The Roles of the Structure and Basic Sites of Sodium Titanates on Transesterification Reactions to Obtain Biodiesel
Abstract
:1. Introduction
2. Results and Discussion
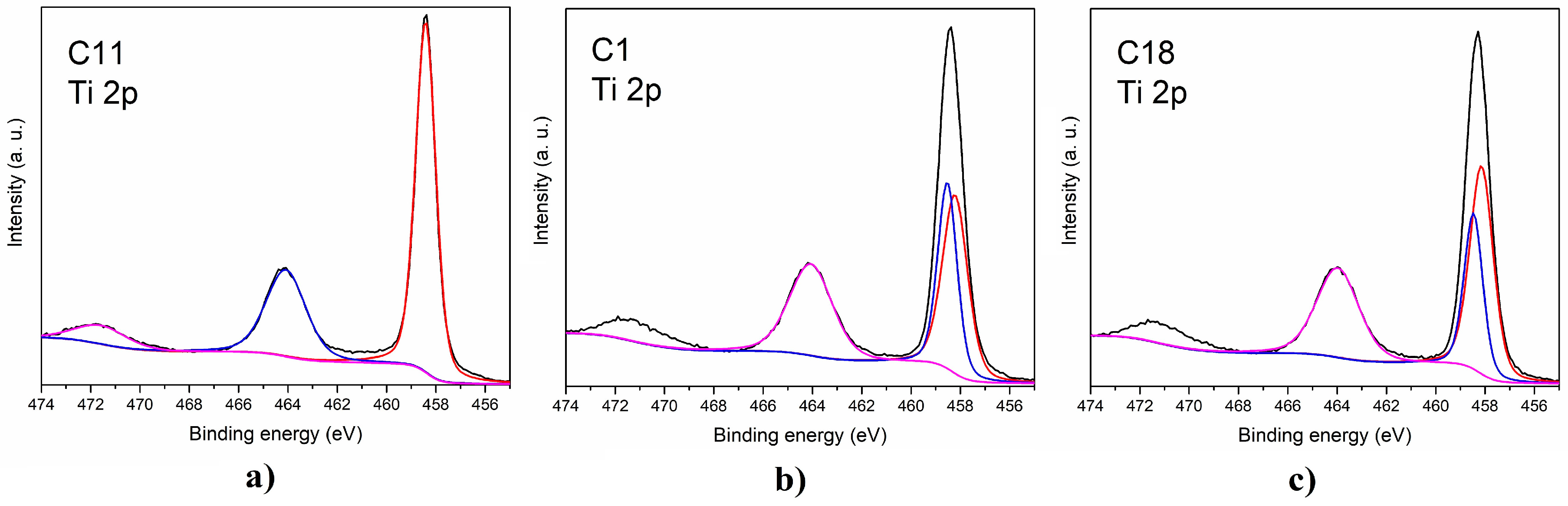
2.1. Characterization and Catalytic Performance of Sodium Titanates
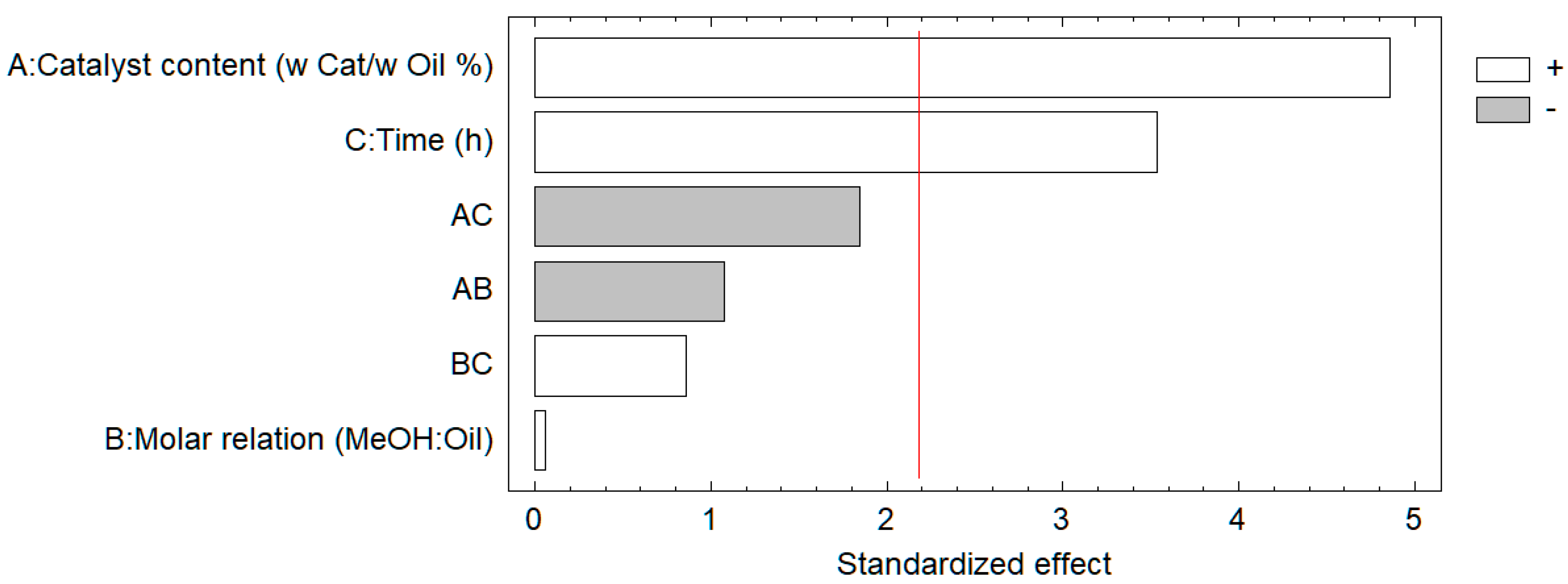
2.2. Biodiesel Conversion Optimization
3. Materials and Methods
3.1. Materials
3.2. Preparation of Catalytic Materials
3.3. Characterization of the Catalytic Materials
3.4. Synthesis and Quantification of Biodiesel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Organisation for Economic Co-operation and Development, Food and Agriculture Organization of the United Nations. Biofuels. In OECD-FAO Agric. Outlook 2015–2024; OECD Publishing: Paris, France, 2015; pp. 126–142. [Google Scholar] [CrossRef]
- Marwaha, A.; Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Waste materials as potential catalysts for biodiesel production: Current state and future scope. Fuel Process. Technol. 2018, 181, 175–186. [Google Scholar] [CrossRef]
- Du, L.; Ding, S.; Li, Z.; Lv, E.; Lu, J.; Ding, J. Transesterification of castor oil to biodiesel using NaY zeolite-supported La2O3 catalysts. Energy Convers. Manag. 2018, 173, 728–734. [Google Scholar] [CrossRef]
- Kouzu, M.; Fujimori, A.; Suzuki, T.; Koshi, K.; Moriyasu, H. Industrial feasibility of powdery CaO catalyst for production of biodiesel. Fuel Process. Technol. 2017, 165, 94–101. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Piraman, S. Biodiesel synthesis by TiO2-ZnO mixed oxide nanocatalyst catalyzed palm oil transesterification process. Bioresour. Technol. 2013, 150, 55–59. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.-T.; Yan, J.; Dahlquist, E. Biodiesel production from waste cooking oil catalyzed by TiO2–MgO mixed oxides. Bioresour. Technol. 2010, 101, 9570–9576. [Google Scholar] [CrossRef]
- Hernández-Hipólito, P.; García-Castillejos, M.; Martínez-Klimova, E.; Juárez-Flores, N.; Gómez-Cortés, A.; Klimova, T.E. Biodiesel production with nanotubular sodium titanate as a catalyst. Catal. Today 2014, 220–222, 4–11. [Google Scholar] [CrossRef]
