Morphology- and Crystalline Composition-Governed Activity of Titania-Based Photocatalysts: Overview and Perspective
Abstract
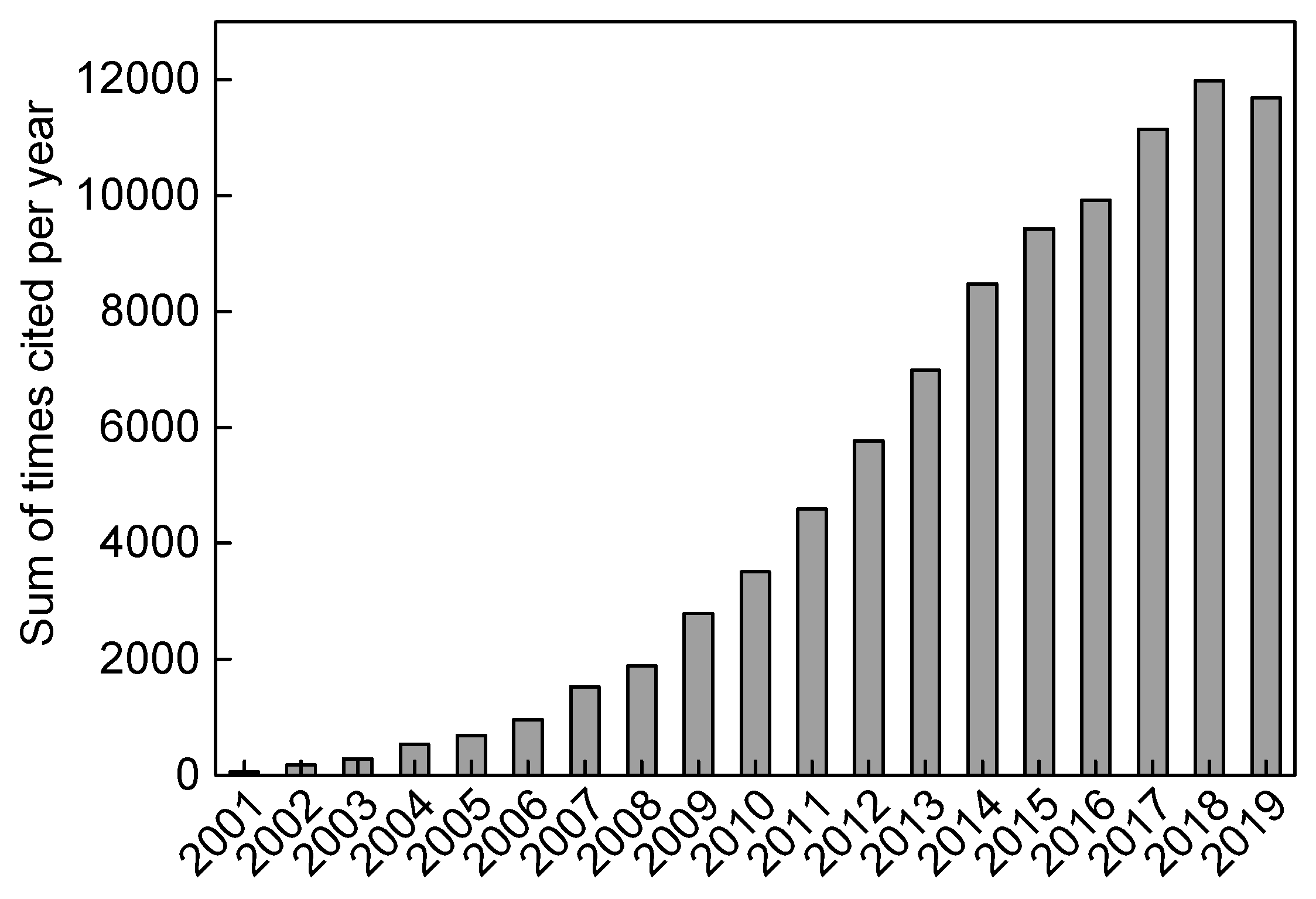
1. Introduction
2. Titania Polymorphs and Mixed-Phase Samples
2.1. Photocatalytic Activity of Titania Polymorphs
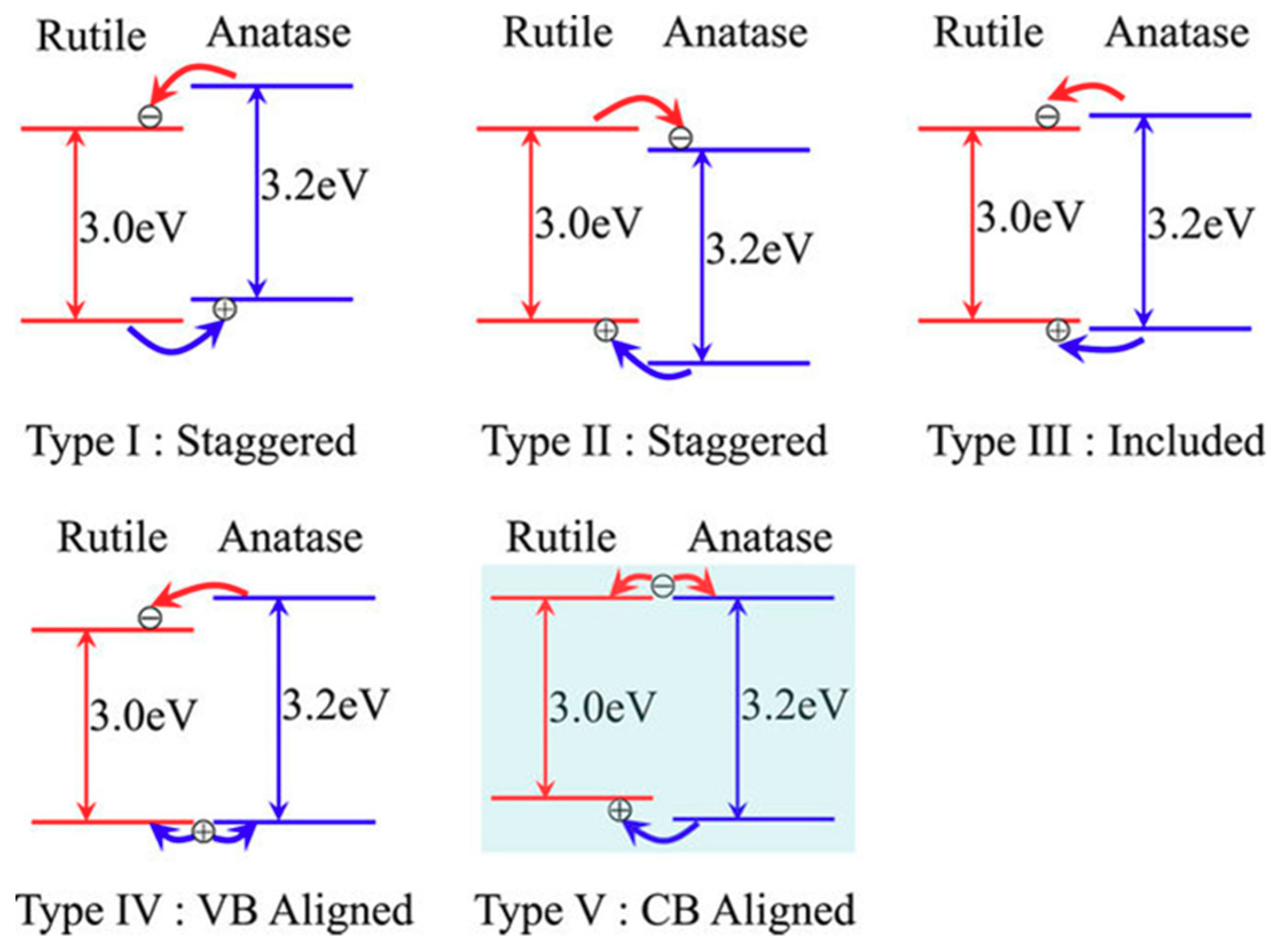
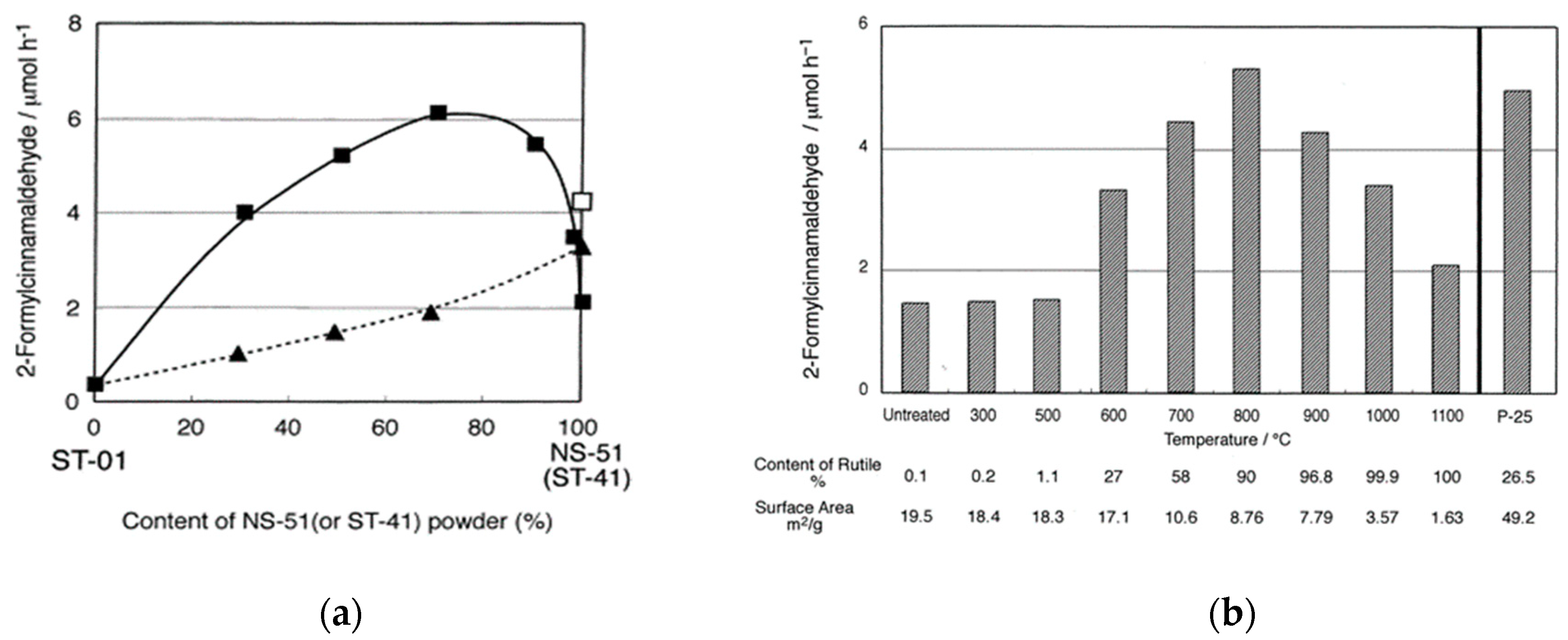
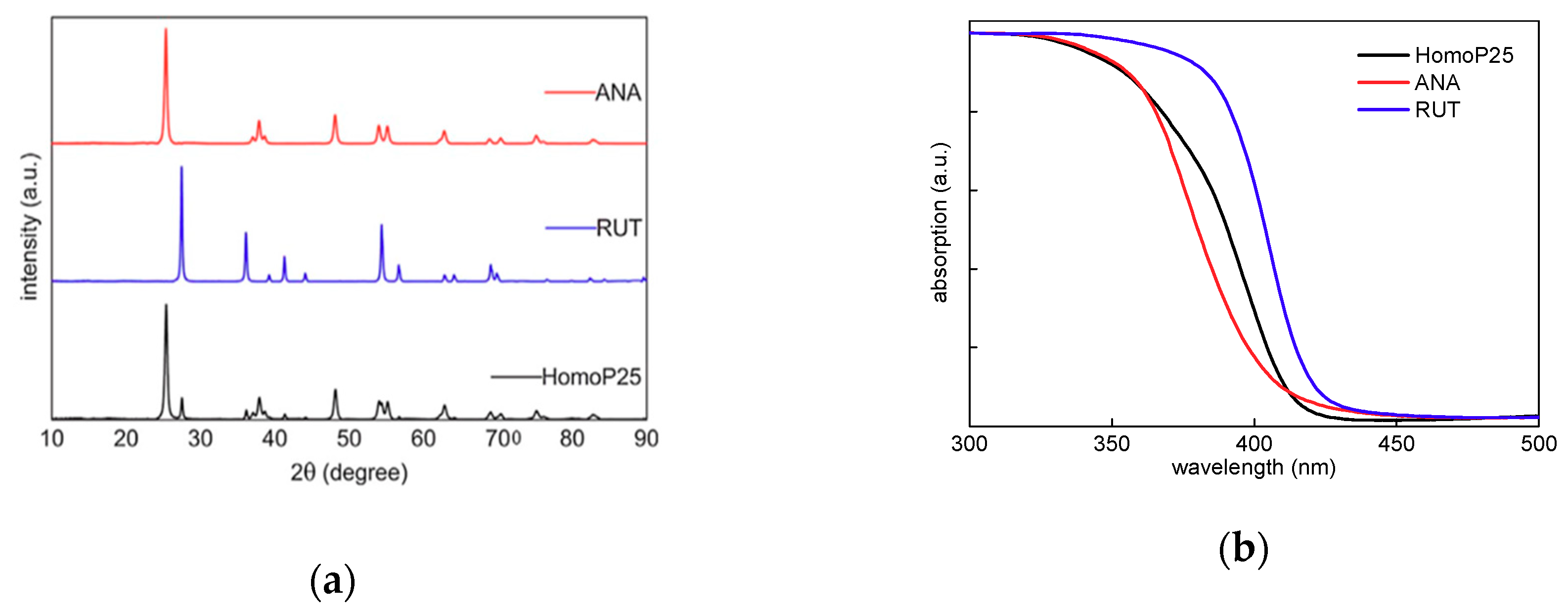
2.2. Photocatalytic Activity of Mixed-Phase Titania
3. Nanostructured Titania: Zero-, One-, Two-, and Three-Dimensional Structures
3.1. Zero-Dimensional Titania
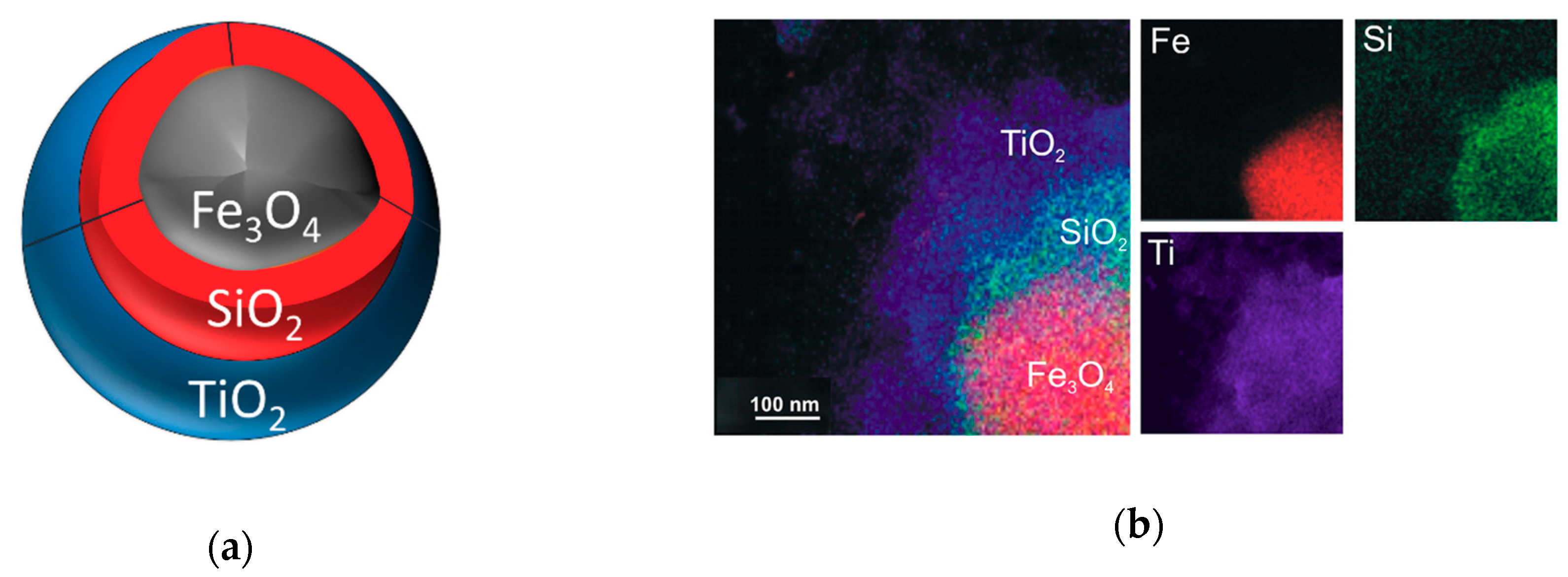
3.1.1. Core–Shell Titania Composites
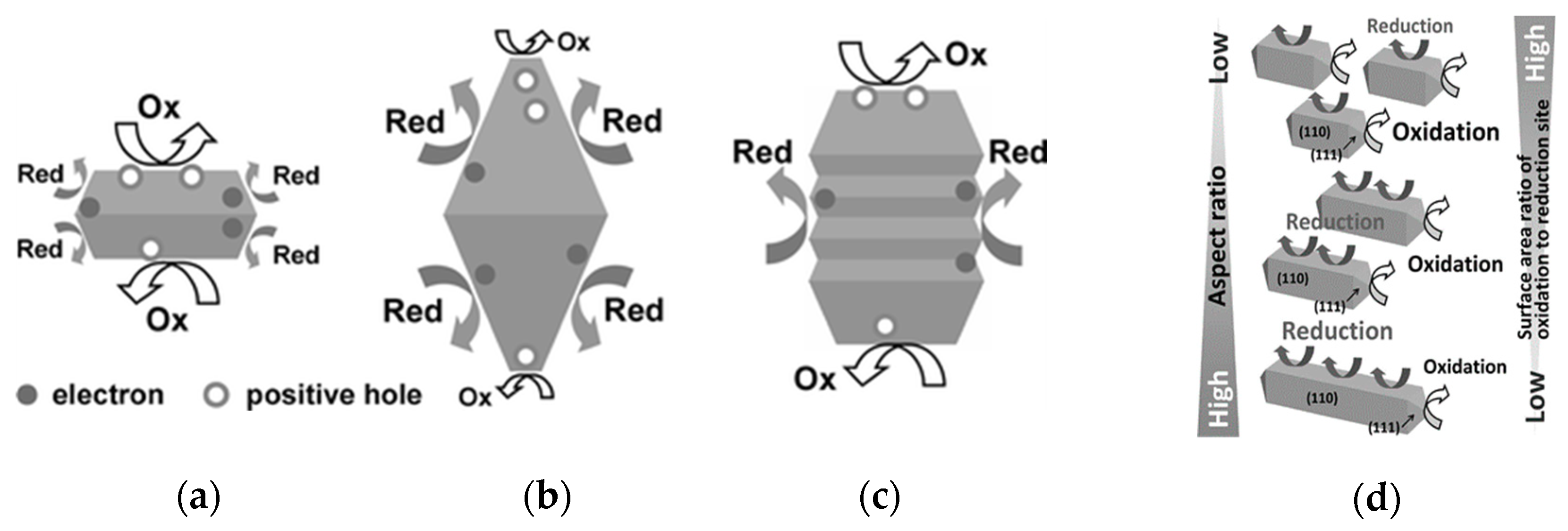
3.1.2. Faceted Titania
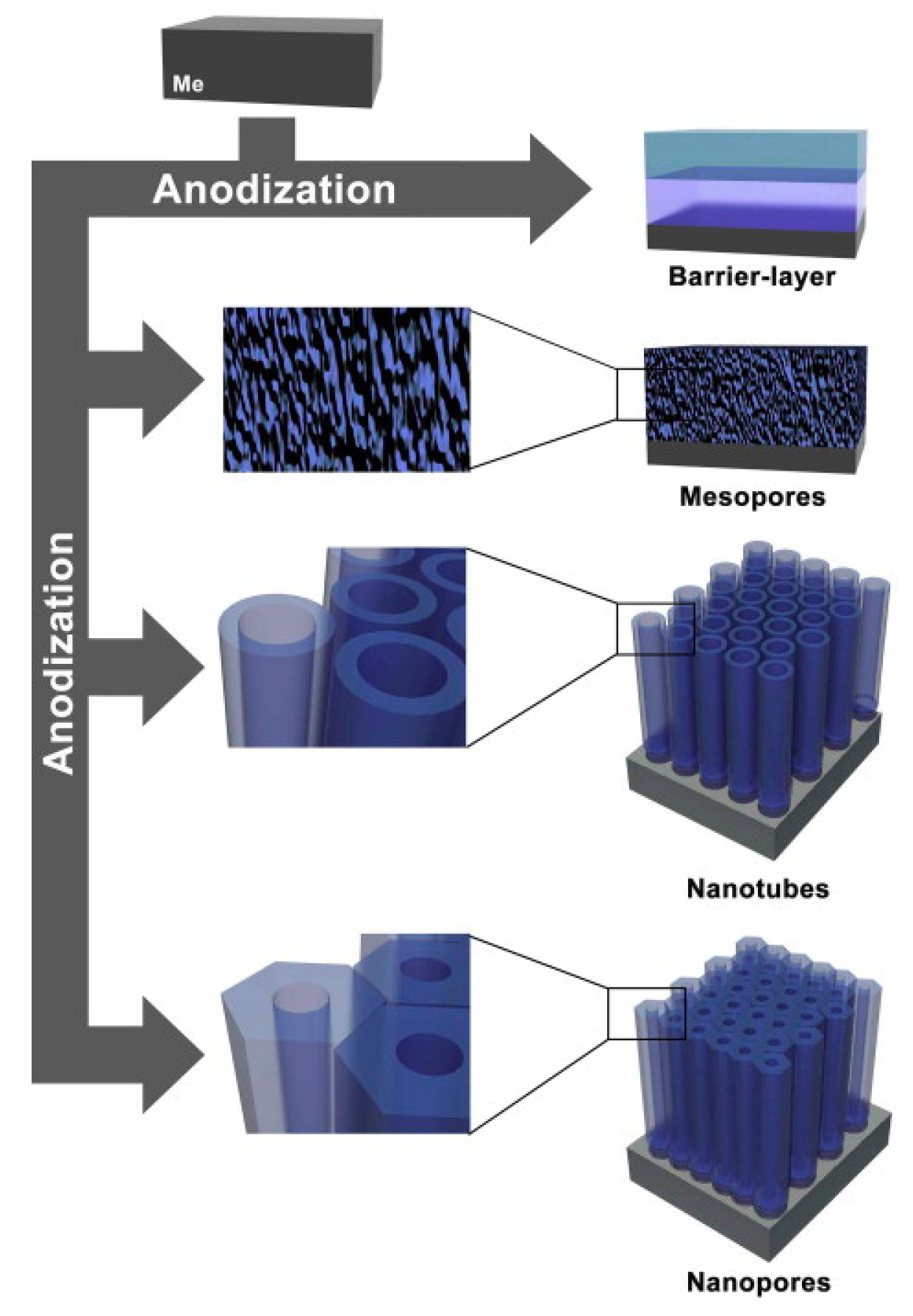
3.2. One-Dimensional Titania
3.3. Two-Dimensional Titania
3.4. Three-Dimensional Titania
3.4.1. Titania Aerogels
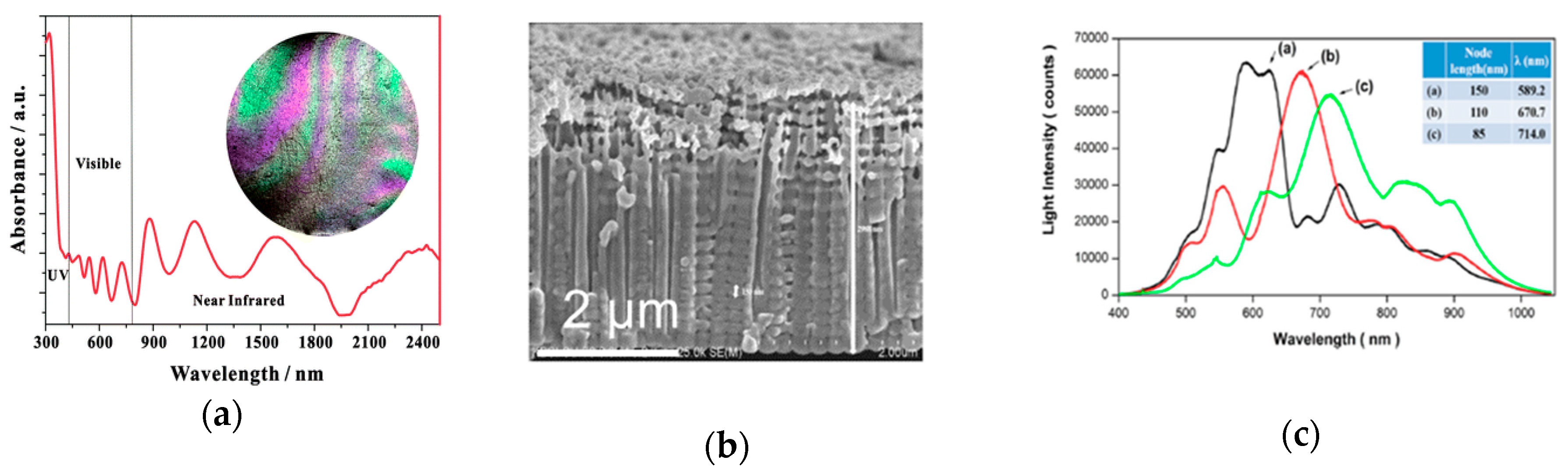
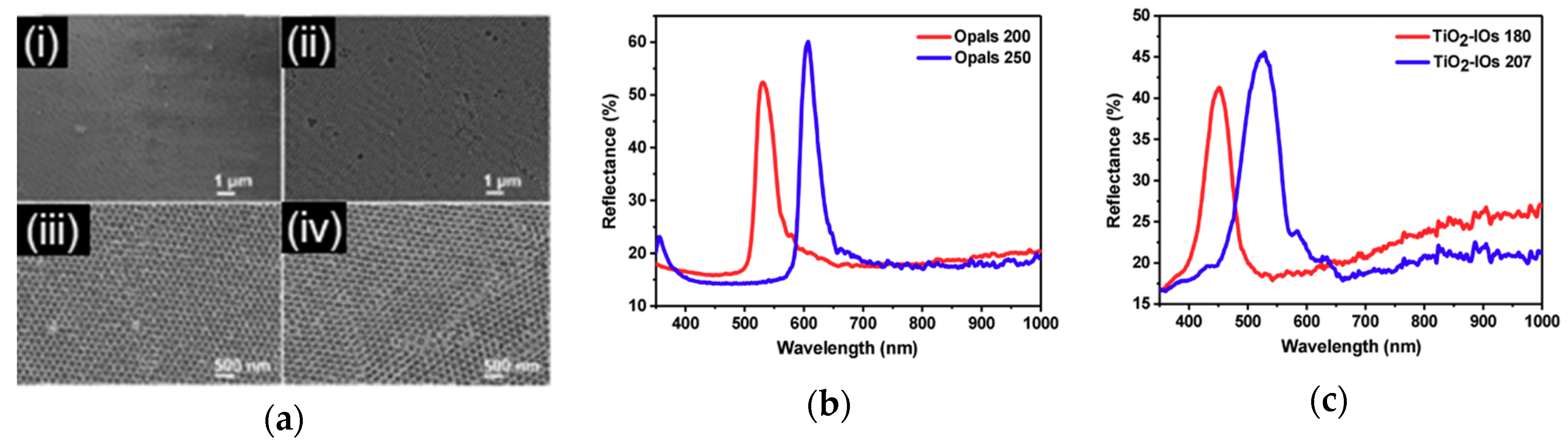
3.4.2. Inverse Opal Photonic Crystals (IO-PCs)
3.4.3. Titania Mesocrystals
4. Summary and Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- El Goresy, A.; Dubrovinsky, L.; Gillet, P.; Graup, G.; Chen, M. Akaogiite: An ultra-dense polymorph of TiO2 with the baddeleyite-type structure, in shocked garnet gneiss from the Ries Crater, Germany. Am. Miner. 2010, 95, 892–895. [Google Scholar] [CrossRef]
- Smalley, R.E. Nanotechnology, Energy and People; MIT Forum: River Oaks, TX, USA, 2003. [Google Scholar]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Li, K.; Zeng, Y.X.; Zhao, W.; Zhang, T.; Zhan, Y.J.; Xie, R.J.; Leung, D.Y.C.; Huang, H.B. Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: Overview and forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Feldhoff, A.; Robben, L.; Dillert, R.; Bahnemann, D.W. Tailored titanium dioxide nanomaterials: Anatase nanoparticles and brookite nanorods as highly active photocatalysts. Chem. Mater. 2010, 22, 2050–2060. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Prieto-Mahaney, O.O.; Murakami, N.; Abe, R.; Ohtani, B. Correlation between photocatalytic activities and structural and physical properties of titanium(IV) oxide powders. Chem. Lett. 2009, 38, 238–239. [Google Scholar] [CrossRef]
- Oyama, T.; Iimura, Y.; Takeuchi, K.; Ishii, T. Synthesis of rutile and anatase TiO2 fine particles by laser-ignited vapour-phase reaction. J. Mater. Sci. Lett. 1996, 15, 594–596. [Google Scholar] [CrossRef]
- Ohno, T.; Tokieda, K.; Higashida, S.; Matsumura, M. Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal. A Gen. 2003, 244, 383–391. [Google Scholar] [CrossRef]
- Murabayashi, M.; Itoh, K.; Furushima, H.; Chen, D. Photocatalytic degradation of chloroform with rutile-anatase mixed TiO2 powder. Denki Kagaku 1991, 59, 524–525. [Google Scholar]
- Hatayama, F.; Ohno, T.; Maruoka, T.; Ono, T.; Miyata, H. Structure and Acidity of Vanadium-Oxide Layered on Titania (Anatase and Rutile). J. Chem. Soc. Faraday Trans. 1991, 87, 2629–2633. [Google Scholar] [CrossRef]
- Augustynski, J. The role of the surface intermediates in the photoelectrochemical behavior of anatase and rutile TiO2. Electrochim. Acta 1993, 38, 43–46. [Google Scholar] [CrossRef]
- Rao, M.V.; Rajeshwar, K.; Verneker, V.R.P.; DuBow, J. Photosynthetic production of hydrogen and hydrogen peroxide on semiconducting oxide grains in aqueous solutions. J. Phys. Chem. 1980, 84, 1987–1991. [Google Scholar] [CrossRef]
- Gratzel, M.; Rotzinger, F.P. The Influence of the crystal-lattice structure on the conduction-band energy of oxides of titanium(IV). Chem. Phys. Lett. 1985, 118, 474–477. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zhu, S.K.; Pan, N. Studying the mechanisms of titanium dioxide as ultraviolet-blocking additive for films and fabrics by an improved scheme. J. Appl. Polym. Sci. 2004, 92, 3201–3210. [Google Scholar] [CrossRef]
- Luo, Z.; Poyraz, A.S.; Kuo, C.H.; Miao, R.; Meng, Y.T.; Chen, S.Y.; Jiang, T.; Wenos, C.; Suib, S.L. Crystalline mixed phase (anatase/rutile) mesoporous titanium dioxides for visible light photocatalytic activity. Chem. Mater. 2015, 27, 6–17. [Google Scholar] [CrossRef]
- Kumar, S.; Mishra, D.K.; Sobral, A.J.F.N.; Kohl, J. CO2 adsorption and conversion of epoxides catalyzed by inexpensive and active mesoporous structured mixed-phase (anatase/brookite) TiO2. J. CO2 Util. 2019, 34, 386–394. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Shao, G.N.; Kim, W.D.; Kim, H.T. Sol-gel synthesis of mesoporous anatase-brookite and anatase-brookite-rutile TiO2 nanoparticles and their photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 1–7. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Buasri, A.; Srilachai, C.; Srimuang, H. The synthesis of microporous and mesoporous titania with high specific surface area using sol-gel method and activated carbon templates. Mater. Lett. 2012, 87, 47–50. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.G.; Ho, W.K.; Zhang, L.Z. Preparation of highly photocatalytic active nano-sized TiO2 particles via ultrasonic irradiation. Chem. Commun. 2001, 1942–1943. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Zhang, L.Z.; Yu, J.G. Direct sonochemical preparation and characterization of highly active mesoporous TiO2 with a bicrystalline framework. Chem. Mater. 2002, 14, 4647–4653. [Google Scholar] [CrossRef]
- Mahoney, L.; Rasalingam, S.; Wu, C.M.; Peng, R.; Koodali, R.T. Aging dependent phase transformation of mesostructured titanium dioxide nanomaterials prepared by evaporation-induced self-assembly process: Implications for solar hydrogen production. AIMS Mater. Sci. 2015, 2, 230–242. [Google Scholar] [CrossRef]
- Masolo, E.; Meloni, M.; Garroni, S.; Mulas, G.; Enzo, S.; Baro, M.D.; Rossinyol, E.; Rzeszutek, A.; Herrmann-Geppert, I.; Pilo, M. Mesoporous titania powders: The role of precursors, ligand addition and calcination rate on their morphology, crystalline structure and photocatalytic activity. Nanomaterials 2014, 4, 583–598. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. One-step synthesis of mesoporous platinum/titania nanocomposites as photocatalyst with enhanced photocatalytic activity for methanol oxidation. Green Chem. 2011, 13, 428–435. [Google Scholar] [CrossRef]
- Atitar, M.F.; Ismail, A.A.; Al-Sayari, S.A.; Bahnemann, D.; Afanasev, D.; Emeline, A.V. Mesoporous TiO2 nanocrystals as efficient photocatalysts: Impact of calcination temperature and phase transformation on photocatalytic performance. Chem. Eng. J. 2015, 264, 417–424. [Google Scholar] [CrossRef]
