Abstract
Since the beginning of the 20th century, numerous research efforts made elegant use of barbituric acid derivatives as building blocks for the elaboration of more complex and useful molecules in the field of pharmaceutical chemistry and material sciences. However, the construction of chiral scaffolds by the catalytic enantioselective transformation of barbituric acid and derivatives has only emerged recently. The specific properties of these rather planar scaffolds, which also encompass either a high Brønsted acidity concerning the native barbituric acid or the marked electrophilic character of alkylidene barbituric acids, required specific developments to achieve efficient asymmetric processes. This review covers the enantioselective catalytic reactions developed for barbituric acid platforms using an organocatalytic and metal-based enantioselective sequences. These achievements currently allow several unique addition and annulation reactions towards the construction of high valued chiral heterocycles from barbituric acid derivatives along with innovative enantioselective developments.
1. Introduction
1.1. Context
Barbituric acid 1, namely 2,4,6-(lH,3H,5H)-pyrimidinetrione, and derivatives are fascinating building blocks in organic synthesis (Figure 1). This story dates back to 1894 when von Baeyer reported on the preparation of barbituric acid [1]. Later, in 1903, Fisher and von Mering highlighted the pharmacological properties of 5,5-diethyl barbituric acid, the so-called barbital, as a potent hypnotic agent [2]. Ever since, barbituric acid derivatives served as starting materials for the elaboration of thousands of complex architectures useful in medicinal chemistry, a field of research covered by several exhaustive reviews [3,4,5,6]. Selected examples of bioactive compounds in this series are depicted in Figure 1, which highlights scaffolds that possess, at least, one stereogenic center or a tetrasubstituted carbon as a source of chemical diversity and structural complexity. Phenobarbital, involved in the treatment of certain types of epilepsy, derived from the original barbital but displays two different substituents at C-5 position (Figure 1a) [7]. The opportunity afforded by the double functionalization of the C-5 position of barbituric acid allowed the construction of several spiro-compounds and, thus, more rigid architectures highlighted by the development of nucleotide mimics [8], inhibitor of matrix metalloproteinases (MMP) [9] and anticancer agents [10], for example (Figure 1b–d) [11,12]. On the other hand, the synthesis of fused-bicycle derivatives led to ingredients possessing either antidiabetic [13] or antituberculosis properties (Figure 1e–f) [14]. Besides medicinal chemistry, the photophysical properties of barbituric acid derivatives were exploited in colorimetric or heat detection [15], and provided promising dyes or fluorogenic probes to name a few [16,17].
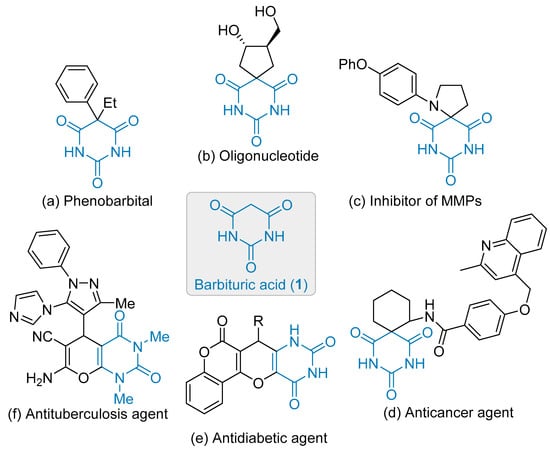
Figure 1.
Barbituric acid scaffolds within bioactive architectures.
The versatile properties of products obtained from barbituric acid elicited the motivation of organic chemists to explore its chemistry [3,4,6], and more recent evolutions have met the development of efficient multicomponent reactions (MCR) [16,18]. Despite great synthetic advances in this field of research, the use of diastereoselective sequences or separation techniques (crystallization or chromatography) of racemic mixtures were required for the elaboration of enantiopure architectures of chiral bioactive compounds as depicted in Figure 1 [19]. At the end of the 1990s, the pioneering research of Brunner’s group, on the palladium-catalyzed enantioselective alkylation of barbituric acid derivatives have shed light on the potential of this starting material in asymmetric synthesis while pointing out challenges, which have to be overcome with this unique architecture [20,21,22,23]. This review intends to cover the literature dealing with the enantioselective catalytic transformations of barbituric acid derivatives, an exercise which is unprecedented to the best of our knowledge. The literature concerning the enantioselective transformations of barbituric acid and alkylidene barbituric acid derivatives will be discussed separately, to focus on specific types of reactions developed for a given barbiturate structure. To give a flavor of the uniqueness of barbituric acid compounds, its properties will first be introduced.
1.2. Properties of Barbituric Acid Derivatives
To gain insight into the reactivity of barbituric acid derivatives, the unique chemical properties of these molecules have to be taken into account. As shown in Figure 2a, barbituric acid 1 displays a rather low pKa value (pKa = 8.4 in DMSO) [24] lying just above Meldrum’s acid 2 well-known for its remarkable acidity (pKa = 7.03 in DMSO) [25,26]. Consequently, barbituric acid 1 and derivatives will be much more prone to deprotonation by soft bases than dimedone 3 or non-cyclic homologues, such as malonate 4, for instance. However, direct correlation between the pKa value and the nucleophilic behavior is not always feasible [27].
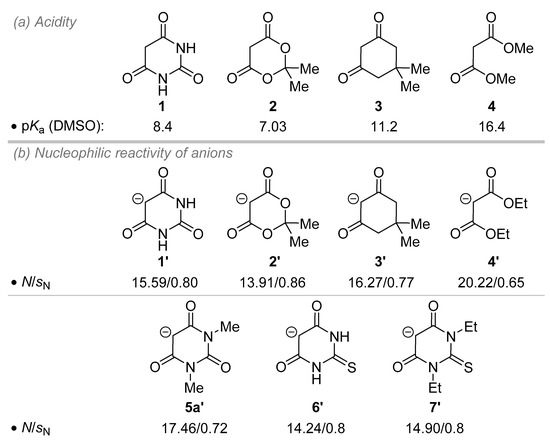
Figure 2.
Properties of barbituric acid derivatives. (a) Acidity given in DMSO. (b) Nucleophilic reactivity parameters of anions.
In 2017, Spange and co-workers embarked on the insightful evaluation of the kinetics of derivatives of barbiturate acid anions thanks to the reaction with specific electrophiles, such as benzhydrylium cations and Michael acceptors [28]. Then, the so-called nucleophilicity parameter N and the corresponding nucleophile-specific susceptibility parameter sN of barbiturate anions 1’ could be determined and compared to related 1,3-dicarbonyl anions 2’-4’ (Figure 2b) [27]. Barbituric acid anion 1’ features a nucleophilic character between Meldrum’s acid 2’ and dimedone 3’ anions but is much less reactive than malonate anion 4’, which actually leads to a rather good correlation with the scale of pKa values. In addition to being more soluble, the 1,3-dimethyl barbituric acid 5a’ reacted faster than the parent anion 1’, a phenomenon attributed to the electron-donating effect of the N-alkyl groups. Nevertheless, a ground state stabilization of the negative charge of 1’ by hydrogen bonding of the NH moieties might also account for this outcome. In comparison, the anionic thiobarbiturate analogues 6’ and 7’ proved to be less nucleophilic due to superior electron withdrawing properties of the thiocarbonyl versus carbonyl of 1’ for instance. Interestingly, during this study, the O-alkylation reaction has never been observed. Accordingly, a computational study showed that the C-alkylation process is favored both from a thermodynamic and a kinetic point of view. This scenario might be different with harder electrophiles, however.
Mayr and co-workers studied the electrophilicity of benzylidene barbituric acid derivatives (Figure 3) [29], which proved to be a potent electrophile in various conjugate addition reactions or cycloaddition processes (vide infra). 5-Arylidene 1,3-dimethylbarbituric acid 8a has an electrophilicity parameter E similar to 5-arylidene Meldrum’s acid 10, which is in line with the reactivity of benzylidenemalononitriles, such as 12. For comparison purposes, the benzylidene barbituric acid derivative 8a is a much more potent electrophile than the malonate counterpart 11 and, thus, affords interesting reactivity to be exploited in catalysis. Another important feature, relies on the modulation of reactivity by moving from barbiturate 8a to thiobarbiturate 9a which displays a superior electrophilicity. The authors attributed this phenomenon to the higher positive polarization of nitrogen in the thiobarbiturate 9a versus barbiturate 8a [29].
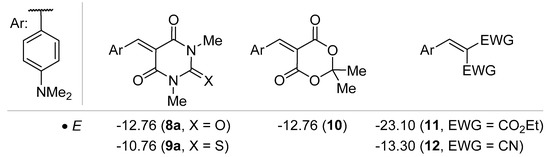
Figure 3.
Reactivity issues.
2. Enantioselective Transformations of Barbituric Acid Derivatives
As aforementioned, barbituric acid derivatives are easily deprotonated with regard to their rather low pKa value. Simple reasoning based upon resonance structures obviously shows that the conjugate base of barbituric acid may undergo either a C-alkylation or an O-alkylation reaction (Figure 4a), an issue known to be dependent on the nature of the electrophile and reaction conditions [28]. Several research groups took advantage of these features to develop enantioselective transformations based on three different strategies (Figure 4b). Barbituric acid derivatives having different N-substituents (R1 ≠ R2) are pro-chiral by nature (if R3 = H) and, consequently, they provide the corresponding alkylated products with a stereocenter inside the ring (strategy A). This approach was mainly applied to the formation of tetrasubstituted products (R3 ≠ H). On the other hand, symmetrical architectures (R1 = R2, strategy B) will furnish products along with the construction of a stereocenter outside the ring after the reaction with a pro-chiral electrophile. Finally, annulation processes may take place, through both a C- and O-alkylation sequence (strategy C), allowing the synthesis of fused ring scaffolds while the initial symmetry of the barbituric acid precursor is broken.
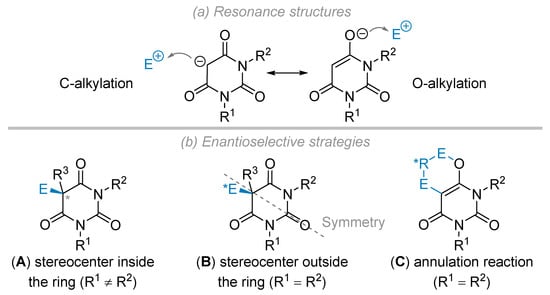
Figure 4.
Reactivity issues and asymmetric synthesis. (a) Resonance structures towards C or O-alkylation reaction. (b) Enantioselective strategies.
