CdS Photocatalysts Modified with Ag: Effect of the Solvothermal Temperature on the Structure and Photoactivity for Hydrogen Production
Abstract
1. Introduction
2. Results
2.1. Physicochemical Characterization
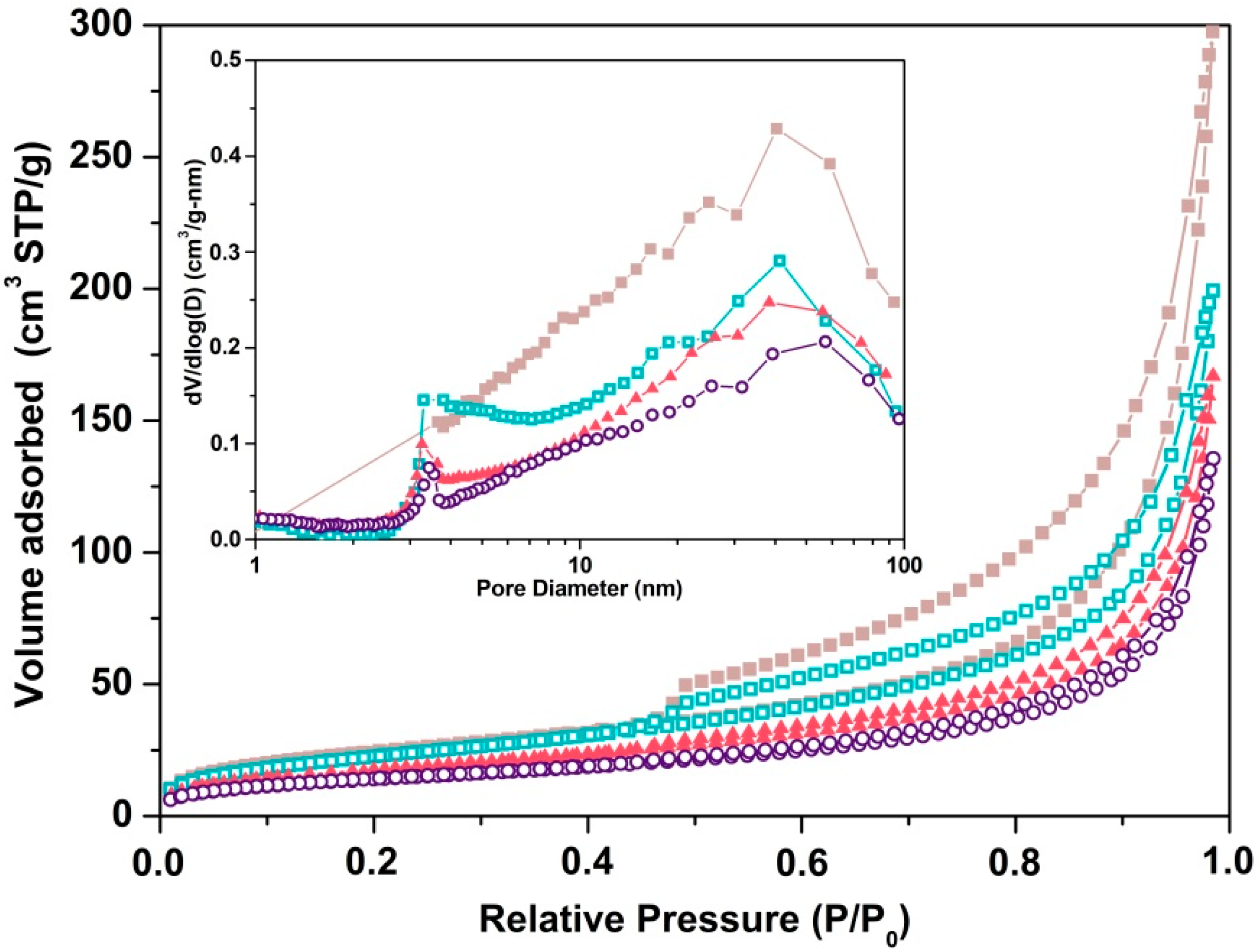
2.1.1. Chemical and Textural Analysis
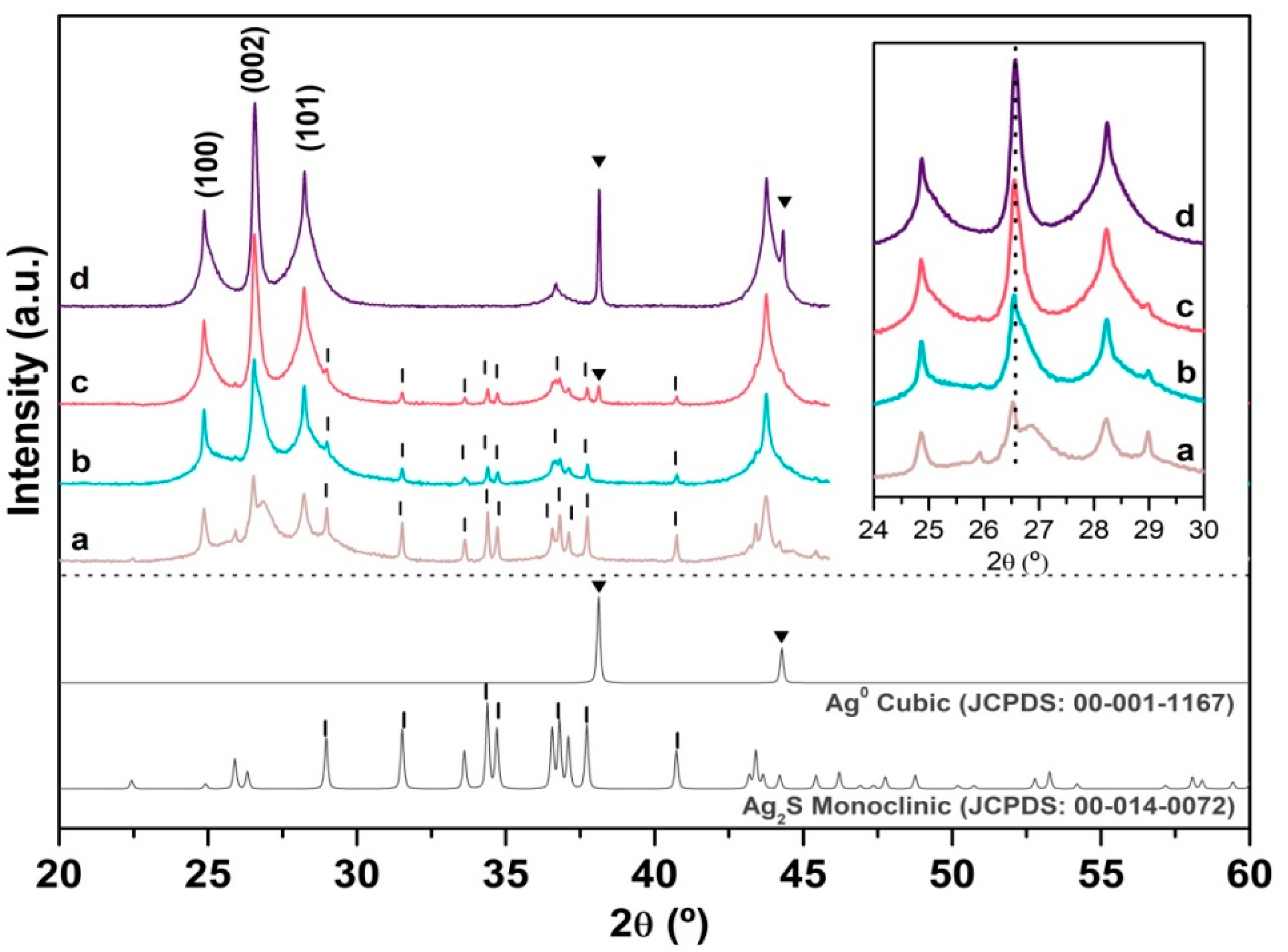
2.1.2. X-ray Diffraction (XRD)
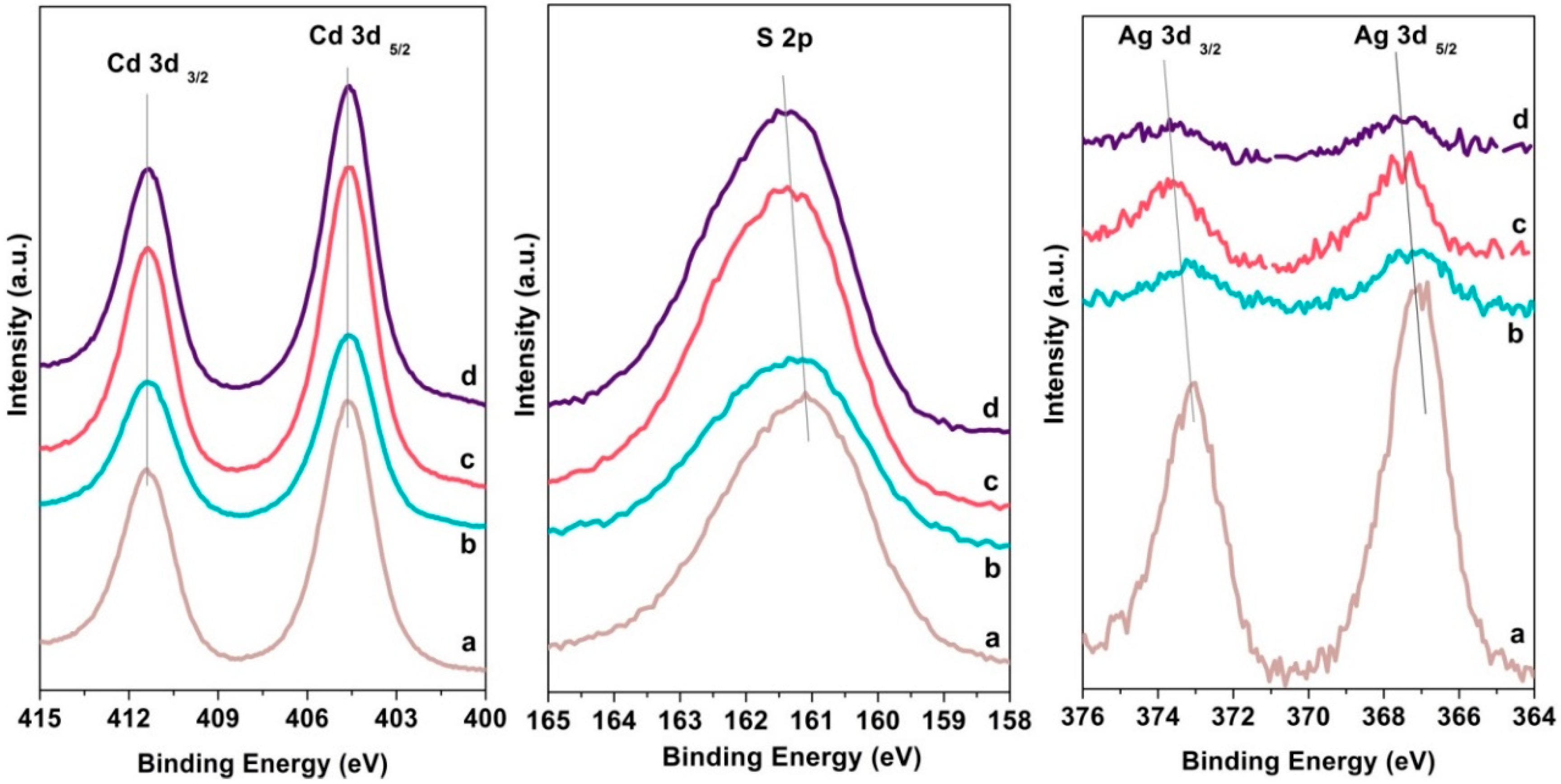
2.1.3. X-ray Photoelectron Spectroscopy (XPS)
2.1.4. UV–vis Spectroscopy (UV–vis)
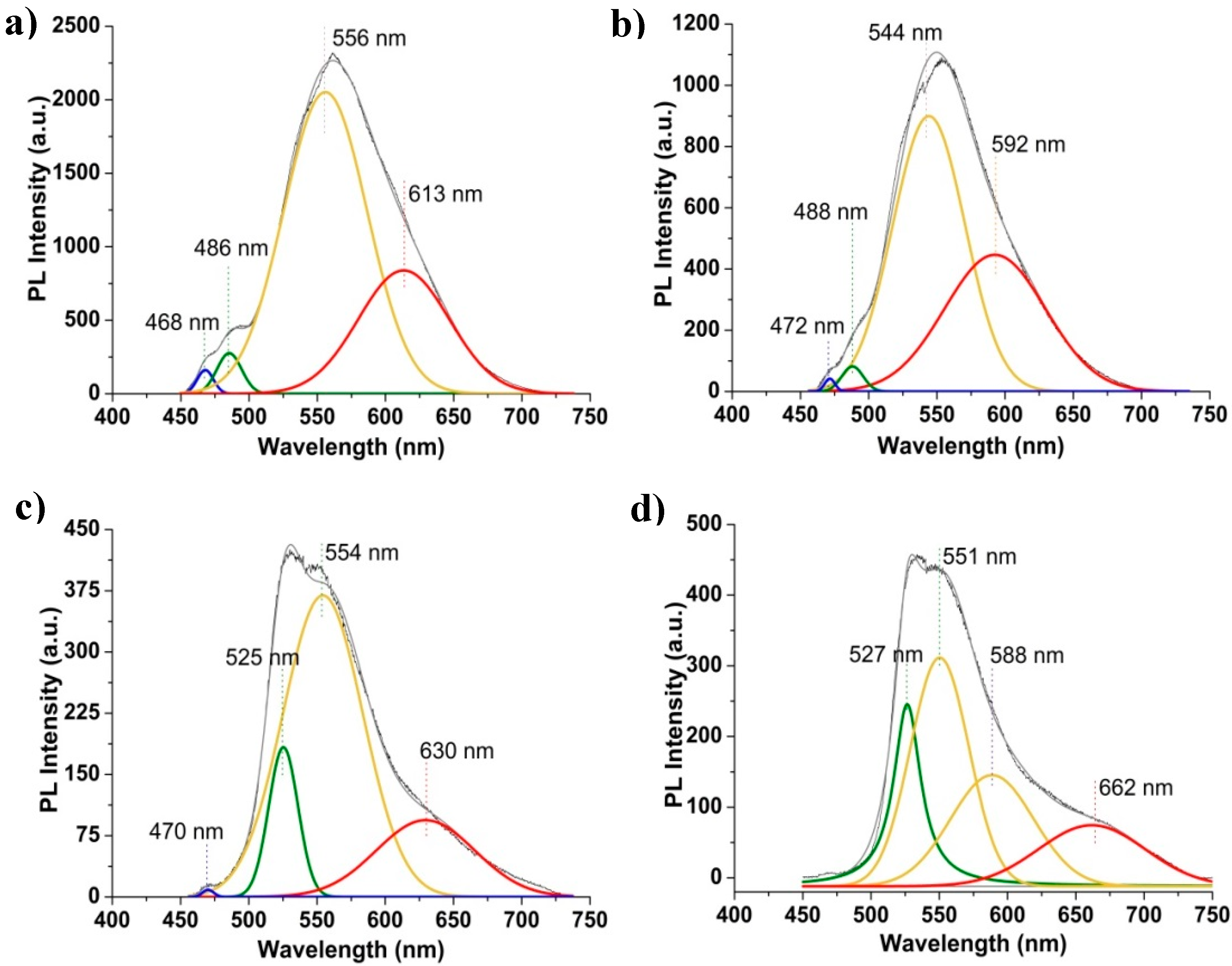
2.1.5. Photoluminescence (PL)
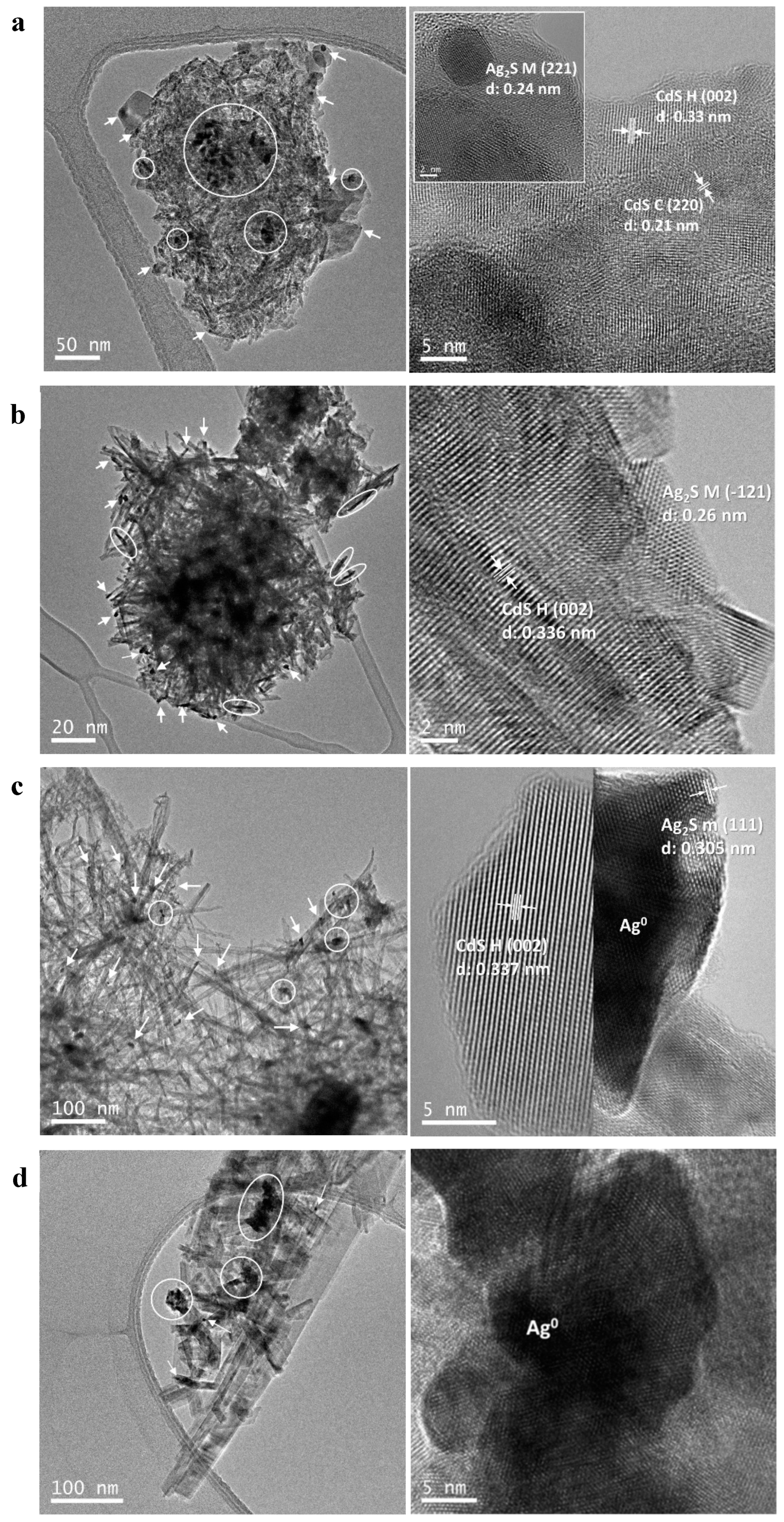
2.1.6. Transmission Electron Microscopy (TEM)
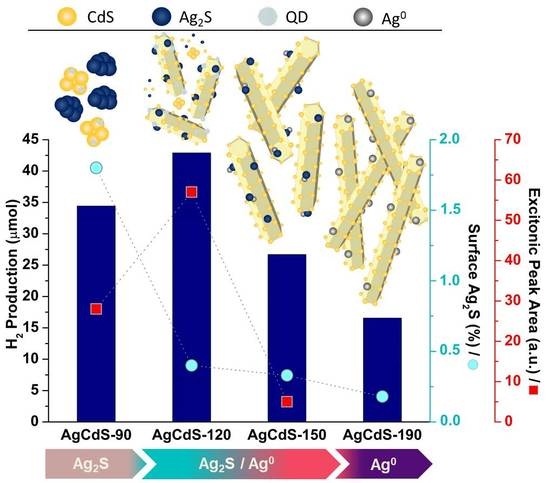
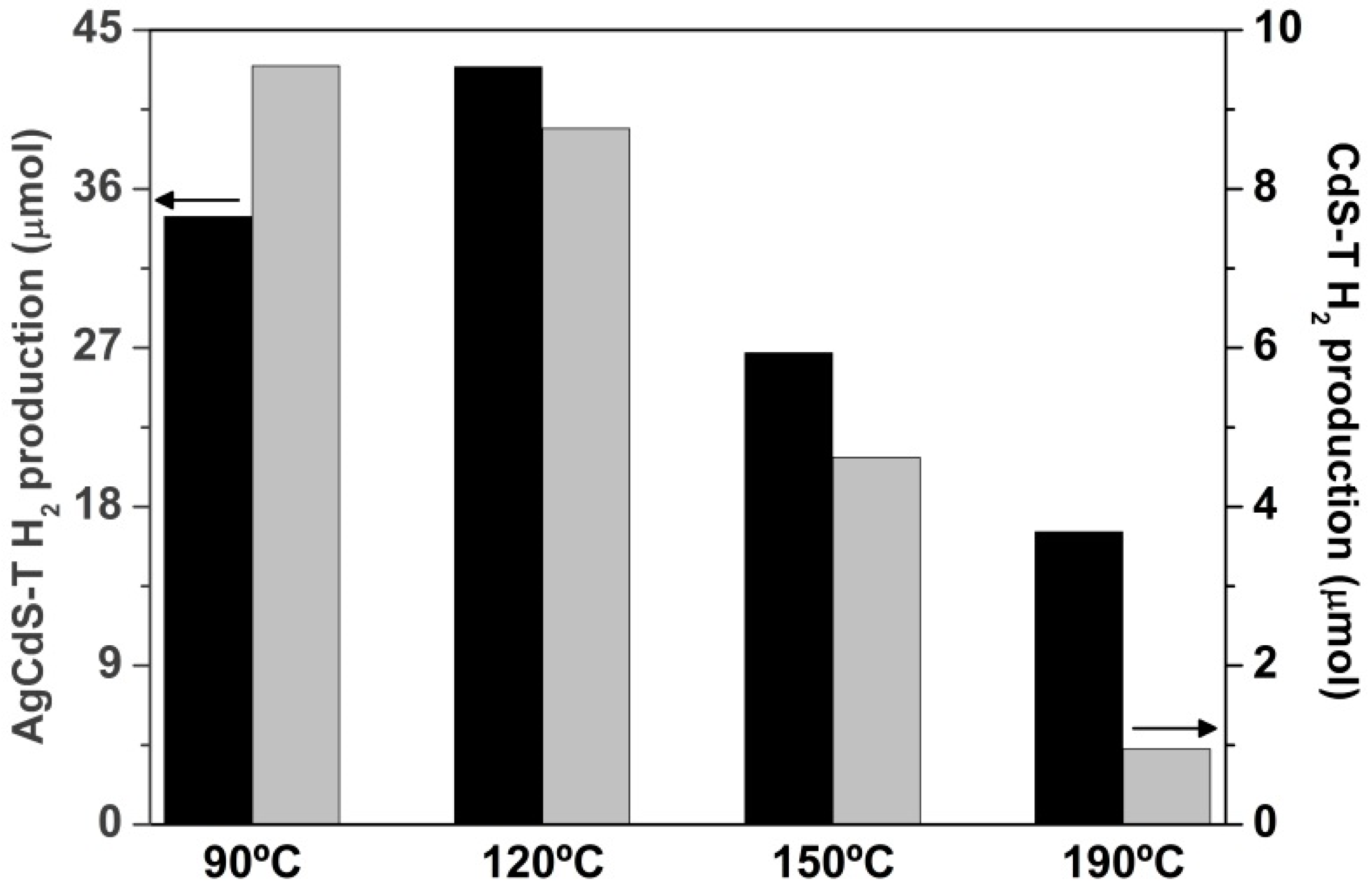
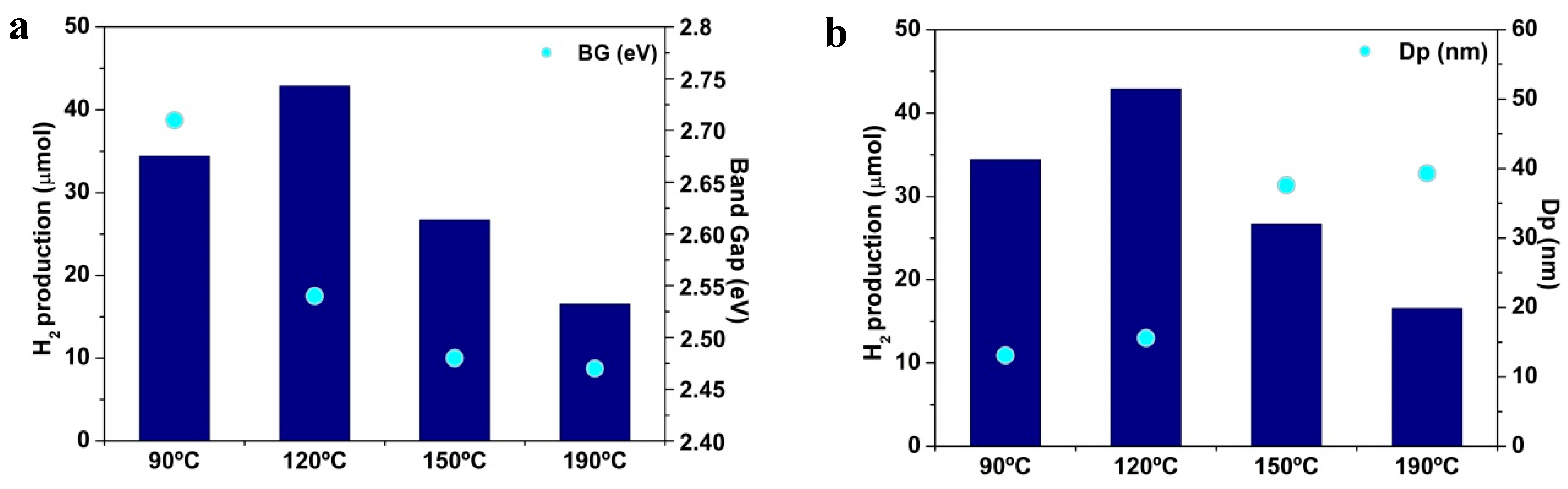
2.2. Photoactivity Tests
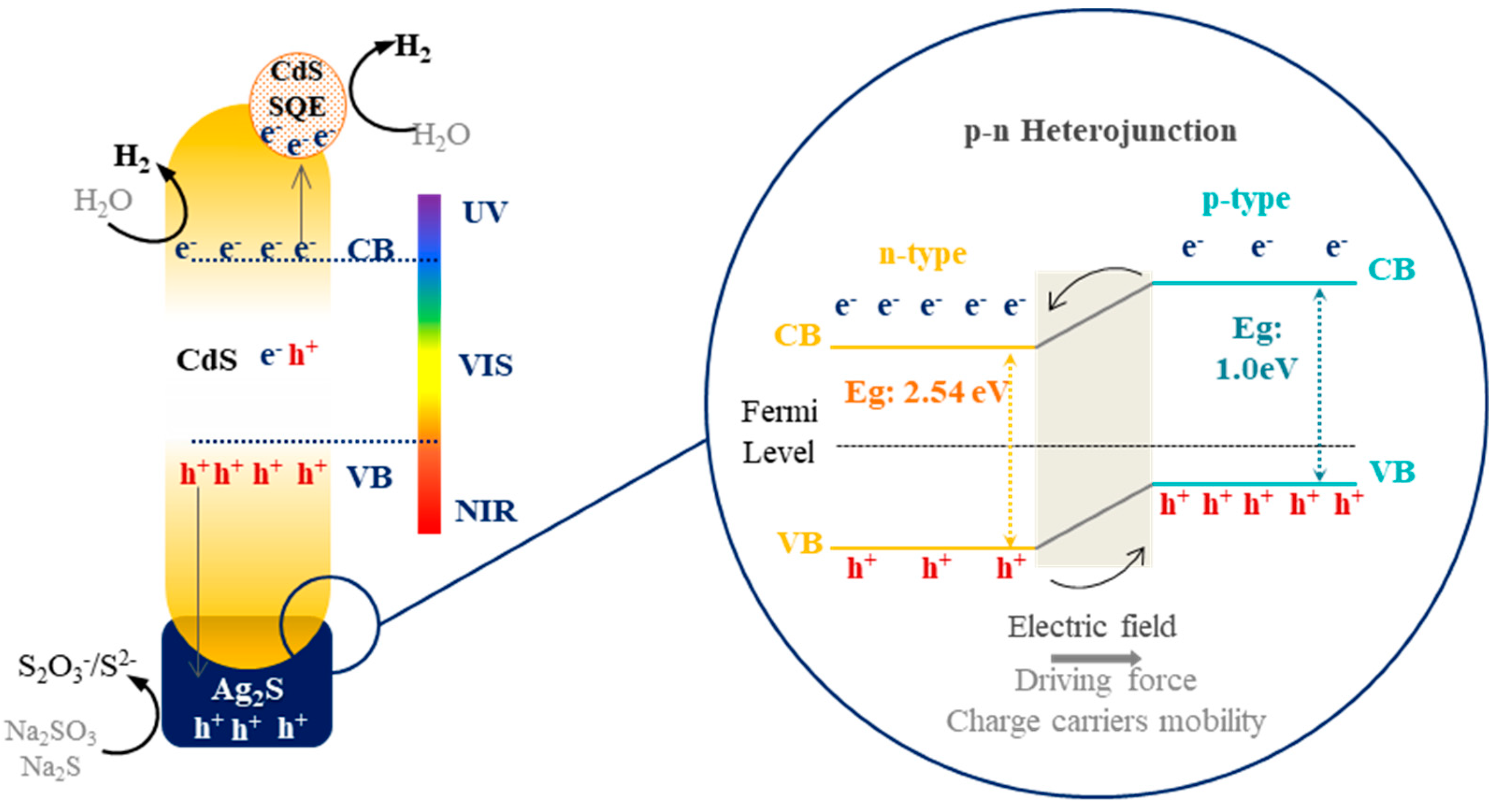
3. Discussion
4. Materials and Methods
4.1. Synthesis of Ag Modified CdS Photocatalysts
4.2. Physicochemical Characterization
4.3. Photocatalytic Activity Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kudo, A. Photocatalyst materials for water splitting. Catal. Surv. Asia 2003, 7, 31–38. