Optimization of Photocatalytic Degradation of Acid Blue 113 and Acid Red 88 Textile Dyes in a UV-C/TiO2 Suspension System: Application of Response Surface Methodology (RSM)
Abstract
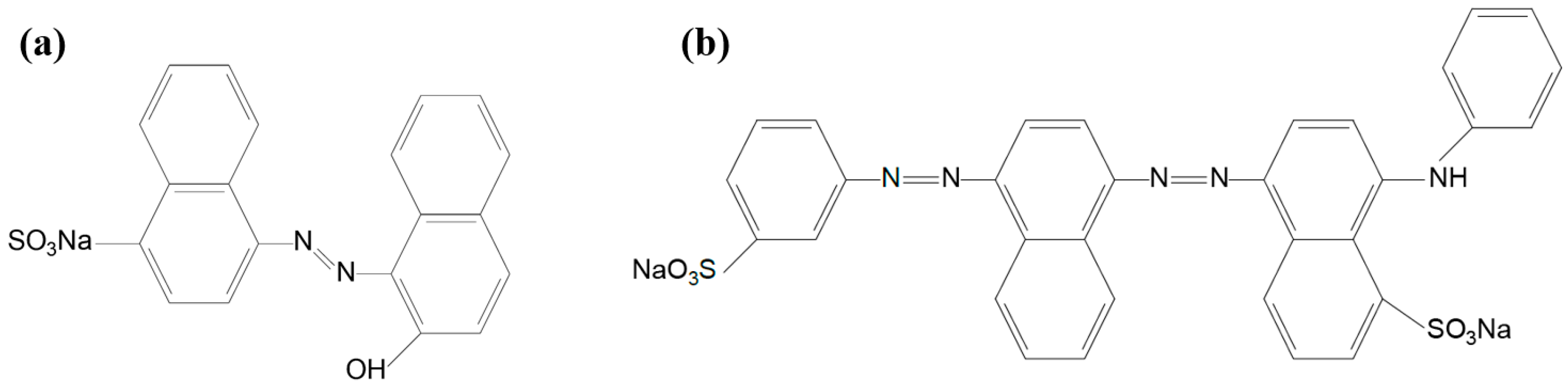
1. Introduction
2. Results and Discussions
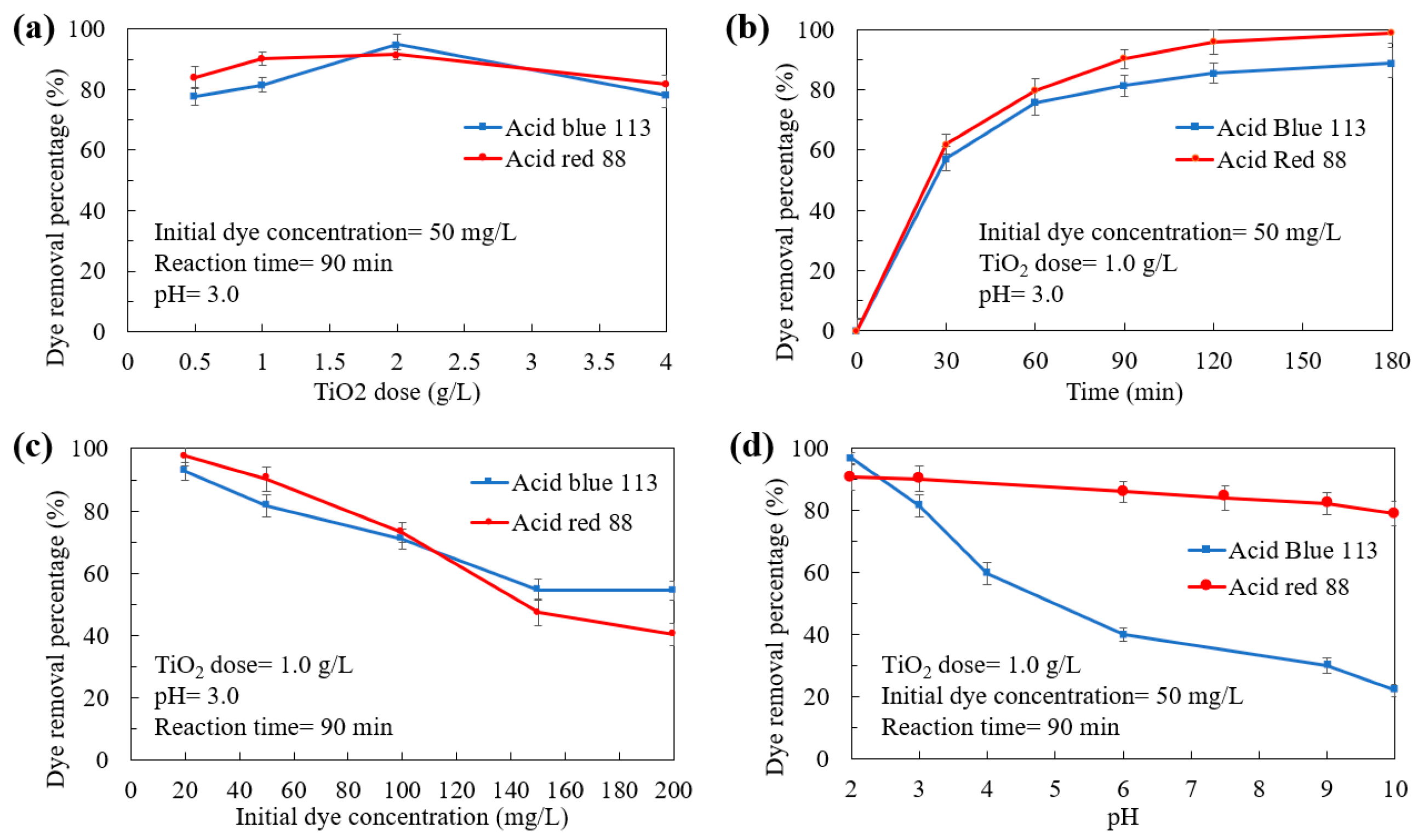
2.1. Stage 1: Preliminary Experiments
2.2. Stage 2: Process Optimization
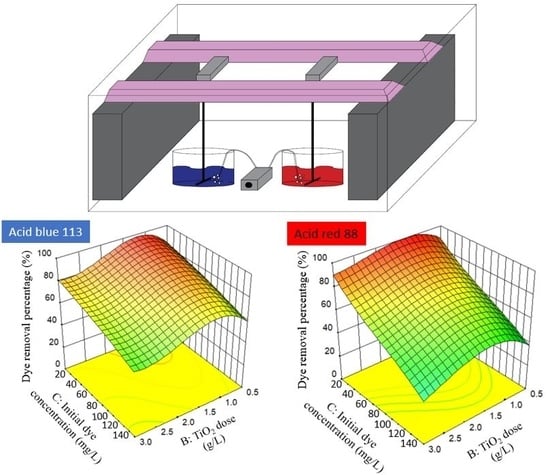
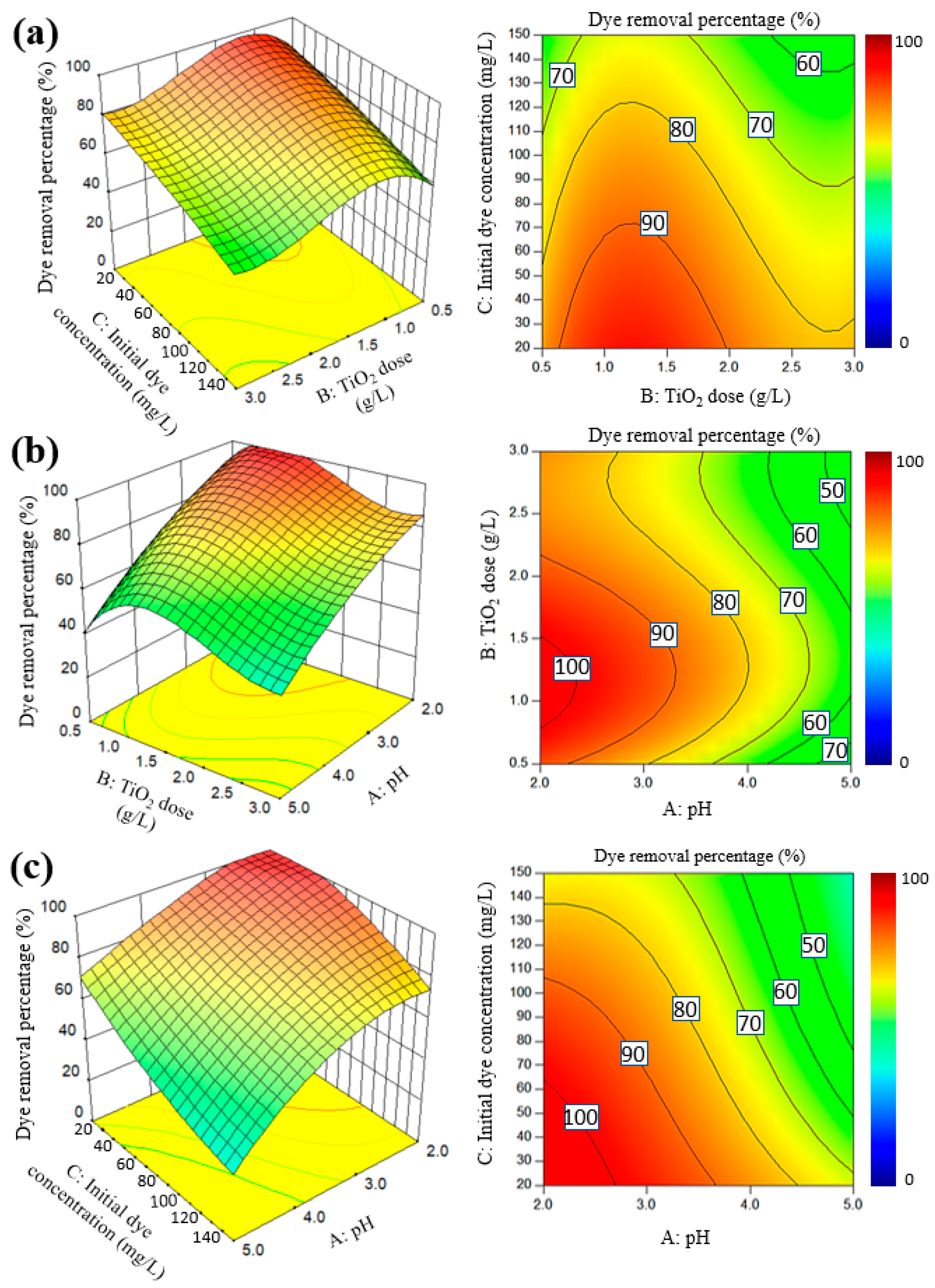
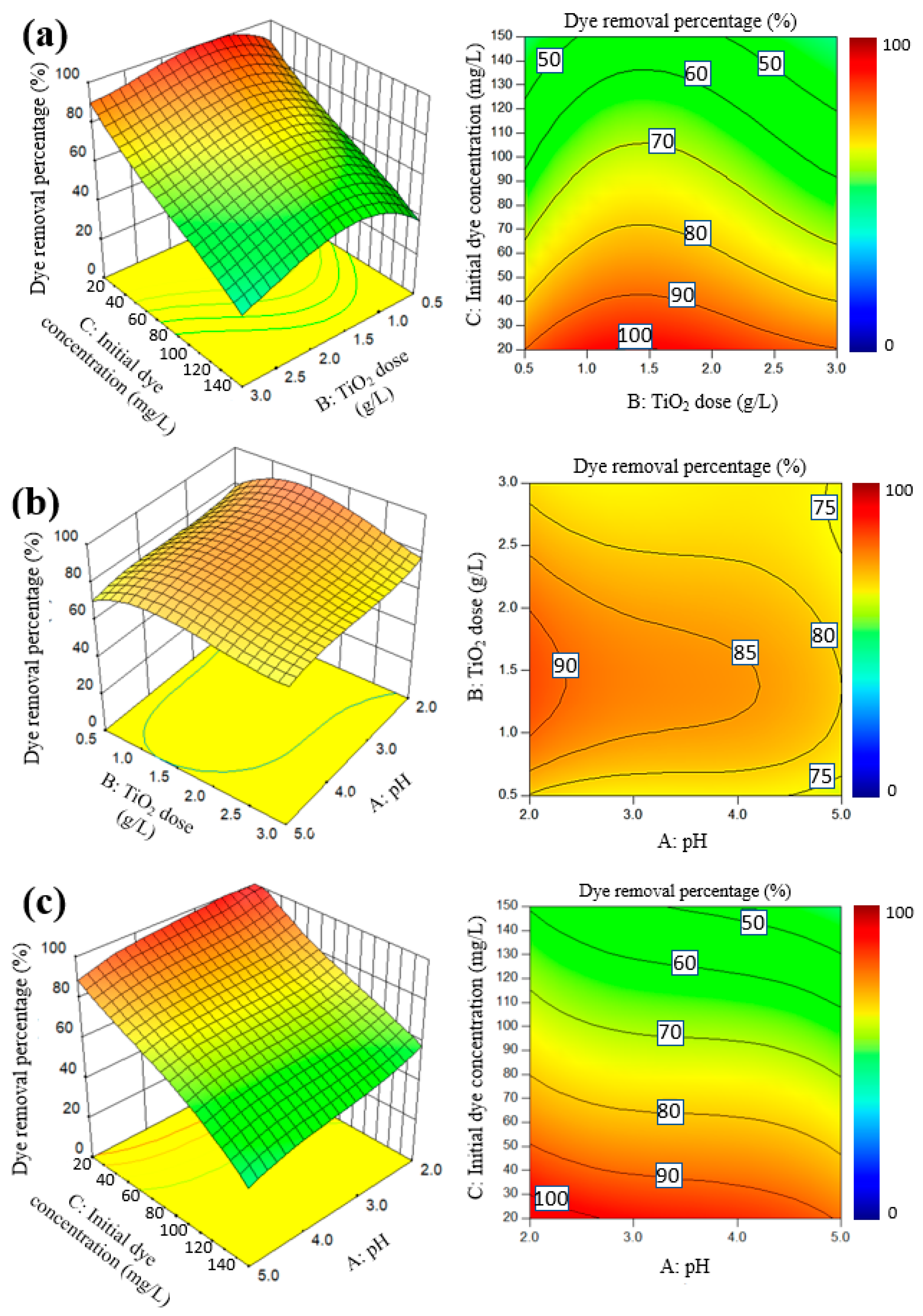
2.2.1. Response Surface Plots, Fitted Models and ANOVA
− 5.14A2C − 4.44AB2 + 5.23AC2 + 16.52B3
3.57AB2 − 3.99AC2 − 3.30B2C − 4.44A3 + 7.18B3 − 3.95C3
2.2.2. Optimization
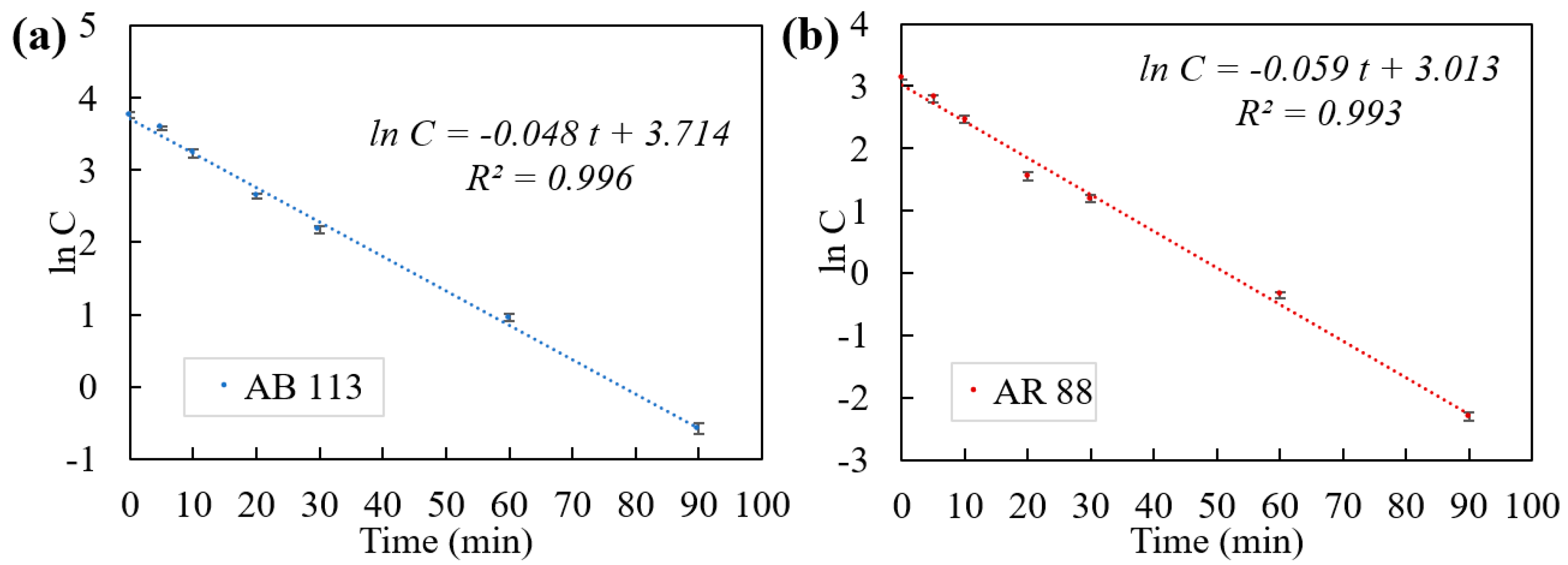
2.3. Stage 3: Kinetics of Photocatalytic Degradation
3. Materials and Methods
3.1. Materials and Equipment
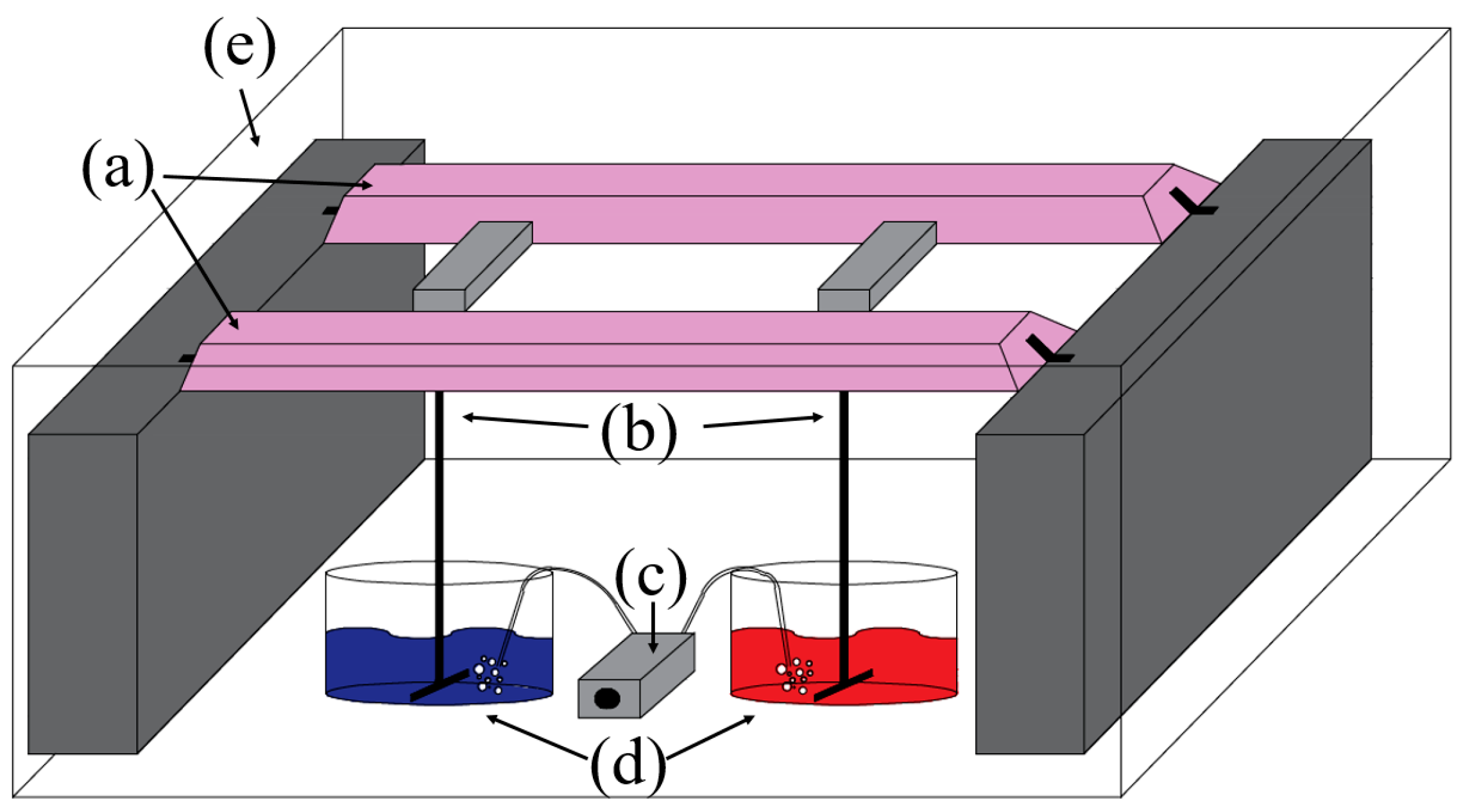
3.2. Photoreactor
3.3. Experimental Procedure and Measurements
3.4. Statistical Analysis
3.5. Experimental Design and Optimization
3.5.1. Preliminary Experiments
3.5.2. Experimental Design Using Response Surface Methodology
3.5.3. Kinetic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sohrabi, M.R.; Ghavami, M. Photocatalytic degradation of Direct Red 23 dye using UV/TiO2: Effect of operational parameters. J. Hazard. Mater. 2008, 153, 1235–1239. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. Available online: https://ac-els-cdn-com.ezproxy.library.unlv.edu/S0926337300002769/1-s2.0-S0926337300002769-main.pdf?_tid=7e7afe96-d229-11e7-85a6-00000aab0f26&acdnat=1511646312_3be9e82c1ae096d6f42aa8199f88814d (accessed on 25 November 2017). [CrossRef]
- Konicki, W.; Sibera, D.; Mijowska, E.; Lendzion-Bieluń, Z.; Narkiewicz, U. Equilibrium and kinetic studies on acid dye Acid Red 88 adsorption by magnetic ZnFe2O4 spinel ferrite nanoparticles. J. Colloid Interface Sci. 2013, 398, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Pal, A.; Sahoo, C. Photocatalytic degradation of a mixture of Crystal Violet (Basic Violet 3) and Methyl Red dye in aqueous suspensions using Ag+ doped TiO2. Dyes Pigments 2006, 69, 224–232. [Google Scholar] [CrossRef]
