H2O and/or SO2 Tolerance of Cu-Mn/SAPO-34 Catalyst for NO Reduction with NH3 at Low Temperature
Abstract
1. Introduction
2. Results and Discussion
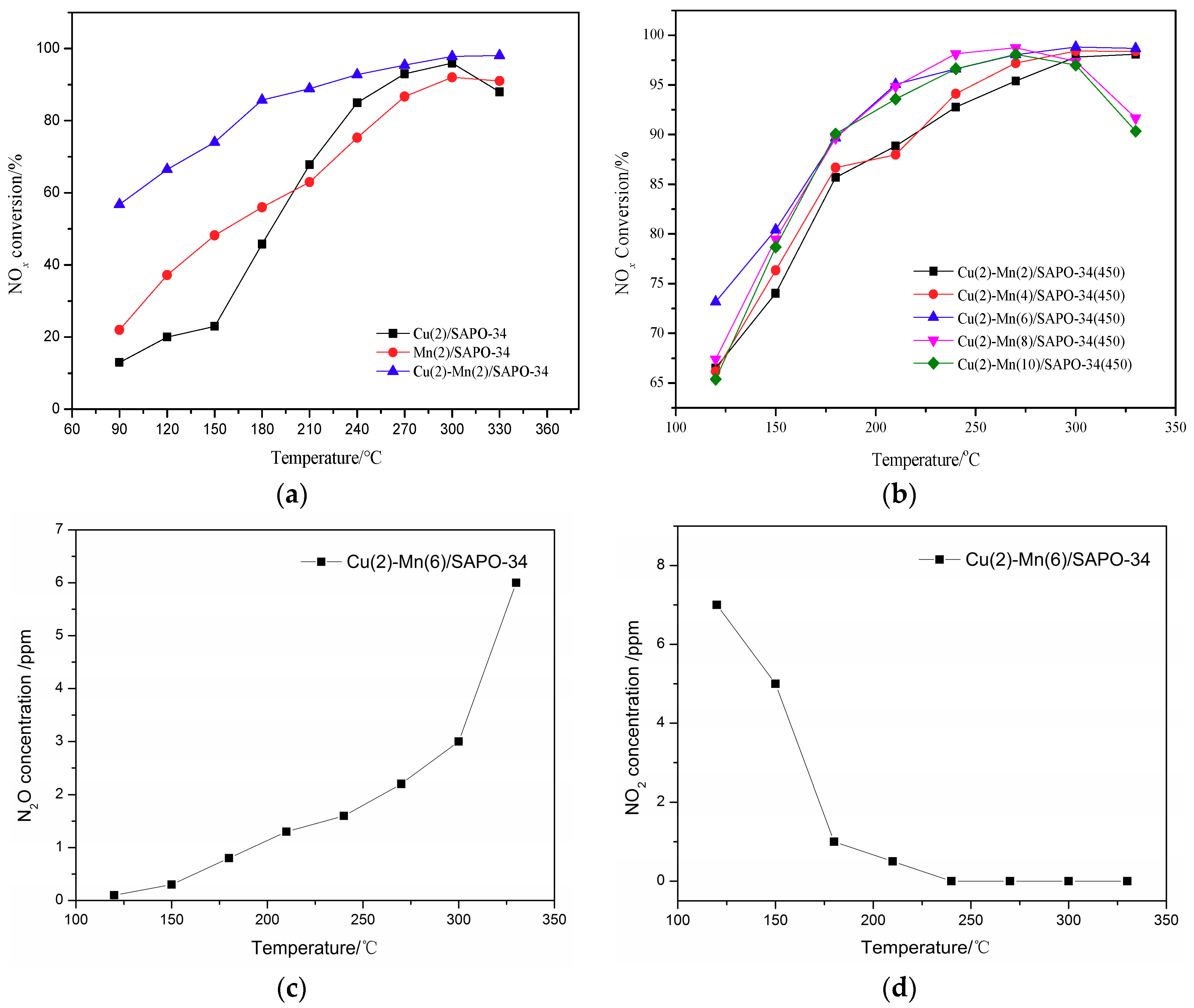
2.1. DeNOx Activity of the Catalysts Without H2O and SO2
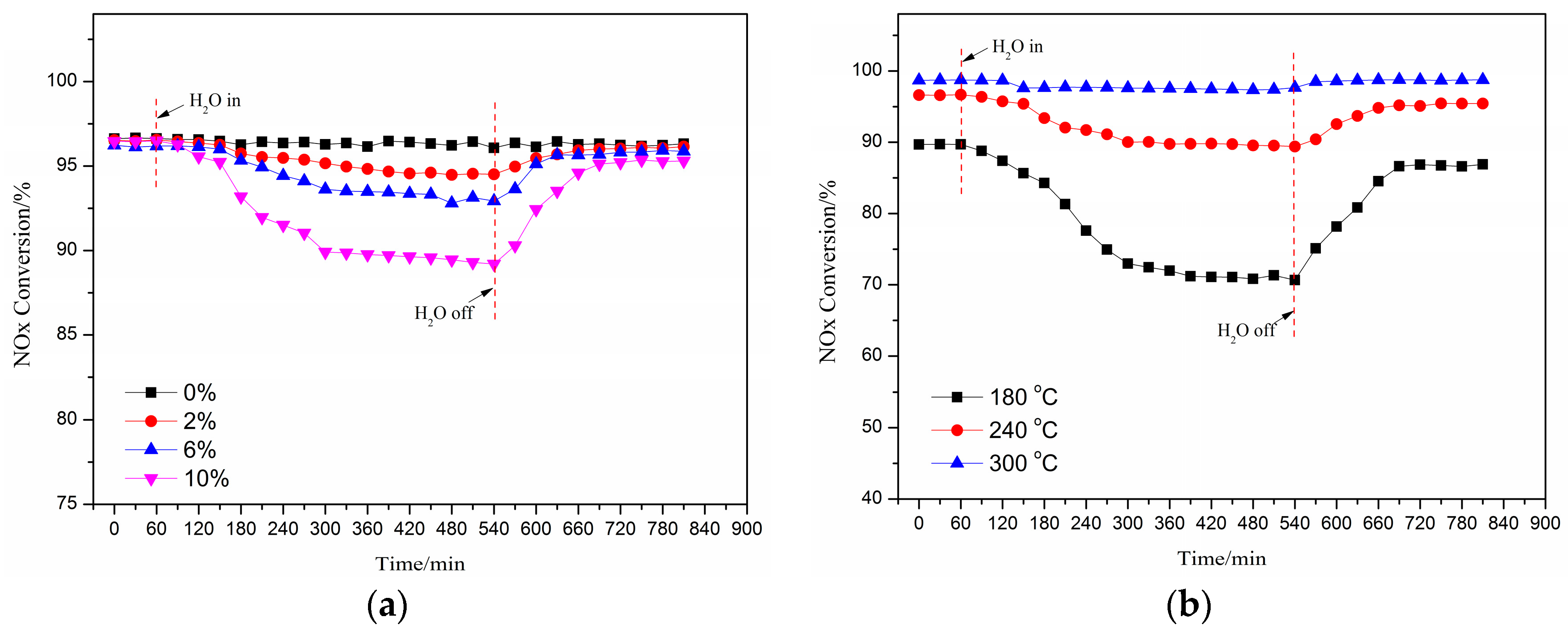
2.2. Effect of H2O on the deNOx Activity of the Catalyst
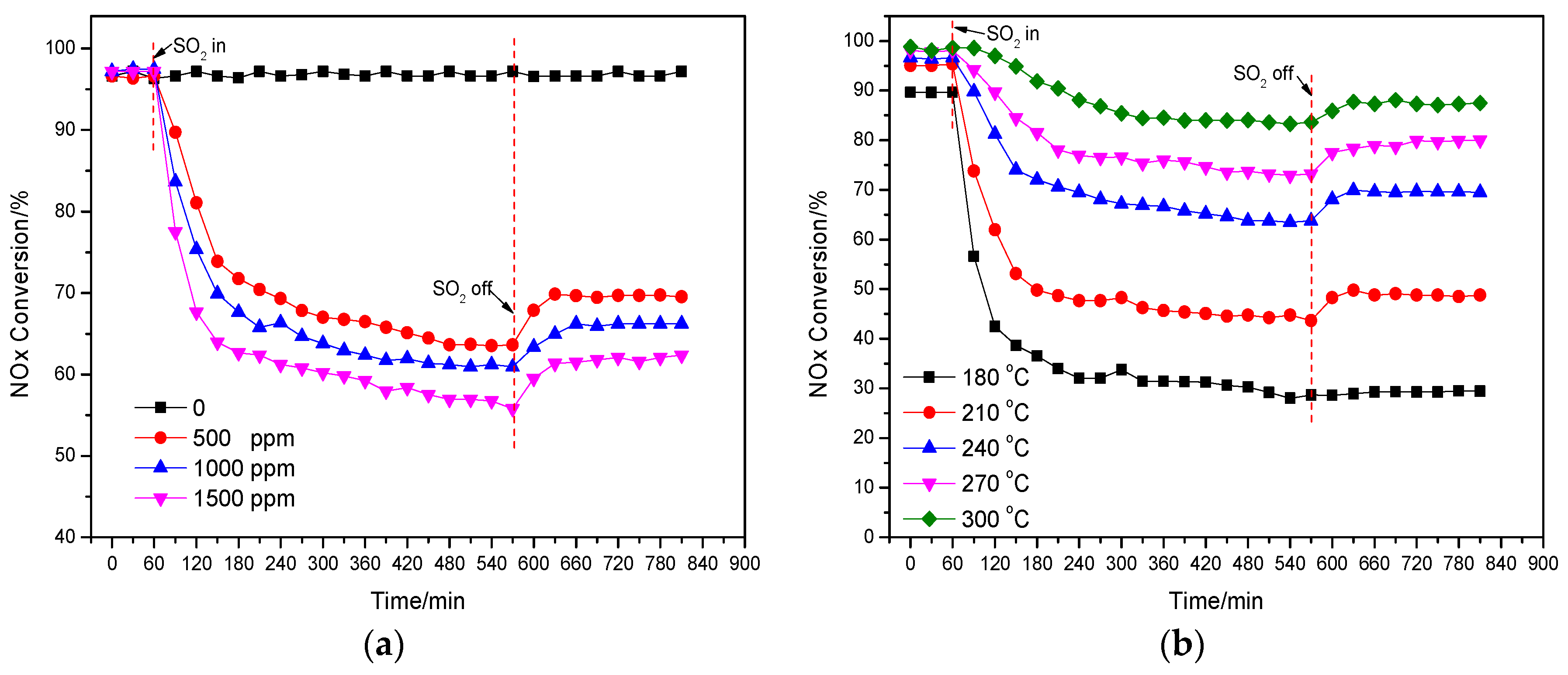
2.3. Effect of SO2 on the deNOx Activity of the Catalyst
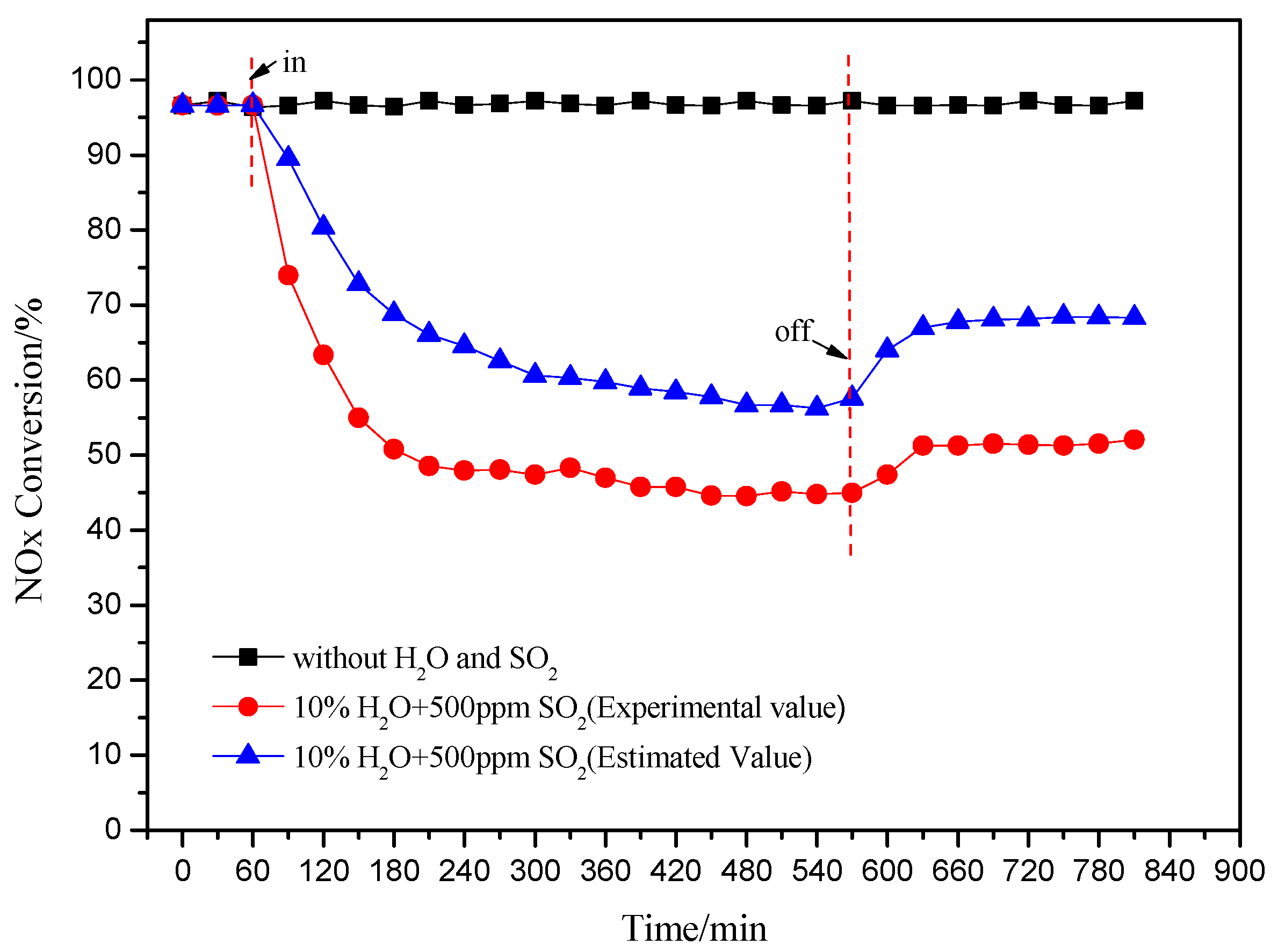
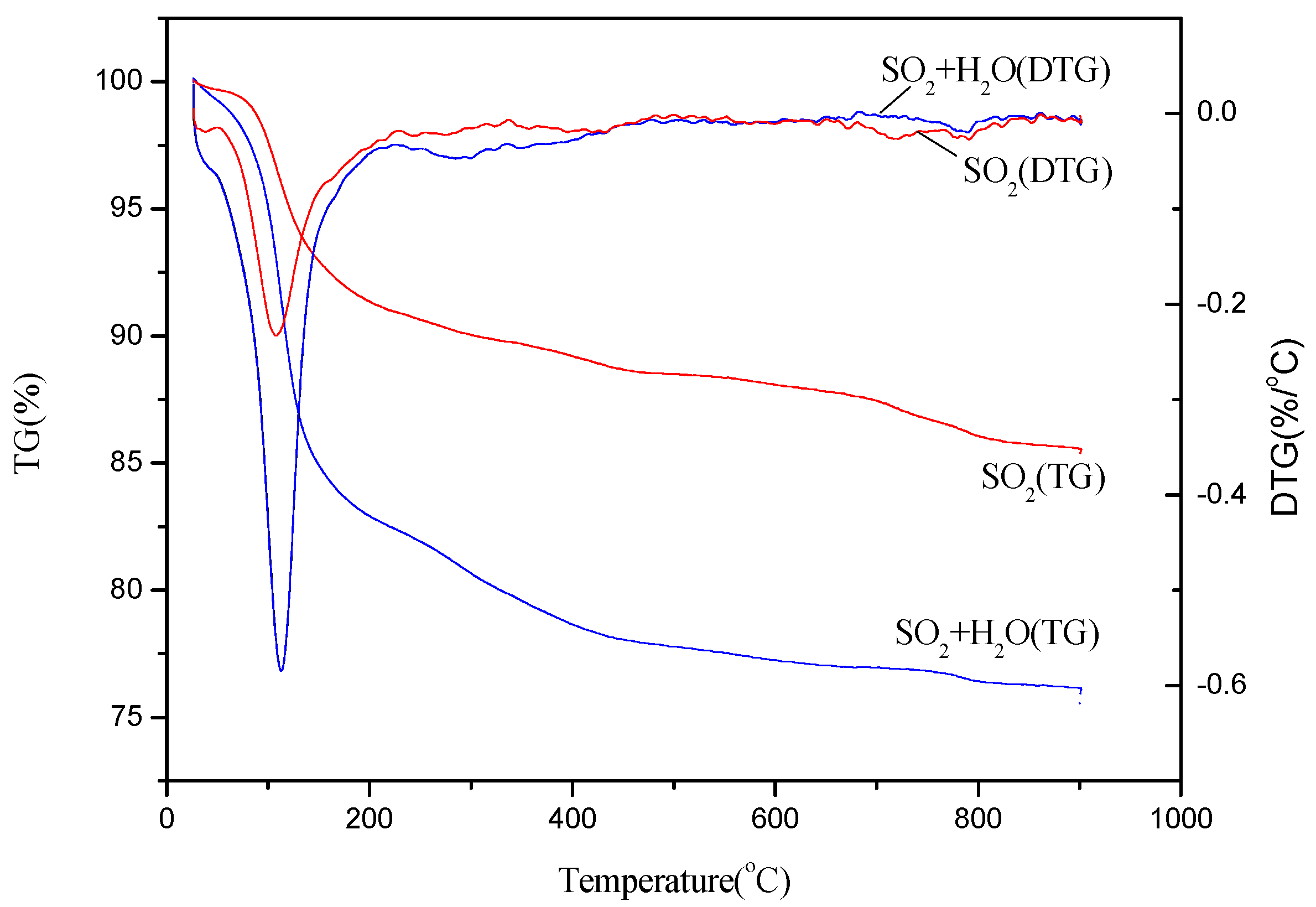
2.4. Effect of Both H2O and SO2 Injection on the deNOx Activity of the Catalyst
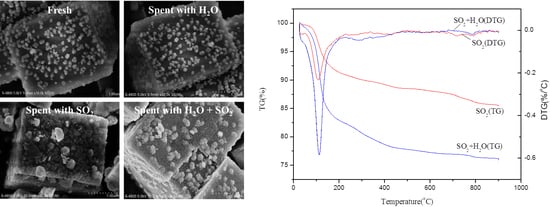
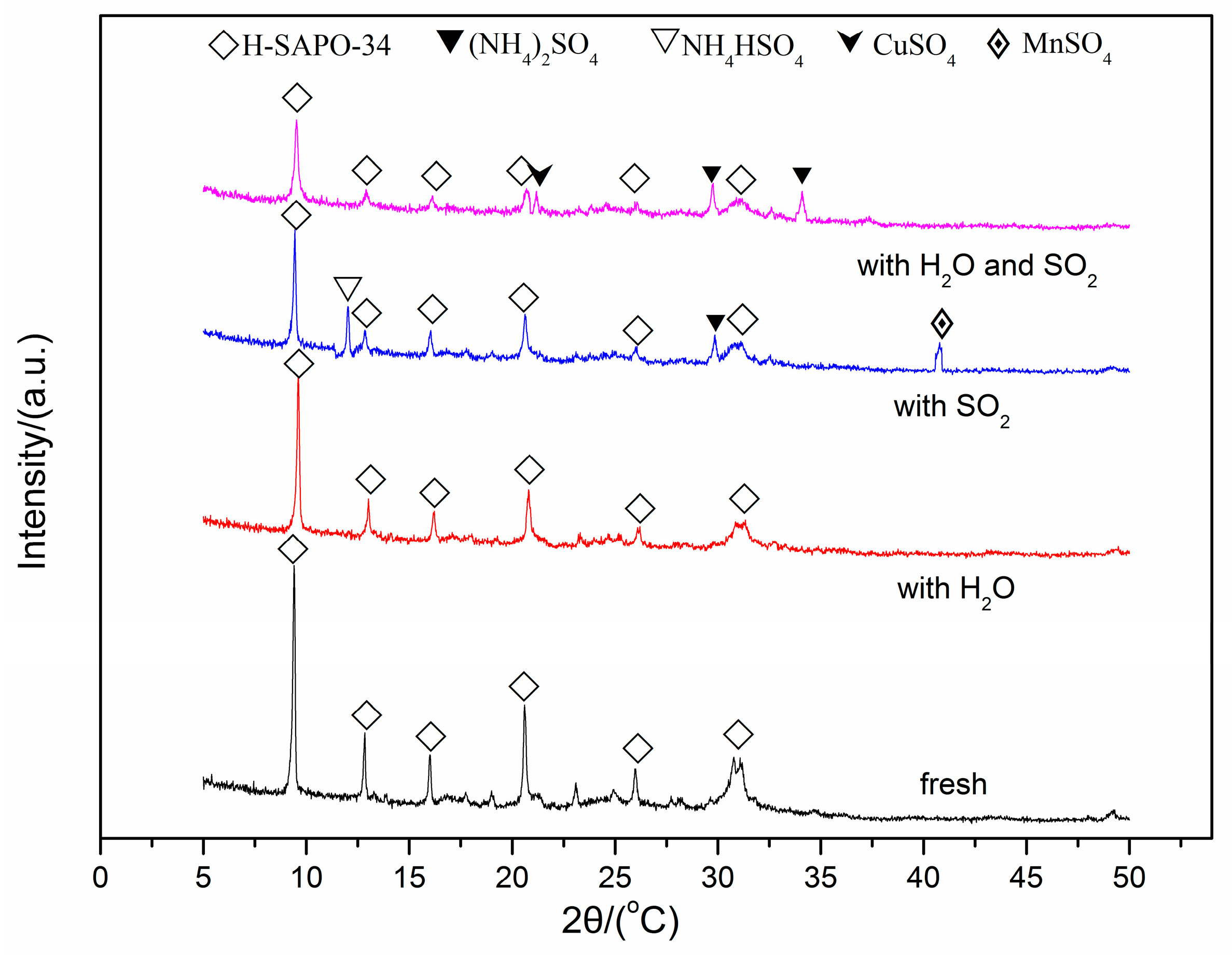
2.5. Mechanism of the Catalyst Poisoning by H2O and/or SO2
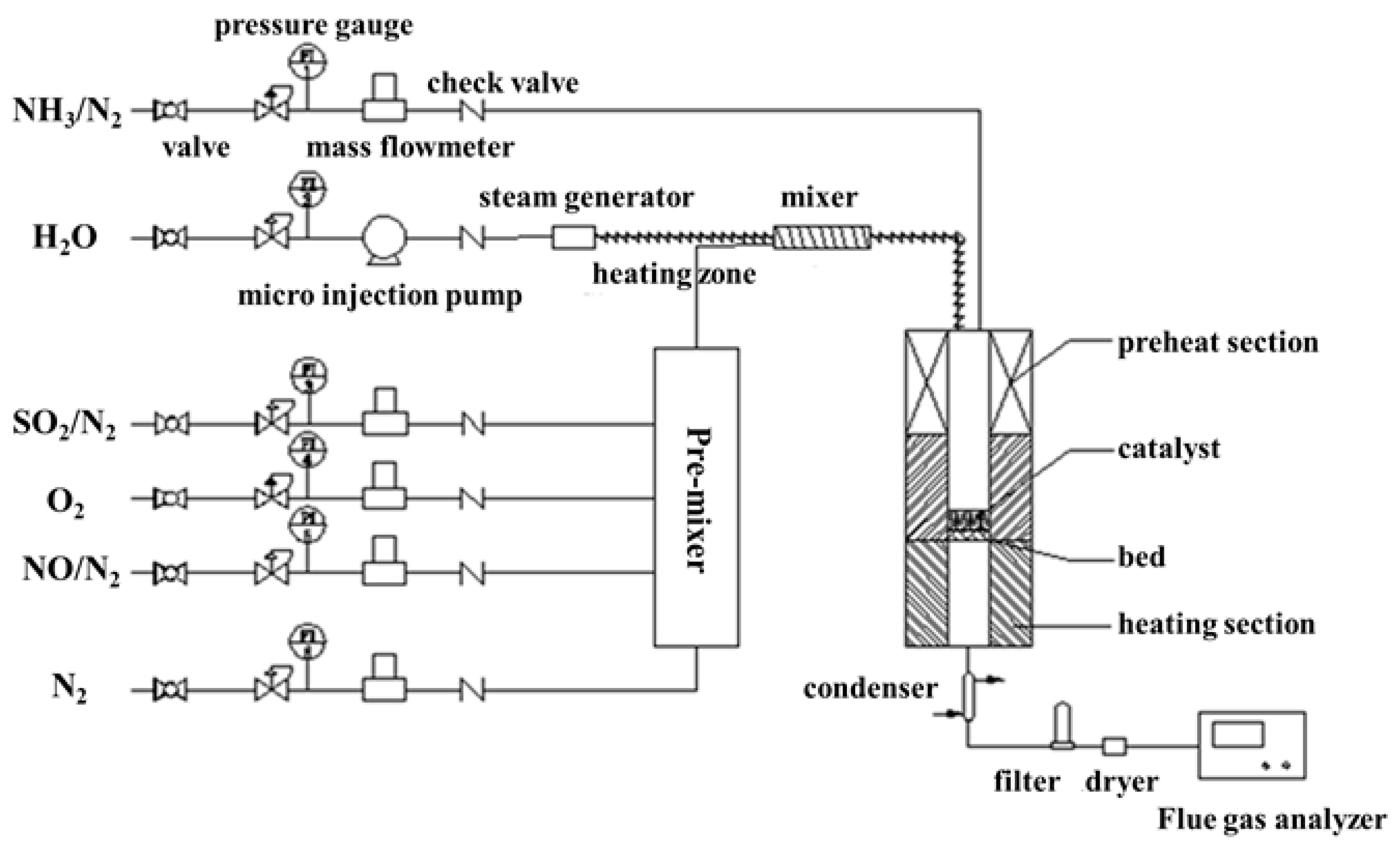
3. Materials and Methods
3.1. Catalyst Preparation
3.2. DeNOx Activity Measurements
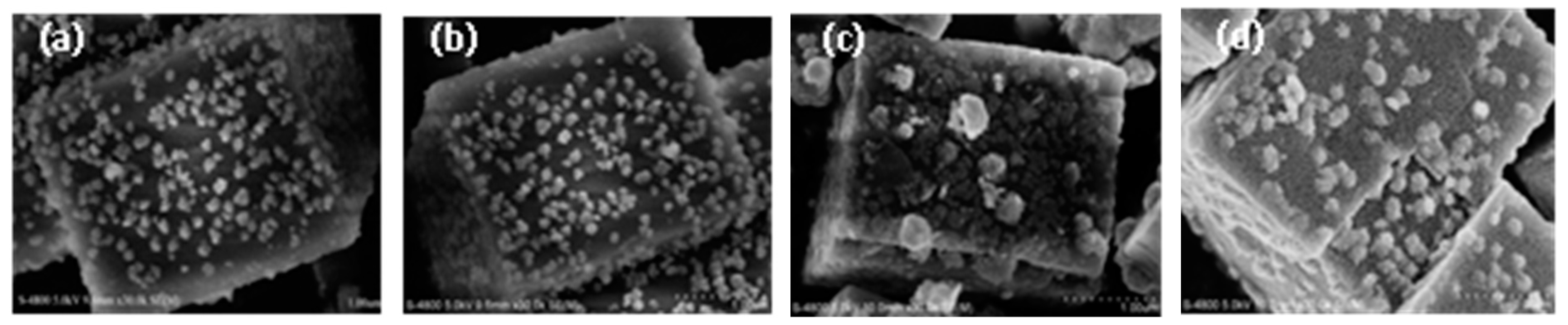
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tie, X.; Huang, R.-J.; Dai, W.; Cao, J.; Long, X.; Su, X.; Zhao, S.; Wang, Q.; Li, G. Effect of heavy haze and aerosol pollution on rice and wheat productions in China. Sci. Rep. 2016, 6, 29612. [Google Scholar] [CrossRef] [PubMed]
- Maher, B.A.; Ahmed, I.A.M.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.A.; Torres-Jardón, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Natl. Acad. Sci. 2016, 113, 10797–10801. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today. 