In the following Section, we will describe the achievement based on these three enantioselective synthetic strategies.
2.1. Addition Reactions with Stereocenters Created Inside the Ring
In the 1990s, the Brunner group published a series of pioneering investigations highlighting the stereoselective alkylation reaction of non-symmetrical barbituric acid derivatives. In 1994 [21], the authors succeeded in the Pd-catalyzed allylation reaction of the 1,5-dimethylbarbituric acid 13 to give the corresponding product 14 in 68% yield and 12.7% ee (Scheme 1), along with the creation of an all-carbon tetrasubstituted stereocenter. The process needed the use of a stoichiometric amount of 1,8-diazabicyclo[5.4.0] undec-7-ene (DBU) as a base to deprotonate the starting material 13. This beautiful early achievement was allowed with the parallel development of novel chelating chiral phosphine imine ligands, such as 15.
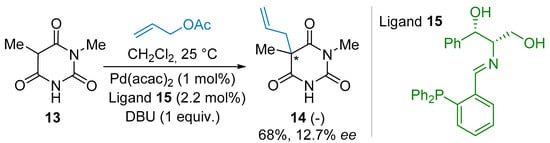
Scheme 1.
Pioneering investigations.
During subsequent investigations, this group of researchers took advantage of the readily available phosphine imines like 17, easily synthesized from the corresponding aldehyde with suitable chiral amines (Scheme 2). Then, 134 various ligands were evaluated in this enantioselective allylation process of 13 [20,23]. The novel phosphine ligand 17 allowed to improve the ee up to 34% for product 14, and another side-product, 16, which originated from the double allylation reaction was observed in these conditions. Based on preliminary theoretical and NMR investigations, the authors have proposed a transition state wherein the (η3-allyl)-palladium complex would possess two phosphine-ligands L featuring π-π stacking events between their phenyl rings. Then, the hydroxyl functional group of the ligand may create hydrogen-bonding interactions with the barbituric acid anion to differentiate the two enantiotopic enolate faces (Scheme 2). These pioneering achievements are noteworthy since the pro-chiral incoming anion, and the palladium complex (having the chiral information) are lying at the opposite faces of allylic activated species, which makes the design of a suited ligand for this asymmetric reaction significantly challenging contrariwise to the one used for a pro-chiral electrophile.
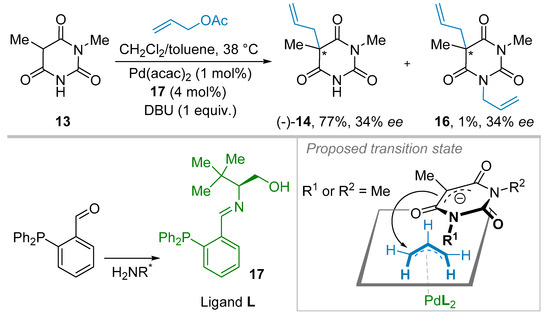
Scheme 2.
Ligand optimization for the Pd-catalyzed allylation reaction.
In 1999, Brunner and colleagues tackled the stereoselective synthesis of methohexital 19, a potent short-time anesthetic (Scheme 3) [22]. As mentioned in the article, the four stereoisomers of barbituric acid derivatives 19 displayed distinct narcotic effects that make asymmetric synthesis a useful constructive-tool in this case. The palladium-promoted allylation reaction was, obviously, a suited approach to 19 but had to challenge the presence of a stereogenic center on 1-methyl-5-(1’-methylpent-2’-ynyl) barbituric acid 18, in so much as this starting material was used as a racemic mixture. The phosphine imine 17 proved to be the best ligand for this reaction, and an advanced investigation revealed salient features. Indeed, the stereoselective allylation of one of the prostereogenic faces of the transient enolate species took place upon the influence of the chiral ligand 17, giving two diastereoisomers (19, (R,R) and (R,S), for instance) for a given enantiomer of 18 (for instance, from the one having the R propargylic-stereocenter). Additionally, this allylation sequence also led to a kinetic resolution process resulting in the faster transformation rate of either (R)-18 or (S)-18 at the propargylic-stereocenter. In line with these two processes (Scheme 3), the reaction stopped after 9 hours and provided two diastereoisomers 19 with 26% yield and one of them was obtained in up to 80% ee (the other one in 13% ee). The remaining starting material 18 was recovered in 79% yield and a combined 5% ee. After 72 hours of reaction, the enantiomeric excess of the first pair of enantiomers decreased to 46% while the other one reached 72%. Nonetheless, the combined ee of the starting material 18 (7%) was improved to 83% in accordance with a kinetic resolution event.
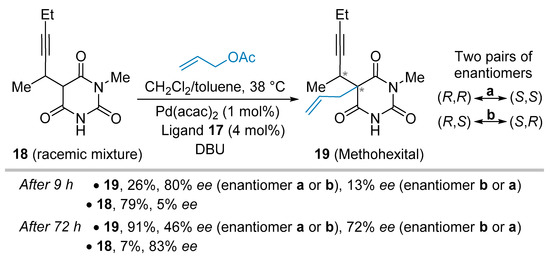
Scheme 3.
Synthesis of stereoisomers of methohexital.
In 2000 the Trost group envisaged the elaboration of a simplified analogue 21 of methohexital, lacking a stereogenic center at the propargylic position (Scheme 4) [30]. The catalyzed asymmetric allylic alkylation reaction (AAA), using the C2-symmetrical cyclohexyldiamine ligands 23 or 24 in the presence of palladium chloride species, allowed the transformation of the starting material 20 into the corresponding allylated product 21 albeit in modest 19% yield and 37% ee. In this case, the more sterically hindered ligand 24 versus 23, having two naphthyl moieties, proved to be the most efficient one. The authors moved to a prochiral cyclopentenyl carbonate as electrophile with the hope to favor the stereodifferentiation of the prochiral faces of the enolate intermediate derived from deprotonation of nucleophile 20. In a similar catalytic system, but in dichloromethane solvent instead of DMSO, the product 22 was obtained in a good 78% yield, modest 2:1 dr but with excellent ees of 93% and 86% for both diastereoisomers, respectively. This constitutes the highest selectivity obtained at that time for a catalytic metal-based transformation of a non-symmetrical barbituric acid. That is noteworthy as this requires the chiral discrimination between an NMe and NH amide moieties of the pro-nucleophilic partner 20.
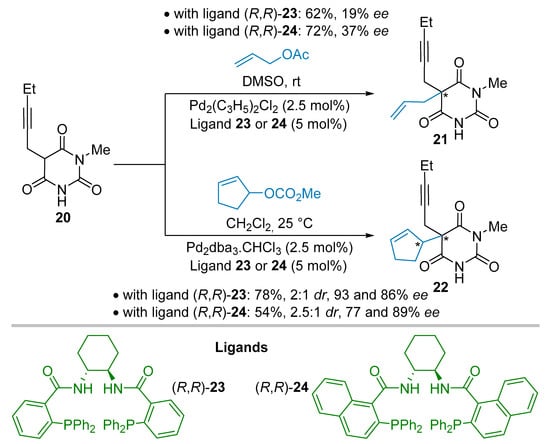
Scheme 4.
Catalyzed asymmetric allylic alkylation reaction.
Recently, Palomo and co-workers tackled the enantioselective alkylation reaction of non-symmetrical barbituric acid derivatives making use of an organocatalytic strategy (Scheme 5) [31]. After the observation of very moderate ees obtained using (thio)barbituric acid nucleophiles, this research established that 2-alkylthio-4,6-dioxopyrimides 25 were competent barbituric acid surrogates for the conjugate addition reaction to vinyl aryl ketones 26. Upon the influence of a newly designed squaramide-derived 9-epi-amino quinine 28a organocatalyst, as a chiral Brønsted base, an array of Michael adducts 27 were synthesized in good yields and ees ranging from 90 to 97% irrespective of the nature of substituents (R1, R2, and Ar) on precursors 25 and 26. To explain the enantioselectivity of this reaction, the authors proposed a transition state in which the bifunctional organocatalyst 28a would create two hydrogen bonds between the squaramide moiety and the corresponding enolate of barbiturate derivative 25 within the ion-pair 29 (Scheme 5), and more precisely with the most accessible oxy-anion. Then, enones 26 would approach the front-face of the barbiturate enolate escorted by a hydrogen bonding allowed by the quinuclidinium cation moiety.
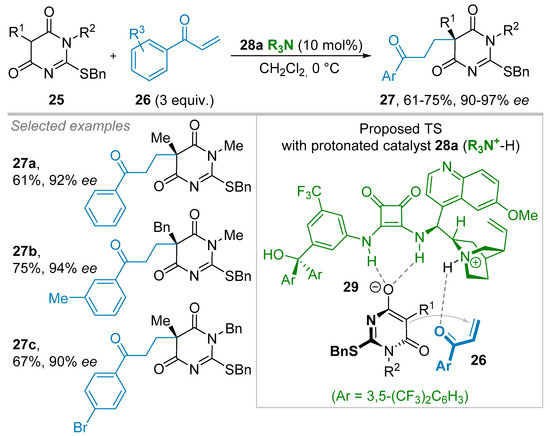
Scheme 5.
Organocatalytic conjugate addition reaction.
Notwithstanding this major achievement in this field of research, the Michael reaction could not be extrapolated to less reactive acrylate derivatives, and the moderately sterically hindered acrolein led to a modest ee of 48% [31]. Nevertheless, this group showed that enone 30 (Scheme 6), a precursor of acrylates or acrolein after oxidative cleavage sequences, was able to undergo the stereoselective conjugate addition of 25 under rather similar organocatalytic conditions to give barbituric acid products 31 with ees between 81% and 94%, after an acidic hydrolysis of the 2-benzylthioether moiety. As depicted in Scheme 6, this reaction tolerates various linear and branched alkyl substituents at C5 (R1), and both alkyl and aryl pendants on nitrogen (R2) of products 31a-f. Curiously, as an exception, the precursors 29 with an iso-propyl (R1) did not furnish any product 31g in these conditions (Scheme 6).
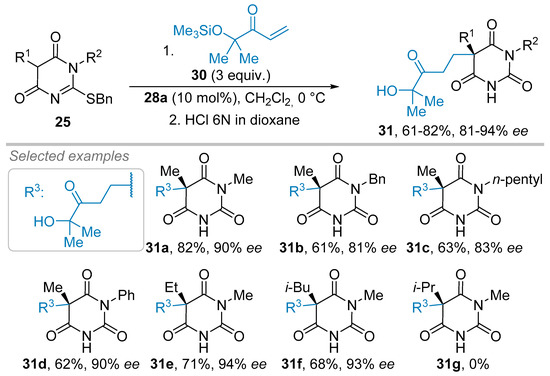
Scheme 6.