- Manique, M.C.; Silva, A.P.; Alves, A.K.; Bergmann, C.P. Application of hydrothermally produced TiO2 nanotubes in photocatalytic esterification of oleic acid. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2016, 206, 17–21. [Google Scholar] [CrossRef]
- Salinas, D.; Guerrero, S.; Araya, P. Transesterification of canola oil on potassium-supported TiO2catalysts. Catal. Commun. 2010, 11, 773–777. [Google Scholar] [CrossRef]
- Salinas, D.; Araya, P.; Guerrero, S. Study of potassium-supported TiO2 catalysts for the production of biodiesel. Appl. Catal. B Environ. 2012, 117–118, 260–267. [Google Scholar] [CrossRef]
- Hernández-Hipólito, P.; Juárez-Flores, N.; Martínez-Klimova, E.; Gómez-Cortés, A.; Bokhimi, X.; Escobar-Alarcón, L.; Klimova, T.E. Novel heterogeneous basic catalysts for biodiesel production: Sodium titanate nanotubes doped with potassium. Catal. Today 2015, 250, 187–196. [Google Scholar] [CrossRef]
- Martínez-Klimova, E.; Hernández-Hipólito, P.; Klimova, T.E. Biodiesel Production with Nanotubular Sodium Titanate Doped with Potassium as a Catalyst. MRS Adv. 2016, 1, 415–420. [Google Scholar] [CrossRef]
- Yahya, N.Y.; Ngadi, N.; Wong, S.; Hassan, O. Transesterification of used cooking oil (UCO) catalyzed by mesoporous calcium titanate: Kinetic and thermodynamic studies. Energy Convers. Manag. 2018, 164, 210–218. [Google Scholar] [CrossRef]
- González, E.A.Z.; García-Guaderrama, M.; Villalobos, M.R.; Dellamary, F.L.; Kandhual, S.; Rout, N.P.; Tiznado, H.; Arizaga, G.G.C. Potassium titanate as heterogeneous catalyst for methyl transesterification. Powder Technol. 2015, 280, 201–206. [Google Scholar] [CrossRef]
- Salinas, D.; Guerrero, S.; Cross, A.; Araya, P.; Wolf, E.E. Potassium titanate for the production of biodiesel. Fuel 2016, 166, 237–244. [Google Scholar] [CrossRef]
- Russbueldt, B.M.E.; Hoelderich, W.F. New rare earth oxide catalysts for the transesterification of triglycerides with methanol resulting in biodiesel and pure glycerol. J. Catal. 2010, 271, 290–304. [Google Scholar] [CrossRef]
