Efficient Biocatalytic Preparation of Optically Pure (R)-1-[4-(Trifluoromethyl)phenyl]ethanol by Recombinant Whole-Cell-Mediated Reduction
Abstract
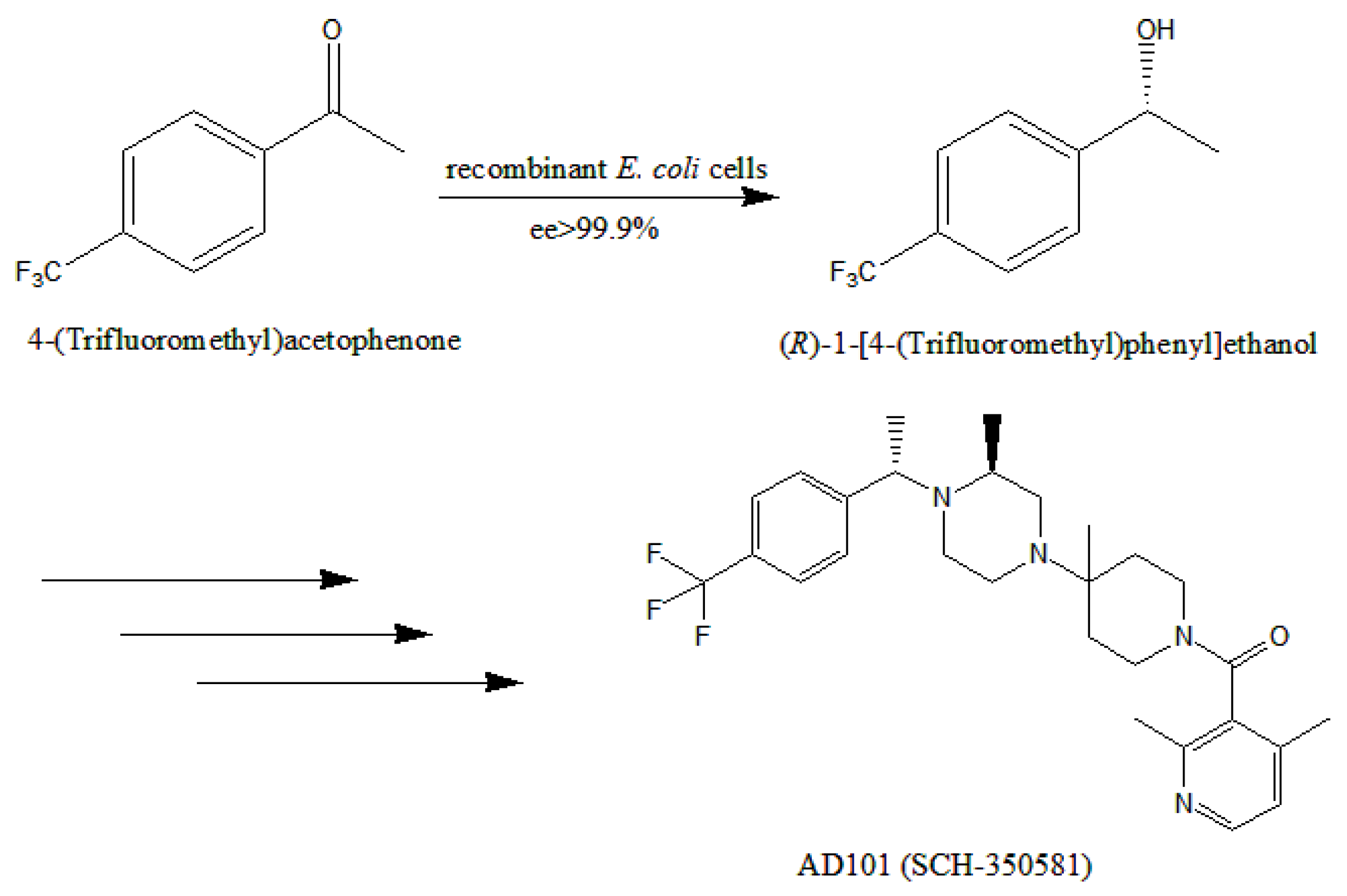
:1. Introduction
2. Results
2.1. Screening of Biocatalyst
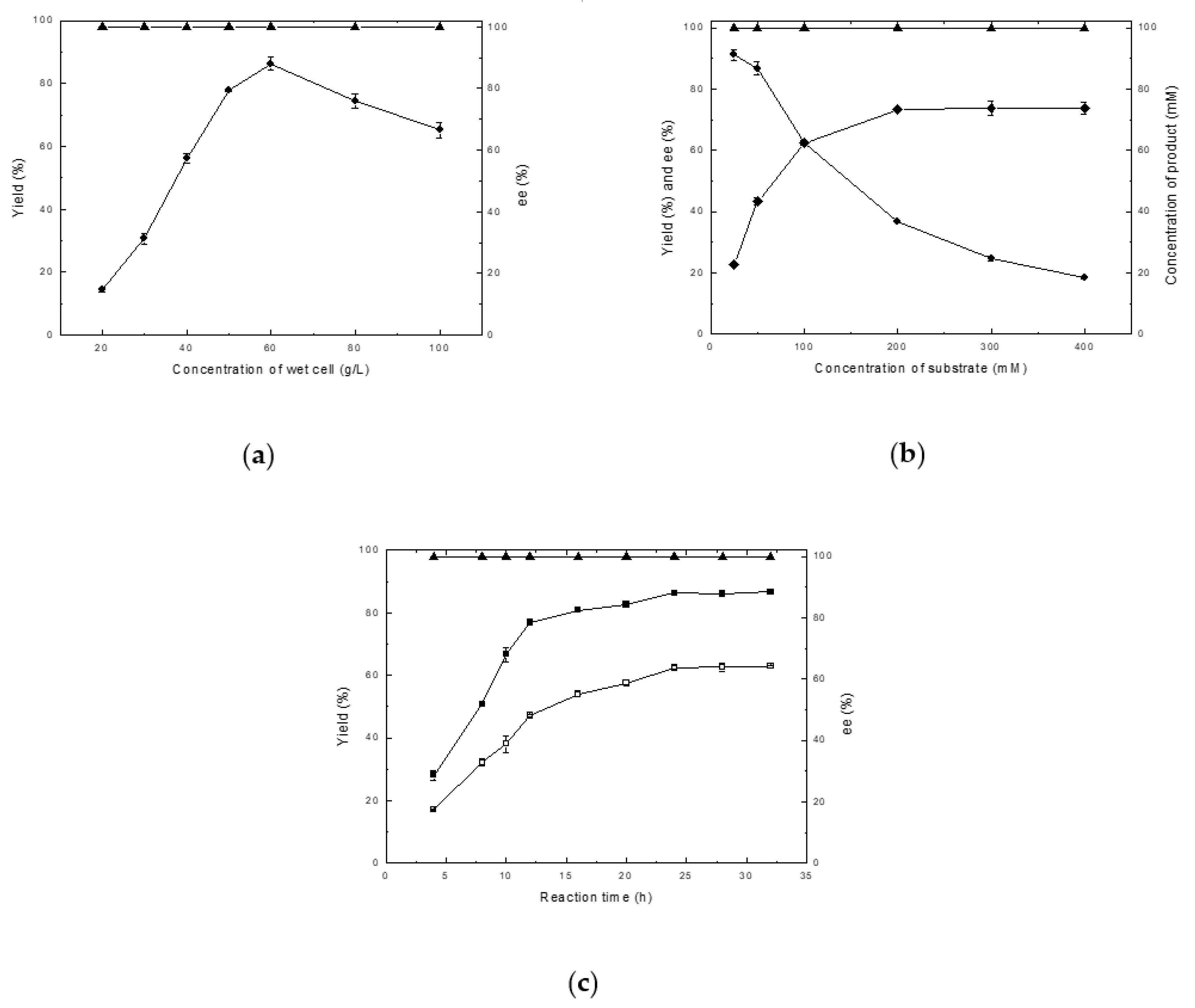
2.2. Effects of Cell Concentration and Substrate Concentration on the Asymmetric Reduction of 4-(trifluoromethyl)acetophenone in Phosphate Buffer
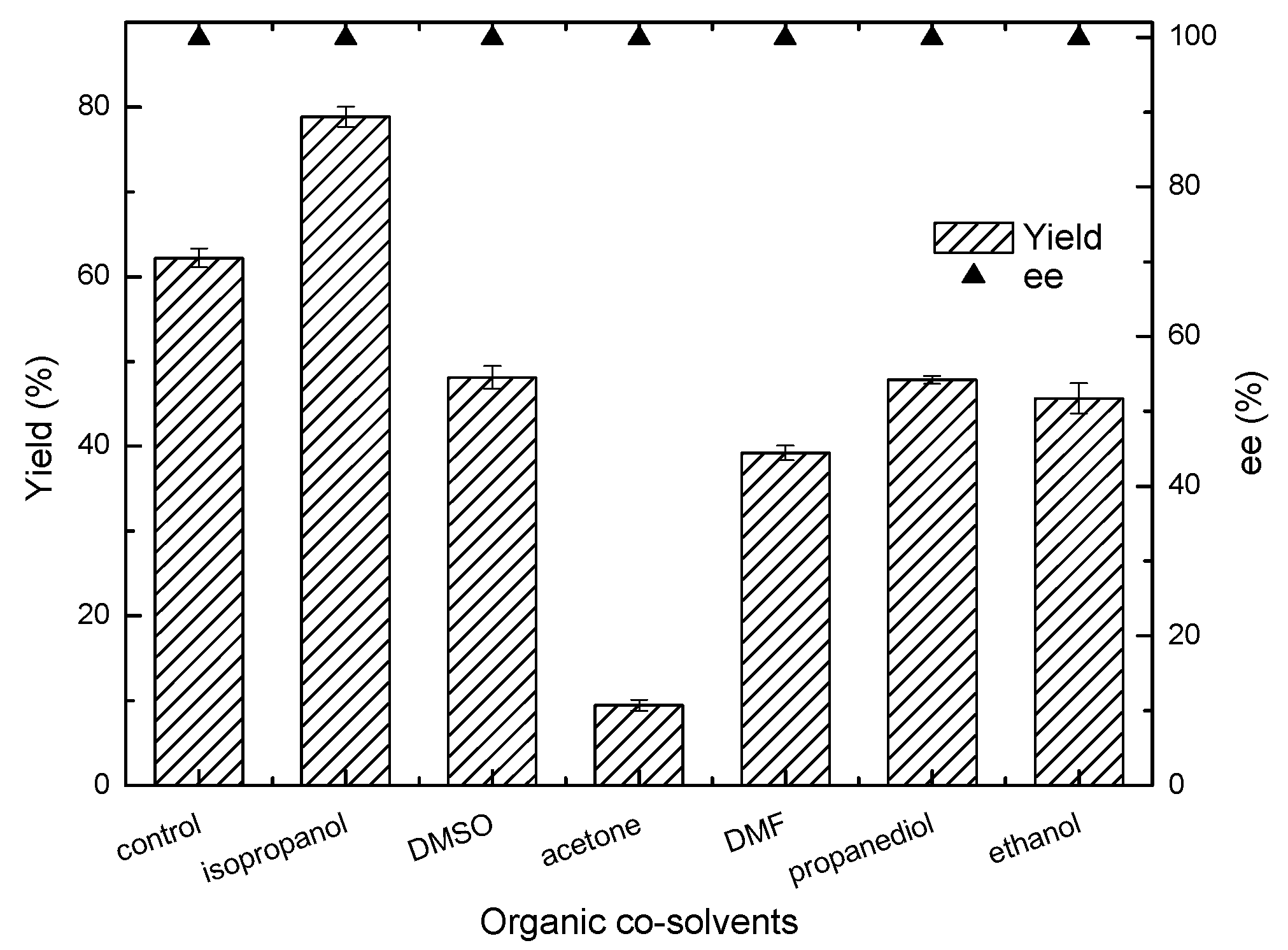
2.3. Effect of Organic Cosolvents on the Asymmetric Reduction of 4-(trifluoromethyl)acetophenone
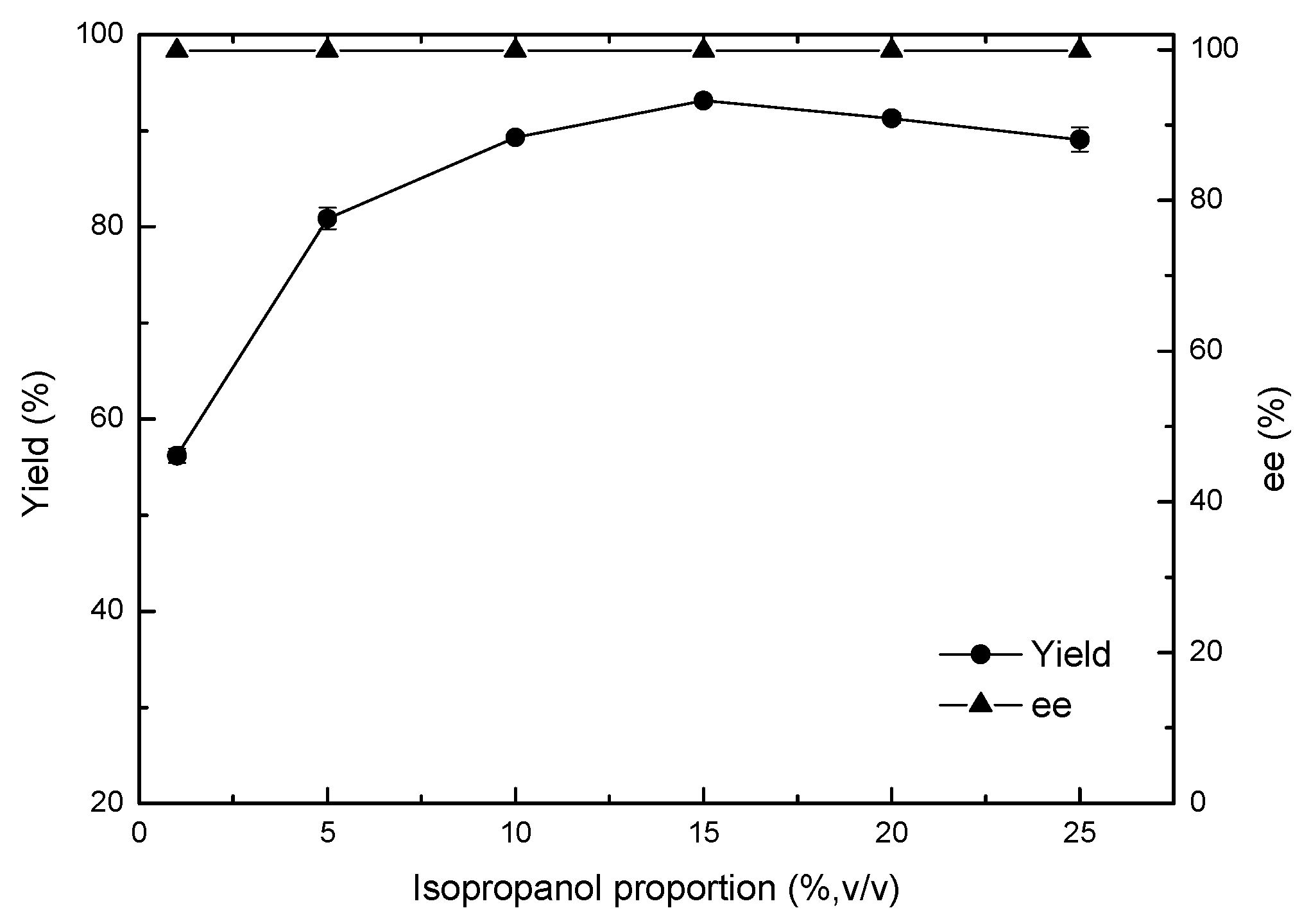
2.4. Effects of Key Variables on Asymmetric Reduction of 4-(trifluoromethyl)acetophenone in Polar Organic Solvent–Aqueous System
2.5. Relationship between Bioreduction and Cosolvent Characteristics
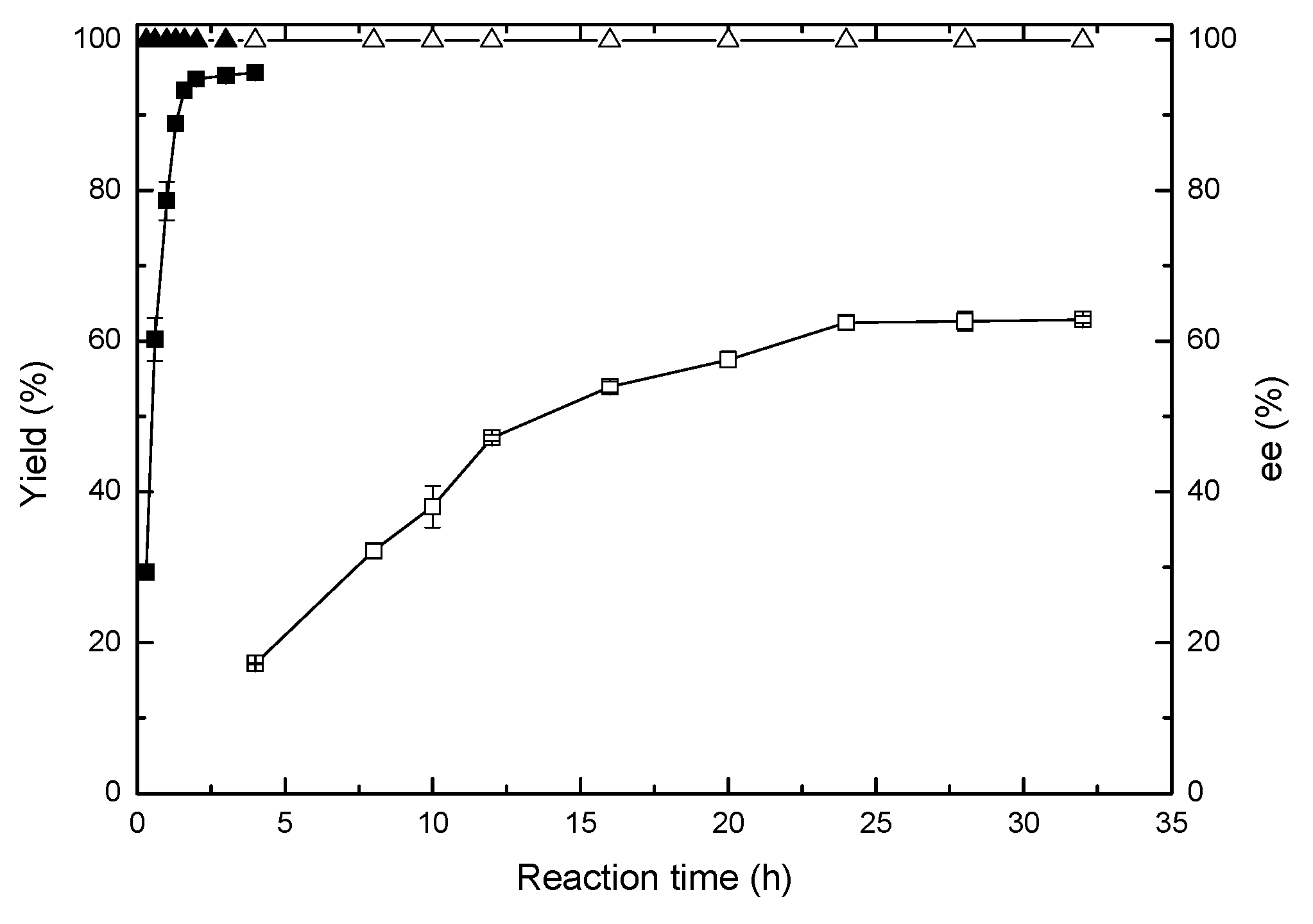
2.6. Comparison of Asymmetric Reduction in an Isopropanol-Aqueous System and Buffer Solution
2.7. Preparative Scale Bioreduction of 4-(trifluoromethyl)acetophenone to (R)-1-[4-(trifluoromethyl)phenyl]ethanol in the Developed Isopropanol-Aqueous System
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Strain and Cultivation
4.3. General Bioreduction Process and Selection of Organic Cosolvents
4.4. GC Analysis
4.5. Cell Membrane Permeability Assay
4.6. Substrate Solubility Assay
4.7. Effects of Key Variables on the Asymmetric Reduction
