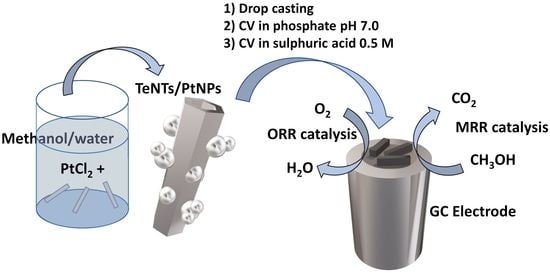
Synthesis and Characterization of Te Nanotubes Decorated with Pt Nanoparticles for a Fuel Cell Anode/Cathode Working at a Neutral pH
Abstract
:1. Introduction
2. Results
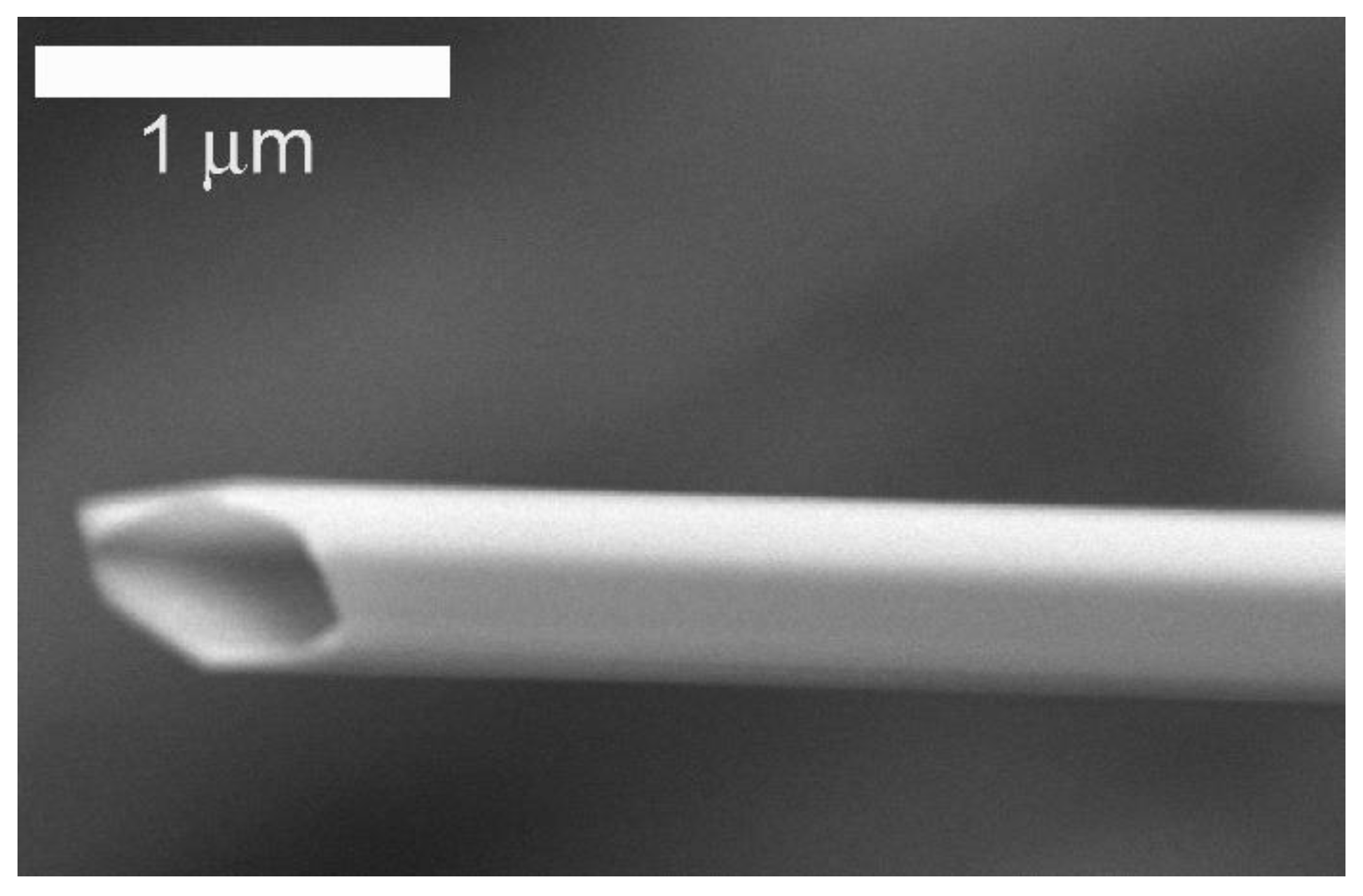
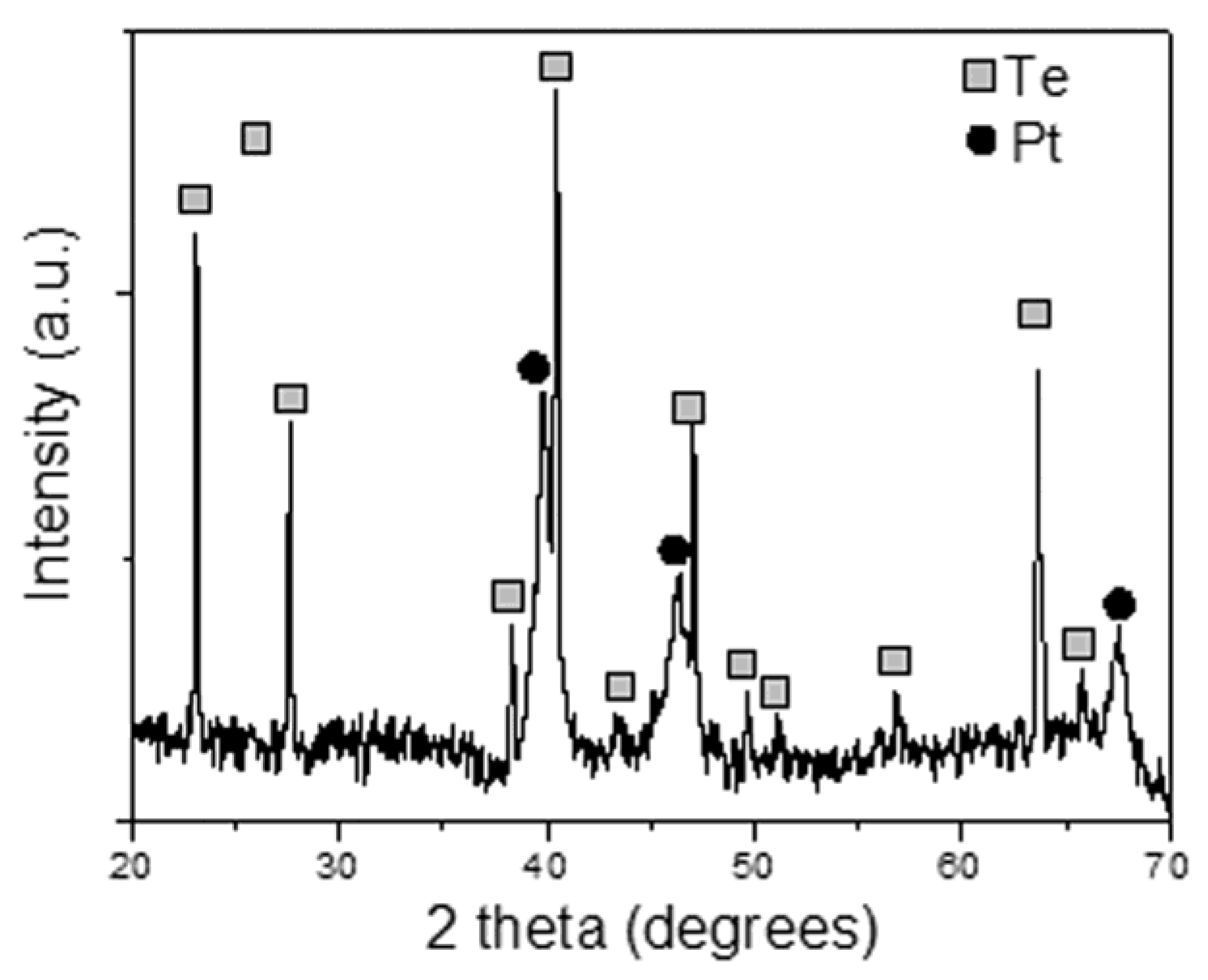
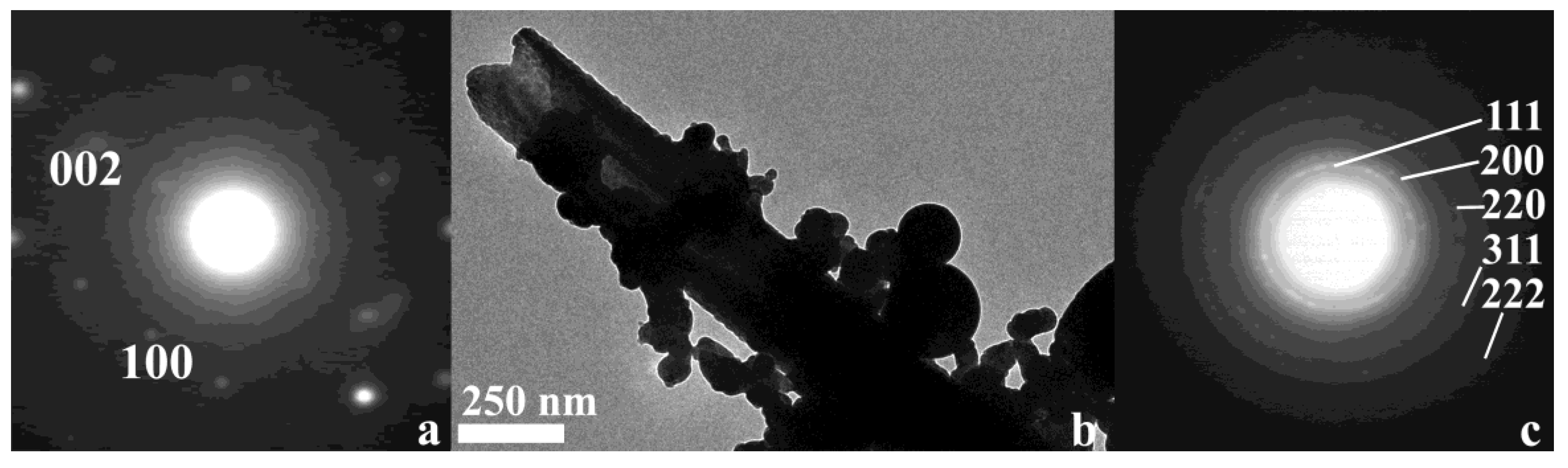
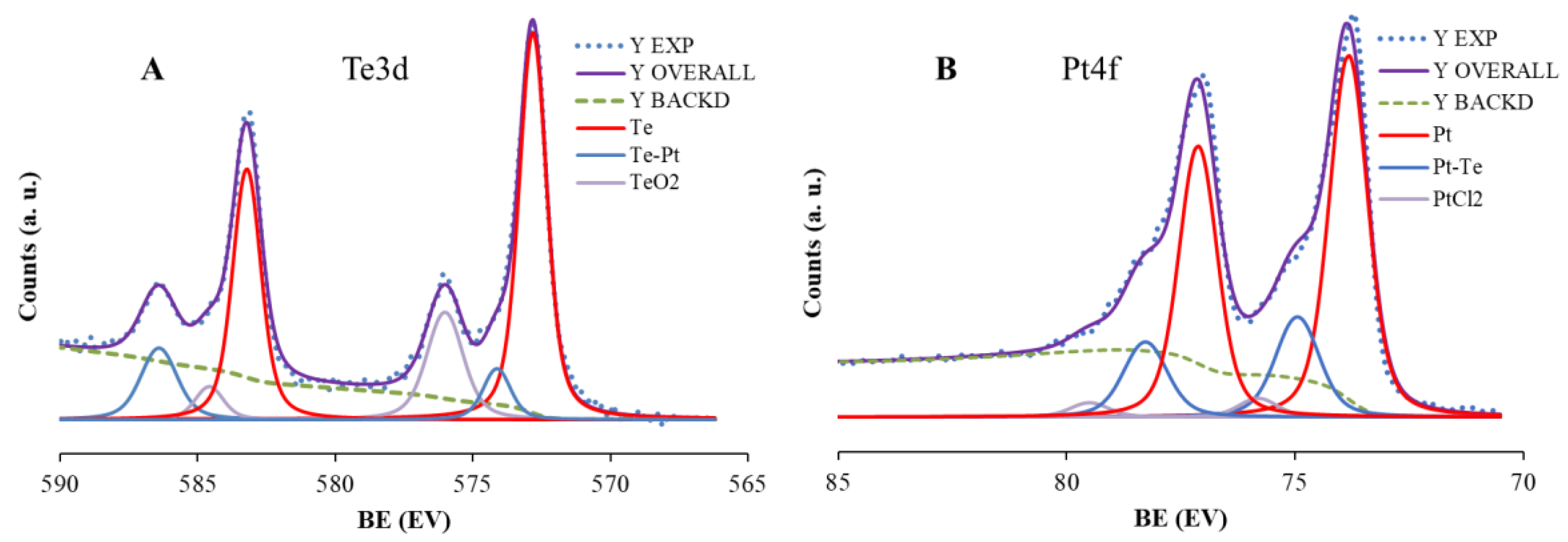
2.1. Morphological and Spectroscopic Characterization
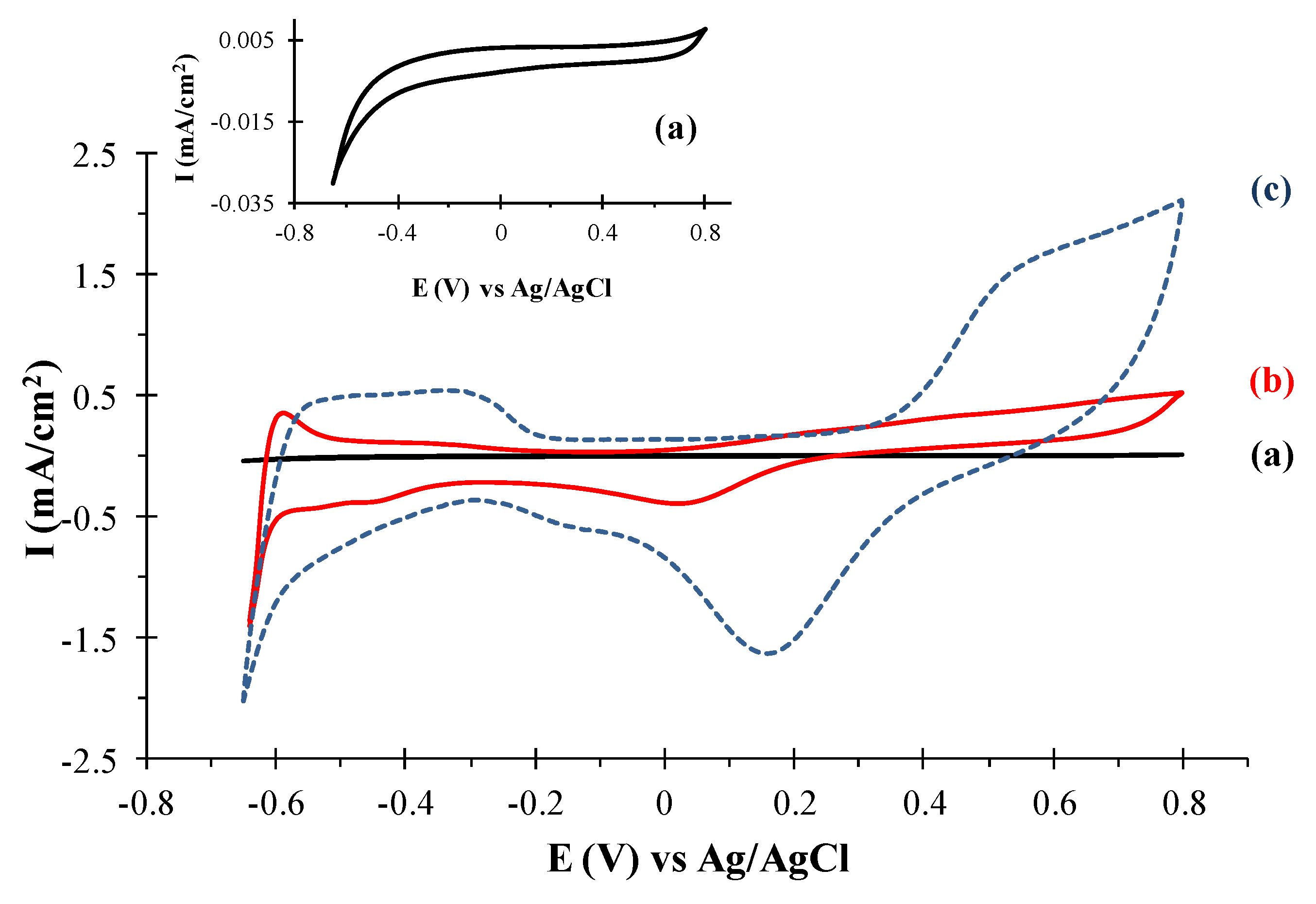
2.2. Electrochemical Characterization of the TeNT/PtNP Materials onto the GC Electrodes
2.3. Electrochemical Characterization of TeNT/PtNP/GC Modified electrodes with Respect to Methanol Oxidation
2.4. Electrochemical Characterization of TeNT/PtNP/GC Modified electrodes with Respect to Oxygen Reduction
3. Materials and Methods
3.1. Chemicals
3.2. Apparatus and Methods
3.3. Synthesis of Tellurium Nanotubes
3.4. Synthesis of Te Nanotubes Decorated with Pt Nanoparticles (TeNTs/PtNPs)
3.5. Preparation of the TeNTs/PtNPs/GC Modified Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rao, C.N.R.; Kulkarni, G.U.; Thomas, P.J.; Edwards, P.P. Size-dependent chemistry: Properties of nanocrystals. Chem. A Eur. J. 2002, 8, 28–35. [Google Scholar] [CrossRef]
- Patzke, G.R.; Krumeich, F.; Nesper, R. Oxidic nanotubes and nanorods—Anisotropic modules for a future nanotechnology. Angew. Chem. Int. Ed. 2002, 41, 2446–2461. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Luo, X.L.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Sakamoto, M.; Takamura, K. Catalytic-oxidation of biological components on platinum-electrodes modified by adsorbed metals—Anodic-oxidation of glucose. Bioelectroch. Bioenerg. 1982, 9, 571–582. [Google Scholar] [CrossRef]
