Electrodeposited Nanostructured CoFe2O4 for Overall Water Splitting and Supercapacitor Applications
Abstract
:1. Introduction
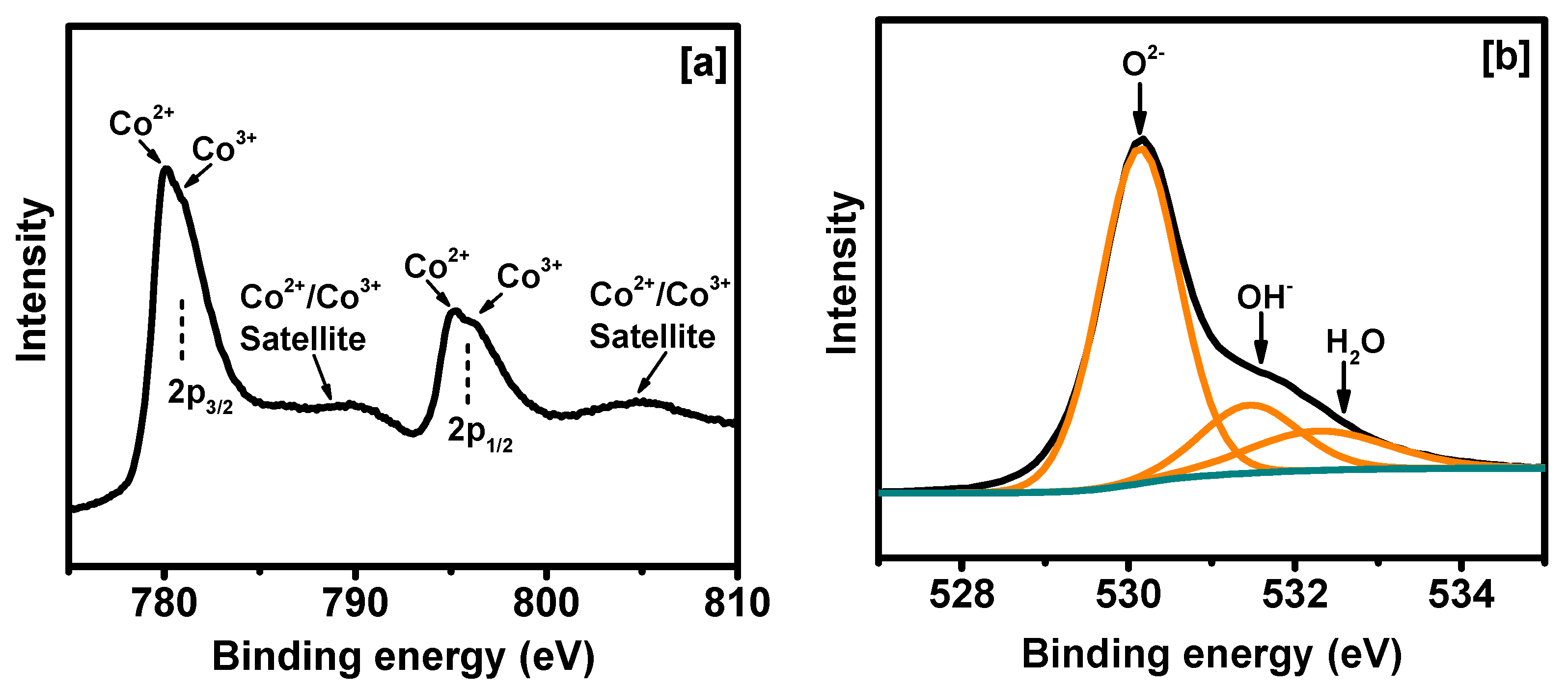
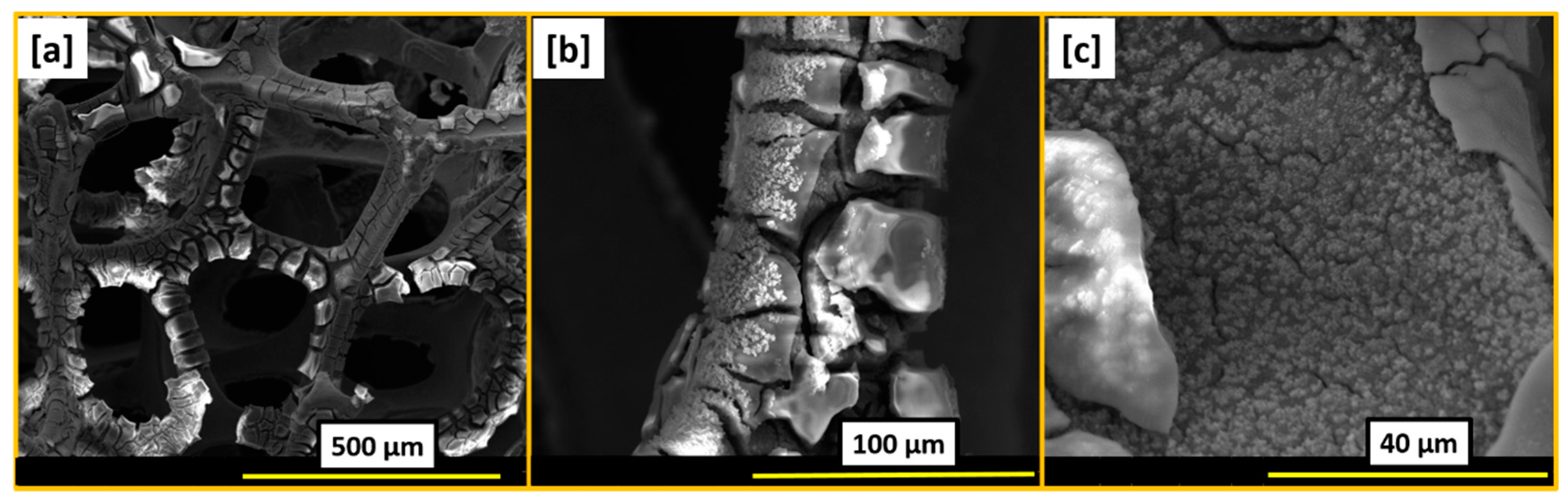
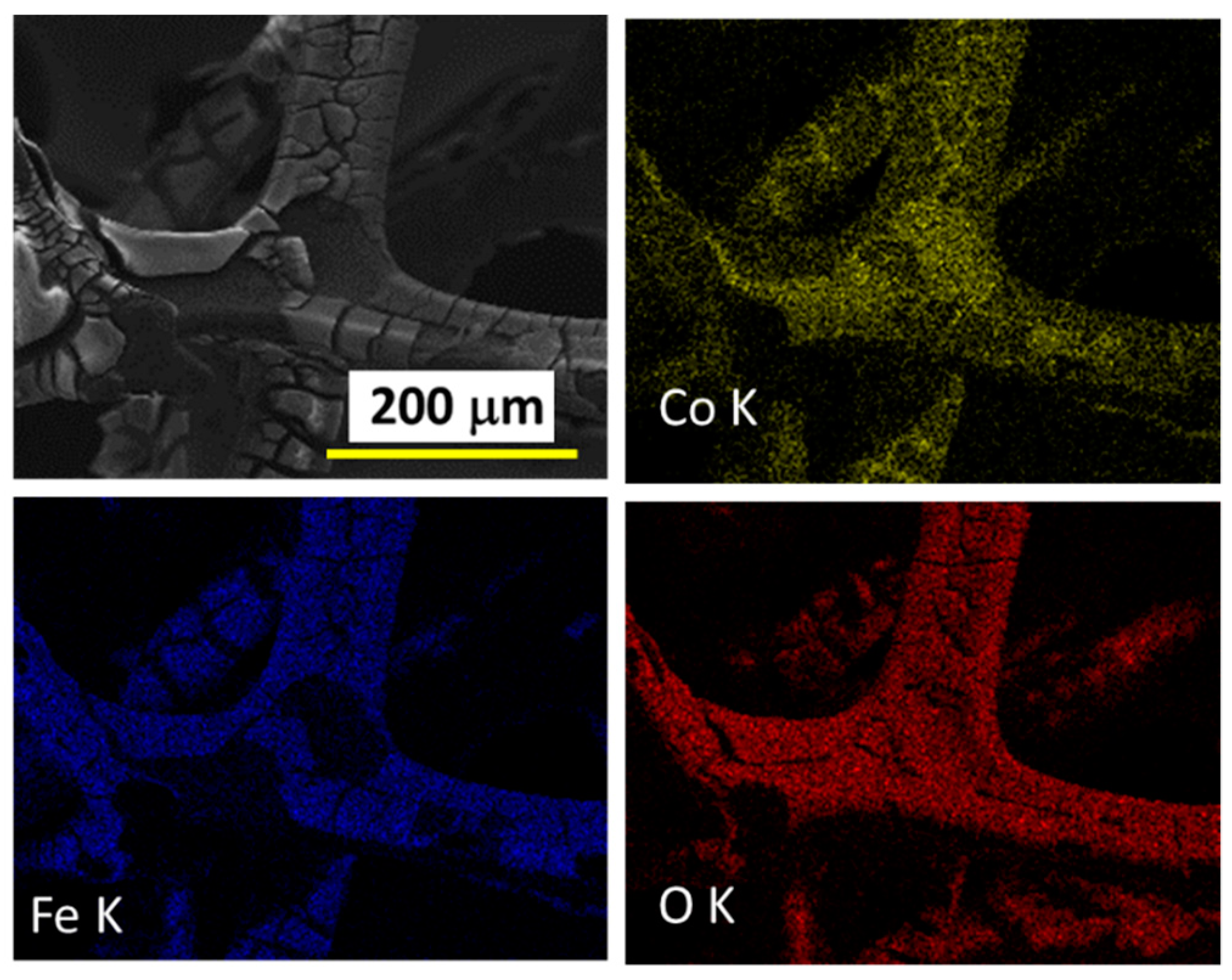
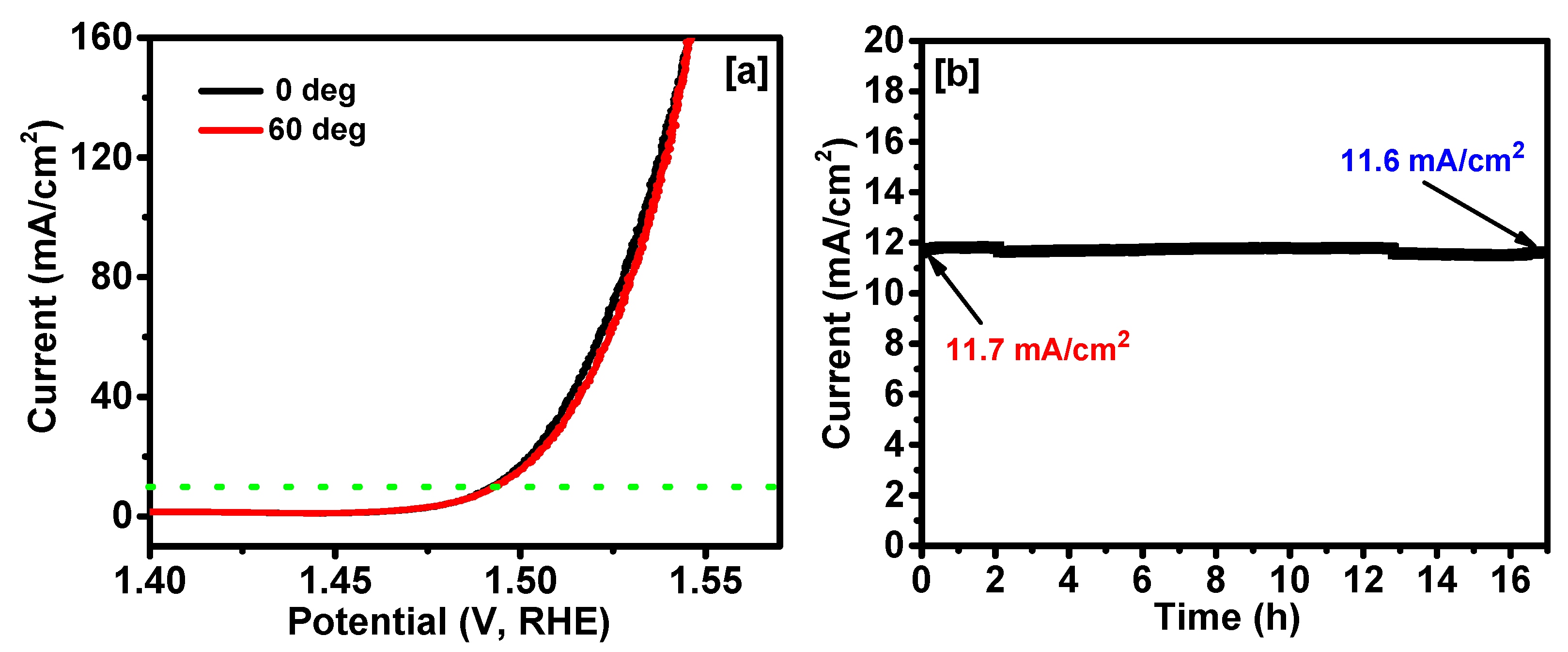
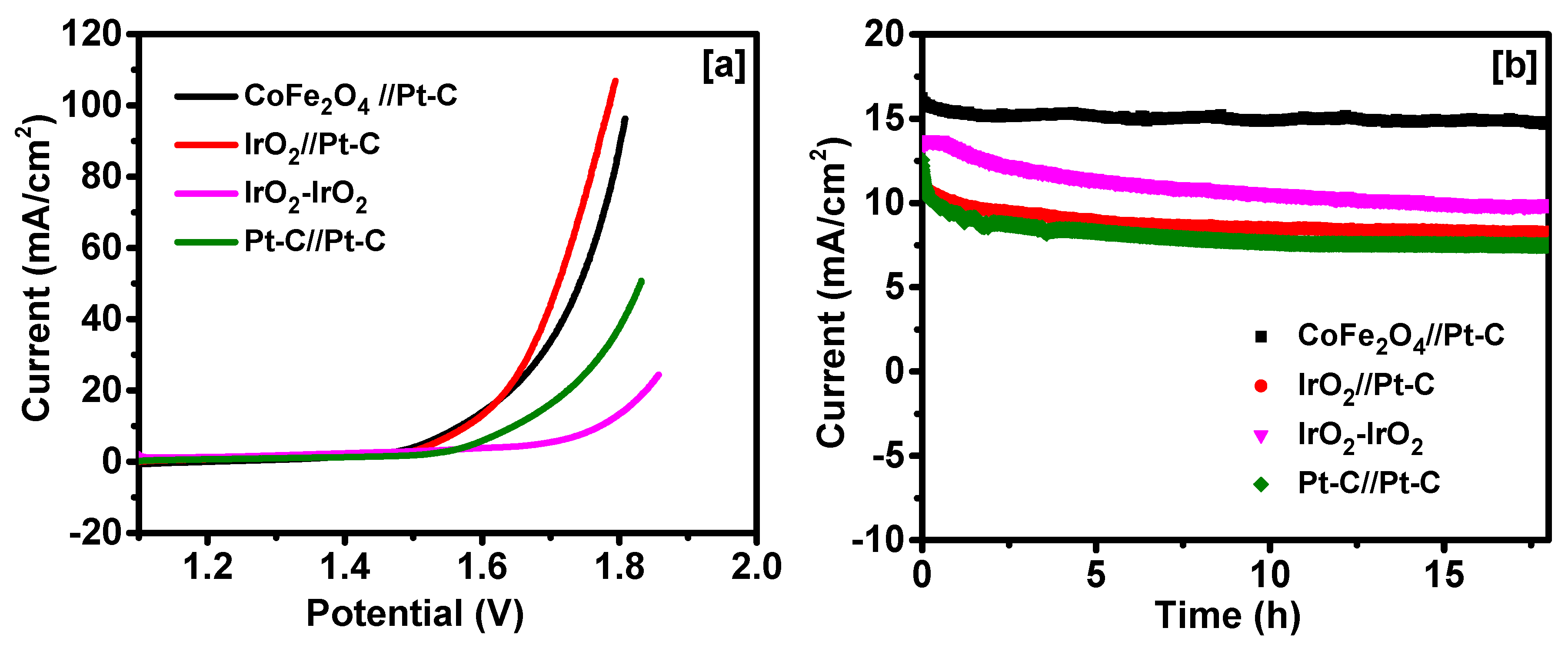
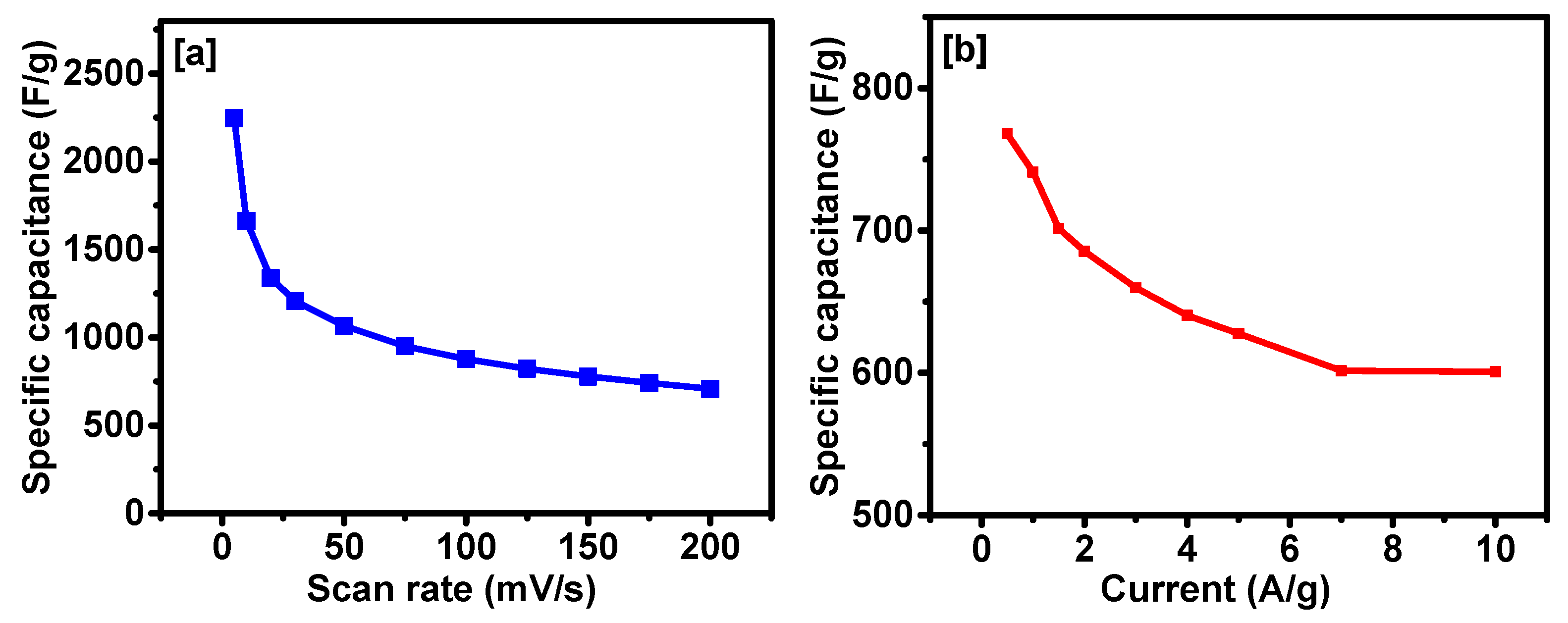
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Electrodeposited CoFe2O4 Electrodes
3.3. Preparation of Dip-Coated CoFe2O4 Electrode
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IRENA; IEA; REN21. Renewable Energy Policies in a Time of Transition. 2018. Available online: https://www.irena.org/publications/2018/Apr/Renewable-energy-policies-in-a-time-of-transition.
- Mitch Jacoby. Fuel-Cell Cars Finally Drive off the Lot. Chem. Eng. News Arch. 2017, 95, 28. [Google Scholar]
- Rissman, J. The Future Of Electric Vehicles In The U.S., Part 1: 65%-75% New Light-Duty Vehicle Sales By 2050. Forbes, 14 September 2017; 1. [Google Scholar]
- Hu, H.; Guan, B.; Xia, B.; Lou, X.W. Designed Formation of Co3O4/NiCo2O4 Double-Shelled Nanocages with Enhanced Pseudocapacitive and Electrocatalytic Properties. J. Am. Chem. Soc. 2015, 137, 5590–5595. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Zhao, C.; Han, W.; Liu, Y.; Zhao, H.; Ma, Y.; Xie, E. Cobalt Sulfide Nanosheets Coated on NiCo2S4 Nanotube Arrays as Electrode Materials for High-Performance Supercapacitors. J. Mater. Chem. A 2015, 3, 10492–10497. [Google Scholar] [CrossRef]