- Han, F.; Kambala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A Gen. 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Juang, R.S.; Lin, S.H.; Hsueh, P.Y. Removal of binary azo dyes from water by UV-irradiated degradation in TiO2 suspensions. J. Hazard. Mater. 2010, 182, 820–826. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef]
- Barakat, M.A. Adsorption and photodegradation of Procion yellow H-EXL dye in textile wastewater over TiO2 suspension. J. Hydro-Environ. Res. 2011, 5, 137–142. [Google Scholar] [CrossRef]
- Soutsas, K.; Karayannis, V.; Poulios, I.; Riga, A.; Ntampegliotis, K.; Spiliotis, X.; Papapolymerou, G. Decolorization and degradation of reactive azo dyes via heterogeneous photocatalytic processes. Desalination 2010, 250, 345–350. [Google Scholar] [CrossRef]
- Anandan, S.; Kumar, P.S.; Pugazhenthiran, N.; Madhavan, J.; Maruthamuthu, P. Effect of loaded silver nanoparticles on TiO2 for photocatalytic degradation of Acid Red 88. Sol. Energy Mater. Sol. Cells 2008, 92, 929–937. [Google Scholar] [CrossRef]
- Zayani, G.; Bousselmi, L.; Pichat, P.; Mhenni, F. Photocatalytic degradation of the Acid Blue 113 textile azo dye in aqueous suspensions of four commercialized TiO(2) samples. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2008, 43, 202–209. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Mittal, A.; Saleh, T.A.; Nayak, A.; Agarwal, S.; Sikarwar, S. Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater. Sci. Eng. C 2012, 32, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Toor, A.T.; Verma, A.; Jotshi, C.K.; Bajpai, P.K.; Singh, V. Photocatalytic degradation of Direct Yellow 12 dye using UV/TiO2 in a shallow pond slurry reactor. Dyes Pigments 2006, 68, 53–60. [Google Scholar] [CrossRef]
