Montmorillonite K10-Catalyzed Solvent-Free Conversion of Furfural into Cyclopentenones
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Methods
3.2. General Experimental Procedure for the Microwave-Assisted Cyclical Rearrangement of Furfural and Amines
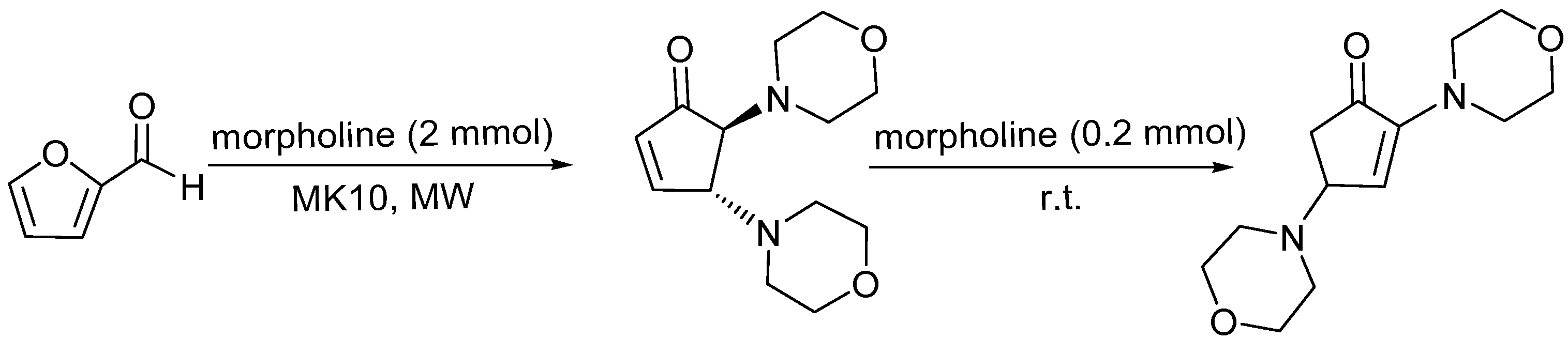
3.3. General Protocol for the Synthesis of 2,4-Diamorpholinecyclopent-2-enones
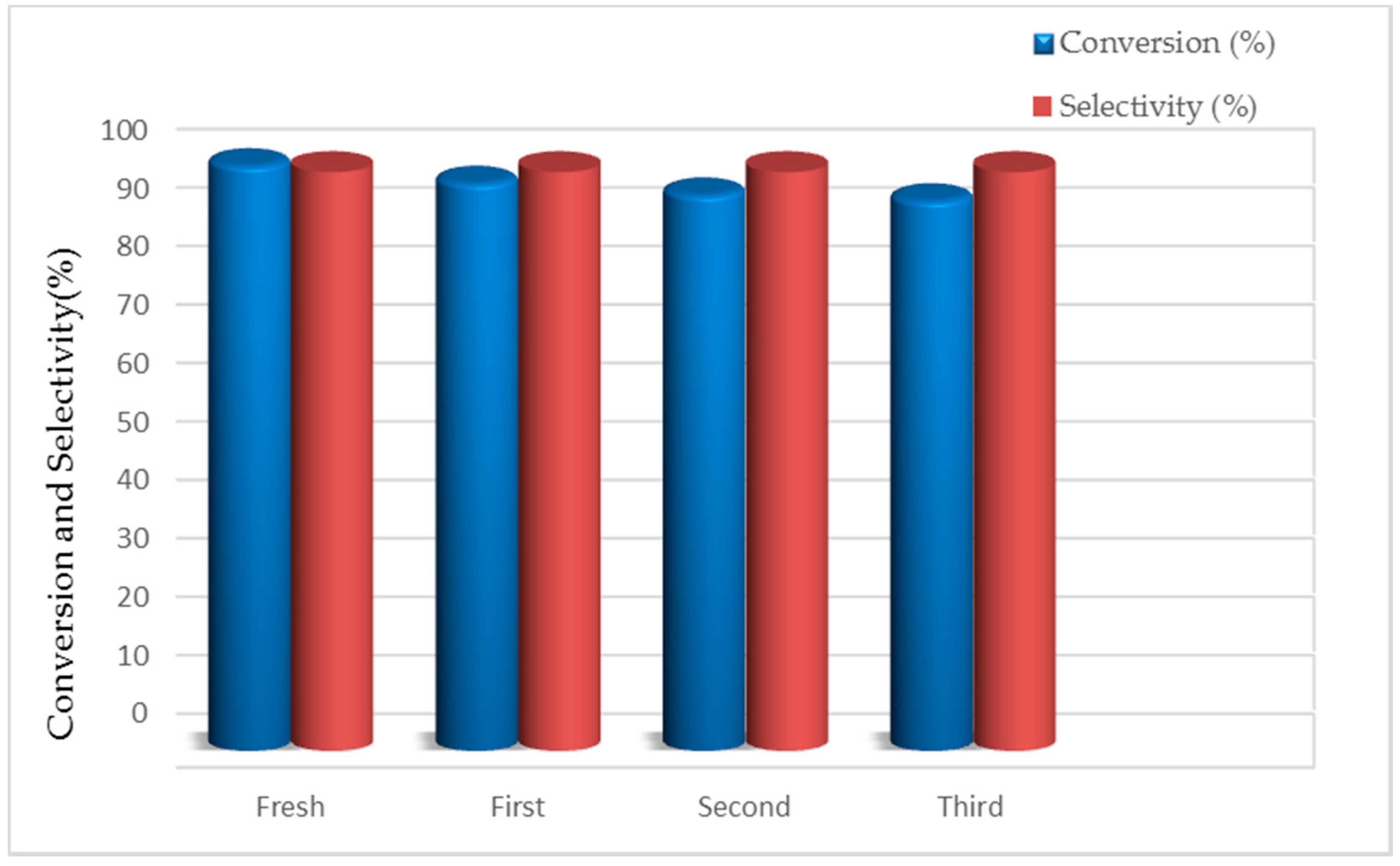
3.4. Catalyst Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thomas, J.M.; Raja, R.; Lewis, D.W. Single-site heterogeneous catalysts. Angew. Chem. Int. Ed. 2005, 44, 6456. [Google Scholar] [CrossRef]
- Ballini, R.; Bosica, G.; Carloni, L.; Maggi, R.; Sartori, G. Zeolite HSZ-360 as a new reusable catalyst for the direct acetylation of alcohols and phenols under solventless conditions. Tetrahedron Lett. 1998, 39, 6049–6052. [Google Scholar] [CrossRef]
- Procopio, A.; De Luca, G.; Nardi, M.; Oliverio, M.; Paonessa, R. General MW-assisted grafting of MCM-41: Study of the dependence on time dielectric heating and solvent. Green Chem. 2009, 11, 770–773. [Google Scholar] [CrossRef]
- Procopio, A.; Cravotto, G.; Oliverio, M.; Costanzo, P.; Nardi, M.; Paonessa, R. An eco-sustainable Erbium(III)-catalysed method for formation/cleavage of O-tert-butoxy carbonates. Green Chem. 2011, 13, 436–443. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Macario, A.; De Luca, G.; Nardi, M.; Procopio, A. A bifuctional heterogeneous catalyst erbium-based: A cooperative route towards C-C bond formation. Molecules 2014, 19, 10218–10229. [Google Scholar] [CrossRef] [PubMed]
- Procopio, A.; Das, G.; Nardi, M.; Oliverio, M.; Pasqua, L. A mesoporous Er(III)-MCM-41 catalyst for the cyanosilylation of aldehydes and ketones under solvent-free conditions. ChemSusChem 2008, 1, 916–919. [Google Scholar] [CrossRef]
- Mikami, K. Green Reaction Media in Organic Synthesis; Blackwell: Hoboken, NJ, USA, 2005. [Google Scholar]
- Nelso, W.M. Green Solvents for Chemistry Perspectives and Practice; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Lindström, U.M. Stereoselective organic reactions in water. Chem. Rev. 2002, 10, 2751–2772. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Tagarelli, A.; Sindona, G. Simple and efficient MW-assisted cleavage of acetals and ketals in pure water. Tetrahedron Lett. 2007, 48, 8623–8627. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Rosati, O. Highly efficient and versatile chemoselective addition of amines to epoxides in water catalyzed by erbium(III) triflate. Tetrahedron Lett. 2008, 49, 2289–2293. [Google Scholar] [CrossRef]