- Chen, X.L.; Pan, H.B.; Liu, H.F.; Du, M. Nonenzymatic glucose sensor based on flower-shaped au@pd core-shell nanoparticles-ionic liquids composite film modified glassy carbon electrodes. Electrochim Acta 2010, 56, 636–643. [Google Scholar] [CrossRef]
- Toshima, N.; Yonezawa, T. Bimetallic nanoparticles—Novel materials for chemical and physical applications. New J. Chem. 1998, 22, 1179–1201. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, A.Q.; Chi, Y.S.; Lin, H.P.; Mou, C.Y. Synergistic effect in an au-ag alloy nanocatalyst: Co oxidation. J. Phys. Chem. B 2005, 109, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, K.Y.; Jeong, G.H.; Jang, J.K.; Han, S.W. Fabrication of au-ag alloy nanoprisms with enhanced catalytic activity. Chem. Lett. 2007, 36, 1350–1351. [Google Scholar] [CrossRef]
- Booth, M.A.; Leveneur, J.; Costa, A.S.; Kennedy, J.; Travas-Sejdic, J. Tailoring the conductivity of polypyrrole films using low-energy platinum ion implantation. J. Phys. Chem. C 2012, 116, 8236–8242. [Google Scholar] [CrossRef]
- Jin, G.P.; Li, J.; Peng, X. Preparation of platinum nanoparticles on polyaniline-coat multi-walled carbon nanotubes for adsorptive stripping voltammetric determination of formaldehyde in aqueous solution. J. Appl. Electrochem. 2009, 39, 1889–1895. [Google Scholar] [CrossRef]
