Comprehensive Comparison between Nanocatalysts of Mn−Co/TiO2 and Mn−Fe/TiO2 for NO Catalytic Conversion: An Insight from Nanostructure, Performance, Kinetics, and Thermodynamics
Abstract
:1. Introduction
2. Results and Discussions
2.1. Physicochemical Properties of the Nanocatalysts
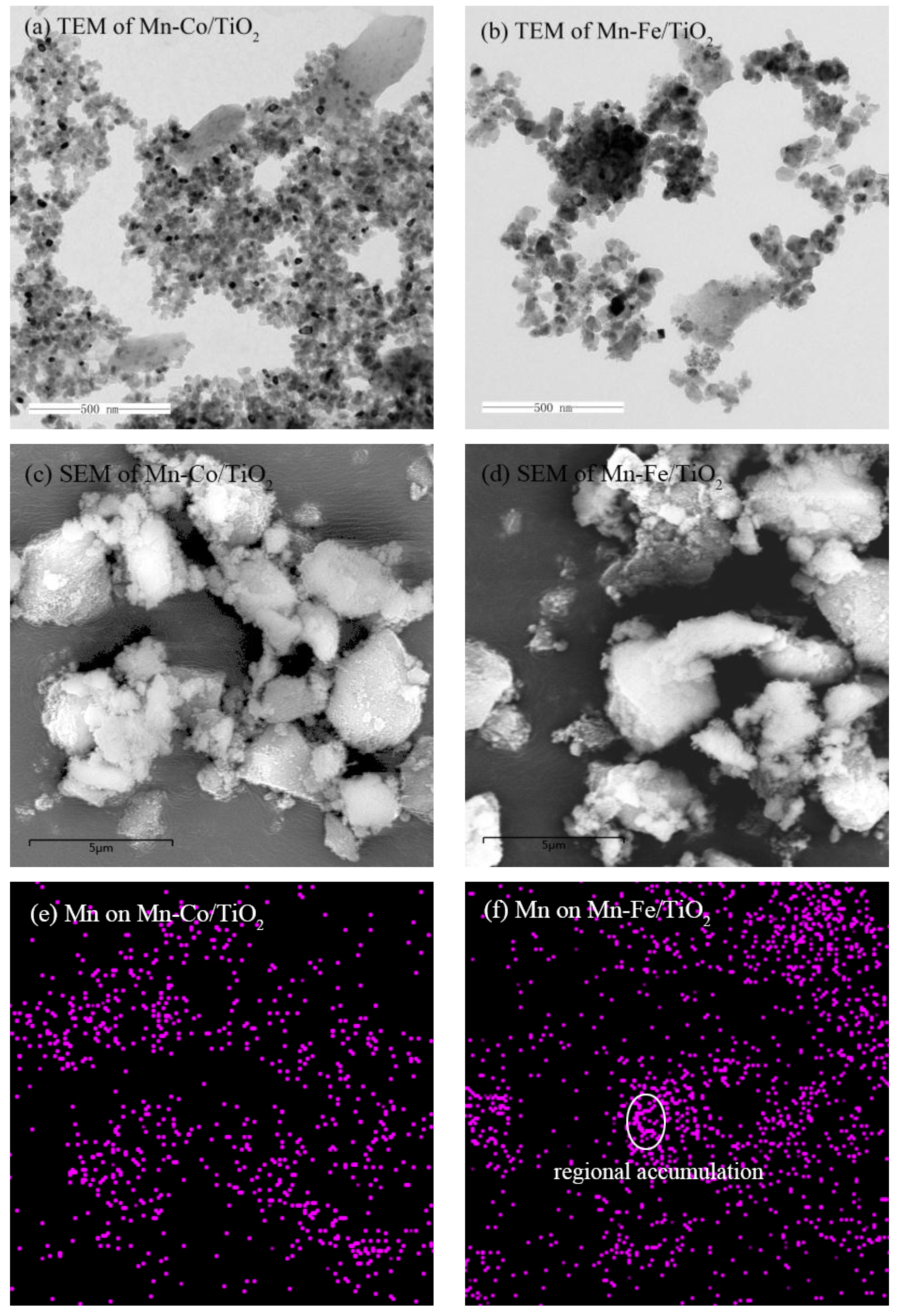
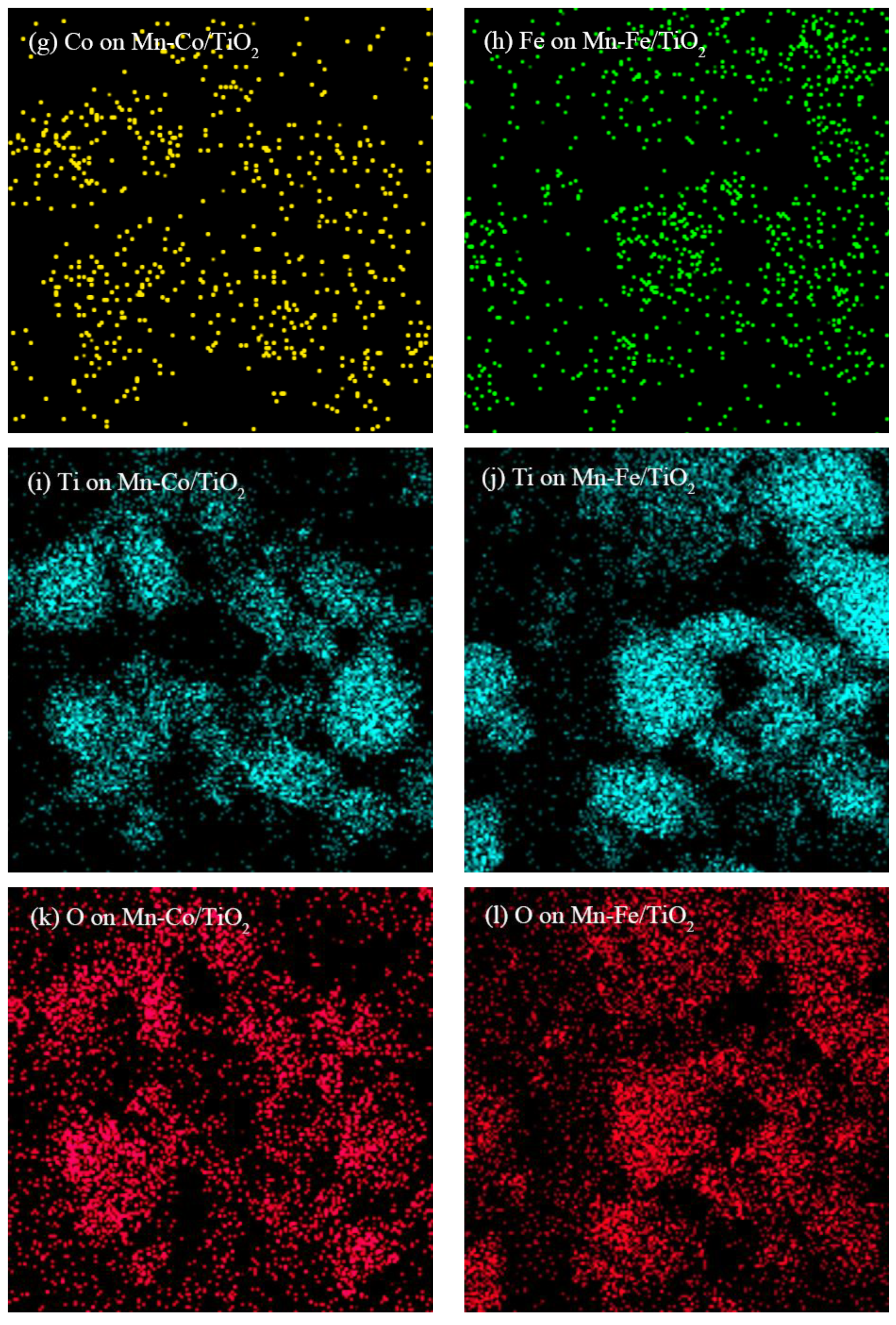
2.1.1. TEM Analysis
2.1.2. BET Analysis
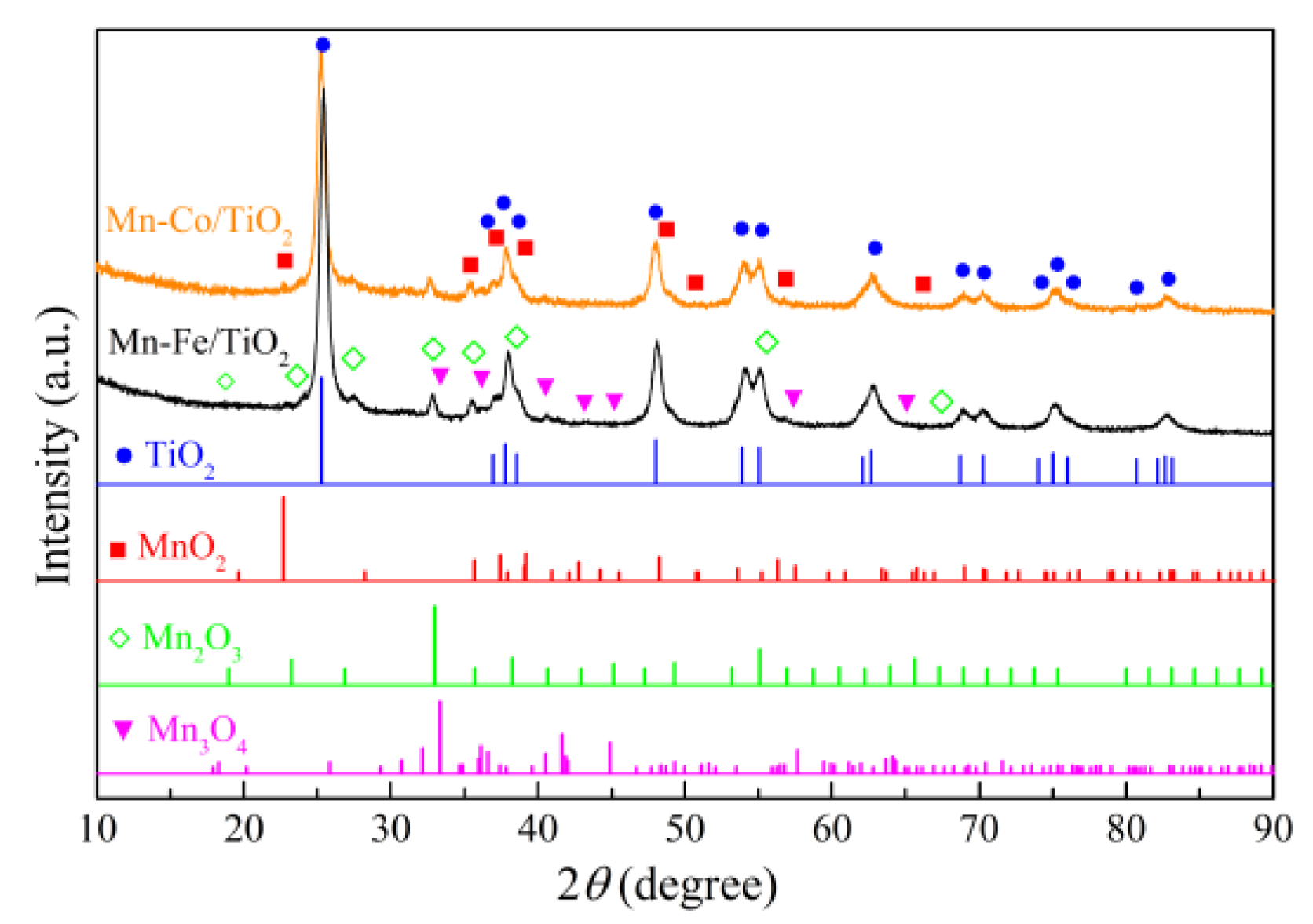
2.1.3. Components Analysis
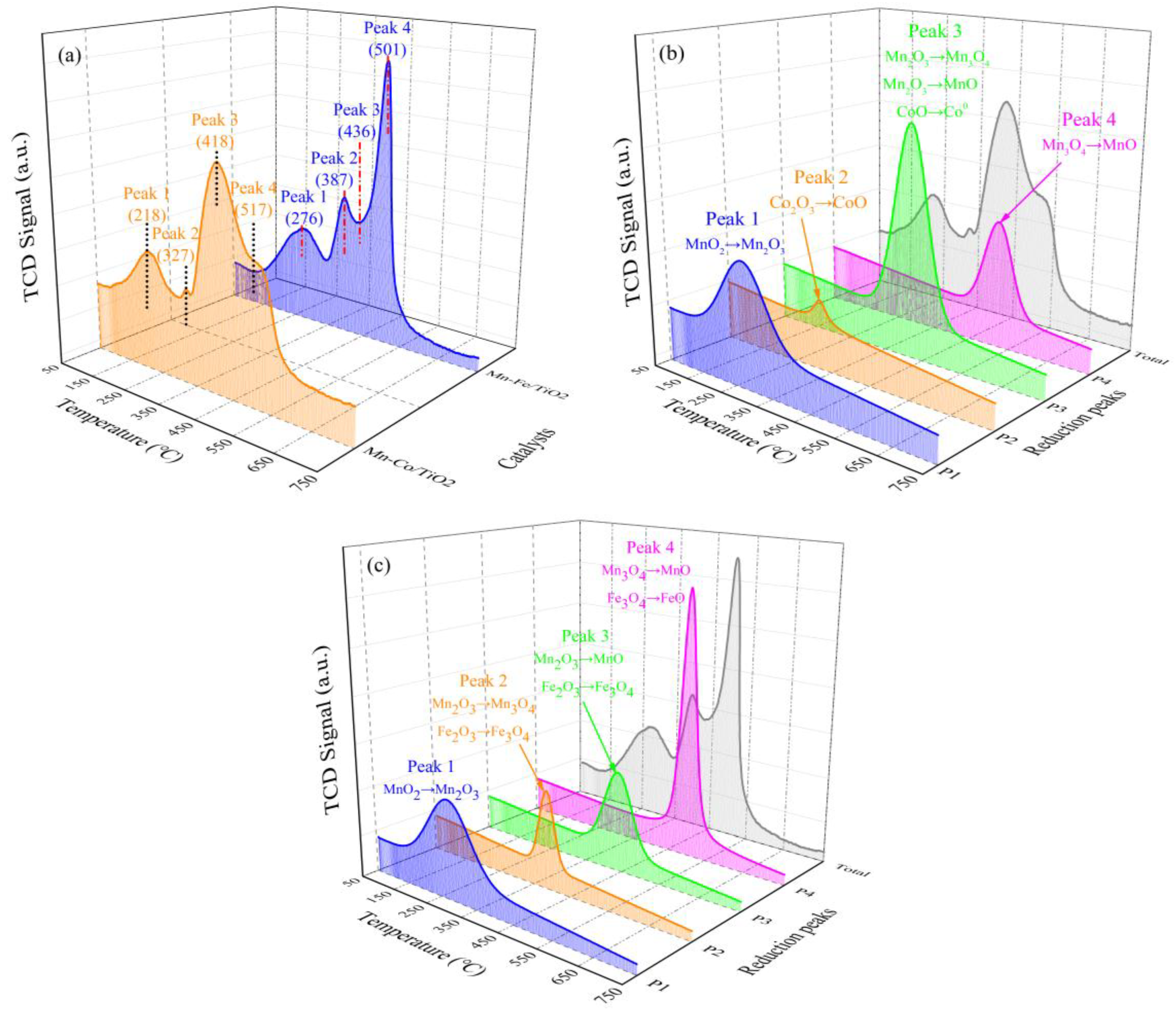
2.1.4. Reducibility Properties
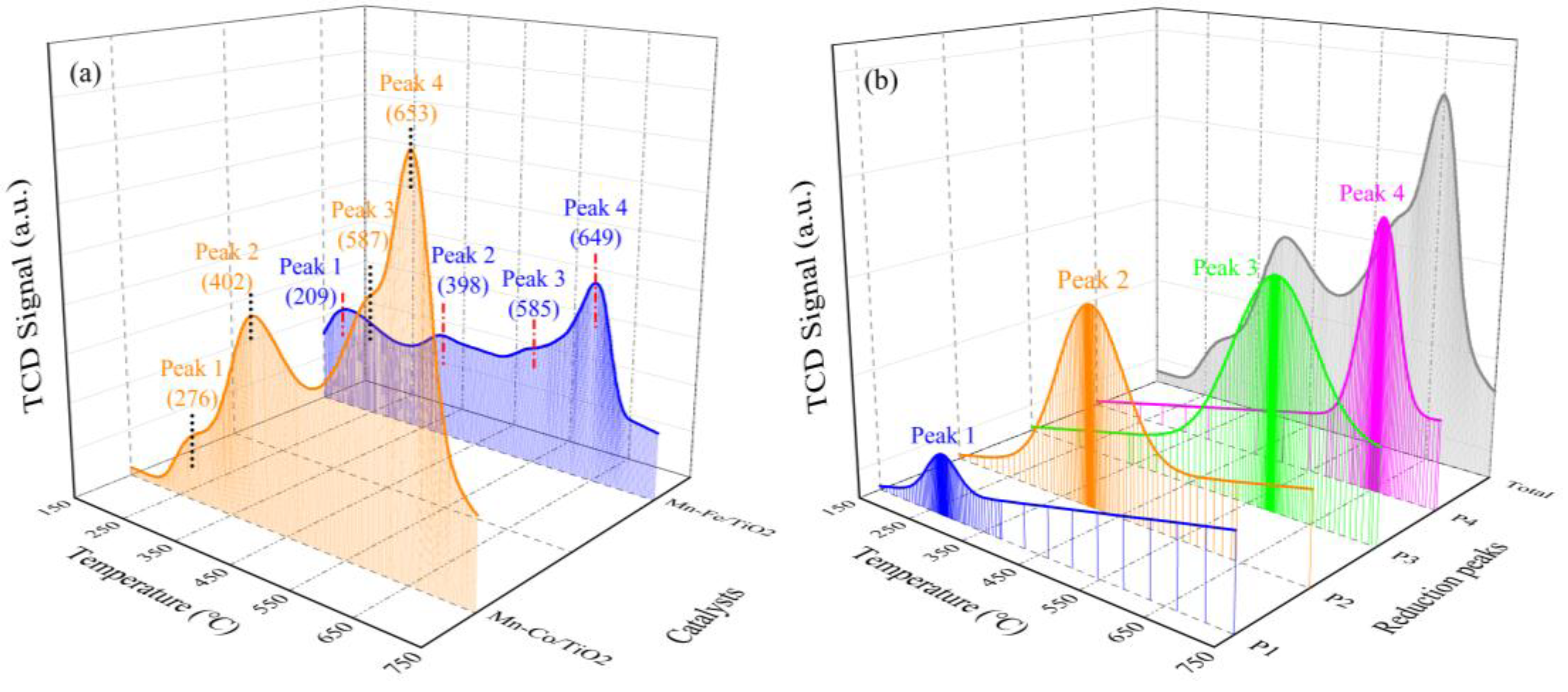
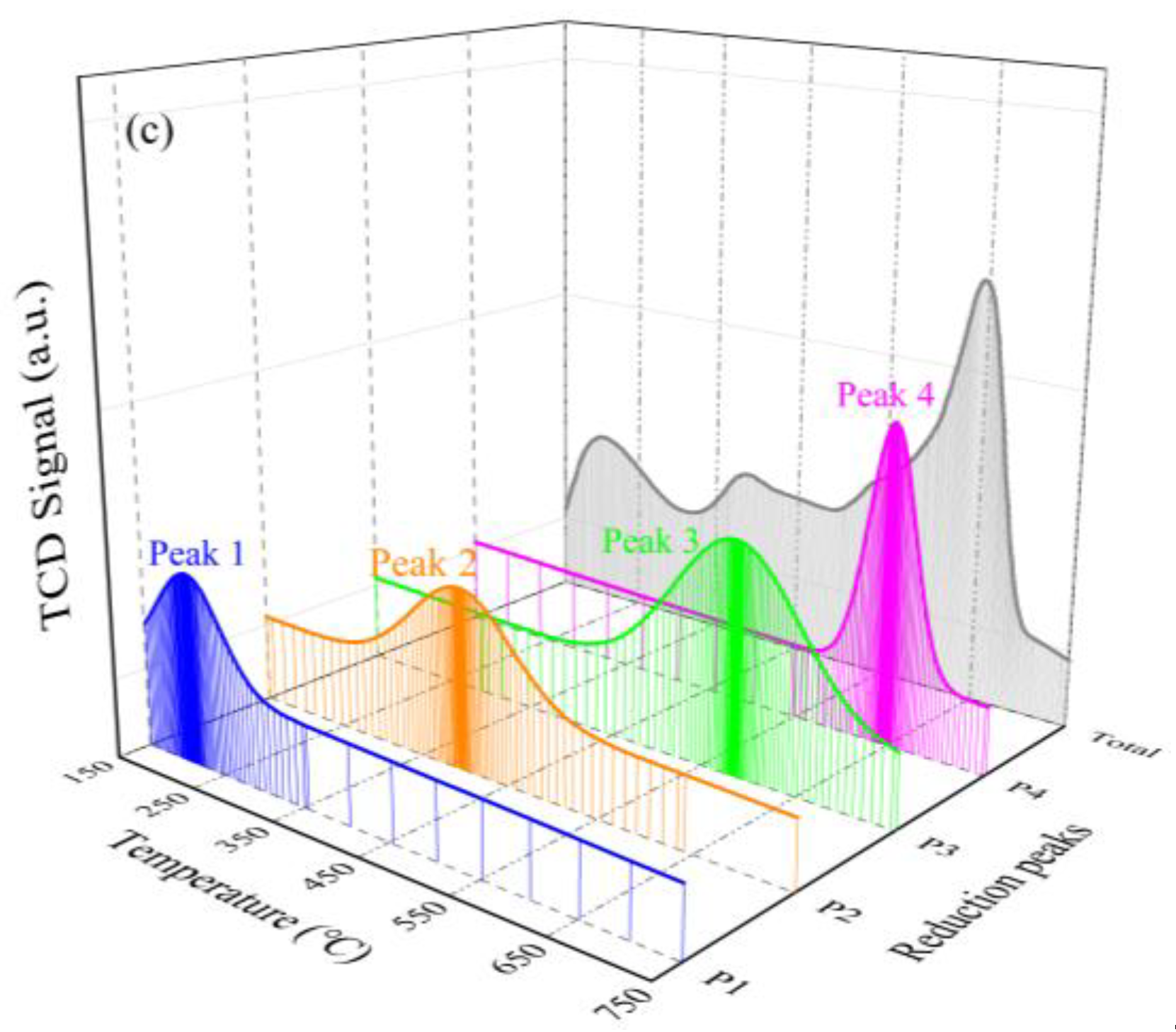
2.1.5. Ammonia Adsorption Properties
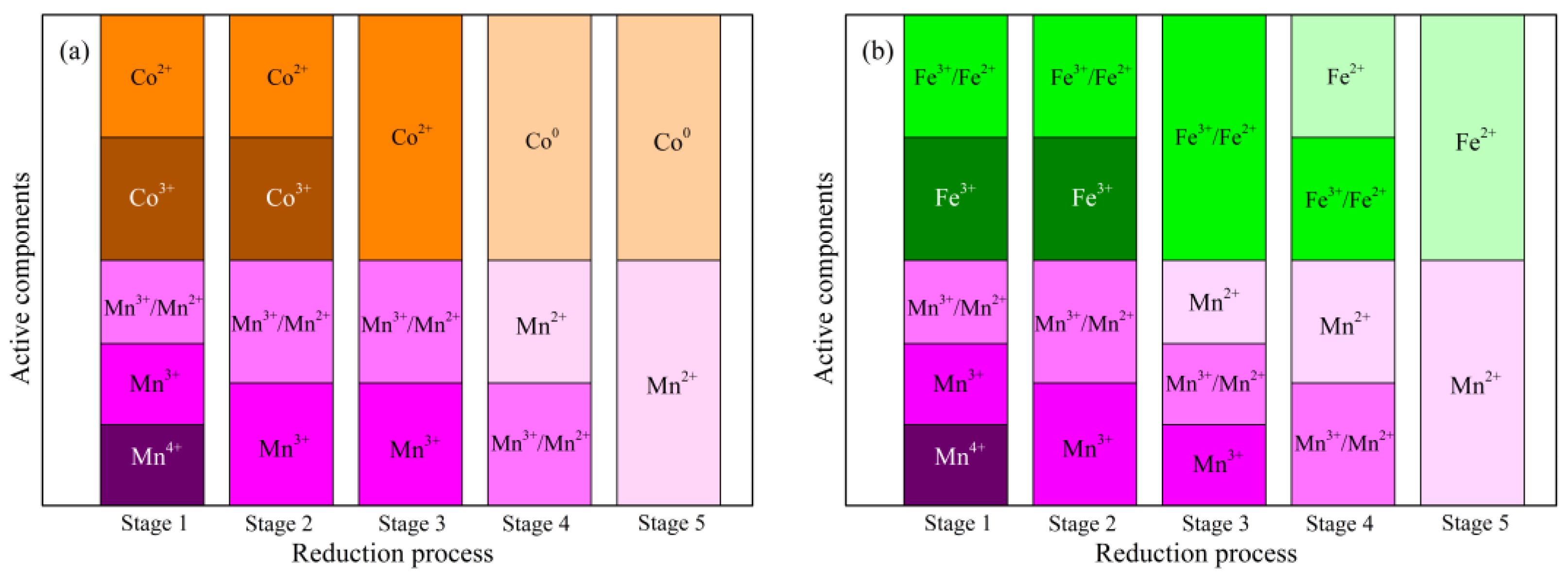
2.1.6. Oxidation States of Active Species
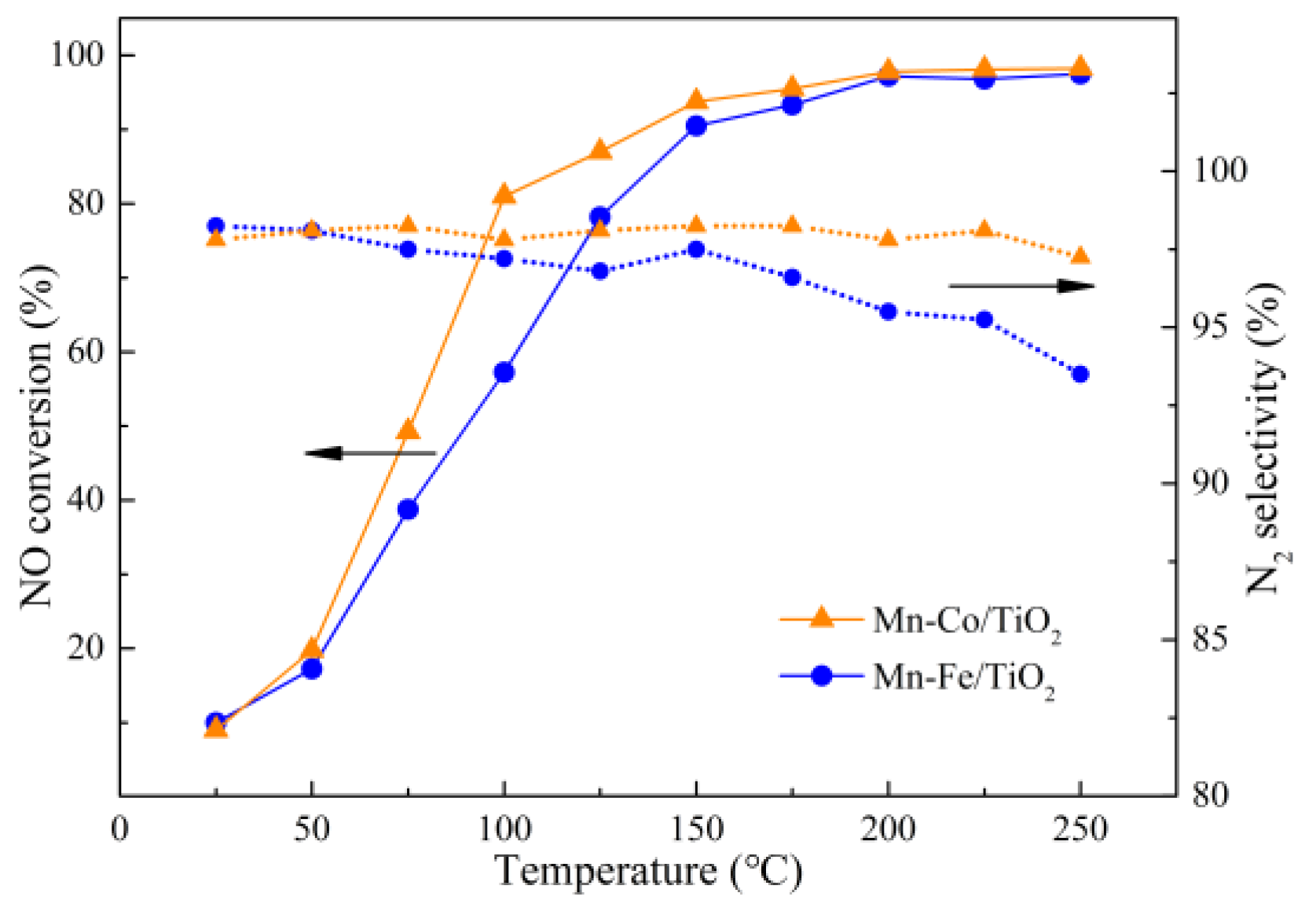
2.2. Catalytic Performance
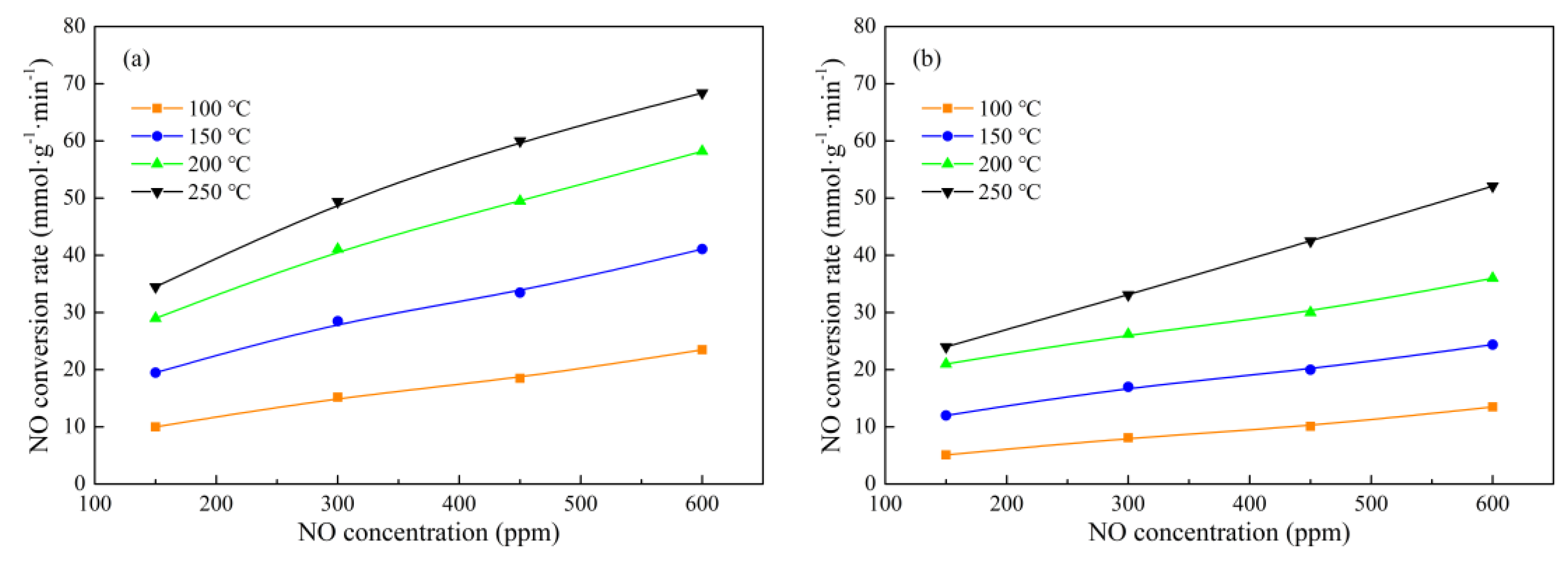
2.3. Reaction Kinetic Study
2.4. Thermodynamic Calculation
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Performance Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.; Guang, J.; Guo, D.; Wang, L.; Li, X. Ceria modified FeMnOx —Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B Environ. 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Gillot, S.; Tricot, G.; Vezin, H.; Dacquin, J.-P.; Dujardin, C.; Granger, P. Induced effect of tungsten incorporation on the catalytic properties of CeVO4 systems for the selective reduction of NOx by ammonia. Appl. Catal. B Environ. 2018, 234, 318–328. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhao, Q.; Ke, J.; Xiao, H.; Lv, X.; Liu, S.; Tadé, M.; Wang, S. Mechanistic investigation of the enhanced NH3-SCR on cobalt-decorated Ce-Ti mixed oxide: In situ FTIR analysis for structure-activity correlation. Appl. Catal. B Environ. 2017, 200, 297–308. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Lu, T.; Cheng, K.; Xu, H. Performance of V2O5-WO3-MoO3/TiO2 Catalyst for Selective Catalytic Reduction of NOx by NH3. Chin. J. Chem. Eng. 2013, 21, 1–7. [Google Scholar] [CrossRef]
