Abstract
The delay in the energy transition, focused in the replacement of fossil diesel with biodiesel, is mainly caused by the need of reducing the costs associated to the transesterification reaction of vegetable oils with methanol. This reaction, on an industrial scale, presents several problems associated with the glycerol generated during the process. The costs to eliminate this glycerol have to be added to the implicit cost of using seed oil as raw material. Recently, several alternative methods to convert vegetable oils into high quality diesel fuels, which avoid the glycerol generation, are being under development, such as Gliperol, DMC-Biod, or Ecodiesel. Besides, there are renewable diesel fuels known as “green diesel”, obtained by several catalytic processes (cracking or pyrolysis, hydrodeoxygenation and hydrotreating) of vegetable oils and which exhibit a lot of similarities with fossil fuels. Likewise, it has also been addressed as a novel strategy, the use of straight vegetable oils in blends with various plant-based sources such as alcohols, vegetable oils, and several organic compounds that are renewable and biodegradable. These plant-based sources are capable of achieving the effective reduction of the viscosity of the blends, allowing their use in combustion ignition engines. The aim of this review is to evaluate the real possibilities that conventional biodiesel has in order to success as the main biofuel for the energy transition, as well as the use of alternative biofuels that can take part in the energy transition in a successful way.
1. Introduction
Over the last three decades, through a series of international treaties it has been decided to address, in a global way, a process of replacing fossil fuels by alternative energy sources of renewable nature. In this way, the world’s governments have negotiated, for the first time in history, a treaty that envisages climate action by many countries, the so-called Paris Agreement [1]. In this connection, many countries have determined the development of its own internal climate and energy policy framework for 2030 and beyond, advancing in decarbonization and fostering low-carbon innovation towards a new climate economy [2]. Among these strategies to reduce anthropogenic greenhouse gas (GHG) emissions, those measures adopted for reducing the high consumption of fossil fuels play a fundamental role. Thus, an unprecedented effort is being made to implement alternatives (photovoltaic, wind, hydrogen, and nuclear energy) [3,4] that allow the gradual replacement of natural gas, coal, and fossil fuels in the field of electricity generation. However, there is no such equivalent in transport, since vehicles capable of using fuel cells or electric motors cannot yet compete with spark ignition (SI) engines or compression ignition (CI) engines, especially in the field of heavy trucks [5], aviation [6,7], or the shipping sector [8]. Consequently, regardless of the growing effort in the introduction of vehicles that incorporate electric or hydrogen engines, the gradual incorporation of biofuels as fossil fuels substitutes continues being an imperative necessity for reducing GHG emissions [9,10]. In this line, considerable challenges for the transport sector in the coming decades must be assumed in order to increase the energy security [11,12].
The transport sector, including road, air, and waterborne transport, which contributes about 20% of the global emissions of greenhouse gases [11,13] is currently carried out by engines developed at the end of the 19th century, which were thought to work with ethanol or vegetable oils as fuels. These engines are usually known by the names of their inventors, Otto or Diesel, as well as by the two different ignition procedures, by spark, spark-ignition engines [1] or by internal compression ignition engines [14]. These engines were improved and adapted for application with fossil fuels during the last century, using various fractions obtained from the distillation of crude oil (or mineral oil) [15]. Thus, gasoline and diesel engines based on these fossil fuels are employed worldwide. Gasoline is the lighter fraction obtained from crude oil at distillation temperatures below the boiling point of water, whereas Diesel is obtained at higher distillation temperatures. Today, the problem arises since there is a necessity to keep using the current fleet of vehicles designed to work with fossil fuels, without modifying these engines, which surpassed a billion-units in the year 2010 [16].
In this scenario, the greatest consensus, in order to find a viable energy transition, is using the current fleet of vehicles in the coming decades, and gradually introducing biofuels in mixtures with these fossil fuels. In this way, biofuels can be easily integrated into the logistic of the global transportation system [17]. In this sense, the objectives pursued by the EU are estimated at 20% of biofuel in the mixture with fossil fuels for the year 2020 and 30% for 2030 [18]. However, despite the fact that these objectives are, apparently, not difficult to achieve, the replacement of the fossil fuel with biofuels is currently considered unattainable in these deadlines. The reason is that the most used biofuels, bioethanol and biodiesel, require huge agricultural resources to comply with these energy purposes [19,20,21,22], being much higher for biodiesel. To get an idea, the 1.6% of these biofuels in respect the total transport fuel worldwide [19], correspond to an ethanol production of almost 50 billion liters and a world biodiesel production of almost four billion liters [2], i.e., a relation lower than 1/10. Therefore, the incorporation of bioethanol has been, until now, easier and more effective than that for biodiesel. It is commonly accepted that bioethanol, produced from sugarcane, corn, or any other cereal, can easily be mixed in any proportion with gasoline to get liquid biofuels for use in spark ignition engines. This is feasible because the rheological properties of bioethanol are very similar to that of gasoline, being especially decisive due to their similar kinematic viscosity values [23].
However, the use of seed oils as biofuels, for the replacement of fossil diesel, is not as easy to achieve as with bioethanol and gasoline. The problem is that the CI engines are built to operate with viscosity values in the interval of 3–5 cSt, which is the one exhibited by fossil diesel fuel. Since seed oils have higher viscosity values, at least in the range of 30–40 cSt, it is necessary to perform some type of oil treatment to reduce their initial viscosity values, before mixing them in different proportions with fossil diesel. Biodiesel is, to date, the liquid biofuel produced in higher amounts for being employed as a substitute of fossil diesel. Nowadays, it is obtained through chemical conversion, mainly homogeneous alkaline transesterification of vegetable oil or animal fat with methanol, giving rise to a mixture of mono-alkyl esters of long chain fatty acids derived [24,25,26,27]. However, the transesterification reaction on an industrial scale has been associated as the main problem, with glycerol being generated in the process (10% by weight of the total biodiesel produced). This glycerol has to be completely removed, since the high temperatures reached in the engines promotes the formation of polymers of glycerol, as well as acrolein, which has a high toxicity. Therefore, to eliminate the glycerol, several washes with water have to be performed, making the process more expensive and tedious.
To solve this existing important barrier that prevents consolidation of conventional biodiesel as a suitable biofuel for the fossil fuel replacement, different alternative methods to convert vegetable oils into high quality diesel fuels while avoiding the generation of glycerol are being investigated. Thus, several oxygenated biofuels that integrate glycerol as soluble derivatives, very similar to biodiesel, have been described, such as Gliperol, DMC-Biod, or Ecodiesel [28]. Likewise, alternative high-quality diesel fuels, generally known as “green diesel”, have also been obtained by several processes, i.e., cracking or pyrolysis, hydrodeoxygenation, and hydrotreating of vegetable oils. All these biofuels can be considered as “renewable diesel”, although their composition is similar to that of a fossil fuel [29,30].
Finally, aware of the economic barriers that delay the process of replacing fossil fuels in current diesel CI engines, the use of straight vegetable oils (SVO) has been addressed as a possible strategy to make the fossil diesel replacement economically viable. Two alternatives are possible for this task. On one hand, the possibility of modifying the engine designs, as well as the operating parameters. On the other hand, the use of additives that allows for the reduction of kinematic viscosity of the blend. The first option, given the large number of cars existing seems very unworkable. Therefore, some researchers have described biofuels, from various plant-based sources such as alcohols, vegetable oils, and several organic compounds, that are renewable and biodegradable, as well as exhibiting adequate viscosity to be blended with vegetable oils in order to achieve a reduction of their kinematic viscosity. Since these compounds have lower octane index values, these low-carbon biofuels, with low viscosity, are considered as less viscous and lower cetane (LVLC) fuels to be employed in mixtures with oils [31,32].
This review aims to evaluate the real possibilities that conventional biodiesel has to respond to the challenge of successfully carrying out the energy transition, considering the current fleet of diesel vehicles. Likewise, this review overviews the alternative processes to successfully achieve this inexcusable current challenge. In such a case, what possibilities and advantages or disadvantages present each one of the methods described recently, in order to reduce the viscosity of triglycerides to be used as biofuels in mixtures with fossil diesel.
2. Biodiesel: The Current Renewable Biofuel in CI Diesel Engines
Once several international conventions (Kyoto and so on) were approved in the last decade of the last century [33], intending to minimize the anthropogenic effect on climate change, it was practically unanimously assumed that vegetable oils would be the raw material for obtaining the biofuels which will replace the current fossil fuels used in CI diesel engines. This solution was very easy to adopt, given that the first fuel used by Rudolf Diesel was a vegetable oil [34]. But with the current fleet of diesel vehicles, its use is not directly feasible, as it results in bioethanol being in gasoline engines. Whereas, the majority of rheological properties of biodiesel are optimal for being employed as biofuel in current diesel engines, the high viscosity that is exhibited constitutes the main problem.
Since the renewal of interest in vegetable oil-derived fuels, during the late 1970s, four possible solutions to the problem of high viscosity were investigated: Transesterification reaction, pyrolysis, dilution with conventional petroleum-derived diesel fuel, and micro-emulsification. The initial researches, on a laboratory scale or in a pilot plant, showed that the mixing of vegetable oil with diesel cannot exceed 10%, since the viscosity rises excessively. Micro-emulsification with short chain alcohols also presents solubility problems. Pyrolysis was considered more complex from a technical point of view. Thus, transesterification with methanol is currently the most common method and leads to methyl esters blend of vegetable oils and fats (FAME), usually called biodiesel. Biodiesel is biodegradable, nontoxic, renewable, has a high cetane number, a high flash point, in-built oxygen content that allows higher combustion, with very low sulfur, or aromatic components as well as other pollutants emissions. In addition, it perfectly fits into existing engines with no modification, being able to be used pure or in mixtures with fossil diesel, in any proportion. Consequently, biodiesel has been the subject of intense research in recent decades which continue to progress despite the level of its development throughout thousands of scientific papers [35]. However, this process presents a serious handicap related to the unavoidable production of glycerol as a byproduct of the transesterification reaction, Figure 1.
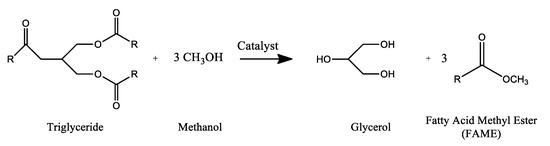
Figure 1.
Transesterification reaction of triglyceride molecules with methanol by conventional base catalysis.
Thus, the high production of biodiesel, mainly in the last three decades, has produced large volumes of waste with glycerol as the major product, being 10–20% of the total volume of biodiesel produced [36,37]. This excess of glycerol has caused a decrease in its commercial value, not only by the huge amount produced but also by its low quality, since it is mixed with other products such as methanol, water, and salts [38]. Since there are currently no industrial processes capable of adsorbing this continuous increase in the production of glycerol, this problem is far from disappearing. In fact, it is expected that by 2020, the global production of crude glycerol will reach 7.66 million tons [39]. To solve this problem, extensive efforts in catalysis research are currently being directed to the development of new and economically viable ways to valorize crude glycerol mass generated in the industrial production of biodiesel [36,37,38,39].
But even in the case of being able to solve the problems associated with the management of the increasing amounts of poor-quality glycerol, the conventional transesterification process also presents other serious problems such as the low atom yield (or atom efficiency) of the process. Atom yield can be defined as the conversion efficiency of a chemical process in terms of all atoms involved and the desired products produced. As such, the atom yield is an important concept of green chemistry, since it is a different concern than chemical yield, because a high-yielding process can still result in substantial by-products, as is the case in biodiesel production. In addition to this problem, the biodiesel obtained has to be cleaned from the residual glycerol, together with the alkaline impurities, from the homogenous catalyst currently used in the industrial transesterification process. This supposes an additional cleaning process, which is usually carried out by successive washing steps with water, requiring a large consumption of water, energy, and time to obtain the elimination of glycerol in order to comply the limits established by the quality standard EN 14214 [39,40,41,42]. Furthermore, the effects of water on the quality of biodiesel are not negligible [40], therefore various types of adsorbents have been proposed to eliminate the residues that contaminate the FAME mixture that constitute biodiesel [41,42].
Therefore, due to the generation of glycerol as a secondary compound in the apparent simplicity of the process, shown in Figure 1, a subsequent very complex design to make possible the industrial production of biodiesel [43] must be taken into consideration, Figure 2.