- Shi, L.; Liang, R.P.; Qiu, J.D. Controllable deposition of platinum nanoparticles on polyaniline-functionalized carbon nanotubes. J. Mater. Chem. 2012, 22, 17196–17203. [Google Scholar] [CrossRef]
- Xia, B.Y.; Wu, H.B.; Wang, X.; Lou, X.W. One-pot synthesis of cubic ptcu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction. J. Am. Chem. Soc. 2012, 134, 13934–13937. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.C.; Liu, J.G.; Zhou, Y.; Liu, W.M.; Gao, J.A.; Xie, Y.; Yin, Y.; Zou, Z.G. Preparation and characterization of pt supported on graphene with enhanced electrocatalytic activity in fuel cell. J. Power Sources 2011, 196, 1012–1018. [Google Scholar] [CrossRef]
- Singh, R.N.; Awasthi, R.; Sharma, C.S. Review: An overview of recent development of platinum-based cathode materials for direct methanol fuel cells. Int. J. Electrochem. Sci. 2014, 9, 5607–5639. [Google Scholar]
- Yang, L.; Ge, J.J.; Liu, C.P.; Wang, G.L.; Xing, W. Approaches to improve the performance of anode methanol oxidation reaction-a short review. Curr. Opin. Electrochem. 2017, 4, 83–88. [Google Scholar] [CrossRef]
- Jeon, T.Y.; Lee, K.S.; Yoo, S.J.; Cho, Y.H.; Kang, S.H.; Sung, Y.E. Effect of surface segregation on the methanol oxidation reaction in carbon-supported pt-ru alloy nanoparticles. Langmuir 2010, 26, 9123–9129. [Google Scholar] [CrossRef]
- He, C.; Desai, S.; Brown, G.; Bollepalli, S. PEM Fuel Cell Catalysts: Cost, Performance, and Durability; The Electrochemical Society Interface: Pennington, NJ, USA, 2005; pp. 41–44. [Google Scholar]
