Tuning the Catalytic Properties of Copper-Promoted Nanoceria via a Hydrothermal Method
Abstract
:1. Introduction
2. Results
2.1. EPR Measurements
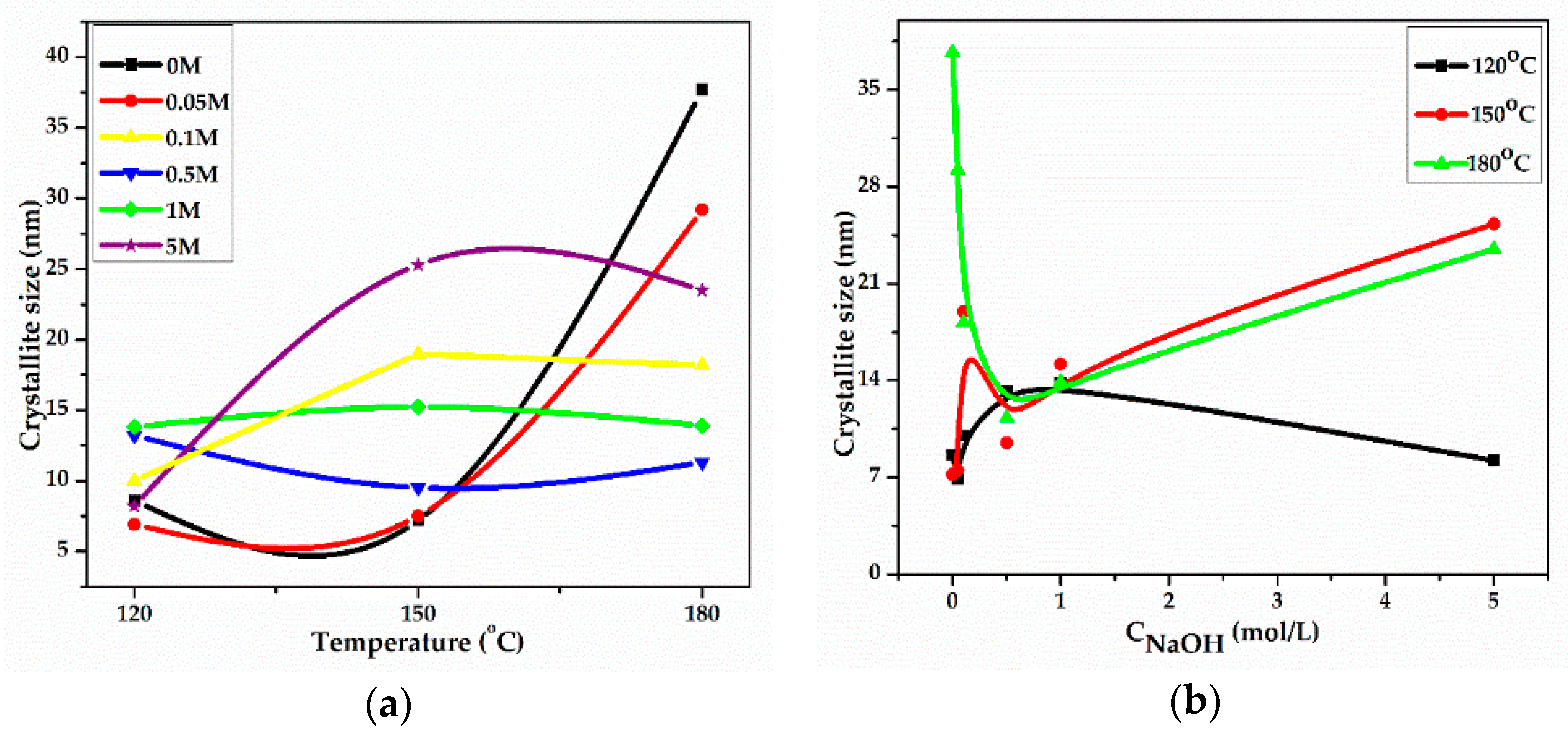
2.2. XRD Measurements
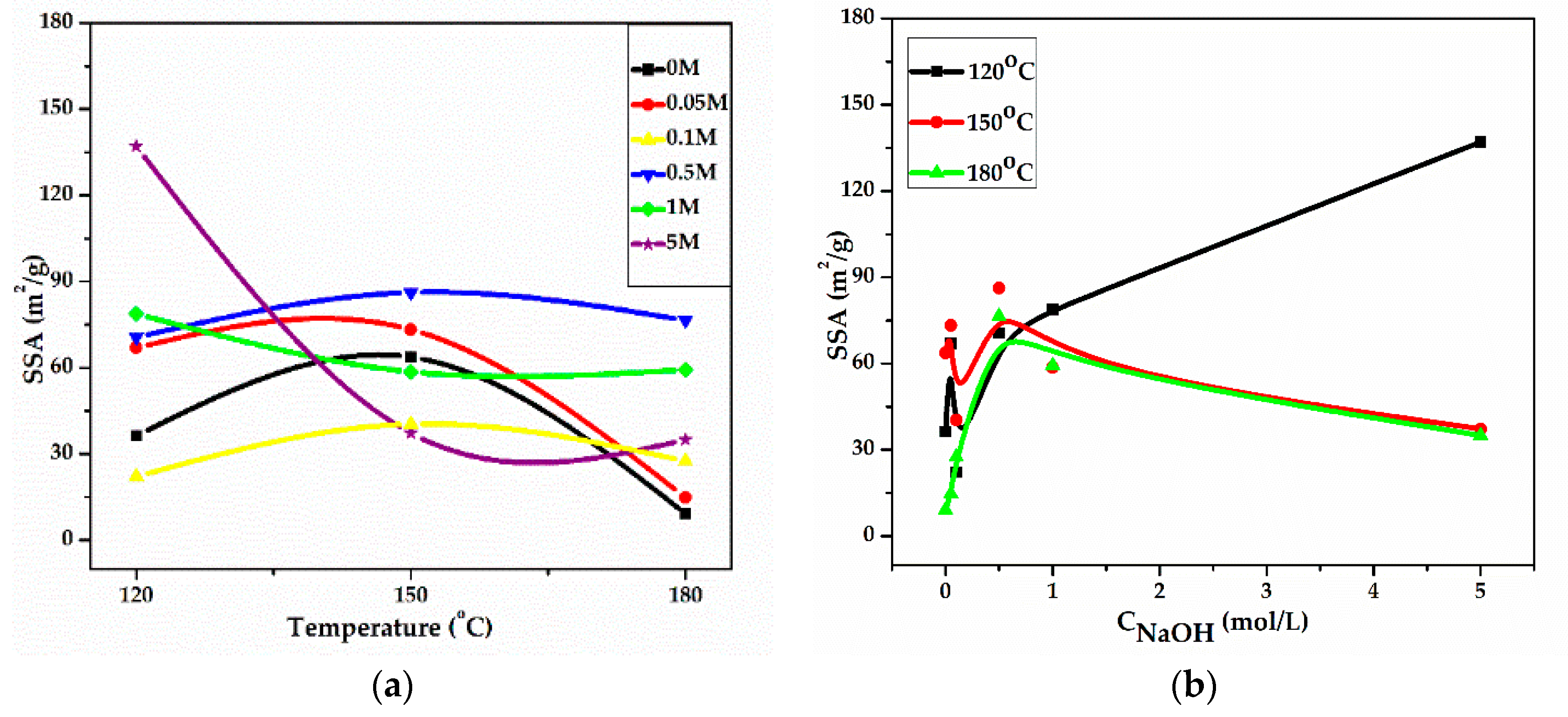
2.3. N2 Adsorption Measurements
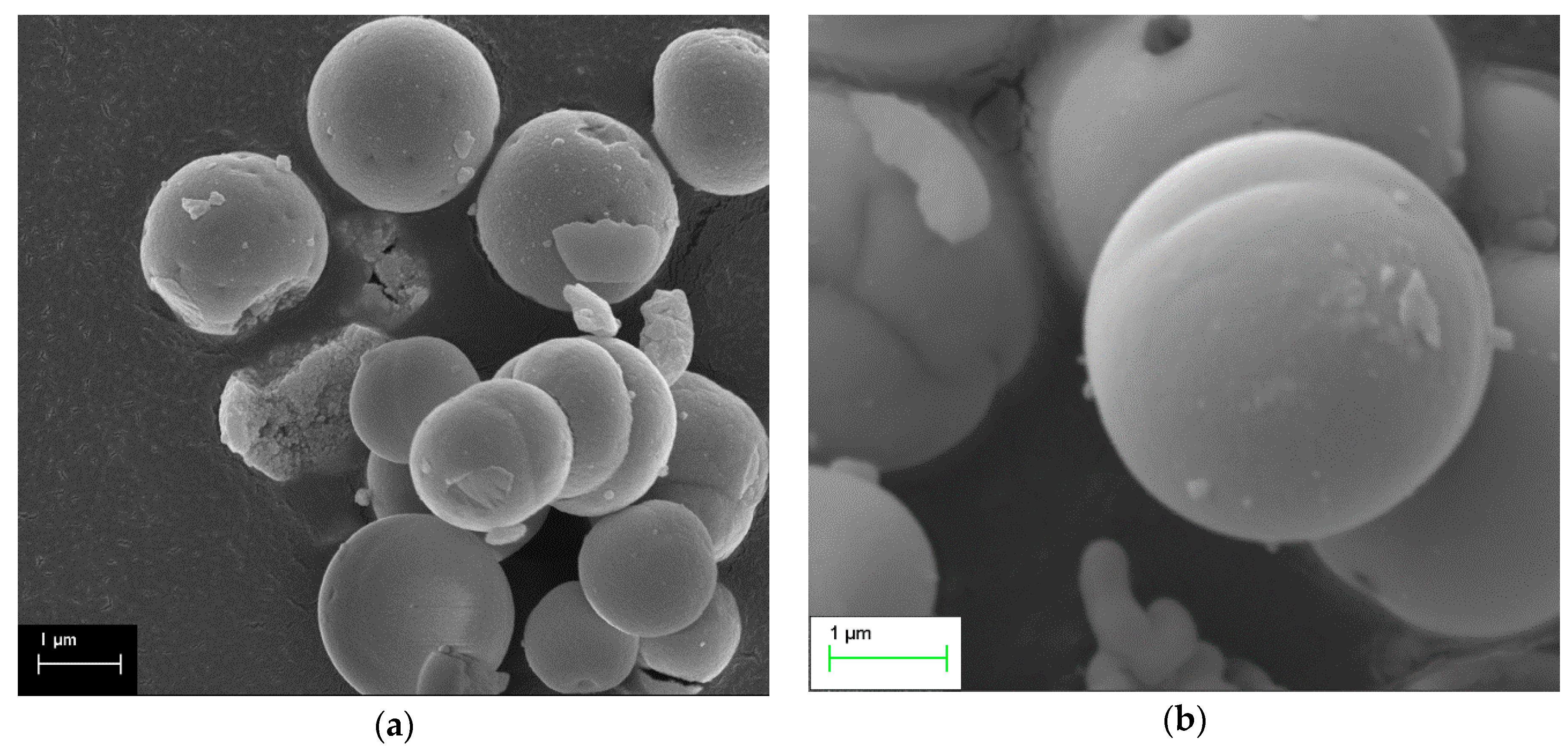
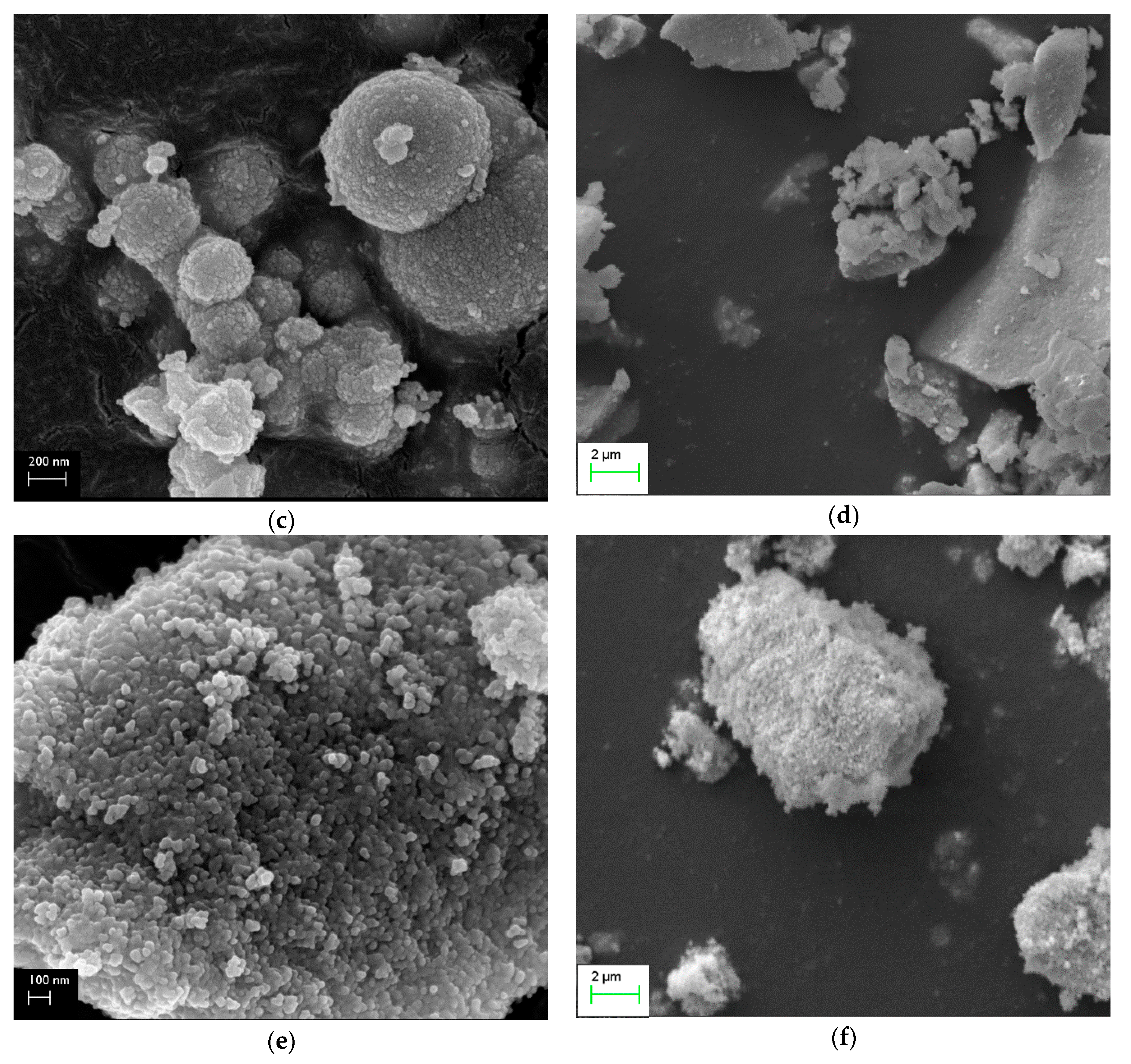
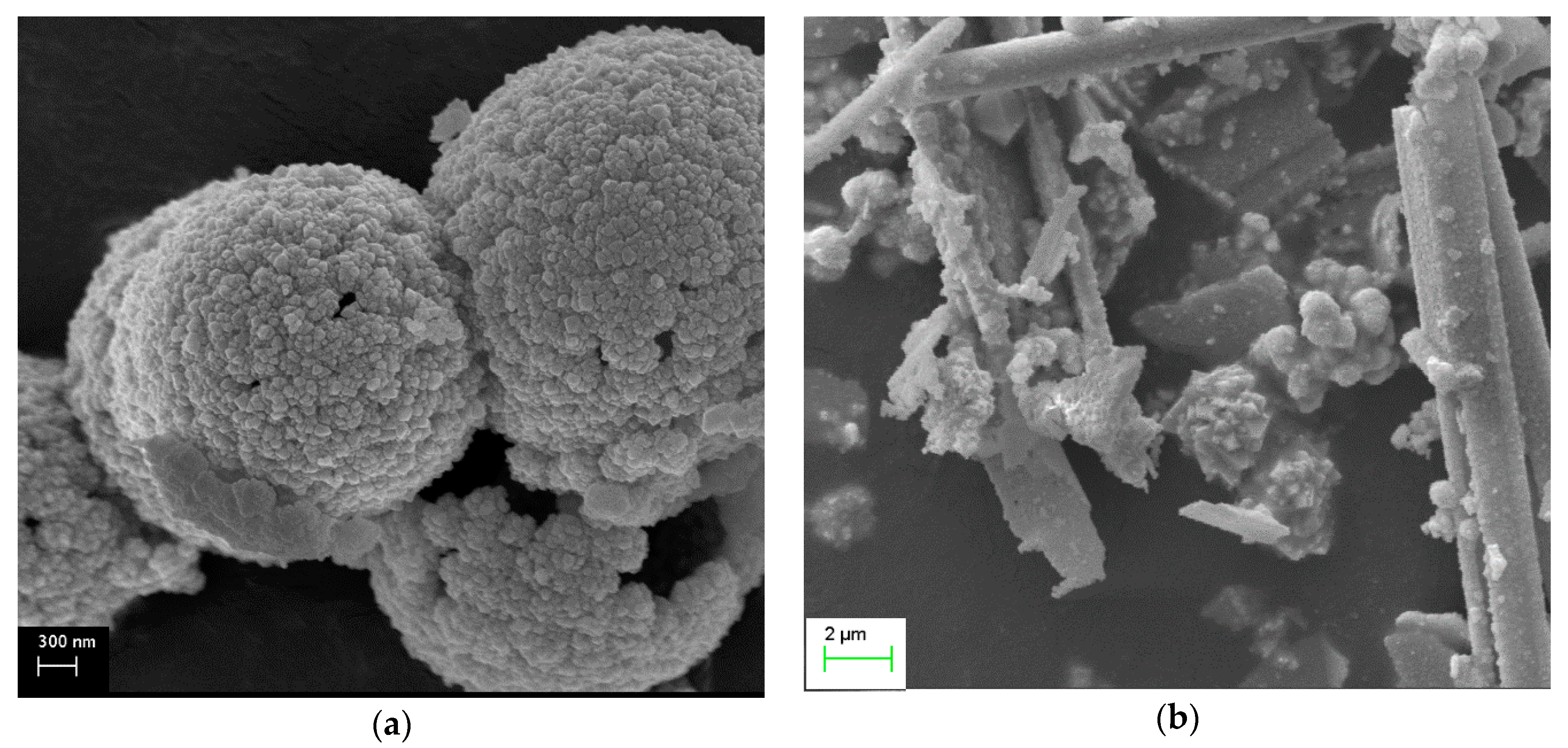
2.4. SEM Measurements
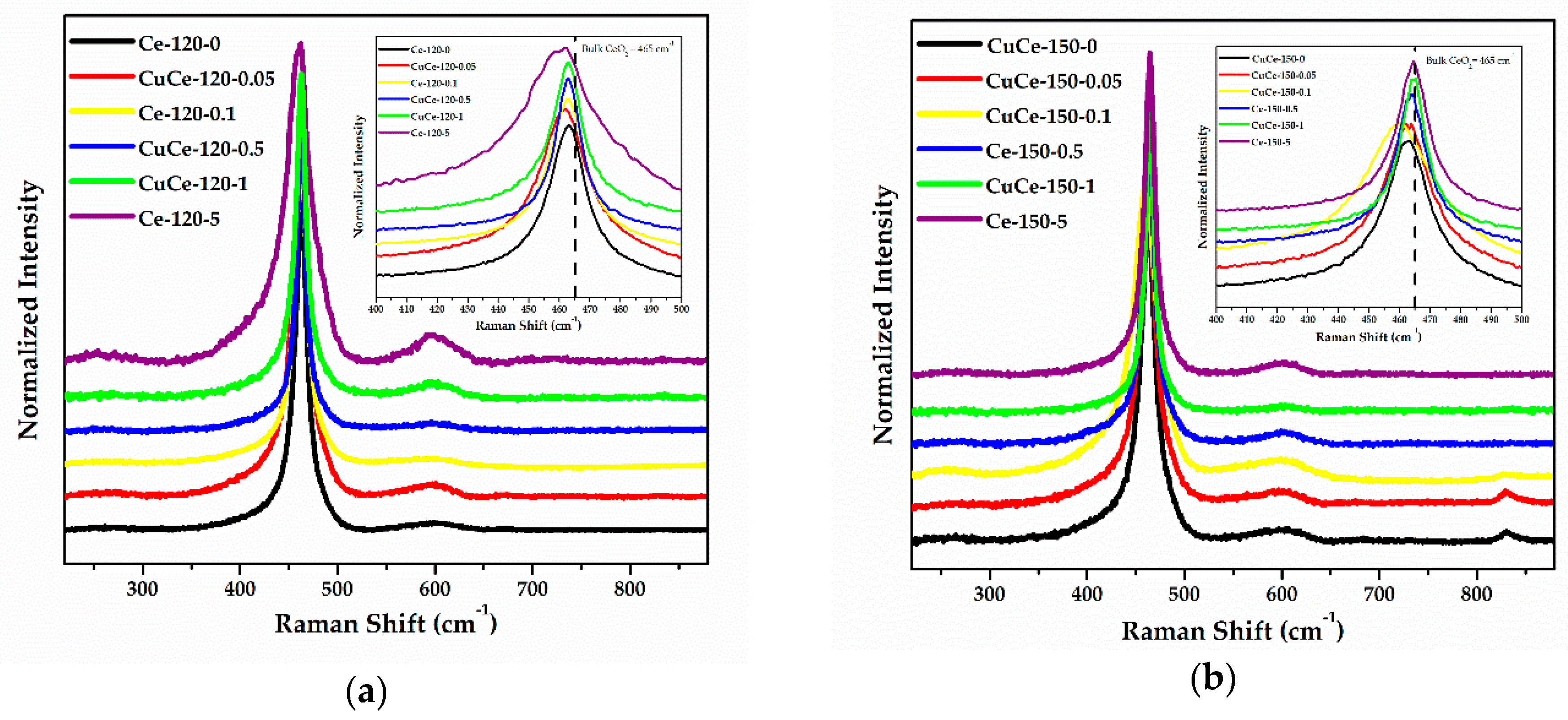
2.5. Raman Measurements
2.6. UV-Vis DRS Measurements
2.7. Formation Mechanism
- The weakening of the complex stability and precipitation of the latter as hydroxide into the solution.
- Depending on the hydrothermal parameters, the leaching and migration of copper species from the copper ring to the solution where their deposition in the ceria phase is taking place.