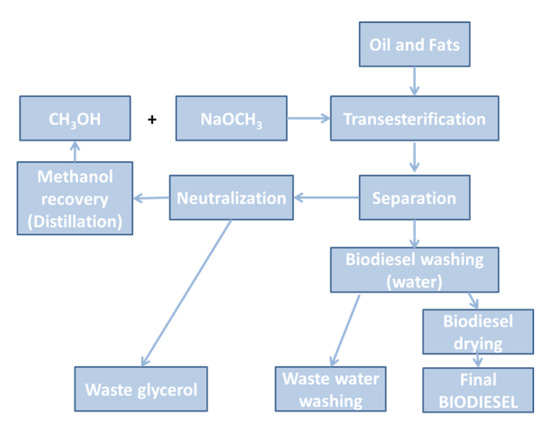
Figure 2.
Standard design diagram of a conventional biodiesel plant.
Generally, the transesterification reaction is carried out in a discontinuous reactor under constant agitation and at 60 °C of the reaction temperature. Then, glycerol is separated by decantation, together with the excess of methanol that will be recovered by distillation. This crude biodiesel contains catalyst residues which must be neutralized and eliminated. Besides, an important amount of glycerol is also present in this primary reaction product. Glycerol cleaning is a very important and laborious step, as abovementioned. According to EN 14214, the amount of glycerol in the refined biodiesel should not exceed the 0.02%, because the reaction of this glycerol with oxygen inside the engine at high temperature, produces acrolein, that by polymerization generates deposits of carbonaceous compounds on the injector nozzles, pistons, and valves in the engines, thus reducing its efficiency and even its service life [30].
Biodiesel purification is usually carried out commonly by neutralization and several washing steps with hot water to remove glycerol as well as all inorganic impurities. After washing the biodiesel, it is dried in an evaporator to remove residual water [40]. Purification of biodiesel may also be obtained by dry washing, employing organic resins, or using starch and cellulose as adsorbents of impurities [41,42].
Therefore, the complexity of the production process, mainly associated to the production of glycerol as a secondary product, greatly increases the production costs of conventional biodiesel [36]. Thus, the high global cost is very unfavorable in comparison to that of conventional fuels. In this way, the cost of biodiesel unit price is 1.5–3.0 times higher than that of fossil diesel fuel, depending on the type of triglyceride used as feedstock [44]. In a recent study on the economic feasibility of biodiesel through its life cycle costing, it was obtained that this biofuel could be competitive with diesel fossil, if the conditions of biodiesel production were optimized enough and a low-cost material feedstock was used. Therefore, economic viability is the main weaknesses that must be considered to ensure the development of this biofuel [45]. One approach that would increase the feasibility of the process is the revalorization of the crude glycerol obtained as by-product. In fact, glycerol is currently considered as one of the main platform molecules derived from biomass and a lot of researches are focused on transforming glycerol into value-added products, such as acrolein, oxygenated fuel additives, biofuels, and polyols (e.g., 1,3-propanediol) [46,47,48].
In conclusion, despite the enormous interest shown worldwide by researchers, industrialists, and governments about the use of biodiesel, its economic feasibility is still one of the major drawbacks that must be taken into account in the decision to continue or not with the development of this biofuel.
3. Biodiesel-Like Biofuels Which Integrate Glycerol as a Derivative Obtained in the Same Transesterification Process
A little over a decade ago, several researchers concluded that the difficulty in managing the glycerol produced during biodiesel synthesis constitutes enough technical and economic problems to be considered as a viable option to replace fossil diesel, at the pace foreseen by the current international agreements. In this context, the production of biofuels, in the same process of obtaining FAMEs, that generate glycerol derivatives instead of glycerol itself, seemed the best option to avoid the huge amounts of glycerol produced in the conventional transesterification process and also avoid the process of separation and cleaning of residual glycerol dissolved from the FAMEs mixture which constitutes the raw biodiesel [28,30,49,50,51,52]. In this respect, among the alternatives described for the integration of glycerol as a biofuel, it is necessary to highlight those that prevent the generation of glycerol in the same biodiesel production process, that is, processes in which together with the corresponding FAMEs mixture, some derivatives of glycerol, such as glycerol triacetate (or Glyperol), glycerol carbonate (or DMC-Biod), or monoglyceride (like Ecodiesel) are also obtained.
This process is possible by substituting the alcohols employed in the conventional process (methanol or ethanol) by esters, for instant. This methodology also exhibits an important advantage in comparison with the traditional transesterification process, an increase of the atom yield of the process (atom efficiency), since no by-products are obtained and, therefore, a significant increase of the reaction products are obtained. In the last decade, several methodologies to prepare esters from lipids using different acyl acceptors which directly afford alternative co-products are being investigated. These interesterification processes using alternative alcohol donors such as methyl or ethyl acetate and dimethyl or diethyl carbonate, instead of using methanol, are performed with the same catalysts employed in the conventional transesterification processes (acid or alkali homogeneous or heterogeneous catalysts, as well as lipases).
3.1. Biodiesel-Like Biofuels that Integrate Glycerol as Glycerol Triacetate, Obtained by Interesterification of Oils or Fats
In 2004, researchers of the Industrial Chemistry Research Institute, Varsow (Poland) patented a new type of biofuel, Gliperol [53]. Gliperol was obtained by interesterification reaction of triglycerides with methyl acetate, in the presence of a strong acid catalyst. The products of the reaction were a mixture of fatty acid methyl esters and glycerol triacetate (triacetin), Figure 3 [53,54,55]. Furthermore, together with triacetin, small amounts of diacetin and monoacetin are also obtained. In any case, these compounds, as well as the excess of methyl acetate, do not imply the need for their separation from the biofuel mixture, since all of them are soluble in fossil diesel. It is only necessary to clean the biofuel obtained in order to eliminate the inorganic salts which are only there if a homogeneous catalyst is employed, as consequence of the neutralization process.
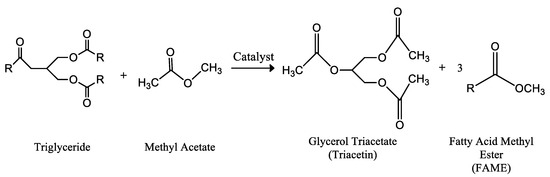
Figure 3.
Interesterification reaction of a triglyceride molecule with methyl acetate obtained by a conventional catalyst, producing a mixture of fatty acid methyl esters (FAME) and glycerol triacetate (triacetin). This mixture is a biodiesel-like biofuel, patented as Gliperol®, by the Research Institute of Industrial Chemistry Varsow (Poland) [55].
Authors studied several reaction conditions, i.e., oil/methyl acetate molar ratio in the range 1:3 to 1:9, and temperatures in the range of 40–200 °C. This biofuel exhibited similar properties as biodiesel, although the obtention process of Gliperol improves the yield, the efficiency, and the economic feasibility of the conventional biodiesel obtention process. After Gliperol was patented, a lot of subsequent studies that use homogeneous basic catalysts, such as potassium hydroxide, potassium methoxide, and polyethylene glycolate have been carried out [56,57,58,59,60,61,62,63,64,65,66,67]. These studies manifested that the reaction conditions to obtain Gliperol are similar to that for the production of Biodiesel. Likewise, this reaction has been studied over heterogeneous acid catalysts [68,69,70,71,72,73,74,75,76,77,78,79] as well as using lipases as biocatalysts, either in solvent-free systems [80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97] or in ionic liquids [98,99,100,101]. Furthermore, many other innovative technologies have also been applied, such as supercritical conditions [102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117] or ultrasonic-assisted interesterification [118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133].
Analogously to the interesterification of TG with methyl acetate, the ethyl acetate can be also considered as a good option to obtain Gliperol. Furthermore, this ethyl acetate has the advantage of its renewable character. However, to this date it has been studied less than methyl acetate [134,135,136,137,138,139].
Regarding the influence of triacetin on engine performance, many studies have been published to date [43,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154]. Thus, added to biodiesel or fossil diesel, triacetin acts as an efficient anti-knock additive in direct injection (DI) diesel engines. In addition, the use of triacetin as an additive for diesel and biodiesel also reduces the emission of pollutant. Regarding the investigations carried out on the blends triacetin/biodiesel and triacetin/diesel, the combination of 10% by weight of triacetin with biodiesel is the one that shows the best results [155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170]. Finally, beneficial effects of triacetin on the cloud point and cold filter plugging point have also been revealed in several studies [171,172,173]. About the economic viability of the process of interesterification of triglycerides with methyl or ethyl esters of acetic acid, there are many economic studies that support their advantages in respect to conventional biodiesel processes [174,175,176].
Therefore, it can be concluded that the interesterification of triglycerides with methyl or ethyl acetate can be a suitable methodology to obtain conventional biodiesel (FAME or fatty acids ethyl ester (FAEE)), also including a certain amount of a well-recognized additive, the triacetin, which improves Biofuel performance, in relation to conventional biodiesel, since it reduces the emissions and greatly simplifies the biofuel synthesis process. Likewise, the process exhibits an atomic efficiency of 100% since triacetin is obtained, which is a more profitable compound compared with the glycerol obtained as a by-product in conventional biodiesel production. Furthermore, the quality of biodiesel seems to be enhanced with the presence of triacetin.
3.2. Biodiesel-Like Biofuels that Integrate the Glycerol as Glycerol Carbonate, Obtained in a Single Process of Interesterification of Oils or Fats
Analogously to methyl acetate, dimethyl carbonate (DMC) can also be used as an interesterification reagent for the obtention of glycerol esters from lipids, directly yielding alternative soluble co-products in biodiesel solutions. The reaction is quite attractive, given that DMC is considered a prototype of a green reagent since it is not harmful to both humans and the environment, in addition to being an environmentally benign molecule (non-toxic, non-corrosive, and non-flammable) and less toxic than methanol. In this regard, it should be noted that the raw materials to produce DMC, that is, methanol and carbon monoxide, are derived from synthesis gas that can be produced from the thermochemical conversion of biomass [177], although the direct synthesis of DMC from methanol and CO2 has also been described [178]. Consequently, its utilization as a reaction medium as well as a phosgene substitute has currently been attracting much interest because of DMCs ability to act on a wide variety of reactions such as polycarbonate synthesis, polyurethane synthesis, carboxylation reagents, alkylating reagents, and polar solvents, not to mention its application as a fuel additive. In fact, DMC is also employed to obtain glycerol carbonate, considered an added-value product to revalorize the glycerol [179,180,181,182,183,184,185,186,187,188,189,190,191].
Therefore, a fuel produced with DMC and vegetable oils or fats as raw materials should be considered as an alternative fuel, totally derived from renewable resources. Thus, dimethyl carbonate operates as an alternative acyl acceptor. In this way, a novel biodiesel-like material (abbreviated as DMC-BioD) was developed by reaction of a vegetable oil with dimethyl carbonate, which avoids the co-production of free glycerol. According to Figure 4, the only difference between DMC-BioD and conventional biodiesel is the presence of fatty acid glycerol carbonate monoesters (FAGCs) in addition to FAMEs [192,193,194,195]. In fact, the process operates with the same basic catalysts described in the production of biodiesel, such as KOH, sodium methylate, sodium hydride, some amines, as well as different alkaline solids, operating as heterogeneous base catalysts at different experimental conditions [196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214]. Given the interest of the process, supercritical conditions [215,216,217,218,219,220,221,222] or the use of different lipases [223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239] have also been studied. DMC has been recently investigated in regards to the synthesis of fatty acid methyl esters via ionic liquids [240,241] or non-catalytic transesterification of vegetable oils with dimethyl carbonate and some amounts of silica loading operating as a dispersing agent [242].
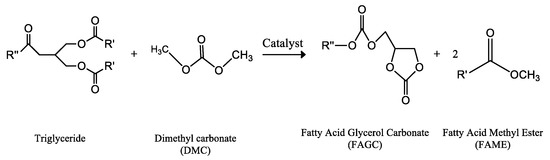
Figure 4.
Alkaline catalysed interesterification reaction of the triglycerides with dimethyl carbonate (DMC), generating a mixture of two moles of FAME and one mole of glycerol carbonate fatty acid (FAGC), a biodiesel-like biofuel, patented by Polimeri Europe (Italy) as DMC-BioD [194].