- Ding, Y.M.; Liu, Y.L.; Rao, G.S.; Wang, G.F.; Zhong, Q.L.; Ren, B.; Tian, Z.Q. Electrooxidation mechanism of methanol at pt-ru catalyst modified gc electrode in electrolytes with different ph using electrochemical and sers techniques. Chin. J. Chem. 2007, 25, 1617–1621. [Google Scholar] [CrossRef]
- Yan, B.; Concannon, N.M.; Milshtein, J.D.; Brushett, F.R.; Surendranath, Y. A membrane-free neutral ph formate fuel cell enabled by a selective nickel sulfide oxygen reduction catalyst. Angew. Chem. Int. Ed. 2017, 56, 7496–7499. [Google Scholar] [CrossRef]
- Tominaka, S.; Nishizeko, H.; Ohta, S.; Osaka, T. On-chip fuel cells for safe and high-power operation: Investigation of alcohol fuel solutions. Energy Environ. Sci. 2009, 2, 849–852. [Google Scholar] [CrossRef]
- Lehmann, K.; Yurchenko, O.; Urban, G. Carbon nanowalls for oxygen reduction reaction in bio fuel cells. J. Phys. Conf. Ser. 2014, 557, 012008. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Huang, M.H.; Wang, F.; Li, L.R.; Guo, Y.L. A novel binary pt3tex/c nanocatalyst for ethanol electro-oxidation. J. Power Sources 2008, 178, 48–52. [Google Scholar] [CrossRef]
- Guascito, M.R.; Chirizzi, D.; Malitesta, C.; Siciliano, M.; Siciliano, T.; Tepore, A. Amperometric non-enzymatic bimetallic glucose sensor based on platinum tellurium microtubes modified electrode. Electrochem. Commun. 2012, 22, 45–48. [Google Scholar] [CrossRef]