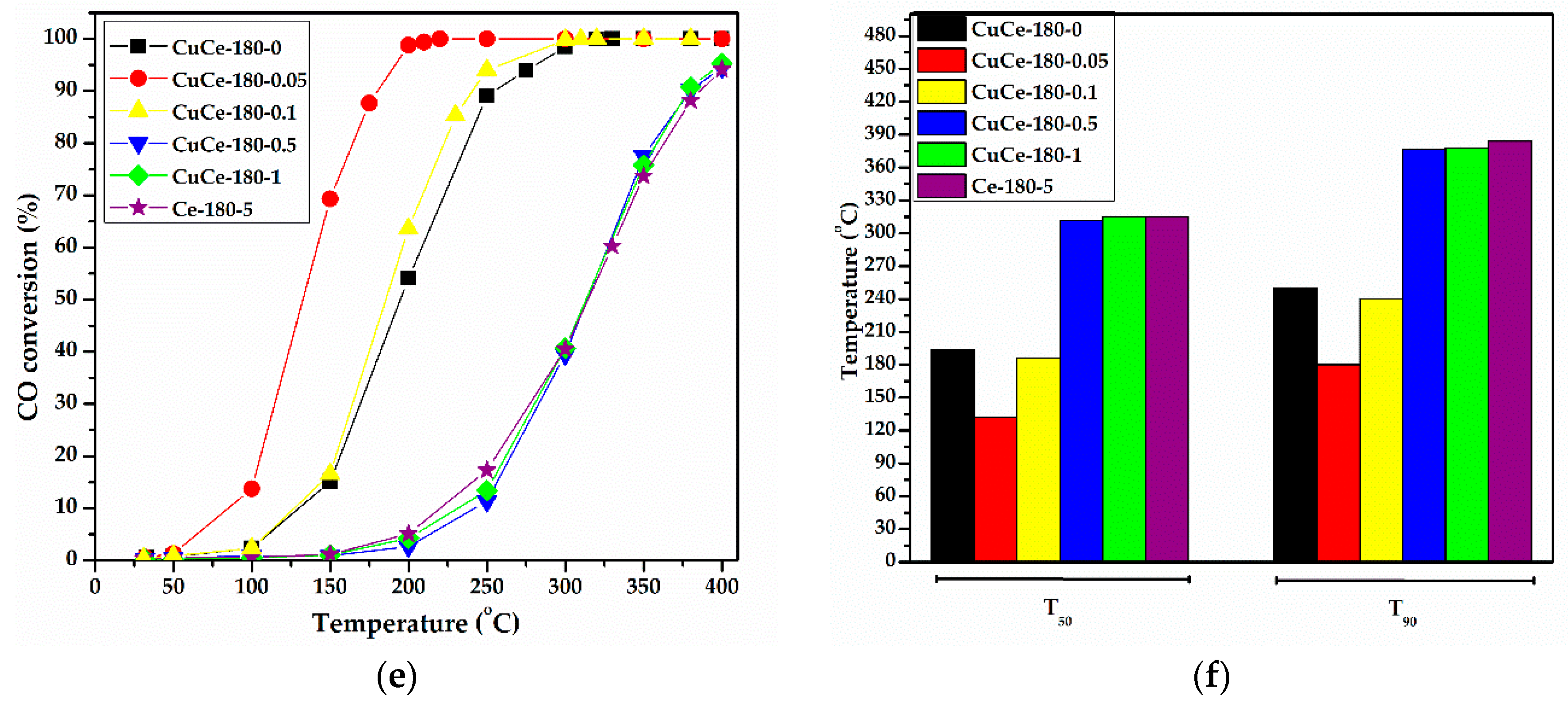
2.8. CO Oxidation Catalytic Studies
3. Discussion
Correlation of the Physicochemical Characteristics with the Catalytic Activity
- The most active samples presented spherical and/or rod-like morphology (samples with concentrations of NaOH ≤0.1 M), which was attributed to the effective combination of hydrothermal parameters. Exception to this trend was the sample Ce-120-0.1, which illustrated rods with high crystallinity. The bulky aggregates which, were revealed by the samples with high concentrations (≥0.5 M), seemed to negatively affect the catalytic activity.
- While the samples with high NaOH concentrations (≥0.5 M) showed high SSA, they could not compete the activities of the samples prepared with low concentrations of NaOH (≤0.1 M). In general, the high SSA is a desirable parameter for enhanced catalytic activity, but in our work, it is not a determining factor, as the observed catalytic performance is a combination of the copper content, copper species nature, morphology, oxygen vacancies and Cu-Ce interactions.
- Two different copper species were detected in ceria-based materials, which promoted the catalytic activity. This effect was more pronounced in the case of Cu2+ clusters as compared with the effect caused by the presence of Cu2+ isolated ions. The former species were favored under high hydrothermal temperatures and low NaOH concentrations.
