Perovskite-type LaFeO3: Photoelectrochemical Properties and Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation
Abstract
1. Introduction
2. Results and Discussion
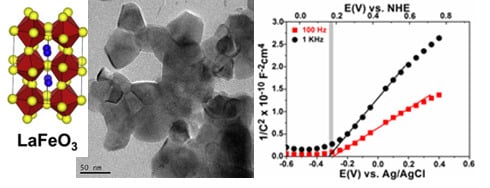
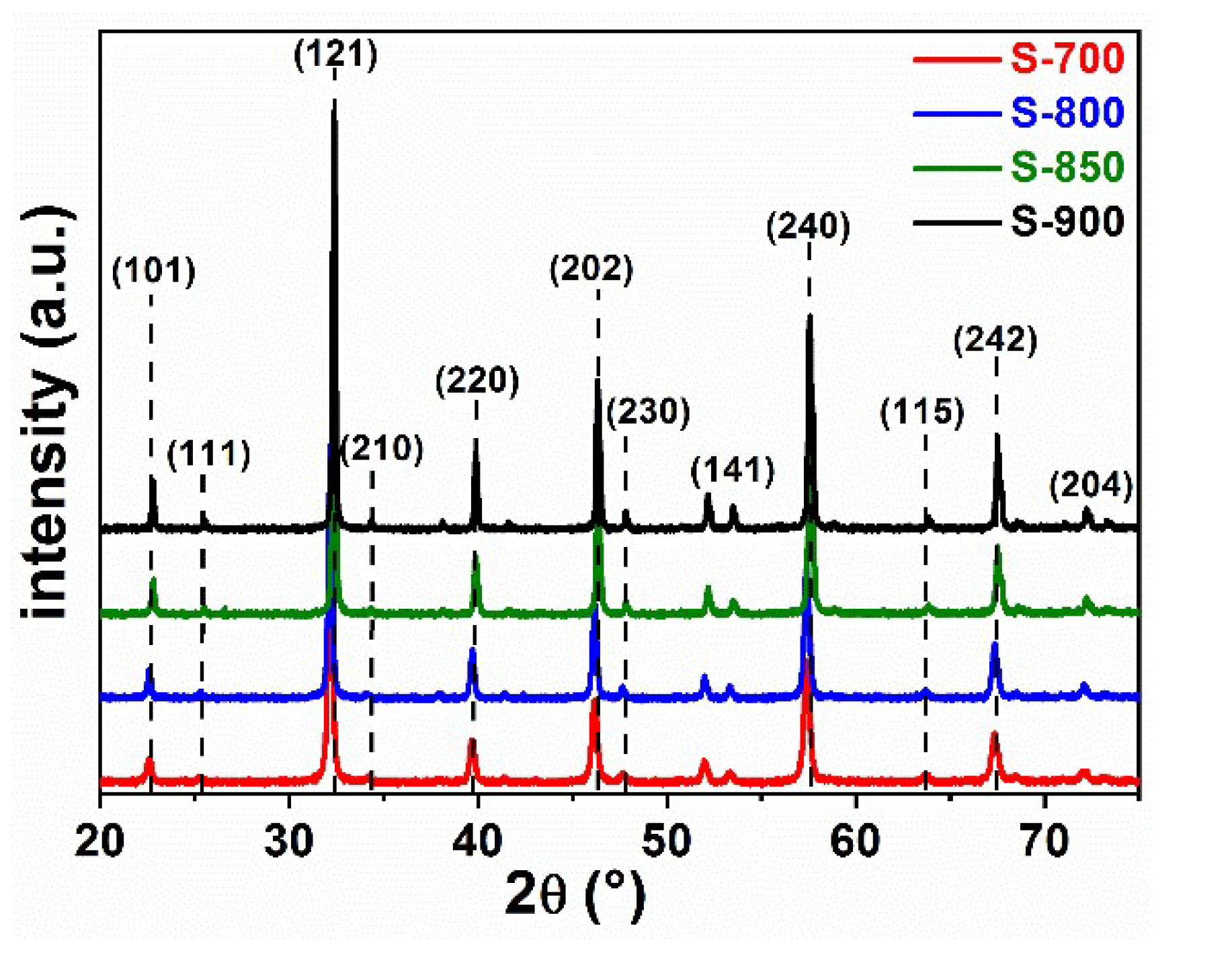
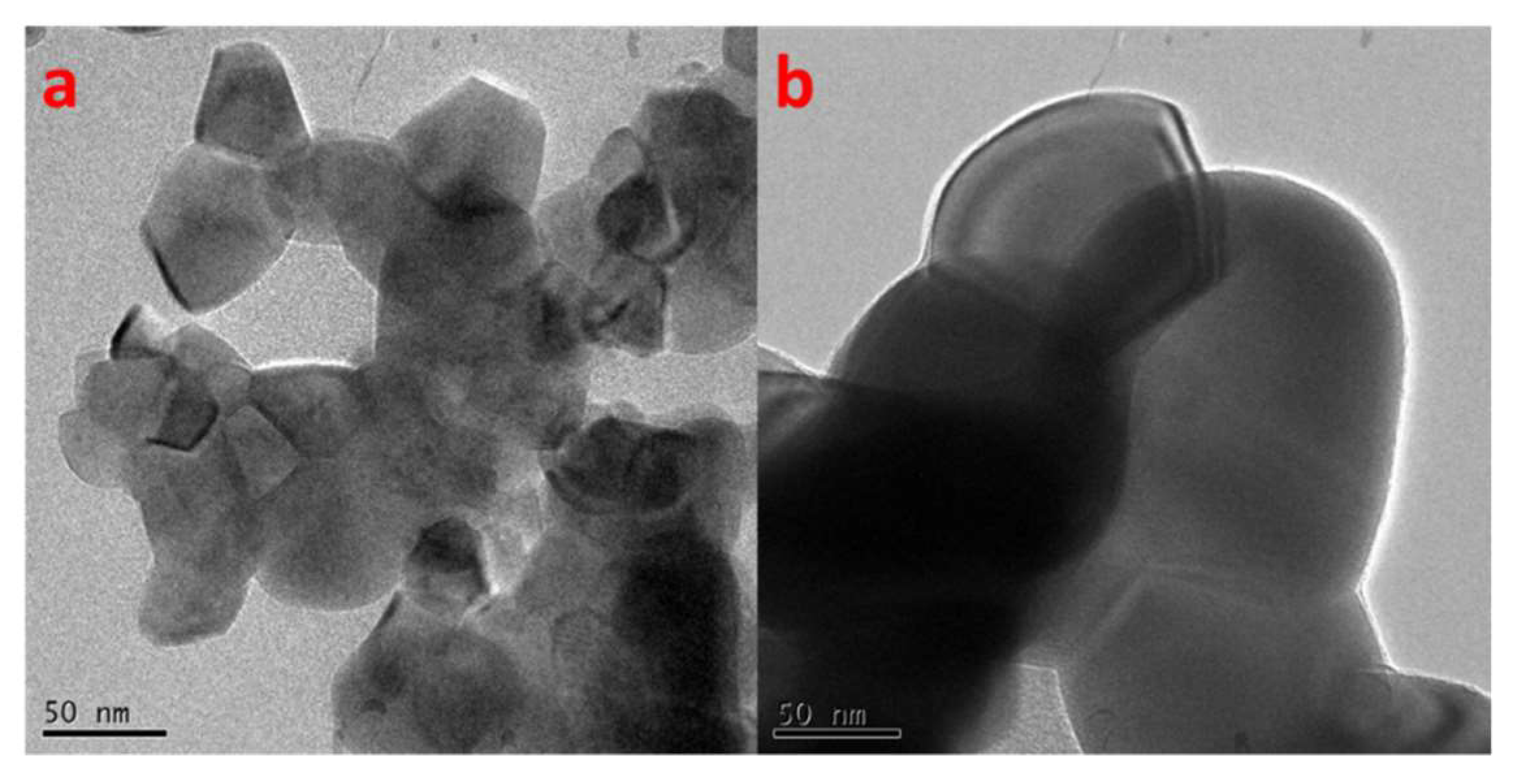
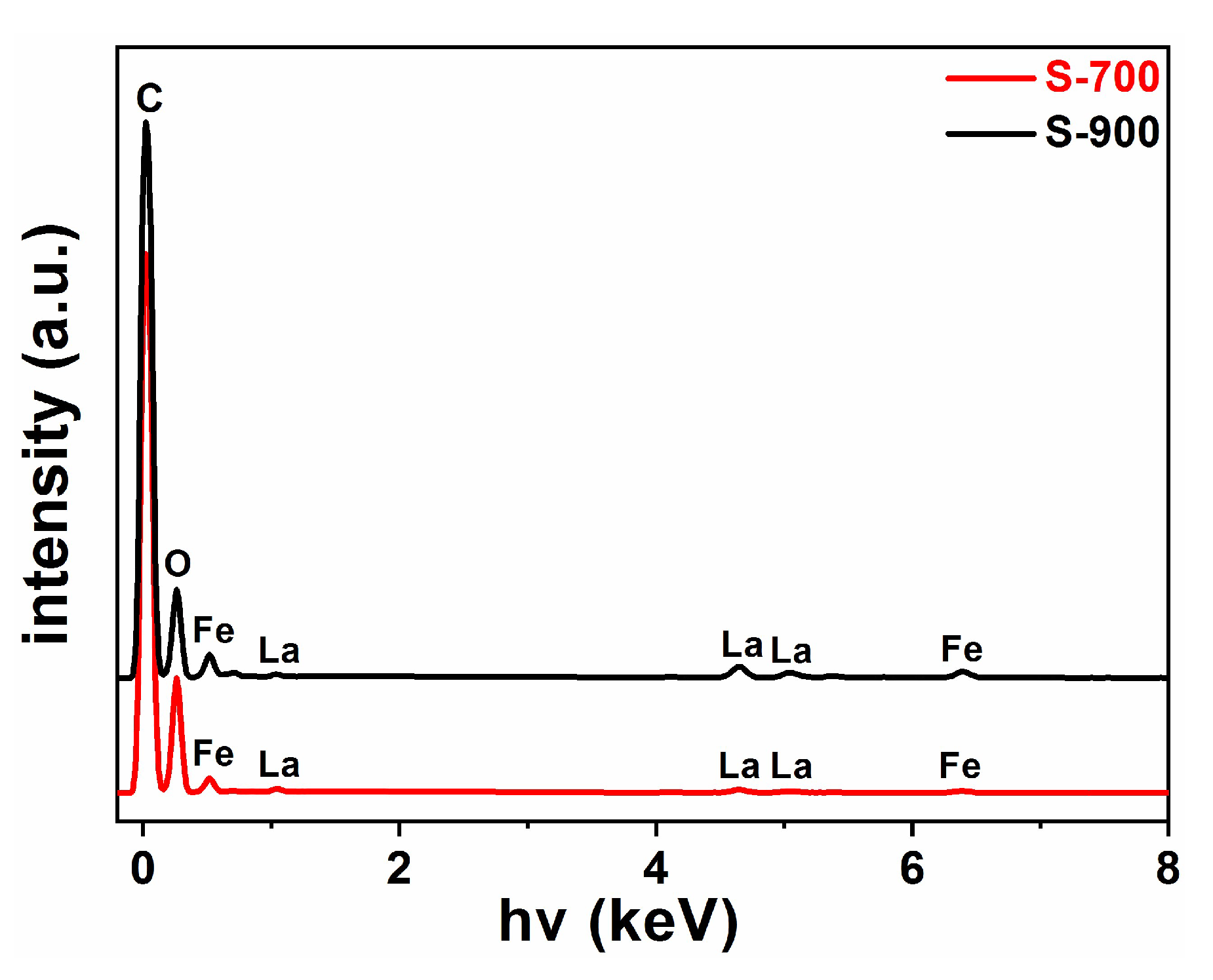
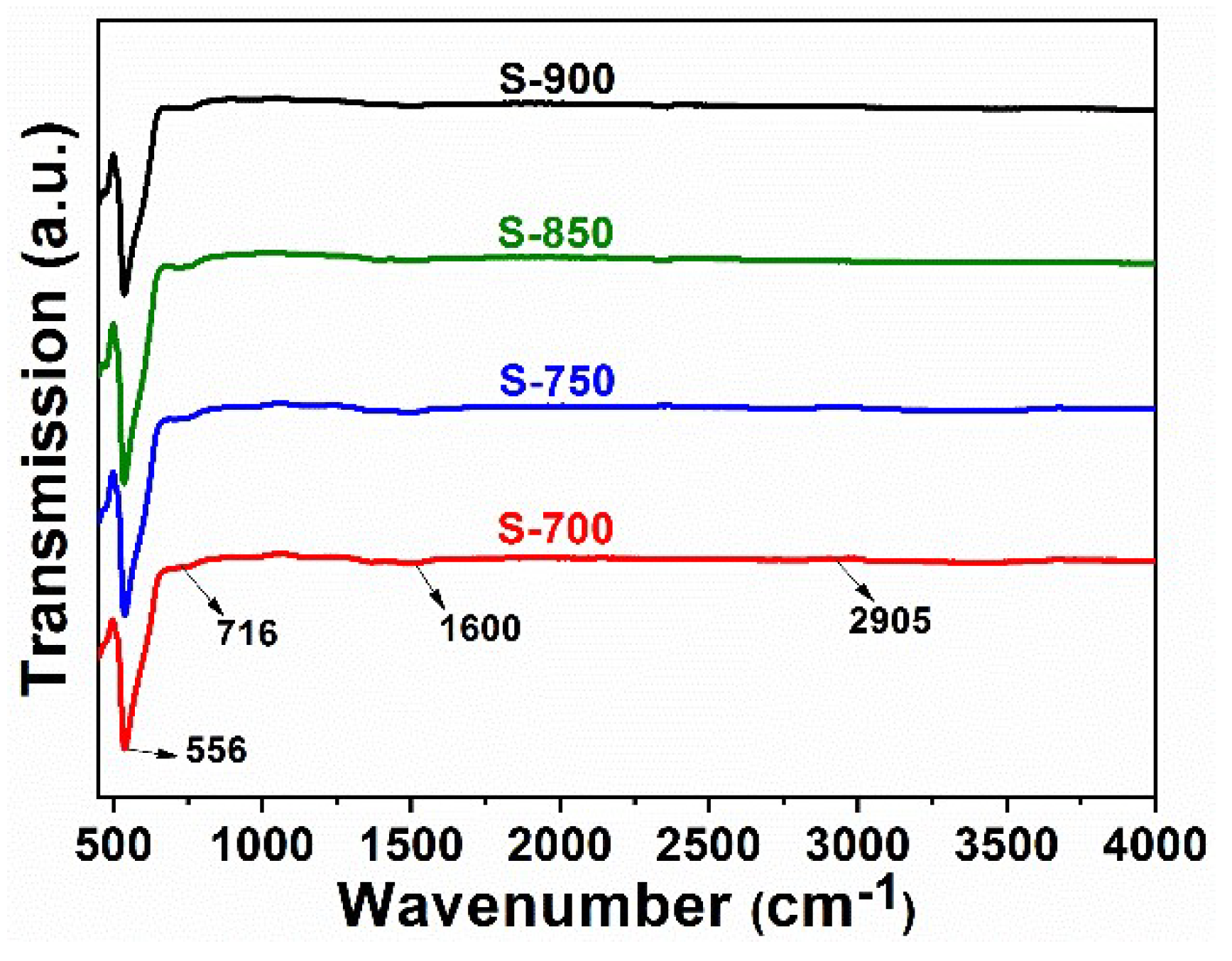
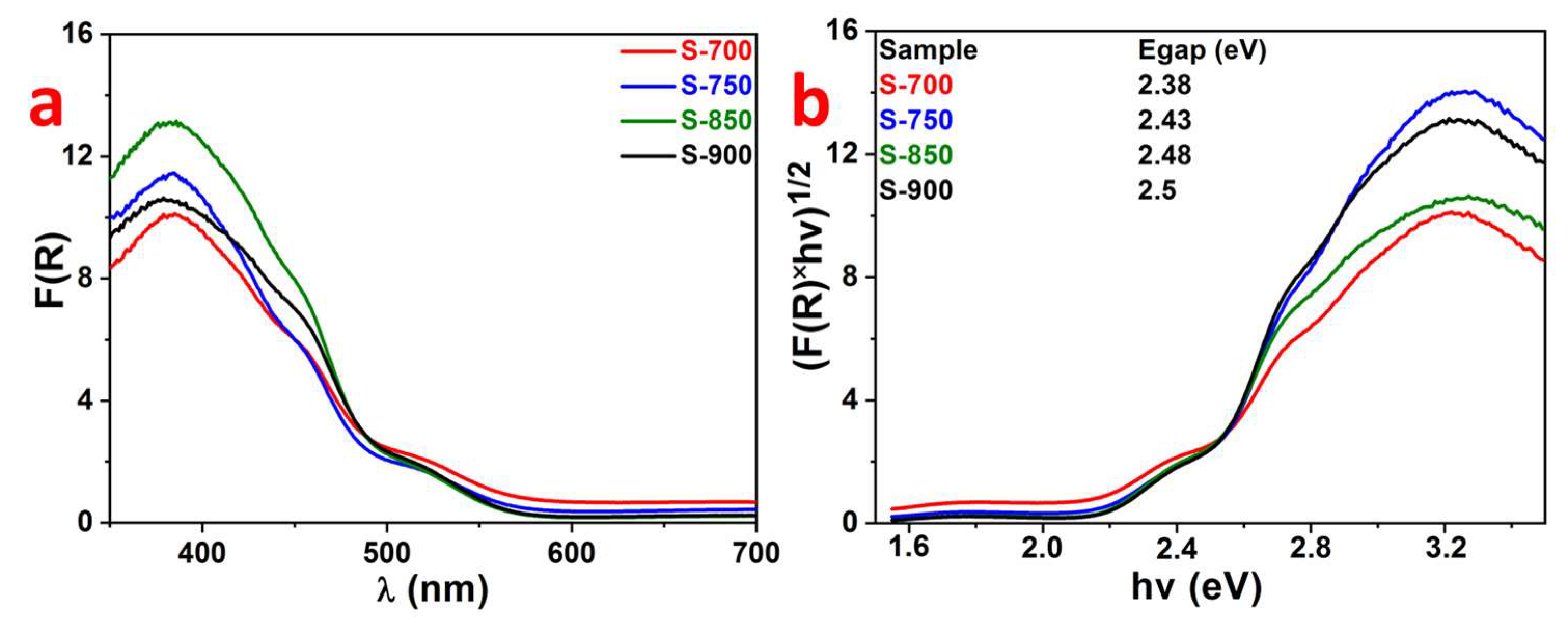
2.1. Structural and Optical Characterization of LaFeO3
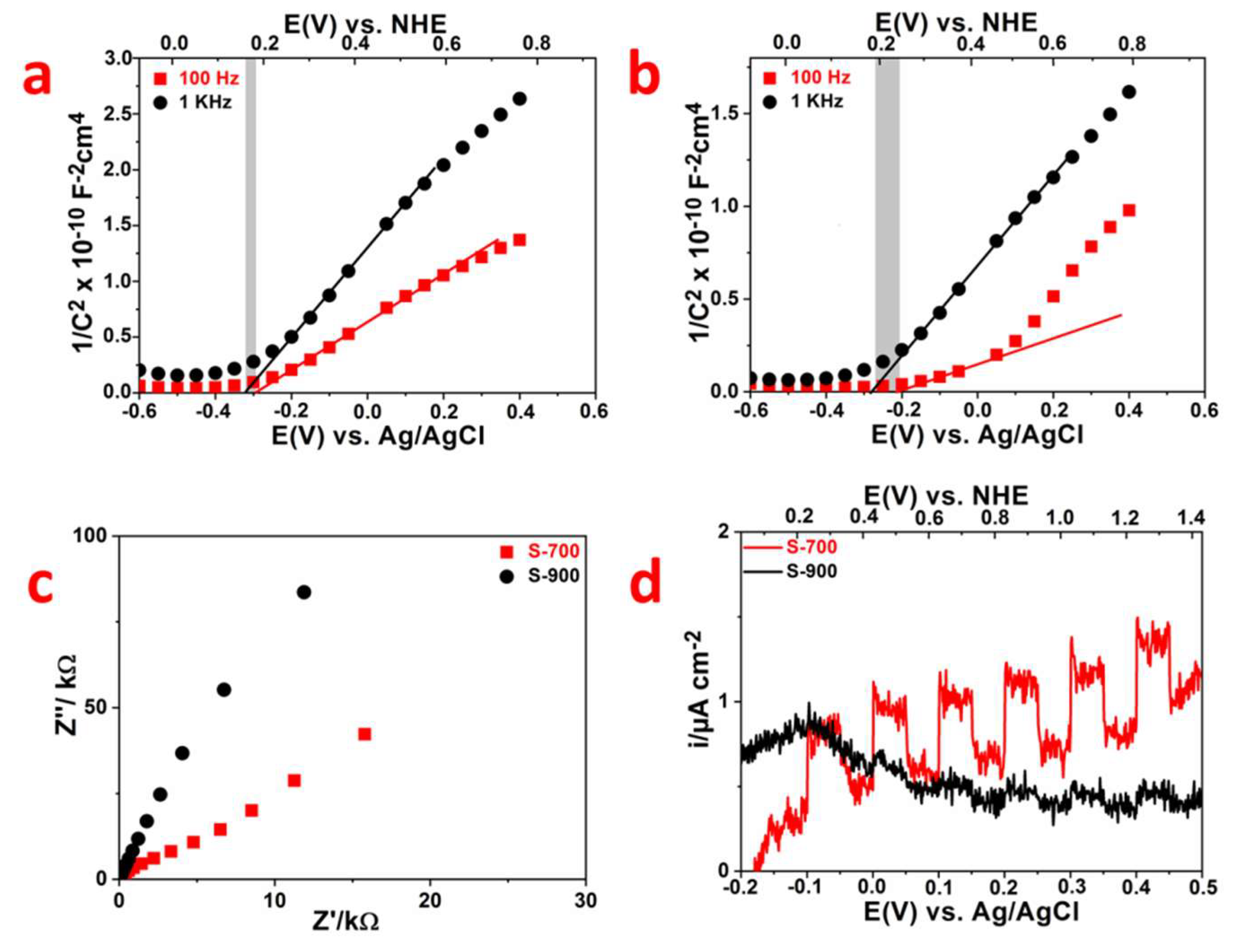
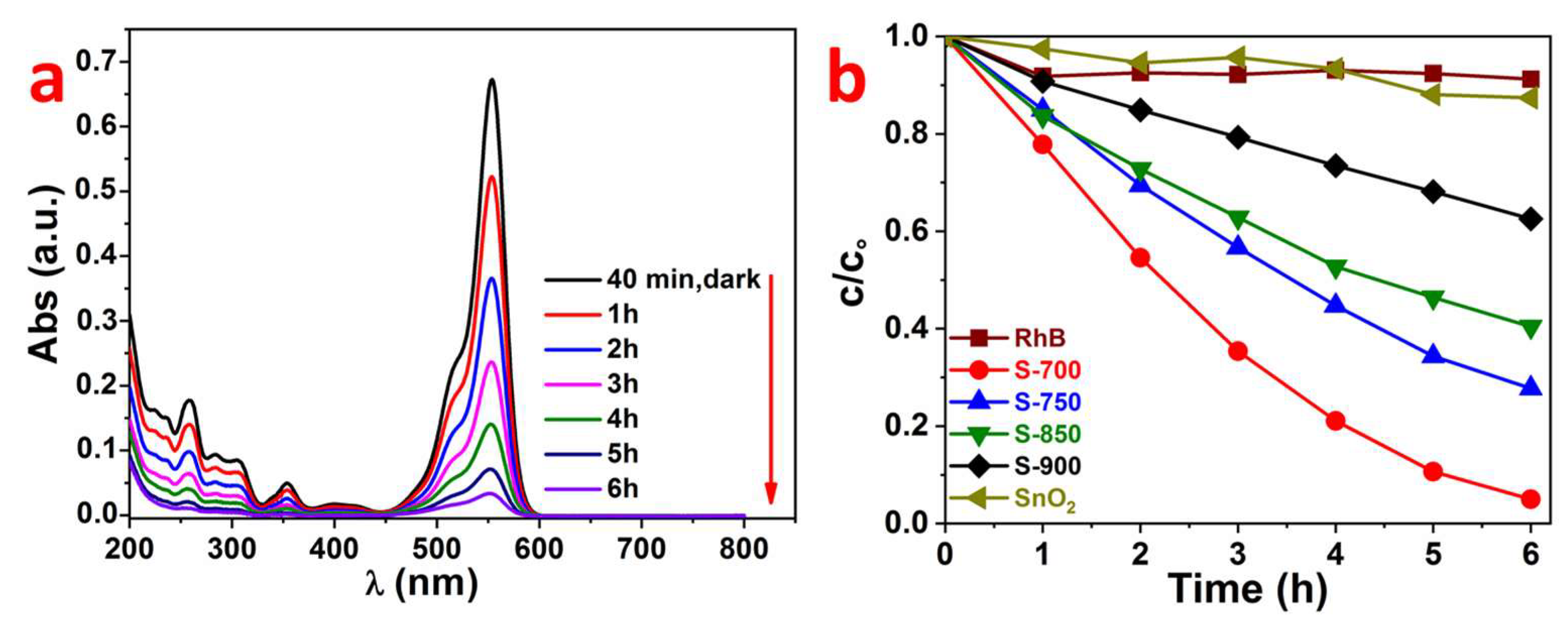
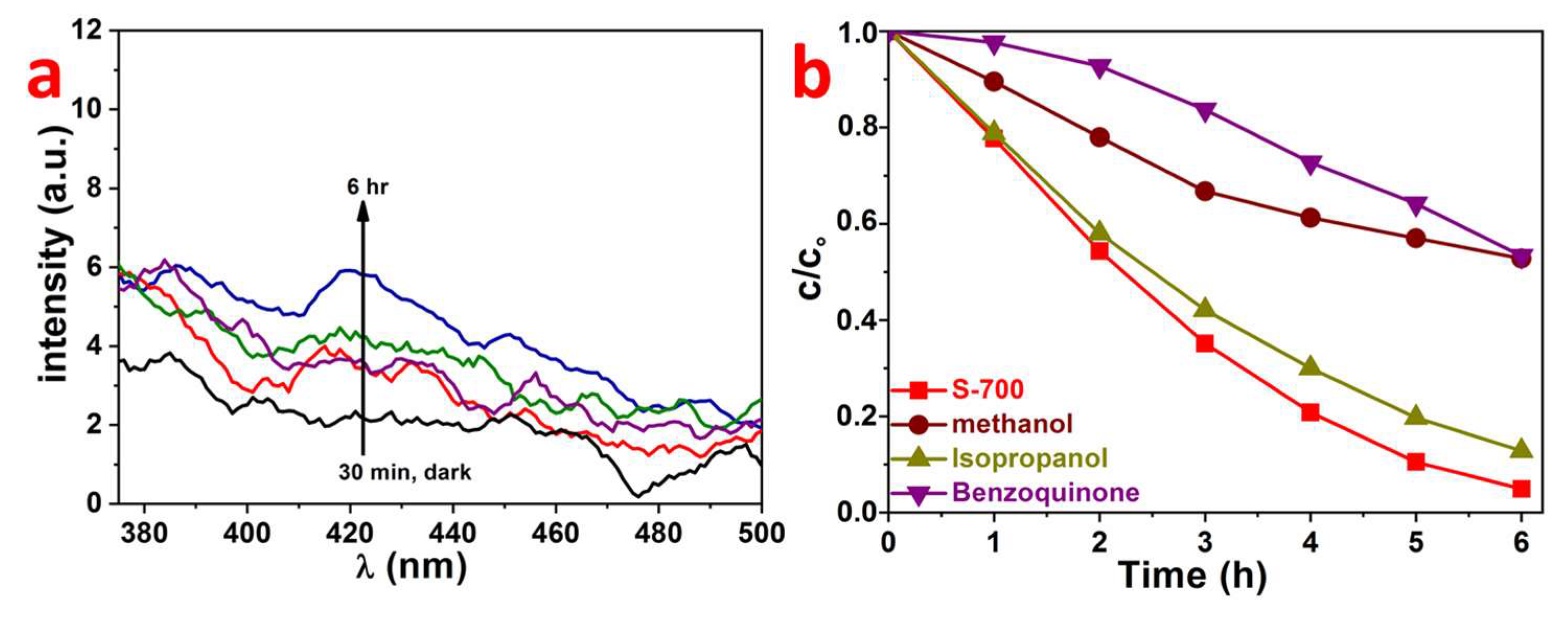
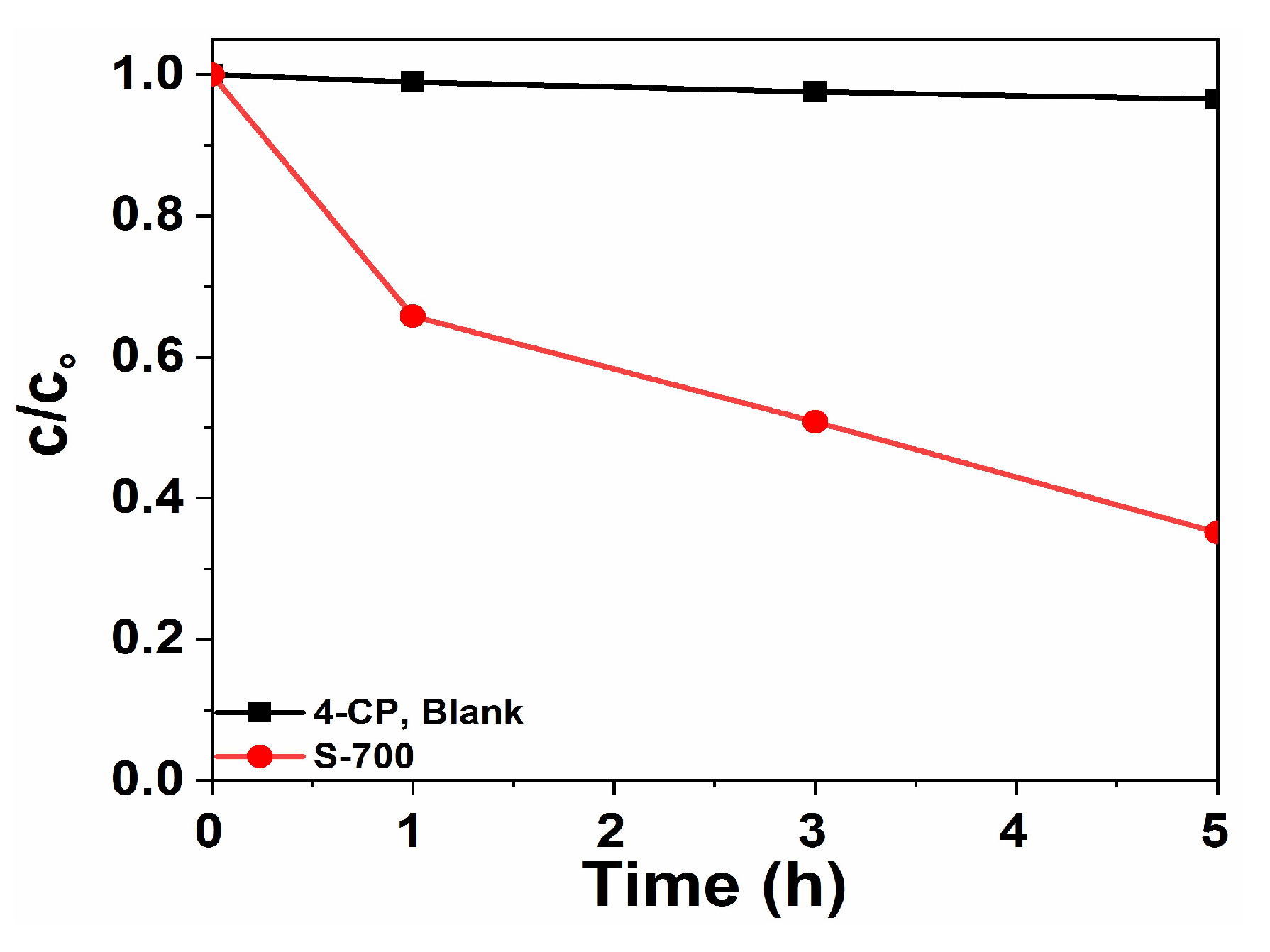
2.2. Photocatalytic Properties
3. Experimental
3.1. Materials
3.2. Synthesis of LaFeO3 by the Citric Acid Assisted Sol-gel Method
3.3. Characterization
3.4. Photocatalytic Degradation Activity and Hydrogen Evolution Measurements
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.M.; Martin, T.S.; Choi, W.; Bahnemann, W.D. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Hu, C.; Hu, X.; Guo, J.; Qu, J. Efficient Destruction of Pathogenic Bacteria with NiO/SrBi2O4 under Visible Light Irradiation. Environ. Sci. Technol. 2006, 40, 5508–5513. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.Y.; Lightkap, V.I.; Goodwin, K.; Matsumura, M.; Kamat, V.P. To What Extent Do Graphene Scaffolds Improve the Photovoltaic and Photocatalytic Response of TiO2 Nanostructured Films? J. Phys. Chem. Lett. 2010, 1, 2222–2227. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight Photocatalysis by Carbon-Modified Titanium Dioxide. Angew. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Zhang, J.; Tian, B.; Chen, F.; He, D. Preparation of Fe3+ doped TiO2 catalysts by controlled hydrolysis of titanium alkoxide and study on their photocatalytic activity for methyl orange degradation. J. Hazard. Mater. 2008, 155, 572–579. [Google Scholar] [CrossRef]
