Layered Double Hydroxides as Bifunctional Catalysts for the Aryl Borylation under Ligand-Free Conditions
Abstract
:1. Introduction
2. Results
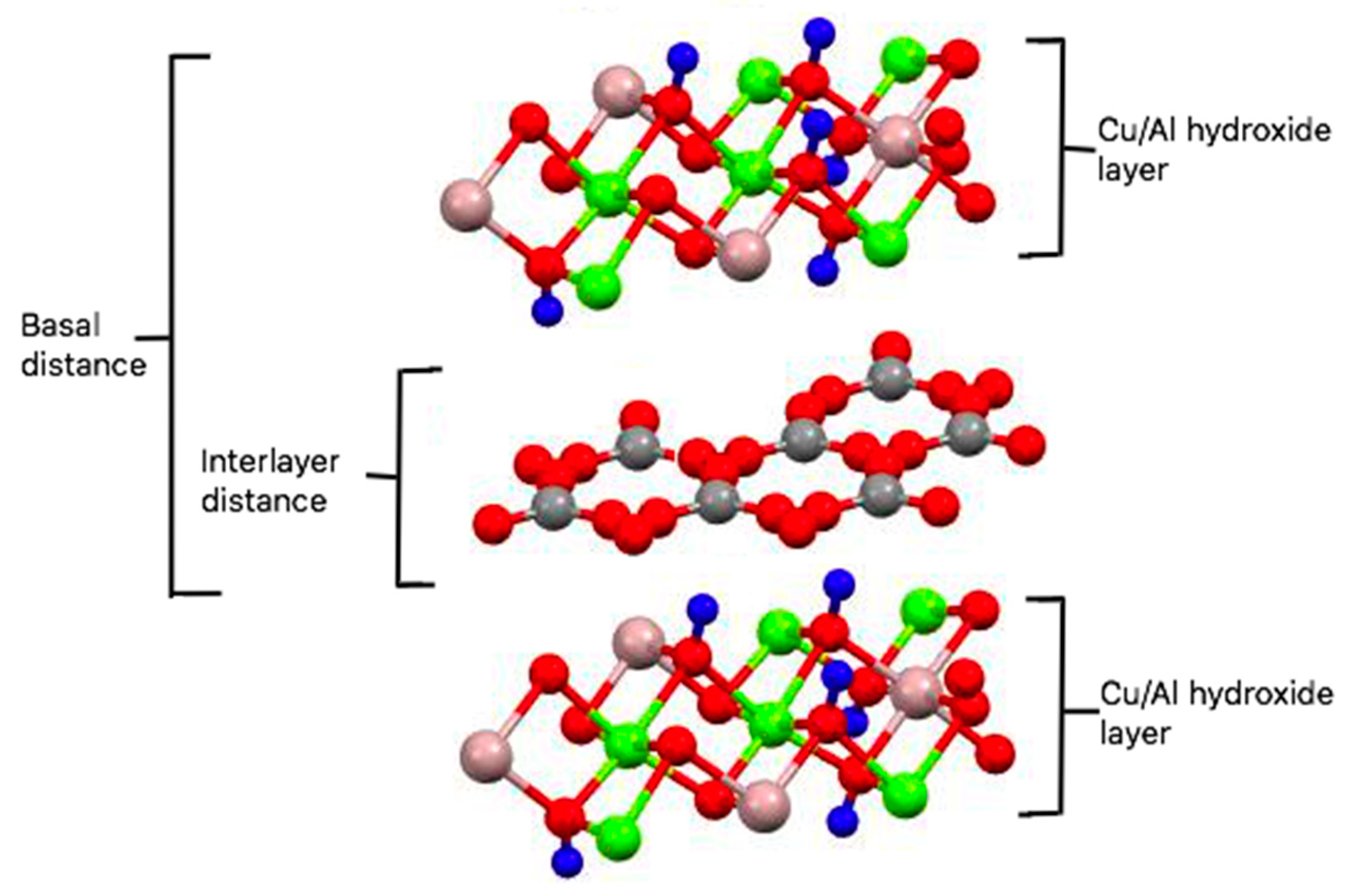
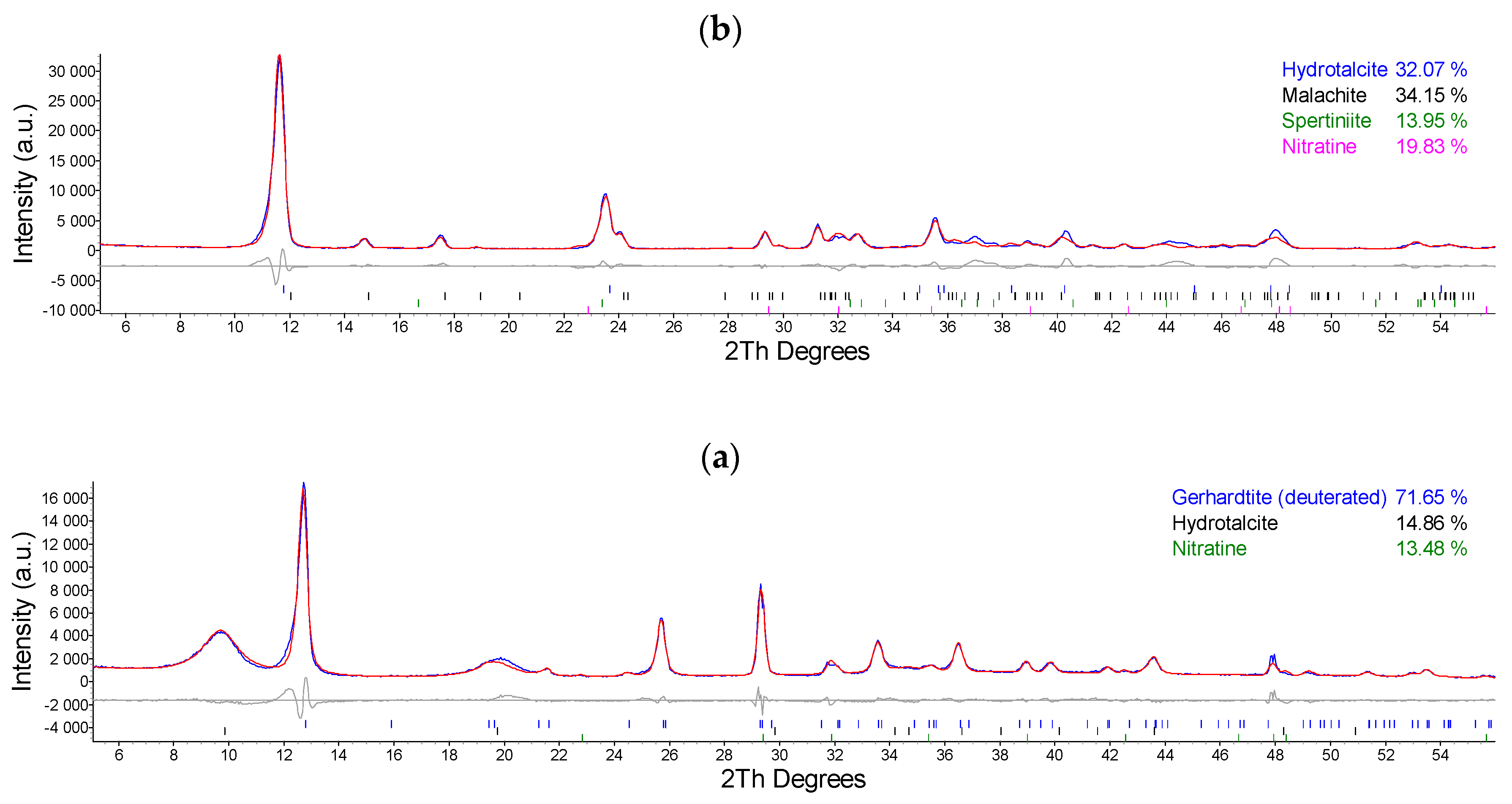
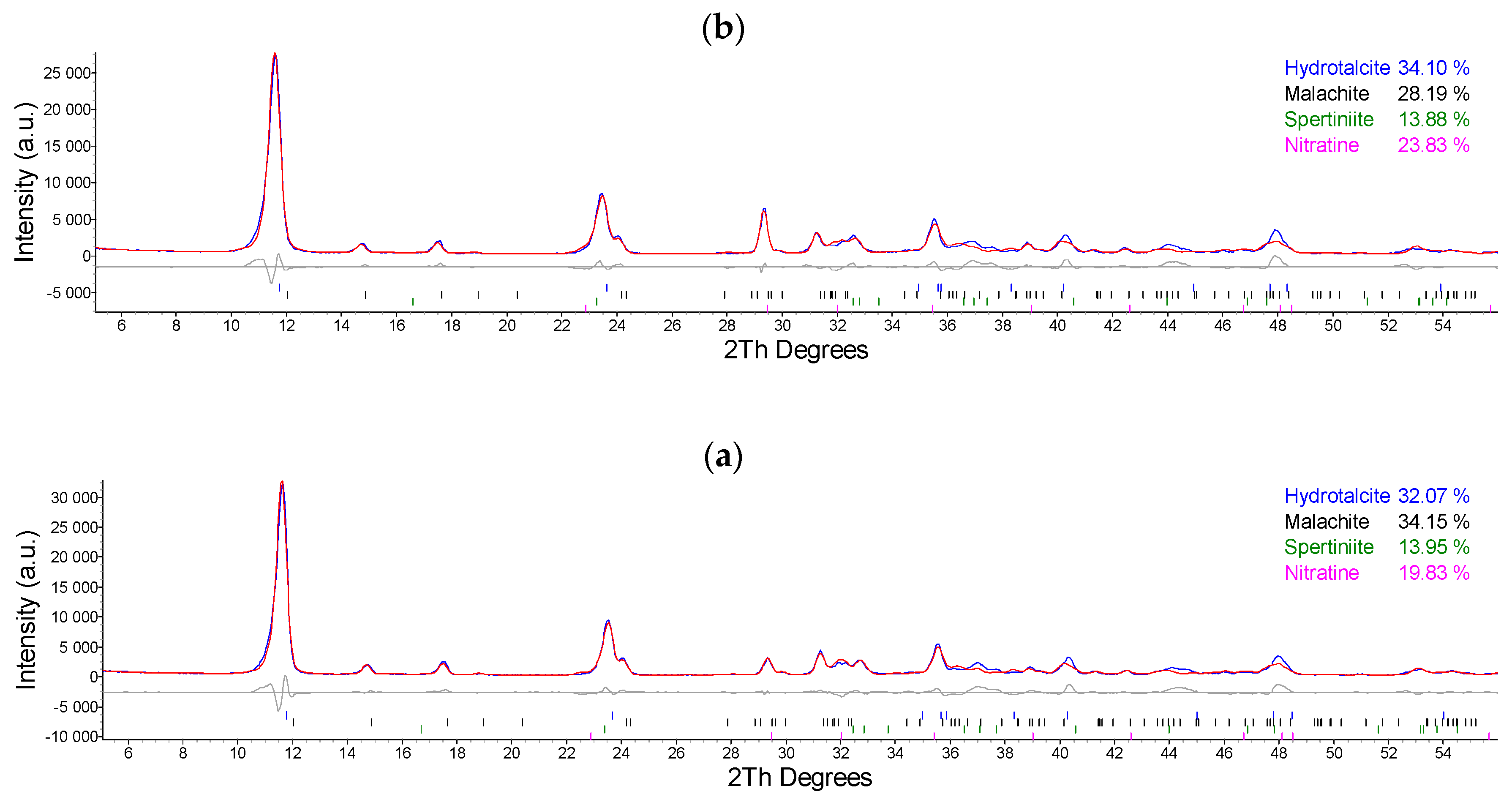
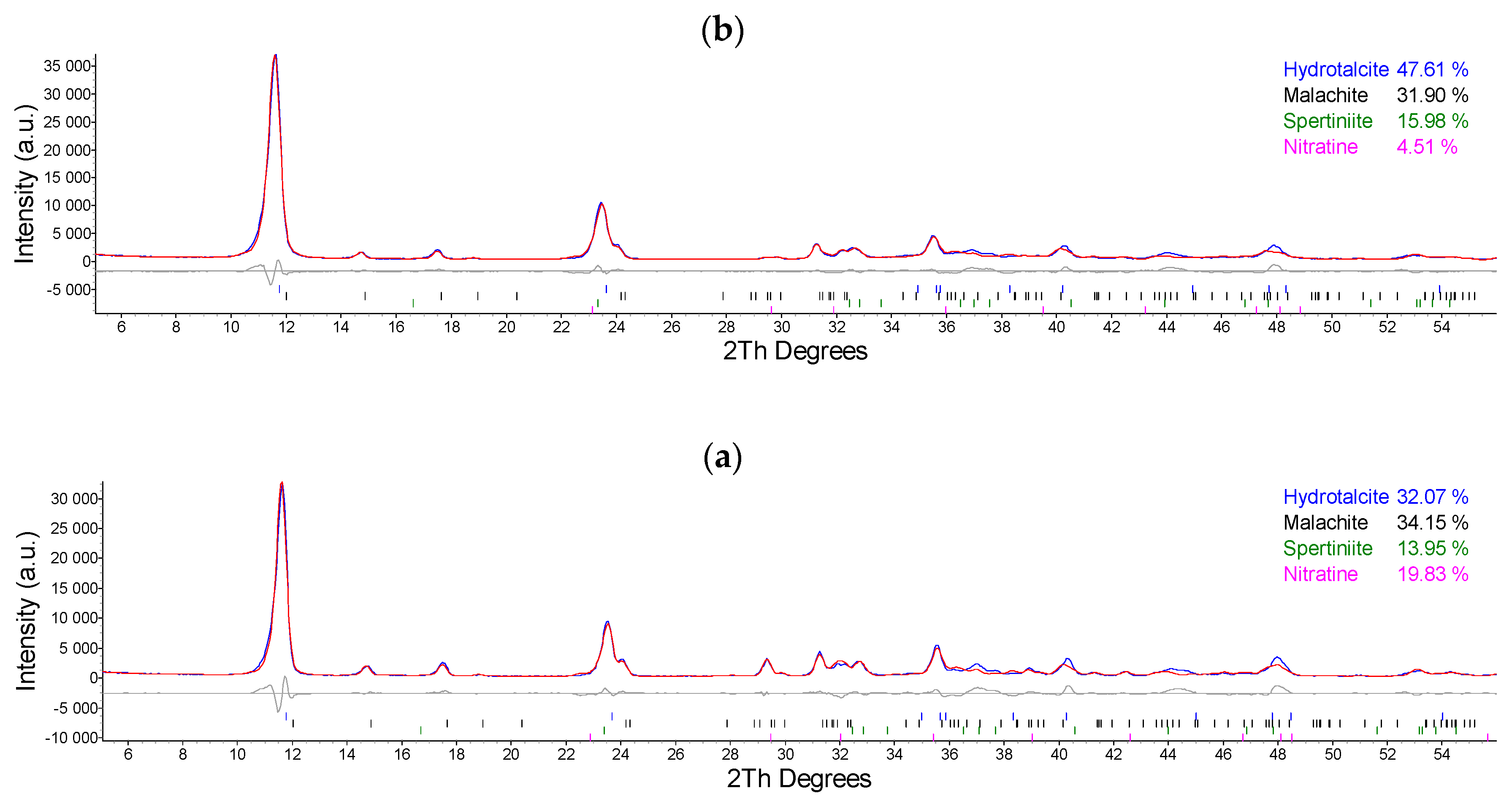
2.1. Synthesis and Characterization of Cu/Al LDH
2.2. Synthesis of Aryl Boronic Esters Employing Cu/Al LDH Catalyst
3. Discussion
4. Materials and Methods
4.1. Synthesis of the Cu/Al Layered Double Hydroxides
4.2. Synthesis of Boronic Esters Employing Palladium Nanoparticles
4.3. Synthesis of Boronic Esters Employing Na2PdCl4/Cu/Al LDH
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Chen, X.X.; Jiang, Y.B. Recent advances in boronic acid-based optical chemosensors. Analyst 2017, 142, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Roll, M.F. Ionic borohydride clusters for the next generation of boron thin-films: Nano-building blocks for electrochemical and refractory materials. J. Mater. Res. 2016, 31, 2736–2748. [Google Scholar] [CrossRef]
