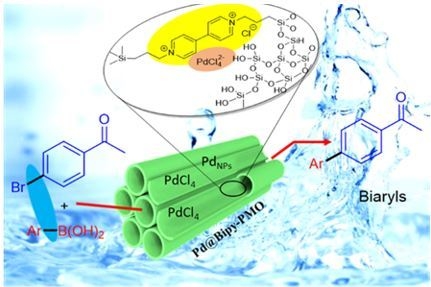
Palladium Comprising Dicationic Bipyridinium Supported Periodic Mesoporous Organosilica (PMO): Pd@Bipy–PMO as an Efficient Hybrid Catalyst for Suzuki–Miyaura Cross-Coupling Reaction in Water
Abstract
:1. Introduction
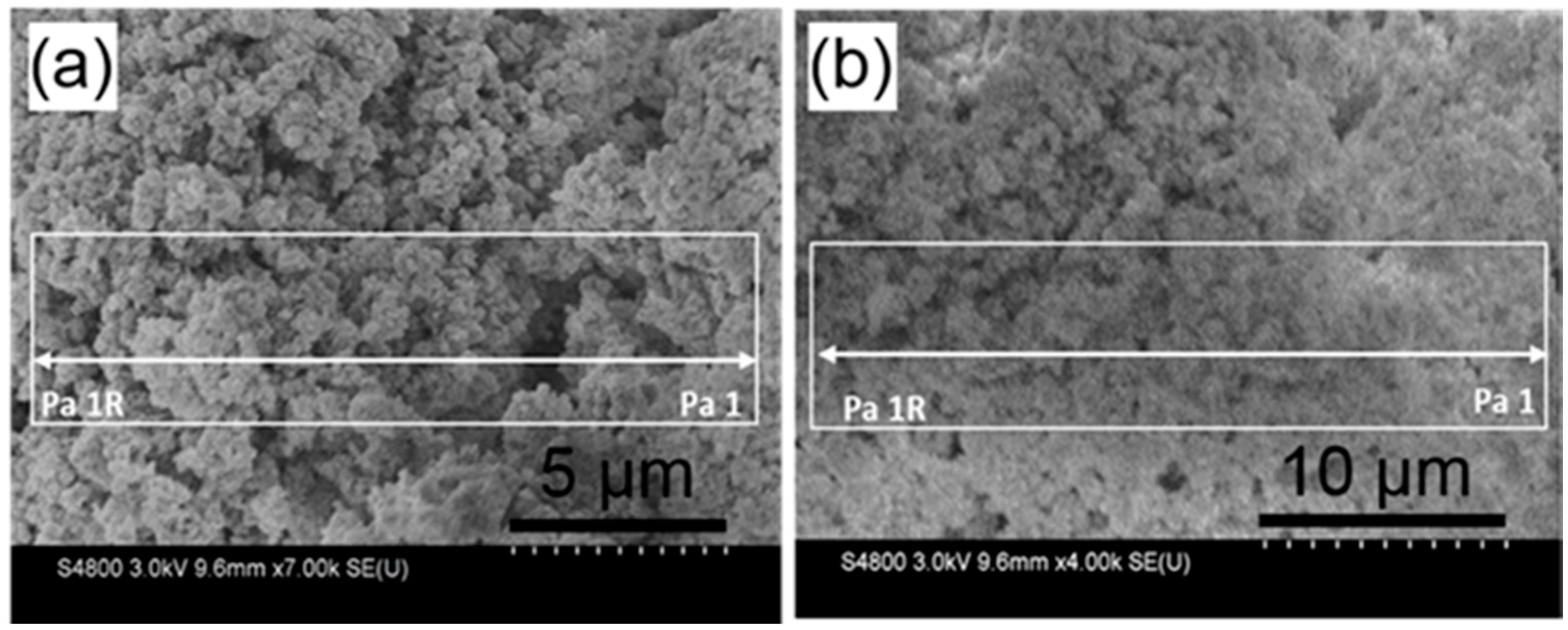
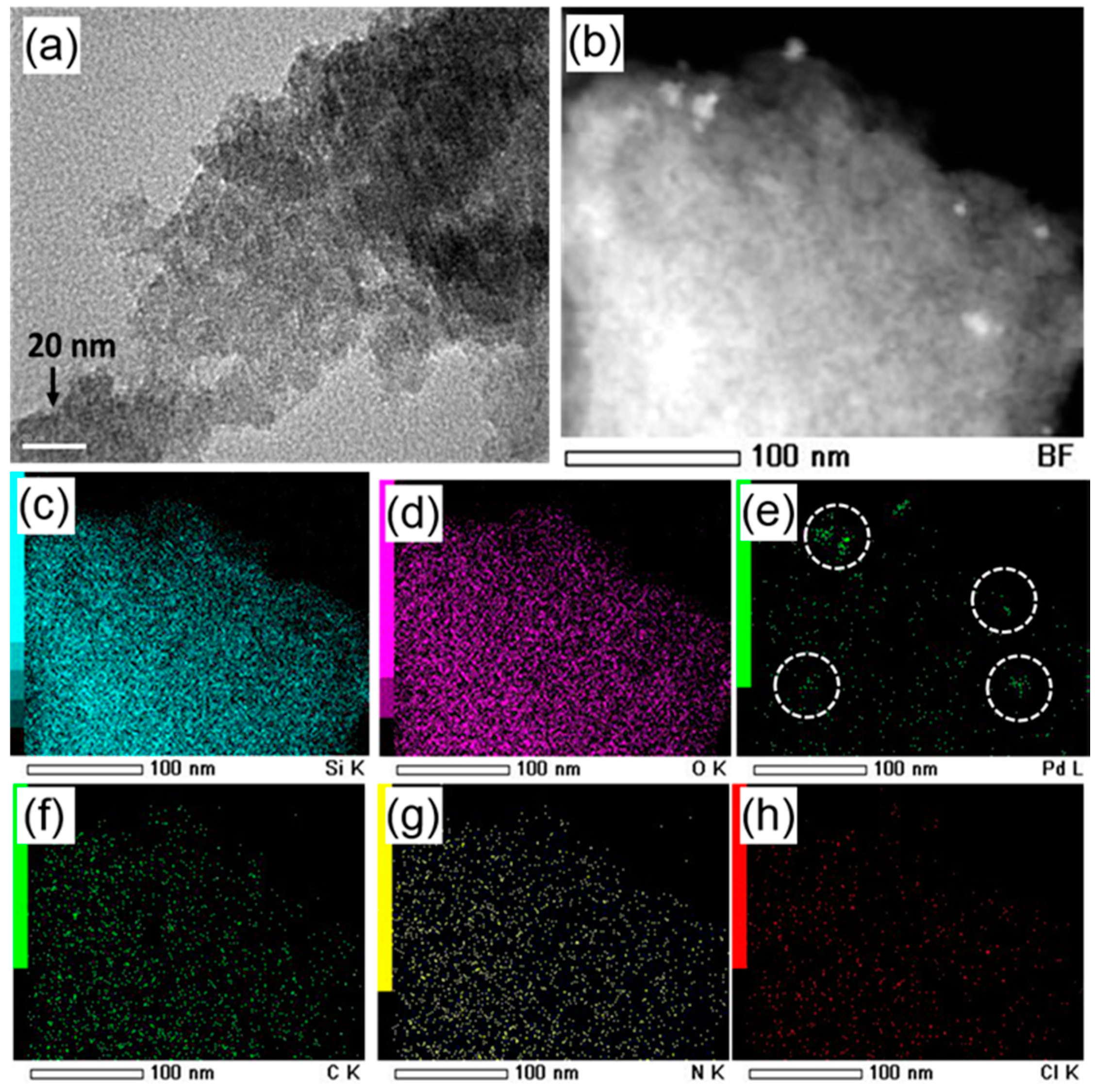
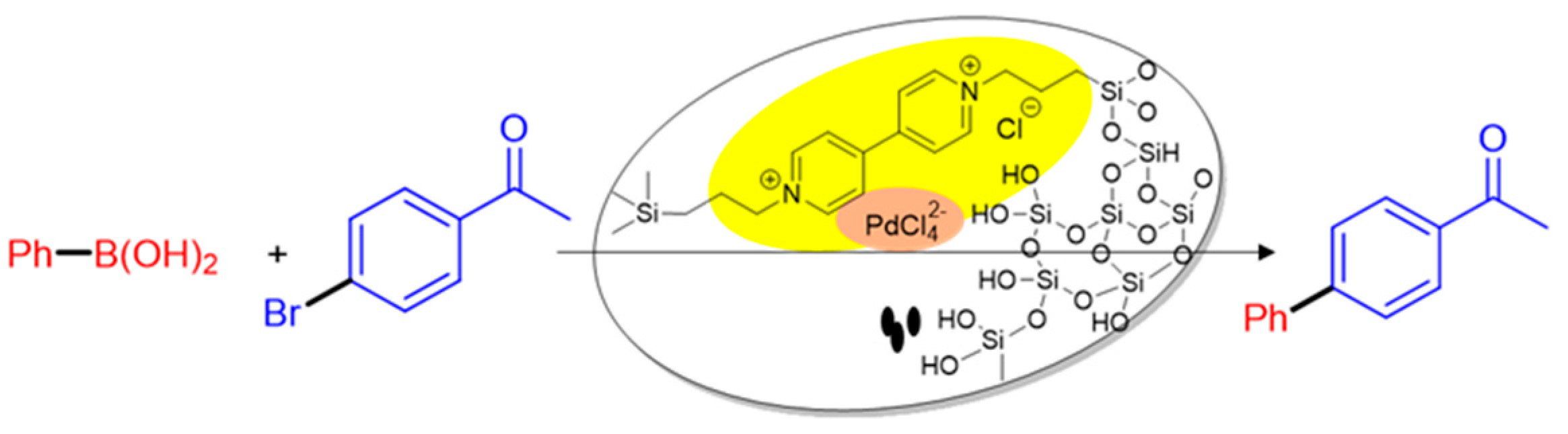
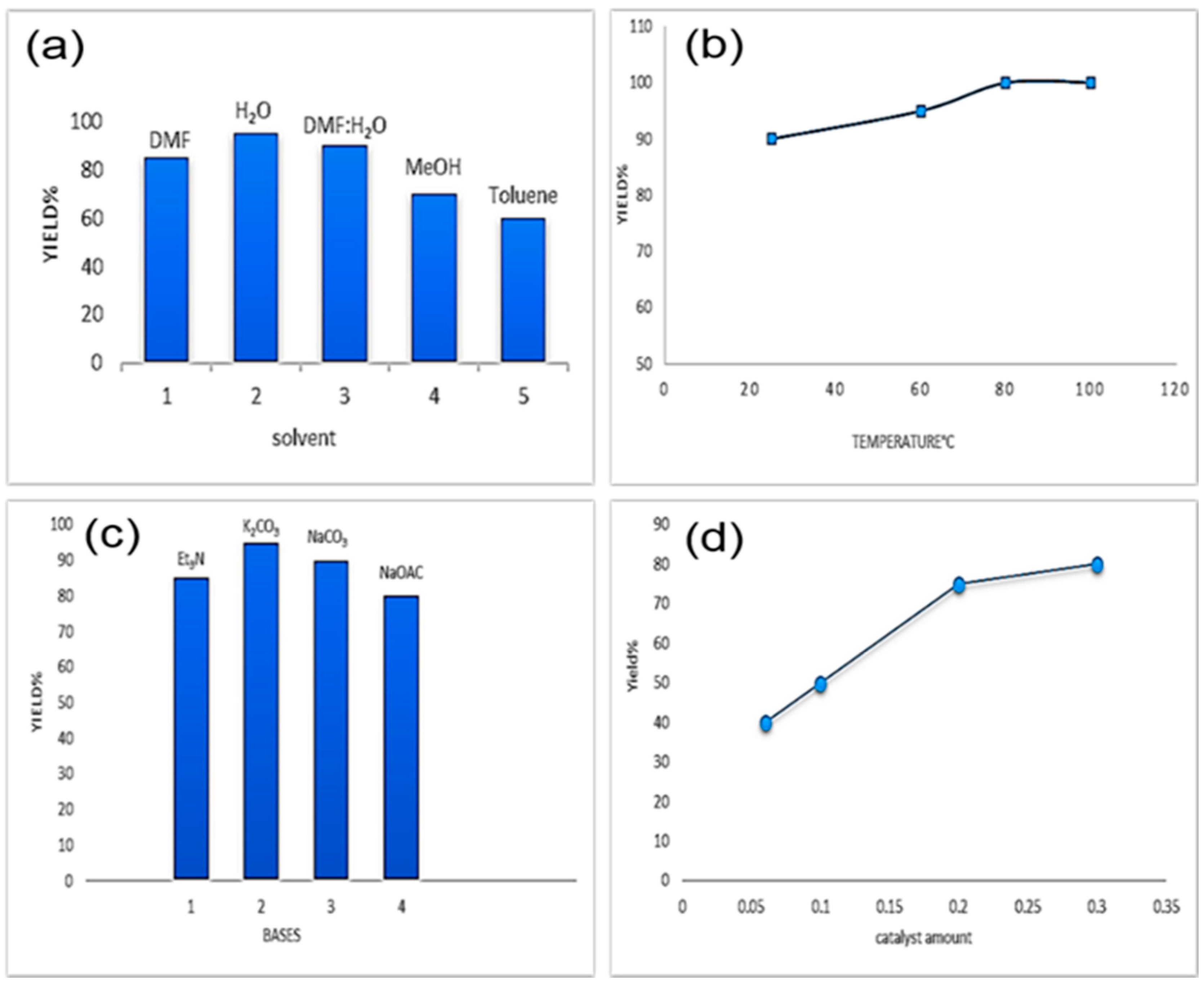
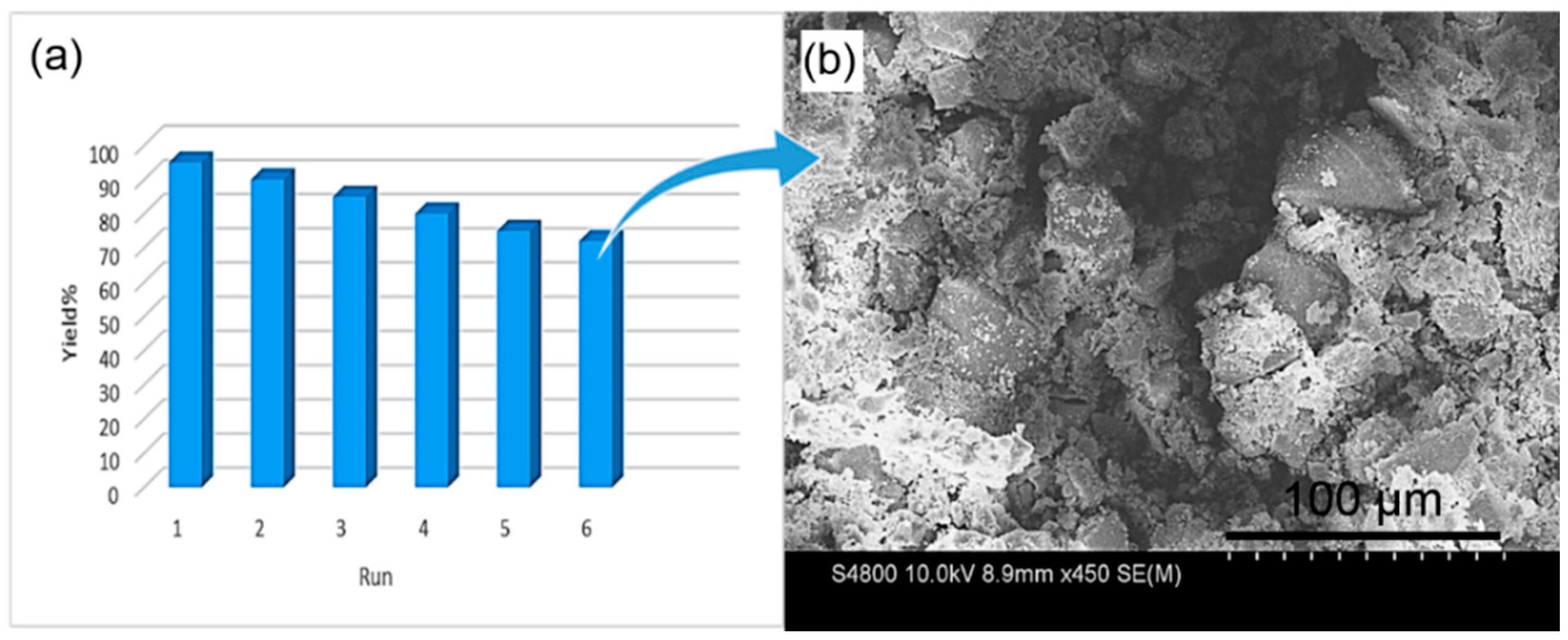
2. Results and Discussion
3. Experimental
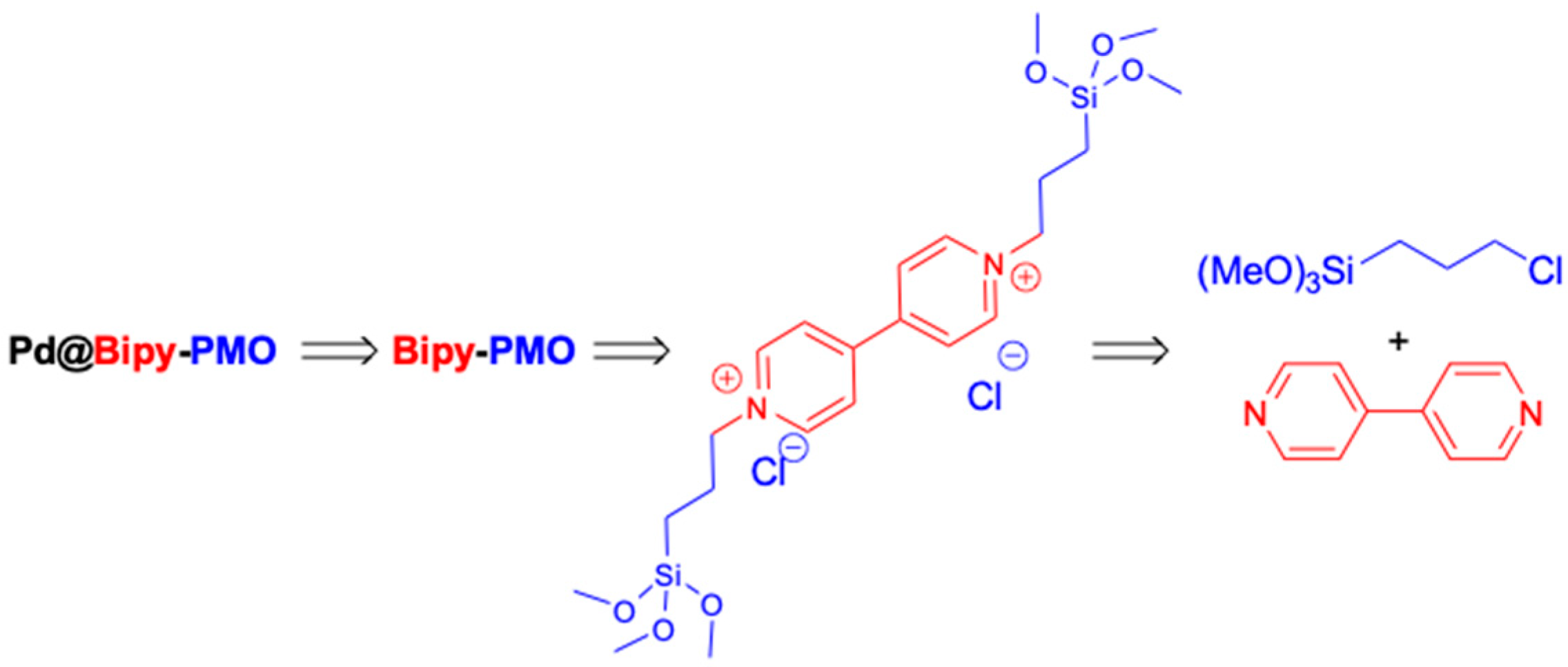
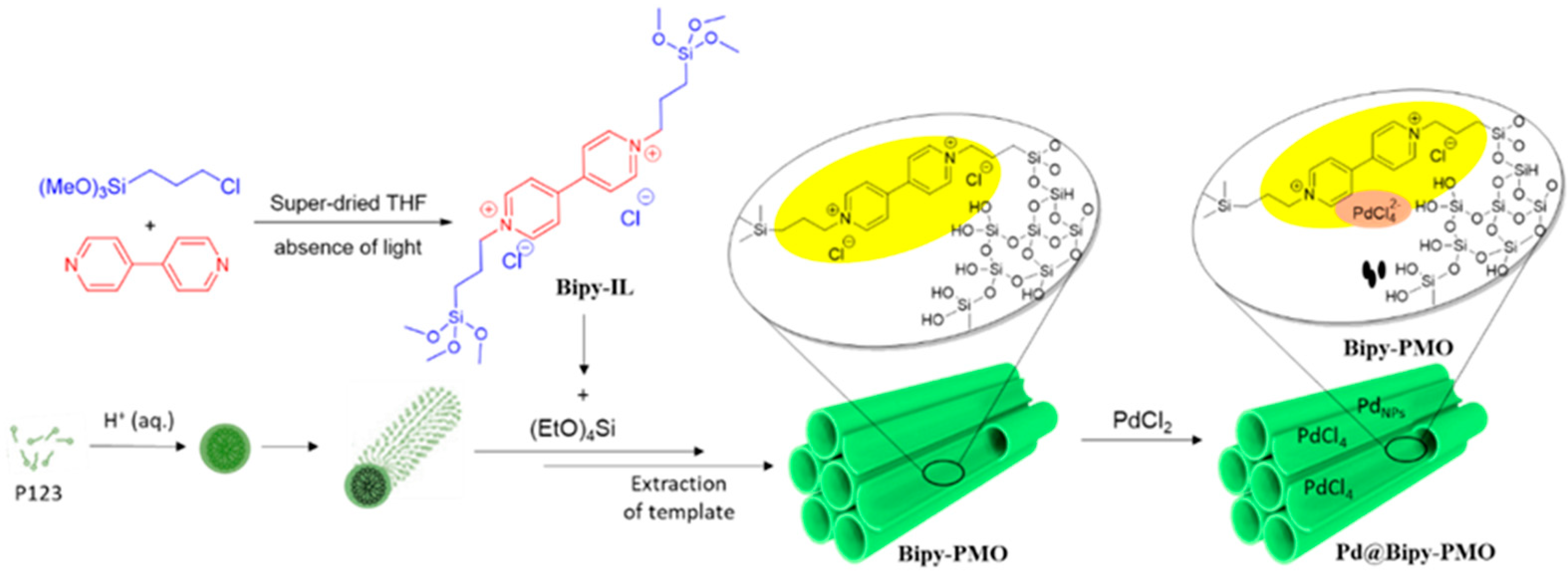
3.1. Synthesis of Bridged Bipyridinium Chloride Ionic Liquid
3.2. Synthesis of Bipy–PMO
3.3. Synthesis of Pd@Bipy–PMO
3.4. Heterogeneous Suzuki–Miyaura cross-coupling reaction
3.5. NMR Data for Selected Compounds
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Phan, M.T.; Sluys, M.V.D.; Jones, C.W. On the nature of the active species in palladium catalyzed Mizoroki–Heck and Suzuki–Miyaura couplings—Homogeneous or heterogeneous catalysis, a critical review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Rostamnia, S.; Kholdi, S. Synthesis of hybrid interfacial silica-based nanospheres composite as a support for ultra–small palladium nanoparticle and application of PdNPs/HSN in Mizoroki–Heck reaction. J. Phys. Chem. Solids 2017, 111, 47–53. