Bioprocess Intensification Using Flow Reactors: Stereoselective Oxidation of Achiral 1,3-diols with Immobilized Acetobacter Aceti
Abstract
:1. Introduction
2. Results
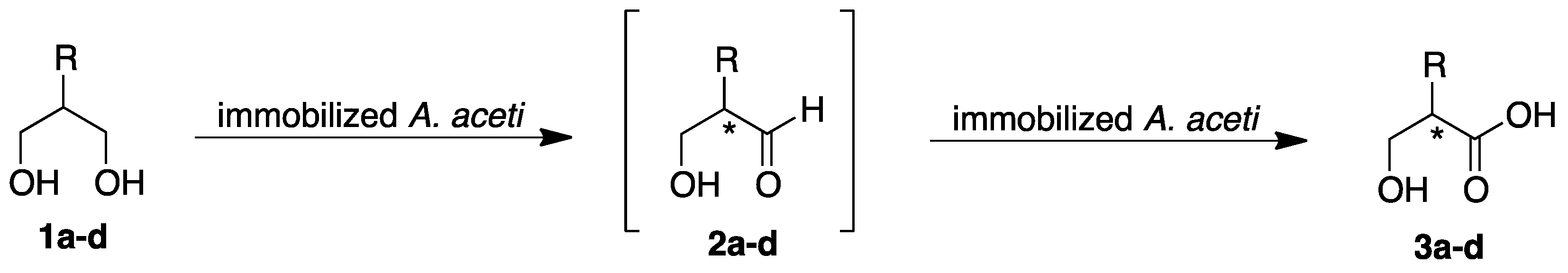
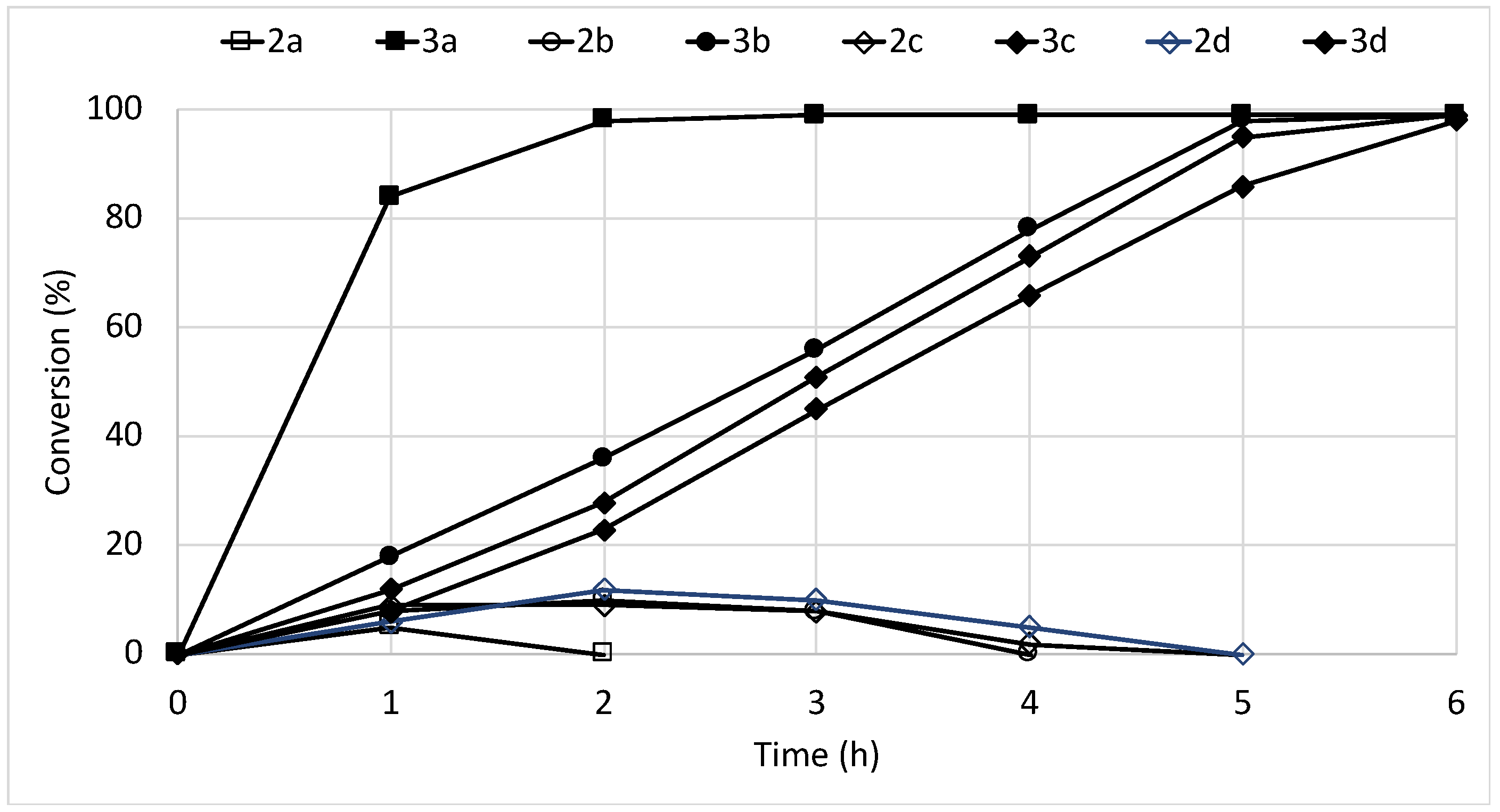
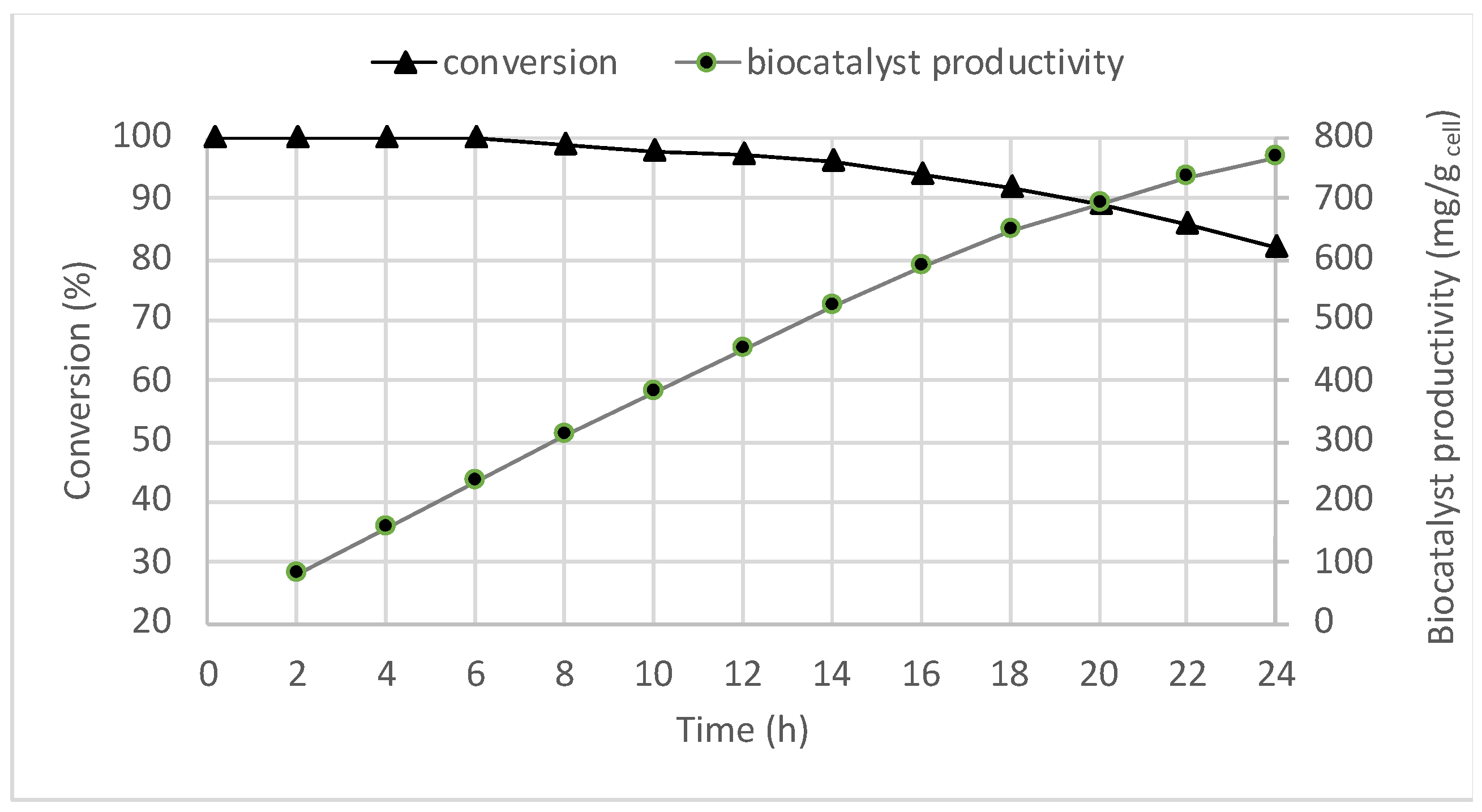
2.1. Batch Biotransformations with Immobilized Cells
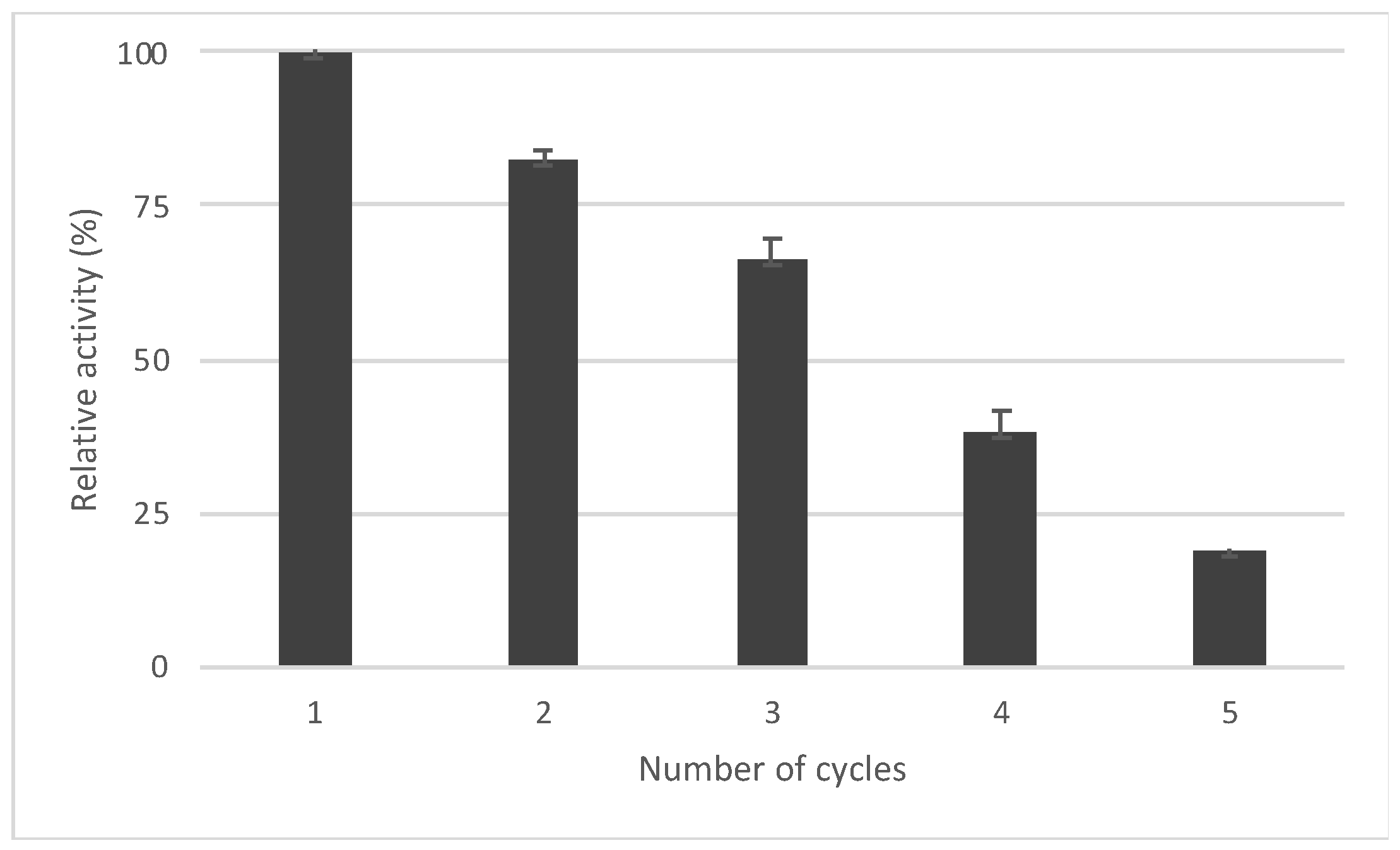
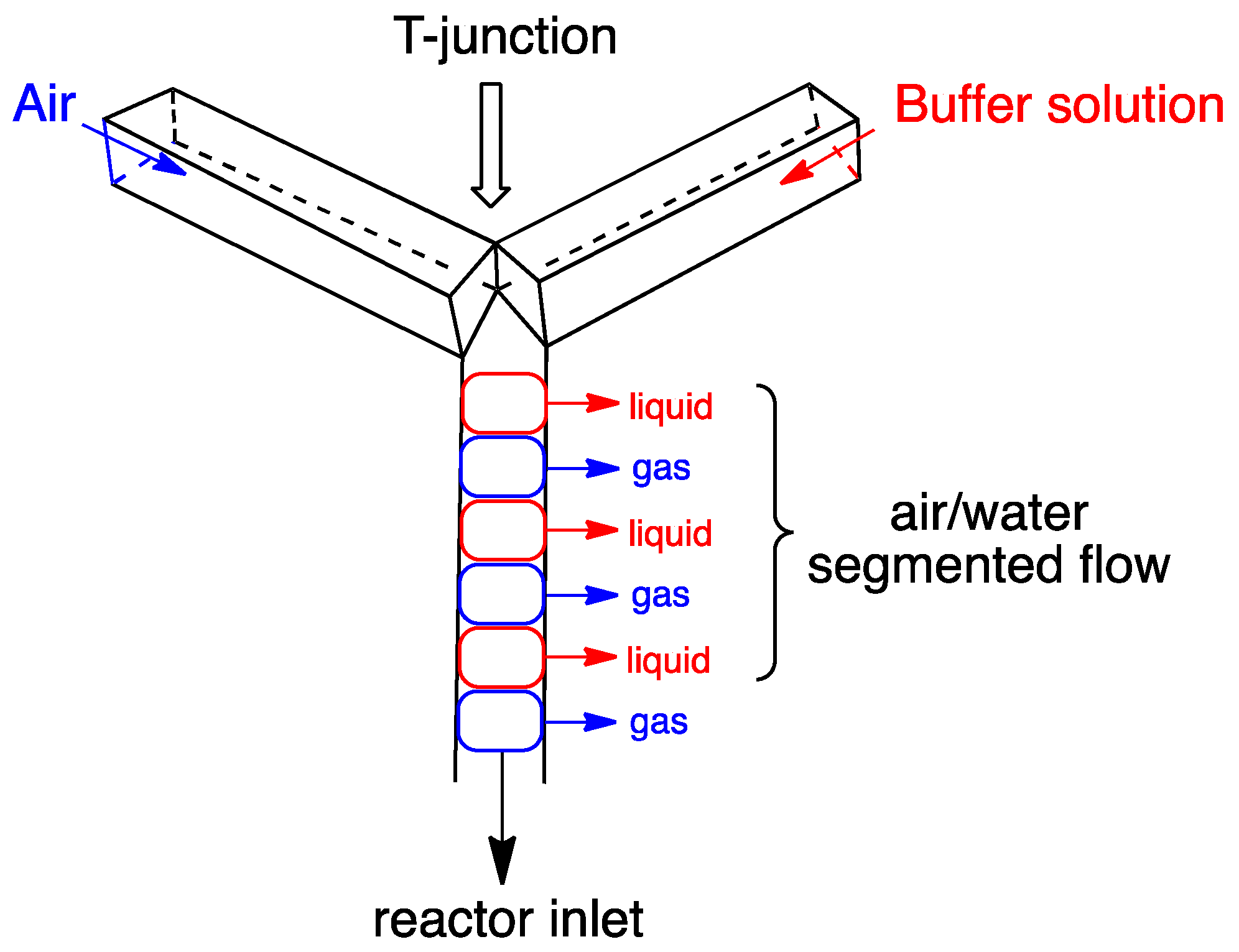
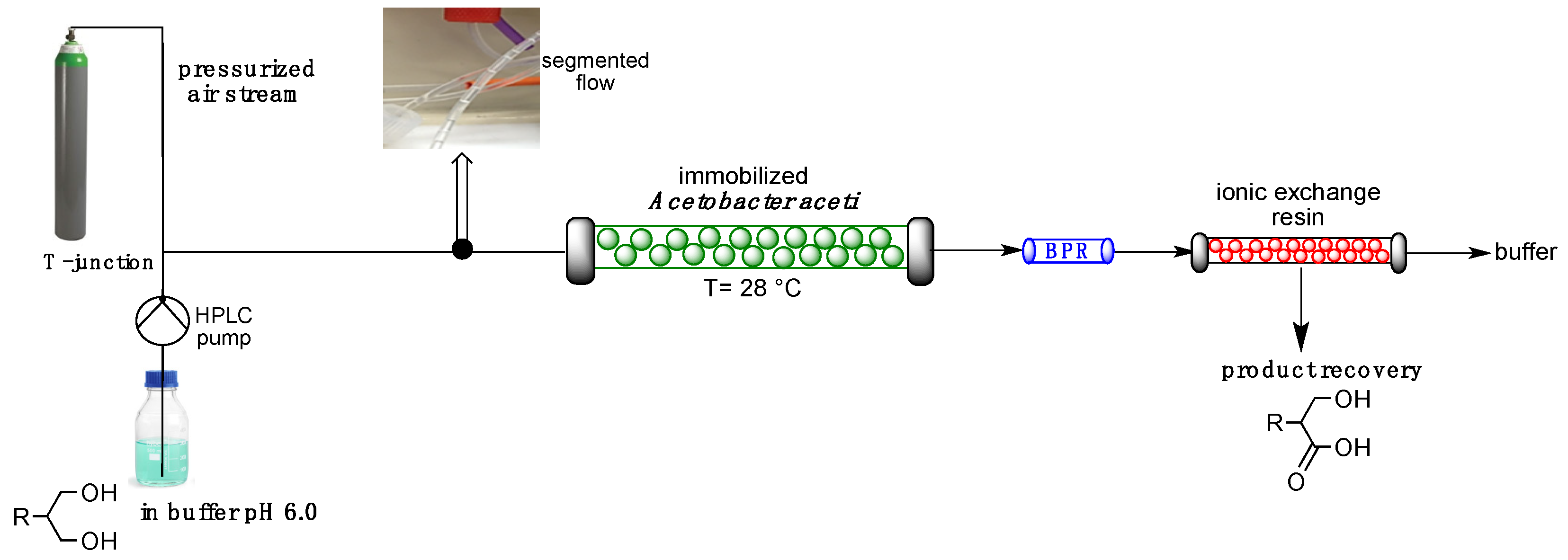
2.2. Oxidation in Flow Reactors
3. Discussion
4. Materials and Methods
4.1. General
4.2. Strain Preparation
4.3. Preparation of Dry Alginate Beads
4.4. General Batch Procedure for the Preparation of Mono-Carboxylic Acids (3a–d)
4.5. General Flow Procedure for the Preparation of Mono-Carboxylic Acids (3a–d)
Author Contributions
Funding
Conflicts of Interest
References
- Hollmann, F.; Arends, I.W.C.E.; Buehler, K.; Schallmey, A.; Bühler, B. Enzyme-mediated oxidations for the chemist. Green Chem. 2011, 13, 226–265. [Google Scholar] [CrossRef]
- Molinari, F. Oxidations with isolated and cell-bound dehydrogenases and oxidases. Curr. Org. Chem. 2006, 10, 1247–1263. [Google Scholar] [CrossRef]
- Gamenara, D.; Seoane, G.A.; Saenz-Mendez, P.; Dominguez de Maria, P. Redox Biocatalysis; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Saichana, N.; Matsushita, K.; Adachi, O.; Frébort, I.; Frebortova, J. Acetic acid