This biofuel, patented with the name of DMC-BIOD [201], is obtained as aforementioned by the interesterification reaction between triglycerides and dimethyl carbonate which produces a mixture of FAMEs and cyclic esters of glycerol carbonate fatty acids (FAGCs). In this mixture, a small amount of glycerol dicarbonate (GDC), and glycerol carbonate (GC) is also obtained, due to the decomposition of FAGC molecules. The short name, glycerol carbonate, is currently used for 4-hydroxymethyl-2-oxo-1, 3-dioxolan, and glycerol dicarbonate for 4-(methoxycarbonyloxymethyl)-1, 3-dioxolan-2-one, that also are obtained in the interesterification process [243].
In summary, the main difference between DMC-BIOD biofuel (obtained by the interesterification of triglycerides with DMC) and conventional biodiesel is the presence of some amounts of fatty acid glycerol carbonate monoesters, glycerol dicarbonate, and glycerol carbonate, in addition to the FAMEs that constitute biodiesel. These glycerol derivatives are completely soluble in the biofuel, meaning that all the reaction products can be used as biofuel, without any additional separation operation, and taking advantage that the 100% of atomic efficiency is reached. It is not even necessary to separate the unreacted DMC, since it can be used as an additive for fossil diesel, due to the high oxygen content of the molecule. In fact, the DMC is attracting the interest of researchers due to the increasing importance of environmental and resource issues to the realization of a sustainable society through a green chemistry activity development [243,244,245,246,247,248,249].
The addition of fuel-born oxygen, in the form of DMC, (a bio-derived with a 53.3% wt. of oxygen) in a conventional pump diesel produced a notable decreased in total hydrocarbons (THCs), carbon monoxide (CO), and particulate maters (PM). These emissions were reduced by up to 50% with a 96% diesel and 4% DMC blend. A DMC and diesel blend may also have potential in the reduction of yet unregulated carcinogenic emissions such as benzene and 1,3-butadiene [250,251,252,253,254]. Similarly to these processes, the use of diethyl carbonate (DEC) instead of DMC has been much less investigate [221,227,234], although the available results in literature [255,256,257,258,259,260,261,262,263,264,265,266] allow us to conclude that they exhibit a behavior very similar to those obtained with DMC.
3.3. Biodiesel-Like Biofuels that Integrate Glycerol as Monoglycerides, Obtained in a Selective 1,3-Transesterification Process
Another strategy to avoid the generation of free glycerol consisted in the partial transesterification of triglycerides, generating only two equivalents molecules of FAMEs, maintaining the third equivalent of fatty acids in the form of monoglyceride (MG). This procedure was initially performed over lipases, taking advantage of its 1,3-selective nature, which allows the process to be “stopped” in the second step of alcoholysis, Figure 5. Thus, several researches have been carried out using pig pancreatic lipase (PPL) in free form [267,268] and immobilized [269,270] in the selective transesterification process. In addition, to avoid the generation of glycerol, it is worth mentioning that the operating conditions of the enzymatic process are much softer and without generation of acidic or alkaline impurities, in comparison with the conventional biodiesel preparation method. Another specific feature of the enzymatic process is that it is necessary to use ethanol, instead of methanol, thus generating a mixture of FAEEs. This biodiesel-like biofuel was patented as Ecodiesel [271], and it is soluble in both, the mixture of FAEEs and in fossil diesel.
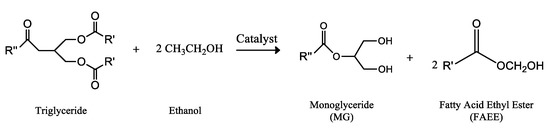
Figure 5.
Biodiesel-like biofuel, patented as Ecodiesel by the University of Cordoba [271], obtained by the 1,3-Selective ethanolysis of a triglyceride molecule through the application of enzymatic catalysis, constituted by two molecules of ethyl esters of fatty acids and a monoglyceride molecule.
An important handicap of this procedure was the very early verification of the economic difficulty of carrying out this process on an industrial scale, due to the high price of PPL lipases, even with their use in immobilized form, which allows their reuse. Therefore, in the last decade, a high number of low-cost lipases, both in free as well as immobilized forms have been evaluated, demonstrating in all cases the technical efficiency of the process [272,273,274,275,276,277,278,279,280,281,282,283]. However, the economic cost of the process is still the main difficulty to guarantee the economic viability at high production level.
The high price of lipases has opened very recently new researches, to search more cost-effective process for producing Ecodiesel, such as the employ of supported KF [284] or CaO [285] as heterogeneous catalysts as well as homogeneous alkaline catalyst such as sodium methoxide [286]. Now, to obtain the same selective alcoholysis process initially achieved with enzymatic catalysis, the reaction is made by operating under kinetic control of the chemical process, i.e., using a less basic catalyst than the alkali metals employed in the biodiesel production and/or operating under softer conditions of temperature and concentration of the alcohol used as a reagent. However, in this case, methanol can also be used, which produces the FAMEs blend. In enzymatic catalysis, ethanol must be mandatory, because methanol inhibits the lipase biocatalysts ability. Thus, the weaker heterogeneous basic sites [284,285], are enough strong to perform the transesterification of primary alcohols (like those in positions one and three of glycerol) but not enough strong to get the methanolysis of secondary alcohols (like those in position two of glycerol) in general much more difficult to achieve. In the case of using homogenous alkaline metal catalysis, selectivity is achieved by operating at sufficiently low temperatures.
In this respect, very recently it has been described that the production of Ecodiesel from castor oil and also from sunflower oil, using sodium methoxide as a homogeneous catalyst, and using a mixture of methanol and ethanol in which ethanol acts as a solvent of methanol to reduce its reactivity [286]. The reaction temperature employed was 30 °C, lower than that used in the conventional biodiesel synthesis, to get the kinetic control of selective transesterification, which allows obtaining a mixture of FAMEs and MG. The behavior of these biofuels was evaluated in a conventional diesel engine, operating as an electricity generator. The emission levels produced were also evaluated based on the opacity values of the generated fumes. With the blend’s diesel/Ecodiesel, a 30% of fossil diesel can be replaced, because the rheological properties of the double blends here studied, such as viscosity, pour point, and cloud point values of the different samples allow their use as biofuels in conventional diesel engines. The results obtained using a compression ignition diesel engine showed that the different biofuels studied had no differences with respect to the power generated, although in some cases a small increase in fuel consumption was obtained in respect to pure fossil diesel. However, with the biofuels used, a significant reduction is obtained, up to 40%, in the emission of pollutants, mainly with the diesel/castor oil mixture [286].
Therefore, the experimental conditions of operation to obtain a selective process that results in a high proportion of MG in the final FAME mixture, not only are much softer, but also there is no need to perform any additional purification operation, since glycerol is not produced, and the unreacted alcohol can be left as part of the mixture that constitutes the biofuel, so that the atomic efficiency of the process is practically 100% because all reagents are integrated in the final biofuel. Furthermore, in recent researches, it has been demonstrated that MG enhance the biodiesel lubricity as well [287,288,289,290,291,292,293,294,295].
In this line, a recent patent proposes the use of excess glycerol, obtained in the conventional biodiesel manufacture, in the production of mixtures of monoglycerides, obtained by transesterification of triglycerides with glycerol [296,297]. The process is shown in Figure 6, where can be verified that dimethoxymethane (DMM), so-called methylal, is employed as a reagent, in addition to glycerol. This reactant is a colorless and flammable liquid with a low boiling point, low viscosity, and excellent dissolving power. It has a chloroform-like smell and a spicy taste and it is soluble in three parts of water and miscible with the most common organic solvents, salts, and other water-soluble wastes.
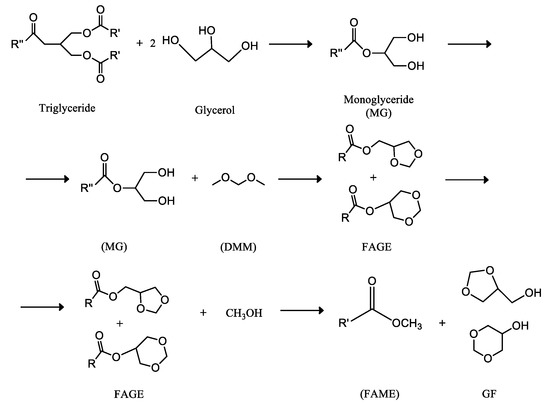
Figure 6.
Glycerol-derived advanced biofuel, fatty acid formal glycerol ester (FAGE) patented by Institut Universitari de Ciència i Tecnologia, SA [296,297]. A Biodiesel-like biofuel, obtained by transesterification of triglyceride molecules with glycerol or its derivates, to obtain a blend of monoglyceride molecules, and subsequent reaction with dimethoxymethane (DMM) to produce FAGE, a mixture of esters of formal glycerols and the formal glycerols (GF) themselves, consisting of the mixture of 5-hydroxy-1, 3-dioxane and 4-hydroxymethyl-1, 3-dioxolane.
This biofuel is proposed to be used after blending with biodiesel, petrol derived diesel, or mixtures of them. These biofuels thus obtained could allow the complete incorporation of the glycerol obtained in the current biodiesel. This new advanced biofuel, fatty acid formal glycerol ester (FAGE)—produced from crude glycerol, after blending with diesel fuel (20% FAGE content)—showed significant benefits in hydrocarbon, carbon monoxide and particle emissions, particle number, and opacity after testing in an automotive engine. Besides, it has been found that it improves the lubricity of the fuel [298,299,300]. In addition, the pour point and the cold filter plugging point were also improved with only a small effect on cloud point and crystallization temperature. A combination of both, acetals and cold-flow improver, is proposed as an optimal method to improve cold flow properties [173]. Thus, this innovative chemical process uses whichever oil and glycerol as reagents and yields esters of glycerol formal FAGE, which can be part of a blending component in diesel fuels. In the case of biodiesel and FAME/FAGE blends in diesel, ratios up to 20% meet all requirements set in current fuel quality standards [300]. The reaction could also be integrated in conventional biodiesel plants for a combined FAME/FAGE product [288].
4. Fossil Diesel-Like Biofuels: Green Diesel from Hydrotreated Vegetable Oils
Green diesel is a renewable fuel composed of fossil fuel-like hydrocarbons. Thus, several processes (cracking or pyrolysis, hydrodeoxygenation or hydrocracking) are currently under development for the conversion of fats and oils to obtain fuels very similar to fossil diesel [29]. In this regard, the catalyst hydrotreatment of vegetable oils, in existing refinery processing installations, is one of the best possible solutions [301]. These procedures would be recommended for application in oils with a high content of free fatty acids, since catalysis could generate high concentrations of soaps and deactivate the catalyst. The pioneering work in this field was carried out in China in the 1930s, where the cracking of various vegetable oils was carried out, giving rise to emergency fuels, capable of replacing conventional petroleum-derived fuels during World War II [302].
Since then, and especially in the last decade, different heterogeneous catalysts have been described that by means of a hydrotreatment process are able to transform different vegetable oils into normal alkanes, with boiling points in the range of gasoline or diesel, capable of providing substitutes of fossil diesel, suitable for application in internal combustion engines, without any modification. Green fuels can be classified as naphtha, jet fuel, and diesel. In the case of green diesel, its increasing demand could reach 900 million tons by 2020 [1]. The technology to obtain green fuels from triglycerides is the same currently used in the hydrotreating of vacuum gas oil. This hydrotreating or hydrofining process involves a non-destructive hydrogenation and is used to improve the quality of petroleum distillates without significant alteration of the boiling range. However, there is an increasing interest in developing their optimal production, by obtaining the best catalysts and the most favorable operating conditions [303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354]. The hydroprocessing of triglycerides consist in the hydrogenation of the double bonds of the fatty acids and the removal of oxygen in esters bonds, Figure 7. The triglycerides are converted into hydrocarbons, mainly to n-paraffins at the temperatures between 300 and 450 °C and hydrogen pressures above 3 MPa releasing CO, CO2, and water as by-products, throughout a complex process of several consecutive steps. The process basically first removes the oxygen, resulting in diesel-range waxy paraffins. A second reaction cracks the paraffins to smaller, highly branched molecules. Oxygen removal from triglycerides is developed through different reactions such as hydrodeoxygenation, decarboxylation, and decarbonylation that determine the final blend of hydrocarbons. Thus, there are two different reactions, hydrocracking and hydrotreating. Hydrocracking is carried out on acid sites of amorphous supports, while hydrotreating takes place on the metal active sites of the catalysts. The hydrotreating with hydrogen of most vegetable oils in the presence of metal oxide catalysts, leads to the production of a liquid blend of C15-C18 hydrocarbons, exhibiting a boiling point within the range of diesel, so that it is commonly called “green diesel”, “renewable diesel”, or “bio-hydrogenated diesel”, and therefore with the same chemical behavior that petroleum-derived diesel.