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E.; Golchin Hossieni, H.; Luque, R. Covalently bonded PIDA on SBA–15 as robust Pd support: Water tolerant designed catalysts for aqueous Suzuki couplings. ChemistrySelect 2017, 2, 329–334. [Google Scholar]
- Rostamnia, S.; Rahmani, T. Ordered mesoporous SBA–15/PrSO3Pd and SBA–15/PrSO3PdNP as active, reusable and selective phosphine–free catalysts in C–X activation Heck coupling process. Appl. Organomet. Chem. 2015, 29, 471–474. [Google Scholar] [CrossRef]
- Piao, Y.; Jang, Y.; Shokouhimehr, M.; Lee, I.S.; Hyeon, T. Facile aqueous-phase synthesis of uniform palladium nanoparticles of various shapes and sizes. Small 2007, 3, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Sydnes, M.O. The Use of Palladium on Magnetic Support as Catalyst for Suzuki–Miyaura Cross-coupling Reactions. Catalysts 2017, 7, 35. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.W.; Shokouhimehr, M.; Lee, Y.S. Polymer–supported N–heterocyclic carbene−palladium complex for heterogeneous Suzuki cross-coupling reaction. J. Org. Chem. 2005, 70, 6714–6720. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M.; Lee, J.E.; Han, S.I.; Hyeon, T. Magnetically recyclable hollow nanocomposite catalysts for heterogeneous reduction of nitroarenes and Suzuki reactions. Chem. Commun. 2013, 49, 4779–4781. [Google Scholar] [CrossRef]
