Abstract
The aim of this paper is to demonstrate the possibilities of anaerobic sludge cells immobilized into poly(vinyl alcohol) cryogel for the methanogenic conversion of various lignocellulosic waste and other media containing antibiotics (ampicillin, kanamycin, benzylpenicillin) or pesticides (chlorpyrifos or methiocarb and its derivatives). It was established that the immobilized cells of the anaerobic consortium can be stored frozen for at least three years while preserving a high level of metabolic activity. The cells after the long-term storage in an immobilized and frozen state were applied for the methanogenesis of a wide number of wastes, and an increase in both methane yield and methane portion in the produced biogas as compared to the conventionally used suspended anaerobic sludge cells, was ensured. It was shown that the “additional” introduction of bacterial Clostridium acetobutylicum, Pseudomonas sp., Enterococcus faecalis cells (also immobilized using same support) improves characteristics of methanogenesis catalyzed by immobilized anaerobic sludge.
1. Introduction
The efficient use of lignocellulose-containing compounds in agricultural, forestry, or food industry waste, or specially grown plant biomass for biogas production via methanogenesis, has several important limitations. Chief among these limitations are the relatively low biogas yield and therefore long period of time (several months) necessary for keeping such substrates in the bioreactor, which negatively influences the economic viability of the process [1].
The difficulties in converting of lignocellulose waste (LCW) into methane are often further aggravated by the presence of xenobiotics in the LCW, which inhibit the metabolic activity of the microorganisms inhabiting the anaerobic sludge [2]. Xenobiotics in the agricultural waste can be represented by antibiotics and pesticides which are used for treating animals and plants [3,4,5]. These xenobiotics are usually not easily biodegradable. Agriculture is currently one of the chief consumers of antibiotics [3,6], therefore the probability of the antibiotics’ presence in the media used for biogas production is rather high. Therefore, we have studied the influence of the antibiotics contained in the potential substrates on the functional activity of the suspended, and the immobilized anaerobic sludge. Therefore, kanamycin, ampicillin, and benzylpenicillin, which are traditionally used in poultry and cattle farming [7,8,9], were added to the methane generating medium. Most of the antibiotics used in veterinary practice are not completely absorbed in the intestines, and 30–90% of these substances are excreted with fecal matter [10]. The maximum concentrations of the antibiotics in manure can be as high as several milligrams per kilogram [11]. LCW, agricultural wastewater, and manure used as substrates in biogas production can also contain a significant quantity of pesticide [5]. Therefore, we have studied the possible use of immobilized anaerobic sludge for biogas production in the presence of carbamate pesticides and their derivatives, namely, methiocarb, methiocarb sulfoxide, methiocarb sulfone and organophosphorus pesticide, namely, chlorpyrifos.
Sulfones and sulfoxides are the intermediate decomposition products of many pesticides such as methiocarb, fenthion, thiometon, etc. Agricultural products, in which the content of the pesticides and their derivatives exceed the permissible County Agricultural Commission (CAC) and European Union (EU) standards, can potentially be used to produce biogas. However, this fact should be investigated. In this regard, it was interesting to assess the effect of such xenobiotics on the process of methanogenesis.
Methiocarb being widely used carbamate pesticide was applied in this investigation. Methiocarb and its derivatives, namely, (3,5-dimethyl-4-methylsulfinylphenyl-N-methylcarbamate) and sulfone (3,5-dimethyl-4-methylsulfonylphenyl-N-methylcarbamate), are suspected to be carcinogens and mutagens. The maximum residue limits of methiocarb in foods of animal origin was established as 0.05 mg/kg [12].
The chlorpyrifos-based chemicals are currently actively used in agriculture in many countries [13]. The toxicity of chlorpiryfos is rather high compared to that of other organophosphorus compounds, and its half-life, depending on several factors, can be as long as 120 days [13]. The non-decomposed chlorpyrifos can accumulate in the agricultural wastes and thereby provoke hazardous situations [14].
Anaerobic conversion of organic wastes into biogas involves several consecutive biochemical processes driven by different bacterial types which ensure the deep decomposition of different specific substrates. The metabolite of one microbial group is a substrate for those of another one in the overall methanogenesis process, which results in methane accumulation as a part of biogas [15]. The main stages of methanogenesis are generally hydrolysis, acetogenesis, and methane formation. Therefore, developing the approaches for preserving the maximum metabolic activity of all participants of the general process is a topical problem in biogas production using various organic wastes.
The increase of the methane yield, and also of the methane content in the total biogas output for a given substrate input in the bioreactor is also one of the priority aims of the studied process involving anaerobic sludge. Various approaches have been analyzed over the recent years for increasing the biogas yield from different renewable resources. Several pretreatment techniques for LCW were suggested, including thermolysis, delignification with alkali, acidic and fermentative hydrolysis, etc. [16,17,18,19,20,21].
Since each way of LCW pre-treatment has both its specific advantages and drawbacks, a combination of several physico-chemical and fermentative techniques are considered to be prospective for producing a substrate maximally suitable for further methanogenesis and containing minimum of toxic substances, which can suppress the activity of the methane-producing cells [19,22,23].
Immobilization allows the acquisition of positive effects in different areas of modern biocatalysis [24,25,26,27,28]. A series of processes involving immobilized microorganism cells has been developed for converting pre-treated LCW into other products (apart from methane), which address the above-mentioned problems [29,30]. Therefore, using artificially immobilized cells in methane production from pre-treated LCW also looks viable. This seems to be quite probable, especially since the anaerobic sludge cells are capable of self-immobilization (via formation of granules and biofilms); however, such natural processes take too long (more than 20 days) for practical purposes [31].
Various carriers for the immobilization of anaerobic sludge have been studied. However, using natural polymer-based supports generally failed due to the premature biodegradation and physico-chemical destruction of the carriers caused, among others, by the pressure of the different gaseous metabolites of the cells accumulated in porous structures [32].
Solving the problem of carrier destruction under anaerobic conditions can be possible via immobilization of the anaerobic sludge cells into macroporous cryogel of poly(vinyl alcohol) (PVA) [33]. As was earlier established in the studies on the production of various biofuel types, PVA cryogel can withstand excess pressure of gases (CO2 and H2) produced by the anaerobic microorganisms and does not prevent either the access of nutrients to the producer cells or removal of their metabolites [30]. This technique of a cells’ inclusion to PVA cryogel implies the freezing/thawing of a suspension of anaerobic sludge in a polymer solution. The advantages of this immobilization method, and the effectiveness of its use have been repeatedly demonstrated earlier in the example of cells of various microorganisms [24,29,30,34].
Known attempts at freezing the sludge led to a grave damage of the cells by the ice crystals formed both in and outside the cells, which causes cell death upon thawing [35]. In case of cell immobilization, the freezing of anaerobic sludge in presence of PVA solution allows the expectation of maintaining cell viability since the PVA is known to play the role of cryoprotector [36].
In order to increase the methanogenesis efficiency and the methane content in the produced biogas, researchers often introduce some additional cells into the methane tank besides the anaerobic sludge. These microorganisms usually stimulate the processes at various stages of the cellulose-containing substrates’ conversion into methane: hydrolysis of complex substrates [1,2,37], additional formation of hydrogen [38] and methane [39].
It was noted, however, that sometimes such additional microorganisms added into the bioreactor fail to cause the desired biogas yield increase, and even lead to an inhibition of methane formation by anaerobic sludge [40,41]. The main reason for this failure was the difficulty in determining and supporting the necessary microbial equilibrium. However, all these results were obtained for suspended anaerobic sludge. No such research has been reported so far for methanogenesis with artificially immobilized cells.
The aim of the present study was comparing the efficiency of methanogenesis when converting various substrates including LCW with suspended cells and prepared samples of anaerobic sludge immobilized in PVA cryogel. Additionally, the possible enhancement of the efficiency of biogas production, and increasing the methane content therein via the introduction of additional bacterial cells (also immobilized in the PVA cryogel) into the methanogenesis process were studied in this work.