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Forzatti, P.; Nova, I.; Tronconi, E.; Kustov, A.; Thøgersen, J.R. Effect of operating variables on the enhanced SCR reaction over a commercial V2O5–WO3/TiO2 catalyst for stationary applications. Catal. Today 2012, 184, 153–159. [Google Scholar] [CrossRef]
- Zhang, X.P.; Shen, B.X.; Chen, J.H.; Cai, J.; He, C.; Wang, K. Mn0·4/Co0·1Ce0·45Zr0·45OX, high performance catalyst for selective catalytic reduction of NO by ammonia. J. Energy Inst. 2013, 86, 119–124. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Liu, Y.; Kamasamudram, K.; Li, L.; Epling, W. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst. Appl. Catal. B Environ. 2014, 156–157, 371–377. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, L. Characterization of commercial Cu-SSZ-13 and Cu-SAPO-34 catalysts with hydrothermal treatment for NH3-SCR of NOx in diesel exhaust. Chem. Eng. J. 2013, 225, 323–330. [Google Scholar] [CrossRef]
- Blakeman, P.G.; Burkholder, E.M.; Chen, H.-Y.; Collier, J.E.; Fedeyko, J.M.; Jobson, H.; Rajaram, R.R. The role of pore size on the thermal stability of zeolite supported Cu SCR catalysts. Catal. Today 2014, 231, 56–63. [Google Scholar] [CrossRef]
- Bates, S.A.; Delgass, W.N.; Ribeiro, F.H.; Miller, J.T.; Gounder, R. Methods for NH3 titration of Brønsted acid sites in Cu-zeolites that catalyze the selective catalytic reduction of NOx with NH3. J. Catal. 2014, 312, 26–36. [Google Scholar] [CrossRef]