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, F.; G Fierro, J.L.; Navarro Yerga, R.M. From nanorods to nanowires of CdS synthesized by a solvothermal method: Influence of the morphology on the photoactivity for hydrogen evolution from water. Molecules 2016, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, G. Growth and optical properties of wurtzite-type CdS nanocrystals. Inorg. Chem. 2006, 45, 5103–5108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Liu, W.; Shang, Y.; Zhu, A.; Tan, P.; Xiong, X.; Pan, J. Facet and morphology dependent photocatalytic hydrogen evolution with CdS nanoflowers using a novel mixed solvothermal strategy. J. Colloid Interface Sci. 2018, 513, 222–230. [Google Scholar] [CrossRef]
- Yu, J.; Yu, Y.; Zhou, P.; Xiao, W.; Cheng, B. Morphology-dependent photocatalytic H2 production activity of CdS. Appl. Catal. B Environ. 2014, 156–157, 184–191. [Google Scholar] [CrossRef]
- Zhai, T.; Fang, X.; Li, L.; Bando, Y.; Golberg, D. One-dimensional CdS nanostructures: Synthesis, properties, and applications. Nanoscale 2010, 2, 168–187. [Google Scholar] [CrossRef]
- Vaquero, F.; Navarro, R.M.; Fierro, J.L.G. Influence of the solvent on the structure, morphology and performance for H2 evolution of CdS photocatalysts prepared by solvothermal method. Appl. Catal. B Environ. 2017, 203, 753–767. [Google Scholar] [CrossRef]