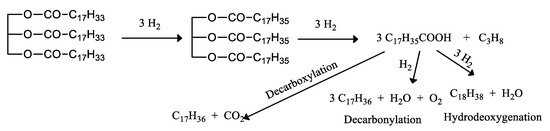
Figure 7.
Set of molecular reactions occurring in the hydroprocessing of triglycerides.
This technology has been extended to the application of hydrocracking treatments to mixtures of vegetable oils with fractions of heavy petroleum crude, of equivalent molecular weights in conventional oil refineries [355,356,357,358,359,360,361,362,363,364,365,366,367,368,369,370,371,372,373,374]. Therefore, this procedure makes possible to transform triglycerides into high quality diesel fuel, with the facilities already available in conventional oil refineries, using specific catalysts. In this way, it is possible to obtain renewable liquid alkanes by treating mixtures of vegetable oils and heavy vacuum gas oil (HVO), in hydrogen streams and the same conventional catalysts currently used in the hydrocracking units, such as the bimetallic sulfides NiMo/Al2O3 and many other conventional ones, operating under usual conditions of 3 MPa and 300–450 °C. However, different by-products are obtained, mainly consisting of H2, CO, CO2, O2, N2, H2S, CH4, and C2-C6 hydrocarbons. In general, liquid hydrocarbons are obtained with a significantly high yield, of the order of 80% by weight. These obtained liquid hydrocarbons have a low acidity index, adequate density and viscosity, and a high cetane index. In this sense, conventional hydrodesulfurization catalysts, consisting of sulfur-supported bimetallic systems, such as NiMo/Al2O3, CoMo/Al2O3, and NiW/Al2O3 are currently being investigated to obtain renewable diesel in conventional oil refinery facilities. To obtain lighter hydrocarbons such as those within the boiling point range of jet fuel or gasoline, catalysts with stronger acid sites (e.g., zeolites) can be used. The zeolite supported catalysts usually contain higher acidity and therefore a more severe cracking activity than those supported on alumina. The acid sites of the catalyst increase also the isomerization degree of the molecules, thus boosting the properties of the green liquid fuels, such as a lower pour point and a higher-octane number.
In summary, the hydrotreatment units of the fossil diesel initially intended to reduce the sulfur content of the fuels to reach the fuel specifications in every countries, has taken advantage for the production of high quality diesel biofuels by treating vegetable oils or animal fats with hydrogen at varying pressures, over the same supported metal catalysts used in the desulfurization of diesel fuels of fossil origin. These high-quality diesel fuels are often referred to as renewable diesel or green diesel which contains the same components as those present in fossil diesel [375,376,377,378,379,380,381,382,383,384,385,386,387,388,389,390].
Regarding the advantages of this methodology, it must be considered that it is especially useful with oils whose content of fatty acids are high. It is also important to highlight that the application of this technology avoids the cleaning operations of the crude mixture of FAMEs obtained with the conventional transesterification process, which constitute the major drawback in obtaining biodiesel, as aforementioned. However, in the synthesis of green diesel there is a decrease in the atomic performance of the process (atomic efficiency), since a significant loss of raw material is eliminated as CO2 and H2O. In practice, the complete loss of matter could be equivalent to glycerol obtained as a by-product in the biodiesel synthesis, with a high difference, in this case it is not required a special manage of these wastes.
When assessing this technology, it is mandatory to consider as a fundamental aspect and the main advantage, the possibility of obtention in the existing refineries [391,392]. Thus, application of the co-processing method of oils and fats of any quality, together to fossil diesel, in hydrocracking facilities, has notable advantages over the conventional methods of biodiesel production currently applied.
5. Blends of Straight Vegetable Oils (SVO) with Less Viscous and Lower Cetane (LVLC) Biofuels
Very recently, in order to reduce as much as possible the cost of manufacturing the biofuels of current diesel engines, instead of performing any chemical treatment of triglycerides, the possibility of using mixtures of vegetable oils with certain solvents of low viscosity is being studied, so that this mixture finally get a suitable value (3–5 cSt) to be used in current diesel engines [393]. Thus, it has been recently described that pine oil (viscosity 1.3 cSt) has been used in mixtures with castor oil to compensate the high viscosity of this oil (226.2 cSt).
In this way, the properties of castor oil and pine oil balance each other, achieving a suitable viscosity for blending in different proportions with fossil diesel [32]. These mixtures exhibit a low cetane number, because of the pine oil, limiting to a 30% by volume the quantity in which they can be blended for being employed in the engine properly. In this way, it has already been described that the use of various compounds, such as eucalyptus oil, orange oil, or camphor oil, which have low viscosity, but also low cetane number or LVLC fuels, for use in double mixtures with oils, or for use in triple mixtures with fossil diesel [394,395]. In this respect, there is an extensive bibliography regarding the use of low viscous vegetable oils blended with several biodiesels to get performance improvement in a diesel engine [396,397,398,399,400,401,402]. Furthermore, low viscous vegetable oils improved the performance of biodiesel-fueled CI engine, due to get better fuel properties. In fact, some of these compounds have demonstrated their ability to operate as biofuels either by themselves or in blends with fossil diesel [403,404,405,406,407].
On the other hand, fuel additives are also blended with LVLC compounds to improve their characteristics, such as better ignition, low emissions, enhance the cetane index of fuels, and solve the cold start problem. In this respect, several cetane enhancers like diethyl ether (DEE), dimethyl carbonate, and diethylene glycol dimethyl ether (DIGLYME) have been studied in blends with LVLC fuels and tested in CI engines [408,409,410,411,412,413].
Within this strategy, the use of quaternary mixtures has been described in blends with diesel fossil. Thus, straight vegetable oils, soybean biodiesel, and higher alcohols, such as propanol and pentanol were evaluated in diesel engines with the purpose of increasing fossil diesel replacement with renewable fuels [414]. Analogously, triple mixtures of vegetable oils, different alcohols, and fossil diesel have been described. Generally, ternary blends of SVO-diesel-alcohol are found to reduce smoke and could be used as potential sources of renewable energy to partially or fully replace diesel fuel [415,416,417,418,419,420,421,422,423,424,425,426,427,428,429,430,431]. The application of bioethanol in the triple mixtures, encounters difficulties due to its limited solubility in both, vegetable oils and fossil diesel. This has been attempted to be resolved with the addition of surfactants [432,433,434,435,436,437,438]. However, these triple mixtures work very well with neat castor oil because it has a high affinity for ethanol, due to the high ricinoleic acid amount in castor oil (about 90%), and hence mixes in any proportion without any phase separation. The blending of bioethanol and diesel enhances the combustion of neat castor oil as a fuel, for compression ignition engines, in spite of their high viscosity. Besides, hydroxyl group in the ricinoleic acid enhance ignitability, lubrication, and solubility characteristics of castor oil/ethanol/diesel blends. Considering the renewable nature of bioethanol, these triple mixtures achieve a high level of replacement of the fossil fuel [439,440,441].
On the other hand, diethyl ether, that can be produced from bioethanol, obtained by a dehydrating process from biomass, may be also considered as a biofuel. This isomer of butanol has several favorable properties for blending with diesel fuel, including very high cetane number, reasonable energy density for on-board storage, high oxygen content, low autoignition temperature, broad flammability limits, and high miscibility with vegetable oils and diesel fossil. Thus, DEE has been recently reported as a low-emission renewable fuel and high-quality combustion improver, for the utilization of several vegetable oils, as a biofuel in blends with diesel fossil [419,442,443,444,445,446,447,448,449,450,451,452,453,454,455,456,457]. Similarly, dimethyl carbonate has been used as a component of triple mixtures to reduce the viscosity of straight vegetable oils [458,459]. The strategy has even been applied for using triple mixtures using non-renewable compounds, such as gasoline, capable of reducing the viscosity of vegetable oils. The strategy has even been applied of using triple mixtures using non-renewable compounds, such as gasoline, capable of reducing the viscosity of vegetable oils. In this way, these triple blends allow the substitution of fossil diesel up to 40% with sunflower oil, and up to 25% with castor oil with a significant reduction in the emission of pollutants has also been obtained with these triple blends. [460,461].
In summary, it has been possible to verify that a suitable strategy to reduce the viscosity of vegetable oils to the values required by current CI engines can be the use of mixtures of these oils with low viscosity compounds as biofuels, although they usually present lower energy density, that is, lower octane index. However, since fossil diesel has a lower viscosity than vegetable oils, there will be an adequate percentage for each oil in the triple blends to comply with regulations of the EN 14214 standard.
6. Summary, Conclusions, Challenges, and Research Outlook
Biofuels have emerged as attractive alternative to current petroleum-based fuels, since they allow the replacement of fossil diesel in engines without any modification of the engines. In addition, they also show a favorable profile in the gases emitted in combustion, producing much less carbon monoxide, sulfur dioxide, and unburned hydrocarbons, than fossil fuel. Therefore, biofuels obtained from vegetable oils could play an essential role in replacing the current petroleum-derived fuels, since by being able to directly replace them, they can easily be integrated into the logistics systems that are currently operating in the global system of transport supply. However, in order to apply the vegetable oils in the current CI diesel engines, it is necessary a previous reduction of the viscosity of the oils.
In the current energy scenario, the complete replacement of petroleum-derived fuels with biofuels is practically impossible considering a short or medium term, from the point of view of the impossibility of producing the raw material, not only due to economic difficulties and the high prices of vegetable oils, but also because of the shortage of agricultural land suitable for it. However, energy insecurity and climate change are driving forces of enough entity to stimulate the development of biofuels throughout the world. In any case, this also implies great potential to stimulate agribusiness. Therefore, it is a priority to proceed with the gradual expansion of the currently unproductive land to devote them to the production of inedible vegetable oils in order to advance the gradual introduction of biofuels based on renewable raw materials.
Three decades ago, at the beginning of the energy transition, the most appropriate procedure to reduce the viscosity of vegetable oils was their transformation into a mixture of methyl esters of fatty acids by transesterification reaction of triglycerides. These mixtures were called biodiesel, or FAME, and they have rheological properties that allow their use as fuels in mixtures with fossil diesel in any proportion.
Throughout the following decade, and after the attempt to scale this process to industrial levels, it was found that the process exhibited a series of technical and environmental difficulties, which made this option practically unfeasible, from the technical and economical point of view. The main disadvantage of the transesterification of triglycerides was related to the production of glycerol as by-product, which implies an exhaustive process of cleaning of the biodiesel as well as the production of a huge amount of glycerol difficult to manage. Thus, several researches were initiated to get biofuels where glycerol were incorporated as a derivative in the biodiesel-like biofuel, to make this way viable for the use of vegetable oils as a raw material for obtaining biofuels applicable to current CI diesel engines.
It is also possible that the introduction of vegetable oils as completely deoxygenated renewable biofuels through their transformation into hydrocarbons, through a catalytic reaction that includes hydro processing and/or decarboxylation/decarbonylating of triglycerides to get the so-called green diesel. Finally, in the last decade the study of the mixtures of vegetable oils with different solvents of low viscosity has gained importance as a method to reduce the viscosity of vegetable oils, to the limits required for the proper functioning of current diesel CI engines. In Table 1 shows the summary of the pros and cons of current methodologies for obtaining biofuels, from different vegetable oils, applicable in current internal combustion diesel engines.

Table 1.
Highlights of the main characteristics of the current available technologies to produce renewable liquid fuels from vegetable oils.
Therefore, the adequate technologies are already available to carry out the process of substitution of fossil fuels, some of them with clear advantages regarding the conventional process of Biodiesel production. In fact, the technology to transform renewable triglycerides into green fuels has been developed in petroleum refineries, throughout the last five decades. However, in the last decade an increasing interest for the optimization of this procedure has been carried out, mainly focused in the search of the best catalysts and the optimal operating conditions for production of hydrogenated green fuels. In this sense, the green diesel can be obtained through the hydroprocessing of oils and fats employing the current facilities of the oil refineries, using the same catalysts and also taking advantage of the same reactors used in the hydrotreating of fossil diesel, therefore becoming a very interesting and economically viable technology. The paraffins thus obtained are high quality compounds, available for distribution immediately, using the current logistics system.
The degree of maturity of the “Green Diesel” obtention process in conventional refineries is quite high. However, this fact should not prevent further researches in the field of biofuels, especially considering the options here discussed for the transformation of triglycerides into renewable diesel without generating glycerol as a byproduct, since a higher atomic yield among other advantages aforementioned can be reached.