- Bates, S.A.; Verma, A.A.; Paolucci, C.; Parekh, A.A.; Anggara, T.; Yezerets, A.; Schneider, W.F.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Identification of the active Cu site in standard selective catalytic reduction with ammonia on Cu-SSZ-13. J. Catal. 2014, 312, 87–97. [Google Scholar] [CrossRef]
- Ettireddy, P.R.; Kotrba, A.; Boningari, T.; Smirniotis, P. Low temperature SCR catalysts optimized for cold-start and low-load engine exhaust conditions. SAE Technical Paper 2015, 01, 1026. [Google Scholar]
- Kim, Y.J.; Kwon, H.J.; Heo, I.; Nam, I.S.; Cho, B.K.; Jin, W.C.; Cha, M.S.; Yeo, G.K. Mn–Fe/ZSM5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust. Appl. Catal. B Environ. 2012, 126, 9–21. [Google Scholar] [CrossRef]
- Nova, I.; Tronconi, E. Urea-SCR Technology for deNOx after Treatment of Diesel Exhausts; Springer: New York, NY, USA, 2014. [Google Scholar]
- Chen, H.-Y.; Wei, Z.; Kollar, M.; Gao, F.; Wang, Y.; Szanyi, J.; Peden, C.H. A comparative study of N2O formation during the selective catalytic reduction of NOx with NH3 on zeolite supported Cu catalysts. J. Catal. 2015, 329, 490–498. [Google Scholar] [CrossRef]
- Wang, J.; Yu, T.; Wang, X.; Qi, G.; Xue, J.; Shen, M.; Li, W. The influence of silicon on the catalytic properties of Cu/SAPO-34 for NOx reduction by ammonia-SCR. Appl. Catal. B Environ. 2012, 127, 137–147. [Google Scholar] [CrossRef]