- Xu, J.J.; Ao, H.Y.; Fu, G.D.; Yuan, W.S. Synthesis of Gd-doped TiO2 nanoparticles under mild condition and their photocatalytic activity. Colloid Surf. A 2009, 334, 107–111. [Google Scholar] [CrossRef]
- Dodd, A.; Mckinley, A.; Tsuzuki, T.; Sauners, M. Optical and photocatalytic properties of nanoparticulate (TiO2)x (ZnO)1−x powders. J. Alloys Compd. 2010, 489, L17–L21. [Google Scholar] [CrossRef]
- Dai, K.; Peng, Y.T.; Ke, N.D.; Wei, Q.B. Photocatalytic hydrogen generation using a nanocomposite of multi-walled carbon nanotubes and TiO2 nanoparticles under visible light irradiation. Nanotechnology 2009, 20, 125603. [Google Scholar] [CrossRef]
- Chai, B.; Peng, Y.T.; Zeng, P.; Mao, J. Synthesis of floriated In2S3 decorated with TiO2 nanoparticles for efficient photocatalytic hydrogen production under visible light. J. Mater. Chem. 2011, 21, 14587–14593. [Google Scholar] [CrossRef]
- Laokiat, L.; Khemthong, P.; Grisdanurak, N.; Sreearunothai, P.; Pattanasiriwisawa, W.; Klysubun, W. Photocatalytic degradation of benzene, toluene, ethylbenzene, and xylene (BTEX) using transition metal-doped titanium dioxide immobilized on fiberglass cloth. Korean J. Chem. Eng. 2012, 29, 377–383. [Google Scholar] [CrossRef]
- Taffa, H.D.; Dillert, R.; Ulpe, C.A.; Bauerfeind, L.C.K.; Bredow, T.; Bahnemann, W.D.; Wark, M. Photoelectrochemical and theoretical investigations of spinel type ferrites (MxFe3-xO4) for water splitting: A mini-review. J. Photon. Energy 2016, 7, 12009. [Google Scholar] [CrossRef]
- Su, H.M.; He, C.; Sharma, K.V.; Abou Asi, M.; Xia, D.; Li, Z.X.; Deng, Q.H.; Xiong, Y. Mesoporous zinc ferrite: Synthesis, characterization, and photocatalytic activity with H2O2/visible light. J. Hazard. Mater. 2012, 211–212, 95–103. [Google Scholar] [CrossRef]