- Chauhan, M.; Reddy, K.P.; Gopinath, C.S.; Deka, S. Copper Cobalt Sulfide Nanosheets Realizing a Promising Electrocatalytic Oxygen Evolution Reaction. ACS Catal. 2017, 7, 5871–5879. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and Beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou, N.; Koczwara, C.; Prehal, C.; Terziyska, V.; Babic, B.; Matovic, B.; Constantinides, G.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.; et al. Nanoporous Activated Carbon Cloth as a Versatile Material for Hydrogen Adsorption, Selective Gas Separation and Electrochemical Energy Storage. Nano Energy 2017, 40, 49–64. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Lee, J.H.; Je, S.H.; Buyukcakir, O.; Kwon, T.; Polychronopoulou, K.; Choi, J.W.; Coskun, A. Chemical Blowing Approach for Ultramicroporous Carbon Nitride Frameworks and Their Applications in Gas and Energy Storage. Adv. Funct. Mater. 2017, 27, 1604658. [Google Scholar] [CrossRef]
- Li, Y.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. From Biomass to High Performance Solar–thermal and Electric–thermal Energy Conversion and Storage Materials. J. Mater. Chem. A 2014, 2, 7759–7765. [Google Scholar] [CrossRef]
- Chen, S.; Bi, J.; Zhao, Y.; Yang, L.; Zhang, C.; Ma, Y.; Wu, Q.; Wang, X.; Hu, Z. Nitrogen-Doped Carbon Nanocages as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reaction. Adv. Mater. 2012, 24, 5593–5597. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Qiu, W.; Yang, H.; Fan, W.; Huang, Y.; Fang, P.; Li, G.; Ji, H.; Tong, Y. A Monolithic Metal-Free Electrocatalyst for Oxygen Evolution Reaction and Overall Water Splitting. Energy Environ. Sci. 2016, 9, 3411–3416. [Google Scholar] [CrossRef]