In this line, in the field of biodiesel-like biofuels which are practically developed in the last decade, it is noteworthy that the atomic efficiency of 100% is always achieved without generating no wastes. Furthermore, the biofuels obtained do not require any cleaning or refining process for being employed in the current engines. This implies a considerable reduction of the economic production cost if we think at the industrial scale. In fact, the prices for their production are comparatively much lower than that of conventional biodiesel. Another interesting aspect is that all the reagents employed to treat the vegetable oils, e.g., ethanol, acetate, or ethyl carbonate are of renewable nature, because in all cases it can be obtained from bioethanol, that is obtained from different agricultural crops. It also represents an interesting opportunity to spend CO2, generated in many industrial processes. These processes are being prioritized to contribute to the minimization of such emissions.
The main drawback regarding the production of the biodiesel-like biofuels is, perhaps, the catalytic systems used, which consist on homogeneous alkaline systems or different lipases. Undoubtedly, an interesting field is still open for the search of heterogeneous catalytic systems which overcome the drawbacks of homogeneous ones (neutralization, separation, and cleaning of the product) and also that reduce the economic price of the lipases.
In this sense, perhaps we would have to redirect the efforts made in the last decades from the subject of the catalytic processes of biodiesel production, which has been a trending topic for researchers for many years, reflected in thousands of researches but without finding effective results. This is probably because the difficulty of the process is not the chemical reaction to obtain the biofuel, but to the disadvantages of the unavoidable treatment of the reaction product. Such interesting results are obtained from the interesterification processes with different ethyl or methyl acetates or carbonates, as well as from the selective transesterification processes.
In summary, it would be of great interest to obtain heterogeneous catalysts capable of efficiently perform the interesterification processes that produce glycerol derivatives, together with the methyl or ethyl esters of fatty acids that constitute oils and fats. This would further implement the technical and economic feasibility of already very competitive processes in respect to the conventional biodiesel production. That is, heterogeneous catalysis will be one of the key factors that allow the production of biodiesel-like biofuels.
Finally, we must consider the possibilities that the most recent strategy opens the door to achieve the application of vegetable oils as biofuels in today’s diesel CI engines. The most important objective is to reduce the kinematic viscosity of oils to the appropriate levels in order to comply with the regulations. This fact can be reached by mixing the vegetable oils with low viscosity compounds of different origin. These compounds normally exhibit low values of energy density as well, so they have been called as LVLC compounds. Despite the novelty of this research field, very interesting results have been already obtained, leading to this methodology becoming one of the best procedures to reduce as much as possible the cost of manufacturing the biofuels of current diesel engines, since it avoids the need to carry out any chemical treatment of vegetable oils.
In this respect, the viability of the process is transferred to the technical and/or the economic accessibility to the LVLC solvent. For this reason, another interesting investigation field is here open. Thus, it has been recently published that even gasoline can operate as an LVLC solvent for castor oil, achieving high fossil diesel replacement values. The incorporation of fossil diesel to these gasoline/oil mixtures produces diesel/gasoline/oil triple blends, which exhibited the suitable rheological properties to be able to operate in conventional diesel engines, allowing the substitution of fossil diesel up to 25%, with a significant reduction in the emission of pollutants.
As a general conclusion, it can be stated that any of the alternative methods proposed in the last decade are able to compete advantageously with conventional biodiesel in order to get a gradual replacement of fossil fuels by others of renewable nature, always considering the necessity of still using the fleet of existing cars, and also taking into account that no design modifications of CI diesel engines will be performed. Therefore, it is mandatory to consider that the biofuel produced, or its mixtures with fossil diesel, must be within the acceptable limits prescribed by ASTM D 6751, currently in force for conventional fossil diesel fuel. It is not consistent to demand a level as demanding as the EN 14214 standard for a biofuel, which would be finally used in a mixture with fossil diesel, with less demanding quality levels. Consequently, it would be advisable that both policy makers and researchers would direct their priorities to the development of these novel processes, instead of the initial alternative, based on the transesterification of triglycerides.
Furthermore, we must not forget that these biofuels will operate in the current engines, although the energy transition have to include vehicles that incorporate electric or hydrogen engines for urban transport. If not, it would be materially impossible to produce the necessary amounts of biofuels to supply the fleet of cars operating with diesel engines, without jeopardizing agricultural resources for feed [462,463]. However, it is not foreseeable even in the long term a scenario in which the explosion or combustion engines are not present operating with biofuels, given that for certain circumstances, electric motors or vehicles capable of using fuel cells cannot compete yet with explosion or combustion engines, especially in the field of heavy trucks, aviation, or the shipping sector [464,465].
Author Contributions
This research article is part of the doctoral thesis of L.A.-D., directed by professors R.E. and F.M.B., who in general way conceived and designed the review and wrote the paper. All coauthors have made substantive intellectual contributions to this study, making substantial contributions to conception and design of it, as well as to the acquisition, analysis and interpretation of the analyzed bibliography. All of them have been also involved in drafting and revising the manuscript, so that everyone has given final approval of the current version to be published in Catalysts Journal.
Funding
This research received no external funding.
Acknowledgments
This research is supported by the MEIC fund (Project ENE 2016-81013-R), Junta de Andalucía and FEDER (P11-TEP-7723), as well as the MINECO project (MAT 2012-31127), that cover the costs to publish in open access.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| GHG | greenhouse gas emissions |
| CI | compression ignition engines |
| SVO | straight vegetable oils |
| LVLC | less viscous and lower cetane fuels |
| FAME | fatty acids methyl esters, components of conventional biodiesel |
| FAEE | fatty acids ethyl esters, components of conventional biodiesel |
| DMC | dimethyl carbonate |
| FAGCs | fatty acid glycerol carbonate esters |
| GDC | glycerol dicarbonate |
| GC | glycerol carbonate |
| PPL | pig pancreatic lipase |
| MG | monoglycerides or monoacylglycerols |
| DG | diacylglycerols |
| TG | triacylglycerols or triglycerides |
| THC | total hydrocarbons |
| CO | carbon monoxide |
| PM | particulate maters |
| HAP | hazardous air pollutants emissions |
| DEC | diethyl carbonate |
| DMM | Dimethoxymethane |
| GF | formal glycerol |
| DEE | diethyl ether |
| DIGLYME | diethylene glycol dimethyl ether |
References
- Obergassel, W.; Arens, C.; Hermwille, L.; Kreibich, N.; Mersmann, F.; Ott, H.E.; Wang-Helmreich, H. Phoenix from the Ashes—An Analysis of the Paris Agreement to the United Nations Framework Convention on Climate Change; Wuppertal Institute for Climate, Environment and Energy: Wuppertal, Germany, 2016. [Google Scholar]
- Oberthü, S. Where to go from Paris? The European Union in climate geopolitics. Glob. Aff. 2016, 2, 119–130. [Google Scholar] [CrossRef]
- Delucchi, M.A.; Jacobson, M.Z. Meeting the world’s energy needs entirely with wind, water, and solar power. Bull. At. Sci. 2013, 69, 30–40. [Google Scholar] [CrossRef]
- Arutyunov, V.S.; Lisichkin, G.V. Energy resources of the 21st century: Problems and forecasts. Can renewable energy sources replace fossil fuels? Russ. Chem. Rev. 2017, 86, 777–804. [Google Scholar] [CrossRef]
- Sen, B.; Ercan, T.; Tatari, O. Does a battery-electric truck make a difference?—Life cycle emissions, costs, and externality analysis of alternative fuel-powered Class 8 heavy-duty trucks in the United States. J. Clean. Prod. 2017, 141, 110–121. [Google Scholar] [CrossRef]
- Nair, S.; Paulose, H. Emergence of green business models: The case of algae biofuel for aviation. Energy Policy 2014, 65, 175–184. [Google Scholar] [CrossRef]
- Noh, H.M.; Benito, A.; Alonso, G. Study of the current incentive rules and mechanisms to promote biofuel use in the EU and their possible application to the civil aviation sector. Transp. Res. Part. D Transp. Environ. 2016, 46, 298–316. [Google Scholar] [CrossRef]
- Winnes, H.; Styhre, L.; Fridell, E. Reducing GHG emissions from ships in port areas. Res. Transp. Bus. Manag. 2015, 17, 73–82. [Google Scholar] [CrossRef]
- Levidow, L. EU criteria for sustainable biofuels: Accounting for carbon, depoliticising plunder. Geoforum 2013, 44, 211–223. [Google Scholar] [CrossRef]
- Creutzig, F.; Jochem, P.; Edelenbosch, O.Y.; Mattauch, L.; van Vuuren, D.P.; McCollum, D.; Minx, J. Transport: A roadblock to climate change mitigation? Science 2015, 350, 911–912. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Fulton, L.M.; Lynd, L.R.; Körner, A.; Greene, N.; Tonachel, L.R. The need for biofuels as part of a low carbon energy future. Biofuels Bioprod. Biorefin. 2015, 9, 476–483. [Google Scholar] [CrossRef]
- Fontana, G.; Galloni, E. Variable valve timing for fuel economy improvement in a small spark-ignition engine. Appl. Energy 2009, 86, 96–105. [Google Scholar] [CrossRef]
- Reitz, R.D.; Duraisamy, G. Review of high efficiency and clean reactivity controlled compression ignition (RCCI) combustion in internal combustion engines. Prog. Energy Combust. Sci. 2015, 46, 12–71. [Google Scholar] [CrossRef]
- Zhoua, D.; Yanga, W.; Lia, J.; Taya, K.L.; Choua, S.K.; Kraftb, M. Efficient Combustion Modelling in RCCI Engine with Detailed Chemistry. Energy Procedia 2017, 105, 1582–1587. [Google Scholar] [CrossRef]
- Petrovi, G.S.; Madi, M.J.; Markovi, D.S.; Mili, P.D.; Stefanovi, G.M. Multiple criteria decision making of alternative fuels for waste collection vehicles in southeast region of Serbia. Therm. Sci. 2016, 20 (Suppl. 5), S1585–S1598. [Google Scholar] [CrossRef]
- Ridjan, I.; Mathiesen, B.V.; Connolly, D.; Dui, N. The feasibility of synthetic fuels in renewable energy systems. Energy 2013, 57, 76–84. [Google Scholar] [CrossRef]