- Pisarev, M.A.; Dagrosa, M.A.; Juvenal, G.J. Boron neutron capture therapy in cancer: Past, present and future. Arq. Bras. Endocrinol. Metab. 2007, 51, 852–856. [Google Scholar] [CrossRef]
- Hall, D.G. Boronic Acids; Wiley: Weinheim, Germany, 2005. [Google Scholar]
- Cheng, Y.; Mgck-Lichtenfeld, C.; Studer, A. Metal-Free Radical Borylation of Alkyl and Aryl Iodides. Angew. Chem. Int. Ed. 2018, 57, 16832–16836. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, L. Pyridine-Catalyzed Radical Borylation of Aryl Halides. J. Am. Chem. Soc. 2017, 139, 607–610. [Google Scholar] [CrossRef]
- Chow, W.K.; Yuen, O.Y.; Choy, P.Y.; So, C.M.; Lau, C.P.; Wong, W.T.; Kwong, F.Y. A decade advancement of transition metal-catalyzed borylation of aryl halides and sulfonates. RSC Adv. 2013, 3, 12518–12539. [Google Scholar] [CrossRef]
- Ishiyama, T.; Murata, M.; Miyaura, N. Palladium(0)-Catalyzed Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters. J. Org. Chem. 1995, 60, 7508–7510. [Google Scholar] [CrossRef]
- Avitia, B.; MacIntosh, E.; Muhia, S.; Kelson, E. Single-flask preparation of polyazatriaryl ligands by a sequential borylation/Suzuki-Miyaura coupling. Tetrahedron Lett. 2011, 52, 1631–1634. [Google Scholar] [CrossRef]
