Mechanochemically Synthesized Supported Magnetic Fe-Nanoparticles as Catalysts for Efficient Vanillin Production
Abstract
:1. Introduction
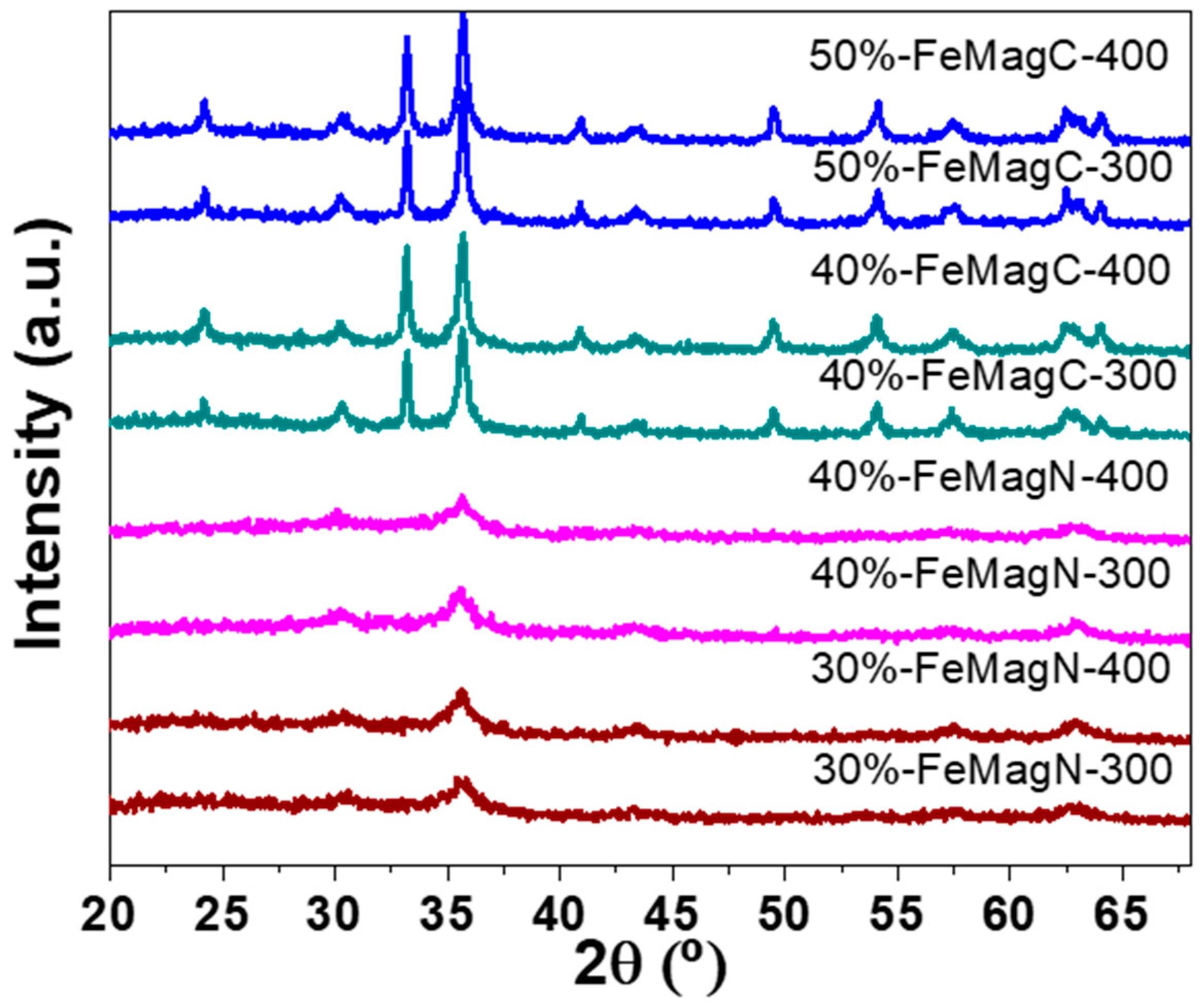
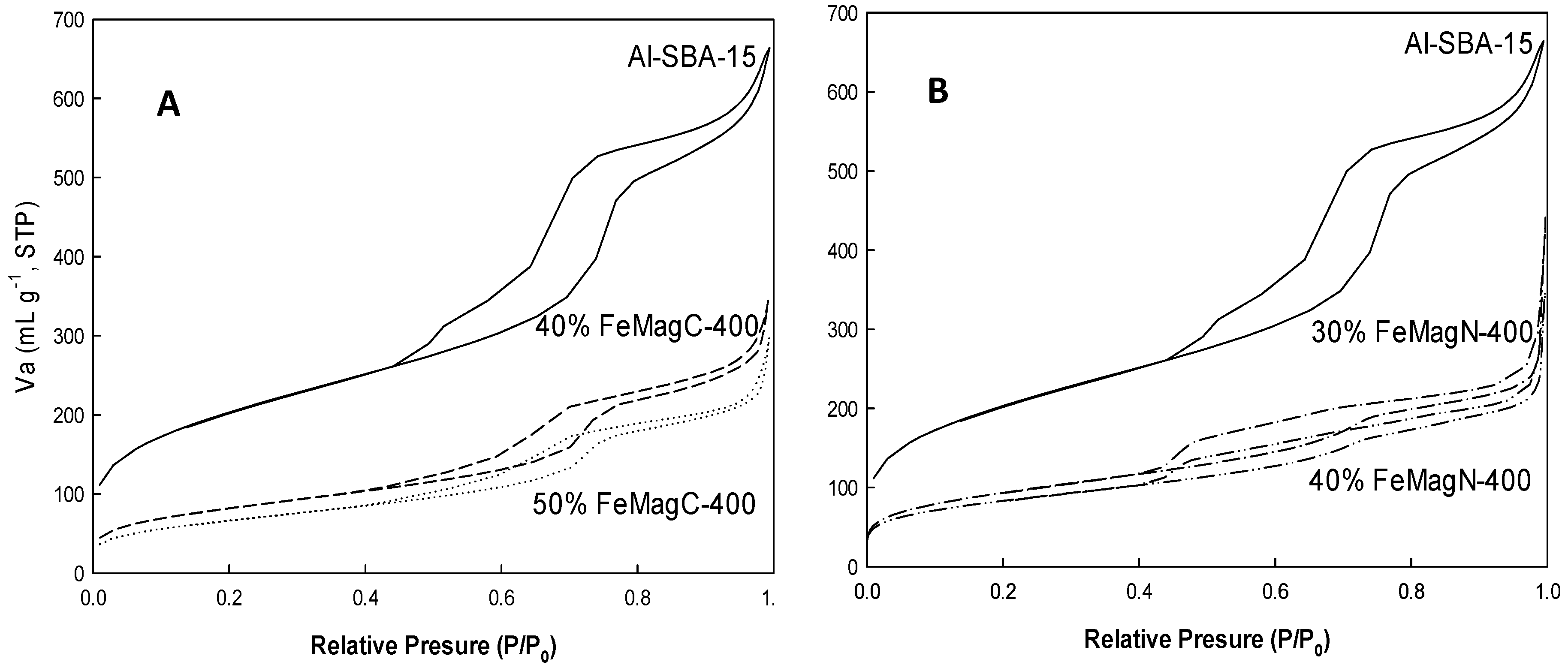
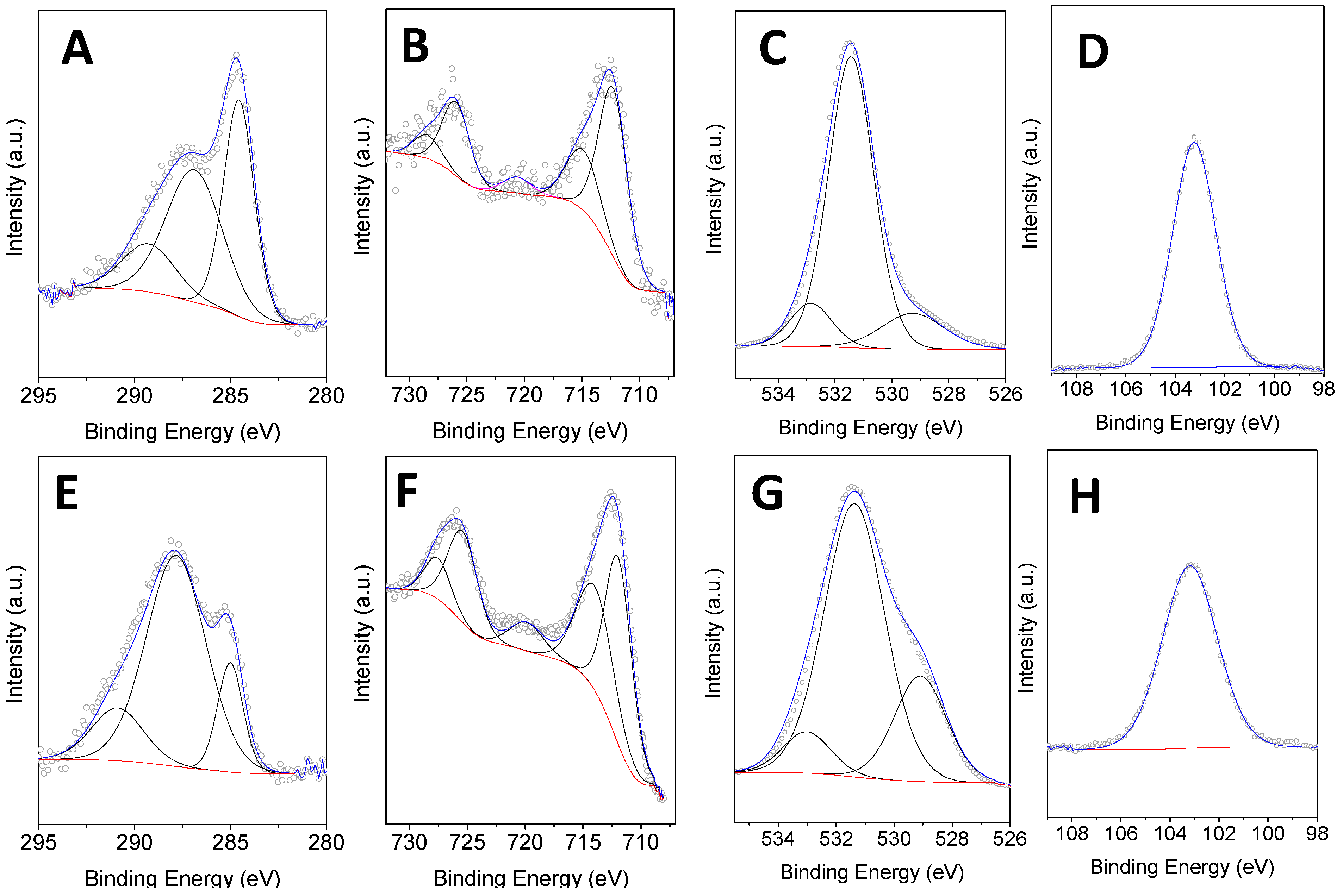
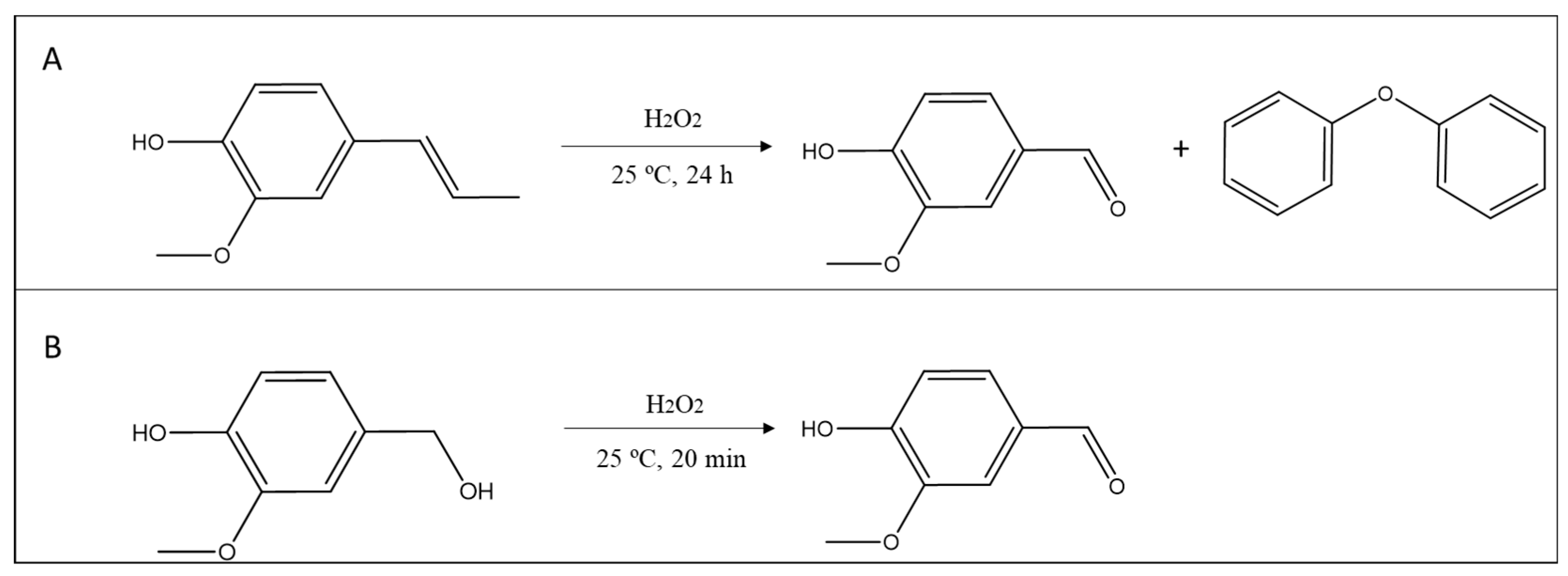
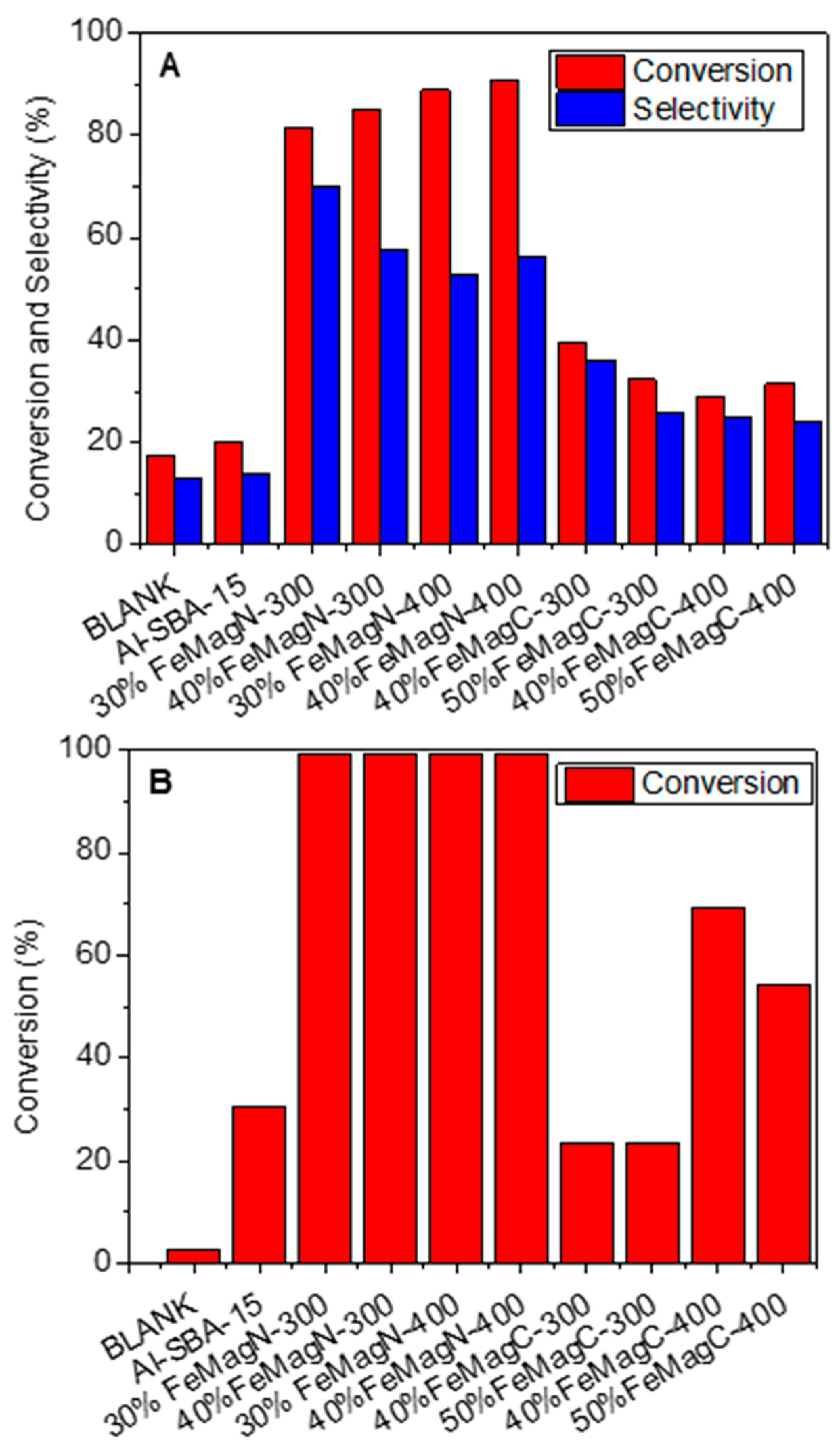
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Al-SBA-15
3.2. Synthesis of Catalysts
3.3. Characterization Techniques
3.4. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sang, W.; Bai, F. Vascular diversity patterns of forest ecosystem before and after a 43-year interval under changing climate conditions in the Changbaishan Nature Reserve, northeastern China. In Forest Ecology; Springer: Berlin, Germany, 2008; Volume 201, pp. 115–130. [Google Scholar]
- Clark, J.H.; Luque, R.; Matharu, A.S. Green chemistry, biofuels, and biorefinery. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 183–207. [Google Scholar] [CrossRef]
- Liao, S.; Wang, F.; Wu, T.; Pan, W. Crude oil price decision under considering emergency and release of strategic petroleum reserves. Energy 2016, 102, 436–443. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef]
- Stöcker, M. Biofuels and biomass to liquid fuels in the biorefinery: Catalytic conversion of lignocellulosic biomass using porous materials. Angew. Chem. Int. Ed. 2008, 47, 9200–9211. [Google Scholar] [CrossRef]