- Dafnomilisa, I.; Hoefnagelsb, R.; Pratamab, Y.W.; Schotta, D.L.; Lodewijksa, G.; Jungingerb, M. Review of solid and liquid biofuel demand and supply in Northwest Europe towards 2030—A comparison of national and regional projections. Renew. Sustain. Energy Rev. 2017, 78, 31–45. [Google Scholar] [CrossRef]
- Nogueira, L.A.H. Does biodiesel make sense? Energy 2011, 36, 3659–3666. [Google Scholar] [CrossRef]
- Rocha, M.H.; Capaz, R.S.; Lora, E.E.S.; Nogueira, L.A.H.; Leme, M.M.V.; Renó, M.L.G.; Olmo, O.A. Life cycle assessment (LCA) for biofuels in Brazilian conditions: A meta-analysis. Renew. Sustain. Energy Rev. 2014, 37, 435–459. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Bulte, E.H.; Conijn, S.G. Can large-scale biofuels production be sustainable by 2020? Agric. Syst. 2009, 101, 197–199. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels securing the planet’s future energy needs. Energy Convers. Manag. 2009, 50, 2239–2249. [Google Scholar] [CrossRef]
- Kumbar, V.; Dostal, P. Temperature dependence density and kinematic viscosity of petrol, bioethanol and their blends. Pak. J. Agric. Sci. 2014, 51, 175–179. [Google Scholar]
- Demirbas, A. Future Energy Sources. In Waste Energy for Life Cycle Assessment; Springer: Cham, Switzerland, 2016; pp. 33–70. [Google Scholar]
- Mythili1, R.; Venkatachalam, P.; Subramanian, P.; Uma, D. Production characterization and efficiency of biodiesel: A review. Int. J. Energy Res. 2014, 38, 1233–1259. [Google Scholar] [CrossRef]
- Siraj, S.; Kale, R.; Deshmukh, S. Effects of Thermal, Physical, and Chemical Properties of Biodiesel and Diesel Blends. Am. J. Mech. Ind. Eng. 2017, 2, 24–31. [Google Scholar] [CrossRef]
- Poonam Singh Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Luque, R.; Herrero-Davila, L.; Campelo, J.M.; Clark, J.H.; Hidalgo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Biofuels: A technological perspective. Energy Environ. Sci 2008, 1, 513–596. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Lee, H.V.; Rashid, U.; Islam, A.; Taufiq-Yap, Y.H. A Review on Thermal Conversion of Plant Oil (Edible and Inedible) into Green Fuel Using Carbon-Based Nanocatalyst. Catalysts 2019, 9, 350. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Berbel, J.; Verdugo-Escamilla, C. An overview on glycerol-free processes for the production of renewable liquid biofuels, applicable in diesel engines. Renew. Sustain. Energy Rev. 2015, 42, 1437–1452. [Google Scholar] [CrossRef]
- Mat, S.C.; Idroas, M.Y.; Teoh, Y.H.; Hamid, M.F. An Investigation of Viscosities, Calorific Values and Densities of Binary Biofuel Blends. In Proceedings of the MATEC Web of Conferences (ICME’17), Langkawi, Malaysis, 22–23 July 2017. [Google Scholar]
- Prakash, T.; Geo, V.E.; Martin, L.J.; Nagalingam, B. Evaluation of pine oil blending to improve the combustion of high viscous (castor oil) biofuel compared to castor oil biodiesel in a CI engine. Heat Mass Transf. 2019, 55, 1491–1501. [Google Scholar] [CrossRef]
- Von Stein, J. The International Law and Politics of Climate Change Ratification of the United Nations Framework Convention and the Kyoto Protocol. J. Confl. Resolut. 2008, 52, 243–268. [Google Scholar] [CrossRef]
- Knothe, G.; Van Gerpen, J.; Krahl, J. (Eds.) The Biodiesel Handbook, 2nd ed.; AOCS Press: Champaign, IL, USA, 2015. [Google Scholar]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An Overview of Recent Research in the Conversion of Glycerol into Biofuels, Fuel Additives and other Bio-Based Chemicals. Rev. Catal. 2019, 9, 15. [Google Scholar] [CrossRef]
- Quispe, C.A.; Coronado, C.J.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Selishcheva, S.A.; Yakovlev, V.A. Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts 2018, 8, 595. [Google Scholar] [CrossRef]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. The effects of water on biodiesel production and refining technologies: A review. Renew. Sustain. Energy Rev. 2012, 16, 3456–3470. [Google Scholar] [CrossRef]
- Garcia-Gomes, M.; Queiroz-Santos, D.; Carlos de Morais, L.; Pasquini, D. Purification of biodiesel by dry washing, employing starch and cellulose as natural adsorbents. Fuel 2015, 155, 1–6. [Google Scholar] [CrossRef]
- Squissato, A.L.; Fernandes, D.V.; Sousa, R.M.F.; Cunha, R.R.; Serqueira, D.S.; Richter, E.M.; Pasquini, P.; Muñoz, R.A.A. Eucalyptus pulp as an adsorbent for biodiesel purification. Cellulose 2015, 22, 1263–1274. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Oha, P.P.; Lau, H.L.N.; Chen, J.; Chong, M.F.; Choo, Y.M. A review on conventional technologies and emerging process intensification (PI) methods for biodiesel production. Renew. Sustain. Energy Rev. 2012, 16, 5131–5145. [Google Scholar] [CrossRef]
- Santori, G.; Di Nicola, G.; Moglie, M.; Polonara, F. A review analyzing the industrial biodiesel production practice starting from vegetable oil refining. Appl. Energy 2012, 92, 109–132. [Google Scholar] [CrossRef]
- Demirbas, A. Importance of biodiesel as transportation fuel. Energy Policy 2007, 35, 4661–4670. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Luna, D.; Bautista, F.M. An Overview of the Production of Oxygenated Fuel Additives by Glycerol Etherification, Either with Isobutene or tert-Butyl Alcohol, over Heterogeneous Catalysts. Energies 2019, 12, 2364. [Google Scholar] [CrossRef]
- Drozdzynska, A.; Leja, K.; Czaczyk, K. Biotechnological production of 1,3-propanediol from crude glycerol. Biotechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2011, 92, 92–100. [Google Scholar]
- Posada, J.A.; Orrego, C.E.; Cardona, C.A. Biodiesel production: Biotechnological approach. Int. Rev. Chem. Eng. 2009, 1, 571–580. [Google Scholar]
- Khang, D.S.; Tan, R.R.; Uy, O.M.; Michael Angelo, B.; Promentilla, M.G.B.; Tuan, P.-D.; Abe, N.; Razon, L.F. A design of experiments approach to the sensitivity analysis of the life cycle cost of biodiesel. Clean Technol. Environ. Policy 2018, 20, 573–580. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Posadillo, A.; Bautista, F.M.; Romero, A.A.; Berbel, J.; Verdugo, C. Technological challenges for the production of biodiesel in arid lands. J. Arid Environ. 2014, 102, 127–138. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Briggs, M. Biodiesel production—Current state of the art and challenges. J. Ind. Microbiol. Biotechnol. 2008, 35, 421–430. [Google Scholar] [CrossRef]
- Okoye, B.P.U.; Hameed, H. Review on recent progress in catalytic carboxylation anda cetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Uthoff, S.; Bröker, D.; Steinbüchel, A. Current state and perspectives of producing biodiesel-like compounds by biotechnology. Microb. Biotechnol. 2009, 2, 551–565. [Google Scholar] [CrossRef][Green Version]
- Kijenski, J.; Lipkowski, A.; Walisiewicz-Niedbalska, W.; Gwardiak, H.; Rożyczki, K.; Pawlak, I. A Biofuel for Compression-Ignition Engines and a Method for Preparing the Biofuel. European Patent EP1580255, 26 March 2004. [Google Scholar]
- Ryms, M.; Lewandowski, W.M.; Januszewicz, K.; Klugmann-Radziemska, E.; Ciunel, K.K. Methods of liquid biofuel production-the biodiesel example. Proc. ECOpole 2013, 7, 511–516. [Google Scholar] [CrossRef]
- Kijenski, J. Biorefineries: From biofuels to the chemicalization of agricultural products. Pol. J. Chem. Technol. 2007, 9, 42–45. [Google Scholar] [CrossRef]
- Casas, A.; Ramos, M.J.; Pérez, A. Product Separation after Chemical Interesterification of Vegetable Oils with Methyl Acetate. Part II: Liquid–Liquid Equilibrium. Ind. Eng. Chem. Res. 2012, 51, 10201–10206. [Google Scholar] [CrossRef]
- Casas, A.; Ramos, M.J.; Pérez, A. Kinetics of chemical interesterification of sunflower oil with methyl acetate for biodiesel and triacetin production. Chem. Eng. J. 2011, 171, 1324–1332. [Google Scholar] [CrossRef]
- Casas, A.; Ramos, M.J.; Perez, A. New trends in biodiesel production: Chemical interesterification of sunflower oil with methyl acetate. Biomass Bioenergy 2011, 35, 1702–1709. [Google Scholar] [CrossRef]
- Casas, A.; Ramos, M.J.; Pérez, A. Methanol-enhanced chemical interesterification of sunflower oil with methyl acetate. Fuel 2013, 106, 869–872. [Google Scholar] [CrossRef]
- Visioli, L.J.; Trentini, C.P.; Castilhos, F.; Silva, C. Esters production in continuous reactor from macauba pulp oil using methyl acetate in pressurized conditions. J. Supercrit. Fluids 2018, 140, 238–247. [Google Scholar] [CrossRef]
- Kampars, V.; Abelniece, Z.; Blaua, S. The Unanticipated Catalytic Activity of Lithium tert-Butoxide/THF in the Interesterification of Rapeseed Oil with Methyl Acetate. Hindawi J. Chem. 2019. [Google Scholar] [CrossRef]
- Sile, E.; Kampars, V.; Sustere, Z. Competitive Interesterification-Transesterification of Rapeseed Oil with Methyl Acetate in Presence of Potassium Metoxide Solutions. Eng. Mater. 2018, 762, 158–162. [Google Scholar] [CrossRef]
- Kampars, V.; Abelniece, Z.; Kampare, R. Biofuel via interesterification of rapeseed oil with methyl acetate in presence of potassium t-butoxide/THF. SWS J. Earth Planet. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Brondani, L.N.; Simões, S.S.; Celante, D.; Castilhos, F. Kinetic Modeling of Supercritical Interesterification with Heterogeneous Catalyst to Produce Methyl Esters Considering Degradation Effects. Ind. Eng. Chem. Res. 2019, 58, 816–827. [Google Scholar] [CrossRef]
- Sustere, Z.; Murnieks, R.; Kampars, V. Chemical interesterification of rapeseed oil with methyl, ethyl, propyl and isopropyl acetates and fuel properties of obtained mixtures. Fuel Process. Technol. 2016, 149, 320–325. [Google Scholar] [CrossRef]
- Singh, D.S.; Tat, T.K. Optimization of biodiesel production via methyl acetate reaction from cerbera odollam. Adv. Energy Res. 2016, 4, 325–337. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Celante, D.; Simoe, S.S.; Bassac, M.M.; Silva, C.; Castilhos, F. Efficiency of heterogeneous catalysts in interesterification reaction from macaw oil (Acrocomia aculeata) and methyl acetate. Fuel 2017, 200, 499–505. [Google Scholar] [CrossRef]
- Māliņš, K.; Kampars, V.; Kampare, R.; Sustere, Z.; Arenta, A. Production of Biodiesel and Triacetin by Interesterification of Rapeseed Oil. Key Eng. Mater. 2018, 762, 129–133. [Google Scholar] [CrossRef]
- Simões, S.S.; Ribeiro, J.S.; Celante, D.; Brondani, L.N.; Castilhos, F. Heterogeneous catalyst screening for fatty acid methyl esters production through interesterification reaction. Renew. Energy 2020, 146, 719–726. [Google Scholar] [CrossRef]
- Tian, Y.; Xiang, J.; Verni, C.C.; Soh, L. Fatty acid methyl ester production via ferric sulfate catalyzed interesterification. Biomass Bioenergy 2018, 115, 82–87. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Celante, D.; Brondani, L.N.; Trojahn, D.O.; Silva, C.; Castilhos, F. Synthesis of methyl esters and triacetin from macaw oil (Acrocomia aculeata) and methyl acetate over γ-alumina. Ind. Crops Prod. 2018, 124, 84–90. [Google Scholar] [CrossRef]
- Gupta, A.R.; Yadav, S.V.; Rathod, V.K. Enhancement in biodiesel production using waste cooking oil and calcium diglyceroxide as a heterogeneous catalyst in presence of ultrasound. Fuel 2015, 158, 800–806. [Google Scholar] [CrossRef]