- Xue, J.; Wang, X.; Qi, G.; Wang, J.; Shen, M.; Li, W. Characterization of copper species over Cu/SAPO-34 in selective catalytic reduction of NOx with ammonia: Relationships between active Cu sites and de-NOx performance at low temperature. J. Catal. 2013, 297, 56–64. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, L.; Yang, R.T. Activity, propene poisoning resistance and hydrothermal stability of copper exchanged chabazite-like zeolite catalysts for SCR of NO with ammonia in comparison to Cu/ZSM-5. Appl. Catal. A Gen. 2012, 427–428, 24–34. [Google Scholar] [CrossRef]
- Deka, U.; Lezcano-Gonzalez, I.; Warrender, S.J.; Picone, A.L.; Wright, P.A.; Weckhuysen, B.M.; Beale, A.M. Changing active sites in Cu–CHA catalysts: deNOx selectivity as a function of the preparation method. Microporous Mesoporous Mater. 2013, 166, 144–152. [Google Scholar] [CrossRef]
- Gao, F.; Kwak, J.H.; Szanyi, J.; Peden, C.H. Current Understanding of Cu-Exchanged Chabazite Molecular Sieves for Use as Commercial Diesel Engine DeNOx Catalysts. Top. Catal. 2013, 56, 1441–1459. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Weng, D.; Si, Z.; Ran, R. Migration, reactivity, and sulfur tolerance of copper species in SAPO-34 zeolite toward NOx reduction with ammonia. RSC Adv. 2017, 7, 37787–37796. [Google Scholar] [CrossRef]
- Shen, M.; Wen, H.; Hao, T.; Yu, T.; Fan, D.; Wang, J.; Li, W.; Wang, J. Deactivation mechanism of SO2 on Cu/SAPO-34 NH3-SCR catalysts: structure and active Cu2+. Catal. Sci. Technol. 2015, 5, 1741–1749. [Google Scholar] [CrossRef]