Organocatalytic Michael reaction to versatile enones.
The scope of this transformation was further broadened during the development of an asymmetric allylation reaction thanks to the potent Cinchona derived organocatalyst 28a (Scheme 7). The reaction proceeded smoothly between barbiturate 32 and the Morita-Baylis-Hillman allyl bromide 33 in the presence of potassium carbonate to neutralize the HBr production. As a representative example, the allylated product 34 was synthesized in 65% yield and 92% ee. The optimization demonstrated that the best results were obtained with electrophile 33 having a tert-butyl ester and K2CO3 proved to be the more suitable base. The authors illustrated the usefulness of the obtained barbiturate derivatives, such as 34, possessing an all-carbon quaternary stereocenter by performing various transformations [31]. For instance, a metathesis reaction on product 34 furnished the cyclopentene 35 in 78% yield (Scheme 7). Eventually, the barbituric acid architecture 36 was formed upon an acidic hydrolysis.
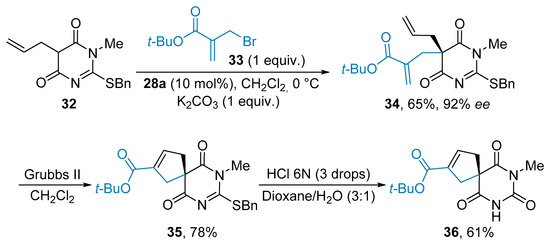
Scheme 7.
Organocatalytic allylation reaction.
These achievements constitute a breakthrough in the field of catalytic enantioselective transformations of non-symmetrical barbituric acid derivatives along with the demonstration of useful transformations of the obtained enantioenriched products. In parallel, the development of catalytic asymmetric addition reactions of symmetrical barbituric acid derivatives to prochiral electrophiles has evolved (see Section below).
2.2. Addition Reactions with Stereocenters Created Outside the Ring
Inspired by previous reports by Brunner et al., dealing with the Pd-catalyzed asymmetric allylic alkylation reaction (AAA) of pro-chiral barbituric acid derivatives [20,21,22,23], Trost et al. reported the AAA reaction of symmetrical barbituric acid derivatives in the course of the synthesis of cyclopentobarbital 38 and pentobarbital 42 that are known to exhibit sedative and hypnotic properties, respectively (Scheme 8) [30]. Initial attempt to synthesize cyclopentobarbital 38 from allylic barbituric acid derivatives 37a (R1 = allyl) and allylic carbonate in the presence of Pd2(dba)3·CHCl3 (2.5 mol%), ligand (S,S)-23 (5 mol%, see Scheme 4 for structure), and Et3N (1 equivalent) in CH2Cl2 at 25 °C allowed the formation of the expected product 38 in high enantiomeric excess (84% ee). However, moderate isolated yield of 39% was obtained due to the presence of significant amounts of di- and tri-alkylated products 40 and 41, respectively (38/40/41 = 4.3:1:1). The presence of polyalkylated products was assigned to the higher solubility and hence reactivity of the first formed monoalkylated product 38 versus barbituric acid 37a. To solve this issue, the reaction was performed without base but in the presence of an ammonium salt (n-Hex4NBr) playing a dual role of (1) providing a more sterically congested nucleophile resulting in the diminution of polyalkylated products, and (2) increasing interactions between the nucleophile and the ligand, thus, improving the enantiomeric excesses. Indeed, by implementing such conditions and by switching to ligand (S,S)-24 (see Scheme 4), a selective formation of the cyclopentobarbital 38 was observed in a good isolated yield of 85% and excellent 91% ee. The (R)-absolute configuration of 38 was proposed using the predictive model previously developed by Trost et al. for such type of ligands and nucleophiles [32]. To synthesize pentobarbital 42 from barbituric acid derivative 37b and allylic carbonate, the reaction conditions were slightly modified. Indeed, by using the same palladium(0) source (Pd2(dba)3·CHCl3, 1 mol%) in the presence of ligand (R,R)-23 (2 mol%), the mono alkylated product 39 was obtained in almost quantitative yield (96%) and 78% ee in 3h providing that 10 mol% of TBAT (tetra n-butylammonium triphenyldifluorosilicate) was used as additive. After a hydrogenation step, the enantioenriched pentobarbital 38 was obtained in a quantitative yield and 81% ee after a simple recrystallization. (R)-42 is known in the literature, the absolute configuration was eventually ascertained by comparison of the optical rotation.
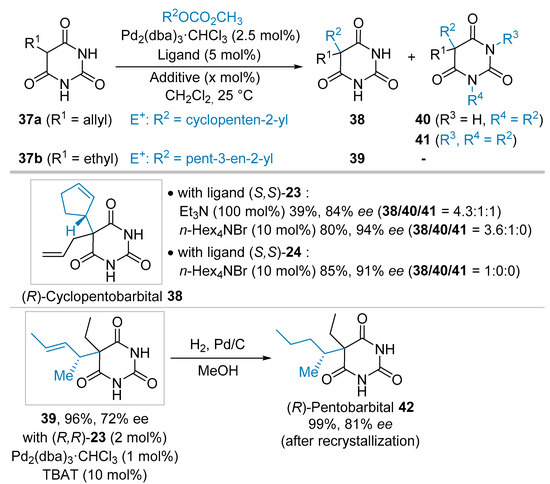
Scheme 8.
Pd-catalyzed allylation of mono-substituted 5-barbituric acid derivatives.
Later, Rawal et al. reported an enantioselective Michael addition of N,N′-disubstituted barbituric acid derivatives 5 to β-nitro olefins 43 using chiral thiosquaramides 44 as a bifunctional organocatalyst (Scheme 9) [33]. To circumvent the known solubility issue of the original squaramide catalysts, the authors have developed an unprecedented and more soluble chiral thiosquaramide version 44. Furthermore, the higher acidity of the N–H bonds of the thiosquaramide moiety was estimated with a lower pKa of 4 to 5 units in comparison to the squaramide counterpart allowing stronger hydrogen bonding activation events. Several chiral thiosquaramides 44, having a chiral cyclohexanediamine scaffold, were synthesized in three steps from commercially available butyl squaramate ester in good yields ranging from 67 to 83%. These catalysts were evaluated in the nucleophilic 1,4-addition reaction of N,N′-diphenyl barbituric acid 5b acid to β-nitro styrene 43a. In each case, complete conversions into adduct 45 were obtained in toluene at room temperature in half an hour along with ees ranging from 45 to 97% even at catalyst loading as low as 0.5 mol%. Interestingly, a comparison of catalyst 44b to its squaramide version 46 revealed that both catalysts are very similar in terms of conversion (above 98%) and enantioselectivity (97% ee vs 95% ee, respectively). It is noteworthy that the reaction could be carried out using only 0.05 mol% of catalyst 44b in toluene without erosion of both conversion and enantioselectivity (>98%, 96% ee). As mentioned by the authors, these reactions were conducted without any precautions under air atmosphere. Thus, by implementing optimized conditions (i.e., 0.5 mol% of catalyst 44b in toluene at room temperature for 0.5 h to 24 h), several addition products 45 have been obtained in excellent isolated yields and ees regardless to the substitution of the barbituric precursors 5 or the β-nitrostyrenes 43 (Scheme 9c). Only for more sterically hindered precursors 5 or 43, longer reaction times (24 h instead of 0.5 h) and higher catalyst loading (5 mol% instead of 0.5 mol%) were required to maintain the level of isolated yields and ees. For HPLC analysis issue (high retention times and broad peaks), the addition products 45 were chlorinated to afford 47 before analysis.
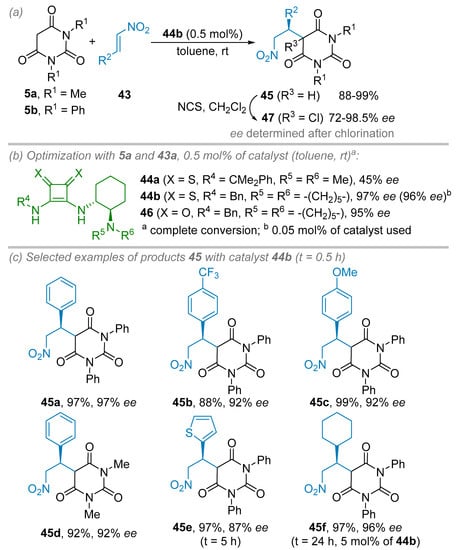
Scheme 9.
Organocatalyzed enantioselective Michael addition of N,N′-disubstituted barbituric acid derivatives to β-nitro olefins. (a) General conditions. (b) Catalyst optimization. (c) Scope and limitations.
The same year, Wang and co-workers reported on the enantioselective organocatalytic Michael addition of N,N′-dialkylbarbituric acid derivatives 5 to β-substituted enones 48 making use of chiral bifunctional squaramide catalyst 28b based upon a 9-epi-aminoquinine scaffold (Scheme 10) [34]. The screening of reaction parameters was performed using N,N′-dimethyl barbituric acid 5a and chalcone 48a as model substrates in toluene at 25 °C. Quinine (QN) provided the corresponding addition product 49a in a good yield of 68%, but ee values did not exceed 15% (Scheme 10a). Drastic improvement of the induction was achieved by switching to bifunctional squaramide catalyst 28b (10 mol%) allowing to obtain product 49a in 78% ee. After an optimization endeavor, the best conditions were set as followed: o-xylene as solvent at room temperature in the presence of 10 mol% of catalyst 28b using N,N′-di-tert-butylbarbituric acid 5c as substrate. Then, a survey of the scope of the reaction was undertaken using various substituted enones 48 and barbituric acid 5c (R1 = t-Bu, Scheme 10c). A large panel of Michael adducts 49 flanked by mono or di-substituted (electron-withdrawing or electron-donating groups) aryl, heteroaryl could be tolerated as long as R2 and R3 substituents are concerned in almost perfect stereoinduction (25 examples, 95–99% ee) with, nonetheless, variable isolated yields (44–99%). Aliphatic R2 group (n-Pr) in association with phenyl R3 group resulted in a slight drop of the ee (92%) whereas enones with R3 = Me, and R2 = Ph or n-Pr remained unreactive in the reaction conditions. The absolute configuration of compounds 49i was attributed as (S) thanks to single-crystal X-ray analysis. To account for the high level of induction observed, the authors proposed a transition state where the squaramide part of the catalyst 28b binds to the carbonyl of the enone while the barbituric acid moiety (under its enol form) is well-positioned by the quinucline Brønsted base part (Scheme 10b). Thus, an addition to the Re-face of the enone is favored.