- Ma, S.; Xie, J.; Wen, J.; He, K.; Li, X.; Liu, W.; Zhang, X. Constructing 2D layered hybrid CdS nanosheets/MoS2 heterojunctions for enhanced visible-light photocatalytic H2 generation. Appl. Surf. Sci. 2017, 391, 580–591. [Google Scholar] [CrossRef]
- Yan, H.; Yang, J.; Ma, G.; Wu, G.; Zong, X.; Lei, Z.; Shi, J.; Li, C. Visible-light-driven hydrogen production with extremely high quantum efficiency on Pt–PdS/CdS photocatalyst. J. Catal. 2009, 266, 165–168. [Google Scholar] [CrossRef]
- Murillo Leo, I.; Soto, E.; Vaquero, F.; Mota, N.; Navarro, R.M.; Fierro, J.L.G. Influence of the reduction of graphene oxide (rGO) on the structure and photoactivity of CdS-rGO hybrid systems. Int. J. Hydrogen Energy 2017, 42, 13691–13703. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Mohamed, M.B.; Abdel-Kader, M.H.; Alhazime, A.A.; Almarashi, J.Q.M. Effect of preparation methods and doping on the structural and tunable emissions of CdS. J. Mol. Struct. 2018, 1155, 666–674. [Google Scholar] [CrossRef]
- Del Valle, F.; Ishikawa, A.; Domen, K.; Villoria de la Mano, J.A.; Sánchez-Sánchez, M.C.; González, I.D.; Herreras, S.; Mota, N.; Rivas, M.E.; Álvarez Galván, M.C. Influence of Zn concentration in the activity of Cd1−xZnxS solid solutions for water splitting under visible light. Catal. Today 2009, 143, 51–56. [Google Scholar] [CrossRef]