2. Results
2.1. PVA Cryogel-Immobilization of Anaerobic Sludge
Samples of various types of suspended anaerobic sludge (Table 1) were taken in order to demonstrate the possible using of artificially immobilized anaerobic active sludge based on PVA cryogel for producing methane. Upon the completion of the cell immobilization procedure the functional activity of the samples was evaluated. The free cells from the initial samples of the suspended sludge were used as control.

Table 1.
Characteristics of the anaerobic sludge samples used in the study.
It was found that the immobilization of suspended anaerobic sludge in PVA cryogel ensures an increase in its methanogenic activity (by 13–55%), and the effect did not depend on the type of the initial suspended anaerobic sludge. However, the effect of cell immobilization in PVA cryogel on the acidogenic activity was negative, i.e. the anaerobic sludge activity decreased by 7–16%; this trend did not depend on the type of the initial suspended sample type.
The effect of immobilization in PVA cryogel was strongest for the anaerobic sludge type I: the maximum increase in the methanogenic activity was 55%, and the maximum decrease in acidogenic one was 16%, compared to the initial suspended biomass of the anaerobic sludge. This sludge type was therefore chosen as the most interesting subject for investigation in further experiments.
2.2. The Influence of the Initial Chemical Oxygen Demand on the Yield and Composition of the Biogas Produced using PVA Cryogel-Immobilized Anaerobic Sludge
The initial COD of the medium is known to essentially influence the characteristics of the methanogenesis process [2]. We have therefore studied the influence of this parameter on the specific productivity of methanogenesis and the methane content in the biogas produced with PVA cryogel-immobilized anaerobic sludge type I using medium based on milk whey (Table 2). As a control, a similar study was performed using suspended cells of the same anaerobic sludge (Table 2). The methane content in the biogas accumulated in the reactor with immobilized anaerobic sludge cells in the milk whey-containing medium was higher than in case of suspended sludge over a wide range of COD (1.0–33.0 g/L). The maximum difference in the methane content in the biogas was observed at COD of 9.0 g/L. The specific methanogenesis productivity in a milk whey-containing medium for COD at the limits of the studied range (namely, 1.0 g/L and 33.0 g/L) was almost the same for the suspended and the immobilized anaerobic sludge cells (Table 2). However, over all of the intermediate COD range (2.5 ± 16.0 g/L) the immobilized anaerobic sludge cells functioned more efficiently than the suspended one. When the COD of medium was 3.0 g/L, the maximum distinction (2.5 times) between the specific methanogenesis productivity of the immobilized and suspended anaerobic sludge was reached (in absolute units the difference was 187.0 mL CH4/g COD).

Table 2.
Characteristics of methanogenesis with suspended and PVA cryogel-immobilized anaerobic sludge cells in medium based on milk whey.
2.3. Dependence of the Metabolic Activity of Immobilized Anaerobic Sludge Cells on the Storage Conditions
The metabolic activity of the immobilized anaerobic sludge was evaluated after its long-term storage at −18 °C and at +35 °C (Figure 1). In order to evaluate the residual metabolic activity of the immobilized cells of the anaerobic sludge the nutrition media based on milk whey, glucose, and acetate with the initial COD of 3 g/L were used. These substrates were chosen because their transformation involved certain groups of microorganisms present in the anaerobic sludge (those with hydrolytic activity, acetogenes, and methanogenes).
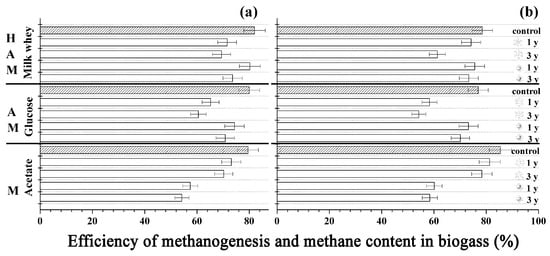
Figure 1.
Methanogenesis efficiency (a) and methane content in the biogas (b) during transformation of the media based on 3 g COD/L of milk whey, 3 g COD/L of glucose, and 3 g COD/L of acetate with immobilized anaerobic sludge cells stored at −18 °C (  ) and +35 °C (
) and +35 °C (  ) for 1–3 years. The data obtained under similar conditions, but with fresh immobilized anaerobic sludge cells are shown for control (shaded bars). Letters indicate the groups of microorganisms of the anaerobic sludge involved into the methanogenesis for each substrate: H-hydrolytically active microorganisms, A-acetogens, M-methanogens.
) for 1–3 years. The data obtained under similar conditions, but with fresh immobilized anaerobic sludge cells are shown for control (shaded bars). Letters indicate the groups of microorganisms of the anaerobic sludge involved into the methanogenesis for each substrate: H-hydrolytically active microorganisms, A-acetogens, M-methanogens.
 ) and +35 °C (
) and +35 °C (  ) for 1–3 years. The data obtained under similar conditions, but with fresh immobilized anaerobic sludge cells are shown for control (shaded bars). Letters indicate the groups of microorganisms of the anaerobic sludge involved into the methanogenesis for each substrate: H-hydrolytically active microorganisms, A-acetogens, M-methanogens.
) for 1–3 years. The data obtained under similar conditions, but with fresh immobilized anaerobic sludge cells are shown for control (shaded bars). Letters indicate the groups of microorganisms of the anaerobic sludge involved into the methanogenesis for each substrate: H-hydrolytically active microorganisms, A-acetogens, M-methanogens.
This research showed that the PVA cryogel-immobilized anaerobic sludge is capable of retaining its characteristics at the level comparable to the initial one for at least three years under the storage conditions used in the study (Figure 1). Storage of the immobilized sludge at negative temperature allowed, upon thawing, the production of biogas in a quantity comparable to that produced with immobilized sludge stored at +35 °C. It was shown that microorganisms functioning at the stage of hydrolysis of biomolecules or acidogenesis favor storage at positive temperatures, whereas those involved in methanogenesis proper prefer negative ones.
2.4. Methanogenesis of Various Hydrolysates of Lignocellulose-Containing Waste with Immobilized Anaerobic Sludge
PVA cryogel-immobilized anaerobic sludge, taken after storage for three years at −18 °C, was studied for methanogenesis of hydrolysates of various LCW types. It was interesting because these substrates are hard to be biodegraded with anaerobic sludge. Similar experiments with suspended cells of the same type of anaerobic sludge were performed for control. The maximum methanogenesis efficiency with both the suspended and the immobilized anaerobic sludge in case of anaerobic fermentation of milk whey was achieved for the initial COD of 3.0 g/L. Therefore, media with this COD value were prepared based on the hydrolysates of various LCWs for further experiments. However, the influence of COD increase above 3.0 g/L was also studied for various substrates (Table 3).

Table 3.
Characteristics of methanogenesis with suspended and PVA cryogel-immobilized anaerobic sludge cells in in media with various lignocelluloses wastes.
Using sawdust hydrolysates as the substrate for methane production was less efficient than with other substrate types; however, the immobilized anaerobic sludge cells generally functioned more efficiently than the suspended ones in this case as well. Total COD (CODtot) contained soluble organic part (CODfilt) and organic solids (CODss). This could be due to the lower ratio of CODfilt in CODtot (40–43.3%) in sawdust hydrolysates. Note that in case of pine sawdust transformation with immobilized anaerobic sludge the increase of the initial CODtot from 3.0 to 8.5 g/L did not influence the methanogenesis efficiency, whereas in case of suspended cells such increase had an inhibiting effect. When transforming aspen sawdust hydrolysates with immobilized anaerobic sludge cells, the optimal initial COD in terms of methanogenesis characteristics was 8.5 g/L.