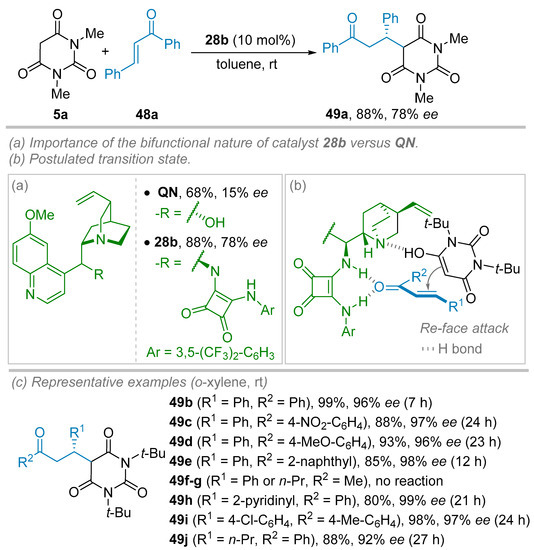
Scheme 10.
Organocatalyzed enantioselective Michael addition of N,N′-disubstituted barbituric acid derivatives to β-substituted enones. (a,b) Importance of the bifunctional nature of the catalyst in the transition state. (c) Scope and limitations.
2.3. Annulation Reactions
Barbituric acid derivatives may also be involved in annulation reactions. This approach was first tackled by Chen and co-workers during the synthesis of tetrahydropyrano bicycles 50 (Scheme 11) [35]. Starting from N,N′-dimethyl barbituric acid 5a and Morita-Baylis-Hillman (MBH) acetate of nitroalkene 51, a domino Michael-oxa-Michael addition reaction was achieved upon organocatalysis. During the reaction investigation, it was found that the original tertiary amine-thiourea bifunctional organocatalyst 52 was superior to the other organocatalysts. Thus, by applying optimized conditions (i.e., 10 mol% of 52, CH2Cl2, 25 °C), the investigation of the scope of the reaction was undertaken. Excellent results were obtained regardless of the substitution pattern on the aromatic ring to give 76–99% isolated yields, dr <19:1 and 86–99% ees. Polyaromatic or heretoaromatic ring pendants of the MBH adduct 51 were tolerated but at the expense of a slight drop of the diastereoisomeric ratio (dr >10:1) or isolated yield (51%), respectively. To account for the high diastereo- and enantioselectivity, the authors proposed a transition state where the amine part of the catalyst would deprotonate the barbituric acid 5a while its thiourea part binds to the nitro group of the MBH derivative 51. This results in a well-defined TS allowing an attack of the enolate to the Re-face of the nitroalkene 51. Then, a 6-endo-trig annulation took place through a 6-membered ring transition state where the nitro group is in pseudo-equatorial position to minimize steric interactions with an aromatic group, thus, leading to product 50 as trans-isomers in high diastereo- and enantioselectivity.
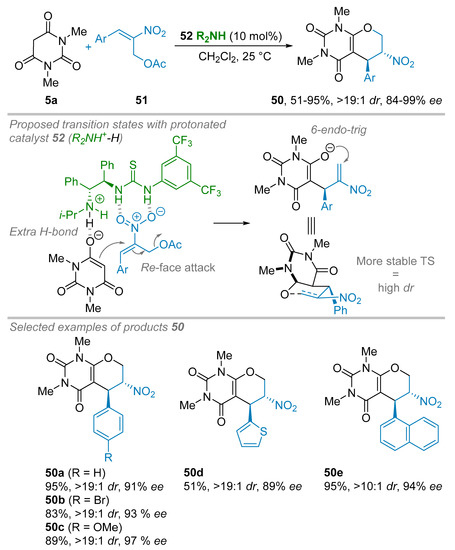
Scheme 11.
Organocatalyzed enantioselective Michael-oxa-Michael addition sequence of N,N′-dimethyl barbituric acid to Morita-Baylis-Hillman adduct.
One year later, the same group reported on a (3+3) annulation reaction consisted of a similar Michael-oxa-Michael sequence starting from N,N′-dimethyl barbituric acid 5a but in the presence of 2-(1-alkynyl)-2-alken-1-ones 53 as electrophilic partners (Scheme 12) [36]. Thus, by implementing the Takemoto’s organocatalyst, possessing an amine-thiourea bifunctional scaffold 54 (10 mol%) in mesitylene at −20 °C, almost 20 cyclic products 55 were obtained in fair to good isolated yields (32–87%) and moderate to high ees of 67–94%. The substitution of Ar1 by either electro-withdrawing or -donating groups proved to have a low impact on the ees being generally above 80% except for nitro-substituted compounds obtained in 67% ee values. This was attributed to possible interactions with the catalyst that hamper the optimal activation process. Contrariwise, substitution of Ar2 was found to have a positive effect on the asymmetric induction leading to ees ranging from 84 to 94%. Generally speaking, the steric effect seems to be detrimental to obtain high yields and enantioselectivties as shown by results obtained with 2,4-dichloro substituent on Ar1 whereas no reaction was observed when cyclohexyl was used instead of Ar1 group or in the presence of N,N′-diethyl barbituric acid 5d. From a mechanistic point of view, the authors proposed the activation of the barbituric acid 5a, under its enol form, by the tertiary amine part of the catalyst meanwhile the thiourea moiety chelates the carbonyl group of the enynone 53 to favor the attack of nucleophile to the Si-face of the C–C double bond of 53. Then, an oxa-Michael addition to the transient allene 56 takes place, thus, affording the (3+3) cycloadduct 55.
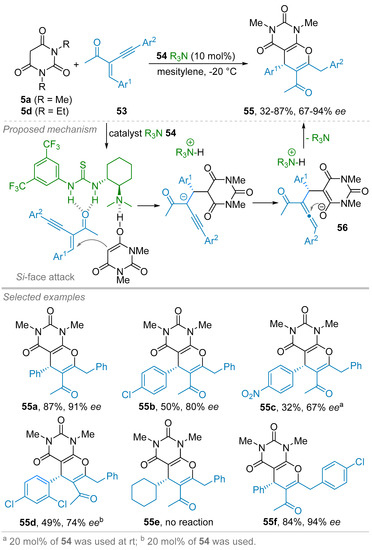
Scheme 12.
Organocatalyzed enantioselective Michael-oxaMichael addition sequence of N,N′-dimethyl barbituric acid to enynones.
An example of annulation reaction consisted of two consecutive C–C bonds was reported in 2015 by Lam and co-workers during the synthesis of a series of spiro-indenes of type 59 with good to excellent ees. However, in this study, a single example was reported which involved the 1-methyl-5-phenylbarbituric acid 57 and alkynes 58 in the presence of chiral rhodium complexes 60 as catalyst and Cu(OAc)2 as oxidant giving rise to the formation of 59 in good isolated yield (76%) but with only 11% ee (Scheme 13) [37]. This low enantioselection was attributed to the fact that both carbonyl functional groups (which act as directing groups for the formation of the rhodacycle 61 in the catalytic cycle) adjacent to the phenyl substituent are electronically and sterically similar. This results in a lack of stereodifferentiation between the two possible transition states (rhodacycles 61 and ent-61) obtained after the coordination and migratory insertion of the alkyne partner.
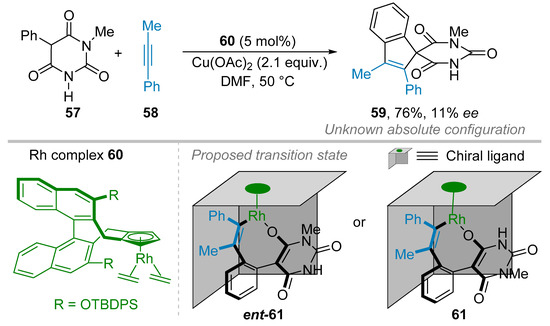
Scheme 13.
Rhodium(III)-catalyzed enantioselective CH-functionalization-spiroannulation sequence.
3. Enantioselective Transformations from Alkylidene Barbituric Acid
As discussed in the introduction (Cf. § 1.2), alkylidene barbituric acids are highly reactive electron-poor alkene derivatives which have been involved into either cycloaddition or annulation reaction (Scheme 14). On the one hand, under asymmetric catalytic conditions, (3+2) and (4+2) cycloaddition or annulation reactions took place on the C–C double bond and have allowed the construction of spiro-compounds (path A). On the other hand, suitably designed dienophiles led to enantioselective Diels–Alder reactions through the concomitant C–C and C–O bond formation to provide chiral fused-compounds (path B).
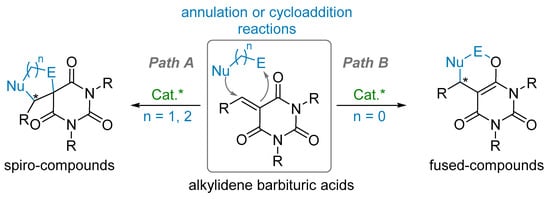
Scheme 14.
Annulation reaction onto alkylidene barbituric acids.
3.1. (3+2) Cycloaddition Reactions
Alkylidene barbiturates have been involved in (3+2) cycloaddition as found by different research groups. First of all, isothiocyanates were used with amine catalysis (Scheme 15a). Then, Morita-Baylis-Hillman (MBH) adduct or ynone starting materials were reported as dipole precursors along with phosphine catalysis (Scheme 15b,c).
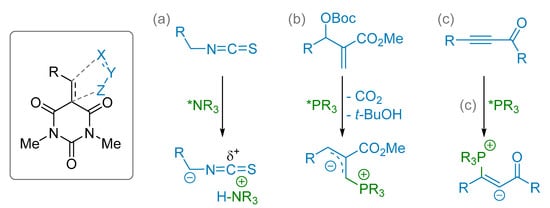
Scheme 15.
Reagents in (3+2) cycloaddition with alkylidene barbiturate.