- Rostamnia, S.; Lamei, K.; Pourhassan, F. Generation uniform and small particle size of palladium onto the SH–decorated SBA–15 pore–walls: SBA–15/(SH)XPd–NPY as a recoverable nanocatalyst for Suzuki–Miyaura coupling reaction in air and water. RSC Adv. 2014, 4, 59626–59631. [Google Scholar] [CrossRef]
- Zhong, R.; Lindhorst, A.C.; Groche, F.J.; Kühn, F.E. Immobilization of N–heterocyclic carbene compounds: A synthetic perspective. Chem. Rev. 2017, 117, 1970–2058. [Google Scholar] [CrossRef]
- Roy, D.; Uozumi, Y. Recent advances in palladium-catalyzed cross-coupling reactions at ppm to ppb molar catalyst loadings. Adv. Synth. Catal. 2018, 360, 602–625. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Pagliaro, M.; Pandarus, V.; Ciriminna, R.; Beland, F.; Cara, P.D. Heterogeneous versus homogeneous palladium catalysts for cross-coupling reactions. ChemCatChem 2012, 4, 432–445. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Kim, J.H.; Lee, Y.S. Heterogeneous Heck reaction catalyzed by recyclable polymer–supported N–heterocyclic carbene–palladium complex. Synlett 2006, 618–620. [Google Scholar] [CrossRef]
- Kim, A.; Rafiaei, S.M.; Abolhosseini, S.; Shokouhimehr, M. Palladium nanocatalysts confined in mesoporous silica for heterogeneous reduction of nitroaromatics. Energy Environ. Focus 2015, 4, 18–23. [Google Scholar] [CrossRef]