- Galia, A.; Centineo, A.; Saracco, G.; Schiavo, B.; Scialdone, O. Interesterification of rapeseed oil catalyzed by tin octoate. Biomass Bioenergy 2014, 67, 193–200. [Google Scholar] [CrossRef]
- Man, I.C.; Soriga, S.G.; Parvulescu, V. Theoretical aspects of methyl acetate and methanol activation on MgO(100) and (501) catalyst surfaces with application in FAME production. Appl. Surf. Sci. 2017, 392, 920–928. [Google Scholar] [CrossRef]
- Man, I.C.; Soriga, S.G.; Parvulescu, V. Effect of Ca and Sr in MgO(100) on the activation of methanol and methyl acetate. Catal. Today 2018, 306, 207–214. [Google Scholar] [CrossRef]
- Alves, M.A.L.; Pinheiro, N.S.C.; Brondani, L.N.; Celante, D.; Ketzer, F.; Castilhos, F. Assessment of niobium phosphate as heterogeneous catalyst in esterification with methyl acetate. Chem. Technol. Biotechnol. 2019, 94, 3172–3179. [Google Scholar] [CrossRef]
- Komintarachat, C.; Tongroon, M.; Chuepeng, S. Biofuel Synthesis from Waste Cooking Oils and Ethyl Acetate via Interesterification under CaO Catalyst from Waste Eggshells. In Proceedings of the 2018 Third International Conference on Engineering Science and Innovative Technology (ESIT), North Bangkok, Thailand, 19–22 April 2018. [Google Scholar]
- Casas, A.; Ramos, M.J.; Pérez, A. Adsorption equilibrium and kinetics of methyl acetate/methanol and methyl acetate/water mixtures on zeolite 5A. Chem. Eng. J. 2013, 220, 337–342. [Google Scholar] [CrossRef]
- Demirbas, A. Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- Xu, Y.; Du, W.; Liu, D.; Zeng, J. A novel enzymatic route for biodiesel production from renewable oils in a solvent-free medium. Biotechnol. Lett. 2003, 25, 1239–1241. [Google Scholar] [CrossRef]
- Xu, Y.; Du, W.; Liu, D. Study on the kinetics of enzymatic interesterification of triglycerides for biodiesel production with methyl acetate as the acyl acceptor. J. Mol. Catal. B Enzym. 2005, 32, 241–245. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.; Liu, D.; Zeng, J. Comparative study on lipase-catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J. Mol. Catal. B Enzym. 2004, 30, 125–129. [Google Scholar] [CrossRef]
- Orçaire, O.; Buisson, P.; Pierre, A.C. Application of silica aerogel encapsulated lipases in the synthesis of biodiesel by transesterification reactions. J. Mol. Catal. B Enzym. 2006, 42, 106–113. [Google Scholar] [CrossRef]
- Usai, E.M.; Gualdi, E.; Solinas, V.; Battistel, E. Simultaneous enzymatic synthesis of FAME and triacetyl glycerol from triglycerides and methyl acetate. Bioresour. Technol. 2010, 101, 7707–7712. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, Y. Lipase-Catalyzed Biodiesel Production with Methyl Acetate as Acyl Acceptor. Z. Nat. C 2008, 63, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Ognjanovic, N.; Bezbradica, D.; Knezevic-Jugovic, Z. Enzymatic conversion of sunflower oil to biodiesel in a solvent-free system: Process optimization and the immobilized system stability. Bioresour. Technol. 2009, 100, 5146–5154. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.M.R.; Das, P.; Fang, T.S.; Wu, J.C. Enhanced enzymatic transesterification of palm oil to biodiesel. Biochem. Eng. J. 2011, 55, 119–122. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Fu, B. Environmentally Sustainable Biofuels: Advances in Biodiesel Research. Waste Biomass Valorization 2010, 1, 47–63. [Google Scholar] [CrossRef]
- Razack, S.A.; Duraiarasan, S. Response surface methodology assisted biodiesel production from waste cooking oil using encapsulated mixed enzyme. Waste Manag. 2016, 47, 98–104. [Google Scholar] [CrossRef]
- Surendhiran, D.; Sirajunnisa, A.R.; Vijay, M. An alternative method for production of microalgal biodiesel using novel Bacillus lipase. 3 Biotech. 2015, 5, 715–725. [Google Scholar] [CrossRef]
- Marx, S. Glycerol-free biodiesel production through transesterification: A review. Fuel Process. Technol. 2016, 151, 139–147. [Google Scholar] [CrossRef]
- Norjannah, B.; Ong, H.C.; Masjuki, H.H.; Juan, J.C.; Chong, W.T. Enzymatic transesterification for biodiesel production: A comprehensive review. RSC Adv. 2016, 6, 60034–60055. [Google Scholar] [CrossRef]
- Van Dammea, S.; Brama, S.; Contino, F. Comparison of biodiesel production scenarios with coproduction of triacetin according to energy and GHG missions. Energy Procedia 2014, 61, 1852–1859. [Google Scholar] [CrossRef][Green Version]
- Karmee, S.K. Lipase Catalyzed Synthesis of Fatty Acid Methyl Esters from Crude Pongamia Oil. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 536–542. [Google Scholar] [CrossRef]
- Ognjanović, N.D.; Šaponjić, S.V.; Bezbradica, D.I.; Knežević, Z.D. Lipase-catalyzed biodiesel synthesis with different acyl acceptors. APTEFF 2008, 39, 161–169. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Chen, S.S.; Su, C.H.; Lin, I.H.; Chien, C.C. Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology. Energy Convers. Manag. 2018, 158, 168–175. [Google Scholar] [CrossRef]
- Alavijeh, R.S.; Tabandeh, F.; Tavakoli, O.; Karkhane, A.; Shariati, P. Enzymatic Production of Biodiesel from Microalgal Oil using Ethyl Acetate as an Acyl Acceptor. J. Oleo Sci. 2015, 64, 69–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruzich, N.I.; Bassi, A.S. Investigation of Enzymatic Biodiesel Production using Ionic Liquid as a Co-solvent. Can. J. Chem. Eng. 2010, 68, 277–284. [Google Scholar] [CrossRef]
- Ruzich, N.I.; Bassi, A.S. Proposed kinetic mechanism of biodiesel production through lipase catalysed interesterification with a methyl acetate acyl acceptor and ionic liquid [BMIM][PF6] co-solvent. Can. J. Chem. Eng. 2011, 89, 166–170. [Google Scholar] [CrossRef]
- Muhammada, N.; Elsheikh, Y.A.; Mutalib, M.I.A.; Bazmi, A.A.; Khan, R.A.; Khan, H.; Rafiq, S.; Man, Z.; Khan, I. An overview of the role of ionic liquids in biodiesel reactions. J. Ind. Eng. Chem. 2015, 21, 1–10. [Google Scholar] [CrossRef]
- Andreani, L.; Rocha, J.D. Use of ionic liquids in biodiesel production: A review. Braz. J. Chem. Eng. 2012, 29, 1–13. [Google Scholar] [CrossRef]
- Saka, S.; Isayama, Y. A new process for catalyst-free production of biodiesel using supercritical methyl acetate. Fuel 2009, 88, 1307–1313. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. A glycerol-free process to produce biodiesel by supercritical methyl acetate technology: An optimization study via Response Surface Methodology. Bioresour. Technol. 2010, 101, 965–969. [Google Scholar] [CrossRef]
- Campanelli, P.; Banchero, M.; Manna, L. Synthesis of biodiesel from edible, non-edible and waste cooking oils via supercritical methyl acetate transesterification. Fuel 2010, 89, 3675–3682. [Google Scholar] [CrossRef]
- Niza, N.M.; Tan, K.; Ahmad, Z.; Lee, K.T. Comparison and optimisation of biodiesel production from Jatropha curcas oil using supercritical methyl acetate and methanol. Chem. Pap. 2011, 65, 721–729. [Google Scholar] [CrossRef]
- Niza, N.M.; Tan, K.T.; Lee, K.T.; Ahmad, Z. Biodiesel production by non-catalytic supercritical methyl acetate: Thermal stability study. Appl. Energy 2013, 101, 198–202. [Google Scholar] [CrossRef]
- Goembira, F.; Matsuura, K.; Saka, S. Biodiesel production from rapeseed oil by various supercritical carboxylate esters. Fuel 2012, 97, 373–378. [Google Scholar] [CrossRef]
- Bernal, M.; Lozano, P.; García-Verdugo, E.; Burguete, M.I.; Sánchez-Gómez, G.; López-López, G.; Pucheault, M.; Vaultier, M.; Luis, S.V. Supercritical Synthesis of Biodiesel. Review. Molecules 2012, 17, 8696–8719. [Google Scholar] [CrossRef] [PubMed]
- Lourinho, G.; Brito, P. Advanced biodiesel production technologies: Novel developments. Rev. Environ. Sci. Biotechnol. 2015, 14, 287–316. [Google Scholar] [CrossRef]
- Komintarachat, C.; Sawangkeaw, R.; Ngamprasertsith, S. Continuous production of palm biofuel under supercritical ethyl acetate. Energy Convers. Manag. 2015, 93, 332–338. [Google Scholar] [CrossRef]
- Goembira, F.; Saka, S. Advanced supercritical Methyl acetate method for biodiesel production from Pongamia pinnata oil. Renew. Energy 2015, 83, 1245–1249. [Google Scholar] [CrossRef]
- Hoang, D.; Bensaid, S.; Saracco, G. Supercritical fluid technology in biodiesel production. Green Process. Synth. 2013, 2, 407–425. [Google Scholar] [CrossRef]
- Farobie, O.; Matsumura, Y. State of the art of biodiesel production under supercritical conditions. Prog. Energy Combust. Sci. 2017, 63, 173–203. [Google Scholar] [CrossRef]
- Patil, P.D.; Reddy, H.; Muppaneni, T.; Deng, S. Biodiesel fuel production from algal lipids using supercritical methyl acetate (glycerin-free) technology. Fuel 2017, 195, 201–207. [Google Scholar] [CrossRef]
- Sootchiewcharn, N.; Attanatho, L.; Reubroycharoen, P. Biodiesel Production from Refined Palm Oil using Supercritical Ethyl Acetate in A Microreactor. Energy Procedia 2015, 79, 697–703. [Google Scholar] [CrossRef]
- Lamba, N.; Gupta, K.; Modak, J.M.; Madras, G. Biodiesel synthesis from Calophyllum inophyllum oil with different supercritical fluids. Bioresour. Technol. 2017, 241, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Maddikeri, G.L.; Pandit, A.B.; Gogate, P.R. Ultrasound assisted interesterification of waste cooking oil and methyl acetate for biodiesel and triacetin production. Fuel Process. Technol. 2013, 116, 241–249. [Google Scholar] [CrossRef]
- Tavares, G.R.; Gonzalves, J.E.; dos Santos, W.D.; da Silvad, C. Enzymatic interesterification of crambe oil assisted by ultrasound. Ind. Crops Prod. 2017, 97, 218–223. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Gogate, P.R. Ultrasound assisted intensification of biodiesel production using enzymatic interesterification. Ultrason. Sonochem. 2016, 29, 67–75. [Google Scholar] [CrossRef]
- Bokhari, A.; Suzana Yusup, S.; Chuah, L.F.; Klemeš, J.J.; Asif, S.; Ali, B.; Akbar, M.M.; Kamil, R.N.M. Pilot scale intensification of rubber seed (Hevea brasiliensis) oil via chemical interesterification using hydrodynamic cavitation technology. Bioresour. Technol. 2017, 242, 272–282. [Google Scholar] [CrossRef]
- Kashyap, S.S.; Gogate, P.R.; Joshi, S.M. Ultrasound assisted synthesis of biodiesel from karanja oil by interesterification: Intensification studies and optimization using RSM. Ultrason. Sonochem. 2019, 50, 36–45. [Google Scholar] [CrossRef]
- Sustere, Z.; Kampars, V. Chemical Interesterification of the Rapeseed Oil with Methyl Acetate in the Presence of Potassium tert-Butoxide in tert-Butanol. Int. J. Eng. Technol. Res. 2015, 3, 226–232. [Google Scholar]
- Kovalenko, G.A.; Perminova, L.V.; Beklemishev, A.B.; Yakovleva, E.Y.; Pykhtina, M.B. Heterogeneous biocatalytic processes of vegetable oil interesterification to biodiesel. Catal. Ind. 2015, 7, 73–81. [Google Scholar] [CrossRef]
- Valdis, K.; Zane, S.; Ruta, K. Biofuel via interesterification of rapeseed oil with methyl acetate in presence of potassium t-butoxide/THF. Min. Ecol. Manag. 2018, 18, 163–170. [Google Scholar] [CrossRef]
- Aldo, M.; Medeiros, A.M.; Santos, E.R.M.; Azevedo, S.H.G.; Jesus, A.A.; Oliveira, H.N.M.; Sousa, E.M.B.D. Chemical interesterification of cotton oil with methyl acetate assisted by ultrasound for biodiesel production. Braz. J. Chem. Eng. 2018, 35, 1005–1018. [Google Scholar] [CrossRef]