- The presence of lattice defects and especially oxygen vacancies, which were certified by the spectroscopic techniques, affected the catalytic behavior, but not following a conventional trend. As shown via EPR spectroscopy, the catalysts, which were prepared with high concentrations of NaOH (≥0.5 M), presented a high concentration of Ce3+ ions (see Table 1), they did not result in a high catalytic activity. On the other hand, the samples with low concentrations of NaOH (≤0.1 M), although possessing small concentrations of Ce3+ ions, they exhibited better catalytic behavior. Strong copper-ceria concentrations were confirmed via DRS analysis for several samples prepared at 150 and 180 °C with low concentrations of NaOH. Conclcuding the above remarks, it can be commented that the formation of a high-defective structure is not a crucial factor for high catalytic activity, but the formation of a structure that will have a suitable concentration of oxygen vacancies. Similar results have been illustrated by Lin et al. [99]. Pulse calorimetry measurements showed that the high concentration of oxygen vacancies promoted the buildup of adsorbed carbonates that prohibit the adsorption and activation of CO, but not O2. According to several studies, oxygen vacancies tend to form oxygen vacancy clusters [100,101]. Wang et al. [102] showed that oxygen vacancy clusters with suitable size and distribution are responsible for high activity. Thus, it is believed that the various morphologies, which were obtained in this study, can present oxygen vacancies with different size and distribution.
4. Materials and Methods
4.1. Catalysts Preparation
4.2. Catalysts Characterization
4.3. Catalytic Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Wen, C.; Guo, Y.; Lu, G.; Wang, Y. Effects of surface area and oxygen vacancies on ceria in CO oxidation: Differences and relationships. J. Mol. Catal. Chem. 2010, 316, 59–64. [Google Scholar] [CrossRef]
- Rao, K.N.; Bharali, P.; Thrimurthulu, G.; Reddy, B.M. Supported copper–ceria catalysts for low temperature CO oxidation. Catal. Commun. 2010, 11, 863–866. [Google Scholar] [CrossRef]
- He, H.; Yang, P.; Li, J.; Shi, R.; Chen, L.; Zhang, A.; Zhu, Y. Controllable synthesis, characterization, and CO oxidation activity of CeO2 nanostructures with various morphologies. Ceram. Int. 2016, 42, 7810–7818. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T.; Matralis, H.K.; Batista, J.; Hocevar, S. CuO-CeO2 mixed oxide catalysts for the selective oxidation of carbon monoxide in excess hydrogen. Catal. Lett. 2001, 73, 33–40. [Google Scholar] [CrossRef]
- Gamarra, D.; Cámara, A.L.; Monte, M.; Rasmussen, S.B.; Chinchilla, L.E.; Hungría, A.B.; Munuera, G.; Gyorffy, N.; Schay, Z.; Corberán, V.C.; et al. Preferential oxidation of CO in excess H2 over CuO/CeO2 catalysts: Characterization and performance as a function of the exposed face present in the CeO2 support. Appl. Catal. B Environ. 2013, 130–131, 224–238. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, R. A new insight into the morphology effect of ceria on CuO/CeO2 catalysts for CO selective oxidation in hydrogen-rich gas. Catal. Sci. Technol. 2016, 6, 3862–3871. [Google Scholar] [CrossRef]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Carbon monoxide (CO) optical gas sensor based on ZnO thin films. Sens. Actuators B Chem. 2017, 250, 679–685. [Google Scholar] [CrossRef]
- Lin, Q.; Han, C.; Su, H.; Sun, L.; Ishida, T.; Honma, T.; Sun, X.; Zheng, Y.; Qi, C. Remarkable enhancement of Fe–V–Ox composite metal oxide to gold catalyst for CO oxidation in the simulated atmosphere of CO2 laser. RSC Adv. 2017, 7, 38780–38783. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Mai, H.-X.; Sun, L.-D.; Zhang, Y.-W.; Si, R.; Feng, W.; Zhang, H.-P.; Liu, H.-C.; Yan, C.-H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef]
- Qin, J.; Lu, J.; Cao, M.; Hu, C. Synthesis of porous CuO–CeO2 nanospheres with an enhanced low-temperature CO oxidation activity. Nanoscale 2010, 2, 2739–2743. [Google Scholar] [CrossRef] [PubMed]
- Lykaki, M.; Pachatouridou, E.; Iliopoulou, E.; Carabineiro, S.A.; Konsolakis, M. Impact of the synthesis parameters on the solid state properties and the CO oxidation performance of ceria nanoparticles. RSC Adv. 2017, 7, 6160–6169. [Google Scholar] [CrossRef]
- Campbell, C.T.; Peden, C.H.F. Oxygen Vacancies and Catalysis on Ceria Surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, K.; Liu, X.; Tian, Q.; Lu, D.; Yang, S. Single-crystalline ceria nanocubes: Size-controlled synthesis, characterization and redox property. Nanotechnology 2007, 18, 185606–185609. [Google Scholar] [CrossRef]
- Xu, J.; Harmer, J.; Li, G.; Chapman, T.; Collier, P.; Longworth, S.; Chi Tsang, S. Size dependent oxygen buffering capacity of ceria nanocrystals. Chem. Commun. 2010, 46, 1887–1889. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Takeda, M.; Tsukimoto, S.; Nakayama, K.S.; Asao, N. Cerium Oxide Nanorods with Unprecedented Low-Temperature Oxygen Storage Capacity. Adv. Mater. 2016, 28, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Sayle, T.X.T.; Parker, S.C.; Catlow, C.R.A. The role of oxygen vacancies on ceria surfaces in the oxidation of carbon monoxide. Surf. Sci. 1994, 316, 329–336. [Google Scholar] [CrossRef]
- Conesa, J. Computer modeling of surfaces and defects on cerium dioxide. Surf. Sci. 1995, 339, 337–352. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.; Sun, X.; Peng, Q.; Li, Y. Enhanced catalytic activity of ceria nanorods from well-defined reactive crystal planes. J. Catal. 2005, 229, 206–212. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Tana; Zhang, M.; Li, J.; Li, H.; Li, Y.; Shen, W. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles. Catal. Today 2009, 148, 179–183. [Google Scholar] [CrossRef]
- Periyat, P.; Laffir, F.; Tofail, S.A.M.; Magner, E. A facile aqueous sol–gel method for high surface area nanocrystalline CeO2. RSC Adv. 2011, 1, 1794–1798. [Google Scholar] [CrossRef]