Bagasse hydrolysates had the lowest CODfilt value in CODtot, and the methanogenesis characteristics were among the lowest observed in this study, as it was in the case with sawdust hydrolysates. However, the specific productivity of the immobilized anaerobic sludge calculated taking into account CODfilt was even higher for bagasse hydrolysates than in the case of using milk whey as CH4-producing medium (Table 3). No similar trend was observed for suspended anaerobic sludge cells.
The characteristics of CH4 production from beet pulp hydrolysates with suspended and immobilized anaerobic sludge cells were similar. The increase of the initial COD from 3.0 to 10.5 g/L resulted in a decrease of the basic methanogenesis characteristics, and the decrease of the methane content in the biogas was essential (46% and 33% for suspended and immobilized anaerobic sludge cells, respectively).
Note the following result of methanogenesis in hydrolysates of Jerusalem artichoke stems: the maximum specific methane productivity of suspended and immobilized anaerobic sludge cells was achieved for the initial COD of 3.0 g/L. Further increase of the initial COD caused a decrease of the process characteristics, which became similar for the suspended and the immobilized anaerobic sludge cells.
An interesting effect was observed in the dependence of methanogenesis characteristics on the initial COD of hydrolysates of Jerusalem artichoke stems treated with suspended and immobilized anaerobic sludge cells. The increase of the initial COD of this medium caused a decrease of the suspended cells’ activity by a factor of 1.4, whereas that of the immobilized anaerobic sludge cells decreased only by a factor of 1.1 (Table 3).
The biogas yield and composition, as well as volatile fatty acids (VFA) concentration in the cultural broth was monitored in the experiments (Table 3, Figure 2 and Figure 3). Independently, on total initial COD, the advantage of the immobilized anaerobic sludge over the suspended was observed in all tested media (Table 2 and Table 3, Figure 3).
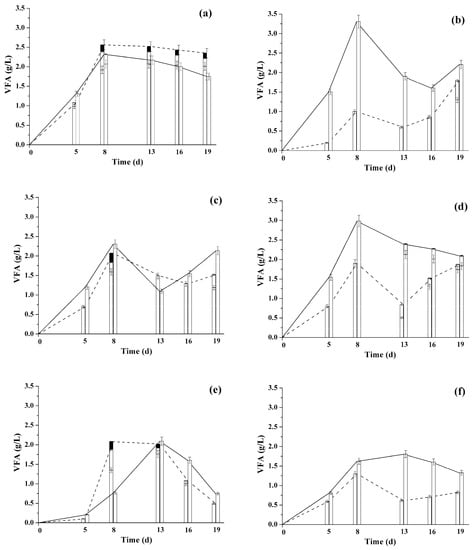
Figure 2.
Current VFA concentration (acetic acid-white, propionic acid- light grey, butyric acid-dark grey) in the liquid phase during methanogenesis of LCW hydrolysates (COD 3.0 ± 0.2 g/L: (a) aspen sawdust; (b) pine sawdust; (c) beet pulp; (d) bagasse; (e) Jerusalem artichoke stems; (f) chicory steam) under the action of suspended cells (dashed line) and immobilized (solid line) anaerobic sludge Type I. Anaerobic immobilized sludge was used after 3 years storage at −18 °C.
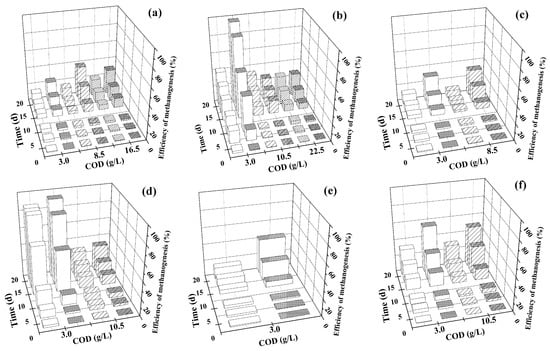
Figure 3.
Efficiency of methanogenesis in LCW hydrolysates-(a) aspen sawdust; (b) Jerusalem artichoke stems; (c) pine sawdust; (d) beet pulp; (e) bagasse (f) chicory steam, with suspended cells (white bars) and immobilized (grey bars) anaerobic sludge Type I. Anaerobic immobilized sludge was used after 3 years storage at −18 °C.
2.5. Methanogenesis Intensification due to Partial Substitution of the Anaerobic Sludge with Immobilized Cells of Individual Bacterial Cultures
The use of immobilized anaerobic sludge cells for methanogenesis of hydrolysates of Jerusalem artichoke stems and beet pulp with initial CODtot of 10.5 g/L did not improve the process characteristics as compared to the case of suspended sludge biomass. In order to intensify methanogenesis, a part of the immobilized anaerobic sludge was replaced with PVA cryogel-immobilized cells of several chosen bacterial cultures (Figure 4):
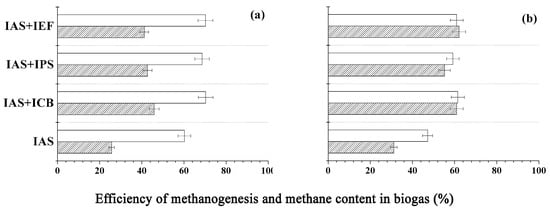
Figure 4.
Methanogenesis efficiency (shaded bars) and methane content in the biogas (blank bars) in the course of transformation of hydrolysates of Jerusalem artichoke stems (a) and beet pulp (b) in the presence of immobilized active sludge alone (IAS) and with its 10% (w/w)-substitution by other immobilized cell types: C. acetobutylicum cells (ICB), Pseudomonas sp. cells (IPS), E. faecalis cells (IEF), respectively.
- (a)
- Clostridium acetobutylicum cells, which are anaerobic producers of cellulases, as well as buthanol, ethanol, acetone and hydrogen, which can be used as substrates for the anaerobic sludge [30,42,43],
- (b)
- Pseudomonas sp. cells, being facultative anaerobic bacteria using a wide range of organic substances as substrates [44],
- (c)
- Enterococcus faecalis cells, which can synthesize a number of hydrolytic enzymes [45].
It was established that by replacing 10% (w/w) of the immobilized anaerobic sludge in the reactor with immobilized bacterial cells of individual species, and using thus obtained biocatalytic combination for the transformation of hydrolysates of Jerusalem artichoke stems ensures the enhancement of methanogenesis efficiency by a factor of 1.7 ± 0.1, and the CH4 content in the biogas increased by 14.0 ± 16.8%; the corresponding parameters for beet pulp were 1.9 ± 0.1 and 25.4 ± 30.3%, respectively (Figure 4). Thus the “quality” of the produced biogas was essentially enhanced, so its composition became similar to that of the natural gas [46].
Further increase of the immobilized bacterial cell concentration up to 20% (w/w) in substitution did not cause either an essential increase of methanogenesis efficiency or a substantial change of methane content in the produced biogas.
2.6. The Influence of Xenobiotics’ Presence in the Agricultural Waste on the Functioning of the Anaerobic both Suspended and Immobilized Sludge
The current studies of the antibiotics’ effect on methanogenesis were performed in the media containing chicken manure with COD of 3.0 g/L. The concentration of antibiotics in the media was 1 g/L (Table 4).

Table 4.
Basic characteristics of the methanogenesis with suspended and immobilized anaerobic sludge in the presence of various xenobiotics.
The antibiotics-caused decrease of the methanogenesis efficiency in the case of suspended anaerobic sludge was 1.3–2.2 times greater than in the case of the immobilized cells. The methane content in the biogas produced with immobilized anaerobic sludge cells was higher than in the case of suspended cells. A comparison of methanogenesis efficiency in the presence of various antibiotics showed that the antibacterial effect of ampicillin on the anaerobic sludge was the greatest, whereas that of the kanamycin was the smallest.