- Filiciotto, L.; Luque, R. Biomass Promises: A Bumpy Road to a Renewable Economy. Curr. Green Chem. 2018, 5, 47–59. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Muñoz-Batista, M.J.; Luque, R. Environmental Catalysis: Present and Future. ChemCatChem 2018, 11, 18–38. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Franco, A.; De, S.; Balu, A.M.; Romero, A.A.; Luque, R. Selective oxidation of isoeugenol to vanillin over mechanochemically synthesized aluminosilicate supported transition metal catalysts. ChemistrySelect 2017, 2, 9546–9551. [Google Scholar] [CrossRef]
- Filiciotto, L.; Balu, A.M.; Romero, A.A.; Rodríguez-Castellón, E.; van der Waal, J.C.; Luque, R. Benign by design preparation of humin-based iron oxide catalytic nanocomposites. Green Chem. 2017, 19, 4423–4434. [Google Scholar] [CrossRef]
- Chen, C.L.; Chang, H.M.; Kirk, T.K. Aromatic acids produced during degradation of lignin in spruce wood by Phanerochaete chrysosporium. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood 1982, 36, 3–9. [Google Scholar]
- Gallage, N.J.; Møller, B.L. Vanillin–bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol. Plant 2015, 8, 40–57. [Google Scholar] [CrossRef]
- Lampman, G.M.; Sharpe, S.D. A phase transfer catalyzed permanganate oxidation: Preparation of vanillin from isoeugenol acetate. J. Chem. Educ. 1983, 60, 503. [Google Scholar] [CrossRef]
- Márquez-Medina, M.D.; Prinsen, P.; Li, H.; Shih, K.; Romero, A.A.; Luque, R. Continuous-Flow Synthesis of Supported Magnetic Iron Oxide Nanoparticles for Efficient Isoeugenol Conversion into Vanillin. ChemSusChem 2018, 11, 389–396. [Google Scholar] [CrossRef]