- Dalvand, P.; Mohammadi, M.R.; Fray, D.J. One-dimensional cadmium sulfide (CdS) nanostructures by the solvothermal process: Controlling crystal structure and morphology aided by different solvents. Mater. Lett. 2011, 65, 1291–1294. [Google Scholar] [CrossRef]
- Bao, C.; Zhu, G.; Yang, J.; Liu, M.; Zhang, R.; Shen, X. Small molecular amine mediated synthesis of hydrophilic CdS nanorods and their photoelectrochemical water splitting performance. Dalton Trans. 2015, 44, 1465–1472. [Google Scholar] [CrossRef]
- Mahdi, M.A.; Hassan, J.J.; Ng, S.S.; Hassan, Z. Growth of CdS nanosheets and nanowires through the solvothermal method. J. Cryst. Growth 2012, 359, 43–48. [Google Scholar] [CrossRef]
- Jang, J.S.; Joshi, U.A.; Lee, J.S. Solvothermal synthesis of CdS nanowires for photocatalytic hydrogen and electricity production. J. Phys. Chem. C 2007, 111, 13280–13287. [Google Scholar] [CrossRef]
- Vaquero, F.; Navarro, R.M.; Fierro, J.L.G. Evolution of the nanostructure of CdS using solvothermal synthesis at different temperature and its influence on the photoactivity for hydrogen production. Int. J. Hydrogen Energy 2016, 41, 11558–11567. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.; Del Valle, F.; Fierro, J.L.G. Photocatalytic hydrogen evolution from CdS–ZnO–CdO systems under visible light irradiation: Effect of thermal treatment and presence of Pt and Ru Cocatalysts. Int. J. Hydrogen Energy 2008, 33, 4265–4273. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, G.; Zhang, L.; Liang, C.; Zhang, J. Catalytic growth of large-scale single crystal CdS nanowires by physical evaporation and their photoluminescence. Chem. Mater. 2002, 14, 1773–1777. [Google Scholar] [CrossRef]
- Song, L.; Li, T.; Zhang, S. Synthesis of rhodium phosphide cocatalyst and remarkably enhanced photocatalytic hydrogen evolution over CdS under visible light radiation. Chem. Eng. J. 2017, 314, 498–507. [Google Scholar] [CrossRef]
- Dweydari, A.W.; Mee, C.H.B. Work function measurements on (100) and (110) surfaces of silver. Phys. Status Solidi 1975, 27, 223–230. [Google Scholar] [CrossRef]
- Kryukov, A.I.; Stroyuk, A.L.; Zin’chuk, N.N.; Korzhak, A.V.; Kuchmii, S.Y. Optical and catalytic properties of Ag2S nanoparticles. J. Mol. Catal. A Chem. 2004, 221, 209–221. [Google Scholar] [CrossRef]
- Wang, J.; Feng, H.; Chen, K.; Fan, W.; Yang, Q. Solution-phase catalytic synthesis, characterization and growth kinetics of Ag2S-CdS matchstick-like heteronanostructures. Dalton Trans. 