- Chew, K.Y.; Tan, W.L.; Bakar, N.H.H.A.; Bakar, M.A. Transesterification of palm cooking oil using barium-containing titanates and their sodium doped derivatives. Int. J. Energy Environ. Eng. 2017, 8, 47–53. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of Titanium Oxide Nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Ji, T.; Tu, R.; Li, L.; Mu, L.; Liu, C.; Lu, X.; Zhu, J. Localizing microwave heat by surface polarization of titanate nanostructures for enhanced catalytic reaction efficiency. Appl. Catal. B Environ. 2018, 227, 266–275. [Google Scholar] [CrossRef]
- Morgado, E.; de Abreu, M.A.S.; Pravia, O.R.C.C.; Marinkovic, B.A.; Jardim, P.M.; Rizzo, F.C.; Araújo, A.S. A study on the structure and thermal stability of titanate nanotubes as a function of sodium content. Solid State Sci. 2006, 8, 888–900. [Google Scholar] [CrossRef]
- Ou, H.H.; Lo, S.L. Review of titania nanotubes synthesized via the hydrothermal treatment: Fabrication, modification, and application. Sep. Purif. Technol. 2007, 58, 179–191. [Google Scholar] [CrossRef]
- Salinas, D.A.; Marchena, C.L.; Pierella, L.B.; Pecchi, G. Catalytic oxidation of 2-(methylthio)-benzothiazole on alkaline earth titanates, ATiO3(A = Ca, Sr, Ba). Mol. Catal. 2017, 438, 76–85. [Google Scholar] [CrossRef]
- Morgado, E.; de Abreu, M.A.S.; Moure, G.T.; Marinkovic, B.A.; Jardim, P.M.; Araujo, A.S. Effects of thermal treatment of nanostructured trititanates on their crystallographic and textural properties. Mater. Res. Bull. 2007, 42, 1748–1760. [Google Scholar] [CrossRef]
- Qamar, M.; Yoon, C.R.; Oh, H.J.; Kim, D.H.; Jho, J.H.; Lee, K.S.; Lee, W.J.; Lee, H.G.; Kim, S.J. Effect of post treatments on the structure and thermal stability of titanate nanotubes. Nanotechnology 2006, 17, 5922–5929. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Synthesis and characterization of ion-exchangeable titanate nanotubes. Chem. Eur. J. 2003, 9, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Aramendía, M.A.; Borau, V.; Jiménez, C.; Marinas, A.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Magnesium-containing mixed oxides as basic catalysts: Base characterization by carbon dioxide TPD-MS and test reactions. J. Mol. Catal. A Chem. 2004, 218, 81–90. [Google Scholar] [CrossRef]