bacteria: A group of bacteria with versatile biotechnological applications Biotechnol. Adv. 2015, 33, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Yakushi, T.; Matsushita, K. Alcohol dehydrogenase of acetic acid bacteria: Structure, mode of action, and applications in biotechnology. Appl. Microbiol. Biotechnol. 2010, 86, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Romano, D.; Villa, R.; Molinari, F. Preparative biotransformations: Oxidation of alcohols. ChemCatChem 2012, 4, 739–749. [Google Scholar] [CrossRef]
- Gandolfi, R.; Cavenago, K.; Gualandris, R.; Sinisterra Gago, J.V.; Molinari, F. Production of 2-phenylacetic acid and phenylacetaldehyde by oxidation of 2-phenylethanol with free immobilized cells of Acetobacter aceti. Process Biochem. 2004, 39, 747–751. [Google Scholar] [CrossRef]
- Zambelli, P.; Pinto, S.; Romano, D.; Crotti, E.; Conti, P.; Tamborini, L.; Villa, R.; Molinari, F. One-pot chemoenzymatic synthesis of aldoximes from primary alcohols in water. Green Chem. 2012, 14, 2158–2161. [Google Scholar] [CrossRef]
- Romano, D.; Contente, M.; Granato, T.; Remelli, W.; Zambelli, P.; Molinari, F. Biocatalytic oxidation of 1,4-diols and γ-lactols into γ-lactones: Application to chemoenzymatic synthesis of drospirenone. Monatsh. Chem. 2013, 144, 735–737. [Google Scholar] [CrossRef]