- Suresh, R.; Ponnuswamy, V.; Mariappan, R. Effect of annealing temperature on the microstructural, optical and electrical properties of CeO2 nanoparticles by chemical precipitation method. Appl. Surf. Sci. 2013, 273, 457–464. [Google Scholar] [CrossRef]
- Kepenekçi, O.; Emirdag-Eanes, M.; Demir, M.M. Effect of alkali metal hydroxides on the morphological development and optical properties of ceria nanocubes under hydrothermal conditions. J. Nanosci. Nanotechnol. 2011, 11, 3565–3577. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, Y.; Sun, W.; Yao, Z.; Liu, B.; Gao, F.; Dong, L. Morphology and nanosize effects of ceria from different precursors on the activity for NO reduction. Catal. Today 2011, 175, 48–54. [Google Scholar] [CrossRef]
- Zdravković, J.; Simović, B.; Golubović, A.; Poleti, D.; Veljković, I.; Šćepanović, M.; Branković, G. Comparative study of CeO2 nanopowders obtained by the hydrothermal method from various precursors. Ceram. Int. 2015, 41, 1970–1979. [Google Scholar] [CrossRef]
- Zhang, W.; Niu, X.; Chen, L.; Yuan, F.; Zhu, Y. Soot Combustion over Nanostructured Ceria with Different Morphologies. Sci. Rep. 2016, 6, 29062. [Google Scholar] [CrossRef]
- Lu, X.; Zhai, T.; Cui, H.; Shi, J.; Xie, S.; Huang, Y.; Liang, C.; Tong, Y. Redox cycles promoting photocatalytic hydrogen evolution of CeO2 nanorods. J. Mater. Chem. 2011, 21, 5569–5572. [Google Scholar] [CrossRef]
- Terribile, D.; Trovarelli, A.; Llorca, J.; de Leitenburg, C.; Dolcetti, G. The Synthesis and Characterization of Mesoporous High-Surface Area Ceria Prepared Using a Hybrid Organic/Inorganic Route. J. Catal. 1998, 178, 299–308. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, D.; Shi, L.; Fang, J. Template-Free Synthesis, Controlled Conversion, and CO Oxidation Properties of CeO2 Nanorods, Nanotubes, Nanowires, and Nanocubes. Eur. J. Inorg. Chem. 2008, 2008, 2429–2436. [Google Scholar] [CrossRef]
- Shan, W.; Guo, H.; Liu, C.; Wang, X. Controllable preparation of CeO2 nanostructure materials and their catalytic activity. J. Rare Earths 2012, 30, 665–669. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, A.B.; Scanlon, D.O.; Watson, G.W. Role of Lattice Distortions in the Oxygen Storage Capacity of Divalently Doped CeO2. Chem. Mater. 2011, 23, 4464–4468. [Google Scholar] [CrossRef]
- Mahammadunnisa, S.; Reddy, P.M.K.; Karuppiah, J.; Subrahmanyam, C. Facile Synthesis of Au/CeO2 Catalyst for Low Temperature CO Oxidation. Adv. Chem. Lett. 2013, 1, 264–271. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.; Meng, D.; Guo, Y.; Guo, Y.; Lu, G. Effect of Ceria Crystal Plane on the Physicochemical and Catalytic Properties of Pd/Ceria for CO and Propane Oxidation. ACS Catal. 2016, 6, 2265–2279. [Google Scholar] [CrossRef]
- Gatla, S.; Aubert, D.; Agostini, G.; Mathon, O.; Pascarelli, S.; Lunkenbein, T.; Willinger, M.G.; Kaper, H. Room-Temperature CO Oxidation Catalyst: Low-Temperature Metal–Support Interaction between Platinum Nanoparticles and Nanosized Ceria. ACS Catal. 2016, 6, 6151–6155. [Google Scholar] [CrossRef]
- Elias, J.S.; Stoerzinger, K.A.; Hong, W.T.; Risch, M.; Giordano, L.; Mansour, A.N.; Shao-Horn, Y. In Situ Spectroscopy and Mechanistic Insights into CO Oxidation on Transition-Metal-Substituted Ceria Nanoparticles. ACS Catal. 2017, 7, 6843–6857. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Yao, Z.; Chen, Z.; Hong, M.; Zhu, R.; Liang, Y.; Zhao, J. Transition-Metal Doped Ceria Microspheres with Nanoporous Structures for CO Oxidation. Sci. Rep. 2016, 6, 23900. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.G.; Ball, I.K.; Azelee, W.; Daniell, W.; Goldfarb, D. Nature and Surface Redox Properties of Copper(II)-Promoted Cerium(IV) Oxide CO-Oxidation Catalysts. Chem. Mater. 2000, 12, 3715–3725. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T. Effect of synthesis parameters on catalytic properties of CuO-CeO2. Appl. Catal. B Environ. 2006, 67, 1–11. [Google Scholar] [CrossRef]
- Elias, J.S.; Artrith, N.; Bugnet, M.; Giordano, L.; Botton, G.A.; Kolpak, A.M.; Shao-Horn, Y. Elucidating the Nature of the Active Phase in Copper/Ceria Catalysts for CO Oxidation. ACS Catal. 2016, 6, 1675–1679. [Google Scholar] [CrossRef]
- Wang, W.-W.; Yu, W.-Z.; Du, P.-P.; Xu, H.; Jin, Z.; Si, R.; Ma, C.; Shi, S.; Jia, C.-J.; Yan, C.-H. Crystal Plane Effect of Ceria on Supported Copper Oxide Cluster Catalyst for CO Oxidation: Importance of Metal–Support Interaction. ACS Catal. 2017, 7, 1313–1329. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Rawski, M.; Vakros, J.; Avgouropoulos, G. A Novel Post-Synthesis Modification of CuO-CeO2 Catalysts: Effect on Their Activity for Selective CO Oxidation. ChemCatChem 2018, 10, 2096–2106. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Vakros, J.; Avgouropoulos, G. Impact of acid treatment of CuO-CeO2 catalysts on the preferential oxidation of CO reaction. Catal. Commun. 2018, 115, 68–72. [Google Scholar] [CrossRef]
- Landi, G.; Barbato, P.S.; Di Benedetto, A.; Lisi, L. Optimization of the preparation method of CuO/CeO2 structured catalytic monolith for CO preferential oxidation in H2-rich streams. Appl. Catal. B Environ. 2016, 181, 727–737. [Google Scholar] [CrossRef]
- Konsolakis, M. The role of Copper-Ceria interactions in catalysis science: Recent theoretical and experimental advances. Appl. Catal. B Environ. 2016, 198, 49–66. [Google Scholar] [CrossRef]
- Gong, X.; Liu, B.; Kang, B.; Xu, G.; Wang, Q.; Jia, C.; Zhang, J. Boosting Cu-Ce interaction in CuxO/CeO2 nanocube catalysts for enhanced catalytic performance of preferential oxidation of CO in H2-rich gases. Mol. Catal. 2017, 436, 90–99. [Google Scholar] [CrossRef]
- Adamski, A.; Zając, W.; Zasada, F.; Sojka, Z. Copper ionic pairs as possible active sites in N2O decomposition on CuOx/CeO2 catalysts. Catal. Today 2012, 191, 129–133. [Google Scholar] [CrossRef]