- Cao, W.S.; Zhu, J.Y.; Cheng, F.G.; Huang, H.Y. ZnFe2O4 nanoparticles: Microwave-hydrothermal ionic liquid synthesis and photocatalytic property over phenol. J. Hazard. Mater. 2009, 171, 431–435. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, K.V.; Li, Z.X. Synthesis and photocatalytic activity of ferrites under visible light. A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Nakanishi, T.; Masuda, Y.; Koumoto, K. Site-Selective Deposition of Magnetite Particulate Thin Films on Patterned Self-assembled Monolayers. Chem. Mater. 2004, 16, 3484–3488. [Google Scholar] [CrossRef]
- Juan, X.W.; Yun, H.S.; Yan, H.T.; Hua, Q.Y. Photocatalytic Degradation of Water-Soluble Azo Dyes by LaFeO3 and YFeO3. Adv. Mater. Res. 2012, 465, 37–43. [Google Scholar]
- Hou, L.; Sun, G.; Liu, K.; Li, Y.; Gao, F. Preparation, characterization and investigation of catalytic activity of Li-doped LaFeO3 nanoparticles. J. Sol-Gel Sci. Technol. 2006, 40, 9–14. [Google Scholar] [CrossRef]
- Tang, P.; Fu, M.; Chen, H.; Cao, F. Synthesis of Nanocrystalline LaFeO3 by Precipitation and its Visible-Light Photocatalytic Activity. Mater. Sci. Forum 2011, 694, 150–154. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Masteralo, R.V.; Ponpandian, N. Photocatalytic degradation of organic dyes under visible light irradiation by floral-like LaFeO3 nanostructures comprised of nanosheet petals. New J. Chem. 2014, 38, 5480–5490. [Google Scholar] [CrossRef]
- Su, H.; Jing, L.; Shi, K.; Yao, C.; Fu, H. Synthesis of large surface area LaFeO3 nanoparticles by SBA-16 template method as high active visible photocatalysts. J. Nanopart. Res. 2010, 12, 967–974. [Google Scholar] [CrossRef]
- Yang, J.; Zhong, H.; Li, M.; Zhang, L.; Zhang, Y. Markedly enhancing the visible-light photocatalytic activity of LaFeO3 by post-treatment in molten salt. React. Kinet. Catal. Lett. 2009, 97, 269–274. [Google Scholar] [CrossRef]