- Geng, L.; Zheng, B.; Wang, X.; Zhang, W.; Wu, S.; Jia, M.; Yan, W.; Liu, G. Fe3O4 nanoparticles anchored on carbon serve the dual role of catalyst and magnetically recoverable entity in the aerobic oxidation of alcohols. ChemCatChem 2016, 8, 805–811. [Google Scholar] [CrossRef]
- Saberi, F.; Rodríguez-Padrón, D.; Doustkhah, E.; Ostovar, S.; Franco, A.; Shaterian, H.R.; Luque, R. Mechanochemically modified aluminosilicates for efficient oxidation of vanillyl alcohol. Catal. Commun. 2019, 118, 65–69. [Google Scholar] [CrossRef]
- Saberi, F.; Rodriguez-Padrón, D.; Garcia, A.; Shaterian, H.R.; Luque, R. Unprecedented Proline-Based Heterogeneous Organocatalyst for Selective Production of Vanillin. Catalysts 2018, 8, 167. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 2015, 4, 35–46. [Google Scholar] [CrossRef]
- Zhang, K.; Hong, K.; Suh, J.M.; Lee, T.H.; Kwon, O.; Shokouhimehr, M.; Jang, H.W. Facile synthesis of monodispersed Pd nanocatalysts decorated on graphene oxide for reduction of nitroaromatics in aqueous solution. Res. Chem. Intermed. 2019, 45, 599–611. [Google Scholar] [CrossRef]
- Zhang, K.; Suh, J.M.; Choi, J.W.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Recent Advances in the Nanocatalyst-Assisted NaBH4 Reduction of Nitroaromatics in Water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef]
- Lu, F.; Ruiz, J.; Astruc, D. Palladium-dodecanethiolate nanoparticles as stable and recyclable catalysts for the Suzuki-Miyaura reaction of aryl halides under ambient conditions. Tetrahedron Lett. 2004, 45, 9443–9445. [Google Scholar] [CrossRef]