It is known that a source of carbon should be introduced into the medium for the biodegradation of organophosphorus pesticides with anaerobic microorganisms [47]. Hydrocarbons present in the hydrolysate of the Jerusalem artichoke stems (with initial COD of 3.0 g/L) were used as carbon source for degradation of 100 mg/L chlorpyrifos or methiocarb (Table 3).
To improve the pesticide biodegradation, an adaptation of the bacterial consortia can be effectively undertaken [48]. According to this adaptation, chlorpyrifos was implemented in the present study. Chlorpyrifos (10 mg/L) was three-weekly introduced to the medium containing suspended anaerobic sludge for six months. No such adaptation was performed for the immobilized anaerobic sludge; and it was directly introduced into the medium containing 100 mg/L chlorpyrifos (Table 4).
It was established that immobilized anaerobic sludge can ensure decomposition chlorpyrifos (probably as a co-substrate) in the medium containing hydrolysate of Jerusalem artichoke stems within 45 days. The specific productivity of methanogenesis was 315.3 ± 14.2 mL CH4/g COD. Introduction of the same concentration of pesticide into the culturing medium with suspended anaerobic sludge completely suppressed biogas formation, and did not cause decomposition of the organophosphorus pesticide. The adapted suspended anaerobic sludge was capable of decomposing only 58 mg/L chlorpyrifos within 45 days. The methane content in the accumulated biogas (54.3 ± 2.5 mL CH4/g COD) was six times less than in the case of non-adapted immobilized anaerobic sludge.
The results of biogas production in the presence of methiocarb were comparable to those obtained for chlorpyrifos (Table 4). In the presence of methiocarb derivatives, methanogenesis efficiency was higher than for pure methiocarb.
The dynamics of chlorpyrifos degradation in the medium containing hydrolysate of Jerusalem artichoke stems during methanogenesis with non-adapted immobilized cells as well as non-adapted and adapted suspended anaerobic sludge is shown in Figure 5.
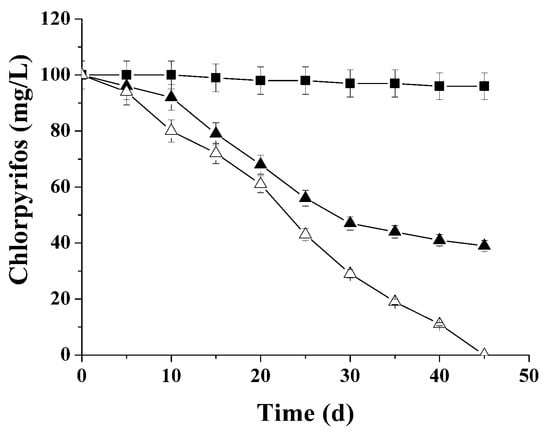
Figure 5.
Chlorpyrifos concentration dynamics in the medium containing hydrolysate of Jerusalem artichoke stems (COD 3.0 g/L) during methanogenesis with non-adapted suspended (■), adapted suspended (▲), and non-adapted immobilized (∆) anaerobic sludge.
3. Discussion
The observed increase in methanogenic activity after the immobilization of suspended anaerobic sludge in PVA cryogel (Table 1) may be due to several factors. The hydrolysis stage for complex substrates is known to be the limiting stage in biogas production; this is especially important in case of LCW [49]. When this limitation is obviated due to the LCW pretreatment, then the rate of methane formation shall predominantly influence the characteristics of the overall process [50]. Therefore, the observed increase of methanogenic activity due to the artificial immobilization of the anaerobic sludge should ensure a higher efficiency of the overall methanogenesis process.
It is known besides that the initial microbial composition of the anaerobic sludge influences its activity, especially the methanogenic one [51,52]. The genetic analysis of the anaerobic sludge type I performed previously has shown the presence of cells of the following genera: Proteobacteria, Bacteroidetes, Chloroflexi, Firmicutes, Verrucomicrobia, Lentisphaerae, Spirochaetales, Planctomycetes, Methanomicrobiales, Methanobacteriales and Methanosarcinales [53]. The anaerobic sludge formed in the course of methanogenesis in manure (active sludge type II sample) contained the major bacteria belonging to the phylum Firmicutes, Clostridia, and Bacteroidetes, whereas the archaeal community was dominated by the methanogenic archaea of the taxa Methanomicrobiales, Methanosarcinales, and Methanobacteria [54]. Thus, the difference in the level of the acidogenic and methanogenic activity of the anaerobic sludge before and after the immobilization in PVA cryogel was due to the different impact of the immobilization procedure itself on the cells inhabiting the active sludge and catalyzing the different stages of methanogenesis.
Freezing of the suspended anaerobic sludge for long-term storage (Figure 1, Table 2 and Table 3) has not been studied before. Only a few papers reported methane tank functioning in cold climate or during winter [55,56]. However, the most solutions suggested in such studies are limited, as a rule, to enhancing the methane tank thermal insulation. For many countries developing the immobilized methanogenesis biocatalysts capable of preserving their activity upon freezing can help extending the geographical range of methane tank application.
The analysis of the obtained results (Table 2 and Table 3, Figure 2 and Figure 3) has shown the following: (i) the specific productivity of the immobilized anaerobic sludge cells was higher than that of the suspended cells for all the types of LCW hydrolysates with the total initial COD of 3.0 g/L. However, the degree of the this parameter’s increase depended strongly on the type of the initial substrate; (ii) the higher was the ratio of the soluble substances’ COD in the total COD of the substrate, the higher was the efficiency of methanogenesis in LCW; (iii) the ratio of methane in the biogas produced with suspended and immobilized cells was either similar (in case of pine sawdust and beet root hydrolysates) or higher (by as much as 23%) in the media containing immobilized sludge; (iv) the current concentrations of VFA in most of the studied media were higher for immobilized anaerobic sludge cells (with the exception of aspen sawdust hydrolysate). The VFA produced with immobilized anaerobic sludge cells include only acetate (Figure 2), whereas propionate and butyrate were also produced in case of free ones. The high VFA concentrations in the medium are known to inhibit the methanogenesis process; however, the immobilized anaerobic sludge cells, despite higher VFA concentrations, still functioned more efficiently than the suspended ones. The reason for this effect was in propionate degrading at much lower rate than acetate did. This, in turn, was caused by acetate being capable of direct conversion into CH4 and CO2, whereas propionate should be degraded to acetate before methane production [57].
Thus, it was shown (Figure 4) that combining the developed methanogenesis biocatalysts with other immobilized biocatalysts based on chosen individual bacterial cultures allows an additional methanogenesis efficiency enhancement. These additional bacterial cultures facilitate destruction of various biomolecules including biopolymers present in the media, and thus cause an increase in the conversion efficiency of LCW hydrolysates to biogas with improved content of methane. This approach is likely to be proposed to improve the efficiency of various processes related to the conversion of renewable raw materials into biogas.
The basic characteristics of methanogenesis in the presence of xenobiotics were better when using immobilized in the PVA cryogel sludge compared to suspended sludge (Table 4). Cell immobilization, as in other studies, noticeably improved the process efficiency on biogas/methane yield [58].
Taking in account the obtained results, it is necessary to note, that the technique of inclusion of the anaerobic sludge cells into the carrier matrix has already been tested previously. Thus, the application of Ca-alginate gel as carrier in this approach for biogas production has been reported [32]. This immobilized anaerobic sludge appeared to be better than the suspended one in terms of biogas yield, as well as the rate and depth of COD utilization. However, as the authors of the research note, the granules of such immobilized sludge tend to be decomposed after 12.2 days of sludge functioning. Therefore Ca-alginate gel cannot be efficiently used for CH4 production.