- Huang, J.; Huang, H.; Jiang, H.; Liu, L. The promotional role of Nd on Mn/TiO2 catalyst for the low-temperature NH3 -SCR of NOx. Catal. Today 2018, 411, 338–346. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, W.; Luo, N.; Li, P.; Lei, Z.; Chen, B. Low-temperature NH3-SCR of NO by lanthanum manganite perovskites: Effect of A-/B-site substitution and TiO2/CeO2 support. Appl. Catal. B Environ. 2014, 146, 94–104. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Mei, D.; Zhang, Z.; Xie, J.; Hu, H. Thermodynamic calculation for the activity and mechanism of Mn/TiO2 catalyst doped transition metals for SCR at low temperature. Catal. Commun. 2014, 52, 45–48. [Google Scholar] [CrossRef]
- Qiu, M.; Zhan, S.; Yu, H.; Zhu, D. Low-temperature selective catalytic reduction of NO with NH3 over ordered mesoporous MnxCo3−xO4 catalyst. Catal. Commun. 2015, 62, 107–111. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Wang, X.; Xu, H.; Sun, K.; Shen, K. Influence of the addition of transition metals (Cr, Zr, Mo) on the properties of MnOx –FeOx catalysts for low-temperature selective catalytic reduction of NOx by Ammonia. J. Colloid Interface Sci. 2013, 392, 319–324. [Google Scholar] [CrossRef]
- Wang, R.; Wu, X.; Zou, C.; Li, X.; Du, Y. NOx Removal by Selective Catalytic Reduction with Ammonia over a Hydrotalcite-Derived NiFe Mixed Oxide. Catalysts 2018, 8, 384. [Google Scholar] [CrossRef]
- Li, W.; Guo, R.-T.; Wang, S.-X.; Pan, W.-G.; Chen, Q.-L.; Li, M.-Y.; Sun, P.; Liu, S.-M. The enhanced Zn resistance of Mn/TiO2 catalyst for NH3 -SCR reaction by the modification with Nb. Fuel Process. Technol. 2016, 154, 235–242. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Chu, C.; Li, N.; Li, J.; Zhao, S. In-situ DRIFTS for the mechanistic studies of NO oxidation over α-MnO2, β-MnO2 and γ-MnO2 catalysts. Chem. Eng. J. 2017, 322, 525–537. [Google Scholar] [CrossRef]
- Hu, H.; Cai, S.; Li, H.; Huang, L.; Shi, L.; Zhang, D. Mechanistic Aspects of deNOx Processing over TiO2 Supported Co–Mn Oxide Catalysts: Structure–Activity Relationships and In Situ DRIFTs Analysis. ACS Catal. 2015, 5, 6069–6077. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, J.; Yan, N.; Ma, L.; Chang, H. Low temperature selective catalytic reduction of NO with NH3 over Mn–Fe spinel: Performance, mechanism and kinetic study. Appl. Catal. B Environ. 