- Simon, M.O.; Li, C.J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Paonessa, R.; Nardi, M.; Procopio, A. Catalyst-free tosylation of lipophilic alcohols in water. RSC Adv. 2013, 3, 2548–2552. [Google Scholar] [CrossRef]
- Nardi, M.; Herrera Cano, N.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Aqueous MW eco-friendly protocol for amino group protection. RSC Adv. 2015, 5, 18751–18760. [Google Scholar] [CrossRef]
- Nardi, M.; Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Olivito, F.; Procopio, A. Selective acetylation of small biomolecules and their derivatives catalyzed by Er(OTf)3. Catalysts 2017, 7, 269. [Google Scholar] [CrossRef]
- Abbott, A.P.; Davies, D.L.; Capper, G.; Rasheed, R.K.; Tambyrajah, V. Ionic Liquids and Their Use As solvents. U.S. Patent 7,183,433, 27 February 2007. [Google Scholar]
- Di Gioia, M.L.; Barattucci, A.; Bonaccorsi, P.; Leggio, A.; Minuti, L.; Romio, E.; Temperini, A.; Siciliano, C. Deprotection/reprotection of the amino group in α-amino acids and peptides. A one-pot procedure in [Bmim][BF4] ionic liquid. RSC Adv. 2014, 4, 2678–2686. [Google Scholar] [CrossRef]
- De Nino, A.; Maiuolo, L.; Merino, P.; Nardi, M.; Procopio, A.; Roca-Lopez, D.; Russo, B.; Algieri, V. Efficient organocatalyst supported on a simple ionic liquid as a recoverable system for the asymmetric diels-alder reaction in the presence of water. ChemCatChem 2015, 7, 830–835. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Gagliardi, A.; Leggio, A.; Leotta, V.; Romio, E.; Liguori, A. N-Urethane protection of amines and amino acids in an ionic liquid. RSC Adv. 2015, 5, 63407–63420. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Nardi, M.; Olivito, F.; Procopio, A. Simple and efficient Fmoc removal in ionic liquid. RSC Adv. 2017, 7, 36482–36491. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Gorke, J.T.; Srienc, F.; Kazlauskas, R.J. Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun. 2008, 10, 1235–1237. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Ginies, C.; Chemat, F. Total lipid extraction of food using d-limonene as an alternative to n-hexane. Chromatographia 2008, 68, 311–313. [Google Scholar] [CrossRef]
- Lapkin, A.; Plucinski, P.K.; Cutler, M. Comparative assessment of technologies for extraction of artemisinin. J. Nat. Prod. 2006, 69, 1653–1664. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- García, J.I.; García-Marín, H.; Pires, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar] [CrossRef]