- Tijare, N.S.; Joshi, V.M.; Padole, S.P.; Manguklar, A.P.; Rayalu, S.S.; Labhsetwar, K.N. Photocatalytic hydrogen generation through water splitting on nano-crystalline LaFeO3 perovskite. Int. J. Hydrog. Energy 2012, 37, 10451–10456. [Google Scholar] [CrossRef]
- Wu, H.; Hu, R.; Zhou, T.; Li, C.; Meng, W.; Yang, J. A novel efficient boron-doped LaFeO3 photocatalyst with large specific surface area for phenol degradation under simulated sunlight. CrystEngComm 2015, 17, 3859–3865. [Google Scholar] [CrossRef]
- Ju, L.; Chen, Z.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Sol-Gel Synthesis and Photo-Fenton-Like Catalytic Activity of EuFeO3 Nanoparticles. J. Am. Ceram. Soc. 2011, 94, 3418–3424. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Ganesh, I.; Mangalaraj, D.; Viswanathan, C.; Balamurugan, A. Controlled synthesis of perovskite LaFeO3 microsphere composed of nanoparticles via self-assembly process and their associated photocatalytic activity. Chem. Eng. J. 2012, 209, 420–428. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, L. Porous structure dependent photoreactivity of graphitic carbon nitride under visible light. J. Mater. Chem. 2012, 22, 1160–1166. [Google Scholar] [CrossRef]
- Li, K.; Wang, D.; Wu, F.; Xie, T.; Li, T. Surface electronic states and photovoltage gas-sensitive characters of nanocrystalline LaFeO3. Mater. Chem. Phys. 2000, 64, 269–272. [Google Scholar] [CrossRef]
- Marschall, R.; Soldat, J.; Wark, M. Enhanced photocatalytic hydrogen generation from barium tantalate composites. Photochem. Photobiol. Sci. 2013, 12, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhou, J.; Yue, Z.; Gui, Z.; Li, L. Auto-combustion synthesis of nanocrystalline LaFeO3. Mater. Chem. Phys. 2002, 78, 25–29. [Google Scholar] [CrossRef]