2011, 110, 71–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Wang, X.; Lu, X. Preparation of Mn–FeOx/CNTs catalysts by redox co-precipitation and application in low-temperature NO reduction with NH3. Catal. Commun. 2015, 62, 57–61. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, W.; Luan, T.; Li, H.; Zhang, W.; Feng, W.; Jiang, H. High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species. Catalysts 2019, 9, 103. [Google Scholar] [CrossRef]
- Qiu, L.; Pang, D.; Zhang, C.; Meng, J.; Zhu, R.; Ouyang, F. In situ IR studies of Co and Ce doped Mn/TiO2 catalyst for low-temperature selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2015, 357, 189–196. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yan, Z.; Liu, L.; Zhang, Z.; Wang, X. Promoting effect of Nd on the reduction of NO with NH3 over CeO2 supported by activated semi-coke: An in situ DRIFTS study. Catal. Sci. Technol. 2015, 5, 2251–2259. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Y.; Li, S.; Li, W. Promotional synergistic effect of Cu and Nb doping on a novel Cu/Ti-Nb ternary oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2018, 220, 234–250. [Google Scholar] [CrossRef]
- Yang, S.; Fu, Y.; Liao, Y.; Xiong, S.; Qu, Z.; Yan, N.; Li, J. Competition of selective catalytic reduction and non selective catalytic reduction over MnOx/TiO2 for NO removal: the relationship between gaseous NO concentration and N2O selectivity. Catal. Sci. Technol. 2014, 4, 224–232. [Google Scholar] [CrossRef]
- Wei, L.; Cui, S.; Guo, H.; Ma, X. Study on the role of Mn species in low temperature SCR on MnOx/TiO2 through experiment and DFT calculation. Mol. Catal. 2018, 445, 102–110. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Zhang, W.; Li, H. The promotional effects of cerium on the catalytic properties of Al2O3-supported MnFeOx for NO oxidation and fast SCR reaction. Res. Chem. Intermed. 2019, 45, 663–686. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Jia, Y.; Liu, X.; Zhong, Q. Selective catalytic oxidation of NO with O2 over Ce-doped MnOx/TiO2 catalysts. J. Nat. Gas Chem. 2012, 21, 17–24. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, H.; Li, Q.; Zheng, J.; Zhang, X. Catalytic reduction of NO by NH3 over Fe–Cu–Ox/CNTs-TiO2 composites at low temperature. Appl. Catal. A Gen. 2012, 427–428, 43–48. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, S.; Sun, H.; Shu, Y.; Quan, X. Low-temperature selective catalytic reduction of NOx with NH3 over hierarchically macro-mesoporous Mn/TiO2. Catal. Commun. 2013, 42, 10–13. [Google Scholar] [CrossRef]