- Khataee, A.R.; Pons, M.N.; Zahraa, O. Photocatalytic degradation of three azo dyes using immobilized TiO2 nanoparticles on glass plates activated by UV light irradiation: Influence of dye molecular structure. J. Hazard. Mater. 2009, 168, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, K.; Venckatesh, R.; Sivaraj, R.; Rajiv, P. TiO2 nanoparticles versus TiO2-SiO2 nanocomposites: A comparative study of photo catalysis on acid red 88, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yap, P.S.; Lim, T.M.; Lim, T.T. Adsorption-photocatalytic degradation of Acid Red 88 by supported TiO2: Effect of activated carbon support and aqueous anions. Chem. Eng. J. 2011, 171, 1098–1107. [Google Scholar] [CrossRef]
- Moon, J.; Yun, C.Y.; Chung, K.W.; Kang, M.S.; Yi, J. Photocatalytic activation of TiO2 under visible light using Acid Red 44. Catal. Today 2003, 87, 77–86. [Google Scholar] [CrossRef]
- Khataee, A.R.; Zarei, M. Photoelectrocatalytic decolorization of diazo dye by zinc oxide nanophotocatalyst and carbon nanotube based cathode: Determination of the degradation products. Desalination 2011, 278, 117–125. [Google Scholar] [CrossRef]
- Cavazzuti, M. Optimization Methods: From Theory to Design Scientific and Technological Aspects in Mechanics; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Antony, J. Taguchi or classical design of experiments: A perspective from a practitioner. Sens. Rev. 2006, 26, 227–230. [Google Scholar] [CrossRef]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology, Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Wei, L.; Zhu, H.; Mao, X.; Gan, F. Electrochemical oxidation process combined with UV photolysis for the mineralization of nitrophenol in saline wastewater. Sep. Purif. Technol. 2011, 77, 18–25. [Google Scholar] [CrossRef]