- Molnar, A. Efficient, selective, and recyclable palladium catalysts in carbon–carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Misono, M. Heterogeneous catalysis. Chem. Rev. 1998, 98, 199–218. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Dekamin, M.G. Green and facile synthesis of 4H-pyran scaffold catalyzed by pure nano-ordered periodic mesoporous organosilica with isocyanurate framework (PMO-ICS). ChemistrySelect 2017, 2, 9236–9243. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Dekamin, M.G.; Arefi, E.; Karimi, B. Propylsulfonic acid–anchored isocyanurate-based periodic mesoporous organosilica (PMO–ICS–Pr–SO3H): A new and highly efficient recoverable nanoporous catalyst for the one–pot synthesis of bis(indolyl) methane derivatives. J. Colloid Interface Sci. 2017, 505, 956–963. [Google Scholar] [CrossRef]
- Karimi, B.; Gholinejad, M.; Khorasani, M. Highly efficient three–component coupling reaction catalyzed by gold nanoparticles supported on periodic mesoporous organosilica with ionic liquid framework. Chem. Commun. 2012, 48, 8961–8963. [Google Scholar] [CrossRef]
- Karimi, B.; Naderi, Z.; Khorasani, M.; Mirzaei, H.M.; Vali, H. Ultrasmall platinum nanoparticles supported inside the nanospaces of periodic mesoporous organosilica with an imidazolium network: An efficient catalyst for the aerobic oxidation of unactivated alcohols in water. ChemCatChem 2016, 8, 906–910. [Google Scholar] [CrossRef]