An method is known to strengthen the immobilized biocatalyst formed with thermophilic anaerobic sludge and Ca-alginate gel via adding carobixymethyl cellulose or chitosan into the composition [59]. Such immobilized anaerobic sludge was used for CH4 production from a mixture containing such carbon sources as acetate, propionate, butyrate, methanol, and glucose. The maximum specific productivity of methanogenesis (30 mL/g COD) was achieved on the sixth day from the start of the process. However, the stability of the biocatalyst was still insufficient, because after six days of functioning the granules started to decompose under the influence of hydrolytically active bacteria present in the methanogenic consortium [60].
Immobilization of anaerobic sludge in carriers based on synthetic polymers should obviously be preferred to that based on the natural ones, because the former are less prone to biodegradation. Thus, using PVA hydrogel formed with an addition of boric acid [60] as carrier allowed to produce immobilized sludge which is better than the suspended sludge of the same composition in terms of all the relevant characteristics. However, the possibility of using such immobilized sludge for biogas production was demonstrated only for synthetic media, and not for any hydrolysates of LCW. As for the PVA cryogel-immobilized anaerobic sludge used in the present study, the application of the chosen carrier has proven its efficiency. In addition, it is known that when cells are immobilized in a PVA gel by borate, there is a negative toxic effect on the cells of microorganisms. [24,61]. This can be avoided in the case of the cryoimmobilization method used in the work. The granules containing the immobilized cells did not decompose, which confirmed the preliminary data on the high mechanical strength of PVA cryogel [29,30]. This strength did not diminish in various media and during various processes. The generality of the studied approach to cell immobilization for CH4 production was confirmed for three different types of anaerobic sludge (Table 1). These results can be used in other processes related to the production of biogas.
4. Materials and Methods
4.1. Materials
Dursban 480 EC containing 480 g of chlorpyrifos (O, O-diethyl O-3, 5, 6-trichloro-2-pyridinyl phosphorothioate, CPY; Chemical Abstract Service number 2921-88-2) per 1 L was purchased from Dow AgroSiences LLC (Indianapolis, IN, USA). Methiocarb (3,5-dimethyl-4-methylthiophenyl-N-methylcarbamate) and its derivatives - sulfoxide (3,5-dimethyl-4-methylsulfinylphenyl-N-methylcarbamate) and sulfone (3,5-dimethyl-4-methylsulfonylphenyl-N-methylcarbamate) were purchased from Sigma (Saint Louis, MO, USA). Poly(vinyl alcohol) 16/1 (84 kDa) was purchased from Sinopec Corp. (Beijing, China). All the antibiotics used in the study were produced by Sintez (Kurgan, Russia). Milk whey was purchased from Product-Service (Voronezh, Russia). The liquid fraction of chicken manure was directly taken from the upper part of the sedimentation reservoir of the industrial farm Oktyabr'skaya located in the Moscow region (Russia). To achieve partial microaerobic preacidification of waste water, the feeding flask was kept open at laboratory ambient temperature (19 ± 1 °C) for 1–2 days.
The following renewable raw materials were used in the study: aspen and pine sawdust (Borovichi-Mebel, Borovichi, Russia), Jerusalem artichoke stems, and beet pulp (Esplanada-Yuzhnaya, Krasnodar region, Russia), chicory stems (Altai-export, Altai region, Russia) and bagasse (Homemart CO, LTD, Ho Chi Minh, Vietnam).
4.2. Pretreatment and Enzymatic Hydrolysis of the Raw Materials
The LCW samples were dried to constant mass at 80 °C and milled to particle size of 100–300 µm using an impeller mill Mikrosilema IM-450 (Techpribor, Schekino, Russia). Pretreatment of the LCWs (200 g/L) was performed in the presence of 2.5 M NaOH at 85 ± 5 °C for 1 h. After that the mixture was cooled and pH was adjusted to 5.5 ± 0.5 with 50% (v/v) sulfuric acid. Insoluble precipitate was centrifuged (10,000 rpm, 10 min), washed with distilled water and dried to constant mass.
A mixture of commercial preparations of cellulases such as Spezyme CP (Dupont, NY, USA) and Novozyme-188 (Novozymes Corp., Copenhagen, Denmark) was used for the enzymatic hydrolysis of the renewable raw materials (the ratio between the enzymatic preparations in the mixture was 3:1). Enzyme preparations were introduced into the reaction medium (10 mg of protein per 1 g of the substrate dry matter). Hydrolysis of the pretreated cellulosic biomass (100 g dry weight/L) was performed in a medium based on 0.05 M citrate buffer (pH 5.0) at 50 °C and with constant stirring at 200 rpm during 24 h.
Total COD (CODtot) contained soluble organic part (CODfilt) and organic solids (CODss). CODtot and CODfilt (soluble filtered part) in the original sample were estimated applying the standard method for COD determination [62]. COD of suspended solids (CODss) was calculated as difference between CODtot and CODfilt (Table 5). Potassium dichromate was used as the oxidizing agent and glucose was used as the control oxidizable substrate. The concentration of the reduction product Cr2O72− was detected spectrophotometrically at 600 nm using an Agilent UV-853 spectrophotometer (Agilent Technologies, Waldbronn, Germany). When necessary, the waste media were diluted with 0.1 M phosphate buffer (pH 7.2).

Table 5.
COD of the media used in the study.
4.3. Microorganisms and Cultivation Conditions
Anaerobic sludge samples used for artificial immobilization into PVA cryogel were taken from various sources (Table 1). Their characteristics were determined according to the published procedure [63]. The both acidogenic and methanogenic activity of the anaerobic sludge samples were evaluated according to the previously described techniques [63,64,65]. Nutrition media based on glucose and acetate, respectively, were used in these experiments. The dry weight, ash content, and volatile suspended solids (VSS) in the biomass were determined as described previously [63,65].
The bacterial strains Clostridium acetobutylicum В1787, Pseudomonas sp. В8621, Enterococcus faecalis В4053 were obtained from the Russian National Collection of Industrial Microorganisms (www.genetika.ru) for immobilization in PVA cryogel and introduction into the methane tank in addition to the anaerobic sludge.
The Clostridium acetobutylicum strain B1787 was cultivated in the following medium (g/L): glucose (20); triptone (10); yeast extract (5, pH 6.8).
The Enterococcus faecalis strain B4053 was cultivated in the following medium (g/L): starch (15); yeast extract (4); KH2PO4 (1); MgSO4x 7 H2O (0.5, pH 7.0).
Cultivation of C. acetobutylicum and E. faecalis cells was performed under anaerobic conditions in an argon atmosphere at 37 °C for 20–24 h and 48 h, respectively.
The Pseudomonas sp. strain B8621 was cultivated in a medium with the following composition (g/L): peptone (10); NaCl (5); beef extract (3, pH 7.0). Cultivation was carried out under aerobic conditions at 28 °C for 16 h.
The biomass of all bacterial cells after their cultivation was centrifuged for 15 min at 8000 rpm.
4.4. Immobilization of the Cells via Inclusion into the PVA Cryogel
The cells of all bacterial cultures and samples of anaerobic sludge were immobilized into PVA cryogel according to the previously developed technique [21]. To fulfil it, the biomass precipitate was thoroughly mixed with 10% (w/v) aqueous PVA solution to obtain the 10% (w/w) concentration of bacterial cells, and 30% (w/w) of anaerobic sludge. It was pipetted into 96-well microplates, which were placed in a freezer at −20 °C for 24 h, and then thawed. The granules of PVA cryogel formed by this method contained cells immobilized by inclusion manner (Figure 6).

Figure 6.
The view of suspended anaerobic sludge Type I before its immobilization (a). Mixture of PVA cryogel-immobilized anaerobic sludge Type I and C. acetobutylicum cells taken in ratio 9:1 (w/w) for methanogenesis (b). Immobilized biocatalysts based on anaerobic sludge Type I (c) and Type II (d) in liquid medium.