- Xie, D.; Rong, L.; Zhang, D.; Hu, J.; Xiao, D.; Li, X.; Xiang, Y.; Jin, W. Palladium-catalyzed borylation of m-dibromobenzene derivative and its applications in one-pot tandem Suzuki-Miyaura arenes synthesis. Tetrahedron 2015, 71, 8871–8875. [Google Scholar] [CrossRef]
- Hemming, D.; Fritzemeier, R.; Westcott, S.A.; Santos, W.L.; Steel, P.G. Copper-boryl mediated organic synthesis. Chem. Soc. Rev. 2018, 47, 7477–7494. [Google Scholar] [CrossRef]
- Ratniyom, J.; Dechnarong, N.; Yotphan, S.; Kiatisevi, S. Convenient Synthesis of Arylboronates through a Synergistic Pd/Cu-Catalyzed Miyaura Borylation Reaction under Atmospheric Conditions. Eur. J. Org. Chem. 2014, 7, 1381–1385. [Google Scholar] [CrossRef]
- Mills, S.J.; Christy, A.G.; Génin, J.-M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: Natural layered double hydroxides. Miner. Mag. 2012, 75, 1289–1336. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Béres, A.; Pálinkó, I.; Kiricsi, I.; Nagy, J.B.; Kiyozumi, Y.; Mizukami, F. Layered double hydroxides and their pillared derivatives—Materials for solid base catalysis; synthesis and characterization. App. Catal. A Gen. 1999, 182, 237–247. [Google Scholar] [CrossRef]