4.7.1. Optimization of Cell Concentration, Substrate Concentration, and Reaction Time in Phosphate Buffer System
4.7.2. Screening of Organic Cosolvents
4.7.3. Effect of Isopropanol Proportion on the Asymmetric Reduction
4.7.4. Optimization of Other Key Variables in Polar Organic Solvent–Aqueous System
4.7.5. Comparison of Asymmetric Reduction in Isopropanol-Buffer Medium and Aqueous System
4.8. Preparative Scale Bioreduction in the Developed Isopropanol-Aqueous System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, H.; Duan, W.D.; de Souza, F.Z.R.; Liu, L.; Chen, B.S. Asymmetric ketone reduction by immobilized Rhodotorula mucilaginosa. Catalysts 2018, 8, 165. [Google Scholar] [CrossRef]
- Tsamis, F.; Gavrilov, S.; Kajumo, F.; Seibert, C.; Kuhmann, S.; Ketas, T.; Trkola, A.; Palani, A.; Clader, J.W.; Tagat, J.R.; et al. Analysis of the mechanism by which the small-Molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J. Virol. 2003, 77, 5201–5208. [Google Scholar] [CrossRef] [PubMed]
- Tagat, J.R.; Steensma, R.W.; McCombie, S.W.; Nazareno, D.V.; Lin, S.I.; Neustadt, B.R.; Cox, K.; Xu, S.; Wojcik, L.; Murray, M.G.; et al. Piperazine-based CCR5 antagonists as HIV-1 inhibitors. II. Discovery of 1-[(2,4-dimethyl-3-pyridinyl)carbonyl]-4- methyl-4-[3(S)-methyl-4-[1(S)-[4-(trifluoromethyl)phenyl]ethyl]-1-piperazinyl]-piperidine N1-Oxide (Sch-350634), an orally bioavailable, potent CCR5 antagonist. J. Med. Chem. 2001, 44, 3343–3346. [Google Scholar]