- Svitel, J.; Sturdik, E. n-Propanol conversion to propionic acid by Gluconobacter oxydans. Enzyme Microb. Technol. 1995, 17, 546–550. [Google Scholar] [CrossRef]
- Habe, H.; Shimada, T.; Yakushi, T.; Hattori, H.; Ano, Y.; Fukuoka, T.; Kitamoto, D.; Itagaki, M.; Watanabe, K.; Yanagishita, H.; et al. Biotransformation of glycerol to d-glyceric acid by Acetobacter tropicalis. Appl. Environ. Microbiol. 2009, 81, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Gandolfi, R.; Nitti, P.; Rollini, M.; Molinari, F. Acetic acid bacteria as enantioselective biocatalysts. J. Mol. Catal. B 2002, 17, 235–240. [Google Scholar] [CrossRef]
- Keliang, G.; Dongzhi, W. Asymmetric oxidation by Gluconobacter oxydans. Appl. Microbiol. Biotechnol. 2006, 70, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Molinari, F.; Gandolfi, R.; Villa, R.; Urban, E.; Kiener, A. Enantioselective oxidation of prochiral 2-methyl-1,3-propandiol by Acetobacter pasteurianus. Tetrahedron Asymmetry 2003, 14, 2041–2043. [Google Scholar] [CrossRef]
- Brenna, E.; Cannavale, F.; Crotti, M.; De Vitis, V.; Gatti, F.G.; Migliazza, G.; Molinari, F.; Parmeggiani, F.; Romano, D.; Santangelo, S. Synthesis of enantiomerically enriched 2-hydroxymethylalkanoic acids by oxidative desymmetrisation of achiral 1,3-diols mediated by Acetobacter aceti. ChemCatChem 2016, 8, 3796–3803. [Google Scholar] [CrossRef]