- Li, W.; Liang, C.; Qiu, J.; Zhou, W.; Han, H.; Wei, Z.; Sun, G.; Xin, Q. Carbon Nanotubes as Support for Cathode Catalyst of a Direct Methanol Fuel Cell. Carbon N. Y. 2002, 40, 791–794. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.Z. Graphitic Carbon Nitride Materials: Controllable Synthesis and Applications in Fuel Cells and Photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731. [Google Scholar] [CrossRef]
- Ranaweera, C.K.; Zhang, C.; Bhoyate, S.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Perez, F.; Gupta, B.K.; Gupta, R.K. Flower-Shaped Cobalt Oxide Nano-Structures as an Efficient, Flexible and Stable Electrocatalyst for the Oxygen Evolution Reaction. Mater. Chem. Front. 2017, 1, 1580–1584. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Li, W.; Gou, X. Fe2O3 Nanotubes in Gas Sensor and Lithium-Ion Battery Applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Bhoyate, S.; Morey, T.; Neria, B.L.; Vasiraju, V.; Gupta, G.; Palchoudhury, S.; Kahol, P.K.; Mishra, S.R.; et al. MoS2 Decorated Carbon Nanofibers as Efficient and Durable Electrocatalyst for Hydrogen Evolution Reaction. C 2017, 3, 33. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; He, G.; Zhang, L.; Li, M. Electrodeposition of High-Capacitance 3D CoS/Graphene Nanosheets on Nickel Foam for High-Performance Aqueous Asymmetric Supercapacitors. J. Mater. Chem. A 2015, 3, 20619–20626. [Google Scholar] [CrossRef]
- Joo, S.H.; Choi, S.J.; Oh, I.; Kwak, J.; Liu, Z.; Terasaki, O.; Ryoo, R. Ordered Nanoporous Arrays of Carbon Supporting High Dispersions of Platinum Nanoparticles. Nature 2001, 412, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Transition-metal-oxide, B.; Carbon, N.S.; Chen, P.; Shen, G.; Shi, Y.; Chen, H.; Zhou, C. Preparation and Characterization of Flexible Asymmetric Supercapacitors. ACS Nano 2010, 4, 4403–4411. [Google Scholar]