- Sheldon, R.A.; Pereira, P.C. Biocatalysis engineering: The big picture. Chem. Soc. Rev. 2017, 46, 2678–2691. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Serencam, H.; Dertli. Whole cell application of Lactobacillus paracasei BD101 to produce enantiomerically pure (S)-cyclohexyl(phenyl)methanol. Chirality 2019, 31, 211–218. [Google Scholar] [CrossRef]
- Vitale, P.; Perna, F.M.; Agrimi, G.; Scilimati, A.; Salomone, A.; Cardellicchioe, C.; Capriati, V. Asymmetric chemoenzymatic synthesis of 1,3-diols and 2,4-disubstituted aryloxetanes by using whole cell biocatalysts. Org. Biomol. Chem. 2016, 14, 11438–11445. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.Q.; Huang, J.; Luo, H.D.; Wang, P.; Li, J. Purification and characterization of a new carbonyl reductase from Leifsonia xyli HS0904 involved in stereoselective reduction of 3,5-bis(trifluoromethyl)acetophenone. J. Mol. Catal. B-Enzym. 2013, 92, 1–6. [Google Scholar] [CrossRef]
- Wang, N.Q.; Sun, J.; Huang, J.; Wang, P. Cloning, expression, and directed evolution of carbonyl reductase from Leifsonia xyli HS0904 with enhanced catalytic efficiency. Appl. Microbiol. Biotechnol. 2014, 98, 8591–8601. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Dong, S.C.; Yin, H.H.; Xue, Y.P.; Tang, X.L.; Zhang, X.J.; He, J.Y.; Zheng, Y.G. Enzymatic synthesis of an ezetimibe intermediate using carbonyl reductase coupled with glucose dehydrogenase in an aqueous-organic solvent system. Bioresour. Technol. 2017, 229, 26–32. [Google Scholar] [CrossRef]
- Zuhse, R.; Leggewie, C.; Hollmann, F.; Kara, S. Scaling-up of “smart cosubstrate” 1,4-butanediol promoted asymmetric reduction of ethyl-4,4,4-trifluoroacetoacetate in organic media. Org. Process Res. Dev. 2015, 229, 369–372. [Google Scholar] [CrossRef]
- Itoh, N.; Isotani, K.; Nakamura, M.; Inoue, K.; Isogai, Y.; Makino, Y. Efficient synthesis of optically pure alcohols by asymmetric hydrogen-transfer biocatalysis: Application of engineered enzymes in a 2-propanol-water medium. Appl. Microbiol. Biotechnol. 2012, 93, 1075–1085. [Google Scholar] [CrossRef]
- Castillo, E.; Casas-Godoy, L.; Sandoval, G. Medium-engineering: A useful tool for modulating lipase activity and selectivity. Biocatalysis 2015, 1, 178–188. [Google Scholar] [CrossRef]
- Kim, P.Y.; Pollard, D.J.; Woodley, J.M. Substrate supply for effective biocatalysis. Biotechnol. Prog. 2007, 23, 74–82. [Google Scholar] [CrossRef]
- Yasohara, Y.; Kizaki, N.; Hasegawa, J.; Wada, M.; Kataoka, M.; Shimizu, S. Stereoselective reduction of alkyl 3-Oxobutanonate by carbonyl reductase from Candida magnoliae. Tetrahedron Asymmetry 2001, 12, 1713–1718. [Google Scholar] [CrossRef]