- Guascito, M.R.; Chirizzi, D.; Malitesta, C.; Siciliano, T.; Tepore, A. Te oxide nanowires as advanced materials for amperometric nonenzymatic hydrogen peroxide sensing. Talanta 2013, 115, 863–869. [Google Scholar] [CrossRef]
- Siciliano, T.; Filippo, E.; Genga, A.; Micocci, G.; Siciliano, M.; Tepore, A. Single-crystalline te microtubes: Synthesis and no(2) gas sensor application. Sens. Actuators B Chem. 2009, 142, 185–190. [Google Scholar] [CrossRef]
- Guascito, M.R.; Chirizzi, D.; Malitesta, C.; Mazzotta, E.; Siciliano, M.; Siciliano, T.; Tepore, A.; Turco, A. Low-potential sensitive H2O2 detection based on composite micro tubular te adsorbed on platinum electrode. Biosens. Bioelectron. 2011, 26, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Nist X-Ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/Default.aspx (accessed on 16 January 2019).
- Mayers, B.; Jiang, X.C.; Sunderland, D.; Cattle, B.; Xia, Y.N. Hollow nanostructures of platinum with controllable dimensions can be synthesized by templating against selenium nanowires and colloids. J. Am. Chem. Soc. 2003, 125, 13364–13365. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Chen, X.M.; Tian, X.T.; Zhao, L.M.; Huang, Z.Y.; Oyama, M. Nonenzymatic sensing of glucose at neutral ph values using a glassy carbon electrode modified with graphene nanosheets and pt-pd bimetallic nanocubes. Microchim. Acta 2014, 181, 783–789. [Google Scholar] [CrossRef]
- Ernst, S.; Heitbaum, J.; Hamann, C.H. The electrooxidation of glucose in phosphate buffer solutions: Part i. Reactivity and kinetics below 350 mv/rhe. J. Electroanal. Chem. Interfacial Electrochem. 1979, 100, 173–183. [Google Scholar] [CrossRef]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef]