- Senguttuvan, P.; Rousse, G.; Seznec, V.; Tarascon, J.M.; Palacín, M.R. Na2Ti3O7: Lowest voltage ever reported oxide insertion electrode for sodium ion batteries. Chem. Mater. 2011, 23, 4109–4111. [Google Scholar] [CrossRef]
- An, Y.; Li, Z.; Xiang, H.; Huang, Y.; Shen, J. First-principle calculations for electronic structure and bonding properties in layered Na2Ti3O7. Cent. Eur. J. Phys. 2011, 9, 1488–1492. [Google Scholar] [CrossRef]
- Cech, O.; Castkova, K.; Chladil, L.; Dohnal, P.; Cudek, P.; Libich, J.; Vanysek, P. Synthesis and characterization of Na2Ti6O13 and Na2Ti6O13/Na2Ti3O7sodium titanates with nanorod-like structure as negative electrode materials for sodium-ion batteries. J. Energy Storage 2017, 14, 391–398. [Google Scholar] [CrossRef]
- Theobald, F.R.; Catlow, C.R.A.; Cormack, A.N. Lattice energy minimization as a complementary technique to refine structures obtained by high-resolution electron microscopy. J. Solid State Chem. 1984, 52, 80–90. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Babu, B.; Feygenson, M.; Neuefeind, J.C.; Shaijumon, M.M. Nanostructured Na2Ti9O19 for Hybrid Sodium-Ion Capacitors with Excellent Rate Capability. ACS Appl. Mater. Interfaces. 2018, 10, 437–447. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Zhang, H.; Yao, X.; Zhao, H. Origin of reactivity diversity of lattice oxygen in titanates. Chem. Phys. Lett. 2011, 511, 82–86. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, T.; Yang, D.; Liu, H.; Zhang, H.; Yao, X.; Zhao, H. Structure, reactivity, photoactivity and stability of Ti-O based materials: A theoretical comparison. Phys. Chem. Chem. Phys. 2012, 14, 2333–2338. [Google Scholar] [CrossRef] [Green Version]
- X-ray Photoelectron Spectroscopy (XPS) Reference Pages: Titanium, (n.d.). Available online: http://www.xpsfitting.com/2008/09/titanium.html (accessed on 26 July 2019).