- Astruc, D. Transition-Metal Nanoparticles in Catalysis: From Historical Background to the State of the Art; Wiley-VCH Verlag Gmbh & Co.: Weinheim, Germany, 2008; Volume 1, pp. 1–48. [Google Scholar]
- Shokouhimehr, M.; Shin, K.-Y.; Lee, J.S.; Hackett, M.J.; Jun, S.W.; Oh, M.H.; Jang, J.; Hyeon, T. Magnetically recyclable core–shell nanocatalysts for efficient heterogeneous oxidation of alcohols. J. Mater. Chem. A 2014, 2, 7593–7599. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Zhang, S.; Ahadi, A.; Rostamnia, S.; Banaei, R.; Li, Z.; Liu, X.; Shokouhimehr, M. Synthesis of bimetallic 4-PySI-Pd@ Cu (BDC) via open metal site Cu-MOF: Effect of metal and support of Pd@ Cu-MOFs in H2 generation from formic acid. Mol. Catal. 2019, 467, 30–37. [Google Scholar] [CrossRef]
- Iranmanesh, M.; Hulliger, J. Magnetic separation: Its application in mining, waste purification, medicine, biochemistry and chemistry. Chem. Soc. Rev. 2017, 46, 5925–5934. [Google Scholar] [CrossRef]
- Kainz, Q.M.; Reiser, O. Polymer and dendrimer coated magnetic nanoparticles as versatile supports for catalysts, scavengers, and reagents. Acc. Chem. Res. 2014, 47, 667–677. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Choi, K.H.; Shokouhimehr, M.; Sung, Y.E. Heterogeneous Suzuki cross-coupling reaction catalyzed by magnetically recyclable nanocatalyst. Bull. Korean Chem. Soc. 2013, 34, 1477–1480. [Google Scholar] [CrossRef]