- Shen, L.; Wang, J.; Xu, G.; Li, H.; Dou, H.; Zhang, X. NiCo2S4 Nanosheets Grown on Nitrogen-Doped Carbon Foams as an Advanced Electrode for Supercapacitors. Adv. Energy Mater. 2015, 5, 2–8. [Google Scholar] [CrossRef]
- 22. Zaquine, C.; Bhoyate, S.; Wang, F.; Li, X.; Siam, K.; Kahol, P.K.; Gupta, R.K. Effect of Solvent for Tailoring the Nanomorphology of Multinary CuCo2S4 for Overall Water Splitting and Energy Storage. J. Alloys Compd. 2019, 784, 1–7. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, Z.; Liu, Q.; Zhu, X.; Liu, L.; Ni, L.; Shen, C. Novel Red Blood Cell Shaped α-Fe2O3 Microstructures and FeO(OH) Nanorods as High Capacity Supercapacitors. RSC Adv. 2015, 5, 91127–91133. [Google Scholar] [CrossRef]
- Guan, C.; Liu, J.; Wang, Y.; Mao, L.; Fan, Z.; Shen, Z.; Zhang, H.; Wang, J. Iron Oxide-Decorated Carbon for Supercapacitor Anodes with Ultrahigh Energy Density and Outstanding Cycling Stability. ACS Nano 2015, 9, 5198–5207. [Google Scholar] [CrossRef] [PubMed]
- Lorkit, P.; Panapoy, M.; Ksapabutr, B. Iron Oxide-Based Supercapacitor from Ferratrane Precursor via Sol–gel-Hydrothermal Process. Energy Procedia 2014, 56, 466–473. [Google Scholar] [CrossRef]
- Liu, J.; Nai, J.; You, T.; An, P.; Zhang, J.; Ma, G.; Niu, X.; Liang, C.; Yang, S.; Guo, L. The Flexibility of an Amorphous Cobalt Hydroxide Nanomaterial Promotes the Electrocatalysis of Oxygen Evolution Reaction. Small 2018, 14, 1703514. [Google Scholar] [CrossRef] [PubMed]
- Numan, A.; Duraisamy, N.; Saiha Omar, F.; Mahipal, Y.K.; Ramesh, K.; Ramesh, S. Enhanced Electrochemical Performance of Cobalt Oxide Nanocube Intercalated Reduced Graphene Oxide for Supercapacitor Application. RSC Adv. 2016, 6, 34894–34902. [Google Scholar] [CrossRef]