- An, H.R.; Park, S.Y.; Kim, H.; Lee, C.Y.; Choi, S.; Lee, S.C.; Seo, S.; Park, E.C.; Oh, Y.K.; Song, C.G.; et al. Advanced nanoporous TiO2 photocatalysts by hydrogen plasma for efficient solar-light photocatalytic application. Sci. Rep. 2016, 6, 29683. [Google Scholar] [CrossRef]
- Shen, J.Y.; Wang, H.; Zhou, Y.; Ye, N.Q.; Li, G.B.; Wang, L.J. Anatase/rutile TiO2 nanocomposite microspheres with hierarchically porous structures for high-performance lithium-ion batteries. RSC Adv. 2012, 2, 9173–9178. [Google Scholar] [CrossRef]
- Qi, L.F.; Ho, W.K.; Wang, J.L.; Zhang, P.Y.; Yu, J.G. Enhanced catalytic activity of hierarchically macro-/mesoporous Pt/TiO2 toward room-temperature decomposition of formaldehyde. Catal. Sci. Technol. 2015, 5, 2366–2377. [Google Scholar] [CrossRef]
- Xie, Y.J.; Zhang, X.; Ma, P.J.; Wu, Z.J.; Piao, L.Y. Hierarchical TiO2 photocatalysts with a one-dimensional heterojunction for improved photocatalytic activities. Nano Res. 2015, 8, 2092–2101. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Asghar, B.H.M.; Muathen, H.A. Facile synthesis of mesoporous bicrystallized TiO2(B)/anatase (rutile) phases as active photocatalysts for nitrate reduction. Catal. Commun. 2012, 28, 58–63. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.L.; Tian, Y.; Lan, K.; Liu, Q.; Wang, C.Y.; Liu, Y.; Elzatahry, A.; Che, R.C.; Li, W.; et al. Synthesis of uniform ordered mesoporous TiO2 microspheres with controllable phase junctions for efficient solar water splitting. Chem. Sci. 2019, 10, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.L.; Shang, M.H.; Wang, L.; Li, W.E.; Tang, B.; Yang, W.Y. Efficient photocatalytic activities of TiO2 hollow fibers with mixed phases and mesoporous walls. Sci. Rep. 2015, 5, 15228. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yao, B.; Cao, Y.; Fan, K. Synthesis of well-ordered mesoporous titania with tunable phase content and high photoactivity. J. Phys. Chem. C 2007, 111, 11849–11853. [Google Scholar] [CrossRef]
- Masolo, E.; Senes, N.; Pellicer, E.; Baro, M.D.; Enzo, S.; Pilo, M.I.; Mulas, G.; Garroni, S. Evaluation of the anatase/rutile phase composition influence on the photocatalytic performances of mesoporous TiO2 powders. Int. J. Hydrog. Energy 2015, 40, 14483–14491. [Google Scholar] [CrossRef]
- Bai, Y.; Li, W.; Liu, C.; Yang, Z.H.; Feng, X.; Lu, X.H.; Chan, K.Y. Stability of Pt nanoparticles and enhanced photocatalytic performance in mesoporous Pt-(anatase/TiO2(B)) nanoarchitecture. J. Mater. Chem. 2009, 19, 7055–7061. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Khedr, T.M.; Hakki, A.; Ismail, A.A.; Badawy, W.A.; Bahnemann, D.W. Visible light activated carbon and co-doped mesoporous TiO2 as efficient photocatalyst for degradation of ibuprofen. Sep. Purif. Technol. 2017, 173, 258–268. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Ismail, A.A.; Kowalska, E.; Bahnemann, D.W. Photodegradation of microcystin-LR using visible light-activated C/N-co-modified mesoporous TiO2 photocatalyst. Materials 2019, 12, 1027. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Ismail, A.A.; Bahnemann, D.W. Photodegradation of 4-aminoantipyrine over nano-titania heterojunctions using solar and LED irradiation sources. J. Environ. Chem. Eng. 2019, 7, 102797. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Hakki, A.; Ismail, A.A.; Badawy, W.A.; Bahnemann, D.W. Highly active non-metals doped mixed-phase TiO2 for photocatalytic oxidation of ibuprofen under visible light. J. Photochem. Photobiol. A 2017, 346, 530–540. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Khedr, T.M.; Zhang, G.S.; Vogiazi, V.; Ismail, A.A.; O’Shea, K.; Dionysiou, D.D. Tailored synthesis of anatase-brookite heterojunction photocatalysts for degradation of cylindrospermopsin under UV-Vis light. Chem. Eng. J. 2017, 310, 428–436. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Abdeldayem, H.M.; Ismail, A.A.; Kowalska, E.; Bahnemann, D.W. A comparative study of microcystin-LR degradation by UV-A, solar and visible light irradiation using bare and C/N/S-modified titania. Catalysts 2019, 9, 877. [Google Scholar] [CrossRef]
- Neppolian, B.; Choi, H.C.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. Solar/UV-induced photocatalytic degradation of three commercial textile dyes. J. Hazard. Mater. 2002, 89, 303–317. [Google Scholar] [CrossRef]
- Mills, A.; Morris, S.; Davies, R. Photomineralisation of 4-chlorophenol sensitised by titanium dioxide: A study of the intermediates. J. Photochem. Photobiol. A Chem. 1993, 70, 183–191. [Google Scholar] [CrossRef]
- Mozia, S.; Tomaszewska, M.; Morawski, A.W. A new photocatalytic membrane reactor (PMR) for removal of azo-dye Acid Red 18 from water. Appl. Catal. B Environ. 2005, 59, 131–137. [Google Scholar] [CrossRef]
- Toepfer, B.; Gora, A.; Puma, G.L. Photocatalytic oxidation of multicomponent solutions of herbicides: Reaction kinetics analysis with explicit photon absorption effects. Appl. Catal. B Environ. 2006, 68, 171–180. [Google Scholar] [CrossRef]
- Grzechulska, J.; Morawski, A.W. Photocatalytic labyrinth flow reactor with immobilized P25 TiO2 bed for removal of phenol from water. Appl. Catal. B Environ. 2003, 46, 415–419. [Google Scholar] [CrossRef]
- Calza, P.; Minero, C.; Hiskia, A.; Papaconstantinou, E.; Pelizzetti, E. Photocatalytic transformations of CCl3Br, CBr3F, CHCl2Br and CH2BrCl in aerobic and anaerobic conditions. Appl. Catal. B Environ. 2001, 29, 23–34. [Google Scholar] [CrossRef]
- Tahiri, H.; Aitichou, Y.; Herrmann, J.M. Photocatalytic degradation of chlorobenzoic isomers in aqueous suspensions of neat and modified titania. J. Photochem. Photobiol. A 1998, 114, 219–226. [Google Scholar] [CrossRef]
- Ferguson, M.A.; Hering, J.G. TiO2-photocatalyzed As(III) oxidation in a fixed-bed, flow-through reactor. Environ. Sci. Technol. 2006, 40, 4261–4267. [Google Scholar] [CrossRef]
- Yao, Z.X.; Wang, M.Q.; Sun, S.H.; Jia, R.B.; Li, H. High performance photocatalysts based on N-doped graphene-P25 for photocatalytic reduction of carbon tetrachloride. J. Inorg. Organomet. Polym. Mater. 2014, 24, 315–320. [Google Scholar] [CrossRef]
- Jiao, Y.C.; Jiang, H.L.; Chen, F. RuO2/TiO2/Pt ternary photocatalysts with epitaxial heterojunction and their application in CO oxidation. ACS Catal. 2014, 4, 2249–2257. [Google Scholar] [CrossRef]
- Thevenet, F.; Guillard, C.; Rousseau, A. Acetylene photocatalytic oxidation using continuous flow reactor: Gas phase and adsorbed phase investigation, assessment of the photocatalyst deactivation. Chem. Eng. J. 2014, 244, 50–58. [Google Scholar] [CrossRef]
- Sakai, H.; Kubota, Y.; Yamaguchi, K.; Fukuoka, H.; Inumaru, K. Photocatalytic decomposition of 2-propanol and acetone in air by nanocomposites of pre-formed TiO2 particles and mesoporous silica. J. Porous Mater. 2013, 20, 693–699. [Google Scholar] [CrossRef]