- Liu, S.L.; Wei, D.Z.; Song, Q.X.; Zhang, Y.W.; Wang, X.D. Effect of organic cosolvent on kinetic resolution of tert-leucine by penicillin G acylase from Kluyvera citrophila. Bioprocess. Biosyst. Eng. 2006, 28, 285–289. [Google Scholar] [CrossRef]
- Lanne, C.; Boeren, S.; Vos, K.; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. [Google Scholar] [CrossRef]
- Honda, K.; Ono, T.; Okano, K.; Miyake, R.; Dekishima, Y.; Kawabata, H. Expression of engineered carbonyl reductase from Ogataea minuta in Rhodococcus opacus and its application to whole-cell bioconversion in anhydrous solvents. J. Biosci. Bioeng. 2019, 127, 145–149. [Google Scholar] [CrossRef]
- Stepankova, V.; Damborsky, J.; Chaloupkova, R. Organic co-solvents affect activity, stability and enantioselectivity of haloalkane dehalogenases. Biotechnol. J. 2013, 8, 719–729. [Google Scholar] [CrossRef]
- Serdakowski, A.L.; Dordick, J.S. Enzyme activation for organic solvents made easy. Trends Biotechnol. 2008, 26, 48–54. [Google Scholar] [CrossRef]
- Yoon, J.H.; Mckenzi, D. A comparison of the activities of three β-galactosidases in aqueous-organic solvent mixtures. Enzyme Microb. Technol. 2005, 36, 439–466. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, P.; Huang, J.; Cai, J.B.; He, J.Y. Efficient enantioselective synthesis of (R)-[3,5-bis(trifluoromethyl)phenyl] ethanol by Leifsonia xyli CCTCC M 2010241 using isopropanol as cosubstrate. J. Microbiol. Biotechnol. 