- Wijayanti, K.; Andonova, S.; Kumar, A.; Li, J.; Kamasamudram, K.; Currier, N.M.; Yezerets, A.; Olsson, L. Impact of sulfur oxide on NH3-SCR over Cu-SAPO-34. Appl. Catal. B: Environ. 2015, 166–167, 568–579. [Google Scholar] [CrossRef]
- Jangjou, Y.; Wang, D.; Kumar, A.; Li, J.; Epling, W.S. SO2 Poisoning of the NH3-SCR Reaction over Cu-SAPO-34: Effect of Ammonium Sulfate versus Other S-Containing Species. ACS Catal. 2016, 6, 6612–6622. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Zhao, S.; Guo, W.; Wang, R.; Ying, M. Characteristics and performance of SAPO-34 catalyst for methanol-to-olefin conversion. Appl. Catal. 1990, 64, 31–40. [Google Scholar] [CrossRef]
- Xu, L.; Du, A.; Wei, Y.; Wang, Y.; Yu, Z.; He, Y.; Zhang, X.; Liu, Z. Synthesis of SAPO-34 with only Si(4Al) species: Effect of Si contents on Si incorporation mechanism and Si coordination environment of SAPO-34. Microporous Mesoporous Mater. 2008, 115, 332–337. [Google Scholar] [CrossRef]
- Buchholz, A.; Wang, W.; Xu, M.; Arnold, A.; Hunger, M. Thermal stability and dehydroxylation of Brønsted acid sites in silicoaluminophosphates H-SAPO-11, H-SAPO-18, H-SAPO-31, and H-SAPO-34 investigated by multi-nuclear solid-state NMR spectroscopy. Microporous Mesoporous Mater. 2002, 56, 267–278. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Yu, T.; Zhu, S.; Shen, M.; Li, W.; Wang, J. The migration of Cu species over Cu–SAPO-34 and its effect on NH3 oxidation at high temperature. Catal. Sci. Technol. 