4.5. Anaerobic Fermentation
The initial inoculum concentration in batch reactors was 10% (v/v) for suspended anaerobic sludge. The quantity of the artificially immobilized anaerobic sludge introduced into the medium was such as to ensure similar concentrations of sludge biomass in the liquid phase. The anaerobic incubation was carried out at 35 °C in all the experiments.
To study the bioconversion of all the substrates to methane, the obtained hydrolysates (55 mL) were diluted to 3.0–22.5 g COD/L with 0.1 M phosphate buffer (pH 7.2) and loaded into hermetically sealable vials (“anaerobic reactors”, 120 mL). The experiments were performed in triplicate.
A control experiment similar to that described above as usual was concurrently conducted to account for the biogas formation due to the possible lysis of the microbial inoculum [63]. The 0.1 M phosphate buffer (pH 7.2) was used instead of the lignocellulose waste. The methane content in the biogas in the experimental control batches was subtracted from that obtained in the corresponding test batches to calculate the methanogenesis efficiency.
The impact of antibiotics on the methanogenesis characteristics was studied as follows. Antibiotics (ampicillin, kanamycin, benzylpenicillin) were added to the chicken manure-based feed medium containing the suspended or immobilized anaerobic sludge cells (Table 3), and the basic characteristics of methanogenesis were monitored. The antibiotic concentration was 1 g/L.
The suspended anaerobic sludge cells used in the study were adapted to the chlorpyrifos via step-wise introduction of chlorpyrifos at 10 mg/L into the medium containing 1g COD/L of milk whey once every three weeks for six months. The initial inoculum concentration of the anaerobic sludge was 10% (v/v). The methiocarb and its derivatives were introduced to media at the same initial concentrations (10 mg/L).
Milk whey solution was added weekly to the medium to ensure the final concentration of 1 g COD/L. Then the supernatant was removed, the anaerobic sludge biomass was washed with 0.1 M phosphate buffer and used for chlorpyrifos degradation.
4.6. Evaluation of the Residual Metabolic Activity of Immobilized Anaerobic Sludge Cells under Different Storage Conditions
The immobilized cells of the anaerobic sludge type I were stored frozen at −18 °C and at +35 °C with periodical (monthly) addition of concentrated milk whey as a feed so that the final concentration was equivalent to 3 g/L COD. After one and three years of storage in such regimes, part of the granules with immobilized cells were arbitrarily taken and placed into a methane tank for biogas accumulation. The feed medium used was a solution (based on 0.1 M phosphate buffer) containing milk whey, acetate, and glucose, so that the COD of the medium was 3 g/L. Then the basic characteristics of the methanogenesis caused by the immobilized anaerobic sludge cells were evaluated for 19 days. A similar methanogenesis process with freshly prepared portion of the immobilized anaerobic sludge (type I) cells was used as control.
To monitor the cell viability of anaerobic sludge and its concentration inside PVA cryogel granules, a bioluminescent method was used to determine the concentration of adenosine triphosphate (ATP) [36]. During cryoimmobilization, storage and use of immobilized cells, the change in ATP concentration did not exceed 10% of the initial value.
4.7. Accumulation of Biogas and Determination of its Content
After 2–5 days we measured the total pressure and biogas concentration in the gas phase of each reactor. Gas measurements were repeated until constant methane content was reached in the gas phase of the reactor.
The content of hydrogen, methane, and carbon dioxide in the gas phase was measured with an LKhM 8 MD chromatograph (Zarya, Dzerzhinsk, Russia) Model 3 with a katharometer (the carrier gas was argon with 20 mL/min flow rate). 2 m long columns were filled with Q porapak (Sigma-Aldrich, MO, USA) [63]. Oven temperature was maintained at 50 °C, the retention times of hydrogen, methane, and carbon dioxide were 43, 67, and 82 s, respectively.
4.8. The Products Formed During Acid Production
Volatile fatty acids (VFA) were analyzed by gas chromatography using an LKhM 8 MD Model 3 chromatograph equipped with a katharometer (the carrier gas was argon, the flow rate of the carrier gas was 30 mL/min [64] and the temperatures of the thermostat of the columns, detector, and evaporator were 190, 210, and 220 °C, respectively).
4.9. Determination of Chlorpyrifos
Samples were analyzed using an HPLC (Knauer Smartline Pump 1000, Knauer Smartline UV Detector 2600, Berlin, Germany) and a Diasfer 110-C18 5 μm, 4.0 × 250 mm reverse-phase chromatography column (Biochemmack CT, Moscow, Russia) with a spectrophotometric detector (274 nm) and isocratic elution. Acetonitrile-water mixture (60:40) was applied as the eluent [64]. The retention time for chlorpyrifos was 31 min. The eluent flow rate was 1 mL min−1 and the detector cell temperature was 25 °C. The sample volume was 20 μL.
4.10. Calculations
The efficiency of methanogenesis E (%) was calculated using the equation:
where Q (mL) is the volume of methane produced in a reactor with a test sample, (Equation (2)):
where C is the methane content in the gas phase (%); Vgph is the volume of the gas phase in the reactor (L); T0 is the temperature under normal conditions, 273 K; T1 is the operating temperature in the reactor (K); P0 is the pressure under normal conditions, 1 atm; and Ptot is the total pressure in the reactor (atm), and Qmax (mL) is the theoretical maximum volume of CH4 (Equation (3)):
where CI is the initial concentration of the organic substances in the used waste sample, (g CODtot/L); Vlph is the volume of the liquid phase in the reactor (L); and 0.35 is the volume of methane produced from 1 g COD at 273 K (L).
Qmax = CIxVlphx0.35x1000
The specific productivity of methanogenesis was calculated as the volume of methane formed from 1.0 g of COD under the influence of anaerobic microbial inoculum introduced into the reactor (mL CH4/g COD).
The data were shown as means of at least three independent experiments ± standard deviation (±SD). Statistical analysis was realized using SigmaPlot 12.5 (ver. 12.5, Systat Software Inc., San Jose, CA, USA).
The significant (p ≤ 0.05) differences between obtained results were estimated by one-way analysis of variance (ANOVA).
5. Conclusions
The present study confirmed the potential of using a wide range of substrates with a high functional stability of the immobilized sludge in media and a high concentration of VFA, antibiotics, and pesticides. A combination of the anaerobic sludge with several individual bacterial cells (also immobilized into PVA cryogel) capable of synthesizing hydrolytic enzymes and intermediate metabolites of the methanogenesis process was also studied. Such a combination was found to be an efficient way of enhancing the characteristics of anaerobic conversion of LCW hydrolysates into CH4. The high metabolic activity of PVA cryogel immobilized anaerobic sludge introduces opportunities for its implementation in biogas production.
Author Contributions
O.S.: M.G. and E.E. designed and administered the experiments. O.S., M.G. and O.M. performed experiments. O.S. and E.E. wrote original draft preparation. O.M. and E.E.—funding acquisition and resources. All authors approved the final version of the manuscript. All authors approved the final version of the manuscript.
Funding
This research was funded by the Russian Foundation for Basic Research, grant number 18-29-05064.