For more than ten years, isothiocyanates have been engaged in catalytic reactions as isothiocyanato amides, esters or phosphonates. In 2015, Liu’s group published on a racemic (3+2) cycloaddition between alkylidene barbiturates 8 and 3-isothiocyanato oxindoles 62 catalyzed by triethylamine (Scheme 16) [38]. In 2016, the same authors reported on an enantioselective version catalyzed by a Cinchona-based thiourea 64 leading to spirobarbiturates 63 in modest to excellent ees and 20:1 dr in most cases (Scheme 16) [39]. The best reaction conditions involved the use of 9-epi-quinine-thiourea 64 (10 mol%), p-toluic acid (10 mol%) and 4 Å molecular sieves in chloroform. According to the proposed mechanism (see TS in Scheme 16), p-toluic acid drives the equilibrium of 62 towards its enol form, which forms a hydrogen bond with the tertiary amine of catalyst 64. The bifunctional nature of organocatalyst 64 allows extra-hydrogen bonding interactions between both the thiourea and barbituric acid moieties, thus, providing a well-organized transition state that favors the selective addition of the nucleophile 62 to one the two prosterogeneic faces of the alkylidene derivative 8. The scope with regard to alkylidene barbiturates 8 was studied and showed that aryl substituents (R1) were well-tolerated irrespective to the substitution pattern on the rings (R2 = R3 = Me: 80–99% yield, 13:1 to >20:1 dr, 75–99% ee). Nevertheless, it was demonstrated that the position of substituents significantly influenced the selectivity as shown with 3-chlorophenyl derivative 63b obtained in 96% ee whereas 4-chlorophenyl derivative 63d was isolated in only 75% ee. Moreover, cycloaddition products 63f-g with 2-naphthyl and iso-propyl pendants were synthesized in high 93% and 75% yields, respectively, albeit with a dramatic drop in ees (31% and 18% ees, respectively), probably due to steric and electronic issues. The scope of 3-isothiocyanato oxindoles 62 has also been surveyed showing that variations of R2 and R3 groups were well-tolerated.
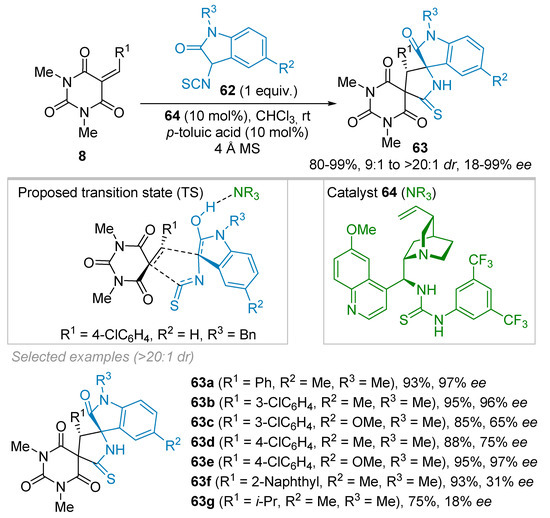
Scheme 16.
Organocatalytic synthesis of dispirobarbiturates from 3-isothiocyanato oxindoles and alkylidenes barbituric acid.
In 2018, the Albrecht group tackled the first use of α-substituted α-amino acid-based isothiocyanates 65 in the annulation reaction with alkylidene barbituric acids 8 leading to the spiranic 2-pyrrolidinethiones 66 (Scheme 17) [40]. The original idea was to combine barbituric acid and quaternary α-amino acid, which are both known as bio-relevant architectures. After the screening of solvents and organocatalysts, the best reaction conditions made use of 9-epi-quinine-squaramide 28b (20 mol%) in toluene, leading to 66a in 95% conversion and 94% ee (Scheme 17b). With their optimized conditions in hand, the authors first studied the scope of the reaction from the alkylidene barbiturates point of view. First of all, the substitution pattern of the aromatic part of the benzylidene barbituric acid 8 (R1 = Ar) had almost no influence on the reaction outcome, and excellent ees were generally obtained independently of the nature or the position of the substituents on the aromatic ring. Interestingly, the alkylidene barbituric acids 8 (R1 = i-Pr) also yielded the corresponding product 66e with excellent 92% ee but at the expense of a lower isolated yield of 40%. To account for the high level of stereoinduction observed, the authors proposed a transition state where the catalyst binds to the alkylidene barbituric acid through hydrogen bonding thanks to the thiourea moiety while the tertiary amine part deprotonates the isothiocyanate 65 (Scheme 17c).
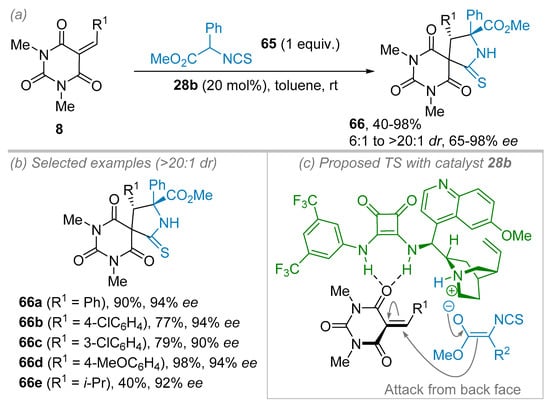
Scheme 17.
Enantioselective synthesis of spiranic 2-pyrrolidinethiones from amino acid isothiocyanate derivatives and alkylidene barbituric acids. (a) General conditions. (b) Scope and limitations. (c) Proposed transition state.
Eventually, the Albrecht group envisioned the use of isothiocyanato α-aminophosphonates 67 for the synthesis of spirobarbiturates 68 (Scheme 18) [40]. By switching the solvent from toluene to THF, the diphenyl derivative 68a was obtained in 99% yield and 98% ee. Albrecht and co-workers then modified the R1 substituent. Various aryl substituted moieties were tolerated without showing a significant impact on the enantiomeric excesses of the reaction, as exemplified with 3-ClC6H4 and 2-ClC6H4 derivatives 68b–68c together with 4-MeOC6H4 derivative 68d providing almost the same ees (92%, 96% and 98%, respectively). Finally, the same trend was observed for the R2 substituents on isothiocyanate nucleophile 67 as underlined by the excellent level of enantioselection obtained either for the 4-ClC6H4 derivative 68e or the 4-MeOC6H4 derivative 68f (96% ee and 94% ee, respectively).
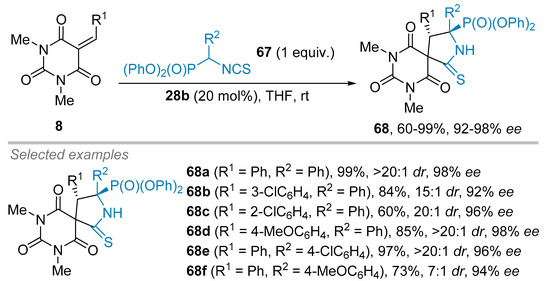
Scheme 18.
Enantioselective spirobarbiturates synthesis from phosphonate isothiocyanate derivatives.
MBH adducts have also been used in (3+2) cycloaddition reaction with alkylidenes barbituric acids 8 as dipolarophile partners. In this reaction, the phosphine catalyst reacts with the MBH adducts 69 to furnish a transient 1,3-dipole giving rise to a (3+2) cycloaddition with the alkylidene barbiturate 8 (Scheme 15b and Scheme 19) [41]. In this vein, Guo et al. described in 2016 the first enantioselective phosphine-catalyzed (3+2) annulation of alkylidene barbiturates 8 with MBH adducts 69 leading to spirobarbiturates 70 in excellent diastereoselectivities (>20:1 dr) and high to excellent enantiomeric excesses of 81 to 99% ees (Scheme 19) [41]. Optimization of the catalyst showed that bifunctional phosphine catalyst 71 afforded the best balance between yield and ee of product 70a for instance (76% yield and 93% ee in PhCF3). It was also noted that the addition of molecular sieves along with 20 mol% of K2CO3 increased the yield of 70a from 76 to 85% while ee reached 96%. It is believed that K2CO3 facilitates the formation of the reactive phosphonium zwitterion (See Scheme 15b). Thereafter, the scope of benzylidene barbituric acids 8 was studied, and it was found that aromatic substituents were, in general, well tolerated and gave rise to the formation of products 70 with 60–98% yields and 91–99% ee. However, the use of a cyclohexyl moiety (R1 = cyclohexyl) on the corresponding alkylidene barbituric acid 70d instead of an aromatic ring decreased the yield of product 70d significantly to 30%, while still affording 81% ee. Guo et al. also showed that the MBH precursors 69 including aromatic substituents (R2 = Ar) furnished a wide range of cycloaddition products 70 in excellent yields (64–99%) and enantioselectivities (85–93% ee), independently of the electronic properties of the substituent on the phenyl ring. However, one should note that the yield decreases when R2 is 3-ClC6H4 (70g, 64%) and that the presence of an aliphatic group (either a cyclohexyl for 70h or an iso-propyl for 70i) on the MBH adduct completely inhibits the reaction.
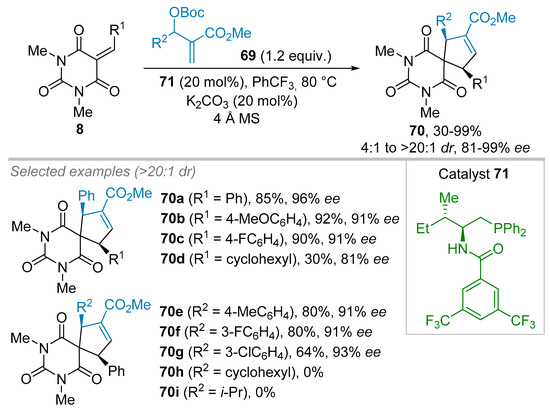
Scheme 19.
(3+2) Cycloaddition between Morita-Baylis-Hillman adducts and alkylidene barbiturates.
In 2017, Guo and colleagues described a (3+2) cycloaddition with alkylidene barbituric acids involving an ynone as dipole precursor (see Scheme 15c) which upon reaction with the phosphine catalyst provides the reactive 1,3-dipole [42]. In their publication, the authors only provided one example of an asymmetric (3+2) cycloaddition between ynone 72 and benzylidene barbituric acid 8b using the chiral phosphine catalyst 71 (Scheme 20). The expected product 73 was, thus, obtained in 49% yield and 40% ee [42]. Remarkably, during the screening of the reaction parameters in racemic fashion, the authors found that using Na2CO3 as a Brønsted base additive (20 mol%) allowed the formation of the expected (3+2) cycloaddition adduct 73 along with equal amount of the (4+2) cycloaddition product 74.