2014, 4, 3004–3012. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Z.; Zhang, X.; Liu, Q. Inhibition effect of H2O on V2O5/AC catalyst for catalytic reduction of NO with NH3 at low temperature. Appl. Catal. B Environ. 2006, 63, 260–265. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, X.; Ma, H.; Yao, Y.; Liu, T. A comparative study of Mn/CeO2, Mn/ZrO2 and Mn/Ce-ZrO2 for low temperature selective catalytic reduction of NO with NH3 in the presence of SO2 and H2O. J. Environ. Sci. 2013, 25, 791–800. [Google Scholar] [CrossRef]
- Notoya, F.; Su, C.; Sasaoka, E.; Nojima, S. Effect of SO2 on the Low-Temperature Selective Catalytic Reduction of Nitric Oxide with Ammonia over TiO2, ZrO2, and Al2O3. Ind. Eng. Chem. Res. 2001, 40, 3732–3739. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, H.; Yuan, Z.; Wang, S. The role of isolated Cu2+ location in structural stability of Cu-modified SAPO-34 in NH3-SCR of NO. Environ. Technol. 2015, 36, 169–177. [Google Scholar] [CrossRef]
- Zhang, R.; Alamdari, H.; Kaliaguine, S. SO2 poisoning of LaFe0.8Cu0.2O3 perovskite prepared by reactive grinding during NO reduction by C3H6. Appl. Catal. A Gen. 2008, 340, 140–151. [Google Scholar] [CrossRef]
- Fan, Y.Z.; Cao, F.H. Thermal Decomposition Kinetics of Ammonium Sulfate. J. Chem. Eng. Chin. Univ. 2011, 25, 341–346. [Google Scholar]
- Shen, B.X.; Liu, T. Deactivation of MnOx-CeOx/ACF Catalysts for Low-Temperature NH3-SCR in the Presence of SO2. Acta Physico-Chimica Sinica 2010, 26, 3009–3016. [Google Scholar]