- Toma, F.L.; Bertrand, G.; Chwa, S.O.; Meunier, C.; Klein, D.; Coddet, C. Comparative study on the photocatalytic decomposition of nitrogen oxides using TiO2 coatings prepared by conventional plasma spraying and suspension plasma spraying. Surf. Coat. Technol. 2006, 200, 5855–5862. [Google Scholar] [CrossRef]
- Nishikawa, N.; Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K.; Yamazaki, E.; Masuyama, K.; Hashimoto, T.; Kamiya, K. Photocatalytic degradation of allergens in water with titanium dioxide. Fresenius Environ. Bull. 2007, 16, 310–314. [Google Scholar]
- Markowska-Szczupak, A.; Wang, K.L.; Rokicka, P.; Endo, M.; Wei, Z.S.; Ohtani, B.; Morawski, A.W.; Kowalska, E. The effect of anatase and rutile crystallites isolated from titania P25 photocatalyst on growth of selected mould fungi. J. Photochem. Photobiol. B 2015, 151, 54–62. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Janda, K.; Wang, K.L.; Morawski, A.W.; Kowalska, E. Effect of water activity and titania P25 photocatalyst on inactivation of pathogenic fungi—Contribution to the protection of public health. Cen. Eur. J. Public Health 2015, 23, 267–271. [Google Scholar]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherischia coli - Effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Catal. B Environ. 2007, 76, 257–263. [Google Scholar] [CrossRef]
- Krishna, V.; Pumprueg, S.; Lee, S.H.; Zhao, J.; Sigmund, W.; Koopman, B.; Moudgil, B.M. Photocatalytic disinfection with titanium dioxide coated multi-wall carbon nanotubes. Process Saf. Environ. 2005, 83, 393–397. [Google Scholar] [CrossRef]
- Thabet, S.; Weiss-Gayet, M.; Dappozze, F.; Cotton, P.; Guillard, C. Photocatalysis on yeast cells: Toward targets and mechanisms. Appl. Catal. B Environ. 2013, 140, 169–178. [Google Scholar] [CrossRef]
- Helali, S.; Polo-Lopez, M.I.; Fernandez-Ibanez, P.; Ohtani, B.; Amano, F.; Malato, S.; Guillard, C. Solar photocatalysis: A green technology for E. coli contaminated water disinfection. Effect of concentration and different types of, suspended catalyst. J. Photochem. Photobiol. A 2014, 276, 31–40. [Google Scholar] [CrossRef]
- Bozzi, A.; Yuranova, T.; Guasaquillo, I.; Laub, D.; Kiwi, J. Self-cleaning of modified cotton textiles by TiO2 at low temperatures under daylight irradiation. J. Photochem. Photobiol. A 2005, 174, 156–164. [Google Scholar] [CrossRef]
- Smits, M.; Chan, C.K.; Tytgat, T.; Craeye, B.; Costarramone, N.; Lacombe, S.; Lenaerts, S. Photocatalytic degradation of soot deposition: Self-cleaning effect on titanium dioxide coated cementitious materials. Chem. Eng. J. 2013, 222, 411–418. [Google Scholar] [CrossRef]
- Vero, N.; Hribernik, S.; Andreozzi, P.; Sfiligoj-Smole, M. Homogeneous self-cleaning coatings on cellulose materials derived from TIP/TiO2 P25. Fiber Polym. 2009, 10, 716–723. [Google Scholar] [CrossRef]
- Antoniadou, M.; Lianos, P. Production of electricity by photoelectrochemical oxidation of ethanol in a PhotoFuelCell. Appl. Catal. B Environ. 2010, 99, 307–313. [Google Scholar] [CrossRef]
- Wang, K.L.; Wei, Z.S.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Wang, G.H.; Xu, L.; Zhang, J.; Yin, T.T.; Han, D.Y. Enhanced photocatalytic activity of TiO2 powders (P25) via calcination treatment. Int. J. Photoenergy 2012, 265760. [Google Scholar]
- Martin, S.T.; Herrmann, H.; Choi, W.; Hoffmann, M.R. Time-resolved microwave conductivity. Part 1. TiO2 photoreactivity and size quantization. J. Chem. Soc. Faraday Trans. 1994, 90, 3315–3322. [Google Scholar] [CrossRef]
- Szczepankiewicz, S.H.; Colussi, A.J.; Hoffmann, M.R. Infrared spectra of photoinduced species on hydroxylated titania surfaces. J. Phys. Chem. B 2000, 104, 9842–9850. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 2002, 26, 1167–1170. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 photocatalyst (Degussa, P 25) consisting of anatase and rutile crystalline phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Hurum, D.C.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Recombination pathways in the Degussa P25 formulation of TiO2: Surface versus lattice mechanisms. J. Phys. Chem. B 2005, 109, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Palmisano, L.; Schiavello, M. Photoreduction of dinitrogen and photooxidation of phenol and nitrophenol isomers as examples of heterogeneous photocatalytic reactions. Res. Chem. Intermed. 1992, 18, 211–226. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Meng, X.; Zeng, N.; Dan, Y.; Jiang, L. Covalent immobilization of TiO2 within macroporous polymer monolith as a facilely recyclable photocatalyst for water decontamination. Colloid Polym. Sci. 2018, 296, 1419–1429. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef]
- Theurich, J.; Lindner, M.; Bahnemann, D.W. Photocatalytic degradation of 4-chlorophenol in aerated aqueous titanium dioxide suspensions: A kinetic and mechanistic study. Langmuir 1996, 12, 6368–6376. [Google Scholar] [CrossRef]
- Wang, C.Y.; Boettcher, C.; Bahnemann, D.W.; Dohrmann, J.K. A comparative study of nanometer sized Fe(III)-doped TiO2 photocatalysts: Synthesis, characterization and activity. J. Mater. Chem. 2003, 13, 2322–2329. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Bickley, R.I.; Gonzalez-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. A structural investigation of titanium dioxide photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Van der Meulen, T.; Mattson, A.; Osterlund, L. A comparative study of the photocatalytic oxidation of propane on anatase, rutile, and mixed-phase anatase-rutile TiO2 nanoparticles: Role of surface intermediates. J. Catal. 2007, 251, 131–144. [Google Scholar] [CrossRef]
- Boehme, M.; Ensinger, W. Mixed Phase Anatase/rutile titanium dioxide nanotubes for enhanced photocatalytic degradation of methylene-blue. Nano Micro Lett. 2011, 3, 236–241. [Google Scholar] [CrossRef]
- Su, R.; Bechstein, R.; So, L.; Vang, R.T.; Sillassen, M.; Esbjornsson, B.; Palmqvist, A.; Besenbacher, F. How the Anatase-to-Rutile Ratio Influences the Photoreactivity of TiO2. J. Phys. Chem. C 2011, 115, 24287–24292. [Google Scholar] [CrossRef]
- Zhu, Q.; Qian, J.S.; Pan, H.; Tu, L.; Zhou, X.F. Synergistic manipulation of micro-nanostructures and composition: Anatase/rutile mixed-phase TiO2 hollow micro-nanospheres with hierarchical mesopores for photovoltaic and photocatalytic applications. Nanotechnology 2011, 22, 395703. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Lian, J.H.; Bai, Y.; Hui, J.; Zhong, J.B.; Li, J.Z.; An, N.; Yu, J.; Wang, F.P. Photocatalytic activity of hydrogen production from water over TiO2 with different crystal structures. Mater. Sci. Semicond. Process. 2015, 40, 418–423. [Google Scholar] [CrossRef]