- Kydd, R.; Teoh, W.Y.; Wong, K.; Wang, Y.; Scott, J.; Zeng, Q.-H.; Yu, A.-B.; Zou, J.; Amal, R. Flame-Synthesized Ceria-Supported Copper Dimers for Preferential Oxidation of CO. Adv. Funct. Mater. 2009, 19, 369–377. [Google Scholar] [CrossRef]
- Wang, F.; Büchel, R.; Savitsky, A.; Zalibera, M.; Widmann, D.; Pratsinis, S.E.; Lubitz, W.; Schüth, F. In Situ EPR Study of the Redox Properties of CuO–CeO2 Catalysts for Preferential CO Oxidation (PROX). ACS Catal. 2016, 6, 3520–3530. [Google Scholar] [CrossRef]
- Knauth, P.; Tuller, H.L. Solute segregation, electrical properties and defect thermodynamics of nanocrystalline TiO2 and CeO2. Solid State Ion. 2000, 136–137, 1215–1224. [Google Scholar] [CrossRef]
- Abi-aad, E.; Bechara, R.; Grimblot, J.; Aboukais, A. Preparation and characterization of ceria under an oxidizing atmosphere. Thermal analysis, XPS, and EPR study. Chem. Mater. 1993, 5, 793–797. [Google Scholar] [CrossRef]
- Mendelovich, L.; Tzehoval, H.; Steinberg, M. The adsorption of oxygen and nitrous oxide on platinum ceria catalyst. Appl. Surf. Sci. 1983, 17, 175–188. [Google Scholar] [CrossRef]
- Fierro, J.L.G.; Soria, J.; Sanz, J.; Rojo, J.M. Induced changes in ceria by thermal treatments under vacuum or hydrogen. J. Solid State Chem. 1987, 66, 154–162. [Google Scholar] [CrossRef]
- Aboukaïs, A.; Bennani, A.; Aïssi, C.F.; Wrobel, G.; Guelton, M.; Vedrine, J.C. Highly resolved electron paramagnetic resonance spectrum of copper(II) ion pairs in CuCe oxide. J. Chem. Soc. Faraday Trans. 1992, 88, 615–620. [Google Scholar] [CrossRef]
- Lamonier, C.; Bennani, A.; D’Huysser, A.; Aboukaïs, A.; Wrobel, G. Evidence for different copper species in precursors of copper–cerium oxide catalysts for hydrogenation reactions. An X-ray diffraction, EPR and X-ray photoelectron spectroscopy study. J. Chem. Soc. Faraday Trans. 1996, 92, 131–136. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y.; Xing, X.; Chen, N.; Deng, D.; Wang, Y. Catalytic activity for CO oxidation of Cu–CeO2 composite nanoparticles synthesized by a hydrothermal method. Anal. Methods 2015, 7, 3238–3245. [Google Scholar] [CrossRef]
- Grigoropoulou, G.; Christoforidis, K.C.; Louloudi, M.; Deligiannakis, Y. Structure-catalytic function relationship of SiO2-immobilized mononuclear Cu complexes: An EPR study. Langmuir 2007, 23, 10407–10418. [Google Scholar] [CrossRef]
- Courcot, D.; Abi-Aad, E.; Capelle, S.; Aboukaïs, A. Investigation of copper-cerium oxide catalysts in the combustion of diesel soot. In Studies in Surface Science and Catalysis; Kruse, N., Frennet, A., Bastin, J.-M., Eds.; Catalysis and Automotive Pollution Control IV.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 116, pp. 625–634. [Google Scholar]
- Aboukaïs, A.; Bennani, A.; Lamonier-Dulongpont, C.; Abi-Aad, E.; Wrobel, G. Redox behaviour of copper(II) species on CuCe oxide catalysts: Electron paramagnetic resonance (EPR) study. Colloids Surf. Physicochem. Eng. Asp. 1996, 115, 171–177. [Google Scholar] [CrossRef]
- Soria, J.; Conesa, J.C.; Martínez-Arias, A.; Coronado, J.M. ESR study of the clustering of Cu ions on the ceria surface in impregnated CuO/CeO2. Solid State Ion. 1993, 63–65, 755–761. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Liu, Q. Ceria- and Cu-Doped Ceria Nanocrystals Synthesized by the Hydrothermal Methods. J. Am. Ceram. Soc. 2008, 91, 2706–2708. [Google Scholar] [CrossRef]
- Sakai, M.; Nagai, Y.; Aoki, Y.; Takahashi, N. Investigation into the catalytic reduction of NOx at copper–ceria interface active sites. Appl. Catal. Gen. 2016, 510, 57–63. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Yuan, S.; Zhang, M.; Ohno, T. Morphology control and characterization of broom-like porous CeO2. Chem. Eng. J. 2015, 260, 126–132. [Google Scholar] [CrossRef]
- Zhou, X.-D.; Huebner, W. Size-induced lattice relaxation in CeO2 nanoparticles. Appl. Phys. Lett. 2001, 79, 3512–3514. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Ishikawa, K.; Li, Z.-Q.; Kawazoe, Y.; Kasuya, A. Origin of Anomalous Lattice Expansion in Oxide Nanoparticles. Phys. Rev. Lett. 2000, 85, 3440–3443. [Google Scholar] [CrossRef]
- Lu, B.; Li, Z.; Kawamoto, K. Synthesis of mesoporous ceria without template. Mater. Res. Bull. 2013, 48, 2504–2510. [Google Scholar] [CrossRef]
- Guo, T.; Du, J.; Li, J. The effects of ceria morphology on the properties of Pd/ceria catalyst for catalytic oxidation of low-concentration methane. J. Mater. Sci. 2016, 51, 10917–10925. [Google Scholar] [CrossRef]
- Araiza, D.G.; Gómez-Cortés, A.; Díaz, G. Partial oxidation of methanol over copper supported on nanoshaped ceria for hydrogen production. Catal. Today 2017, 282 Pt 2, 185–194. [Google Scholar] [CrossRef]
- McBride, J.R.; Hass, K.C.; Poindexter, B.D.; Weber, W.H. Raman and X-ray studies of Ce1−xRExO2−y, where RE = La, Pr, Nd, Eu, Gd, and Tb. J. Appl. Phys. 1994, 76, 2435–2441. [Google Scholar] [CrossRef]
- Spanier, J.E.; Robinson, R.D.; Zhang, F.; Chan, S.-W.; Herman, I.P. Size-dependent properties of CeO2−y nanoparticles as studied by Raman scattering. Phys. Rev. B 2001, 64, 245407. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- Choi, Y.M.; Abernathy, H.; Chen, H.-T.; Lin, M.C.; Liu, M. Characterization of O2-CeO2 Interactions Using In Situ Raman Spectroscopy and First-Principle Calculations. ChemPhysChem 2006, 7, 1957–1963. [Google Scholar] [CrossRef]
- Matei-Rutkovska, F.; Postole, G.; Rotaru, C.G.; Florea, M.; Pârvulescu, V.I.; Gelin, P. Synthesis of ceria nanopowders by microwave-assisted hydrothermal method for dry reforming of methane. Int. J. Hydrogen Energy 2016, 41, 2512–2525. [Google Scholar] [CrossRef]
- López, J.M.; Gilbank, A.L.; García, T.; Solsona, B.; Agouram, S.; Torrente-Murciano, L. The prevalence of surface oxygen vacancies over the mobility of bulk oxygen in nanostructured ceria for the total toluene oxidation. Appl. Catal. B Environ. 2015, 174–175, 403–412. [Google Scholar] [CrossRef]
- Tok, A.I.Y.; Du, S.W.; Boey, F.Y.C.; Chong, W.K. Hydrothermal synthesis and characterization of rare earth doped ceria nanoparticles. Mater. Sci. Eng. A 2007, 466, 223–229. [Google Scholar] [CrossRef]