2014, 43, 3990–3998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xu, Z. Controllable growth of semiconductor heterostructure mediated by bifunctional Ag2S nanocrystals as catalyst or source-host. J. Am. Chem. Soc. 2011, 133, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, F.; Liu, Y.; Liu, X.; Zhang, Z.; Qian, Y. Double-dentate solvent-directed growth of multi-armed CdS nanorod-based semiconductors. Mater. Res. Bull. 2005, 40, 2180–2188. [Google Scholar] [CrossRef]
- Khorsand Zak, A.; Majid, W.H. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Moulder, F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of Standard Spectra for Identification and Interpretation of XPS Data; Perkin Elmer Corporation: Waltham, MA, USA, 1995. [Google Scholar]
- Hammond, J.S.; Gaarenstroom, S.W.; Winograd, N. X-ray Photoelectron spectroscopic studies of cadmium and silver oxygen surfaces. Anal. Chem. 1975, 47, 2193–2199. [Google Scholar] [CrossRef]
- Schön, G. Studies of Ag, Ag2O and AgO. Acta Chem. Scand. 1973, 27, 2623–2633. [Google Scholar] [CrossRef]
- Wagner, A.V.; Naumkin, A.; Kraut-Vass, J.W.; Allison, C.J.; Powell, P.; Rumble, J.R. NIST X-ray Photoelectron Spectroscopy Database; Version 3.3; NIST Standarad Reference Database: Gaithersburg, MD, USA, 2003.