- Gong, P.; Xie, J.; Fang, D.; Han, D.; He, F.; Li, F.; Qi, K. Effects of surface physicochemical properties on NH3-SCR activity of MnO2 catalysts with different crystal structures. Chin. J. Catal. 2017, 38, 1925–1934. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Ettireddy, P.R.; Kotrba, A.; Smirniotis, P.G. Influence of SiO2 on M/TiO2 (M = Cu, Mn, and Ce) Formulations for Low-Temperature Selective Catalytic Reduction of NOx with NH3: Surface Properties and Key Components in Relation to the Activity of NOx Reduction. Ind. Eng. Chem. Res. 2015, 54, 2261–2273. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, W.; Xie, A.; Wu, F.; Yao, C.; Li, X.; Zuo, S.; Liu, T. Effect of MnO2 polymorphs structure on the selective catalytic reduction of NOx with NH3 over TiO2–Palygorskite. Chem. Eng. J. 2016, 286, 291–299. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, C.; Wang, H.; Zhang, M. Synthesis of highly efficient MnOx catalyst for low-temperature NH3-SCR prepared from Mn-MOF-74 template. Mater. Lett. 2016, 168, 17–19. [Google Scholar] [CrossRef]
- Meng, D.; Xu, Q.; Jiao, Y.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G.; Zhan, W. Spinel structured CoaMnbOx mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2018, 221, 652–663. [Google Scholar] [CrossRef]
- Stanciulescu, M.; Caravaggio, G.; Dobri, A.; Moir, J.; Burich, R.; Charland, J.P.; Bulsink, P. Low-temperature selective catalytic reduction of NOx with NH3 over Mn-containing catalysts. Appl. Catal. B Environ. 2012, 123–124, 229–240. [Google Scholar] [CrossRef]
- Kong, F.; Qiu, j.; Liu, H.; Zhao, R.; Zeng, H. Effect of NO/SO2 on Elemental Mercury Adsorption by Nano-Fe2O3. Korean J. Chem. Eng. 2010, 30, 43–48. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Zhang, M.; Zhang, W.; Feng, W. Structure–Activity Relationship Study of Mn/Fe Ratio Effects on Mn−Fe−Ce−Ox/γ-Al2O3 Nanocatalyst for NO Oxidation and Fast SCR Reaction. Catalysts 2018, 8, 642. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, T.; Liu, H.; Chen, D.; Xu, B.; Qing, C. Low temperature SCR reaction over Nano-Structured Fe-Mn Oxides: Characterization, performance, and kinetic study. Appl. Surf. Sci. 2018, 457, 1116–1125. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wu, Z.; Wang, H.; Gu, T. Low-temperature selective catalytic reduction of NO with NH3 over MnCe oxides supported on TiO2 and Al2O3: A comparative study. Chemosphere 2010, 78, 1160–1166. [Google Scholar] [CrossRef]
- Xiong, Z.-b.; Liu, J.; Zhou, F.; Liu, D.-y.; Lu, W.; Jin, J.; Ding, S.-f. Selective catalytic reduction of NOx with NH3 over iron-cerium-tungsten mixed oxide catalyst prepared by different methods. Appl. Surf. Sci. 2017, 406, 218–225. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C.; Shan, W.; Shi, X. Mechanism of the selective catalytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst. Catal. Today 2011, 175, 18–25. [Google Scholar] [CrossRef]
- Fan, X.; Qiu, F.; Yang, H.; Tian, W.; Hou, T.; Zhang, X. Selective catalytic reduction of NOX with ammonia over Mn–Ce–OX/TiO2-carbon nanotube composites. Catal. Commun. 2011, 12, 1298–1301. [Google Scholar] [CrossRef]
- Guan, D.S.; Wang, Y. Synthesis and growth mechanism of multilayer TiO2 nanotube arrays. Nanoscale 2012, 4, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shen, C.; Zhang, Z.; Barrios, E.; Zhai, L. Core–Shell Composite Fibers for High-Performance Flexible Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 4041–4049. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Zhu, M.; Wang, X.; Dan, J.; Zhang, J.; Cao, P.; Dai, B. Microspherical MnO2-CeO2-Al2O3 mixed oxide for monolithic honeycomb catalyst and application in selective catalytic reduction of NOx with NH3 at 50–150 °C. Chem. Eng. J. 2018, 346, 182–192. [Google Scholar] [CrossRef]
- Deng, L.; Ding, Y.; Duan, B.; Chen, Y.; Li, P.; Zhu, S.; Shen, S. Catalytic deep combustion characteristics of benzene over cobalt doped Mn-Ce solid solution catalysts at lower temperatures. Mol. Catal. 2018, 446, 72–80. [Google Scholar] [CrossRef]