- Doustkhah, E.; Rostamnia, S.; Zeynizadeh, B.; Kim, J.; Yamauchi, Y.; Ide, Y. Efficient H2 generation using thiourea-based periodic mesoporous organosilica with Pd nanoparticles. Chem. Lett. 2018, 47, 1243–1245. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E.; Bulgar, R.; Zeynizadeh, B. Supported palladium ions inside periodic mesoporous organosilica with ionic liquid framework (Pd@ IL–PMO) as an efficient green catalyst for S–arylation coupling. Microporous Mesoporous Mater. 2016, 225, 272–279. [Google Scholar] [CrossRef]
- Doustkhah, E.; Rostamnia, S.; Imura, M.; Ide, Y.; Mohammadi, S.; Hyland, C.J.T.; You, J.; Tsunoji, N.; Zeynizadeh, B.; Yamauchi, Y. Thiourea bridged periodic mesoporous organosilica with ultra–small Pd nanoparticles for coupling reactions. RSC Adv. 2017, 7, 56306–56310. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Doustkhah, E.; Rostamnia, S. Covalently bonded sulfonic acid magnetic graphene oxide: Fe3O4@GO–Pr–SO3H as a powerful hybrid catalyst for synthesis of indazolophthalazinetriones. J. Colloid Interface Sci. 2016, 478, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, X.; Zhu, X.; Wang, H.; Rostamnia, S.; Han, J. Well–shaped sulfonic organosilica nanotubes with high activity for hydrolysis of cellobiose. Catalysis 2017, 7, 127. [Google Scholar] [CrossRef]