- Roberts, M.W.; Tomellini, M. Mixed oxidation states of titanium at the metal-oxide interface. Catal. Today 1992, 12, 443–452. [Google Scholar] [CrossRef]
- Pacchioni, G. Oxygen Vacancy: The Invisible Agent on Oxide Surfaces. Chem. Phys. Chem. 2003, 4, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Ali, G.; Chung, K.Y.; Yoon, C.S.; Yashiro, H.; Sun, Y.K.; Lu, J.; Amine, K.; Myung, S.T. Anatase titania nanorods as an intercalation anode material for rechargeable sodium batteries. Nano Lett. 2014, 14, 416–422. [Google Scholar] [CrossRef]
- Wu, L.; Bresser, D.; Buchholz, D.; Giffin, G.A.; Castro, C.R.; Ochel, A.; Passerini, S. Unfolding the Mechanism of Sodium Insertion in Anatase TiO2 Nanoparticles. Adv. Energy Mater. 2015, 5, 1401142. [Google Scholar] [CrossRef]
- Liu, H.; Yang, D.; Zheng, Z.; Ke, X.; Waclawik, E.; Zhu, H.; Frost, R.L. A Raman spectroscopic and TEM study on the structural evolution of Na2Ti3O7 during the transition to Na2Ti6O13. J. Raman Spectrosc. 2010, 41, 1331–1337. [Google Scholar] [CrossRef]
- Turki, A.; Kochkar, H.; Guillard, C.; Berhault, G.; Ghorbel, A. Effect of Na content and thermal treatment of titanate nanotubes on the photocatalytic degradation of formic acid. Appl. Catal. B Environ. 2013, 138–139, 401–415. [Google Scholar] [CrossRef]
- XPS Interpretation of Titanium, (n.d.). Available online: https://xpssimplified.com/elements/titanium.php (accessed on 26 July 2019).
- Montgomery, D.C. Design-and-Analysis-of-Experiments, 5th ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2001; p. 679. [Google Scholar]
- ASTM D6584—17 Standard Test Method for Determination of Total Monoglycerides, Total Diglycerides, Total Triglycerides, and Free and Total Glycerin in B-100 Biodiesel Methyl Esters by Gas Chromatography, (n.d.). Available online: https://www.astm.org/Standards/D6584.htm (accessed on 4 March 2019).