- Sahu, J.N.; Acharya, J.; Meikap, B.C. Response surface modeling and optimization of chromium(VI) removal from aqueous solution using Tamarind wood activated carbon in batch process. J. Hazard. Mater. 2009, 172, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.; Mortazavian, S.; James, D.E.; Hasheminejad, H. Optimization of Collaborative Photo-Fenton Oxidation and Coagulation for the Treatment of Petroleum Refinery Wastewater with Scrap Iron. Water Air Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Saber, A.; Hasheminejad, H.; Taebi, A.; Ghaffari, G. Optimization of Fenton-based treatment of petroleum refinery wastewater with scrap iron using response surface methodology. Appl. Water Sci. 2014, 4, 283–290. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, J.; Liu, R.; Chen, L. Optimization of fenton and electro-fenton oxidation of biologically treated coking wastewater using response surface methodology. Sep. Purif. Technol. 2011, 81, 444–450. [Google Scholar] [CrossRef]
- Benatti, C.T.; Tavares, C.R.G.; Guedes, T.A. Optimization of Fenton’s oxidation of chemical laboratory wastewaters using the response surface methodology. J. Environ. Manag. 2006, 80, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Vahabzadeh, F.; Bonakdarpour, B.; Mofarrah, E.; Mehranian, M. Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. J. Hazard. Mater. 2005, 123, 187–195. [Google Scholar] [CrossRef]
- Bianco, B.; de Michelis, I.; Vegliò, F. Fenton treatment of complex industrial wastewater: Optimization of process conditions by surface response method. J. Hazard. Mater. 2011, 186, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.R.; Ghaedi, M.; Asfaram, A.; Bazrafshan, A.A.; Jannesar, R. Comparative study on ultrasonic assisted adsorption of dyes from single system onto Fe3O4 magnetite nanoparticles loaded on activated carbon: Experimental design methodology. Ultrason. Sonochem. 