- Nardi, M.; Oliverio, M.; Costanzo, P.; Sindona, G.; Procopio, A. Eco-friendly stereoselective reduction of α,β-unsaturated carbonyl compounds by Er(OTf)3/NaBH4 in 2-MeTHF. Tetrahedron 2015, 71, 1132–1135. [Google Scholar] [CrossRef]
- Nardi, M.; Herrera Cano, N.; De Nino, A.; Di Gioia, M.L.; Maiuolo, L.; Oliverio, M.; Santiago, A.; Sorrentino, D.; Procopio, A. An eco-friendly tandem tosylation/ferrier N-glycosylation of amines catalyzed by Er(OTf)3 in 2-MeTHF. Tetrahedron Lett. 2017, 58, 1721–1726. [Google Scholar] [CrossRef]
- Jaselnik, M.; Varma, R.S.; Polanca, S.; Kocevar, M. Catalyst-free reactions under solvent-free conditions: Microwave-assisted synthesis of heterocyclic hydrazones below the melting points of neat reactants. Chem. Commun. 2001, 18, 1716–1717. [Google Scholar] [CrossRef]
- Procopio, A.; Dalpozzo, R.; De Nino, A.; Maiuolo, L.; Nardi, M.; Russo, B. Synthesis of acetonides from epoxides catalyzed by erbium(III) triflate. Adv. Synth. Catal. 2005, 347, 1447–1450. [Google Scholar] [CrossRef]
- Procopio, A.; De Nino, A.; Nardi, M.; Oliverio, M.; Paonessa, R.; Pasceri, R. A new microwave-assisted organocatalytic solvent-free synthesis of optically enriched Michael adducts. Synlett 2010, 12, 1849–1853. [Google Scholar] [CrossRef]
- Procopio, A.; Costanzo, P.; Curini, M.; Nardi, M.; Oliverio, M.; Paonessa, R. An eco-sustainable erbium(III) triflate catalyzed formation and cleavage of tert-butyl ethers. Synthesis 2011, 1, 73–78. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Nardi, M.; Calandruccio, C.; Salerno, R.; Procopio, A. Tunable microwave-assisted method for the solvent-free and catalyst-free peracetylation of natural products. Beilstein J. Org. Chem. 2016, 12, 2222–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiuolo, L.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Delso, I.; Tallarida, M.A.; De Nino, A. Nitrones and nucleobase-containing spiro-isoxazolidines derived from isatin and indanone: Solvent-free microwave-assisted stereoselective synthesis and theoretical calculations. RSC Adv. 2017, 7, 48980–48988. [Google Scholar] [CrossRef]
- Mason, T.J. Sonochemistry: Current uses and future prospects in the chemical and processing industries. Philos. Trans. Royal Soc. Lond. Ser. A 1999, 357, 355–369. [Google Scholar] [CrossRef]