- Golchin Hossieni, H.; Rostamnia, S. Postsynthetic modified SBA–15 with NH2–coordinately immobilized iron–oxine: SBA–15/NH2–FeQ3 as a Fenton-like hybrid catalyst for selective oxidation of organic sulfides. New J. Chem. 2018, 42, 619–627. [Google Scholar] [CrossRef]
- Doustkhah, E.; Heidarizadeh, M.; Rostamnia, S.; Hassankhani, A.; Kazemi, B.; Liu, X. Copper (II) immobilization on carboxylic acid–rich Fe3O4–Pectin: Cu2+@Fe3O4–Pectin a superparamagnetic carbohydrate source for click reaction. Mater. Lett. 2018, 216, 139–143. [Google Scholar] [CrossRef]
- Rostamnia, S.; Gholipour, B. Immobilization of the iron (III) tris(8–quinolinolato–N,O) onto the silica coted Magnetite: Fe3O4@SiO2–FeQ3 as a nanomagnetically interfacial catalyst for waste–free green oxidation of sulfides. J. Colloid Intterface Sci. 2018, 511, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Rostamnia, S.; Mohsenzad, F. Nanoarchitecturing of open metal site Cr–MOFs for oxodiperoxo molybdenum complexes [MoO(O2)2@En/MIL–100(Cr)] as a promising and bifunctional catalyst for selective thioether oxidation. Mol. Catal. 2018, 445, 12–20. [Google Scholar] [CrossRef]