2017, 34, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, S.; Aziz, H.A.; Isa, M.H.; Zahed, M.A.; Adlan, M.N. Statistical optimization of process parameters for landfill leachate treatment using electro-Fenton technique. J. Hazard. Mater. 2010, 176, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, B.; Figueredo, M.; Cobo, M. Response Surface Methodology and Aspen Plus Integration for the Simulation of the Catalytic Steam Reforming of Ethanol. Catalysts 2017, 7, 15. [Google Scholar] [CrossRef]
- Li, L.; Ma, Q.; Wang, S.; Song, S.; Li, B.; Guo, R.; Cheng, X.; Cheng, Q. Photocatalytic Performance and Degradation Mechanism of Aspirin by TiO2 through Response Surface Methodology. Catalysts 2018, 8, 118. [Google Scholar] [CrossRef]
- Inger, M.; Dobrzyńska-Inger, A.; Rajewski, J.; Wilk, M. Optimization of Ammonia Oxidation Using Response Surface Methodology. Catalysts 2019, 9, 249. [Google Scholar] [CrossRef]
- Aljuboury, D.a.A.; Palaniandy, P.; Aziz, H.B.A.; Feroz, S.; Amr, S.S.A. Evaluating photo-degradation of COD and TOC in petroleum refinery wastewater by using TiO2/ZnO photo-catalyst. Water Sci. Technol. 2016, 74, 1312–1325. [Google Scholar] [CrossRef]
- Mohammadzadeh, S.; Olya, M.E.; Arabi, A.M.; Shariati, A.; Nikou, M.R.K. Synthesis, characterization and application of ZnO-Ag as a nanophotocatalyst for organic compounds degradation, mechanism and economic study. J. Environ. Sci. 2015, 35, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Kandiel, T.A.; Hussein, F.H.; Dillert, R.; Bahnemann, D.W. Enhancing the photocatalytic activity of TiO2 by pH control: A case study for the degradation of EDTA. Catal. Sci. Technol. 2013, 3, 3216. [Google Scholar] [CrossRef]
- Hu, C.; Yu, J.C.; Hao, Z.; Wong, P.K. Effects of acidity and inorganic ions on the photocatalytic degradation of different azo dyes. Appl. Catal. B Environ. 2003, 46, 35–47. [Google Scholar] [CrossRef]