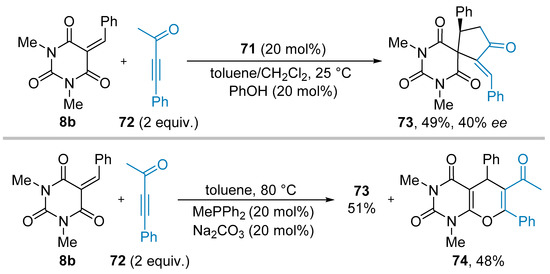
Scheme 20.
Phosphine-catalyzed (3+2) cycloaddition between ynones and alkylidene barbituric acids.
Guo’s team is not the only research group to have studied (4+2) cycloadditions involving alkylidene barbituric acids. Others have published their work on asymmetric (4+2) cycloadditions, the details of which will follow in the next part of this Section.
3.2. (4+2) Cycloaddition Reactions
Enantioselective Diels–Alder reaction involving alkylidene dienophile intermediates, such as 78a, derived from barbituric acid 6, was first tackled by Tolstikov and colleagues in 2009 (Scheme 21a,b) [43]. Indeed, several chiral Diels–Alder adducts 77 were obtained using cyclic sulfone 75 as diene in the presence of chiral amino-acid or amino-alcohol organocatalysts. While several enantioselective examples (based upon optical rotation measurement) were reported in the publication, the enantiomeric excesses were only provided for the product 77a. Accordingly, several chiral catalysts were evaluated for the reaction between cyclic sulfone 75 and benzylidene 78a, generated in situ from thiobarbituric acid 6 and 2-methoxybenzaldehyde 76a in dioxane-water (15:3 v/v) at 130 °C to afford the spiranic adduct 77a (Scheme 21c). Whereas (S)-prolinol provided the cycloaddition product 77a under a racemic form as a 5:1 mixture of diastereoisomer, the use of L-4-(tert-butyldimethylsiloxy)-proline (L-4-(OTBDMS)-proline) or (-)-ephedrine resulted in a spectacular improvement of the enantiomeric excesses (up to 80% ee and 86% ee, respectively) while affording acceptable isolated yields (68 and 60%, respectively) in 77a, which was obtained as a single stereoisomer. The absolute configuration of the cycloadduct 77a was ascertained by X-ray diffraction analysis.
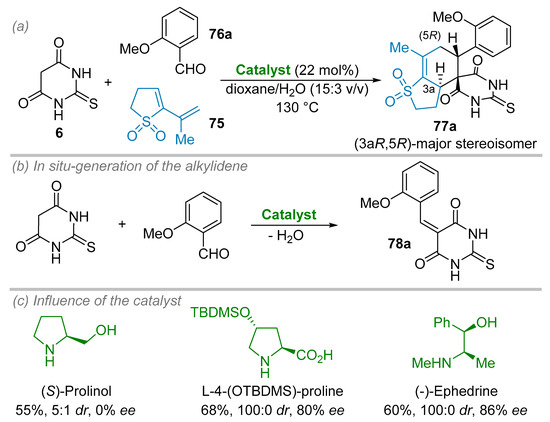
Scheme 21.
Enantioselective Diels–Alder reaction involving in situ-generated alkylidene barbituric acid as dienophile. (a) General conditions. (b) Formation of the alkylidene barbituric acid intermediate. (c) Catalysts evaluation.
In 2016, Guo, Zhou and co-workers have reported on a (4+2) annulation reaction between alkylidene barbituric acid 8, 9 or 79 and allenoates 80 in the presence of a chiral phosphine 81 as a nucleophilic organocatalyst. Chiral spirocycloalkenes 82 were, thus, provided even though architectures are difficult to obtain through regular Diels–Alder reaction (Scheme 22) [44]. Among all the conditions and chiral phosphines tested, the ones displaying a spiranic architecture and more particularly 81, previously reported by the same authors, gave the best level of diastereo-, enantioselectivity and yields providing that 4 Å molecular sieves (MS) were used as an additive (Scheme 22b). The organocatalyst 81 (20 mol%) was elected to study the scope and limitations of this methodology (4 Å MS, toluene, 60 °C). Both substitutions on allenoate 80 and alkylidene 8 partners were evaluated. Generally speaking, excellent ees (above 90%) along with high isolated yields were obtained for a wide range of functional groups covering simple hydrogen atom to diversely substituted aromatic or heteroaromatic rings. Even other alkylidene barbituric acid derivatives 79 and 9 possessing an N-N′-diethyl barbituric acid (R2 = Et, X = O) or N-N′-dimethyl thiobarbituric acid (R2 = Me, X = S) moiety, respectively, were successfully engaged providing also excellent enantiomeric excesses (97% ee and 95% ee, respectively). To underline the synthetic utility of the spirocyclohexene products 82, the authors performed two chemical transformations starting from enantioenriched 82a (R1 = Ph, R2 = Me, R3 = Ph, X = O, 94% ee, Scheme 22c). These encompassed an epoxidation of the double bond of the cyclohexene part to give 83a (87%, 94% ee) and a nitration reaction of the aromatic ring to furnish 84a (78%, 94% ee) without racemization event.
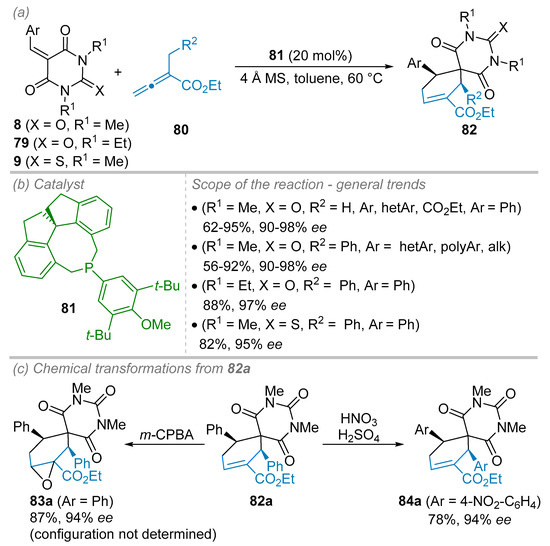
Scheme 22.
Enantioselective (4+2) Cycloaddition Reaction. (a) General conditions. (b) Scope and limitations. (c) Synthetic transformations.
Another application of alkylidene barbituric acids in (4+2) cycloaddition reaction was reported recently by Zhao and colleagues during the synthesis of barbiturate-fused spirotetrahydroquinolines 87 from vinyl benzoxazinanones 86 in the presence of chiral palladium(0)-ligand complex (Scheme 23) [45]. Thus, a chiral ligand (20 mol%) derived from BINOL in association with Pd2(dba)3·CHCl3 (5 mol%) as Pd(0) source in a 4:1 ratio in CH2Cl2 at room temperature were selected as the best conditions to obtain optimal levels of ee and yields (Scheme 23b,c). By applying the above-mentioned conditions, the scope and limitations of the reaction were investigated. By using simple N-Ts lactam 86a (R3 = R5 = H), high level of ee ranging from 89% to 96% was observed for a wide range of mono- or poly-substituted phenyl rings and hetero- or poly-aromatic rings along with variable isolated yields (45–89%). Of note, no reaction was detected with benzylidene barbituric acid having NH moiety (85a, R1 = Ph, R2 = H) or alkylidene barbituric acid having an NR moiety (8c, R1 = i-Pr, R2 = Me). The substitution of the vinyl benzoxazinanone partner 86 was also possible providing that R5 substituent remains a hydrogen atom and R4 substituent a tosyl group. In this case, however, somewhat lower ees were obtained (35–96% ee). Finally, the best results in terms of ee were obtained by introducing substituents on both aromatic parts of the benzylidene barbituric acid 85 and vinyl benzoxazinanones 86, thus, providing the corresponding spirotetrahydroquinolines 87 in up to 97% ee. It should be mentioned that all the spirotetrahydroquinolines 87 were obtained as a single diastereoisomer where vinyl and R1 group exhibited a cis relationship. To account for this excellent diastereoselectivity, the authors proposed a plausible reaction intermediate where Ts and R1 groups are pseudo-trans to each other to avoid a steric clash in the intermediate resulted from a reversible aza-Michael addition of 88 to 85 or 8c (Scheme 23d). Then, the intramolecular addition of the carbanion of the barbiturate occurs at the Si-face of the π-allyl-palladium-ligand complex to provide the cis-adduct. This cyclisation step was proposed as the rate-limiting and enantio-determining step.
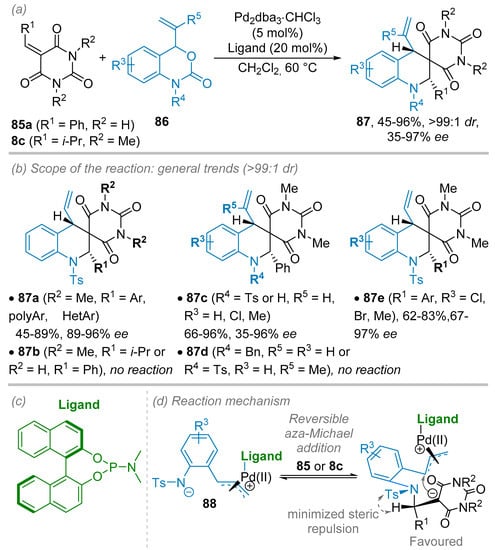
Scheme 23.
Enantioselective palladium-catalyzed (4+2) cycloaddition reaction between vinyl benzoxazinanones and benzylidene N,N′-dimethyl barbituric acids. (a) General conditions. (b) Scope and limitations. (c) Ligand structure. (d) Proposed reaction mechanism.
In 2017, the Myrboh group studied the multi-component reaction involving aromatic aldehydes 76, 1,3-cyclohexanediones 89 and barbituric acid 1 catalyzed by simple L-proline catalyst (15 mol%) to afford chromano[2,3-d]pyrimidine-triones 90 in high yields (70–96%) and low to excellent enantiomeric excesses (8–96% ee, Scheme 24) [46]. The strength of this methodology relies on the user- and the eco-friendly protocol. Indeed, water was used as a solvent at room temperature and pure products 90 were obtained after a simple filtration and recrystallization in ethanol. With these conditions in hand, the authors studied the scope and limitations of this reaction (Scheme 24c). Regarding the 1,3-dione partners, both dimedone (3, R2 = Me) or 1,3-cyclohexanedione (89, R2 = H) were tested; the latter providing slightly better results in terms of ee. Nevertheless, this gem-dimethyl substituent seems to have a strong, erratic impact on the stereoinduction, but this phenomenon was not explained. For example, 4-fluoro benzaldehyde gave excellent results (96% yield, 96% ee) when reacting with 1,3-cyclohexanedione 89 whereas its reaction with dimedone 3 resulted in a dramatic drop in efficacy (80% yield, 30% ee). The opposite trend could be observed when 4-methyl benzaldehyde was used (88% yield, 72% ee with dimedone vs 75% yield, 8% ee with 1,3-cyclohexanedione). Regarding the aldehyde partner 76, most successful examples involve aromatic aldehydes (substituted by electron-withdrawing or -donating groups at different positions) tested while only two examples in the aliphatic or heteroaromatic series were reported with high ee (propanal, 96% ee and 1-naphthaldehyde, 92% ee, respectively). The authors proposed a mechanism where the first step is the formation of the alkylidene barbituric acid 85 catalyzed by proline (Scheme 24b). These Michael acceptor species then react with the enamine formed in situ between the catalyst and the 1,3-diketones 3 or 89 to give the intermediate 91 that cyclizes to provide the desired chromano[2,3-d]pyrimidine-triones 90 thanks to an oxa-Michael addition along with the regeneration of the catalyst.