2013, 23, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Wu, L.; Zheng, L.; Wang, W.Z.; Zhang, X.J.; Jin, L.Q.; Zhang, Y.G. Biosynthesis of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate by carbonyl reductase from Rhodosporidium toruloides in mono and biphasic media. Bioresour. Technol. 2018, 249, 161–167. [Google Scholar] [CrossRef]
- Wachtmeister, J.; Rother, D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Curr. Opin. Chem. Biol. 2016, 42, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.C.; Mutti, F.G.; Kroutil, W. Biocatalytic synthesis of enantiopure building blocks for pharmaceuticals. Drug Discov. Today Technol. 2013, 10, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauska, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef]
- Li, H.M.; Moncecchi, J.; Truppo, M.D. Development of an immobilized ketoreductase for enzymatic (R)-1-(3,5-bis(trifluoromethyl)phenyl)ethanol production. Org. Process Res. Dev. 2015, 19, 695–700. [Google Scholar] [CrossRef]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef]
- Berkowitz, D.B.; Hartung, R.E.; Choi, S. Hydrolytic enzymatic transformation of advanced synthetic intermediates: On the choice of the organic cosolvent. Tetrahedron Asymmetry 1999, 10, 4513–4520. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.; Huang, J. Rhodococcus erythropolis XS1012 and Its Application in the Preparation of Chiral Alcohol. C.N. Patent 103773724 A, 7 May 2014. [Google Scholar]