- Rostamnia, S.; Xin, H. Simultaneously application of ultrasonic irradiation and immobilized ionic liquid onto the SBA–15 nanoreactor (US/[MPIm]Cl@SBA–15): A robust, recyclable and useful combined catalytic system for selective and waste–free Kabachnik–Fields reaction. J. Mol. Liq. 2014, 195, 30–34. [Google Scholar] [CrossRef]

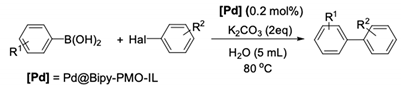
| Entry | Halide | R1 | R2 | Time (h) | Yield (%)2 |
|---|---|---|---|---|---|
| 1 | Br | H | H | 4 | 98 |
| 2 | Br | 4–t–Bu | H | 4 | 98 |
| 3 | Br | H | 4–Me | 3 | 95 |
| 4 | Br | H | 4–OMe | 3 | 90 |
| 5 | Br | H | 2–NO2 | 4 | 93 |
| 6 | Br | H | 4–COMe | 4 | 98 |
| 7 | Br | 3–Me | 4–COMe | 4 | 95 |
| 8 | Br | H | 3–NO2 | 4 | 96 |
| 9 | Br | H | 2–CHO | 4 | 83 |
| 10 | Br | 3–Me | 2–CHO | 4 | 86 |
| 11 | I | H | H | 0.5 | quant. |
| 12 | I | H | 4–COMe | 0.5 | quant. |
| 13 | I | 4–t–Bu | H | 1.5 | 96 |
| 14 | I | H | 2–OMe | 2 | 97 |
| 15 | I | 3–Me | 2–OMe | 1 | 96 |
| 16 | I | 3–Me | 2–NH2 | 1 | 91 |
| 17 | Cl | H | 4–COMe | 6 | 18 3 |
| 18 | Cl | H | 4–Me | 6 | 12 3 |
| 19 | Cl | H | 4–COMe | 12 | 34 3 |
| 20 | Cl | H | 2–i–Pr | 6 | 8 3 |
| 21 | Cl | 3–Me | 2–i–Pr | 6 | 6 3 |
| 22 | I | H | 2–t–Bu | 6 | 12 |