- Polakovic, M.; Svitel, J.; Bucko, M.; Filip, J.; Nedela, V.; Ansorge-Schumacher, M.B.; Gemeiner, P. Progress in biocatalysis with immobilized viable whole cells: Systems development, reaction engineering and applications. Biotechnol. Lett. 2017, 39, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Mignot, L.; Junter, G.A. Diffusion in immobilized-cell agar layers: Influence of microbial burden and cell morphology on the diffusion coefficients of l-malic acid and glucose. Appl. Microbiol. Biotechnol. 1990, 32, 418–423. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Wiesbauer, J.; Nidetzky, B. Biotransformations in microstructured reactors: More than flowing with the stream? Trends Biotechnol. 2011, 29, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow bioreactors as complementary tools for biocatalytic process intensification. Trends Biotechnol. 2018, 36, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Šalic, A.; Zelic, B. ADH-catalysed hexanol oxidation with fully integrated NADH regeneration performed in microreactors connected in series. RSC Adv. 2014, 4, 41714–41721. [Google Scholar] [CrossRef]
- Dall’Oglio, F.; Contente, M.L.; Conti, P.; Molinari, F.; Monfredi, D.; Pinto, A.; Romano, D.; Ubiali, D.; Tamborini, L.; Serra, I. Flow-based stereoselective reduction of ketones using an immobilized ketoreductase/glucose dehydrogenase mixed bed system. Catal. Commun. 2017, 93, 29–32. [Google Scholar] [CrossRef]