- Kadirgan, F.; Beden, B.; Leger, J.M.; Lamy, C. Synergistic effect in the electrocatalytic oxidation of methanol on platinum+palladium alloy electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1981, 125, 89–103. [Google Scholar] [CrossRef]
- Filippo, E.; Baldassarre, F.; Tepore, M.; Guascito, M.R.; Chirizzi, D.; Tepore, A. Characterization of hierarchical alpha-moo3 plates toward resistive heating synthesis: Electrochemical activity of alpha-moo3/pt modified electrode toward methanol oxidation at neutral ph. Nanotechnology 2017, 28, 215601. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Schroder, U.; Scholz, F. Investigation of the electrocatalytic oxidation of formate and ethanol at platinum black under microbial fuel cell conditions. J. Solid State Electr. 2006, 10, 872–878. [Google Scholar] [CrossRef]
- Sadhukhan, M.; Kundu, M.K.; Bhowmik, T.; Barman, S. Highly dispersed platinum nanoparticles on graphitic carbon nitride: A highly active and durable electrocatalyst for oxidation of methanol, formic acid and formaldehyde. Int. J. Hydrog. Energy 2017, 42, 9371–9383. [Google Scholar] [CrossRef]
- Jing, S.C.; Guo, X.L.; Tan, Y.W. Branched pd and pd-based trimetallic nanocrystals with highly open structures for methanol electrooxidation. J. Mater. Chem. A 2016, 4, 7950–7961. [Google Scholar] [CrossRef]