- Kappe, O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Desai, K.R. Green Chemistry Microwave Synthesis, 1st ed.; Himalaya Publication House: New Delhi, India, 2005; p. 20. [Google Scholar]
- Cravotto, G.; Cintas, P. The combined use of microwaves and ultrasound: Improved tools in process chemistry and organic synthesis. Chem. Eur. J. 2007, 13, 1902–1909. [Google Scholar] [CrossRef]
- Bortolini, O.; D’Agostino, M.; De Nino, A.; Maiuolo, L.; Nardi, M.; Sindona, G. Solvent-free, microwave assisted 1,3-cycloaddition of nitrones with vinyl nucleobases for the synthesis of N,O-nucleosides. Tetrahedron 2008, 64, 8078–8081. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Romeo, R. MW-assisted Er(OTf)3-catalyzed mild cleavage of isopropylidene acetals in Tricky substrates. Tetrahedron Lett. 2008, 49, 1961–1964. [Google Scholar] [CrossRef]
- Oliverio, M.; Nardi, M.; Cariati, L.; Vitale, E.; Bonacci, S.; Procopio, A. “On water” mw-assisted synthesis of hydroxytyrosol fatty esters. ACS Sustainable Chem. Eng. 2016, 4, 661–665. [Google Scholar] [CrossRef]
- Maiuolo, L.; De Nino, A.; Algieri, V.; Nardi, M. Microwave-assisted 1,3-dipolar cyclo-addition: Recent advances in synthesis of isoxazolidines. Mini Rev. Org. Chem. 2017, 14, 136–142. [Google Scholar] [CrossRef]
- Summerton, L.; Sneddon, H.F.; Jones, L.C.; Clark, J.H. Green and Sustainable Medicinal Chemistry: Methods, Tools and Strategies for the 21st Century Pharmaceutical Industry; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Nardi, M.; Bonacci, S.; De Luca, G.; Maiuolo, J.; Oliverio, M.; Sindona, G.; Procopio, A. Biomimetic synthesis and antioxidant evaluation of 3,4-DHPEA-EDA [2-(3,4-hydroxyphenyl) ethyl (3S,4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate]. Food Chem. 2014, 162, 89–93. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; Cariati, L.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Synthesis and antioxidant evaluation of lipophilic oleuropein aglycone derivatives. Food Funct. 2017, 8, 4684–4692. [Google Scholar] [CrossRef]
- Costanzo, P.; Calandruccio, C.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. First multicomponent reaction exploiting glycerol carbonate synthesis. J. Clean Prod. 2018, 202, 504–509. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Zheng, M.; Li, S.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Industrially scalable and cost-effective synthesis of 1,3-cyclopentanediol with furfuryl alcohol from lignocellulose. Green Chem. 2016, 18, 3607–3613. [Google Scholar] [CrossRef]