- Jun, S.W.; Shokouhimehr, M.; Lee, D.J.; Jang, Y.; Park, J.; Hyeon, T. One-pot synthesis of magnetically recyclable mesoporous silica supported acid–base catalysts for tandem reactions. Chem. Commun. 2013, 49, 7821–7823. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Lee, J.E.; Han, S.I.; Hyeon, T. Magnetically recyclable hollow nanocomposite catalysts for heterogeneous reduction of nitroarenes and Suzuki reactions. Chem. Commun. 2013, 49, 4779–4781. [Google Scholar] [CrossRef]
- Rafiaei, S.M.; Kim, A.; Shokouhimehr, M. Gadolinium triflate immobilized on magnetic nanocomposites as recyclable Lewis acid catalyst for acetylation of phenols. Nanosci. Nanotechnol. Lett. 2014, 6, 309–313. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Balu, A.M.; Romero, A.A.; Luque, R. New bio-nanocomposites based on iron oxides and polysaccharides applied to oxidation and alkylation reactions. Beilstein J. Org. Chem. 2017, 13, 1982. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Luque, R. Mechanochemistry: Toward sustainable design of advanced nanomaterials for electrochemical energy storage and catalytic applications. ACS Sustain. Chem. Eng. 2018, 6, 9530–9544. [Google Scholar] [CrossRef]
- Ouyang, W.; Yépez, A.; Romero, A.A.; Luque, R. Towards industrial furfural conversion: Selectivity and stability of palladium and platinum catalysts under continuous flow regime. Catal. Today 2018, 308, 32–37. [Google Scholar] [CrossRef]
- Pineda, A.; Balu, A.M.; Campelo, J.M.; Romero, A.A.; Carmona, D.; Balas, F.; Santamaria, J.; Luque, R. A Dry Milling Approach for the Synthesis of Highly Active Nanoparticles Supported on Porous Materials. ChemSusChem 2011, 4, 1561–1565. [Google