- Semicond, C.; Ji, H.B. Comparison of TiO2 Degussa P25 with anatase and rutile crystalline phases for methane combustion. Chem. Eng. J. 2014, 243, 254–264. [Google Scholar]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the relationship between surface phases and photocatalytic activity of TiO2. Angew. Chem. Int. Ed. 2008, 47, 1766–1769. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; Van Langenhove, H.; Sercu, B. Gas-solid adsorption of selected volatile organic compounds on titanium dioxide Degussa P25. Chem. Eng. Sci. 2003, 58, 2255–2267. [Google Scholar] [CrossRef]
- Hurum, D.C.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Photoinitiated reactions of 2,4,6 TCP on Degussa P25 formulation TiO2: Wavelength-sensitive decomposition. J. Phys. Chem. B 2004, 108, 16483–16487. [Google Scholar] [CrossRef]
- Mi, Y.; Weng, Y.X. Band alignment and controllable electron migration between rutile and anatase TiO2. Sci. Rep. 2015, 5, 11482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.C.; Zakaria, R.; Ying, J.Y. Role of particle size in nanocrystalline TiO2-based photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Li, A.L.; Wang, Z.L.; Yin, H.; Wang, S.Y.; Yan, P.L.; Huang, B.K.; Wang, X.L.; Li, R.G.; Zong, X.; Han, H.X.; et al. Understanding the anatase-rutile phase junction in charge separation and transfer in a TiO2 electrode for photoelectrochemical water splitting. Chem. Sci. 2016, 7, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Z.; Manawan, M.; Feng, T.; Qian, R.F.; Zhao, T.; Zhou, G.D.; Kong, F.T.; Wang, Q.; Dai, S.Y.; Pan, J.H. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Jiang, D.L.; Zhang, S.Q.; Zhao, H.J. Photocatalytic degradation characteristics of different organic compounds at TiO2 nanoporous film electrodes with mixed anatase/rutile phases. Environ. Sci. Technol. 2007, 41, 303–308. [Google Scholar] [CrossRef]
- Xiong, Z.G.; Wu, H.; Zhang, L.H.; Gu, Y.; Zhao, X.S. Synthesis of TiO2 with controllable ratio of anatase to rutile. J. Mater. Chem. A 2014, 2, 9291–9297. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.J.; Feng, Z.C.; Chen, J.; Li, C. UV Raman spectroscopic study on TiO2. I. Phase transformation at the surface and in the bulk. J. Phys. Chem. B 2006, 110, 927–935. [Google Scholar] [CrossRef]
- Ohtani, B.; Azuma, Y.; Li, D.; Ihara, T.; Abe, R. Isolation of anatase crystallites from anatase-rutile mixed particles by dissolution with aqueous hydrogen peroxide and ammonia. Trans. Mater. Res. Soc. Jpn. 2007, 32, 401–404. [Google Scholar]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Photocatalytic activities of pure rutile particles isolated from TiO2 powder by dissolving the anatase component in HF solution. J. Phys. Chem. B 2001, 105, 2417–2420. [Google Scholar] [CrossRef]
- Macounova, K.; Urban, J.; Krysova, H.; Krysa, J.; Jirkovsky, J.; Ludvik, J. Photodegradation of metamitron (4-amino-6-phenyl-3-methyl-1,2,4-triazin-5(4H)-one) on TiO2. J. Photochem. Photobiol. A 2001, 140, 93–98. [Google Scholar] [CrossRef]
- Brigden, C.T.; Poulston, S.; Twigg, M.V.; Walker, A.P.; Wilkins, A.J.J. Photooxidation of short-chain hydrocarbons over titania. Appl. Catal. B Environ. 2001, 32, 63–71. [Google Scholar] [CrossRef]
- Tryba, B.; Morawski, A.W.; Inagaki, M. Application of TiO2-mounted activated carbon to the removal of phenol from water. Appl. Catal. B Environ. 2003, 41, 427–433. [Google Scholar] [CrossRef]
- Zielinska-Jurek, A.; Klein, M.; Hupka, J. Enhanced visible light photocatalytic activity of Pt/I-TiO2 in a slurry system and supported on glass packing. Sep. Purif. Technol. 2017, 189, 246–252. [Google Scholar] [CrossRef]
- Hupka, J.; Zaleska, A.; Janczarek, M.; Kowalska, E.; Gorska, P.; Aranowski, R. UV/VIS light-enhanced photocatalysis for water treatment and protection. In Soil and Water Pollution Monitoring, Protection and Remediation; Earth and Environmental Sciences; Twardowska, I., Allen, H.E., Häggblom, M.M., Stefaniak, S., Eds.; Springer: Berlin, Germany, 2006; Volume Nato Science Series: IV; pp. 151–166. [Google Scholar]
- Kowalska, E. Investigation of Photochemical Degradation of Organic Compounds. Ph.D. Thesis, Gdansk University of Technology, Gdansk, Poland, 2004. [Google Scholar]
- Kowalska, E.; Rau, S. Photoreactors for wastewater treatment: A review. Recent Pat. Eng. 2010, 4, 242–266. [Google Scholar] [CrossRef]
- Zielinska-Jurek, A.; Bielan, Z.; Wysocka, I.; Strychalska, J.; Janczarek, M.; Klimczuk, T. Magnetic semiconductor photocatalysts for the degradation of recalcitrant chemicals from flow back water. J. Environ. Manag. 2017, 195, 157–165. [Google Scholar] [CrossRef]
- Zielinska-Jurek, A.; Bielan, Z.; Dudziak, S.; Wolak, I.; Sobczak, Z.; Klimczuk, T.; Nowaczyk, G.; Hupka, J. Design and application of magnetic photocatalysts for water treatment. The effect of particle charge on surface functionality. Catalysts 2017, 7, 360. [Google Scholar] [CrossRef]
- Wysocka, I.; Kowalska, E.; Trzcinski, K.; Lapinski, M.; Nowaczyk, G.; Zielinska-Jurek, A. UV-Vis-Induced degradation of phenol over magnetic photocatalysts modified with Pt, Pd, Cu and Au nanoparticles. Nanomaterials 2018, 8, 28. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Vittadini, A.; Selloni, A.; Rotzinger, F.P.; Gratzel, M. Structure and energetics of water adsorbed at TiO2 anatase (101) and (001) surfaces. Phys. Rev. Lett. 1998, 81, 2954–2957. [Google Scholar] [CrossRef]
- Liu, G.; Yu, J.C.; Lu, G.Q.; Cheng, H.M. Crystal facet engineering of semiconductor photocatalysts: Motivations, advances and unique properties. Chem. Commun. 2011, 47, 6763–6783. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.H.; Lu, C.H.; Wang, J.; Chen, Y.K.; Xu, Z.Z.; Varma, R.S. Selectivity enhancement in heterogeneous photocatalytic transformations. Chem. Rev. 2017, 117, 1445–1514. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B 2001, 63, 155409. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H.M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, M.; Vanderbilt, A. First-principles calculations of the energetics of stoichiometric TiO2 surfaces. Phys. Rev. B 1994, 49, 16721–16727. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Q.; Selloni, A. First-principles study of the structures and energetics of stoichiometric brookite TiO2 surfaces. Phys. Rev. B 2007, 76, 235307. [Google Scholar] [CrossRef]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with dominant {001} facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef]