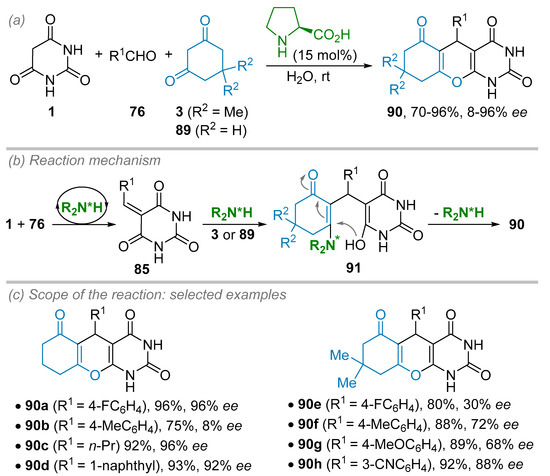
Scheme 24.
(S)-Proline-catalyzed multicomponent synthesis of chromano [2,3-d]pyrimidine-triones from aldehydes, 1,3-cyclohexanediones and barbituric acid. (a) General conditions. (b) Proposed reaction mechanism. (c) Scope and limitations.
3.3. Other Cycloaddition Reactions
Guo and colleagues recently tackled the challenging higher-order cycloaddition reactions both in racemic and enantioselective fashion starting from a heptafulvene barbituric acid platform 92 and functionalized allenoates 80 (Scheme 25) [47]. Upon phosphine catalysis a formal (8+2) cycloaddition process occurred to furnish the corresponding bicyclo [5.3.0] decanes 93 with excellent ees ranging from 86% to 97%. The authors observed that a rather activated ester moiety on allenoate 80 was required to secure a good reactivity with sterically hindered chiral phosphine catalyst 94. Thus, for examples, the arylic esters 93a–93d were obtained with high stereoinduction (86–92% ee) and isolated yields ranging from 61% to 65%, together with derivatives 93e–93f having either a fluorinated (93e, 92% ee) or chlorinated (93f, 97% ee) alkyl substituents on the ester moiety.
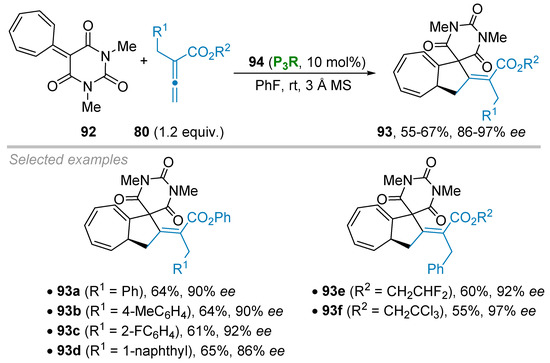
Scheme 25.
Phosphine-catalyzed (8+2) annulation of barbituric acid heptafulvenes.
Actually, a screening of chiral phosphines revealed that the best results were achieved using Kwon’s endo phosphine 94 (Scheme 26). Regarding the mechanism, the authors proposed a formal (8+2) cycloaddition triggered by the addition reaction of the phosphine organocatalyst 94 to the allenoate 80 to form the phosphonium betaine intermediate 95. Then, an addition reaction takes place to the heptafulvene barbituric acid 92 to give 96, giving rise to a cyclization process that leads to the transient cyclopentane 97. This charged intermediate undergoes the final elimination event allowing the formation of product 93 along with the concomitant regeneration of phosphine organocatalyst 94.
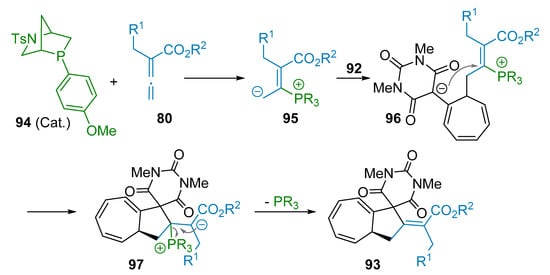
Scheme 26.
Proposed mechanism of the (8+2) Annulation reaction.
4. Conclusions
Despite the long-standing interest in barbituric acid derivatives as useful building blocks for the elaboration of both bio-relevant products or architectures having interesting properties in material science, the enantioselective catalytic synthesis of chiral non-racemic barbiturates-derived products slowly emerged in the 1990s. The marked Brønsted acidity of barbituric acid derivatives or electrophilicity of alkylidene counterparts have required the development of specific catalysts or catalytic approaches to manage these unique properties. Furthermore, as far as non-symmetrical barbituric acid scaffolds are concerned, the challenging stereo-differentiation of rather similar functionality on both nitrogen atoms required ingenious methodological approaches to be carried out. Nonetheless, both metal- and organic catalysts are currently able to engage barbituric derivatives into highly enantioselective addition and cycloaddition reactions to provide original chiral linear, fused, and spiro-bicyclic architectures. The panel of enantioselective reactions carried out on barbituric acids remains, however, rather limited and many opportunities to develop new processes still exist. By giving an overview of the state-of-the-art of catalytic enantioselective transformations, we hope this review will provide a springboard to elicit new developments in this field of research.
Author Contributions
writing—original draft preparation, C.S., A.L., V.L., S.O., J.-F.B.; supervision, J.-F.B.
Acknowledgements
This work has been partially supported by INSA Rouen, Rouen University, CNRS, EFRD, Labex SynOrg (ANR-11-LABX-0029) and region Normandie (CRUNCh network). This work was also supported by the French National Research Agency (ANR) as part of the ANR-16-CE07-0011-01 project.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BINOL | 1,1′-Bi-2-naphthol |
| DMSO | dimethyl sulfoxide |
| HPLC | High Performance Liquid Chromatography |
| THF | tetrahydrofuran |
| TS | Transition state |
References
- Baeyer, A. Mittheilungen aus dem organischen Laboratorium des Gewerbeinstitutes in Berlin: Untersuchungen über die Harnsäuregruppe. Justus Liebigs Ann. Chem. 1864, 130, 129–175. [Google Scholar] [CrossRef]
- Fischer, E.; von, M. New class of narcotics. Ther. Ggw. 1903, 44, 97–101. [Google Scholar]
- Moussier, N.; Bruche, L.; Viani, F.; Zanda, M. Fluorinated Barbituric Acid Derivatives: Synthesis and Bio-activity. Curr. Org. Chem. 2003, 7, 1071–1080. [Google Scholar] [CrossRef]
- Bojarski, J.T.; Mokrosz, J.L.; Barton, H.J.; Paluchowska, M.H. Recent progress in barbituric acid chemistry. Adv. Heterocycl. Chem. 1985, 38, 229–297. [Google Scholar] [CrossRef]
- Sans, R.G.; Chozas, M.G. Historical aspects and applications of barbituric acid derivatives. A review. Pharmazie 1988, 43, 827–829. [Google Scholar]
- Nikoofar, K.; Khademi, Z. Barbituric Acids in Organic Transformations, An Outlook to the Reaction Media. Mini-Rev. Org. Chem. 2017, 14, 143–173. [Google Scholar] [CrossRef]
- Bialer, M. How did phenobarbital’s chemical structure affect the development of subsequent antiepileptic drugs (AEDs)? Epilepsia 2012, 53, 3–11. [Google Scholar] [CrossRef]
- Renard, A.; Lhomme, J.; Kotera, M. Synthesis and Properties of Spiro Nucleosides Containing the Barbituric Acid Moiety. J. Org. Chem. 2002, 67, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Pudzianowski, A.T.; Leavitt, K.J.; Barbosa, J.; McDonnell, P.A.; Metzler, W.J.; Rankin, B.M.; Liu, R.; Vaccaro, W.; Pitts, W. Structure-based design of potent and selective inhibitors of collagenase-3 (MMP-13). Bioorg. Med. Chem. Lett. 2005, 15, 1101–1106. [Google Scholar] [CrossRef]
- Fessenden, R.J.; Larsen, J.G.; Coon, M.D.; Fessenden, J.S. Silicon Heterocyclic Compounds. III. Silicon-Substituted Spirobarbiturates. J. Med. Chem. 1964, 7, 695–698. [Google Scholar] [CrossRef]
- Duan, J.J.W.; Chen, L.; Lu, Z.; Jiang, B.; Asakawa, N.; Sheppeck, J.E.; Liu, R.-Q.; Covington, M.B.; Pitts, W.; Kim, S.-H.; et al. Discovery of low nanomolar non-hydroxamate inhibitors of tumor necrosis factor-α converting enzyme (TACE). Bioorg. Med. Chem. Lett. 2007, 17, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Ingle, V.N.; Gaidhane, P.K.; Dutta, S.S.; Naha, P.P.; Sengupta, M.S. Synthesis of Novel Galactopyranosyl-Derived Spiro Barbiturates. J. Carbohydr. Chem. 2006, 25, 661–671. [Google Scholar] [CrossRef]
- Hese, S.V.; Meshram, R.J.; Kamble, R.D.; Mogle, P.P.; Patil, K.K.; Kamble, S.S. Antidiabetic and allied biochemical roles of new chromeno-pyrano pyrimidine compounds: Synthesis, in vitro and in silico analysis. Med. Chem. Res. 2017, 26, 805–818. [Google Scholar] [CrossRef]
- Kalaria, P.N.; Satasia, S.P.; Raval, D.K. Synthesis, characterization and biological screening of novel 5-imidazopyrazole incorporated fused pyran motifs under microwave irradiation. New J. Chem. 2014, 38, 1512–1521. [Google Scholar] [CrossRef]
- Gomes, R.F.A.; Coelho, J.A.S.; Afonso, C.A.M. Synthesis and Applications of Stenhouse Salts and Derivatives. Chem. Eur. J. 2018, 24, 9170–9186. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Ziarani, G.; Aleali, F.; Lashgari, N. Recent applications of barbituric acid in multicomponent reactions. RSC Adv. 2016, 6, 50895–50922. [Google Scholar] [CrossRef]
- Schade, A.; Schreiter, K.; Rüffer, T.; Lang, H.; Spange, S. Interactions of Enolizable Barbiturate Dyes. Chem. Eur. J. 2016, 22, 5734–5748. [Google Scholar] [CrossRef] [PubMed]