- Rossi, A.; Kapahi, P.; Natoli, G.; Takahashi, T.; Chen, Y.; Karin, M.; Santoro, M.G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 2000, 403, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, C.H.; Straus, D.S. Cyclopentenone causes cell cycle arrest and represses cyclin D1 promoter activity in MCF-7 breast cancer cells. Oncogene 2002, 21, 2212–2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozera, C.; Carattoli, A.; De Marco, A.; Amici, C.; Giorgi, C.; Santoro, M.G. Inhibition of HIV-1 replication by cyclopentenone prostaglandins in acutely infected human cells. J. Clin. Investig. 1996, 97, 1795–1803. [Google Scholar] [CrossRef]
- Santoro, M.G. Antiviral activity of cyclopentenone prostanoids. Trends Microbiol. 1997, 5, 276–281. [Google Scholar] [CrossRef]
- Griffiths, K.; Kumar, P.; Mattock, J.D.; Abdul-Sada, A.; Pitak, M.B.; Coles, S.J.; Navarro, O.; Vargas, A.; Kostakis, G.E. Efficient Ni2IILn2III electrocyclization catalysts for the synthesis of trans-4,5-diaminocyclopent-2-enones from 2-furaldehyde and primary or secondary amines. Inorg. Chem. 2016, 55, 6988–6994. [Google Scholar] [CrossRef]
- Li, S.W.; Batey, R.A. Mild lanthanide (III) catalyzed formation of 4,5-diaminocyclopent-2-enones from 2-furaldehyde and secondary amines: A domino condensation/ring-opening/electrocyclization process. Chem. Commun. 2007, 36, 3759–3761. [Google Scholar] [CrossRef]
- Gomes, R.F.A.; Esteves, N.R.; Coelho, J.A.S.; Afonso, C.A.M. Copper (II) triflate as a reusable catalyst for the synthesis of trans-4,5-diamino-cyclopent-2-enones in water. J. Org. Chem. 2018, 83, 7509–7513. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.P.M.; Afonso, C.A.M.; Caddick, S. Synthesis of 2,4-bifunctionalised cyclopentenones from 2-furaldehyde. RSC Adv. 2013, 3, 14975–14978. [Google Scholar] [CrossRef]
- Procopio, A.; Costanzo, P.; Curini, M.; Nardi, M.; Oliverio, M.; Sindona, G. Erbium(III) chloride in ethyl lactate as a smart ecofriendly system for efficient and rapid stereoselective synthesis of trans-4,5-diaminocyclopent-2-enones. ACS Sustain. Chem. Eng. 2013, 1, 541–544. [Google Scholar] [CrossRef]
- Ramesh, D.; Reddy, T.S.; Narasimhulu, M.; Rajaram, S.; Suryakiran, N.; Mahesh, K.C.; Venkateswarlu, Y. Efficient and rapid stereoselective synthesis of trans-4,5-diaminocyclopent-2-enones by acidic ionic liquid under solvent-free conditions. Chem. Lett. 2009, 38, 586–587. [Google Scholar]
- Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Olivito, F.; Sindona, G.; Procopio, A. Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfural. Green Chem. 2017, 19, 5403–5411. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Nardi, M.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. Biorenewable deep eutectic solvent for selective and scalable conversion of furfural into cyclopentenone derivatives. Molecules 2018, 23, 1891. [Google Scholar] [CrossRef] [PubMed]
- Estevão, M.S.; Afonso, C.A.M. Synthesis of trans-4,5-diaminocyclopent-2-enones from furfural catalyzed by Er(III) immobilized on silica. Tetrahedron Lett. 2017, 58, 302–304. [Google Scholar] [CrossRef]
- Estevão, M.S.; Martins, R.J.V.; Afonso, C.A.M. Synthesis of trans-4,5-bis-dibenzylaminocyclopent-2-enone from furfural catalyzed by ErCl3·6H2O. J. Chem. Educ. 2017, 94, 1587–1589. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, R. Heterogeneous MOF catalysts for the synthesis of trans-4,5-diaminocyclopent-2-enones from furfural and secondary amines. Catal. Commun. 2019, 120, 11–16. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Kaur, N.; Kishore, D. Montmorillonite: An efficient, heterogeneous and green catalyst for organic synthesis. J. Chem. Pharm. Res. 2012, 4, 991–1015. [Google Scholar]
- Kumar, B.S.; Dhakshinamoorthy, A.; Pitchumani, K. K10 montmorillonite clays as environmentally benign catalysts for organic reactions. Catal. Sci. Technol. 2014, 4, 2378–2396. [Google Scholar] [CrossRef]
- Hechelski, M.; Ghinet, A.; Brice Louvel, B.; Dufrenoy, P.; Rigo, B.; Daich, A.; Waterlot, C. From conventional lewis acids to heterogeneous montmorillonite K10: Eco-friendly plant-based catalysts used as green lewis acids. ChemSusChem 2018, 11, 1249–1277. [Google Scholar] [CrossRef]
- Li, J.-T.; Xing, C.-Y.; Li, T.-S. An efficient and environmentally friendly method for synthesis of arylmethylenemalononitrile catalyzed by Montmorillonite K10–ZnCl2 under ultrasound irradiation. J. Chem. Technol. Biotechnol. 2004, 79, 1275–1278. [Google Scholar] [CrossRef]
- Nardi, M.; Cozza, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. 1,5-benzoheteroazepines through eco-friendly general condensation reactions. Tetrahedron Lett. 2011, 52, 4827–4834. [Google Scholar] [CrossRef]
- Nardi, M.; Cozza, A.; De Nino, A.; Oliverio, M.; Procopio, A. One-pot synthesis of dibenzo[b,e][1,4]diazepin-1-ones. Synthesis 2012, 44, 800–804. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Nardi, M.; Rivalta, I.; Procopio, A. Facile ecofriendly synthesis of monastrol and its structural isomers via biginelli reaction. ACS Sustain. Chem. Eng. 2014, 2, 1228–1233. [Google Scholar] [CrossRef]