- Rida, K.; Benabbas, A.; Bouremmad, F.; Pena, A.M.; Sastre, E.; Martinez, A. Effect of calcination temperature on the structural characteristics and catalytic activity for propene combustion of sol-gel derived lanthanum chromite perovskite. Appl. Catal. A 2007, 327, 173–179. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Hebalkar, Y.N.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Shape evolution of perovskite LaFeO3 nanostructures: A systematic investigation of growth mechanism, properties and morphology dependent photocatalytic activity. RSC Adv. 2013, 3, 7549–7561. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Feng, Z.; Chen, J.; Li, C. UV Raman spectroscopic study on TiO2. I. Phase transformation at the surface and in the bulk. J. Phys. Chem. B 2006, 110, 927–935. [Google Scholar] [CrossRef]
- Wu, W.; Liang, S.; Shen, L.; Ding, Z.; Zheng, H.; Su, W.; Wu, L. Preparation, characterization and enhanced visible light photocatalytic activities of polyaniline/Bi3NbO7 nanocomposites. J. Alloys Compd. 2012, 520, 213–219. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Li, G.; Cao, Y.; Shao, Y.; Li, D. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B Environ. 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Ismael, M.; Elhaddad, E.; Taffa, H.D.; Wark, M. Synthesis of Phase Pure Hexagonal YFeO3 Perovskite as Efficient Visible Light Active Photocatalyst. Catalysts 2017, 7, 326. [Google Scholar] [CrossRef]
- Parida, M.K.; Reddy, H.K.; Martha, S.; Das, P.D.; Biswal, N. Fabrication of nanocrystalline LaFeO3: An efficient sol-gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int. J. Hydrog. Energy 2010, 35, 12161–12168. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D. Enhanced Photocatalytic Hydrogen Production from Glucose on Rh-Doped LaFeO3. Chem. Eng. Trans. 2017, 60, 235–240. [Google Scholar]