| 23 | F | H | 4–COMe | 72 | trace |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahadi, A.; Rostamnia, S.; Panahi, P.; Wilson, L.D.; Kong, Q.; An, Z.; Shokouhimehr, M. Palladium Comprising Dicationic Bipyridinium Supported Periodic Mesoporous Organosilica (PMO): Pd@Bipy–PMO as an Efficient Hybrid Catalyst for Suzuki–Miyaura Cross-Coupling Reaction in Water. Catalysts 2019, 9, 140. https://doi.org/10.3390/catal9020140
Ahadi A, Rostamnia S, Panahi P, Wilson LD, Kong Q, An Z, Shokouhimehr M. Palladium Comprising Dicationic Bipyridinium Supported Periodic Mesoporous Organosilica (PMO): Pd@Bipy–PMO as an Efficient Hybrid Catalyst for Suzuki–Miyaura Cross-Coupling Reaction in Water. Catalysts. 2019; 9(2):140. https://doi.org/10.3390/catal9020140
Chicago/Turabian StyleAhadi, Arefeh, Sadegh Rostamnia, Paria Panahi, Lee D. Wilson, Qingshan Kong, Zengjian An, and Mohammadreza Shokouhimehr. 2019. "Palladium Comprising Dicationic Bipyridinium Supported Periodic Mesoporous Organosilica (PMO): Pd@Bipy–PMO as an Efficient Hybrid Catalyst for Suzuki–Miyaura Cross-Coupling Reaction in Water" Catalysts 9, no. 2: 140. https://doi.org/10.3390/catal9020140
APA StyleAhadi, A., Rostamnia, S., Panahi, P., Wilson, L. D., Kong, Q., An, Z., & Shokouhimehr, M. (2019). Palladium Comprising Dicationic Bipyridinium Supported Periodic Mesoporous Organosilica (PMO): Pd@Bipy–PMO as an Efficient Hybrid Catalyst for Suzuki–Miyaura Cross-Coupling Reaction in Water. Catalysts, 9(2), 140. https://doi.org/10.3390/catal9020140