- Murakami, N.; Kurihara, Y.; Tsubota, T.; Ohno, T. Shape-controlled anatase titanium(IV) oxide particles prepared by hydrothermal treatment of peroxo Titanic Acid in the Presence of Polyvinyl Alcohol. J. Phys. Chem. C 2009, 113, 3062–3069. [Google Scholar] [CrossRef]
- Han, X.G.; Wang, X.; Xie, S.F.; Kuang, Q.; Ouyang, J.J.; Xie, Z.X.; Zheng, L.S. Carbonate ions-assisted syntheses of anatase TiO2 nanoparticles exposed with high energy (001) facets. RSC Adv. 2012, 2, 3251–3253. [Google Scholar] [CrossRef]
- Murakami, N.; Katayama, S.; Nakamura, M.; Tsubota, T.; Ohno, T. Dependence of photocatalytic activity on aspect ratio of shape-controlled rutile titanium(IV) oxide nanorods. J. Phys. Chem. C 2011, 115, 419–424. [Google Scholar] [CrossRef]
- Dinh, C.T.; Nguyen, T.D.; Kleitz, F.; Do, T.O. Shape-controlled synthesis of highly crystalline titania nanocrystals. ACS Nano 2009, 3, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Amano, F.; Prieto-Mahaney, O.O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral single-crystalline particles of anatase titanium(IV) oxide with high photocatalytic activity. Chem. Mater. 2009, 21, 2601–2603. [Google Scholar] [CrossRef]
- Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for crystal-face-dependent TiO2 photocatalysis from single-molecule imaging and kinetic analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takashima, M.; Takase, M.; Ohtani, B. Mechanistic study on facet-dependent deposition of metal nanoparticles on decahedral-shaped anatase titania photocatalyst particles. Catalysts 2018, 8, 542. [Google Scholar] [CrossRef]
- Wei, Z.; Kowalska, E.; Verrett, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication-hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef]
- Wei, Z.S.; Kowalska, E.; Ohtani, B. Enhanced photocatalytic activity by particle morphology: Preparation, characterization, and photocatalytic activities of octahedral anatase titania particles. Chem. Lett. 2014, 43, 346–348. [Google Scholar] [CrossRef]
- Kowalski, D.; Schmuki, P. Polypyrrole self-organized nanopore arrays formed by controlled electropolymerization in TiO2 nanotube template. Chem. Commun. 2010, 46, 8585–8587. [Google Scholar] [CrossRef]
- Kozak, M.; Mazierski, P.; Zebrowska, J.; Kobylanski, M.; Klimczuk, T.; Lisowski, W.; Trykowski, G.; Nowaczyk, G.; Zaleska-Medynska, A. Electrochemically obtained TiO2/CuxOy nanotube arrays presenting a photocatalytic response in processes of pollutants degradation and bacteria inactivation in aqueous phase. Catalysts 2018, 8, 237. [Google Scholar] [CrossRef]
- Mazierski, P.; Malankowska, A.; Kobylanski, M.; Diak, M.; Kozak, M.; Winiarski, M.J.; Klimczuk, T.; Lisowski, W.; Nowaczyk, G.; Zaleska-Medynska, A. Photocatalytically active TiO2/Ag2O nanotube arrays interlaced with silver nanoparticles obtained from the one-step anodic oxidation of Ti-Ag alloys. ACS Catal. 2017, 7, 2753–2764. [Google Scholar] [CrossRef]
- Nischk, M.; Mazierski, P.; Wei, Z.S.; Siuzdak, K.; Kouame, N.A.; Kowalska, E.; Remita, H.; Zaleska-Medynska, A. Enhanced photocatalytic, electrochemical and photoelectrochemical properties of TiO2 nanotubes arrays modified with Cu, AgCu and Bi nanoparticles obtained via radiolytic reduction. Appl. Surf. Sci. 2016, 387, 89–102. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Imai, H.; Takei, Y.; Shimizu, K.; Matsuda, M.; Hirashima, H. Direct preparation of anatase TiO2 nanotubes in porous alumina membranes. J. Mater. Chem. 1999, 9, 2971–2972. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Beranek, R.; Tsuchiya, H.; Sugishima, T.; Macak, J.M.; Taveira, L.; Fujimoto, S.; Kisch, H.; Schmuki, P. Enhancement and limits of the photoelectrochemical response from anodic TiO2 nanotubes. Appl. Phys. Lett. 2005, 87, 243114. [Google Scholar] [CrossRef]
- Balaur, E.; Macak, J.M.; Taveira, L.; Schmuki, P. Tailoring the wettability of TiO2 nanotube layers. Electrochem. Commun. 2005, 7, 1066–1070. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
- Shankar, K.; Basham, J.I.; Allam, N.K.; Varghese, O.K.; Mor, G.K.; Feng, X.J.; Paulose, M.; Seabold, J.A.; Choi, K.S.; Grimes, C.A. Recent advances in the use of TiO2 nanotube and nanowire arrays for oxidative photoelectrochemistry. J. Phys. Chem. C 2009, 113, 6327–6359. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem. Commun. 2009, 20, 2791–2808. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Rohani, S. Modified TiO2 nanotube arrays (TNTAs): Progressive strategies towards visible light responsive photoanode, a review. Energy Environ. Sci. 2011, 4, 1065–1086. [Google Scholar] [CrossRef]
- Yablonovitch, E. Photonic Band-Gap Structures. J. Opt. Soc. Am. B 1993, 10, 283–295. [Google Scholar] [CrossRef]
- Lopez, C. Materials aspects of photonic crystals. Adv. Mater. 2003, 15, 1679–1704. [Google Scholar] [CrossRef]
- Kim, W.T.; Choi, W.Y. Fabrication of TiO2 photonic crystal by anodic oxidation and their optical sensing properties. Sens. Actuators A Phys. 2017, 260, 178–184. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the photonic crystal properties of TiO2 nanotube arrays to enhance photocatalytic hydrogen production. ACS Catal. 2016, 6, 1345–1353. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yang, X.L.; Hedhili, M.N.; Ahmed, E.; Shi, L.; Wang, P. Microwave-assisted self-doping of TiO2 photonic crystals for efficient photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2014, 6, 691–696. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wu, H.J. Multiple band light trapping in ultraviolet, visible and near infrared regions with TiO2 based photonic materials. Chem. Commun. 2014, 50, 14179–14182. [Google Scholar] [CrossRef]
- Li, Z.Z.; Xin, Y.M.; Wu, W.L.; Fu, B.H.; Zhang, Z.H. Phosphorus cation doping: A new strategy for boosting photoelectrochemical performance on TiO2 nanotube photonic crystals. ACS Appl. Mater. Interfaces 2016, 8, 30972–30979. [Google Scholar] [CrossRef]
- Deng, X.Q.; Zhu, X.B.; Sun, Z.G.; Li, X.S.; Liu, J.L.; Shi, C.; Zhu, A.M. Exceptional activity for photocatalytic mineralization of formaldehyde over amorphous titania nanofilms. Chem. Eng. J. 2016, 306, 1001–1009. [Google Scholar] [CrossRef]
- Baba, K.; Bulou, S.; Quesada-Gonzalez, M.; Bonot, S.; Collard, D.; Boscher, N.D.; Choquet, P. Significance of a noble metal nanolayer on the UV and visible light photocatalytic activity of anatase TiO2 thin films grown from a scalable PECVD/PVD approach. ACS Appl. Mater. Interfaces 2017, 9, 41200–41209. [Google Scholar] [CrossRef]