- Xu, W.; He, H.; Yu, Y. Deactivation of a Ce/TiO2 catalyst by SO2 in the selective catalytic reduction of NO by NH3. J. Phys. Chem. C 2009, 113, 4426–4432. [Google Scholar] [CrossRef]
- Pan, S.; Luo, H.; Li, L.; Wei, Z.; Huang, B. H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3. J. Mol. Catal. A Chem. 2013, 377, 154–161. [Google Scholar] [CrossRef]
- Kiyoura, R.; Urano, K. Mechanism, Kinetics, and Equilibrium of Thermal Decomposition of Ammonium Sulfate. Ind. Eng. Chem. Proc. Dev. 1970, 9, 489–494. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Wu, Z.B.; Liu, Y.; Lee, S.C.; Ho, W.K. DRIFT Study of the SO2 Effect on Low-Temperature SCR Reaction over Fe−Mn/TiO2. J. Phys. Chem. C 2010, 114, 4961–4965. [Google Scholar] [CrossRef]
| Catalyst | BET Surface Area | Pore Volume | Pore Diameter |
|---|---|---|---|
| (m2g−1) | (cm3g−1) | (nm) | |
| Fresh catalyst | 457 | 0.23 | 2.08 |
| Catalyst exposed to 500 ppm SO2 at 180 °C for 8 h | 307 | 0.14 | 2.42 |
| Catalyst exposed to 500 ppm SO2, at 210 °C for 8 h | 314 | 0.16 | 2.29 |
| Catalyst exposed to 500 ppm SO2, at 240 °C for 8 h | 331 | 0.16 | 2.15 |
| Catalyst exposed to 500 ppm SO2, at 270 °C for 8 h | 367 | 0.19 | 2.09 |
| Catalyst exposed to 500 ppm SO2, at 300 °C for 8 h | 372 | 0.18 | 2.09 |
| Catalyst exposed to 1000 ppm SO2, at 240 °C for 8 h | 321 | 0.16 | 2.14 |
| Catalyst exposed to 1500 ppm SO2, at 240 °C for 8 h | 320 | 0.16 | 2.28 |