- Andrade, L.H.; Kroutil, W.; Jamison, T.F. Continuous flow synthesis of chiral amines in organic solvents: Immobilization of E. coli cells containing both ω-transaminase and PLP. Org. Lett. 2014, 16, 6092–6095. [Google Scholar] [CrossRef] [PubMed]
- Planchestainer, M.; Contente, M.L.; Cassidy, J.; Molinari, F.; Tamborini, L.; Paradisi, F. Continuous flow biocatalysis: Production and in-line purification of amines by immobilised transaminase from Halomonas elongata. Green Chem. 2017, 19, 372–376. [Google Scholar] [CrossRef]
- Contente, M.L.; Dall’Oglio, F.; Tamborini, L.; Molinari, F.; Paradisi, F. Highly efficient oxidation of amines to aldehydes with flow-based biocatalysis. ChemCatChem 2017, 9, 3843–3848. [Google Scholar] [CrossRef]
- Tomaszewski, B.; Lloyd, R.C.; Warr, A.J.; Buehler, K.; Schmid, A. Regioselective biocatalytic aromatic hydroxylation in a gas–liquid multiphase tube-in-tube reactor. ChemCatChem 2014, 6, 2567–2576. [Google Scholar] [CrossRef]
- Willrodt, C.; Halan, B.; Karthaus, L.; Rehdorf, J.; Julsing, M.K.; Buehler, K.; Schmid, A. Continuous multistep synthesis of perillic acid from limonene by catalytic biofilms under segmented flow. Biotechnol. Bioeng. 2017, 114, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Tribulato, M.A.; Petrasek, Z.; Nidetzky, B. Let the substrate flow, not the enzyme: Practical immobilization of d-amino acid oxidase in a glass microreactor for effective biocatalytic conversions. Biotechnol. Bioeng. 