- Kumbhar, V.S.; Jagadale, A.D.; Shinde, N.M.; Lokhande, C.D. Chemical Synthesis of Spinel Cobalt Ferrite (CoFe2O4) Nano-Flakes for Supercapacitor Application. Appl. Surf. Sci. 2012, 259, 39–43. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Luo, D.; Xie, L.; Yu, F.; Bao, J.; Li, Y.; Yu, Y.; Chen, S.; Ren, Z. Hierarchical Cu@CoFe Layered Double Hydroxide Core-Shell Nanoarchitectures as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. Nano Energy 2017, 41, 327–336. [Google Scholar] [CrossRef]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin Iron-Cobalt Oxide Nanosheets with Abundant Oxygen Vacancies for the Oxygen Evolution Reaction. Adv. Mater. 2017, 29, 1606793-1–1606793-7. [Google Scholar] [CrossRef]
- Yan, W.; Bian, W.; Jin, C.; Tian, J.H.; Yang, R. An Efficient Bi-Functional Electrocatalyst Based on Strongly Coupled CoFe2O4/Carbon Nanotubes Hybrid for Oxygen Reduction and Oxygen Evolution. Electrochim. Acta 2015, 177, 65–72. [Google Scholar] [CrossRef]
- Yan, W.; Cao, X.; Tian, J.; Jin, C.; Ke, K.; Yang, R. Nitrogen/Sulfur Dual-Doped 3D Reduced Graphene Oxide Networks-Supported CoFe2O4 with Enhanced Electrocatalytic Activities for Oxygen Reduction and Evolution Reactions. Carbon N. Y. 2016, 99, 195–202. [Google Scholar] [CrossRef]
- Lee, S.; Kang, J.S.; Leung, K.T.; Lee, W.; Kim, D.; Han, S.; Yoo, W.; Yoon, H.J.; Nam, K.; Sohn, Y. Unique Multi-Phase Co/Fe/CoFe2O4 by Water–gas Shift Reaction, CO Oxidation and Enhanced Supercapacitor Performances. J. Ind. Eng. Chem. 2016, 43, 69–77. [Google Scholar] [CrossRef]
- Lv, L.; Xu, Q.; Ding, R.; Qi, L.; Wang, H. Chemical Synthesis of Mesoporous CoFe2O4 Nanoparticles as Promising Bifunctional Electrode Materials for Supercapacitors. Mater. Lett. 2013, 111, 35–38. [Google Scholar] [CrossRef]