- Shirzad-Siboni, M.; Jafari, S.J.; Giahi, O.; Kim, I.; Lee, S.M.; Yang, J.K. Removal of acid blue 113 and reactive black 5 dye from aqueous solutions by activated red mud. J. Ind. Eng. Chem. 2014, 20, 1432–1437. [Google Scholar] [CrossRef]
- Lima, E.C.; Royer, B.; Vaghetti, J.C.P.; Simon, N.M.; da Cunha, B.M.; Pavan, F.A.; Benvenutti, E.V.; Cataluña-Veses, R.; Airoldi, C. Application of Brazilian pine-fruit shell as a biosorbent to removal of reactive red 194 textile dye from aqueous solution. Kinetics and equilibrium study. J. Hazard. Mater. 2008, 155, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.H.; Vosooghian, H. Photocatalytic degradation of some organic sulfides as environmental pollutants using titanium dioxide suspension. J. Photochem. Photobiol. A Chem. 2005, 174, 45–52. [Google Scholar] [CrossRef]
- Venkatachalam, N.; Palanichamy, M.; Murugesan, V. Sol-gel preparation and characterization of alkaline earth metal doped nano TiO2: Efficient photocatalytic degradation of 4-chlorophenol. J. Mol. Catal. A Chem. 2007, 273, 177–185. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Ma, C.-M.; Hong, G.-B.; Chen, H.-W.; Hang, N.-T.; Shen, Y.-S. Photooxidation Contribution Study on the Decomposition of Azo Dyes in Aqueous Solutions by VUV-Based AOPs. Int. J. Photoenergy 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Saharan, V.K.; Pandit, A.B.; Kumar, P.S.S.; Anandan, S. Hydrodynamic cavitation as an advanced oxidation technique for the degradation of Acid Red 88 dye. Ind. Eng. Chem. Res. 2012, 51, 1981–1989. [Google Scholar] [CrossRef]
- Spiess, A.N. qpcR package V1.4-0, Modeling and Analysis of Real-Time PCR Data. 2018. Available online: https://www.rdocumentation.org/packages/qpcR/versions/1.4-0 (accessed on 31 May 2018).
- Konyar, M.; Yildiz, T.; Aksoy, M.; Yatmaz, H.C.; Öztürk, K. Reticulated ZnO Photocatalyst: Efficiency Enhancement in Degradation of Acid Red 88 Azo Dye by Catalyst Surface Cleaning. Chem. Eng. Commun. 2017, 204, 711–716. [Google Scholar] [CrossRef]