- Silva, A.C.; Senra, J.D.; de Souza, A.L.F.; Malta, L.F.B. A Ternary Catalytic System for the Room Temperature Suzuki-Miyaura Reaction in Water. Sci. World J. 2013. [Google Scholar] [CrossRef]
- Sreedhar, B.; Arundhathi, R.; Reddy, P.L.; Reddy, M.A.; Kantam, M.L. Cu-Al Hydrotalcite: An efficient and reusable ligant-free catalyst for the coupling of aryl chlorides with aliphatic, aromatic, and N(H)-heterocyclic amines. Synthesis 2009, 15, 2517–2522. [Google Scholar] [CrossRef]
- Neves, V.A.; Costa, M.V.; Senra, J.D.; Aguiar, L.C.S.; Malta, L.F.B. Thermal behavior of LDH 2CuAl.CO3 and 2CuAl.CO3/Pd. J. Therm. Anal. Calorim. 2017, 130, 689–694. [Google Scholar] [CrossRef]
- Senra, J.D.; Malta, L.F.B.; Michel, R.C.; Cordeiro, Y.; Simão, R.A.; Simas, A.B.C.; Aguiar, L.C.S. Hydrophilic cyclodextrin protected Pd nanoclusters: Insights into their size control and host–guest behavior. J. Mater. Chem. 2011, 21, 13516–13523. [Google Scholar] [CrossRef]