- Li, H.H.; Zhao, S.; Gong, M.; Cui, C.H.; He, D.; Liang, H.W.; Wu, L.; Yu, S.H. Ultrathin ptpdte nanowires as superior catalysts for methanol electrooxidation. Angew. Chem. Int. Ed. 2013, 52, 7472–7476. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Z.H.; Li, H.H.; Liu, J.W.; Yu, S.H. Templating synthesis of ternary ptpdte nanowires with tunable diameter for methanol electrooxidation. CrystEngComm 2016, 18, 4038–4041. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Mecheri, B.; Iannaci, A.; D’Epifanio, A.; Licoccia, S. Iron/polyindole-based electrocatalysts to enhance oxygen reduction in microbial fuel cells. Electrochim. Acta 2016, 190, 388–395. [Google Scholar] [CrossRef]
- Jin, Y.D.; Shen, Y.; Dong, S.J. Electrochemical design of ultrathin platinum-coated gold nanoparticle monolayer films as a novel nanostructured electrocatalyst for oxygen reduction. J. Phys. Chem. B 2004, 108, 8142–8147. [Google Scholar] [CrossRef]
- Zhong, H.X.; Zhang, H.M.; Liang, Y.M.; Zhang, J.L.; Wang, M.R.; Wang, X.L. A novel non-noble electrocatalyst for oxygen reduction in proton exchange membrane fuel cells. J. Power Sources 2007, 164, 572–577. [Google Scholar] [CrossRef]
- Salvi, A.M.; Castle, J.E. The intrinsic asymmetry of photoelectron peaks: dependence on chemical state and role in curve fitting. J. Electron. Spectros. Relat. Phenomena 1998, 95, 45–56. [Google Scholar] [CrossRef]

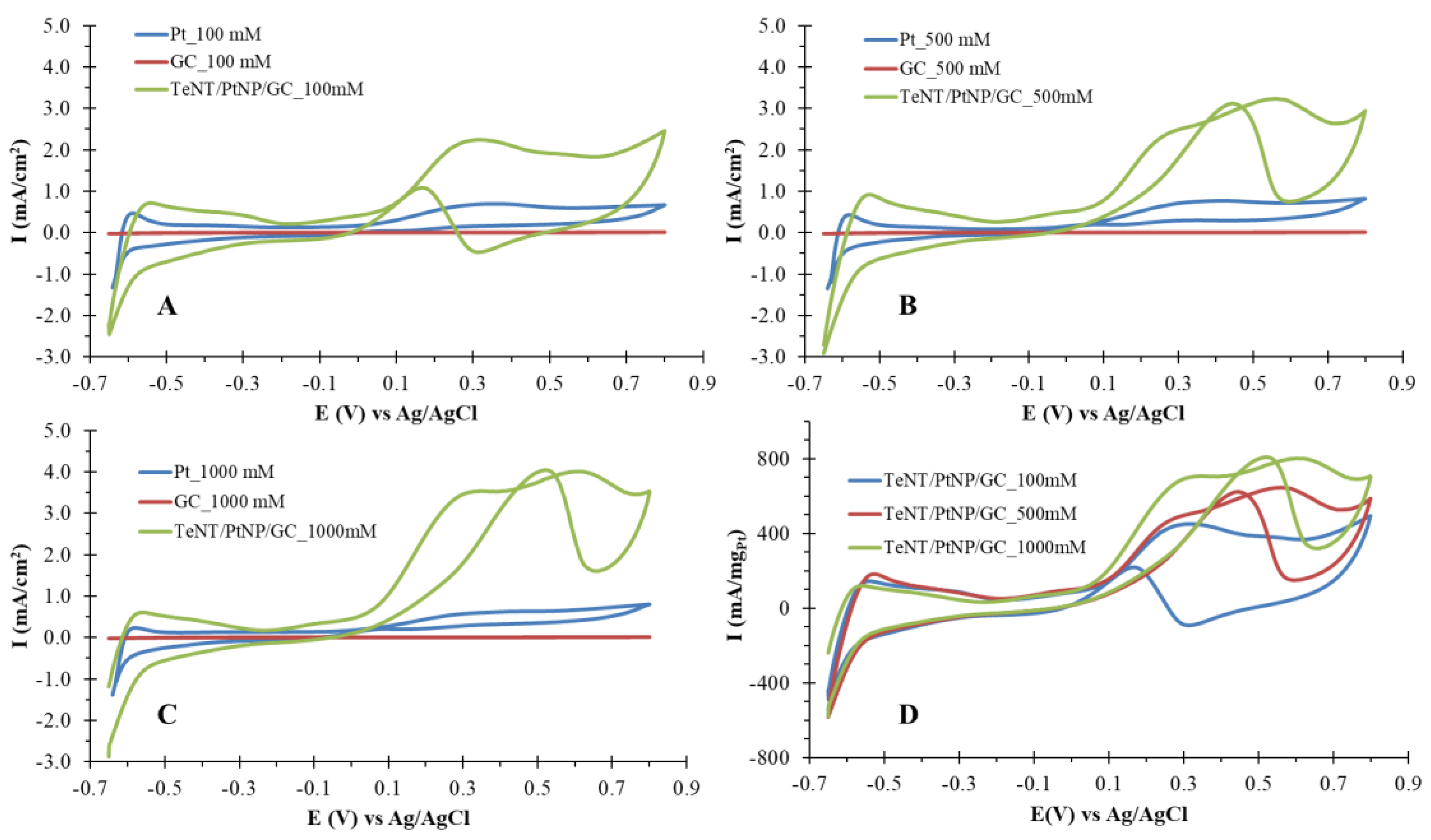

| Electrode | Ipa (mAcm−2)/Epa (mV) [MeOH] 100 mM | Ipa (mAcm−2)/Epa (mV) [MeOH] 500 mM | Ipa (mAcm−2)/Epa (mV) [MeOH] 1000 mM |
|---|---|---|---|