- Shaker, R.M.; Ishak, E.A. Barbituric acid utility in multicomponent reactions. Z. Naturforsch. B J. Chem. Sci. 2011, 66, 1189–1201. [Google Scholar] [CrossRef]
- Bojarski, J. Chiral barbiturates: Synthesis, chromatographic resolutions, and biological activity. Chem. Anal. (New York) 1997, 142, 201–234. [Google Scholar]
- Brunner, H.; Deml, I.; Dirnberger, W.; Nuber, B.; Reißer, W. Enantioselective Palladium-Catalysed Allylation of 1,5-Dimethylbarbituric Acid. Eur. J. Inorg. Chem. 1998. [Google Scholar] [CrossRef]
- Brunner, H.; Fürst, J. Enantioselective allylation of 1,5-dimethylbarbituric acid with Pd catalysts and new optically active PN ligands. Inorg. Chim. Acta 1994, 220, 63–66. [Google Scholar] [CrossRef]
- Brunner, H.; Deml, I.; Dirnberger, W.; Ittner, K.-P.; Reißer, W.; Zimmermann, M. Synthesis of the Stereoisomers of Methohexital by Palladium-Catalyzed Allylation. Eur. J. Inorg. Chem. 1999. [Google Scholar] [CrossRef]
- Brunner, H.; Rückert, T. Enantioselektive Katalyse, 121. Mitt. [1]: Chirale Phosphanliganden mit zusätzlichen Sauerstoffunktionalitäten. Monatshefte Chem./Chem. Mon. 1998, 129, 339–354. [Google Scholar] [CrossRef]
- Bordwell, F.G. Equilibrium acidities in dimethyl sulfoxide solution. Acc. Chem. Res. 1988, 21, 456–463. [Google Scholar] [CrossRef]
- Pair, E.; Cadart, T.; Levacher, V.; Brière, J.-F. Meldrum’s Acid: A Useful Platform in Asymmetric Organocatalysis. ChemCatChem 2016, 8, 1882–1890. [Google Scholar] [CrossRef]
- Dumas, A.M.; Fillion, E. Meldrum’s Acids and 5-Alkylidene Meldrum’s Acids in Catalytic Carbon−Carbon Bond-Forming Processes. Acc. Chem. Res. 2009, 43, 440–454. [Google Scholar] [CrossRef]
- Bug, T.; Mayr, H. Nucleophilic Reactivities of Carbanions in Water: The Unique Behavior of the Malodinitrile Anion. J. Am. Chem. Soc. 2003, 125, 12980–12986. [Google Scholar] [CrossRef]
- Schade, A.; Tchernook, I.; Bauer, M.; Oehlke, A.; Breugst, M.; Friedrich, J.; Spange, S. Kinetics of Electrophilic Alkylations of Barbiturate and Thiobarbiturate Anions. J. Org. Chem. 2017, 82, 8476–8488. [Google Scholar] [CrossRef]
- Seeliger, F.; Berger, S.T.A.; Remennikov, G.Y.; Polborn, K.; Mayr, H. Electrophilicity of 5-Benzylidene-1,3-dimethylbarbituric and -thiobarbituric Acids. J. Org. Chem. 2007, 72, 9170–9180. [Google Scholar] [CrossRef]
- Trost, B.M.; Schroeder, G.M. Palladium-Catalyzed Asymmetric Allylic Alkylation of Barbituric Acid Derivatives: Enantioselective Syntheses of Cyclopentobarbital and Pentobarbital. J. Org. Chem. 2000, 65, 1569–1573. [Google Scholar] [CrossRef]
- del Pozo, S.; Vera, S.; Oiarbide, M.; Palomo, C. Catalytic Asymmetric Synthesis of Quaternary Barbituric Acids. J. Am. Chem. Soc. 2017, 139, 15308–15311. [Google Scholar] [CrossRef]
- Trost, B.M.; Van Vranken, D.L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422. [Google Scholar] [CrossRef]
- Rombola, M.; Sumaria, C.S.; Montgomery, T.D.; Rawal, V.H. Development of Chiral, Bifunctional Thiosquaramides: Enantioselective Michael Additions of Barbituric Acids to Nitroalkenes. J. Am. Chem. Soc. 2017, 139, 5297–5300. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Duan, H.-X.; Wanyan, D.-Y.; Wang, Y.-Q. Enantioselective organocatalytic Michael additions of N,N′-dialkylbarbituric acids to enones. Org. Biomol. Chem. 2017, 15, 8669–8679. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, G.; Du, Y.; Yang, Z.; Li, Y.; Chen, L. Michael–Michael Addition Reactions Promoted by Secondary Amine-Thiourea: Stereocontrolled Construction of Barbiturate-Fused Tetrahydropyrano Scaffolds and Pyranocoumarins. J. Org. Chem. 2017, 82, 13594–13601. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Zhang, J.; Yu, J.; Yan, L.; Li, Y.; Chen, L.; Yan, X. An Organocatalytic Synthesis of Chiral Pyrano[2,3-d]pyrimidines through [3 + 3] Annulation of 1,3-Dimethyl-barbituric Acid with 2-(1-Alkynyl)-2-alken-1-ones. Eur. J. Org. Chem. 2018, 2018, 347–354. [Google Scholar] [CrossRef]
- Reddy|Chidipudi, S.; Burns, D.J.; Khan, I.; Lam, H.W. Enantioselective Synthesis of Spiroindenes by Enol-Directed Rhodium(III)-Catalyzed C-H Functionalization and Spiroannulation. Angew. Chem. Int. Ed. 2015, 54, 13975–13979. [Google Scholar] [CrossRef]
- Zhao, H.-W.; Tian, T.; Li, B.; Yang, Z.; Pang, H.-L.; Meng, W.; Song, X.-Q.; Chen, X.-Q. Diastereoselective Synthesis of Dispirobarbiturates through Et3N-Catalyzed [3 + 2] Cycloaddition of Barbiturate-Based Olefins with 3-Isothiocyanato Oxindoles. J. Org. Chem. 2015, 80, 10380–10385. [Google Scholar] [CrossRef]
- Zhao, H.-W.; Tian, T.; Pang, H.-L.; Li, B.; Chen, X.-Q.; Yang, Z.; Meng, W.; Song, X.-Q.; Zhao, Y.-D.; Liu, Y.-Y. Organocatalytic [3 + 2] Cycloadditions of Barbiturate-Based Olefins with 3-Isothiocyanato Oxindoles: Highly Diastereoselective and Enantioselective Synthesis of Dispirobarbiturates. Adv. Synth. Catal. 2016, 358, 2619–2630. [Google Scholar] [CrossRef]
- Frankowski, S.; Gajda, T.; Albrecht, Ł. Isothiocyanate Strategy for the Synthesis of Quaternary α-Amino Acids Bearing a Spirocyclic Ring System. Adv. Synth. Catal. 2018, 360, 1822–1832. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, W.; Wu, Y.; Mao, B.; Gao, X.; Liu, H.; Sun, Z.; Xiao, Y.; Guo, H. Asymmetric Construction of Highly Functionalized Spirobarbiturate-Cyclopentenes through Chiral Phosphine-Catalyzed [3 + 2] Annulation of Morita–Baylis–Hillman Carbonates with Barbiturate-Derived Alkenes. Adv. Synth. Catal. 2016, 358, 2867–2872. [Google Scholar] [CrossRef]
- Gao, X.; Li, Z.; Yang, W.; Liu, Y.; Chen, W.; Zhang, C.; Zheng, L.; Guo, H. Phosphine-catalyzed [3 + 2] and [4 + 2] annulation reactions of ynones with barbiturate-derived alkenes. Org. Biomol. Chem. 2017, 15, 5298–5307. [Google Scholar] [CrossRef]
- Shul’ts, E.E.; Andreev, G.N.; Shakirov, M.M.; Komarova, N.I.; Bagryanskaya, I.Y.; Gatilov, Y.V.; Tolstikov, G.A. Diels-Alder reactions with cyclic sulfones: VIII. Organic catalysis in the synthesis of spiro[1-benzothiophene-4,5’-pyrimidine]-2’,4’,6’-trione 1,1-dioxides and 2’-thioxospiro[1-benzothiophene-4,5’-pyrimidine]-4’,6’-dione 1,1-dioxides. Russ. J. Org. Chem. 2009, 45, 87–101. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Yuan, C.; Wang, G.-P.; Zhu, S.-F.; Wu, Y.; Wang, B.; Sun, Z.; Xiao, Y.; Zhou, Q.-L.; et al. Enantioselective Synthesis of Spirobarbiturate-Cyclohexenes through Phosphine-Catalyzed Asymmetric [4 + 2] Annulation of Barbiturate-Derived Alkenes with Allenoates. Org. Lett. 2016, 18, 1302–1305. [Google Scholar] [CrossRef]
- Zhao, H.-W.; Feng, N.-N.; Guo, J.-M.; Du, J.; Ding, W.-Q.; Wang, L.-R.; Song, X.-Q. Diastereoselective and Enantioselective Synthesis of Barbiturate-Fused Spirotetrahydroquinolines via Chiral Palladium(0)/Ligand Complex Catalyzed [4 + 2] Cycloaddition of Vinyl Benzoxazinanones with Barbiturate-Based Olefins. J. Org. Chem. 2018, 83, 9291–9299. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Sutradhar, D.; Chandra, A.K.; Myrboh, B. l-proline as an efficient asymmetric induction catalyst in the synthesis of chromeno[2,3-d]pyrimidine-triones, xanthenes in water. Tetrahedron 2017, 73, 3497–3504. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, C.; Zhou, L.; Yuan, C.; Xiao, Y.; Guo, H. Phosphine-Catalyzed [8 + 2]-Annulation of Heptafulvenes with Allenoates and Its Asymmetric Variant: Construction of Bicyclo[5.3.0]decane Scaffold. Org. Lett. 2018, 20, 4302–4305. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).