- Herrera Cano, N.; Uranga, J.G.; Nardi, M.; Procopio, A.; Wunderlin, D.A.; Santiago, A.N. Selective and eco-friendly procedures for the synthesis of benzimidazole derivatives. The role of the Er(OTf)3 catalyst in the reaction selectivity. Beilstein J. Org. Chem. 2016, 12, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- De Nino, A.; Maiuolo, L.; Nardi, M.; Pasceri, R.; Procopio, A.; Russo, B. Development of one-pot three component reaction for the synthesis of N′-aryl-N-cyanoformamidines, essential precursors of formamidine pesticides family. Arab. J. Chem. 2016, 9, 32–37. [Google Scholar] [CrossRef]
- Costanzo, P.; Bonacci, S.; Cariati, L.; Nardi, M.; Oliverio, M.; Procopio, A. Simple and efficient sustainable semi-synthesis of oleacein [2-(3,4-hydroxyphenyl) ethyl (3S,4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate] as potential additive for edible oils. Food Chem. 2018, 245, 410–414. [Google Scholar] [CrossRef]
- Paonessa, R.; Nardi, M.; Di Gioia, M.L.; Olivito, F.; Oliverio, M.; Procopio, A. Eco-friendly synthesis of lipophilic EGCG derivatives and antitumor and antioxidant evaluation. Nat. Prod. Commun. 2018, 9, 1117–1122. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Belsito, E.L.; Leggio, A.; Leotta, V.; Romio, E.; Siciliano, C.; Liguori, A. Reduction of amide carbonyl group and formation of modified amino acids and dipeptides. Tetrahedron Lett. 2015, 56, 2062–2066. [Google Scholar] [CrossRef]
- Belsito, E.L.; De Marco, R.; Di Gioia, M.L.; Liguori, A.; Perri, F.; Viscomi, M.C. N-(4-nitrophenylsulfonyl)- and N-(fluorenylmethoxycarbonyl)-N-ethyl amino acid methyl esters—A practical approach. Eur. J. Org. Chem. 2010, 22, 4245–4252. [Google Scholar] [CrossRef]
- Duspara, P.A.; Batey, R.A. A short total synthesis of the marine sponge pyrrole-2- aminoimidazole alkaloid ( )-agelastatin, A. Angew. Chem. Int. Ed. 2013, 52, 10862–10866. [Google Scholar] [CrossRef] [PubMed]
- Verrier, C.; Moebs-Sanchez, S.; Queneaua, Y.; Popowycz, F. The Piancatelli reaction and its variants: Recent applications to high added-value chemicals and biomass valorization. Org. Biomol. Chem. 2018, 16, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Lewis, K.G.; Mulquiney, C.E. Aspects of the formation and use of stenhouse salts and related compounds. Tetrahedron 1977, 33, 463–475. [Google Scholar] [CrossRef]
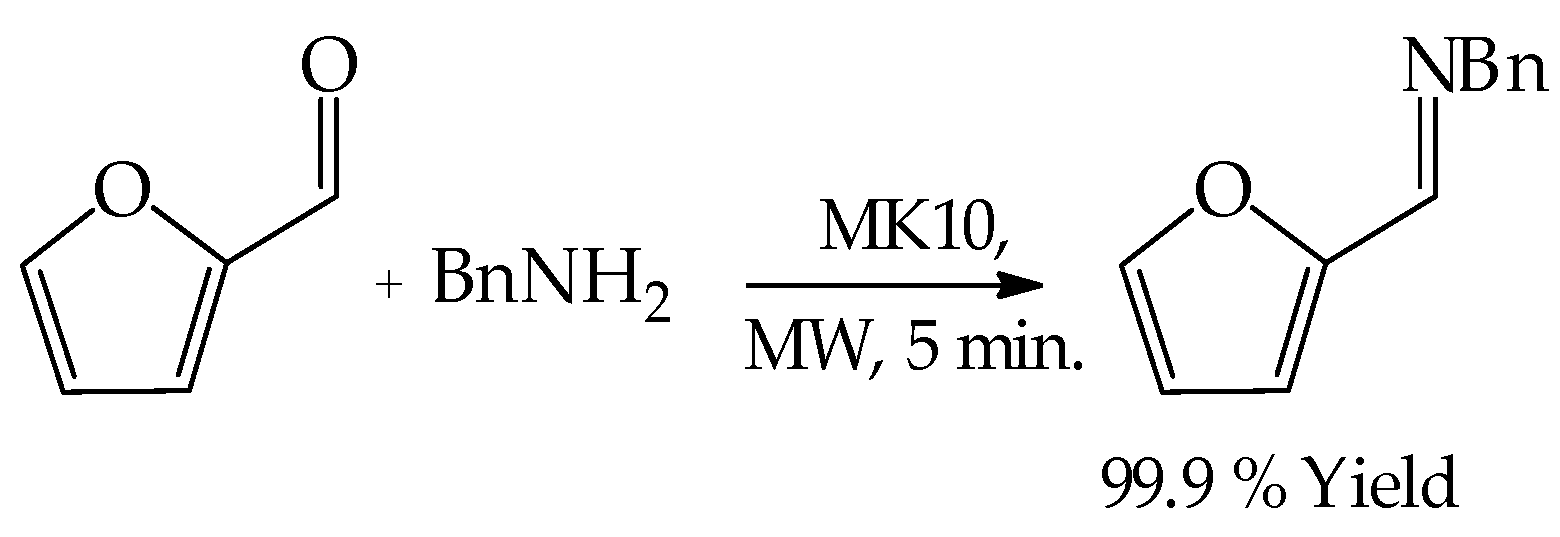
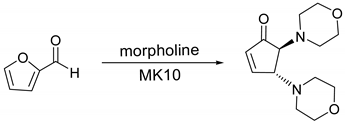