- Das, S.K.; Schwanke, C.; Pfuch, A.; Seeber, W.; Bock, M.; Steinmeyer, G.; Elsaesser, T.; Grunwald, R. Highly efficient THG in TiO2 nanolayers for third-order pulse characterization. Opt. Express 2011, 19, 16985–16995. [Google Scholar] [CrossRef] [PubMed]
- Habazaki, H.; Oikawa, Y.; Fushimi, K.; Shimizu, K.; Nagata, S.; Skeldon, P.; Thompson, G.E. Formation of porous anodic films on Ti-Si alloys in hot phosphate-glycerol electrolyte. Electrochim. Acta 2007, 53, 1775–1781. [Google Scholar] [CrossRef][Green Version]
- Darko, S.A.; Maxwell, E.; Park, S. Photocatalytic activity of TiO2 nanofilms deposited onto polyvinyl chloride and glass substrates. Thin Solid Films 2010, 519, 174–177. [Google Scholar] [CrossRef]
- Guo, W.; Feng, Q.Q.; Tao, Y.F.; Zheng, L.J.; Han, Z.Y.; Ma, J.M. Systematic investigation on the gas-sensing performance of TiO2 nanoplate sensors for enhanced detection on toxic gases. Mater. Res. Bull. 2016, 73, 302–307. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent advances in graphene based TiO2 nanocomposites (GTiO2Ns) for photocatalytic degradation of synthetic dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Morawski, A.W. TiO2/graphene-based nanocomposites for water treatment: A brief overview of charge carrier transfer, antimicrobial and photocatalytic performance. Appl. Catal. B Environ. 2019, 253, 179–186. [Google Scholar] [CrossRef]
- Schneider, M.; Baiker, A. High-surface-area titania aerogels—Preparation and structural-properties. J. Mater. Chem. 1992, 2, 587–589. [Google Scholar] [CrossRef]
- Campbell, L.K.; Na, B.K.; Ko, E.I. Synthesis and characterization of titania aerogels. Chem. Mater. 1992, 4, 1329–1333. [Google Scholar] [CrossRef]
- Malinowska, B.; Walendziewski, J.; Robert, D.; Weber, J.V.; Stolarski, M. Titania aerogels: Preparation and photocatalytic tests. Int. J. Photoenergy 2003, 5, 147–152. [Google Scholar] [CrossRef]
- Subrt, J.; Plizingrova, E.; Palkovska, M.; Bohacek, J.; Klementova, M.; Kupcik, J.; Bezdicka, P.; Sovova, H. Titania aerogels with tailored nano and microstructure: Comparison of lyophilization and supercritical drying. Pure Appl. Chem. 2017, 89, 501–509. [Google Scholar] [CrossRef]
- Kowalska, E. Plasmonic photocatalysis. In Gold Nanoparticles for Physics, Chemistry and Biology, 2nd ed.; Louis, C., Pluchery, O., Eds.; World Scientific: Singapore, 2017; pp. 319–364. [Google Scholar]
- DeSario, P.A.; Pietron, J.J.; DeVantier, D.E.; Brintlinger, T.H.; Stroud, R.M.; Rolison, D.R. Plasmonic enhancement of visible-light water splitting with Au-TiO2 composite aerogels. Nanoscale 2013, 5, 8073–8083. [Google Scholar] [CrossRef] [PubMed]
- Panayotov, D.A.; DeSario, P.A.; Pietron, J.J.; Brintlinger, T.H.; Szymczak, L.C.; Rolison, D.R.; Morris, J.R. Ultraviolet and visible photochemistry of methanol at 3D mesoporous networks: TiO2 and Au-TiO2. J. Phys. Chem. C 2013, 117, 15035–15049. [Google Scholar] [CrossRef]
- Likodimos, V. Photonic crystal-assisted visible light activated TiO2 photocatalysis. Appl. Catal. B Environ. 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.G.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Chiang, C.C.; Tuyen, L.D.; Ren, C.R.; Chau, L.K.; Wu, C.Y.; Huang, P.J.; Hsu, C.C. Fabrication of titania inverse opals by multi-cycle dip-infiltration for optical sensing. Photonics Nanostructures Fundam. Appl. 2016, 19, 48–54. [Google Scholar] [CrossRef]
- Lu, X.Y.; Zhu, Y.; Cen, T.Z.; Jiang, L. Centimeter-scale colloidal crystal belts via robust self-assembly strategy. Langmuir 2012, 28, 9341–9346. [Google Scholar] [CrossRef]
- Jiang, P.; Bertone, J.F.; Hwang, K.S.; Colvin, V.L. Single-crystal colloidal multilayers of controlled thickness. Chem. Mater. 1999, 11, 2132–2140. [Google Scholar] [CrossRef]
- Li, H.; Vienneau, G.; Jones, M.; Subramanian, B.; Robichaud, J.; Djaoued, Y. Crack-free 2D-inverse opal anatase TiO2 films on rigid and flexible transparent conducting substrates: Low temperature large area fabrication and electrochromic properties. J. Mater. Chem. C 2014, 2, 7804–7810. [Google Scholar] [CrossRef]
- Mayoral, R.; Requena, J.; Moya, J.S.; Lopez, C.; Cintas, A.; Miguez, H.; Meseguer, F.; Vazquez, L.; Holgado, M.; Blanco, A. 3D long-range ordering in an SiO2 submicrometer-sphere sintered superstructure. Adv. Mater. 1997, 9, 257–260. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Quan, M.H.; Zhao, W.D.; Yang, Z.; Wang, D.; Cao, H.; He, W.L. Preferential self-assembly behavior of polydisperse silica particles under negative pressure. Colloid Surf. A 2017, 529, 832–839. [Google Scholar] [CrossRef]
- Zhang, J.H.; Liu, H.Y.; Wang, Z.L.; Ming, N.B. Assembly of high-quality colloidal crystals under negative pressure. J. Appl. Phys. 2008, 103, 013517. [Google Scholar] [CrossRef]
- Kubrin, R.; Pasquarelli, R.M.; Waleczek, M.; Lee, H.S.; Zierold, R.; do Rosario, J.J.; Dyachenko, P.N.; Moreno, J.M.M.; Petrov, A.Y.; Janssen, R.; et al. Bottom-up fabrication of multilayer stacks of 3D photonic crystals from titanium dioxide. ACS Appl. Mater. Interfaces 2016, 8, 10466–10476. [Google Scholar] [CrossRef] [PubMed]
- Curti, M.; Robledo, G.L.; Claro, P.C.D.; Ubogui, J.H.; Mendive, C.B. Characterization of titania inverse opals prepared by two distinct infiltration approaches. Mater. Res. Bull. 2018, 101, 12–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Su, F.Y.; Cai, Z.Y.; Liu, J.X.; Wu, X.W.; He, H.L.; Yin, Z.; Wang, L.H.; Wang, B.; et al. Electrically switchable photonic crystals based on liquid-crystal-infiltrated TiO2-inverse opals. Opt. Express 2019, 27, 15391–15398. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Thiyagarajan, P.; Ahn, H.J.; Kim, S.I.; Jang, J.H. Optimization for visible light photocatalytic water splitting: Gold-coated and surface-textured TiO2 inverse opal nano-networks. Nanoscale 2013, 5, 6254–6260. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, P.; Liu, L.X.; Cheng, B.Y. Study on the sedimentation self-assembly of colloidal SiO2 particles under gravitational field. Colloid Surf. A 2005, 253, 169–174. [Google Scholar] [CrossRef]
- Hua, C.X.; Xu, H.B.; Zhang, P.P.; Chen, X.Y.; Lu, Y.Y.; Gan, Y.; Zhao, J.P.; Li, Y. Process optimization and optical properties of colloidal self-assembly via refrigerated centrifugation. Colloid Polym. Sci. 2017, 295, 1655–1662. [Google Scholar] [CrossRef]
- Miguez, H.; Meseguer, F.; Lopez, C.; Blanco, A.; Moya, J.S.; Requena, J.; Mifsud, A.; Fornes, V. Control of the photonic crystal properties of fcc-packed submicrometer SiO2 spheres by sintering. Adv. Mater. 1998, 10, 480–483. [Google Scholar] [CrossRef]