- He, P.; Yang, K.; Wang, W.; Dong, F.; Du, L.; Deng, Y. Reduced Graphene Oxide-CoFe2O4 Composites for Supercapacitor Electrode. Russ. J. Electrochem. 2013, 49, 359–364. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of Hierarchically Structured Three-Dimensional Nickel–iron Electrodes for Efficient Oxygen Evolution at High Current Densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef]
- Gu, D.; Jia, C.-J.; Weidenthaler, C.; Bongard, H.-J.; Spliethoff, B.; Schmidt, W.; Schüth, F. Highly Ordered Mesoporous Cobalt-Containing Oxides: Structure, Catalytic Properties, and Active Sites in Oxidation of Carbon Monoxide. J. Am. Chem. Soc. 2015, 137, 11407–11418. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and Characterization of Cobalt Hydroxide, Cobalt Oxyhydroxide, and Cobalt Oxide Nanodiscs. J. Phys. Chem. C 2010, 114, 111–119. [Google Scholar] [CrossRef]
- Sagu, J.S.; Wijayantha, K.G.U.; Tahir, A.A. The Pseudocapacitive Nature of CoFe2O4 Thin Films. Electrochim. Acta 2017, 246, 870–878. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W.D. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chemie Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Kargar, A.; Yavuz, S.; Kim, T.K.; Liu, C.H.; Kuru, C.; Rustomji, C.S.; Jin, S.; Bandaru, P.R. Solution-Processed CoFe2O4 Nanoparticles on 3D Carbon Fiber Papers for Durable Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2015, 7, 17851–17856. [Google Scholar] [CrossRef] [PubMed]
- Shanmugavani, A.; Kalpana, D.; Selvan, R.K. Electrochemical Properties of CoFe2O4 Nanoparticles as Negative and Co(OH)2 and Co2Fe(CN)6 as Positive Electrodes for Supercapacitors. Mater. Res. Bull. 2015, 71, 133–141. [Google Scholar] [CrossRef]