- Wang, P.; Su, H.Z.; Sun, L.M.; He, J.Y.; Lu, Y.P. Asymmetric bioreduction of 3,5-bis(trifluoromethyl) acetophenone to its corresponding alcohol by Candida tropicalis. Chin. J. Chem. Eng. 2011, 19, 1028–1032. [Google Scholar] [CrossRef]
- Wang, N.Q.; Li, J.; Sun, J.; Huang, J.; Wang, P. Bioreduction of 3,5-bis(trifluoromethyl)acetophenone using ionic liquid as a co-solvent catalyzed by recombinant Escherichia coli cells. Biochem. Eng. J. 2015, 101, 119–125. [Google Scholar] [CrossRef]
- Wang, L.F.; Shen, Y.B.; Liu, M.J.; Tang, R.; Wang, M. Influence of imidazolium-based ionic liquids on steroid biotransformation by Arthrobacter simplex. J. Chem. Technol. Biotechnol. 2018, 93, 426–431. [Google Scholar] [CrossRef]

| Strain | Yield (%) | ee (%) |
|---|---|---|
| CY 1-1 | 14.9 | 99.9 (S) |
| HZ 1-3 | 21.8 | 99.9 (S) |
| XA 1-4 | 30.1 | 99.9 (S) |
| AF 3-4 | 23.9 | 99.9 (S) |
| XM 7-1 | 45.9 | 60.2 (S) |
| CQ 8-1 | 34.7 | 55.7 (S) |
| Escherichia coli BL21(DE3)/pET-28a(+)-LXCAR-S154Y | 65.4 | 99.9 (R) |
| Geotrichum candidum ZJPH1704 | 47.3 | 60.9 (R) |
| Candida tropicalis 104 | 25.0 | 38.9 (R) |
| Rhodococcus erythropolis XS1012 | 9.5 | 35.6 (R) |
| Medium | Net OD260nm | Net OD280nm | Solubility (mg/L) |
|---|---|---|---|
| 15% (v/v) Isopropanol-buffer | 2.560 | 1.225 | 663.6 |