- Xu, K.; Feng, J. Superior photocatalytic performance of LaFeO3/gC3N4 heterojunction nanocomposites under visible light irradiation. RSC Adv. 2017, 7, 45369–45376. [Google Scholar] [CrossRef]
- Yang, H.; Yan, J.; Lu, Z.; Cheng, X.; Tang, Y. Photocatalytic activity evaluation of tetragonal CuFe2O4 nanoparticles for the H2 evolution under visible light irradiation. J. Alloys Compd. 2009, 476, 715–719. [Google Scholar] [CrossRef]
- Rekhila, G.; Bessekhouad, Y.; Trari, M. Visible light hydrogen production on the novel ferrite NiFe2O4. Int. J. Hydrog. Energy 2013, 38, 6335–6343. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Yang, S.; Wu, H.; Wu, Y.; Wu, Y.; Pan, J.; Xiong, X. In situ construction of a SnO2/g-C3N4 heterojunction for enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 68953–68963. [Google Scholar] [CrossRef]
- He, M.Y.; Cai, J.; Zhang, H.L.; Wang, X.X.; Lin, J.H.; Teng, T.B.; Zhao, H.L.; Weng, Z.W.; Wan, L.H.; Fan, M. Comparing Two New Composite Photocatalysts, t-LaVO4/g-C3N4 and m-LaVO4/g-C3N4, for Their Structures and Performances. Ind. Eng. Chem. Res. 2014, 53, 5905–5915. [Google Scholar] [CrossRef]
- Yu, T.H.; Quan, X.; Chen, S.; Zhao, M.H.; Zhang, B.Y. TiO2–carbon nanotube heterojunction arrays with a controllable thickness of TiO2 layer and their first application in photocatalysis. J. Photochem. Photobiol. A Chem. 2008, 200, 301–306. [Google Scholar] [CrossRef]
- Lim, J.; Monllor-Satocaa, D.; Jang, S.J.; Lee, S.; Choi, W. Visible light photocatalysis of fullerol-complexed TiO2 enhanced by Nb doping. Appl. Catal. B Environ. 2014, 152–153, 233–240. [Google Scholar] [CrossRef]
- Bi, P.Y.; Quyang, X.S.; Cao, Y.J.; Ye, H.J. Facile synthesis of rhombic dodecahedral AgX/Ag3PO4 (X = Cl, Br, I) heterocrystals with enhanced photocatalytic properties and stabilities. Phys. Chem. Chem. Phys. 2011, 13, 10071–10075. [Google Scholar] [CrossRef]