- Li, K.; Tang, X.; Yi, H.; Ning, P.; Kang, D.; Wang, C. Low-temperature catalytic oxidation of NO over Mn–Co–Ce–Ox catalyst. Chem. Eng. J. 2012, 192, 99–104. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Li, X.; Liu, Y.; Liu, Y.; Ma, J. Fabrication of hollow carbon nanospheres introduced with Fe and N species immobilized palladium nanoparticles as catalysts for the semihydrogenation of phenylacetylene under mild reaction conditions. Appl. Surf. Sci. 2017, 404, 398–408. [Google Scholar] [CrossRef]
- Wang, T.; Wan, Z.; Yang, X.; Zhang, X.; Niu, X.; Sun, B. Promotional effect of iron modification on the catalytic properties of Mn-Fe/ZSM-5 catalysts in the Fast SCR reaction. Fuel Process. Technol. 2018, 169, 112–121. [Google Scholar] [CrossRef]
- Marbán, G.; Valdés-Solı́s, T.; Fuertes, A.B. Mechanism of low-temperature selective catalytic reduction of NO with NH3 over carbon-supported Mn3O4: Role of surface NH3 species: SCR mechanism. J. Catal. 2004, 226, 138–155. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, J.; Li, J.; Ma, L.; Woo, S.I. Novel Mn–Ce–Ti Mixed-Oxide Catalyst for the Selective Catalytic Reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2014, 6, 14500–14508. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Liao, Y.; Xiao, X.; Dang, H.; Yang, S. Novel Effect of H2O on the Low Temperature Selective Catalytic Reduction of NO with NH3 over MnOx-CeO2: Mechanism and Kinetic Study. J. Phys. Chem. C 2015, 119, 4180–4187. [Google Scholar] [CrossRef]
- Lu, J.J.; Murray, N.V.; Rosenthal, E. Signed formulas and annotated logics. In Proceedings of the [1993] Proceedings of the Twenty-Third International Symposium on Multiple-Valued Logic; Institute of Electrical and Electronics Engineers (IEEE): Piscatville, NJ, USA, 2002; pp. 48–53. [Google Scholar]
- Lee, J.D. A New Concise Inorganic Chemistry; Van Nostrand Reinhold: Berkshire, UK, 1991; ISBN 0-412-40290-4. [Google Scholar]
- Cooksy, A. Physical Chemistry: Thermodynamics, Statistical Mechanics, and Kinetics; PEARSON EDUCATION: Boston, MA, USA, 2012; ISBN 0-321-81415-0. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 90th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1439820773. [Google Scholar]