| GC | - | - | - |
| Pt | 0.58/+370 | 0.74/+370 | 0.69/+370 |
| TeNT/PtNP/GC | 2.24/+298 | 3.22/+547 | 4.04/+615 |
| Electrode | Ipc (mAcm−2)/Epc (mV) N2-saturated | Ipc (mAcm−2)/Epc (mV) atmospheric O2 | Ipc (mAcm−2)/Epc (mV) O2-saturated |
|---|---|---|---|
| GC | - | - | - |
| Pt | - | −0.20/−115 | −0.30/−150 |
| TeNT/PtNP/GC | - | −0.30/+150 | −0.70/+50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guascito, M.R.; Chirizzi, D.; Filippo, E.; Milano, F.; Tepore, A. Synthesis and Characterization of Te Nanotubes Decorated with Pt Nanoparticles for a Fuel Cell Anode/Cathode Working at a Neutral pH. Catalysts 2019, 9, 328. https://doi.org/10.3390/catal9040328
Guascito MR, Chirizzi D, Filippo E, Milano F, Tepore A. Synthesis and Characterization of Te Nanotubes Decorated with Pt Nanoparticles for a Fuel Cell Anode/Cathode Working at a Neutral pH. Catalysts. 2019; 9(4):328. https://doi.org/10.3390/catal9040328
Chicago/Turabian StyleGuascito, Maria Rachele, Daniela Chirizzi, Emanuela Filippo, Francesco Milano, and Antonio Tepore. 2019. "Synthesis and Characterization of Te Nanotubes Decorated with Pt Nanoparticles for a Fuel Cell Anode/Cathode Working at a Neutral pH" Catalysts 9, no. 4: 328. https://doi.org/10.3390/catal9040328
APA StyleGuascito, M. R., Chirizzi, D., Filippo, E., Milano, F., & Tepore, A. (2019). Synthesis and Characterization of Te Nanotubes Decorated with Pt Nanoparticles for a Fuel Cell Anode/Cathode Working at a Neutral pH. Catalysts, 9(4), 328. https://doi.org/10.3390/catal9040328