| 15% (v/v) DMF-buffer | 1.394 | 0.651 | 1416.4 |
| 15% (v/v) DMSO-buffer | 1.614 | 0.597 | 709.3 |
| 15% (v/v) Ethanol-buffer | 1.648 | 0.837 | 641.0 |
| 15% (v/v) Propylene glycol-buffer | 0.954 | 0.520 | 535.5 |
| 15% (v/v) Acetone-buffer | 2.969 | 2.339 | 1067.7 |
| Phosphate buffer | 0.914 | 0.485 | 410.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xia, N.; Liu, Y.; Wang, P. Efficient Biocatalytic Preparation of Optically Pure (R)-1-[4-(Trifluoromethyl)phenyl]ethanol by Recombinant Whole-Cell-Mediated Reduction. Catalysts 2019, 9, 391. https://doi.org/10.3390/catal9040391
Chen Y, Xia N, Liu Y, Wang P. Efficient Biocatalytic Preparation of Optically Pure (R)-1-[4-(Trifluoromethyl)phenyl]ethanol by Recombinant Whole-Cell-Mediated Reduction. Catalysts. 2019; 9(4):391. https://doi.org/10.3390/catal9040391
Chicago/Turabian StyleChen, Ying, Nana Xia, Yuewang Liu, and Pu Wang. 2019. "Efficient Biocatalytic Preparation of Optically Pure (R)-1-[4-(Trifluoromethyl)phenyl]ethanol by Recombinant Whole-Cell-Mediated Reduction" Catalysts 9, no. 4: 391. https://doi.org/10.3390/catal9040391
APA StyleChen, Y., Xia, N., Liu, Y., & Wang, P. (2019). Efficient Biocatalytic Preparation of Optically Pure (R)-1-[4-(Trifluoromethyl)phenyl]ethanol by Recombinant Whole-Cell-Mediated Reduction. Catalysts, 9(4), 391. https://doi.org/10.3390/catal9040391