- Hong, S.; Lee, S.; Jang, S.J.; Lee, S.J. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef]
- Li, X.; Ye, J. Photocatalytic Degradation of Rhodamine B over Pb3Nb4O13/Fumed SiO2 Composite under Visible Light Irradiation. J. Phys. Chem. C 2007, 111, 13109–13116. [Google Scholar] [CrossRef]
- Merka, O.; Yarovyi, V.; Bahnemann, D.W.; Wark, M. pH-Control of the Photocatalytic Degradation Mechanism of Rhodamine B over Pb3Nb4O13. J. Phys. Chem. C 2011, 115, 8014–8023. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Lin, H.; Nong, Q.; Wu, Y.; Wu, T.; He, Y. Synthesis and characterization of ZrO2/g-C3N4 composite with enhanced visible-light photoactivity for rhodamine degradation. RSC Adv. 2014, 4, 40029–40035. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z- what we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, C.; Chen, S. Chem. Facile synthesis of g-C3N4/ZnO composite with enhanced visible light photooxidation and photoreduction properties. Chem. Eng. J. 2012, 209, 386–393. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Cheng, B. Effect of Crystallization Methods on Morphology and Photocatalytic Activity of Anodized TiO2 Nanotube Array Films. J. Phys. Chem. C 2010, 114, 19378–19385. [Google Scholar] [CrossRef]
- He, M.Y.; Cai, J.; Li, T.T.; Wu, Y.; Lin, J.H.; Zhao, H.L.; Luo, F.M. Efficient degradation of RhB over GdVO4/g-C3N4 composites under visible-light irradiation. Chem. Eng. J. 2013, 215–216, 721–730. [Google Scholar] [CrossRef]
- Yang, X.; Cui, H.; Li, Y.; Qin, J.; Zhang, R.; Tang, H. Fabrication of Ag3PO4-Graphene Composites with Highly Efficient and Stable Visible Light Photocatalytic Performance. ACS Catal. 2013, 3, 363–369. [Google Scholar] [CrossRef]
- Wang, F.D.; Kako, T.; Ye, H.J. Efficient Photocatalytic Decomposition of Acetaldehyde over a Solid-Solution Perovskite (Ag0.75Sr0.25)(Nb0.75Ti0.25)O3 under Visible-Light Irradiation. J. Am. Chem. Soc. 2008, 130, 2724–2725. [Google Scholar] [CrossRef]