- Segal, S.R.; Carrado, K.A.; Marshall, C.L.; Anderson, K.B. Catalytic decomposition of alcohols, including ethanol, for in situ H2 generation in a fuel stream using a layered double hydroxide-derived catalyst. Appl. Catal. A Gen. 2003, 248, 33–45. [Google Scholar] [CrossRef]
- Manivannan, R.; Pandurangan, A. Formation of ethyl benzene and styrene by side chain methylation of toluene over calcined LDHs. Appl. Clay Sci. 2009, 44, 137–143. [Google Scholar] [CrossRef]
- Lwin, Y.; Yarmo, M.A.; Yaakob, Z.; Mohamad, A.B.; Daud, W.R.W. Synthesis and characterization of Cu/Al layered double hydroxides. Mater. Res. Bull. 2001, 36, 193–198. [Google Scholar] [CrossRef]
- Muñoz, V.; Zotin, F.M.Z.; Palacio, L.A. Copper-aluminum hydrotalcite type precursors for NOx abatement. Catal. Today 2015, 250, 173–179. [Google Scholar] [CrossRef]
- Gao, P.; Xie, R.; Wang, H.; Zhong, L.; Xia, L.; Zhang, Z.; Wei, W.; Sun, Y. Cu/Zn/Al/Zr catalyst via phase-pure hydrotalcite-like compounds for methanol synthesis from carbon dioxide. J. CO2 Util. 2015, 11, 41–48. [Google Scholar] [CrossRef]