- Koebel, M.; Elsener, M. Selective catalytic reduction of NO over commercial DeNOx-catalysts: Experimental determination of kinetic and thermodynamic parameters. Chem. Eng. Sci. 1998, 53, 657–669. [Google Scholar] [CrossRef]
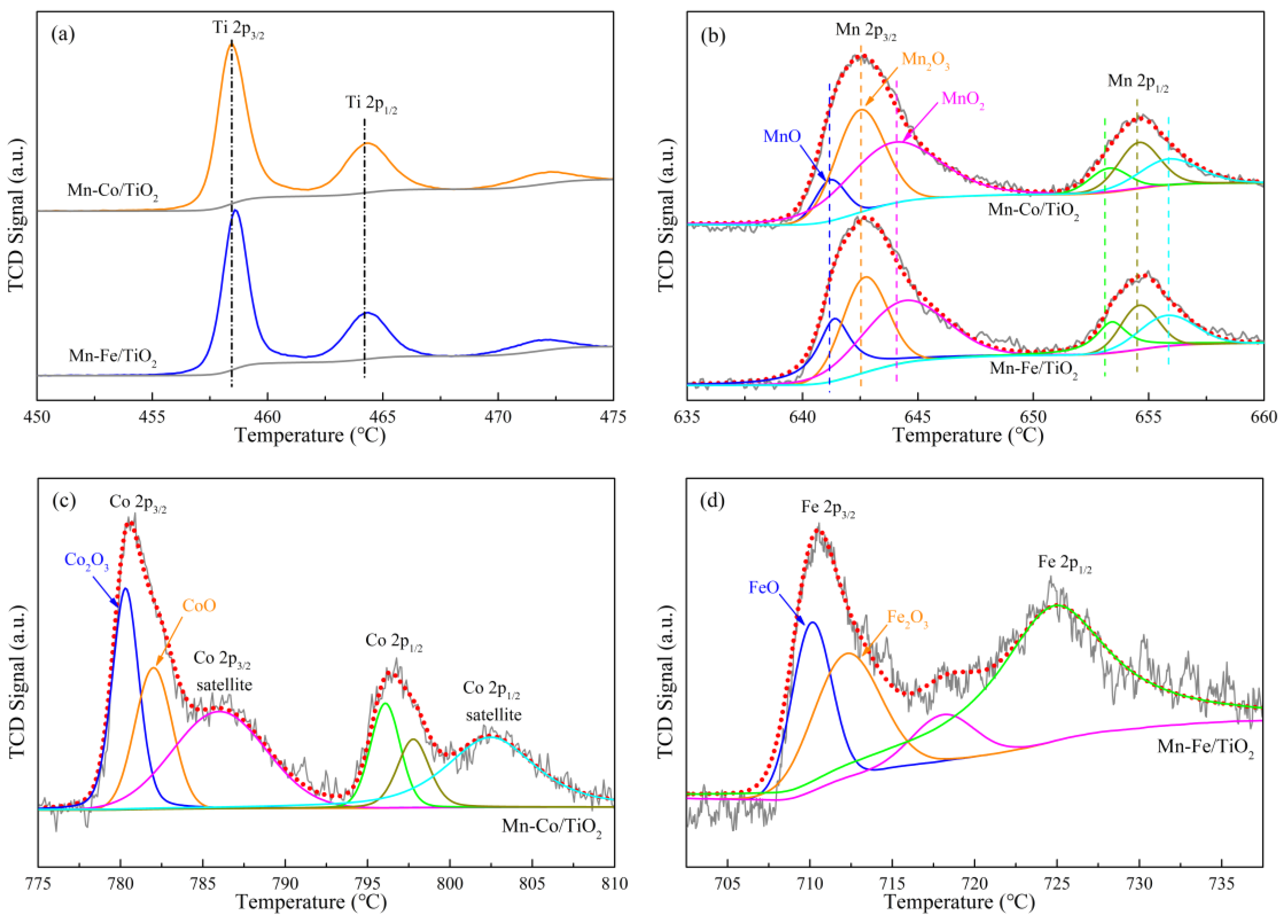
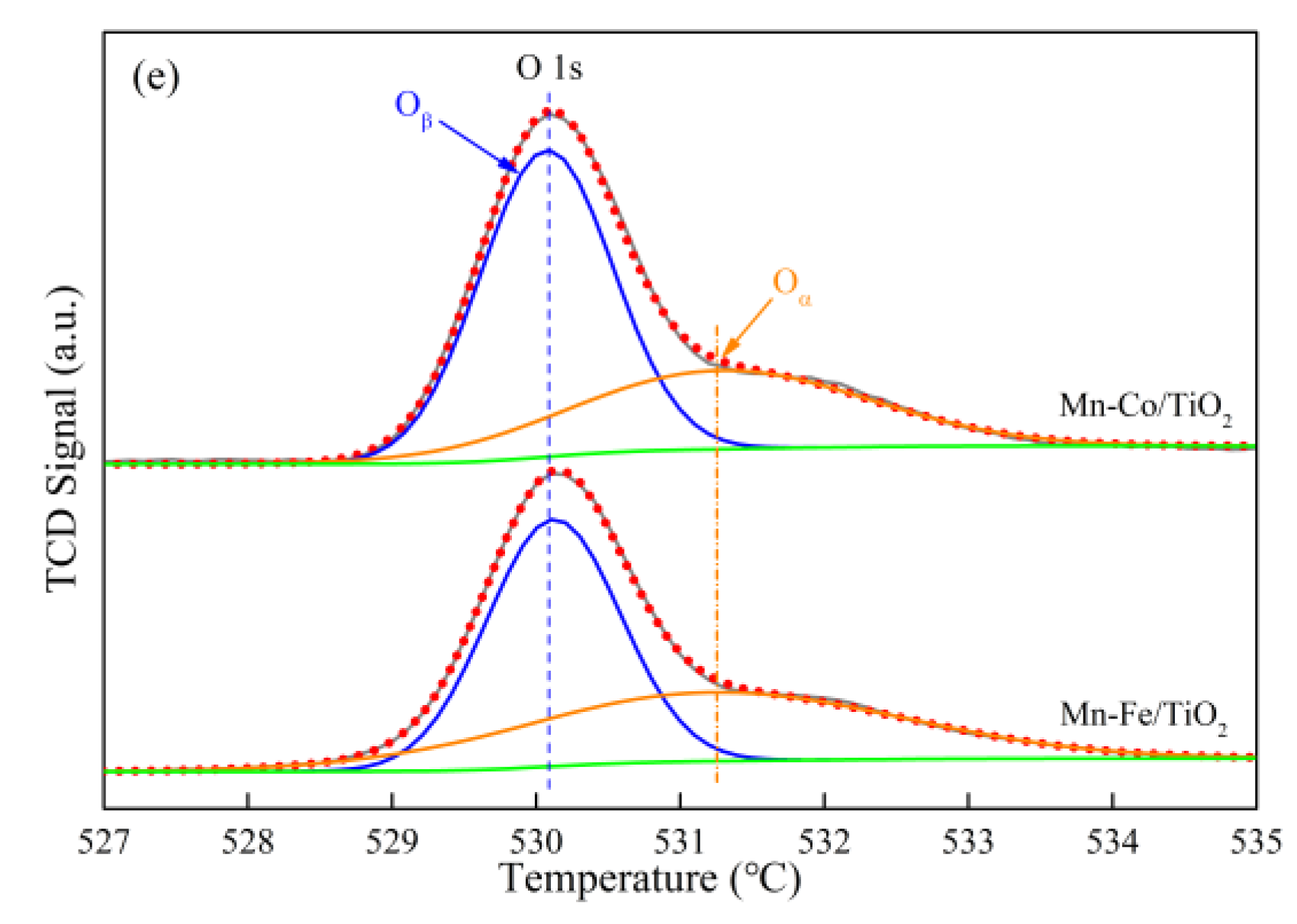

| Samples | Specific Surface Aarea (m2·g−1) | Total Pore Volume (cm3·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| Mn−Co/TiO2 | 189.9 | 0.531 | 33.06 |
| Mn−Fe/TiO2 | 104.6 | 0.424 | 54.85 |
| Samples | Temperature (°C) / H2 Consumption (mmol·g−1) | ||||
|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | Peak 4 | Total | |
| Mn−Co/TiO2 | 218/1.14 | 327/0.21 | 418/2.12 | 517/0.96 | - /4.43 |
| Mn−Fe/TiO2 | 276/0.83 | 387/0.41 | 436/0.54 | 501/1.59 | - /3.37 |
| Samples | Temperature (°C) / NH3 composition (mmol·g−1) | ||||
|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | Peak 4 | Total | |
| Mn−Co/TiO2 | 276/0.11 | 402/0.49 | 587/0.52 | 653/0.32 | --/1.44 |
| Mn−Fe/TiO2 | 209/0.14 | 398/0.24 | 585/0.39 | 649/0.20 | --/0.97 |
| Samples | Binding Energy (eV) / Atomic Composition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn | Fe | Co | O | ||||||
| Mn2+ | Mn3+ | Mn4+ | Fe2+ | Fe3+ | Co2+ | Co3+ | Oα | Oβ | |
| Mn−Co/TiO2 | 641.2/ 13.8 | 642.6/ 39.4 | 644.1/ 46.8 | -/- | -/- | 779.6 39.3 | 782.1/ 60.7 | 531.4/ 33.7 | 530.3/ 66.3 |
| Mn−Fe/TiO2 | 641.4/ 21.3 | 642.8/ 38.5 | 644.6/ 40.2 | 709.6/ 43.4 | 711.7/ 56.6 | -/- | -/- | 531.6/ 28.2 | 530.3/ 71.8 |
| Sample | Temperature (°C) | kSCR-ER/106 | kSCR-LH | R2 | kSCRa |
|---|---|---|---|---|---|
| Mn−Co/TiO2 | 100 | 0.029 | 5.75 | 0.993 | 23.5 |
| 150 | 0.046 | 13.25 | 0.994 | 41.1 | |
| 200 | 0.064 | 20.50 | 0.988 | 58.2 | |
| 250 | 0.075 | 25.01 | 0.984 | 68.4 | |
| Mn−Fe/TiO2 | 100 | 0.018 | 2.35 | 0.989 | 13.5 |
| 150 | 0.027 | 8.25 | 0.990 | 24.4 | |
| 200 | 0.033 | 16.01 | 0.991 | 36.0 | |
| 250 | 0.062 | 14.50 | 0.998 | 52.1 |
| Cp J mol−1 K | △fH0 kJ mol−1 | S0 J mol−1 K | △fG0 kJ mol−1 | |
|---|---|---|---|---|
| Mn | 26.3 | 0.0 | 32.0 | - |
| MnO | 45.4 | −385.2 | 59.7 | −362.9 |
| Mn3O4 | 139.7 | −1387.8 | 155.6 | -1283.2 |
| Mn2O3 | 107.7 | −959.0 | 110.5 | −881.1 |
| MnO2 | 54.1 | −520.0 | 53.1 | −465.1 |
| Co | 24.8 | 0.0 | 30.0 | - |