| Catalyst exposed to 10% H2O, at 180 °C for 8 h | 383 | 0.18 | 2.03 |
| Catalyst exposed to 10% H2O, at 240 °C for 8 h | 408 | 0.19 | 2.02 |
| Catalyst exposed to 10% H2O, at 300 °C for 8 h | 441 | 0.21 | 2.04 |
| Catalyst exposed to 2% H2O, at 240 °C for 8 h | 425 | 0.20 | 2.03 |
| Catalyst exposed to 6% H2O, at 240 °C for 8 h | 413 | 0.19 | 1.95 |
| Catalyst exposed to 10% H2O+500 ppmSO2, at 240 °C for 8 h | 276 | 0.17 | 2.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Zhang, W.; He, P.; Guan, S.; Yuan, B.; Li, R.; Sun, Y.; Shen, D. H2O and/or SO2 Tolerance of Cu-Mn/SAPO-34 Catalyst for NO Reduction with NH3 at Low Temperature. Catalysts 2019, 9, 289. https://doi.org/10.3390/catal9030289
Liu G, Zhang W, He P, Guan S, Yuan B, Li R, Sun Y, Shen D. H2O and/or SO2 Tolerance of Cu-Mn/SAPO-34 Catalyst for NO Reduction with NH3 at Low Temperature. Catalysts. 2019; 9(3):289. https://doi.org/10.3390/catal9030289
Chicago/Turabian StyleLiu, Guofu, Wenjie Zhang, Pengfei He, Shipian Guan, Bing Yuan, Rui Li, Yu Sun, and Dekui Shen. 2019. "H2O and/or SO2 Tolerance of Cu-Mn/SAPO-34 Catalyst for NO Reduction with NH3 at Low Temperature" Catalysts 9, no. 3: 289. https://doi.org/10.3390/catal9030289
APA StyleLiu, G., Zhang, W., He, P., Guan, S., Yuan, B., Li, R., Sun, Y., & Shen, D. (2019). H2O and/or SO2 Tolerance of Cu-Mn/SAPO-34 Catalyst for NO Reduction with NH3 at Low Temperature. Catalysts, 9(3), 289. https://doi.org/10.3390/catal9030289