| Entry | MK10 wt (%) b | Temp (°C) | Time (min) | Conversion (%) c | Selectivity (%) d |
|---|---|---|---|---|---|
| 1 | 10 | rt | 120 | 99.9 | 55.0 |
| 2 | 10 | 60 | 35 | 99.9 | 61.1 |
| 3 | 10 | 80 | 35 | 99.9 | 65.3 |
| 4 | 20 | 80 | 35 | 99.9 | 75.0 |
| 5 | 20 | 100 | 20 | 99.9 | 78.3 |
| 6 | - | 100 | 20 | 60.0 | 49.0 |
| 7 e | 20 | 80 | 5 | 99.9 | 99.9 |
| 8 e | 20 | 60 | 5 | 99.9 | 99.9 |
| 9 e | 20 | 60 | 10 | 99.9 | 99.9 |
| Entry | Amine | Product | Conversion (%) | Selectivity (%) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | 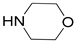 | 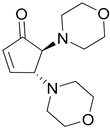 | 99.9 | 99.9 | 95.0 |
| 2 |  | 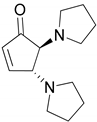 | 95.9 | 99.7 | 96.6 |
| 3 | 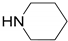 | 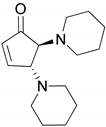 | 99.9 | 99.7 | 99.6 |
| 4 |  | 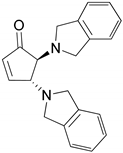 | 91.0 | 99.3 | 90.8 |
| 5 | 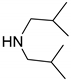 | 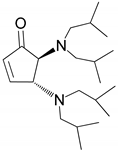 | 97.8 | 99.0 | 94.8 |
| 6 |  | 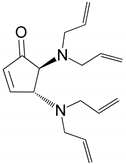 | 96.8 | 99.0 | 94.1 |
| 7 | 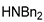 | 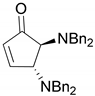 | 90.6 | 99.0 | 98.3 |
| 8 |  | 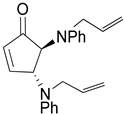 | 99.7 | 99.8 | 97.8 |
| 9 | 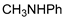 | 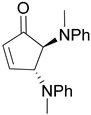 | 99.9 | 99.6 | 95.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonacci, S.; Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Oliverio, M.; Procopio, A. Montmorillonite K10-Catalyzed Solvent-Free Conversion of Furfural into Cyclopentenones. Catalysts 2019, 9, 301. https://doi.org/10.3390/catal9030301
Bonacci S, Nardi M, Costanzo P, De Nino A, Di Gioia ML, Oliverio M, Procopio A. Montmorillonite K10-Catalyzed Solvent-Free Conversion of Furfural into Cyclopentenones. Catalysts. 2019; 9(3):301. https://doi.org/10.3390/catal9030301
Chicago/Turabian StyleBonacci, Sonia, Monica Nardi, Paola Costanzo, Antonio De Nino, Maria Luisa Di Gioia, Manuela Oliverio, and Antonio Procopio. 2019. "Montmorillonite K10-Catalyzed Solvent-Free Conversion of Furfural into Cyclopentenones" Catalysts 9, no. 3: 301. https://doi.org/10.3390/catal9030301
APA StyleBonacci, S., Nardi, M., Costanzo, P., De Nino, A., Di Gioia, M. L., Oliverio, M., & Procopio, A. (2019). Montmorillonite K10-Catalyzed Solvent-Free Conversion of Furfural into Cyclopentenones. Catalysts, 9(3), 301. https://doi.org/10.3390/catal9030301