- Naganuma, T.; Traversa, E. Stability of the Ce3+ valence state in cerium oxide nanoparticle layers. Nanoscale 2012, 4, 4950–4953. [Google Scholar] [CrossRef]
- Ho, C.; Yu, J.C.; Kwong, T.; Mak, A.C.; Lai, S. Morphology-Controllable Synthesis of Mesoporous CeO2 Nano- and Microstructures. Chem. Mater. 2005, 17, 4514–4522. [Google Scholar] [CrossRef]
- Rakai, A.; Bensalem, A.; Muller, J.C.; Tessier, D.; Bozon-Verduraz, F. Revisiting Diffuse Reflectance Spectroscopy for the Characterization of Metal and Semiconducting Oxide Catalysts. In Studies in Surface Science and Catalysis; Guczi, L., Solymosi, F., Tétényi, P., Eds.; New Frontiers in Catalysis—Proceedings of the 10th International Congress on Catalysis, Budapest, Hungary, 19–24 July 1992; Elsevier: Amsterdam, The Netherlands, 1993; Volume 75, pp. 1875–1878. [Google Scholar]
- Bensalem, A.; Muller, J.C.; Bozon-Verduraz, F. From bulk CeO2 to supported cerium-oxygen clusters: A diffuse reflectance approach. J. Chem. Soc. Faraday Trans. 1992, 88, 153–154. [Google Scholar] [CrossRef]
- Binet, C.; Badri, A.; Lavalley, J.-C. A Spectroscopic Characterization of the Reduction of Ceria from Electronic Transitions of Intrinsic Point Defects. J. Phys. Chem. 1994, 98, 6392–6398. [Google Scholar] [CrossRef]
- Aguila, G.; Guerrero, S.; Araya, P. Effect of the preparation method and calcination temperature on the oxidation activity of CO at low temperature on CuO–CeO2/SiO2 catalysts. Appl. Catal. Gen. 2013, 462–463, 56–63. [Google Scholar] [CrossRef]
- Pintar, A.; Batista, J.; Hočevar, S. TPR, TPO, and TPD examinations of Cu0.15Ce0.85O2−y mixed oxides prepared by co-precipitation, by the sol–gel peroxide route, and by citric acid-assisted synthesis. J. Colloid Interface Sci. 2005, 285, 218–231. [Google Scholar] [CrossRef]
- Mena, E.; Rey, A.; Rodríguez, E.M.; Beltrán, F.J. Nanostructured CeO2 as catalysts for different AOPs based in the application of ozone and simulated solar radiation. Catal. Today 2017, 280 Pt 1, 74–79. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Fukuda, T.; Kasuya, A. Blue shift in ultraviolet absorption spectra of monodisperse CeO2−x nanoparticles. J. Appl. Phys. 2000, 87, 1318–1321. [Google Scholar] [CrossRef]
- Itoh, T.; Uchida, T.; Izu, N.; Matsubara, I.; Shin, W. Effect of Core–Shell Ceria/Poly(vinylpyrrolidone) (PVP) Nanoparticles Incorporated in Polymer Films and Their Optical Properties. Materials 2013, 6, 2119–2129. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, D.; Shi, L. CTAB assisted hydrothermal synthesis, controlled conversion and CO oxidation properties of CeO2 nanoplates, nanotubes, and nanorods. J. Solid State Chem. 2008, 181, 1298–1306. [Google Scholar] [CrossRef]
- Taniguchi, T.; Katsumata, K.; Omata, S.; Okada, K.; Matsushita, N. Tuning Growth Modes of Ceria-Based Nanocubes by a Hydrothermal Method. Cryst. Growth Des. 2011, 11, 3754–3760. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, X. Hydrothermal Synthesis and Upconversion Properties of α-NaYF4:Yb3+, Er3+ Nanocrystals Using Citric Acid as Chelating Ligand and NaNO3 as Mineralizer. J. Nanosci. Nanotechnol. 2015, 15, 9656–9664. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H. Preparing micro/nano core-shell sphere CeO2 via a low temperature route for improved lithium storage performance. Mater. Lett. 2016, 168, 80–82. [Google Scholar] [CrossRef]
- Jiang, T.; Qin, W.; Di, W.; Yang, R.; Liu, D.; Zhai, X.; Qin, G. Citric acid-assisted hydrothermal synthesis of α-NaYF4: Yb3+, Tm3+ nanocrystals and their enhanced ultraviolet upconversion emissions. CrystEngComm 2012, 14, 2302–2307. [Google Scholar] [CrossRef]
- Levien, B.J. A Physicochemical Study of Aqueous Citric Acid Solutions. J. Phys. Chem. 1955, 59, 640–644. [Google Scholar] [CrossRef]
- Masui, T.; Hirai, H.; Imanaka, N.; Adachi, G.; Sakata, T.; Mori, H. Synthesis of cerium oxide nanoparticles by hydrothermal crystallization with citric acid. J. Mater. Sci. Lett. 2002, 21, 489–491. [Google Scholar] [CrossRef]
- Ma, J.; Qian, K.; Huang, W.; Zhu, Y.; Yang, Q. Facile One-Step Synthesis of Double-Shelled CeO2 Hollow Spheres and Their Optical and Catalytic Properties. Bull. Chem. Soc. Jpn. 2010, 83, 1455–1461. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.; Jing, C.; Qin, Y. Formation and Thermal Decomposition of Cerium-Organic Precursor for Nanocrystalline Cerium Oxide Powder Synthesis. J. Dispers. Sci. Technol. 2007, 28, 1053–1058. [Google Scholar] [CrossRef]
- Wu, N.-C.; Shi, E.-W.; Zheng, Y.-Q.; Li, W.-J. Effect of pH of Medium on Hydrothermal Synthesis of Nanocrystalline Cerium(IV) Oxide Powders. J. Am. Ceram. Soc. 2002, 85, 2462–2468. [Google Scholar] [CrossRef]
- Lin, M.; Fu, Z.Y.; Tan, H.R.; Tan, J.P.Y.; Ng, S.C.; Teo, E. Hydrothermal Synthesis of CeO2 Nanocrystals: Ostwald Ripening or Oriented Attachment? Cryst. Growth Des. 2012, 12, 3296–3303. [Google Scholar] [CrossRef]
- Bezkrovnyi, O.S.; Lisiecki, R.; Kepinski, L. Relationship between morphology and structure of shape-controlled CeO2 nanocrystals synthesized by microwave-assisted hydrothermal method. Cryst. Res. Technol. 2016, 51, 554–560. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Li, L.; Wang, A.; Zhang, W.; Wang, X.; Zhang, T. Activation of an Ir-in-CeO2 catalyst by pulses of CO: The role of oxygen vacancy and carbonates in CO oxidation. Catal. Today 2012, 180, 155–160. [Google Scholar] [CrossRef]
- Nörenberg, H.; Briggs, G.A.D. Defect Structure of Nonstoichiometric CeO2 (111) Surfaces Studied by Scanning Tunneling Microscopy. Phys. Rev. Lett. 1997, 79, 4222–4225. [Google Scholar] [CrossRef]