Scholar] [CrossRef]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martínez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Asl, M.S.; Mazinani, B. Modulated large-pore mesoporous silica as an efficient base catalyst for the Henry reaction. Res. Chem. Intermed. 2018, 44, 1617–1626. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Peters, C.; Dekkers, M.J. Selected room temperature magnetic parameters as a function of mineralogy, concentration and grain size. Phys. Chem. Earth Parts A/B/C 2003, 28, 659–667. [Google Scholar] [CrossRef]
- Jia, C.; Sun, L.; Yan, Z.; You, L.; Luo, F.; Han, X.; Pang, Y.; Zhang, Z.; Yan, C. Single-crystalline iron oxide nanotubes. Angew. Chem. 2005, 117, 4402–4407. [Google Scholar] [CrossRef]
- Park, J.; Lee, E.; Hwang, N.; Kang, M.; Kim, S.C.; Hwang, Y.; Park, J.; Noh, H.; Kim, J.; Park, J. One nanometer scale size controlled synthesis of monodisperse magnetic Iron oxide nanoparticles. Angew. Chem. 2005, 117, 2932–2937. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. A Surface Area and Porosity; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Rajabi, F.; Fayyaz, F.; Luque, R. Cytosine-functionalized SBA-15 mesoporous nanomaterials: Synthesis, characterization and catalytic applications. Microporous Mesoporous Mater. 2017, 253, 64–70. [Google Scholar] [CrossRef]