2016, 113, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Gemoets, H.P.L.; Su, Y.; Shang, M.; Hessel, V.; Luque, R.; Noel, T. Liquid phase oxidation chemistry in continuous- flow microreactors. Chem. Soc. Rev. 2016, 45, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Contente, M.L.; Guidi, B.; Serra, I.; De Vitis, V.; Romano, D.; Pinto, A.; Lenna, R.; Pinheiro de Souza Oliveira, R.; Molinari, F. Development of a high-yielding bioprocess for 11-α hydroxylation of canrenone under conditions of oxygen-enriched air supply. Steroids 2016, 116, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.R.; Cosgrove, S.C.; Turner, N.J.; Kapur, N.; Blacker, A.J. Highly productive oxidative biocatalysis in continuous flow by enhancing the aqueous equilibrium solubility of oxygen. Angew. Chem. Int. Ed. 2010, 49, 2182–2184. [Google Scholar]
- Gunther, A.; Jhunjhunwala, M.; Thalmann, M.; Schmidt, M.A.; Jensen, K.F. Micromixing of miscible liquids in segmented gas-liquid flow. Langmuir 2005, 21, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- De Vitis, V.; Dall’Oglio, F.; Pinto, A.; De Micheli, C.; Molinari, F.; Conti, P.; Romano, D.; Tamborini, L. Chemoenzymatic synthesis in flow reactors: A rapid and convenient preparation of Captopril. Chem. Open 2017, 6, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, P.; Tamborini, L.; Cazzamalli, S.; Pinto, A.; Arioli, S.; Balzaretti, S.; Plou, F.J.; Fernandez-Arrojo, L.; Molinari, F.; Conti, P.; et al. An efficient continuous flow process for the synthesis of a non-conventional mixture of fructooligosaccharides. Food Chem. 2016, 190, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Romano, D.; Gandolfi, R.; Guglielmetti, S.; Molinari, F. Enzymatic hydrolysis of capsaicins for the production of vanillylamine using ECB deacylase from Actinoplanes utahensis. Food Chem. 2011, 124, 1096–1098. [Google Scholar] [CrossRef]
- Csajagi, C.; Szatzker, G.; Toke, E.R.; Urge, L.; Darvasa, F.; Poppe, L. Enantiomer selective acylation of racemic alcohols by lipases in continuous-flow bioreactors. Tetrahedron Asymmetry 2008, 19, 237–246. [Google Scholar] [CrossRef]
| Entry | Substrate [mM] | Flow Rate a (μL min−1) | rf (μmol min−1gdry cells−1) | c b (%) |
|---|---|---|---|---|
| 1 | 12 | 15 | 1.80 | >97 |
| 2 | 12 | 30 | 3.60 | >97 |
| 3 | 12 | 60 | 6.12 | 85 |
| 4 | 24 | 15 | 3.60 | >97 |
| 5 | 24 | 30 | 7.20 | >97 |
| 6 | 24 | 60 | 7.78 | 54 |
| 7 | 36 | 15 | 4.86 | 90 |
| 8 | 36 | 30 | 8.21 | 76 |
| 9 | 36 | 60 | 11.88 | 55 |
| Entry | Substrate | c (%) | ee (%) [a] | rf (μmol min−1gdry cells−1) |
|---|---|---|---|---|
| 1 | 1a | >97 | 96 (R) | 7.20 |
| 2 | 1b | >97 | 59 (R) | 7.20 |
| 3 | 1c | >97 | 80 (S) | 7.20 |