- Kaushik, V.K. XPS core level spectra and auger parameters for some silver compounds. J. Electron. Spectrosc. Relat. Phenom. 1991, 56, 273–277. [Google Scholar] [CrossRef]
- Abe, T.; Kashiwaba, Y.; Baba, M.; Imai, J.; Sasaki, H. XPS analysis of p-type Cu-doped CdS thin films. Appl. Surf. Sci. 2001, 175–176, 549–554. [Google Scholar] [CrossRef]
- Yang, J.; Deng, D.W.; Yu, J.S. Transfer from trap emission to band-edge one in water-soluble CdS nanocrystals. J. Colloid Interface Sci. 2013, 394, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Jayakrishnan, R.; Ayyub, P. Effect of the size-induced structural transformation on the band gap in CdS nanoparticles. J. Phys. Condens. Matter 2000, 12, 10647–10654. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Semiconductor clusters, nanoscrystals and quantum dots. Sci. New Ser. 1996, 271, 933–937. [Google Scholar]
- Zhu, G.; Bao, C.; Liu, Y.; Shen, X.; Xi, C.; Xu, Z.; Ji, Z. Self-regulated route to ternary hybrid nanocrystals of Ag-Ag2S-CdS with near-infrared photoluminescence and enhanced photothermal conversion. Nanoscale 2014, 6, 11147–11156. [Google Scholar] [CrossRef]
- O’Leary, S.K.; Zukotynski, S.; Perz, J.M. Disorder and optical absoprtion in amorphous silicon and amorphous germanium. J. Non-Cryst. Solids 1997, 210, 249–253. [Google Scholar] [CrossRef]
- Chandran, A.; George, K.C. Phase instability and defect induced evolution of optical properties in Cd rich-CdS nanoparticles. J. Appl. Phys. 2014, 115, 164309. [Google Scholar] [CrossRef]
- Mercy, A.; Sakthi Murugesan, K.; Milton Boaz, B.; Jesper Anandhi, A.; Kanagadurai, R. Synthesis and structural and optical characterization of Mn2+ doped cadmium sulphide nanoparticles stabilized in DETA matrix. J. Alloy. Compd. 2013, 554, 189–194. [Google Scholar] [CrossRef]
- Huang, L.; Yang, J.; Wang, X.; Han, J.; Han, H.; Li, C. Effects of surface modification on photocatalytic activity of CdS nanocrystals studied by photoluminescence spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Morales, R.; Zelaya-Angel, O.; Torres-Delgado, G. On the yellow-band emission in CdS films. Appl. Phys. A Mater. Sci. Process. 2001, 73, 61–65. [Google Scholar] [CrossRef]
- Liu, Z.; Ji, G.; Guan, D.; Wang, B.; Wu, X. Enhanced charge-carrier transfer by CdS and Ag2S quantum dots co-sensitization for TiO2 nanotube arrays. J. Colloid Interface Sci. 2015, 457, 1–8. [Google Scholar] [CrossRef]
- López, I.; Gómez, I. Improvement of the luminescent properties of cadmium sulfide quantum dots by a post-synthesis modification. Phys. B Condens. Matter 2014, 453, 81–85. [Google Scholar] [CrossRef]
- Karimipour, M.; Izadian, L.; Molaei, M. Effect of CdS growth time on the optical properties of one-pot preparation of CdS-Ag2S binary compounds. J. Electron. Mater. 2017, 47, 1322–1329. [Google Scholar]
- Ramsden, J.; Gräetzel, M. Photoluminescence of small cadmium sulphide particles. J. Chem. Soc. Faraday Trans. 1984, 1, 919–933. [Google Scholar] [CrossRef]
- Shen, S.; Guo, L.; Chen, X.; Ren, F.; Mao, S.S. Effect of Ag2S on solar-driven photocatalytic hydrogen evolution of nanostructured CdS. Int. J. Hydrogen Energy 2010, 35, 7110–7115. [Google Scholar] [CrossRef]