- Bhoyate, S.; Kahol, P.K.; Sapkota, B.; Mishra, S.R.; Perez, F.; Gupta, R.K. Polystyrene Activated Linear Tube Carbon Nanofiber for Durable and High-Performance Supercapacitors. Surf. Coatings Technol. 2018, 345, 113–122. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ranaweera, C.K.; Zhang, C.; Morey, T.; Hyatt, M.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Gupta, R.K. Eco-Friendly and High Performance Supercapacitors for Elevated Temperature Applications Using Recycled Tea Leaves. Glob. Challenges 2017, 1, 1700063. [Google Scholar] [CrossRef] [Green Version]
- Bhoyate, S.; Mensah-Darkwa, K.; Kahol, P.K.; Gupta, R.K. Recent Development on Nanocomposites of Graphene for Supercapacitor Applications. Curr. Graphene Sci. 2017, 1, 26–43. [Google Scholar] [CrossRef]
- Adhikari, H.; Ghimire, M.; Ranaweera, C.K.; Bhoyate, S.; Gupta, R.K.; Alam, J.; Mishra, S.R. Synthesis and Electrochemical Performance of Hydrothermally Synthesized Co3O4 Nanostructured Particles in Presence of Urea. J. Alloys Compd. 2017, 708, 628–638. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Hao, X.D.; Diao, Z.P.; Li, J.; Guan, Y.M. One-Pot Controllable Synthesis of Flower-like CoFe2O4/FeOOH Nanocomposites for High-Performance Supercapacitors. Mater. Lett. 2014, 123, 229–234. [Google Scholar] [CrossRef]
- Zhang, C.; Bhoyate, S.; Kahol, P.K.; Siam, K.; Poudel, T.P.; Mishra, S.R.; Perez, F.; Gupta, A.; Gupta, G.; Gupta, R.K. Highly Efficient and Durable Electrocatalyst based on Nanowires of Cobalt Sulfide for Overall Water Splitting. Chem. Nano Mat. 2018, 4, 1240–1246. [Google Scholar] [CrossRef]