- Jovic, V.; Idriss, H.; Waterhouse, G.I.N. Slow photon amplification of gas-phase ethanol photo-oxidation in titania inverse opal photonic crystals. Chem. Phys. 2016, 479, 109–121. [Google Scholar] [CrossRef]
- Cheng, C.W.; Karuturi, S.K.; Liu, L.J.; Liu, J.P.; Li, H.X.; Su, L.T.; Tok, A.I.Y.; Fan, H.J. Quantum-dot-sensitized TiO2 inverse opals for photoelectrochemical hydrogen generation. Small 2012, 8, 37–42. [Google Scholar] [CrossRef]
- Liu, L.J.; Karuturi, S.K.; Su, L.T.; Tok, A.I.Y. TiO2 inverse-opal electrode fabricated by atomic layer deposition for dye-sensitized solar cell applications. Energy. Environ. Sci. 2011, 4, 209–215. [Google Scholar] [CrossRef]
- Li, X.H.; Wu, Y.; Shen, Y.H.; Sun, Y.; Yang, Y.; Xie, A.J. A novel bifunctional Ni-doped TiO2 inverse opal with enhanced SERS performance and excellent photocatalytic activity. Appl. Surf. Sci. 2018, 427, 739–744. [Google Scholar] [CrossRef]
- Moon, J.H.; Cho, Y.S.; Yang, S.M. Room temperature chemical vapor deposition for fabrication of titania inverse opals: Fabrication, morphology analysis and optical characterization. Bull. Korean Chem. Soc. 2009, 30, 2245–2248. [Google Scholar]
- Curti, M.; Mendive, C.B.; Grela, M.A.; Bahnemann, D.W. Stopband tuning of TiO2 inverse opals for slow photon absorption. Mater. Res. Bull. 2017, 91, 155–165. [Google Scholar] [CrossRef]
- Sordello, F.; Duca, C.; Maurino, V.; Minero, C. Photocatalytic metamaterials: TiO2 inverse opals. Chem. Commun. 2011, 47, 6147–6149. [Google Scholar] [CrossRef]
- Wu, M.; Liu, J.; Jin, J.; Wang, C.; Huang, S.Z.; Deng, Z.; Li, Y.; Su, B.L. Probing significant light absorption enhancement of titania inverse opal films for highly exalted photocatalytic degradation of dye pollutants. Appl. Catal. B Environ. 2014, 150, 411–420. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, H.T.; Chen, S.; Quan, X.; Zhao, H.M. Integrating plasmonic nanoparticles with TiO2 photonic crystal for enhancement of visible-light-driven photocatalysis. Environ. Sci. Technol. 2012, 46, 1724–1730. [Google Scholar] [CrossRef]
- Curti, M.; Zvitco, G.; Grela, M.A.; Mendive, C.B. Angle dependence in slow photon photocatalysis using TiO2 inverse opals. Chem. Phys. 2018, 502, 33–38. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Yang, B.F.; Xu, J.; Fu, Z.P.; Wu, M.; Li, F. Facile synthesis of Ag nanoparticles supported on TiO2 inverse opal with enhanced visible-light photocatalytic activity. Thin Solid Films 2012, 520, 3515–3522. [Google Scholar] [CrossRef]
- Ye, J.; He, J.H.; Wang, S.; Zhou, X.J.; Zhang, Y.; Liu, G.; Yang, Y.F. Nickel-loaded black TiO2 with inverse opal structure for photocatalytic reduction of CO2 under visible light. Sep. Purif. Technol. 2019, 220, 8–15. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, J.; Gao, X.N.; Shen, Y.H.; Xie, A.J. Ordered CdSe-sensitized TiO2 inverse opal film as multifunctional surface-enhanced Raman scattering substrate. Appl. Surf. Sci. 2019, 463, 357–362. [Google Scholar] [CrossRef]
- Zheng, X.Z.; Li, D.Z.; Li, X.F.; Chen, J.; Cao, C.S.; Fang, J.L.; Wang, J.B.; He, Y.H.; Zheng, Y. Construction of ZnO/TiO2 photonic crystal heterostructures for enhanced photocatalytic properties. Appl. Catal. B Environ. 2015, 168, 408–415. [Google Scholar] [CrossRef]
- Srinivasan, M.; White, T. Degradation of methylene blue by three-dimensionally ordered macroporous titania. Environ. Sci. Technol. 2007, 41, 4405–4409. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, J.; Wang, X.; Xu, H.; Yuan, S.; Zhang, Q.; Zhang, M. Preparation of inverse opal titanium dioxide for photocatalytic performance research. Opt. Mater. 2019, 96, 109287. [Google Scholar] [CrossRef]
- Rahul, T.K.; Sandhyarani, N. Nitrogen-fluorine co-doped titania inverse opals for enhanced solar light driven photocatalysis. Nanoscale 2015, 7, 18259–18270. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Z.; Li, D.Z.; Li, X.F.; Yu, L.H.; Wang, P.; Zhang, X.Y.; Fang, J.L.; Shao, Y.; Zheng, Y. Photoelectrocatalytic degradation of Rhodamine B on TiO2 photonic crystals. Phys. Chem. Chem. Phys. 2014, 16, 15299–15306. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, G.I.N.; Wahab, A.K.; Al-Oufi, M.; Jovic, V.; Anjum, D.H.; Sun-Waterhouse, D.; Llorca, J.; Idriss, H. Hydrogen production by tuning the photonic band gap with the electronic band gap of TiO2. Sci. Rep. 2013, 3, 2849. [Google Scholar] [CrossRef]
- Rahul, T.K.; Sandhyarani, N. In situ gold-loaded fluorinated titania inverse opal photocatalysts for enhanced solar-light-driven hydrogen production. ChemNanoMat 2017, 3, 503–510. [Google Scholar] [CrossRef]
- Cai, J.M.; Wu, M.Q.; Wang, Y.T.; Zhang, H.; Meng, M.; Tian, Y.; Li, X.G.; Zhang, J.; Zheng, L.R.; Gong, J.L. Synergetic enhancement of light harvesting and charge separation over surface-disorder-engineered TiO2 photonic crystals. Chem 2017, 2, 877–892. [Google Scholar] [CrossRef]
- Zhou, L.; O’Brien, P. Mesocrystals—Properties and Applications. J. Phys. Chem. Lett. 2012, 3, 620–628. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.Y. Organic small molecule-assisted synthesis of high active TiO2 rod-like mesocrystals. CrystEngComm 2010, 12, 2073–2078. [Google Scholar] [CrossRef]
- Sturm, E.V.; Cölfen, H. Mesocrystals: Structural and morphogenetic aspects. Chem. Soc. Rev. 2016, 45, 5821–5833. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, E.; Minnekhanov, A.; Beltiukov, A.; Ivanov, V.; Sutherland, A.J.; Boytsova, O. Unveiling point defects in titania mesocrystals: A combined EPR and XPS study. New J. Chem. 2018, 42, 15184–15189. [Google Scholar] [CrossRef]
- Hu, H.; Yang, Y.; Ou, Y.; Liu, B.; Li, J.; Yuan, W.; Wang, Y.; Zhang, Z. Early stage growth of rutile titania mesocrystals. Cryst. Growth Des. 2018, 18, 4209–4214. [Google Scholar]
- Bian, Z.F.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 superstructure-based plasmonic photocatalysts exhibiting efficient charge separation and unprecedented activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef]
- Ao, Y.H.; Xu, J.L.; Gao, Y.Y.; Wang, P.F.; Wang, C.; Hou, J.; Qian, J. Preparation of Ag nanoparticles loaded TiO2 nanoplate arrays on activated carbon fibers with enhanced photocatalytic activity. Catal. Commun. 2014, 53, 21–24. [Google Scholar] [CrossRef]







| Rutile | Anatase | Brookite | ||
|---|---|---|---|---|
| atomic spacing (Å) a | a = 4.596 c = 2.958 | a = 3.793 c = 9.510 | a = 5.456 b = 9.182 c = 5.143 | |
| axial ratios | a:c = 1:0.64388 | a:c = 1:2.50725 | a:b:c = 0.5942:1:0.5601 | |
| molecule/unit cell a | 2 | 4 | 8 | |
| density (g cm−3) a | 4.25 | 3.88 | 4.12 | |
