar] [CrossRef]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.; Joannopoulos, J. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Matsushita, Y.-I.; Nakamura, K.; Oshiyama, A. Comparative study of hybrid functionals applied to structural and electronic properties of semiconductors and insulators. Phys. Rev. B 2011, 84, 075205. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).