- Zhang, C.; Bhoyate, S.; Hyatt, M.; Neria, B.L.; Siam, K.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Perez, F.; Gupta, R.K. Nitrogen-Doped Flexible Carbon Cloth for Durable Metal Free Electrocatalyst for Overall Water Splitting. Surf. Coatings Technol. 2018, 347, 407–413. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Bhoyate, S.; Zhao, C.; Kahol, P.K.; Kostoglou, N.; Mitterer, C.; Hinder, S.J.; Baker, M.A.; Constantinides, G.; Polychronopoulou, K.; et al. Electrodeposited Nanostructured CoFe2O4 for Overall Water Splitting and Supercapacitor Applications. Catalysts 2019, 9, 176. https://doi.org/10.3390/catal9020176
Zhang C, Bhoyate S, Zhao C, Kahol PK, Kostoglou N, Mitterer C, Hinder SJ, Baker MA, Constantinides G, Polychronopoulou K, et al. Electrodeposited Nanostructured CoFe2O4 for Overall Water Splitting and Supercapacitor Applications. Catalysts. 2019; 9(2):176. https://doi.org/10.3390/catal9020176
Chicago/Turabian StyleZhang, Chunyang, Sanket Bhoyate, Chen Zhao, Pawan K. Kahol, Nikolaos Kostoglou, Christian Mitterer, Steven J. Hinder, Mark A. Baker, Georgios Constantinides, Kyriaki Polychronopoulou, and et al. 2019. "Electrodeposited Nanostructured CoFe2O4 for Overall Water Splitting and Supercapacitor Applications" Catalysts 9, no. 2: 176. https://doi.org/10.3390/catal9020176
APA StyleZhang, C., Bhoyate, S., Zhao, C., Kahol, P. K., Kostoglou, N., Mitterer, C., Hinder, S. J., Baker, M. A., Constantinides, G., Polychronopoulou, K., Rebholz, C., & Gupta, R. K. (2019). Electrodeposited Nanostructured CoFe2O4 for Overall Water Splitting and Supercapacitor Applications. Catalysts, 9(2), 176. https://doi.org/10.3390/catal9020176