- Kormali, P.; Triantis, T.; Dimotikali, D.; Hiskia, A.; Papaconstantinou, E. On the photooxidative behavior of TiO2 and PW12O403−: OH radicals versus holes. Appl. Catal. B 2006, 68, 139–146. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Zhang, D.-W. A Simple Strategy for the preparation of g-C3N4/SnO2 Nanocomposite Photocatalysts. Sci. Adv. Mater. 2014, 6, 1091–1098. [Google Scholar] [CrossRef]
- Pirzada, M.B.; Kunchala, K.R.P.; Naidu, S.B. Synthesis of LaFeO3/Ag2CO3 Nanocomposites for Photocatalytic Degradation of Rhodamine B and p-Chlorophenol under Natural Sunlight. ACS Omega 2019, 4, 2618–2629. [Google Scholar] [CrossRef]
- Hu, R.; Li, C.; Wang, X.; Sun, Y.; Jia, H.; Su, H.; Zhang, Y. Photocatalytic activities of LaFeO3 and La2FeTiO6 in p-chlorophenol degradation under visible light. Catal. Commun. 2012, 29, 35–39. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismael, M.; Wark, M. Perovskite-type LaFeO3: Photoelectrochemical Properties and Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation. Catalysts 2019, 9, 342. https://doi.org/10.3390/catal9040342
Ismael M, Wark M. Perovskite-type LaFeO3: Photoelectrochemical Properties and Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation. Catalysts. 2019; 9(4):342. https://doi.org/10.3390/catal9040342
Chicago/Turabian StyleIsmael, Mohammed, and Michael Wark. 2019. "Perovskite-type LaFeO3: Photoelectrochemical Properties and Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation" Catalysts 9, no. 4: 342. https://doi.org/10.3390/catal9040342
APA StyleIsmael, M., & Wark, M. (2019). Perovskite-type LaFeO3: Photoelectrochemical Properties and Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation. Catalysts, 9(4), 342. https://doi.org/10.3390/catal9040342