- Camarillo, R.; Rincón, J. Photocatalytic Discoloration of Dyes: Relation between Effect of Operating Parameters and Dye Structure. Chem. Eng. Technol. 2011, 34, 1675–1684. [Google Scholar] [CrossRef]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Visible light-induced photocatalytic degradation of Acid Orange 7 in aqueous TiO2 suspensions. Appl. Catal. B Environ. 2004, 47, 189–201. [Google Scholar] [CrossRef]
- Madhavan, J.; Kumar, P.S.S.; Anandan, S.; Grieser, F.; Ashokkumar, M. Degradation of acid red 88 by the combination of sonolysis and photocatalysis. Sep. Purif. Technol. 2010, 74, 336–341. [Google Scholar] [CrossRef]
- Trotma, E.R. Dyeing and Chemical Technology of Textile Fibres; Griffin: London, UK, 1970; Available online: https://www.scribd.com/doc/101550453/Dyeing-and-Chemical-Technology-of-Textile-Fibres (accessed on 3 May 2017).
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Shu, H.Y.; Chang, M.C.; Chen, C.C.; Chen, P.E. Using resin supported nano zero-valent iron particles for decoloration of Acid Blue 113 azo dye solution. J. Hazard. Mater. 2010, 184, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R. Encyclopedia of Physical Science and Technology; Academic Press: New York, NY, USA, 2002. [Google Scholar]
- Zumel, N. Estimating Generalization Error with the PRESS statistic|Win-Vector Blog, (2014) 12. Available online: http://www.win-vector.com/blog/2014/09/estimating-generalization-error-with-the-press-statistic/ (accessed on 2 April 2019).
- Anderson, M.J.; Whitcomb, P.J. RSM Simplified: Optimizing Processes Using Response Surface Methods for Design of Experiments; Productivity Press: New York, NY, USA, 2005; Available online: https://www.crcpress.com/RSM-Simplified-Optimizing-Processes-Using-Response-Surface-Methods-for/Whitcomb-Anderson/p/book/9781563272974 (accessed on 3 May 2017).