- Ichikawa, S.; Miyazoe, S.; Matsuoka, O. A highly efficient Cu/Al(OH)3 catalyst for the hydration of acrylonitrile to acrylamide. Chem. Lett. 2011, 40, 512–514. [Google Scholar] [CrossRef]
- Qu, J.; He, X.; Chen, M.; Hu, H.; Zhang, Q.; Liu, X. Mechanochemical synthesis of Cu/Al and methyl orange intercalated Cu/Al layered double hydroxides. Mater. Chem. Phys. 2017, 191, 173–180. [Google Scholar] [CrossRef]
- Neves, V.A. Nanostructured Materials Based on Cu/Al Layered Double Hydroxides, Pd and Cyclodextrin as Catalysts for Cross-Coupling Reactions. Master’s Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 2018. [Google Scholar]
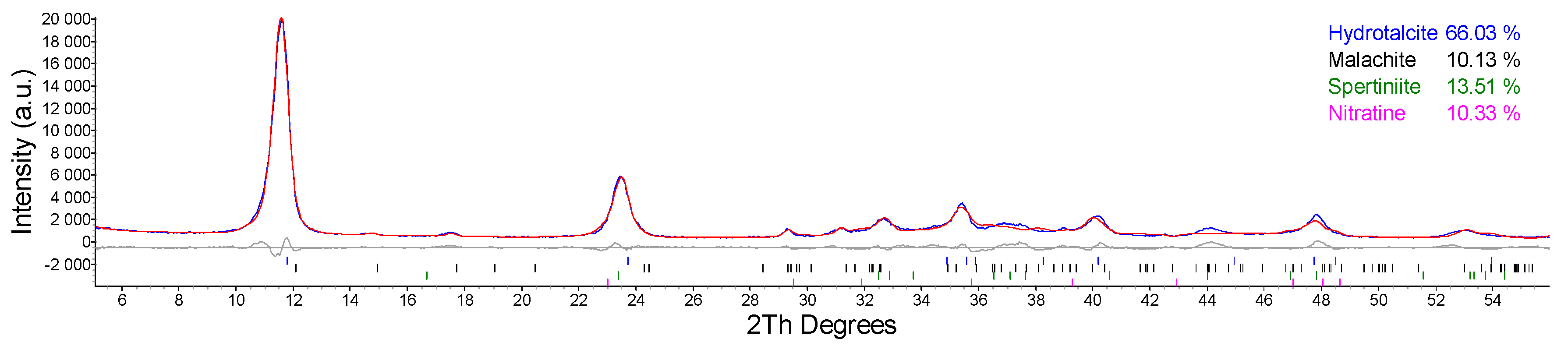
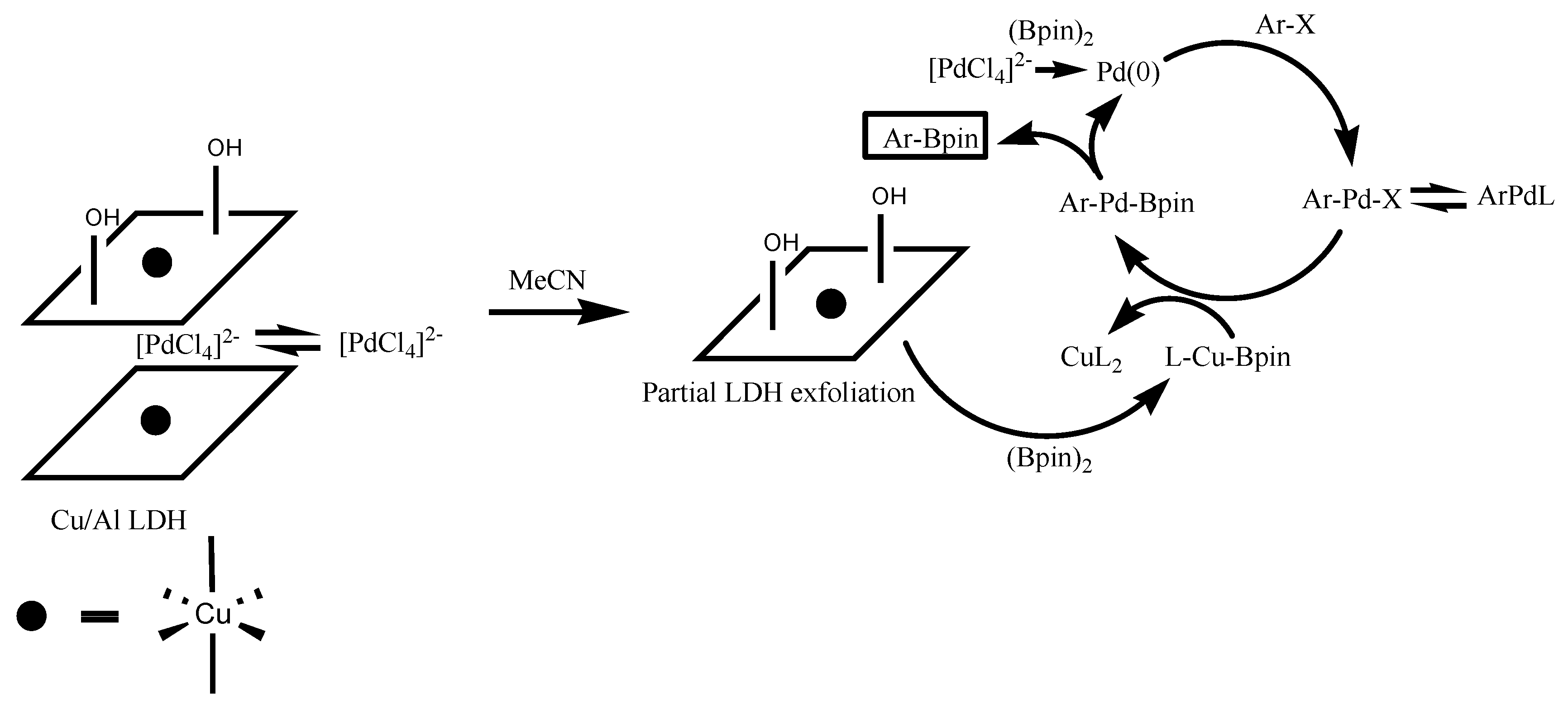
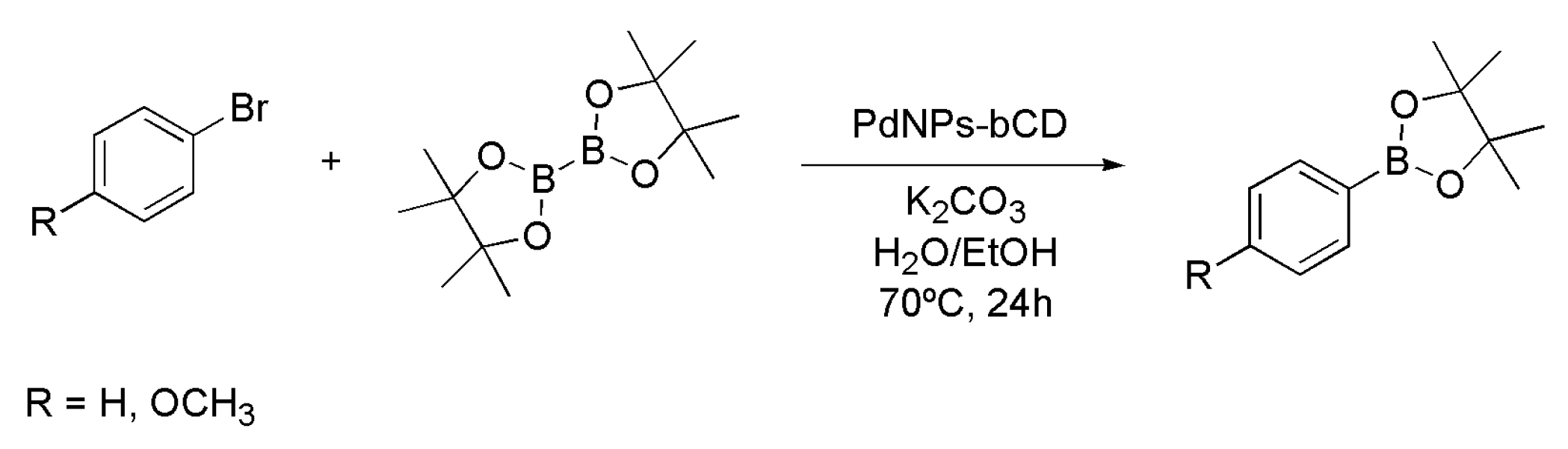
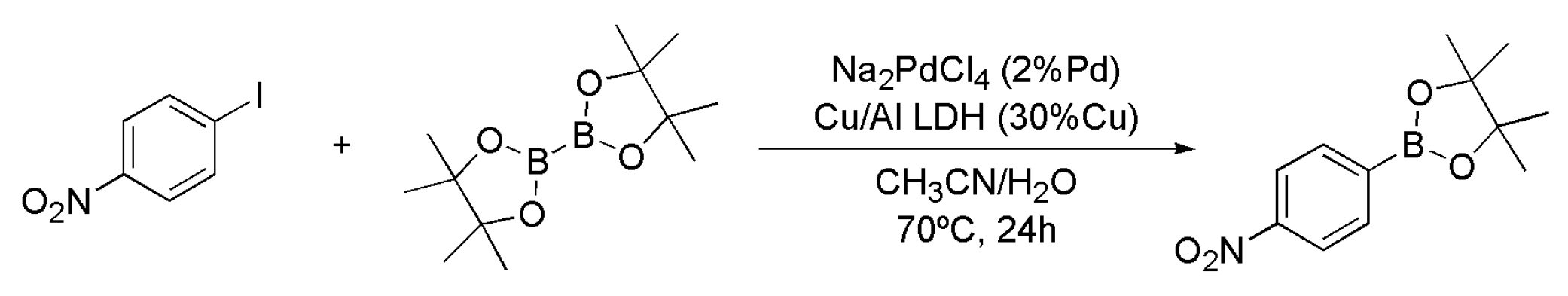
| Entry | Catalyst System | Cu (mol%) | Pd (mol%) | Yield (%) 1 of 3 |
|---|---|---|---|---|
| 1 | LDH | 30 | - | 29% |
| 2 | Malachite | 30 | - | <5% |
| 3 | Na2PdCl4 | - | 2 | <5% |
| 4 | PdNPs-CD | - | 2 | <5% |
| 5 | PdNPs-CD | - | 2 | <5% |
| 6 | Na2PdCl4/CuSO4 | 30 | 2 | <5% |
| 7 | Na2PdCl4/LDH | 30 | 2 | 98% |
| 8 | Na2PdCl4/CuSO4/LDH 2 | 30 | 2 | 77% |
| 9 | Na2PdCl4/LDH 3 | 30 | 2 | 35% |