- EN 14103—European Standards, (n.d.). Available online: https://www.en-standard.eu/csn-en-14103-fat-and-oil-derivatives-fatty-acid-methyl-esters-fame-determination-of-ester-and-linolenic-acid-methyl-ester-contents/ (accessed on 4 March 2019).

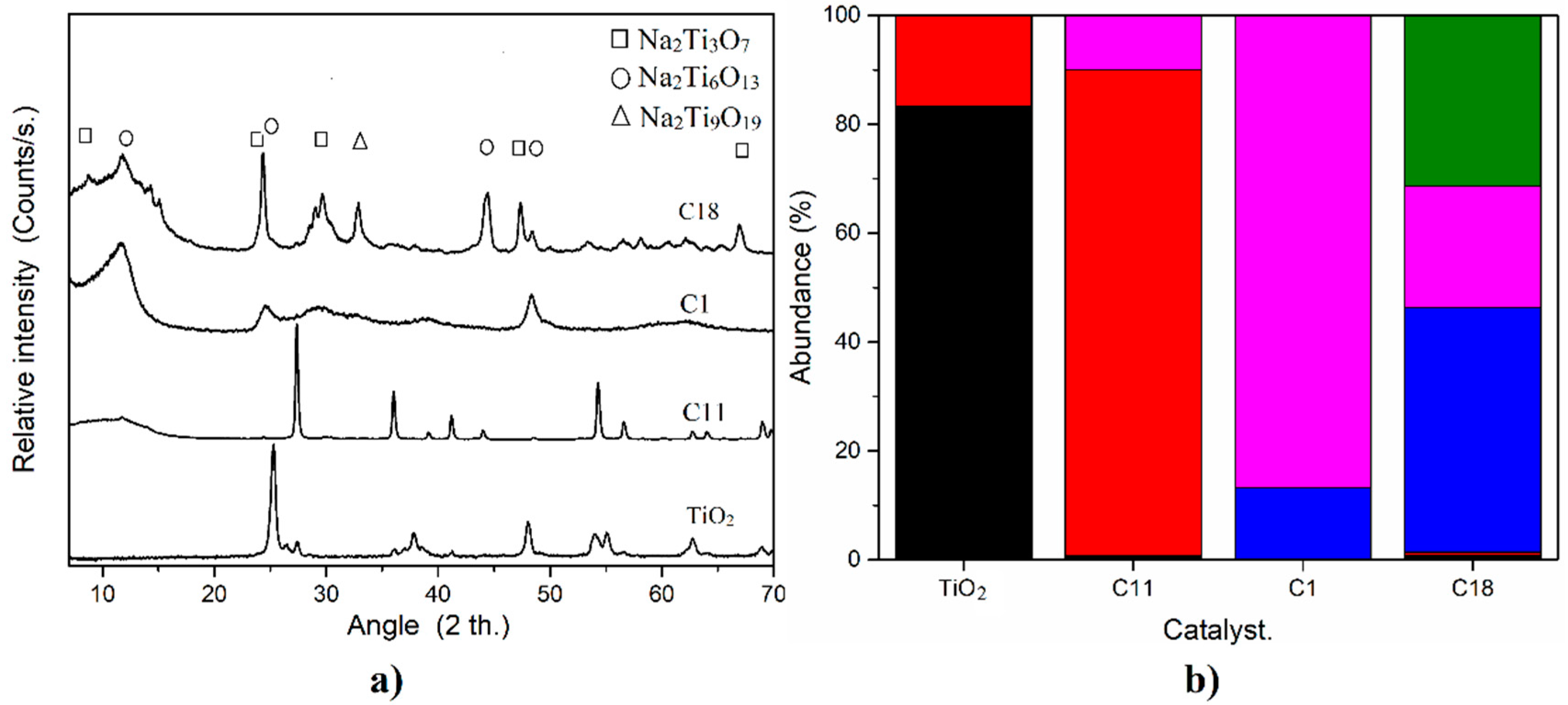

| Catalyst. | NaOH Concentration (mol/L) | Hydrothermal Temperature (°C) | Amount of TiO2 (g/mL) | Calcination Temperature (°C) | Biodiesel Conversion (Mass/Mass %) | |
|---|---|---|---|---|---|---|
| TiO2 | - | - | - | - | 4 | |
| C1 | 7.5 | 170 | 0.12 | 400 | 10 | |
| C2 | 7.5 | 170 | 0.06 | 400 | 9.5 | |
| C3 | 7.5 | 170 | 0.06 | 800 | 1.8 | |
| C4 | 7.5 | 130 | 0.06 | 800 | 1.7 | |
| C5 | 2.5 | 130 | 0.12 | 400 | 5.7 | |
| C6 | 7.5 | 170 | 0.12 | 800 | 6.5 | |
| C7 | 7.5 | 130 | 0.12 | 400 | 11 | |
| C8 | 2.5 | 170 | 0.06 | 400 | 11.6 | |
| C9 | 7.5 | 130 | 0.12 | 800 | 4.8 | |
| C10 | 7.5 | 130 | 0.06 | 400 | 10 | |
| C11 | 2.5 | 170 | 0.06 | 800 | 0.2 | |
| C12 | 2.5 | 130 | 0.06 | 400 | 5.7 | |
| C13 | 2.5 | 130 | 0.12 | 800 | 0.3 | |
| C14 | 2.5 | 170 | 0.12 | 800 | 0.3 | |
| C15 | 2.5 | 170 | 0.12 | 400 | 9.8 | |
| C16 | 2.5 | 130 | 0.06 | 800 | 0.3 | |
| Central points | C17 | 5 | 150 | 0.09 | 600 | 30.3 |
| C18 | 5 | 150 | 0.09 | 600 | 35.5 | |
| C19 | 5 | 150 | 0.09 | 600 | 35 | |
| C20 | 5 | 150 | 0.09 | 600 | 28.6 |
| Catalyst. | Ti (III) | Ti (IV) | ||
|---|---|---|---|---|
| BE (eV) | Area (%) | BE (eV) | Area (%) | |
| C1 | 458.2 | 37.5 | 458.5 464.1 | 30.0 32.5 |
| C18 | 458.1 | 42.2 | 458.4 463.9 | 25.3 32.5 |
| C11 | - | - | 458.4 464.1 | 67.4 32.6 |
| Run. | Cat. Weight (Cat/Oil %) | M. R. (MeOH:Oil) | Time (h) | Biodiesel Conversion (Mass/Mass %) | |
|---|---|---|---|---|---|
| 1 | 5.5 | 60 | 2 | 70 | |
| 2 | 5.5 | 60 | 8 | 91 | |
| 3 | 5.5 | 20 | 2 | 86 | |
| 4 | 5.5 | 20 | 8 | 88 | |
| 5 | 1.8 | 60 | 2 | 33 | |
| 6 | 1.8 | 60 | 8 | 74 | |
| 7 | 1.8 | 20 | 2 | 28 | |
| 8 | 1.8 | 20 | 8 | 63 | |
| Central points | 9 | 3.6 | 40 | 5 | 90 |
| 10 | 3.6 | 40 | 5 | 95 | |
| 11 | 3.6 | 40 | 5 | 93 | |
| 12 | 3.6 | 40 | 5 | 95 |