- Saber, A.; Tafazzoli, M.; Mortazavian, S.; James, D.E. Investigation of kinetics and absorption isotherm models for hydroponic phytoremediation of waters contaminated with sulfate. J. Environ. Manag. 2018, 207, 276–291. [Google Scholar] [CrossRef] [PubMed]
| Source | F-Statistic Value | p-Value |
|---|---|---|
| Model | 86.33 | <0.0001 |
| A-pH | 129.56 | <0.0001 |
| B-TiO2 dose (g/L) | 18.05 | 0.0005 |
| C-Initial dye concentration (mg/L) | 44.81 | <0.0001 |
| AB | 4.11 | 0.0578 |
| AC | 4.58 | 0.0462 |
| A2 | 20.69 | 0.0002 |
| B2 | 50.53 | <0.0001 |
| A2C | 5.13 | 0.0360 |
| AB2 | 4.56 | 0.0467 |
| AC2 | 5.87 | 0.0261 |
| B3 | 14.68 | 0.0012 |
| Lack of Fit | 0.51 | 0.8499 |
| Source | F-Statistic Value | p-Value |
|---|---|---|
| Model | 217.009 | <0.0001 |
| A-pH | 0.773 | 0.392 |
| B-TiO2 dose (g/L) | 14.338 | 0.0016 |
| C-Initial dye concentration (mg/L) | 92.905 | <0.0001 |
| AC | 9.611 | 0.0069 |
| BC | 3.301 | 0.088 |
| B2 | 90.316 | <0.0001 |
| ABC | 4.961 | 0.0406 |
| AB2 | 6.835 | 0.0188 |
| AC2 | 6.427 | 0.0221 |
| B2C | 5.822 | 0.0282 |
| A3 | 3.379 | 0.0847 |
| B3 | 10.334 | 0.0054 |
| C3 | 3.466 | 0.0811 |
| Lack of Fit | 1.312 | 0.4046 |
| Item | AB 113 | AR 88 | ||
|---|---|---|---|---|
| Initial Cubic Model | Reduced Cubic Model | Initial Cubic Model | Reduced Cubic Model | |
| Standard deviation | 3.90 | 3.78 | 2.35 | 2.27 |
| Mean | 64.24 | 64.24 | 65.35 | 65.35 |
| Coefficient of variation, % | 6.06 | 5.88 | 3.60 | 3.47 |
| PRESS | 1205.01 | 664 | 1780.39 | 265.73 |
| R2 | 0.9890 | 0.9814 | 0.9962 | 0.994 |
| R2adj | 0.9682 | 0.9700 | 0.9890 | 0.990 |
| Adequate precision | 26.392 | 34.834 | 33.969 | 42.061 |
| pH | Initial Dye Concentration (mg/L) | TiO2 Dose (g/L) | Predicted Removal Efficiency (%) | Achieved Removal Efficiency (%) | |
|---|---|---|---|---|---|
| AB 113 | 2.21 | 43.13 | 0.98 | 100% | 98.7% |
| AR 88 | 2.36 | 22.40 | 1.22 | 100% | 99.6% |
| k1 (min−1) | p-Value for k1 | Co, model | p-Value for ln Co | R2 | RMSE (mg/L) | |
|---|---|---|---|---|---|---|
| AB 113 | 0.048 | 2.13 × 10−7 | 41 | 1.13 × 10−8 | 0.996 | 1.72 |
| AR 88 | 0.059 | 8.60 × 10−7 | 20.4 | 3.70 × 10−7 | 0.993 | 1.12 |
| Variables | Levels | ||||
|---|---|---|---|---|---|
| pH | 2.0 | 3.0 * | 6.0 | 9.0 | 10.0 |
| Initial dye concentration (mg/L) | 20 | 50 * | 100 | 150 | 200 |
| TiO2 dose (g/L) | 0.5 | 1.0 * | 2.0 | 4.0 | - |
| Reaction time (min) | 30 | 60 | 90 * | 120 | 180 |
| Factors | Levels | |||||
|---|---|---|---|---|---|---|
| A: pH | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | 5.0 |
| B: TiO2 dose (g/L) | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
| C: Initial dye concentration (mg/L) | 20 | 50 | 60 | 80 | 115 | 150 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortazavian, S.; Saber, A.; James, D.E. Optimization of Photocatalytic Degradation of Acid Blue 113 and Acid Red 88 Textile Dyes in a UV-C/TiO2 Suspension System: Application of Response Surface Methodology (RSM). Catalysts 2019, 9, 360. https://doi.org/10.3390/catal9040360
Mortazavian S, Saber A, James DE. Optimization of Photocatalytic Degradation of Acid Blue 113 and Acid Red 88 Textile Dyes in a UV-C/TiO2 Suspension System: Application of Response Surface Methodology (RSM). Catalysts. 2019; 9(4):360. https://doi.org/10.3390/catal9040360
Chicago/Turabian StyleMortazavian, Soroosh, Ali Saber, and David E. James. 2019. "Optimization of Photocatalytic Degradation of Acid Blue 113 and Acid Red 88 Textile Dyes in a UV-C/TiO2 Suspension System: Application of Response Surface Methodology (RSM)" Catalysts 9, no. 4: 360. https://doi.org/10.3390/catal9040360
APA StyleMortazavian, S., Saber, A., & James, D. E. (2019). Optimization of Photocatalytic Degradation of Acid Blue 113 and Acid Red 88 Textile Dyes in a UV-C/TiO2 Suspension System: Application of Response Surface Methodology (RSM). Catalysts, 9(4), 360. https://doi.org/10.3390/catal9040360