Acknowledgments
The authors would like to thank Dr. N. Stepanov (MSU) for advices on the preparation of images for Figure 6.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martin-Ryals, A.; Schideman, L.; Li, P.; Wilkinson, H.; Wagner, R. Improving anaerobic digestion of a cellulosic waste via routine bioaugmentation with cellulolytic microorganisms. Bioresour. Technol. 2015, 189, 62–70. [Google Scholar] [CrossRef]
- Nzila, A. Mini review: Update on bioaugmentation in anaerobic processes for biogas production. Anaerobe 2017, 46, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Rusanowska, P.; Zieliński, M.; Dębowski, M.; Harnisz, M.; Korzeniewska, E.; Amenda, E. Inhibition of Methane Fermentation by Antibiotics Introduced to Municipal Anaerobic Sludge. MDPI Proceed. 2018, 2, 1274. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Otero, L.; Lema, J.M.; Omil, F. The effect and fate of antibiotics during the anaerobic digestion of pig manure. Bioresour. Technol. 2010, 101, 8581–8586. [Google Scholar] [CrossRef]
- Rossetto, M.R.M.; Vianello, F.; Rocha, S.A.; Lima, G.P.P. Antioxidant substances and pesticide in parts of beet organic and conventional manure. Afr. J. Plant Sci. 2009, 3, 245–253. [Google Scholar]
- Durso, L.M.; Cook, K.L. Impacts of antibiotic use in agriculture: What are the benefits and risks? Curr. Opin. Microbiol. 2014, 19, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xie, K.Z.; Guo, H.S.; Li, A.H.; Xie, X.; Zhang, G.X. Residue depletion of ampicillin in eggs. J. Vet. Pharmacol. Ther. 2015, 38, 508–512. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Eremin, S.A.; Yakup, O.; Yao, G.; Zhang, X. Detection of kanamycin and gentamicin residues in animal-derived food using IgY antibody based ic-ELISA and FPIA. Food Chem. 2017, 227, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Akimenko, Y.V.; Kazeev, K.S.; Kolesnikov, S.I. Impact assessment of soil contamination with antibiotics (for example, an ordinary chernozem). Am. J. Appl. Sci. 2015, 12, 80–88. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environments. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, S.; Niu, T.; Guo, Y.; Qi, S.; Han, X.; Liu, D.; Pan, F. Distribution of antibiotic-resistant bacteria in chicken manure and manure-fertilized vegetables. Environ. Sci. Pollut. Res. 2014, 21, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; El-Aty, A.A.; Na, T.W.; Park, J.S.; Kabir, M.H.; Chung, H.S.; Lee, H.S.; Shin, H.-C.; Shim, J.H. Simultaneous quantification of methiocarb and its metabolites, methiocarb sulfoxide and methiocarb sulfone, in five food products of animal origin using tandem mass spectrometry. J. Chromatogr. B 2017, 1060, 387–394. [Google Scholar] [CrossRef] [PubMed]
- John, E.; Shaike, J. Chlorpyrifos: Pollution and remediation. Environ. Chem. Lett. 2015, 13, 269–291. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Merlin Christy, P.; Gopinath, L.R.; Divya, D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew. Sustain. Energy Rev. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Dima, A.; Boura, K.; Bekatorou, A.; Stergiou, P.Y.; Foukis, A.; Gkini, O.A.; Kandylisa, P.; Pissaridia, K.; Kanellakia, M.; Papamichaelb, E.M.; Koutinas, A.A. Scale-up for esters production from straw whiskers for biofuel applications. Bioresour. Technol. 2017, 242, 109–112. [Google Scholar] [CrossRef]
- Koutinas, A.; Kanellaki, M.; Bekatorou, A.; Kandylis, P.; Pissaridi, K.; Dima, A.; Boura, K.; Lappa, K.; Tsafrakidou, P.; Stergiou, P.-Y.; Foukis, A.; Gkini, O.A.; Papamichael, E.M. Economic evaluation of technology for a new generation biofuel production using wastes. Bioresour. Technol. 2016, 200, 178–185. [Google Scholar] [CrossRef]
- Wang, S.; Ma, F.; Ma, W.; Wang, P.; Zhao, G.; Lu, X. Influence of Temperature on Biogas Production Efficiency and Microbial Community in a Two-Phase Anaerobic Digestion System. Water 2019, 11, 133. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Yadvika, S.; Sreekrishnan, T.R.; Kohli, S.; Rana, V. Enhancement of biogas production from solid substrates using different techniques - a review. Bioresour. Technol. 2004, 95, 1–10. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, N.; García-Bernet, D.; Domínguez, J.M. Effects of enzymatic hydrolysis and ultrasounds pretreatments on corn cob and vine trimming shoots for biogas production. Bioresour. Technol. 2016, 221, 130–138. [Google Scholar]
- Pérez-Rodríguez, N.; García-Bernet, D.; Domínguez, J.M. Faster methane production after sequential extrusion and enzymatic hydrolysis of vine trimming shoots. Environ. Chem. Lett. 2018, 16, 295–299. [Google Scholar]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sust. Energ. Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Efremenko, E.N. Immobilized Cells: Biocatalysts and Processes; RIOR: Moscow, Russia, 2018. [Google Scholar]
- Amin, H.A.; Secundo, F.; Amer, H.; Mostafa, F.A.; Helmy, W.A. Improvement of Aspergillus flavus saponin hydrolase thermal stability and productivity via immobilization on a novel carrier based on sugarcane bagasse. Biotechnol. Rep. 2018, 17, 55–62. [Google Scholar] [CrossRef]
- Cattò, C.; Secundo, F.; James, G.; Villa, F.; Cappitelli, F. α-Chymotrypsin Immobilized on a Low-Density Polyethylene Surface Successfully Weakens Escherichia coli Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 4003. [Google Scholar] [CrossRef]
- Foukis, A.; Gkini, O.A.; Stergiou, P.Y.; Sakkas, V.A.; Dima, A.; Boura, K.; Koutinas, A.; Papamichael, E.M. Sustainable production of a new generation biofuel by lipase-catalyzed esterification of fatty acids from liquid industrial waste biomass. Bioresour. Technol. 2017, 238, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Belhacene, K.; Elagli, A.; Vivien, C.; Treizebré, A.; Dhulster, P.; Supiot, P.; Froidevaux, R. Investigation of the effect of plasma polymerized siloxane coating for enzyme immobilization and microfluidic device conception. Catalysts 2016, 6, 209. [Google Scholar] [CrossRef]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SFF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N.; Nikolskaya, A.B.; Lyagin, I.V.; Senko, O.V.; Makhlis, T.A.; Stepanov, N.A.; Maslova, O.V.; Mamedova, F.; Varfolomeyev, S.D. Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour. Technol. 2012, 114, 342–348. [Google Scholar] [CrossRef]
- Sudmalis, D.; Gagliano, M.C.; Pei, R.; Grolle, K.; Plugge, C.M.; Rijnaarts, H.H.M.; Zeeman, G.; Temmink, H. Fast anaerobic sludge granulation at elevated salinity. Water Res. 2018, 128, 293–303. [Google Scholar] [CrossRef]
- Chirchir, A.; Aoyi, A.; Kiriamiti, K.; Kumar, A. Improved biogas production over immobilized methanogenic consortia. Proceed. Sustain. Res. Innovat. 2015, 294–303. [Google Scholar]
- Magrí, A.; Vanotti, M.B.; Szögi, A.A. Anammox sludge immobilized in polyvinyl alcohol (PVA) cryogel carriers. Bioresour. Technol. 2012, 114, 231–240. [Google Scholar]
- Senko, O.; Maslova, O.; Efremenko, E. Optimization of the use of His6-OPH-based enzymatic biocatalysts for the destruction of Chlorpyrifos in soil. Int. J. Environ. Res. Public Health 2017, 14, 1438. [Google Scholar] [CrossRef]