- Yépez, A.; De, S.; Climent, M.S.; Romero, A.A.; Luque, R. Microwave-assisted conversion of levulinic acid to γ-valerolactone using low-loaded supported iron oxide nanoparticles on porous silicates. Appl. Sci. 2015, 5, 532–543. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F. Handbook of X-ray Photoemission Spectra; Perkin-Elmer: Waltham, MA, USA, 1976. [Google Scholar]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Caballero, A.; Balu, A.M.; Romero, A.A.; Luque, R. Highly efficient direct oxygen electro-reduction by partially unfolded laccases immobilized on waste-derived magnetically separable nanoparticles. Nanoscale 2018, 10, 3961–3968. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Romero, A.A.; Luque, R. Solventless mechanochemical preparation of novel magnetic bioconjugates. Chem. Commun. 2017, 53, 7635–7637. [Google Scholar] [CrossRef]
- Balu, A.M.; Pineda, A.; Yoshida, K.; Campelo, M.; Gai, P.L.; Angel, A. Fe/Al synergy in Fe2O3 nanoparticles supported on porous aluminosilicate materials: Excelling activities in oxidation reactions. Chem. Commun. 2010, 46, 7825–7827. [Google Scholar] [CrossRef]
- Mounzer, H. Heterogeneous Oxidation of Alcohols. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2009. [Google Scholar]


| Catalyst | S BET (m2∙g−1) | Average Pore Diameter (nm) | Average Pore Volume (mL∙g−1) | SEM–EDX | ||
|---|---|---|---|---|---|---|
| %Al | %Si | %Fe | ||||
| Al-SBA-15 | 736 | 8.5 | 0.8 | - | - | - |
| 40% FeMagC-400 | 297 | 7.5 | 0.4 | 3.0 | 76.1 | 20.9 |
| 50% FeMagC-400 | 242 | 7.6 | 0.3 | 2.5 | 70.7 | 26.8 |
| 30% FeMagN-400 | 339 | 6.3 | 0.2 | 2.6 | 72.3 | 25.1 |
| 40% FeMagN-400 | 300 | 6.4 | 0.2 | 2.3 | 70.6 | 27.1 |
| Sample | (Fe/Si)XPS | (Fe/Si)EDX | (Fe/Si)XPS/(Fe/Si)EDX |