| 10 | Na2PdCl4/LDH | 15 | 2 | 54% |
| 11 | Na2PdCl4/LDH | 7.5 | 2 | 42% |
| 12 | Na2PdCl4/LDH | 3.75 | 2 | 26% |
| 13 | Na2PdCl4/LDH | 30 | 1 | 46% |
| 14 | Na2PdCl4/LDH | 30 | 0.5 | 42% |
| 15 | Na2PdCl4/LDH | 30 | 0.05 | 23% |
| 16 | Na2PdCl4/LDH 4 | 30 | 2 | <10% |
| 17 | Na2PdCl4/LDH 5 | 30 | 2 | <10% |
| 18 | Na2PdCl4/LDH 6 | 30 | 2 | <5% |
| Entry | Aryl Halide | Product | Yield (%) |
|---|---|---|---|
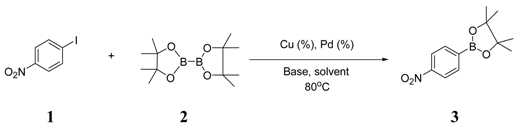
| 1 | 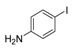 | 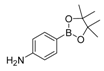 4 | <10 |
| 2 |  | 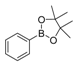 5 | <10 |
| 3 | 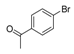 | 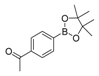 6 | 55 |
| 4 | 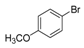 | 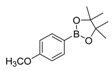 7 | 98 |
| 5 | 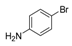 |  4 | <10 |
| 6 |  | 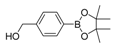 8 | 30 |
| 7 |  | 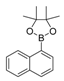 9 | 45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.C.L.L.F.; Neves, V.A.; Ramos, V.S.; Silva, R.S.F.; Campos, J.B.d.; Silva, A.A.d.; Malta, L.F.B.; Senra, J.D. Layered