| 4 | 1d | >97 | 88 (S) | 7.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vitis, V.; Dall’Oglio, F.; Tentori, F.; Contente, M.L.; Romano, D.; Brenna, E.; Tamborini, L.; Molinari, F. Bioprocess Intensification Using Flow Reactors: Stereoselective Oxidation of Achiral 1,3-diols with Immobilized Acetobacter Aceti. Catalysts 2019, 9, 208. https://doi.org/10.3390/catal9030208
De Vitis V, Dall’Oglio F, Tentori F, Contente ML, Romano D, Brenna E, Tamborini L, Molinari F. Bioprocess Intensification Using Flow Reactors: Stereoselective Oxidation of Achiral 1,3-diols with Immobilized Acetobacter Aceti. Catalysts. 2019; 9(3):208. https://doi.org/10.3390/catal9030208
Chicago/Turabian StyleDe Vitis, Valerio, Federica Dall’Oglio, Francesca Tentori, Martina Letizia Contente, Diego Romano, Elisabetta Brenna, Lucia Tamborini, and Francesco Molinari. 2019. "Bioprocess Intensification Using Flow Reactors: Stereoselective Oxidation of Achiral 1,3-diols with Immobilized Acetobacter Aceti" Catalysts 9, no. 3: 208. https://doi.org/10.3390/catal9030208
APA StyleDe Vitis, V., Dall’Oglio, F., Tentori, F., Contente, M. L., Romano, D., Brenna, E., Tamborini, L., & Molinari, F. (2019). Bioprocess Intensification Using Flow Reactors: Stereoselective Oxidation of Achiral 1,3-diols with Immobilized Acetobacter Aceti. Catalysts, 9(3), 208. https://doi.org/10.3390/catal9030208