| CoO | 55.2 | −237.9 | 53.0 | −214.2 |
| Co3O4 | 123.4 | −891.0 | 102.5 | −774.0 |
| Fe | 25.1 | 0.0 | 27.3 | - |
| FeO | - | −272.0 | - | - |
| Fe3O4 | 143.4 | −1118.4 | 146.4 | −1015.4 |
| Fe2O3 | 103.9 | −824.2 | 87.4 | −742.2 |
| NH3 | - | 45.9 | 192.8 | - |
| N2 | 29.1 | 0.0 | 191.6 | - |
| H2O (g) | 33.6 | −241.8 | 188.8 | −228.6 |
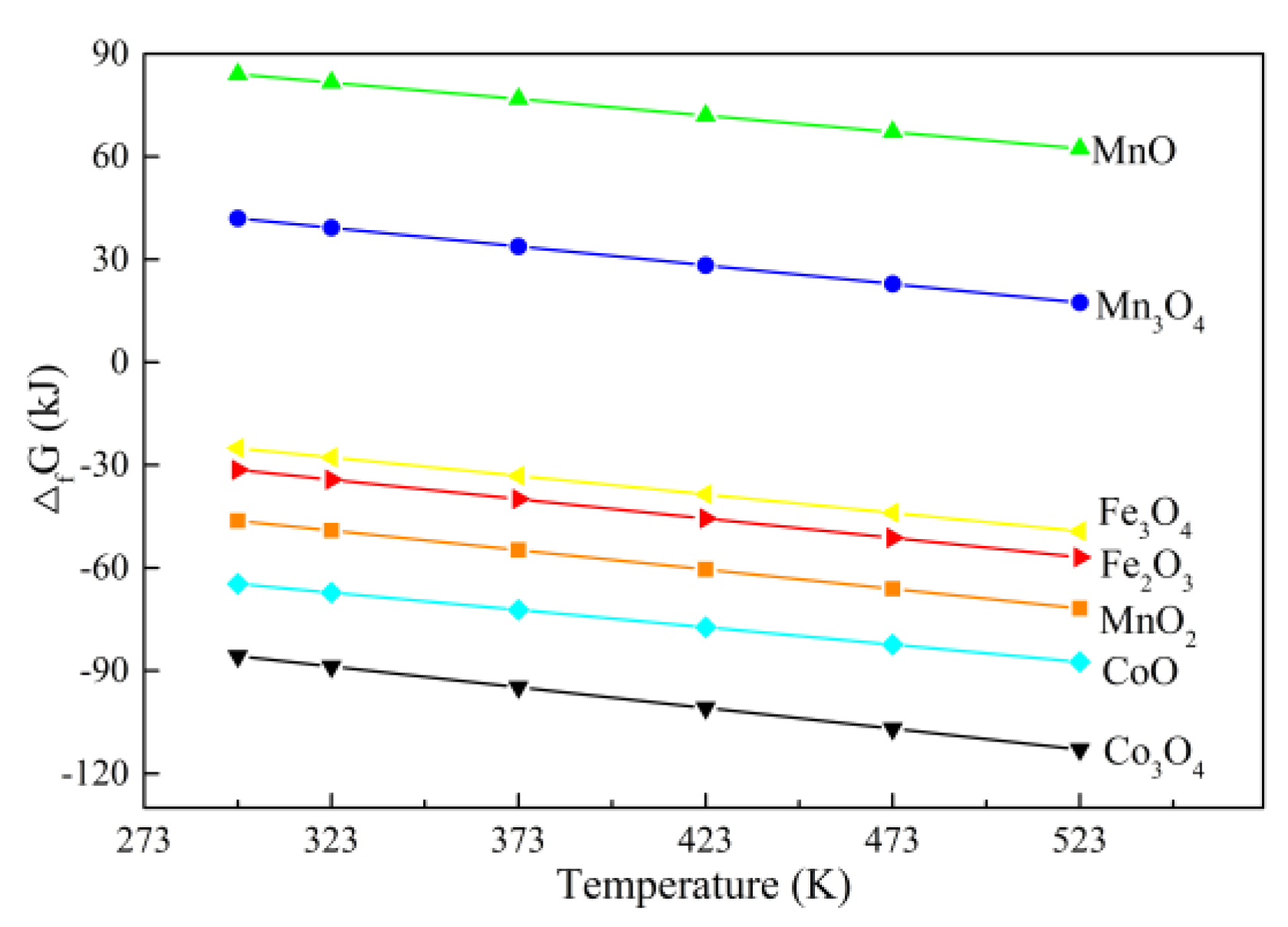
| Substance | △fG /kJ | |||||
|---|---|---|---|---|---|---|
| 298 K | 323 K | 373 K | 423 K | 473 K | 523 K | |
| MnO2 | −46.25 | −49.09 | −54.77 | −60.45 | −66.12 | −71.80 |
| Mn3O4 | 42.00 | 39.27 | 33.81 | 28.34 | 22.88 | 17.42 |
| MnO | 84.06 | 81.65 | 76.83 | 72.01 | 67.19 | 62.37 |
| Co3O4 a | −85.71 | −88.74 | −94.79 | −100.84 | −106.89 | −112.94 |
| CoO | −64.64 | −67.17 | −72.22 | −77.28 | −82.34 | −87.39 |
| Fe2O3 | −31.40 | −34.23 | −39.89 | −45.55 | −51.21 | −56.87 |
| Fe3O4 | −24.99 | −27.69 | −33.09 | −38.49 | −43.89 | −49.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Luan, T.; Zhang, S.; Jiang, W.; Feng, W.; Jiang, H. Comprehensive Comparison between Nanocatalysts of Mn−Co/TiO2 and Mn−Fe/TiO2 for NO Catalytic Conversion: An Insight from Nanostructure, Performance, Kinetics, and Thermodynamics. Catalysts 2019, 9, 175. https://doi.org/10.3390/catal9020175
Gao Y, Luan T, Zhang S, Jiang W, Feng W, Jiang H. Comprehensive Comparison between Nanocatalysts of Mn−Co/TiO2 and Mn−Fe/TiO2 for NO Catalytic Conversion: An Insight from Nanostructure, Performance, Kinetics, and Thermodynamics. Catalysts. 2019; 9(2):175. https://doi.org/10.3390/catal9020175
Chicago/Turabian StyleGao, Yan, Tao Luan, Shitao Zhang, Wenchao Jiang, Wenchen Feng, and Haolin Jiang. 2019. "Comprehensive Comparison between Nanocatalysts of Mn−Co/TiO2 and Mn−Fe/TiO2 for NO Catalytic Conversion: An Insight from Nanostructure, Performance, Kinetics, and Thermodynamics" Catalysts 9, no. 2: 175. https://doi.org/10.3390/catal9020175
APA StyleGao, Y., Luan, T., Zhang, S., Jiang, W., Feng, W., & Jiang, H. (2019). Comprehensive Comparison between Nanocatalysts of Mn−Co/TiO2 and Mn−Fe/TiO2 for NO Catalytic Conversion: An Insight from Nanostructure, Performance, Kinetics, and Thermodynamics. Catalysts, 9(2), 175. https://doi.org/10.3390/catal9020175