| Values Used in the Experimental Design | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaOH Concentration (mol/L) | Hydrothermal Synthesis Temperature (°C) | Amount of TiO2 (g/mL) | Calcination Temperature (°C) | |||||||||
| Coded level | −1 | 0 | 1 | −1 | 0 | 1 | −1 | 0 | 1 | −1 | 0 | 1 |
| 2.5 | 5 | 7.5 | 130 | 150 | 170 | 0.06 | 0.09 | 0.12 | 400 | 600 | 800 | |
| Range of Factors Used | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Catalyst Weight (Cat/Oil %) | Molar Relation (MeOH:Oil) | Reaction Time (h) | |||||||
| Coded level | −1 | 0 | 1 | −1 | 0 | 1 | −1 | 0 | 1 |
| 1.8 | 3.6 | 5.5 | 20:1 | 40:1 | 60:1 | 2 | 5 | 8 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machorro, J.J.; Lazaro, A.L.; Espejel-Ayala, F.; Coutiño-Gonzalez, E.; Chavarria-Hernandez, J.C.; Godínez, L.A.; Rodríguez-Valadez, F.J. The Roles of the Structure and Basic Sites of Sodium Titanates on Transesterification Reactions to Obtain Biodiesel. Catalysts 2019, 9, 989. https://doi.org/10.3390/catal9120989
Machorro JJ, Lazaro AL, Espejel-Ayala F, Coutiño-Gonzalez E, Chavarria-Hernandez JC, Godínez LA, Rodríguez-Valadez FJ. The Roles of the Structure and Basic Sites of Sodium Titanates on Transesterification Reactions to Obtain Biodiesel. Catalysts. 2019; 9(12):989. https://doi.org/10.3390/catal9120989
Chicago/Turabian StyleMachorro, Josue J., Ana L. Lazaro, Fabricio Espejel-Ayala, Eduardo Coutiño-Gonzalez, Juan C. Chavarria-Hernandez, Luis. A. Godínez, and Francisco J. Rodríguez-Valadez. 2019. "The Roles of the Structure and Basic Sites of Sodium Titanates on Transesterification Reactions to Obtain Biodiesel" Catalysts 9, no. 12: 989. https://doi.org/10.3390/catal9120989
APA StyleMachorro, J. J., Lazaro, A. L., Espejel-Ayala, F., Coutiño-Gonzalez, E., Chavarria-Hernandez, J. C., Godínez, L. A., & Rodríguez-Valadez, F. J. (2019). The Roles of the Structure and Basic Sites of Sodium Titanates on Transesterification Reactions to Obtain Biodiesel. Catalysts, 9(12), 989. https://doi.org/10.3390/catal9120989