|---|---|---|---|
| 40% FeMagC at 400 °C | 0.03 | 0.3 | 0.1 |
| 40% FeMagN at 400 °C | 0.19 | 0.4 | 0.5 |
| Sample | Surface Acidity at 300 °C/µmol∙g−1 | |
|---|---|---|
| PY (Total Acidity) | DMPY (Brønsted Acidity) | |
| Si-SBA-15 | - | - |
| Al-SBA-15 | 82 | 61 |
| 40% FeMagN at 400 °C | 290 | 143 |
| 40% FeMagC at 400 °C | 155 | 164 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Medina, M.D.; Rodríguez-Padrón, D.; Balu, A.M.; Romero, A.A.; Muñoz-Batista, M.J.; Luque, R. Mechanochemically Synthesized Supported Magnetic Fe-Nanoparticles as Catalysts for Efficient Vanillin Production. Catalysts 2019, 9, 290. https://doi.org/10.3390/catal9030290
Márquez-Medina MD, Rodríguez-Padrón D, Balu AM, Romero AA, Muñoz-Batista MJ, Luque R. Mechanochemically Synthesized Supported Magnetic Fe-Nanoparticles as Catalysts for Efficient Vanillin Production. Catalysts. 2019; 9(3):290. https://doi.org/10.3390/catal9030290
Chicago/Turabian StyleMárquez-Medina, María Dolores, Daily Rodríguez-Padrón, Alina M. Balu, Antonio A. Romero, Mario J. Muñoz-Batista, and Rafael Luque. 2019. "Mechanochemically Synthesized Supported Magnetic Fe-Nanoparticles as Catalysts for Efficient Vanillin Production" Catalysts 9, no. 3: 290. https://doi.org/10.3390/catal9030290
APA StyleMárquez-Medina, M. D., Rodríguez-Padrón, D., Balu, A. M., Romero, A. A., Muñoz-Batista, M. J., & Luque, R. (2019). Mechanochemically Synthesized Supported Magnetic Fe-Nanoparticles as Catalysts for Efficient Vanillin Production. Catalysts, 9(3), 290. https://doi.org/10.3390/catal9030290