- Esch, F.; Fabris, S.; Zhou, L.; Montini, T.; Africh, C.; Fornasiero, P.; Comelli, G.; Rosei, R. Electron Localization Determines Defect Formation on Ceria Substrates. Science 2005, 309, 752–755. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; He, H.; Zhang, Y.; Qin, X.; Wang, B. Oxygen vacancy clusters essential for the catalytic activity of CeO2 nanocubes for o-xylene oxidation. Sci. Rep. 2017, 7, 12845. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Stathi, P.; Gournis, D.; Deligiannakis, Y.; Rudolf, P. Stabilization of phenolic radicals on graphene oxide: An XPS and EPR study. Langmuir 2015, 31, 10508–10516. [Google Scholar] [CrossRef] [PubMed]
- Scullane, M.; White, L.; Chasteen, N. An efficient approach to computer simulation of EPR spectra of high-spin Fe(III) in rhombic ligand fields. J. Magn. Reson. 1969, 47, 383–397. [Google Scholar] [CrossRef]
- Knijnenburg, J.T.N.; Seristatidou, E.; Hilty, F.M.; Krumeich, F.; Deligiannakis, Y. Proton-promoted iron dissolution from nanoparticles and the influence by the local iron environment. J. Phys. Chem. C 2014, 118, 24072–24080. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T.; Papadopoulou, C.; Batista, J.; Hocevar, S.; Matralis, H.K. A comparative study of Pt/γ-Al2O3, Au/α-Fe2O3 and CuO–CeO2 catalysts for the selective oxidation of carbon monoxide in excess hydrogen. Catal. Today 2002, 75, 157–167. [Google Scholar] [CrossRef]
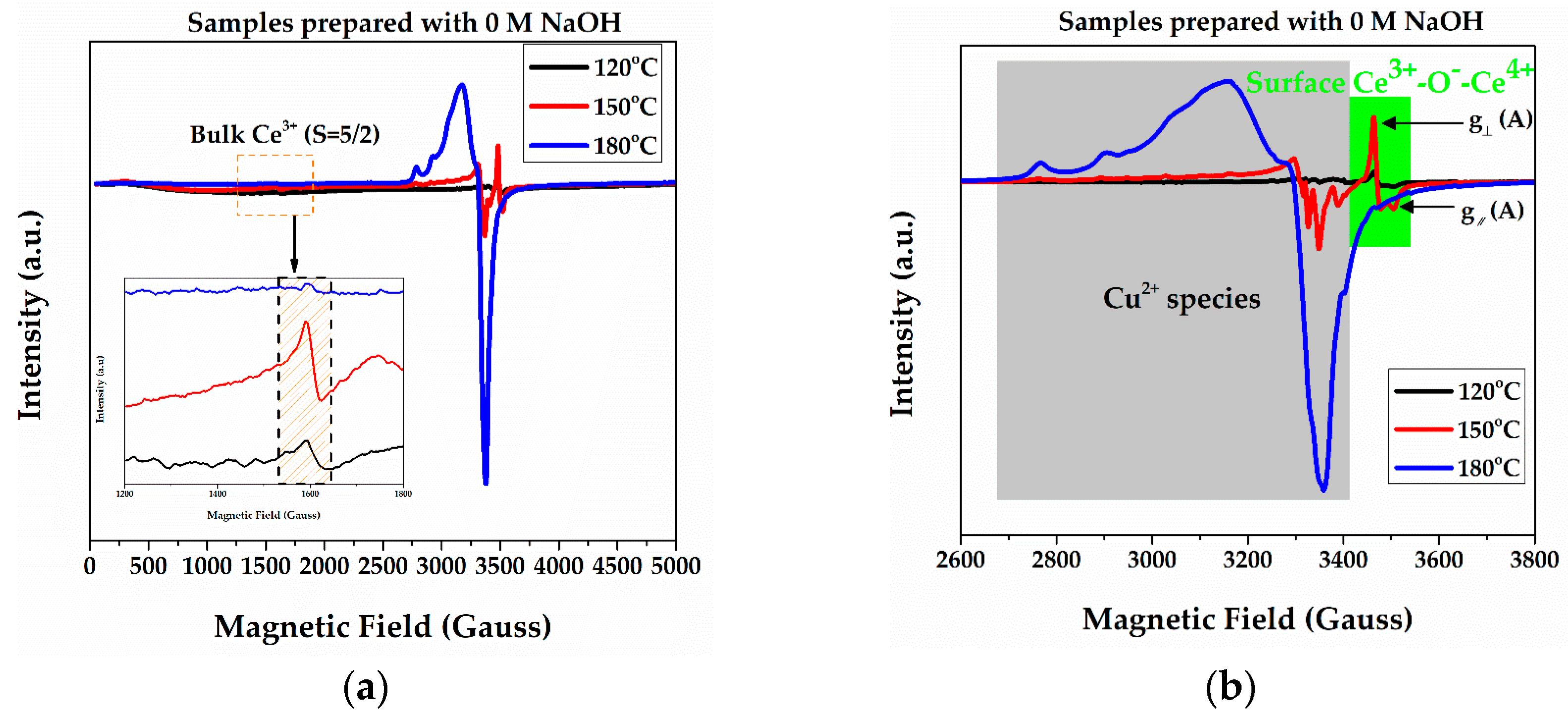
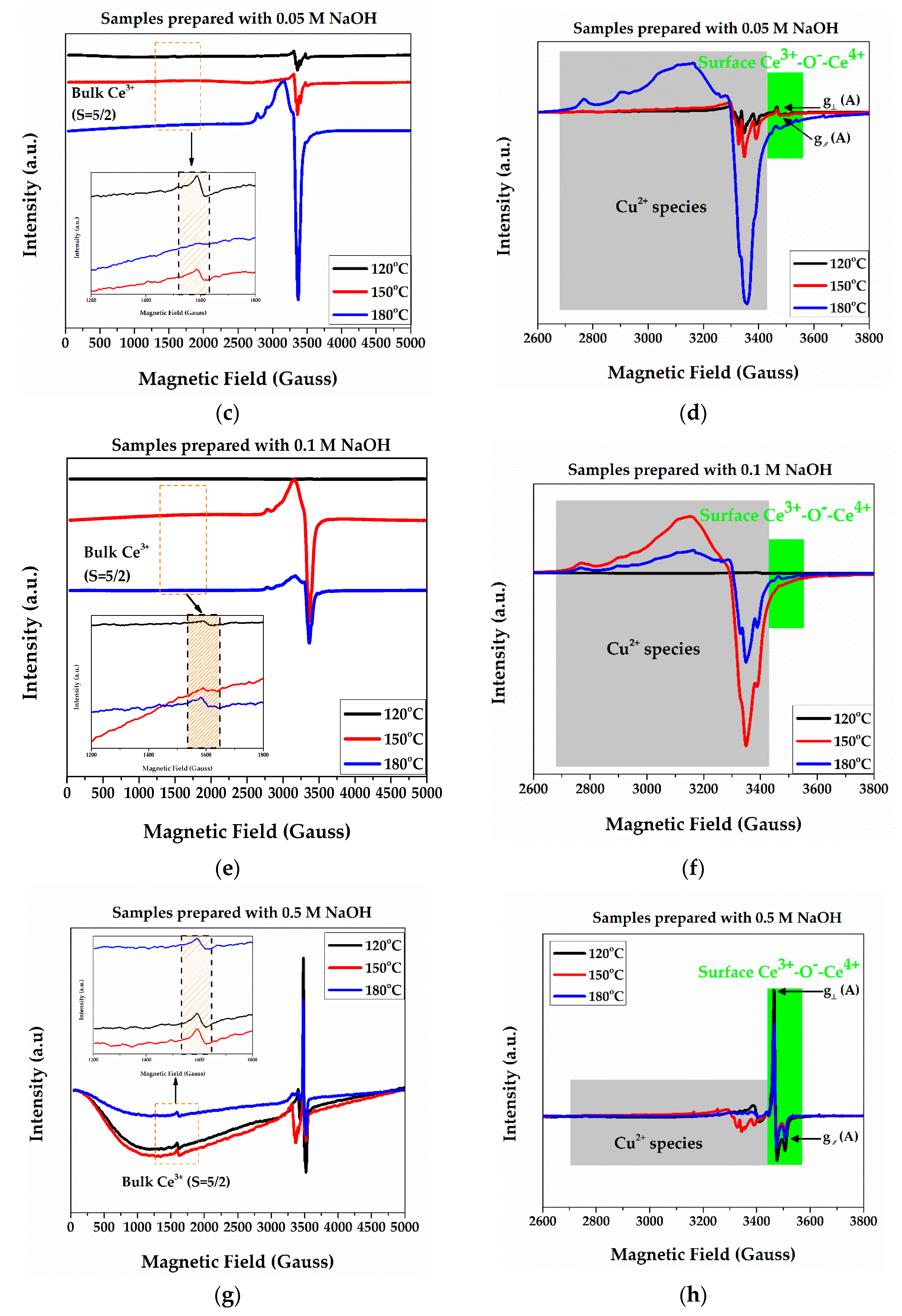







| Samples | Bulk Ce3+ (μM/gr) | Surface Ce3+ (μM/gr) | Cu2+ Species (wt %) |
|---|---|---|---|
| Ce-120-0 | 0.43 | 0.1 | N.D. |
| CuCe-120-0.05 | 0.50 | 1.3 | 0.20 (isolated ions) |
| Ce-120-0.1 | 0.40 | 0.4 | N.D. |
| CuCe-120-0.5 | 0.50 | 4.0 | 0.002 (isolated ions) |
| CuCe-120-1 | 0.29 | 4.0 | 0.02 (isolated ions) |
| Ce-120-5 | 0.50 | 4.0 | N.D. |
| CuCe-150-0 | 1.00 | 1.3 | 0.15 (isolated ions) |
| CuCe-150-0.05 | 0.20 | 1.1 | 0.3 (>95% clusters) |
| CuCe-150-0.1 | 0.40 | 0.8 | 3.9 (>95% clusters) |
| Ce-150-0.5 | 0.50 | 3.0 | N.D. |
| CuCe-150-1 | 0.27 | 4.0 | 0.002 (isolated ions) |
| Ce-150-5 | 0.50 | 4.0 | N.D. |
| CuCe-180-0 | 0.02 | <0.05 | 2.1 (>95% clusters) |
| CuCe-180-0.05 | N.D. 1 | <0.05 | 2.8 (>95% clusters) |
| CuCe-180-0.1 | 0.40 | 0.1 | 1.4 (>95% clusters) |
| CuCe-180-0.5 | 0.50 | 3.0 | 0.02 (isolated ions) |
| CuCe-180-1 | 0.25 | 3.0 | 0.01 (isolated ions) |
| Ce-180-5 | 0.40 | 4.0 | N.D. |
| Samples | d111 (nm) | α (nm) |
|---|---|---|
| Ce-120-0 | 8.6 | 0.54078 |
| CuCe-120-0.05 | 6.9 | 0.54228 |
| Ce-120-0.1 | 10.0 | 0.54136 |
| CuCe-120-0.5 | 13.2 | 0.54118 |
| CuCe-120-1 | 13.8 | 0.54123 |
| Ce-120-5 | 8.2 | 0.54185 |
| CuCe-150-0 | 7.2 | 0.53966 |
| CuCe-150-0.05 | 7.5 | 0.54147 |
| CuCe-150-0.1 | 19.0 | 0.54211 |
| Ce-150-0.5 | 9.5 | 0.54164 |
| CuCe-150-1 | 15.2 | 0.54127 |
| Ce-150-5 | 25.3 | 0.54133 |
| CuCe-180-0 | 37.7 | 0.54168 |
| CuCe-180-0.05 | 29.2 | 0.54168 |
| CuCe-180-0.1 | 18.2 | 0.54142 |
| CuCe-180-0.5 | 11.3 | 0.54134 |
| CuCe-180-1 | 13.9 | 0.54138 |
| Ce-180-5 | 23.5 | 0.54159 |
| Samples | SSA (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| Ce-120-0 | 36.3 | 0.0860 | 10.34 |
| CuCe-120-0.05 | 67.0 | 0.0920 | 5.33 |
| Ce-120-0.1 | 22.2 | 0.0420 | 10.02 |
| CuCe-120-0.5 | 70.6 | 0.1286 | 5.17 |
| CuCe-120-1 | 78.8 | 0.1447 | 5.63 |
| Ce-120-5 | 137.1 | 0.2294 | 5.55 |
| CuCe-150-0 | 63.7 | 0.1070 | 5.51 |
| CuCe-150-0.05 | 73.3 | 0.1050 | 4.80 |
| CuCe-150-0.1 | 40.4 | 0.0320 | 5.23 |
| Ce-150-0.5 | 86.2 | 0.1462 | 4.97 |
| CuCe-150-1 | 58.6 | 0.1390 | 5.88 |
| Ce-150-5 | 37.3 | 0.2114 | 18.06 |
| CuCe-180-0 | 9.2 | 0.0450 | 15.30 |
| CuCe-180-0.05 | 14.8 | 0.0530 | 12.30 |
| CuCe-180-0.1 | 27.8 | 0.0730 | 10.00 |
| CuCe-180-0.5 | 76.5 | 0.1437 | 5.50 |
| CuCe-180-1 | 59.4 | 0.1343 | 6.50 |
| Ce-180-5 | 35.0 | 0.1952 | 16.80 |
| Samples | F2g (cm−1) | FWHM (cm−1) | ID/IF2g | Eg (eV) |
|---|---|---|---|---|
| Ce-120-0 | 463.0 | 14.8 | 0.027 | 3.05 |
| CuCe-120-0.05 | 462.3 | 22.8 | 0.046 | 3.03 |
| Ce-120-0.1 | 463.1 | 17.7 | 0.023 | 3.13 |
| CuCe-120-0.5 | 463.0 | 13.3 | 0.035 | 3.18 |
| CuCe-120-1 | 463.0 | 12.5 | 0.060 | 3.24 |
| Ce-120-5 | 462.3 | 36.5 | 0.106 | 3.12 |
| CuCe-150-0 | 463.0 | 24.2 | 0.031 | 3.12 |
| CuCe-150-0.05 | 462.3 | 26.7 | 0.050 | 3.06 |
| CuCe-150-0.1 | 460.7 | 36.0 | 0.046 | 3.07 |
| Ce-150-0.5 | 463.8 | 17.6 | 0.032 | 3.20 |
| CuCe-150-1 | 464.6 | 12.7 | 0.014 | 3.28 |
| Ce-150-5 | 464.6 | 17.0 | 0.046 | 3.29 |
| CuCe-180-0 | 463.7 | 22.8 | 0.047 | 3.11 |
| CuCe-180-0.05 | 463.0 | 23.2 | 0.080 | 3.00 |
| CuCe-180-0.1 | 461.4 | 28.1 | 0.035 | 3.21 |
| CuCe-180-0.5 | 463.7 | 15.8 | 0.038 | 3.20 |
| CuCe-180-1 | 464.5 | 13.1 | 0.020 | 3.25 |
| Ce-180-5 | 463.8 | 14.8 | 0.034 | 3.29 |
| Samples | pH (Ce3+) 2 | pH (CA) 3 | pH (Ce3+ + CA) 4 | pH (NaOH) 5 | pH Total 6 |
|---|---|---|---|---|---|
| 0 M 1 | 3.75 | 1.56 | 0.95 | 0 | 1.68 |
| 0.05 M | 3.75 | 1.56 | 0.95 | 12.76 | 2.10 |
| 0.1 M | 3.75 | 1.56 | 0.95 | 13.03 | 2.52 |
| 0.5 M | 3.75 | 1.56 | 0.95 | 13.35 | 13.11 |
| 1 M | 3.75 | 1.56 | 0.95 | 13.51 | 13.46 |
| 5 M | 3.75 | 1.56 | 0.95 | 13.91 | 13.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kappis, K.; Papadopoulos, C.; Papavasiliou, J.; Vakros, J.; Georgiou, Y.; Deligiannakis, Y.; Avgouropoulos, G. Tuning the Catalytic Properties of Copper-Promoted Nanoceria via a Hydrothermal Method. Catalysts 2019, 9, 138. https://doi.org/10.3390/catal9020138
Kappis K, Papadopoulos C, Papavasiliou J, Vakros J, Georgiou Y, Deligiannakis Y, Avgouropoulos G. Tuning the Catalytic Properties of Copper-Promoted Nanoceria via a Hydrothermal Method. Catalysts. 2019; 9(2):138. https://doi.org/10.3390/catal9020138
Chicago/Turabian StyleKappis, Konstantinos, Christos Papadopoulos, Joan Papavasiliou, John Vakros, Yiannis Georgiou, Yiannis Deligiannakis, and George Avgouropoulos. 2019. "Tuning the Catalytic Properties of Copper-Promoted Nanoceria via a Hydrothermal Method" Catalysts 9, no. 2: 138. https://doi.org/10.3390/catal9020138
APA StyleKappis, K., Papadopoulos, C., Papavasiliou, J., Vakros, J., Georgiou, Y., Deligiannakis, Y., & Avgouropoulos, G. (2019). Tuning the Catalytic Properties of Copper-Promoted Nanoceria via a Hydrothermal Method. Catalysts, 9(2), 138. https://doi.org/10.3390/catal9020138