- Bhattad, U.; Venkiteshwaran, K.; Cherukuri, K.; Maki, J.S.; Zitomer, D.H. Activity of methanogenic biomass after heat and freeze drying in air. Environ. Sci. Water Res. Technol. 2017, 3, 462–471. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Tatarinova, N.Y. The effect of long-term preservation of bacterial cells immobilized in poly(vinyl alcohol) cryogel on their viability and biosynthesis of target metabolites. Microbiology 2007, 76, 336–341. [Google Scholar] [CrossRef]
- Poszytek, K.; Ciezkowska, M.; Sklodowska, A.; Drewniak, L. Microbial consortium with high cellulolytic activity (MCHCA) for enhanced biogas production. Front. Microbiol. 2016, 7, 324. [Google Scholar] [CrossRef]
- Acs, N.; Bagi, Z.; Rakhely, G.; Minarovics, J.; Nagy, K.; Kovacs, K.L. Bioaugmentation of biogas production by a hydrogen-producing bacterium. Bioresour. Technol. 2015, 186, 286–293. [Google Scholar] [CrossRef]
- Neumann, L.; Scherer, P. Impact of bioaugmentation by compost on the performance and ecology of an anaerobic digester fed with energy crops. Bioresour. Technol. 2011, 102, 2931–2935. [Google Scholar] [CrossRef]
- Costa, J.C.; Barbosa, S.G.; Sousa, D.Z. Effects of pre-treatment and bioaugmentation strategies on the anaerobic digestion of chicken feathers. Bioresour. Technol. 2012, 120, 114–119. [Google Scholar] [CrossRef]
- Nkemka, V.N.; Gilroyed, B.; Yanke, J.; Gruninger, R.; Vedres, D.; McAllister, T. Bioaugmentation with an anaerobic fungus in a two-stage process for biohydrogen and biogas production using corn silage and cattail. Bioresour. Technol 2015, 185, 79–88. [Google Scholar] [CrossRef]
- Theuerl, S.; Klang, J.; Prochnow, A. Process disturbances in agricultural biogas production—Causes, mechanisms and effects on the biogas microbiome: A review. Energies 2019, 12, 365. [Google Scholar] [CrossRef]
- Sabathé, F.; Bélaïch, A.; Soucaille, P. Characterization of the cellulolytic complex (cellulosome) of Clostridium acetobutylicum. FEMS Microbiol. Lett. 2002, 217, 15–22. [Google Scholar] [CrossRef]
- Liang, L.; Song, X.; Kong, J.; Shen, C.; Huang, T.; Hu, Z. Anaerobic biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by a facultative anaerobe Pseudomonas sp. JP1. Biodegradation 2014, 25, 825–833. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Bøhle, L.A.; Gåseidnes, S.; Dalhus, B.; Bjørås, M.; Mathiesen, G. Characterization of the chitinolytic machinery of Enterococcus faecalis V583 and high-resolution structure of its oxidative CBM33 enzyme. J. Mol. Biol. 2012, 416, 239–254. [Google Scholar] [CrossRef]
- Kakaee, A.H.; Paykani, A.; Ghajar, M. The influence of fuel composition on the combustion and emission characteristics of natural gas fueled engines. Renew Sustain. Energ. Rev. 2014, 38, 64–78. [Google Scholar] [CrossRef]
- Savadogo, P.W.; Savago, A.; Ouattara, A.S.; Sedogo, M.P.; Traore, A.S. Anaerobic biodegradation of sumithion an organophosphorus insecticide used in Burkina Faso agriculture by acclimatized indigenous bacteria. Pak. J. Biol. Sci. 2007, 10, 1896–1905. [Google Scholar]
- Krishna, K.R.; Philip, L. Biodegradation of mixed pesticides by mixed pesticide enriched cultures. J. Environ. Sci. Health B 2008, 44, 18–30. [Google Scholar] [CrossRef]
- Arbeli, Z.; Brenner, A.; Abeliovich, A. Treatment of high-strength dairy wastewater in an anaerobic deep reservoir: Analysis of the methanogenic fermentation pathway and the rate-limiting step. Water Res. 2006, 40, 3653–3659. [Google Scholar] [CrossRef]
- Ma, J.; Frear, C.; Wang, Z.W.; Yu, L.; Zhao, Q.; Li, X.; Chen, X. A simple methodology for rate-limiting step determination for anaerobic digestion of complex substrates and effect of microbial community ratio. Bioresour. Technol. 2013, 134, 391–395. [Google Scholar] [CrossRef]
- Regueiro, L.; Veiga, P.; Figueroa, M.; Alonso-Gutierrez, J.; Stams, A.J.; Lema, J.M.; Carballa, M. Relationship between microbial activity and microbial community structure in six full-scale anaerobic digesters. Microbiol. Res. 2012, 167, 581–589. [Google Scholar] [CrossRef]
- Cai, M.; Wilkins, D.; Chen, J.; Ng, S.K.; Lu, H.; Jia, Y.; Lee, P.K.H. Metagenomic reconstruction of key anaerobic digestion pathways in municipal sludge and industrial wastewater biogas-producing systems. Front. Microbiol. 2016, 7, 778. [Google Scholar] [CrossRef]
- Kalyuzhnyi, S.V.; Shestakova, N.M.; Tourova, T.P.; Poltaraus, A.B.; Gladchenko, M.A.; Trukhina, A.I.; Nazina, T.N. Phylogenetic analysis of a microbial community involved in anaerobic oxidation of ammonium nitrogen. Microbiology 2010, 79, 237–246. [Google Scholar] [CrossRef]
- Treu, L.; Kougias, P.G.; Campanaro, S.; Bassani, I.; Angelidaki, I. Deeper insight into the structure of the anaerobic digestion microbial community; the biogas microbiome database is expanded with 157 new genomes. Bioresour. Technol. 2016, 216, 260–266. [Google Scholar] [CrossRef]
- Dhaked, R.K.; Singh, P.; Singh, L. Biomethanation under psychrophilic conditions: A review. Waste Manag. 2010, 30, 2490–2496. [Google Scholar] [CrossRef]
- Wu, B.; Bibeau, E.L. Development of 3-D anaerobic digester heat transfer model for cold weather applications. Trans. ASABE 2006, 49, 749–757. [Google Scholar] [CrossRef]
- Ye, J.; Li, D.; Sun, Y.; Wang, G.; Yuan, Z.; Zhen, F.; Wang, Y. Improved biogas production from rice straw by co-digestion with kitchen waste and pig manure. Waste Manag. 2013, 33, 2653–2658. [Google Scholar] [CrossRef]
- A Pilarska, A.; Wolna-Maruwka, A.; Pilarski, K. Kraft lignin grafted with polyvinylpyrrolidone as a novel microbial carrier in biogas production. Energies 2018, 11, 3246. [Google Scholar] [CrossRef]
- Youngsukkasem, S.; Rakshit, S.K.; Taherzadeh, M.J. Biogas production by encapsulated methane-producing bacteria. BioResources 2012, 7, 56–65. [Google Scholar]
- Hanaki, K.; Hirunmasuwan, S.; Masuo, T. Protection of methanogenic bacteria from low pH and toxic materials by immobilization using polyvinyl alcohol. Water Res. 1994, 28, 877–885. [Google Scholar] [CrossRef]
- Takei, T.; Ikeda, K.; Ijima, H.; Kawakami, K. Fabrication of poly (vinyl alcohol) hydrogel beads crosslinked using sodium sulfate for microorganism immobilization. Process Biochem. 2011, 46, 566–571. [Google Scholar] [CrossRef]
- Dubber, D.; Gray, N. Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J. Environ. Sci. Health A 2010, 45, 1595–1600. [Google Scholar] [CrossRef]
- Gladchenko, M.A.; Kovalev, D.A.; Kovalev, A.A.; Litti, Y.V.; Nozhevnikova, A.N. Methane production by anaerobic digestion of organic waste from vegetable processing facilities. Appl. Biochem. Microbiol. 2017, 53, 242–249. [Google Scholar] [CrossRef]
- Kalyuzhnyi, S.; Gladchenko, M.; Starostina, E.; Shcherbakov, S.; Versprille, A. Combined biological and physico-chemical treatment of baker’s yeast wastewater. Water Sci. Technol. 2005, 52, 175–181. [Google Scholar] [CrossRef]
- Gladchenko, M.A.; Gaydamaka, S.N.; Murygina, V.P.; Varfolomeev, S.D. The optimization of the conversion of agricultural waste into volatile fatty acids under anaerobic conditions. Moscow Univ. Chem. Bull 2014, 69, 187–193. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).