Double Hydroxides as Bifunctional Catalysts for the Aryl Borylation under Ligand-Free Conditions. Catalysts 2019, 9, 302. https://doi.org/10.3390/catal9040302
Silva LCLLF, Neves VA, Ramos VS, Silva RSF, Campos JBd, Silva AAd, Malta LFB, Senra JD. Layered Double Hydroxides as Bifunctional Catalysts for the Aryl Borylation under Ligand-Free Conditions. Catalysts. 2019; 9(4):302. https://doi.org/10.3390/catal9040302
Chicago/Turabian StyleSilva, Lorenna C. L. L. F., Vinícius A. Neves, Vitor S. Ramos, Raphael S. F. Silva, José B. de Campos, Alexsandro A. da Silva, Luiz F. B. Malta, and Jaqueline D. Senra. 2019. "Layered Double Hydroxides as Bifunctional Catalysts for the Aryl Borylation under Ligand-Free Conditions" Catalysts 9, no. 4: 302. https://doi.org/10.3390/catal9040302
APA StyleSilva, L. C. L. L. F., Neves, V. A., Ramos, V. S., Silva, R. S. F., Campos, J. B. d., Silva, A. A. d., Malta, L. F. B., & Senra, J. D. (2019). Layered Double Hydroxides as Bifunctional Catalysts for the Aryl Borylation under Ligand-Free Conditions. Catalysts, 9(4), 302. https://doi.org/10.3390/catal9040302