| crystal system a | tetragonal (ditetragonal dipyramidal) | tetragonal (ditetragonal dipyramidal) | orthorombic (dipyramidal) | |
| bandgap (eV) | 3.02 d | 3.23 d | 3.14 e | |
| absorption edge (nm) | calculated b | 410 | 384 | 395 |
| measured c | 413 f | 388 f | 395 e | |
| Ti Precursor | Method | Crystalline Structure a | BET b | Application | Ref. |
|---|---|---|---|---|---|
| TIP | sol–gel | A(21%–86%) R(14%–79%) | 50–94 | degradation of MB, phenol, and 4ClP | [18] |
| TIP | sol–gel | A(60%–83%)/B(17%–40%) | 115 | conversion of CO2 | [19] |
| TIP | sol–gel | A(76.2%–84.6%)/B(12.8%–3.8%)/R(0%–2.6%) | 48–276 | degradation of MB | [20] |
| TIP | sol–gel | A(53%–96%)/R(4%–47%) | 62–90 | - | [21] |
| TIP | sol–gel (+ US) | A(79.6%–91.6%)/B(9.4%–20.4) | 75.5 | oxidation of acetone | [22] |
| TIP | sonochemical | A(49.5%–60%)/B(40%–50.5%) | 112–172 | n-pentane oxidation | [23] |
| TIP | EISA | A(29%–95%)/R(5%–71%) | 145–161 | generation of H2 | [24] |
| TIP/HCl | EISA | A(36%–70%) R(19%–61%)/B(3%–25%) | 96.1–77.8 | water splitting | [25] |
| TBu | sol-gel | A(65.5%–80.1%)/R(17.7%–34%) | 134–169 | CH3OH oxidation | [26] |
| TBu | sol-gel | A(18.1%–73.3%/R(16.8%–52.7%) | 35–165 | phenol degradation | [27] |
| TBu | sol-gel and H2 plasma | A/B | 271.8–427.5 | degradation of phenol, RhB and RB5 | [28] |
| TBu | hydrothermal | A(72%)/R(28%) | 54.1 | lithium-ion batteries | [29] |
| TBu | hydrothermal | A/B | 129–174 | HCHO degradation | [30] |
| TBu | hydrothermal | A(64.9%)/R(35.1%) | 260 | MB degradation, H2 evolution | [31] |
| TBu | hydrothermal self-assembly | A(32–61.4 R(0%–20%)/B’(0%–48%) | 104.3–129.9 | reduction of nitrate | [32] |
| TBu | coordination- self-assembly | A(40%–77%) R(23%–60%) | 63.6–78.4 | water splitting | [33] |
| TBu | electrospinning | A(94.6%) R(5.4%) | 27.2 | H2 generation and RhB degradation | [34] |
| TBu/TiCl4 | EISA | A(73%–98%)/R(2%–27%) | 92–135 | phenol degradation | [35] |
| TBu/TiCl4 | EISA | A(64%–91%)/R(9%–30%) | 90–118 | water splitting | [25] |
| TBu/TiCl4 | EISA | A(74%–92%)/R(8%–26%) | 73 | H2 generation | [36] |
| K2Ti2O5 | soft-chemistry template-free | A/B’ | 129 | degradation of CHCl3 and H2 generation | [37] |
| Ti2(SO4)3 | hydrothermal | A(64%–87%)/B(13%–36%) | 30–72 | IBU degradation | [38] |
| Ti2(SO4)3 | hydrothermal | C/N-A/B | 20.5–66 | MC-LR degradation | [39] |
| Ti2(SO4)3 | hydrothermal | A(61.8%) B(38.2%) | 62.3 | IBU, 4AAP, CYN, and MC-LR degradation | [40,41,42,43] |
| IO-PCs | Opal/Template | Infiltration Method | Photocatalytic Tests | Findings | Ref. |
|---|---|---|---|---|---|
| IO film | PMMA | CF | SA degr. at 400 nm (0–50°) | PBG max = 490 nm most effective at 40° | [192] |
| IO films | PS | CF | MB degr.; λ = 450 nm CH3CHO(g) degr. at 365 nm | high activity | [188] |
| IO films | PS | ALD | IO as photoanode (DSSCs) | PCE of 2.22% | [185] |
| IO films | PS | CF | RhB degr. at 250800 nm | 95% degr. at 0° | [190] |
| IO films | PS | SW | RhB degr. λ = 320–800 nm | VSDA | [200] |
| LP-IO film | PMMA | CF | MB degr. at 400 nm | high film stability | [177] |
| IO film with LC | PS | SW-vacuum | - | PBGmax = 452 and 528 nm switchable PCs | [178] |
| IO powd. and films | PMMA | vacuum CF | ethanol(g) oxidation λmax = 365 nm | VSDA | [183] |
| IO powd. | PS | CF | MB degr. λ >400 nm | VSDA | [198] |
| IO powd. | PMMA | vacuum | phenol degr. λmax = 365 nm | VSDA | [189] |
| Au/ IO powd. | PMMA | CF | water splitting (H2) under UV and solar | Au/IO—H2 generation | [201] |
| Au/IO film | PS | CF | PEC water splitting | Au/st-IO with high PD | [179] |
| Ag/IO film | SiO2 | dip coating | MB degr. λ = 254 nm and λ = 400–760 nm | Ag-enhanced activity | [193] |
| CdSe/IO | PS | dip coating | SERS for MO PEC measurement | CdSe-IO as SERS substrate with a high sensitivity | [195] |
| CdS/IO film | PS | ALD | PEC; solar | CdS/IO with high PD | [184] |
| Ni/ IO film | PS | spin coating | SERS for 4-MBA MO degr. λ >400 nm | Ni/IO for SERS with high detection ability | [186] |
| BIO/Ni film | PS | SW and CF | CO evolution; λ >400 nm | high rate of CO generation | [194] |
| Au/F/IO film | PS | SW and CF | H2 evolution; λ >400 nm | high rate of H2 generation | [202] |
| (N-F)/IO film | PS | SW aided by CF | RhB degr.; solar | PBG max = 336 and 850 nm 100% RhB degr. | [199] |
| IO/Au | PS | LPD | 2,4-DCP degr.; λ >420 nm | VSDA | [191] |
| H/IO powder | PS | forced impregn. | H2 evolution; solar | VSDA | [203] |
| ZnO/IO film | PS | SW | MO degr.; λ = 320–800 nm | VSDA | [196] |
| Dimension | Morphology | Preparation | Schemes | Applications | References |
|---|---|---|---|---|---|
| 0D | nanorice, core–shells, NPs faceted particles | sol–gel, hydrothermal, solvothermal, gas-phase |  | antifogging, antiseptic, self-cleaning surfaces, photocatalysts | [78,112,113,114,115,116,117,118,119,120,121,122] |
| 1D | nanotubes, nanowires, nanorods | anodization, hydrothermal |  | solar cells, batteries, photocatalysts | [131,132,133,134,135,136,137,138,139,140,141,142,143,144,145] |
| 2D | nanoplates, nanosheets, nanolayers | hydrothermal, solvothermal, CVD, PECVD, SILAR |  | sensors for toxic gas, photocatalysts, solar cells | [150,151,152,153,154,155,210] |
| 3D | aerogels, inverse-opal photonic crystals, mesocrystals | Sol–gel, template, self-assembling, hydrothermal |  | photocatalysts, solar cells, energy storage, | [158,159,160,161], [163,164], [168,169,170], [202,203,204] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Janczarek, M.; Wei, Z.; Raja-Mogan, T.; Endo-Kimura, M.; Khedr, T.M.; Ohtani, B.; Kowalska, E. Morphology- and Crystalline Composition-Governed Activity of Titania-Based Photocatalysts: Overview and Perspective. Catalysts 2019, 9, 1054. https://doi.org/10.3390/catal9121054
Wang K, Janczarek M, Wei Z, Raja-Mogan T, Endo-Kimura M, Khedr TM, Ohtani B, Kowalska E. Morphology- and Crystalline Composition-Governed Activity of Titania-Based Photocatalysts: Overview and Perspective. Catalysts. 2019; 9(12):1054. https://doi.org/10.3390/catal9121054
Chicago/Turabian StyleWang, Kunlei, Marcin Janczarek, Zhishun Wei, Tharishinny Raja-Mogan, Maya Endo-Kimura, Tamer M. Khedr, Bunsho Ohtani, and Ewa Kowalska. 2019. "Morphology- and Crystalline Composition-Governed Activity of Titania-Based Photocatalysts: Overview and Perspective" Catalysts 9, no. 12: 1054. https://doi.org/10.3390/catal9121054
APA StyleWang, K., Janczarek, M., Wei, Z., Raja-Mogan, T., Endo-Kimura, M., Khedr, T. M., Ohtani, B., & Kowalska, E. (2019). Morphology- and Crystalline Composition-Governed Activity of Titania-Based Photocatalysts: Overview and Perspective. Catalysts, 9(12), 1054. https://doi.org/10.3390/catal9121054