- Reber, J.F.; Rusek, M. Photochemical hydrogen production with platinized suspensions of cadmium sulphide and cadmium zinc sulphide modified by silver sulphide. J. Phys. Chem. 1986, 90, 824–834. [Google Scholar] [CrossRef]
- Dalvand, P.; Mohammadi, M.R. Controlling morphology and structure of nanocrystalline cadmium sulfide (CdS) by tailoring solvothermal processing parameters. J. Nanopart. Res. 2011, 13, 3011–3018. [Google Scholar] [CrossRef]
- Ikhmayies, S.J.; Ahmad-Bitar, R.N. A study of the optical bandgap energy and Urbach tail of spray-deposited CdS:In thin films. J. Mater. Res. Technol. 2013, 2, 221–227. [Google Scholar] [CrossRef]
- Haj Lakhdar, M.; Larbi, T.; Ouni, B.; Amlouk, M. Optical and structural investigations on Sb2S2O new kermesite alloy for optoelectronic applications. J. Alloy. Compd. 2013, 579, 198–204. [Google Scholar] [CrossRef]

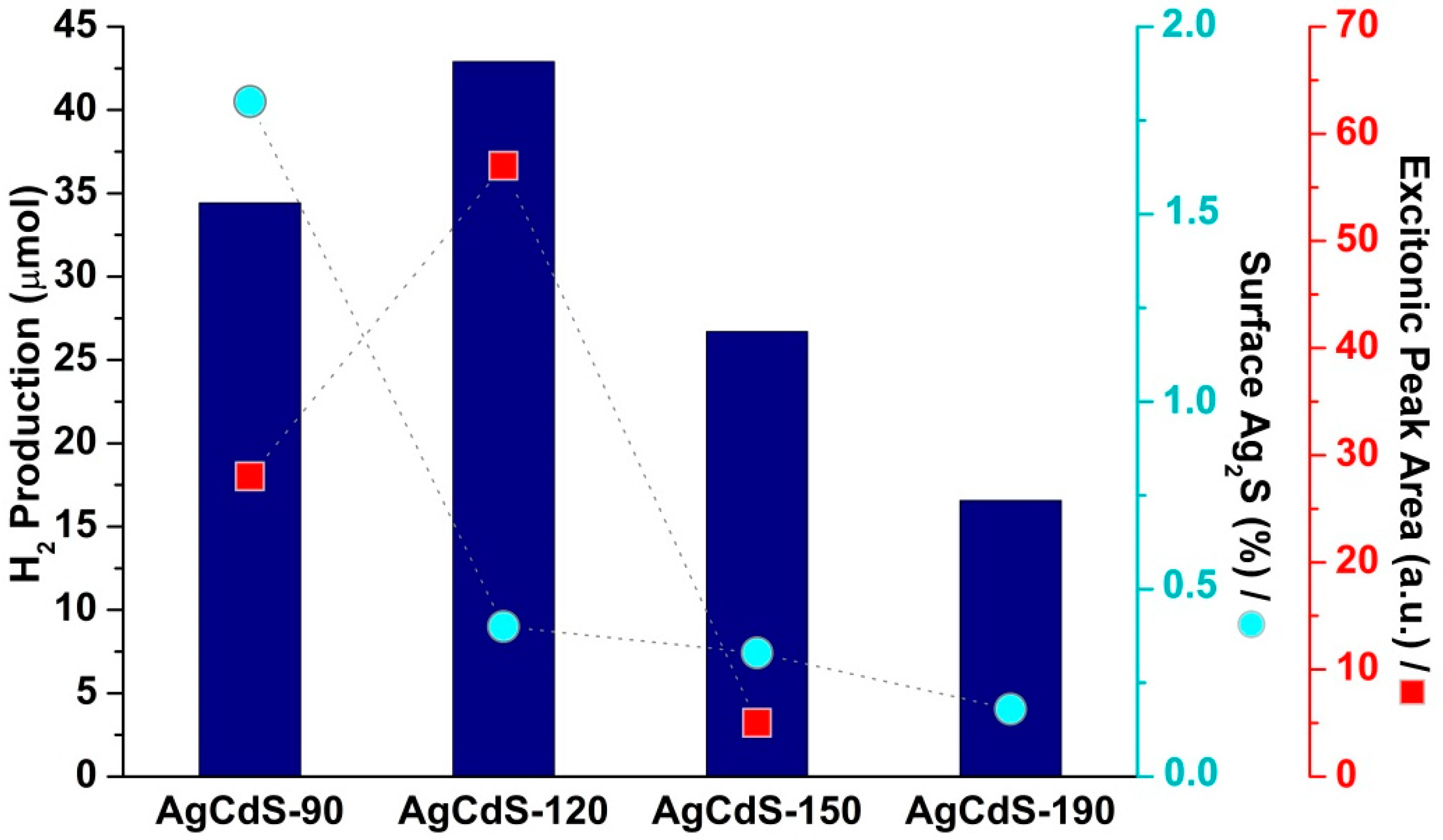
| % Cd | % S | % Ag | Cd/S | Ag/S | BET (m2/g) | Pore Volume (cm3/g) | |
|---|---|---|---|---|---|---|---|
| AgCdS-90 | 43.8 | 48.7 | 7.5 | 0.9 | 0.15 | 87.9 | 0.43 |
| AgCdS-120 | 45.8 | 52.1 | 2.1 | 0.88 | 0.04 | 84.6 | 0.29 |
| AgCdS-150 | 46.6 | 50.4 | 3 | 0.92 | 0.06 | 65.2 | 0.25 |
| AgCdS-190 | 46.2 | 51.3 | 2.5 | 0.9 | 0.05 | 52.2 | 0.21 |
| Dp (nm) | I(100)/(002) | Lattice Strain (ε) | |
|---|---|---|---|
| AgCdS-90 | 13.1 | 0.614 | - |
| AgCdS-120 | 15.6 | 0.594 | −0.0032 |
| AgCdS-150 | 37.6 | 0.492 | −0.0021 |
| AgCdS-190 | 39.3 | 0.473 | −0.0022 |
| Cd 3d5/2 | Ag 3d5/2 | S 2p | % Cd | % S | % Ag | |
|---|---|---|---|---|---|---|
| AgCdS-90 | 404.7 | 367.1 | 161.2 | 41.3 | 56.9 | 1.8 |
| AgCdS-120 | 404.6 | 367.2 | 161.3 | 52.1 | 47.5 | 0.4 |
| AgCdS-150 | 404.6 | 367.6 (81.4)/369 (18.6) 1 | 161.4 | 53.8 | 45.8 | 0.4 |
| AgCdS-190 | 404.6 | 367.5 (91)/368.4 (9) 1 | 161.4 | 52.9 | 46.9 | 0.2 |
| BG (eV) | Excitonic Peak (Rel. Intensity) | Eu (meV) | |
|---|---|---|---|
| AgCdS-90 | 2.71/2.36 | 0.49 | 177.5 |
| AgCdS-120 | 2.54 | 1 | 92.2 |
| AgCdS-150 | 2.48 | 0.08 | 62.6 |
| AgCdS-190 | 2.47 | - | 50.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, E.; Vaquero, F.; Mota, N.; Navarro, R.M.; Fierro, J.L.G. CdS Photocatalysts Modified with Ag: Effect of the Solvothermal Temperature on the Structure and Photoactivity for Hydrogen Production. Catalysts 2019, 9, 110. https://doi.org/10.3390/catal9020110
Soto E, Vaquero F, Mota N, Navarro RM, Fierro JLG. CdS Photocatalysts Modified with Ag: Effect of the Solvothermal Temperature on the Structure and Photoactivity for Hydrogen Production. Catalysts. 2019; 9(2):110. https://doi.org/10.3390/catal9020110
Chicago/Turabian StyleSoto, Erika, Fernando Vaquero, Noelia Mota, Rufino Manuel Navarro, and Jose Luis G. Fierro. 2019. "CdS Photocatalysts Modified with Ag: Effect of the Solvothermal Temperature on the Structure and Photoactivity for Hydrogen Production" Catalysts 9, no. 2: 110. https://doi.org/10.3390/catal9020110
APA StyleSoto, E., Vaquero, F., Mota, N., Navarro, R. M., & Fierro, J. L. G. (2019). CdS Photocatalysts Modified with Ag: Effect of the Solvothermal Temperature on the Structure and Photoactivity for Hydrogen Production. Catalysts, 9(2), 110. https://doi.org/10.3390/catal9020110