- Ketzer, F.; Celante, D.; de Castilhos, F. Catalytic performance and ultrasonic-assisted impregnation effects on WO3/USY zeolites in esterification of oleic acid with methyl acetate. Microporous Mesoporous Mater. 2020, 291, 109704. [Google Scholar] [CrossRef]
- Kashyap, S.S.; Gogate, P.R.; Joshi, S.M. Ultrasound assisted intensified production of biodiesel from sustainable source as karanja oil using interesterification based on heterogeneous catalyst (γ-alumina). Chem. Eng. Process. Process. Intensif. 2019, 136, 11–16. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Transesterification of Waste Frying Oil and Soybean Oil by Combi-lipases Under Ultrasound-Assisted Reactions. Appl. Biochem. Biotechnol. 2018, 186, 576–589. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.B.; Garcia, V.A.S.; Arroyo, P.A.; da Silva, C. Ultrasound-assisted extraction of radish seed oil with methyl acetate for biodiesel production. Can. J. Chem. Eng. 2017, 95, 2142–2147. [Google Scholar] [CrossRef]
- Gusniah, A.; Veny, H.; Hamzah, F. Ultrasonic Assisted Enzymatic Transesterification for Biodiesel Production. Ind. Eng. Chem. Res. 2019, 58, 581–589. [Google Scholar] [CrossRef]
- Pukale, D.D.; Maddikeri, G.L.; Gogate, P.R.; Pandit, A.B.; Pratap, A.P. Ultrasound assisted transesterification of waste cooking oil using heterogeneous solid catalyst. Ultrason. Sonochem. 2015, 22, 278–286. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Pristiyani, R.; Dewajani, H. A New Route of Biodiesel Production through Chemical Interesterification of Jatropha Oil using Ethyl Acetate. Int. J. ChemTech Res. 2016, 9, 627–634. [Google Scholar]
- Leonardo Interrante, L.; Bensaid, S.; Galletti, C.; Pirone, R.; Schiavo, B.; Scialdone, O.; Galia, A. Interesterification of rapeseed oil catalysed by a low surface area tin (II) oxide heterogeneous catalyst. Fuel Process. Technol. 2018, 177, 336–344. [Google Scholar] [CrossRef]
- Kim, S.J.; Jung, S.M.; Park, Y.C.; Park, K. Lipase catalyzed transesterification of soybean oil using ethyl acetate, an alternative acyl acceptor. Biotechnol. Bioprocess. Eng. 2007, 12, 441–445. [Google Scholar] [CrossRef]
- Chuepeng, S.; Komintarachat, C. Interesterification optimization of waste cooking oil and ethyl acetate over homogeneous catalyst for biofuel production with engine validation. Appl. Energy 2018, 232, 728–739. [Google Scholar] [CrossRef]
- Jeong, G.T.; Park, D.H. Synthesis of Rapeseed Biodiesel Using Short-Chained Alkyl Acetates as Acyl Acceptor. Appl. Biochem. Biotechnol. 2010, 161, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Miesiac, I.; Rogalinski, A.; Jozwiak, P. Transesterification of triglycerides with ethyl acetate. Fuel 2013, 105, 169–175. [Google Scholar] [CrossRef]
- Melero, J.A.; Vicente, G.; Morales, G.; Paniagua, M.; Bustamante, J. Oxygenated compounds derived from glycerol for biodiesel formulation: Influence on EN 14214 quality parameters. Fuel 2010, 89, 2011–2018. [Google Scholar] [CrossRef]
- Gonzalves, V.L.C.; Pinto, B.P.; Silva, J.C.; Mota, C.J.A. Acetylation of glycerol catalyzed by different solid acids. Catal. Today 2008, 133, 673–677. [Google Scholar] [CrossRef]
- Liao, X.; Zhua, Y.; Wang, S.G.; Li, Y. Producing triacetylglycerol with glycerol by two steps: Esterification and acetylation. Fuel Process. Technol. 2009, 90, 988–993. [Google Scholar] [CrossRef]
- Balaraju, M.; Nikhitha, P.; Jagadeeswaraiah, K.; Srilatha, K.; Sai Prasad, P.S.; Lingaiah, N. Acetylation of glycerol to synthesize bioadditives over niobic acid supported tungstophosphoric acid catalysts. Fuel Process. Technol. 2010, 91, 249–253. [Google Scholar] [CrossRef]
- Rezayat, M.; Ghaziaskar, H.S. Continuous synthesis of glycerol acetates in supercritical carbon dioxideusing Amberlyst 15®. Green Chem. 2009, 11, 710–715. [Google Scholar] [CrossRef]
- Galana, M.I.; Bonet., J.; Sire, R.; Reneaume, J.M.; Plesu, A.E. From residual to useful oil: Revalorization of glycerine from the biodiesel synthesis. Bioresour. Technol. 2009, 100, 3775–3778. [Google Scholar] [CrossRef]
- Bonet, J.; Costa, J.; Sire, R.; Reneaume, J.M.; Plesu, A.E.; Plesu, V.; Bozga, G. Revalorization of glycerol: Comestible oil from biodiesel synthesis. Food Bioprod. Process. 2009, 87, 171–178. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Acetylation of glycerol over heteropolyacids supported on activated carbon. Catal. Commun. 2011, 12, 573–576. [Google Scholar] [CrossRef]
- Dosuna-Rodríguez, I.; Gaigneaux, E.M. Glycerol acetylation catalysed by ion exchange resins. Catal. Today 2012, 195, 14–21. [Google Scholar] [CrossRef]
- Testa, M.L.; La Parola, V.; Liotta, L.F.; Venezia, A.M. Screening of different solid acid catalysts for glycerol acetylation. J. Mol. Catal. A Chem. 2013, 367, 69–76. [Google Scholar] [CrossRef]
- Costa, B.O.D.; Decolattia, H.P.; Legnoverde, M.S.; Querini, C.A. Influence of acidic properties of different solid acid catalysts for glycerol acetylation. Catal. Today 2017, 289, 222–230. [Google Scholar] [CrossRef]
- Huang, M.Y.; Han, X.X.; Hung, C.T.; Lin, J.C.; Wuc, P.H.; Wud, J.C.; Liu, S.B. Heteropolyacid-based ionic liquids as efficient homogeneous catalysts for acetylation of glycerol. J. Catal. 2014, 320, 42–51. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Triwahyono, S.; Hameed, B.H.; Jalil, A.A. Improved production of fuel oxygenates via glycerol acetylation with acetic acid. Chem. Eng. J. 2014, 243, 473–484. [Google Scholar] [CrossRef]
- Betiha, M.A.; Hassan, H.M.A.; El-Sharkawy, E.A.; Al-Sabagh, A.M.; Menoufy, M.F.; Abdelmoniem, H.-E.M. A new approach to polymer-supported phosphotungstic acid: Application for glycerol acetylation using robust sustainable acidic heterogeneous–homogenous catalyst. Appl. Catal. B Environ. 2016, 182, 15–25. [Google Scholar] [CrossRef]
- Sandesh, S.; Manjunathan, P.; Halgeri, A.B.; Shanbhag, G.V. Glycerol acetins: Fuel additive synthesis by acetylation and esterification of glycerol using cesium phosphotungstate catalyst. RSC Adv. 2015, 5, 104354–104362. [Google Scholar] [CrossRef]
- Kong, P.S.; Aroua, M.K.; Daud, W.M.A.W.; Lee, H.V.; Cognet, P.; Peres-Lucchese, Y. Catalytic role of solid acid catalysts in glycerol acetylation for the production of bio-additives: A review. RSC Adv. 2016, 6, 68885–68905. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. A review on recent developments and progress in the kinetics and deactivation of catalytic acetylation of glycerol—A byproduct of biodiesel. Renew. Sustain. Energy Rev. 2017, 74, 387–401. [Google Scholar] [CrossRef]
- Casas, A.; Ruiz, J.R.; Ramos, M.J.; Perez, A. Effects of triacetin on biodiesel quality. Energy Fuels 2010, 24, 4481–4489. [Google Scholar] [CrossRef]
- Rao, P.V.; Rao, B.V.A. Performance, Emission and Cylinder Vibration studies of DI-Diesel Engine with COME-Triacetin Additive Blends. Int. J. Therm. Technol. 2011, 1, 100–109. [Google Scholar]
- Rao, P.V.; Rao, B.V.A.; Radhakrishna, D. Experimental Analysis of DI Diesel Engine Performance with Triacetin Blend Fuels of Oxygenated Additive and COME Biodiesel. Iran. J. Energy Environ. 2012, 3, 109–117. [Google Scholar] [CrossRef]
- Zare, A.; Bodisco, T.A.; Nabi, M.N.; Hossain, F.M.; Ristovski, Z.D.; Brown, R.J. Engine Performance during Transient and Steady-State Operation with Oxygenated Fuels. Energy Fuels 2017, 31, 7510–7522. [Google Scholar] [CrossRef]
- Zare, A.; Nabi, M.N.; Bodisco, T.A.; Hossain, F.M.; Rahman, M.M.; Ristovski, Z.D.; Brown, R.J. The effect of triacetin as a fuel additive to waste cooking biodiesel on engine performance and exhaust emissions. Fuel 2016, 182, 640–649. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Chakraborty, R. Effects of Bioglycerol Based Fuel Additives on Diesel Fuel Property, Engine Performance and Emission Quality: A Review. Energy Procedia 2015, 79, 671–676. [Google Scholar] [CrossRef]
- Hedayat, F.; Stevanovic, S.; Milic, A.; Miljevic, B.; Nabi, M.N.; Zare, A.; Bottle, S.E.; Brown, R.J.; Ristovski, Z.D. Influence of oxygen content of the certain types of biodiesels on particulate oxidative potential. Sci. Total Environ. 2016, 545, 381–388. [Google Scholar] [CrossRef]
- Rachel, C.; Senra, M.; Soh, L. Cold Flow Properties of Fatty Acid Methyl Ester Blends with and without Triacetin. Energy Fuels 2016, 30, 7400–7409. [Google Scholar] [CrossRef]
- Lacerda, C.V.; Carvalho, M.J.S.; Ratton, A.R.; Soares, I.P.; Borgesa, L.E.P. Synthesis of Triacetin and Evaluation on Motor. J. Braz. Chem. Soc. 2015, 26, 1625–1631. [Google Scholar] [CrossRef]
- Zare, A.; Bodisco, T.; Nabi, M.N.; Farhad, H.M.; Rahman, M.M.; Stuart, D.; Ristovski, Z.; Brown, R.J. Impact of Triacetin as an oxygenated fuel additive to waste cooking biodiesel: Transient engine performance and exhaust emissions. In Proceedings of the Australian Combustion Symposium, Melbourne, Australia, 7–9 December 2015. [Google Scholar]
- Rao, P.V.; Rao, B.V.A. Performance and emission characteristics of diesel engine with COME-Triacetin additive blends as fuel. Int. J. Energy Environ. 2012, 3, 629–638. [Google Scholar]
- Rao, P.V. Role of Triacetin additive in the performance of single cylinder D I diesel engine with COME biodiesel. Int. J. Adv. Eng. Res. Sci. 2018, 5, 253–260. [Google Scholar] [CrossRef][Green Version]
- Meisam Tabatabaei, M.; Aghbashlo, M.; Najafi, B.; Hosseinzadeh-Bandbafha, H.; Ardabili, S.F.; Akbarian, E.; Khalife, E.; Mohammadi, P.; Rastegari, H.; Ghaziaskar, H.S. Environmental impact assessment of the mechanical shaft work produced in a diesel engine running on diesel/biodiesel blends containing glycerol-derived triacetin. J. Clean. Prod. 2019, 223, 466–486. [Google Scholar] [CrossRef]
- Rao, P.V.; Rao, B.V.A. Heat release rate, performance and vibration analysis of diesel engine operating with biodiesel-triacetin additive blend fuels. Int. J. Automob. Eng. Res. Dev. 2018, 8, 11–22. [Google Scholar]
- Zare, A.; Bodisco, T.A.; Nabi, M.N.; Hossain, F.M.; Rahman, M.M.; Ristovski, Z.D.; Brown, R.J. The influence of oxygenated fuels on transient and steady-state engine emissions. Energy 2017, 121, 841–853. [Google Scholar] [CrossRef]
- Panda, K.; Sastry, G.R.K.S.; Rai, R.N. Experimental analysis of performance and emission on DI diesel engine fueled with diesel-palm kernel methyl ester-triacetin blends: A Taguchi fuzzy-based optimization. Environ. Sci. Pollut. Res. 2018, 25, 22035–22051. [Google Scholar] [CrossRef]
- Sakdasri, W.; Ngamprasertsith, S.; Daengsanun, S.; Sawangkeaw, R. Lipid-based biofuel synthesized from palm-olein oil by supercritical ethyl acetate in fixed-bed reactor. Energy Convers. Manag. 2019, 182, 215–223. [Google Scholar] [CrossRef]
- Lapuerta, M.; González-García, I.; Céspedes, I.; Estévez, C.; Bayarri, N. Improvement of cold flow properties of a new biofuel derived from glycerol. Fuel 2019, 242, 794–803. [Google Scholar] [CrossRef]
- Żółtowski, A.; Grzelak, P.L. Emissions from engines fuelled with biofuels. J. Kones Powertrain Transp. 2018, 25, 533–539. [Google Scholar] [CrossRef]
- Ang, G.T.; Tan, K.T.; Lee, K.T. Recent development and economic analysis of glycerol-free processes via supercritical fluid transesterification for biodiesel production. Renew. Sustain. Energy Rev. 2014, 31, 61–70. [Google Scholar] [CrossRef]
- Kralisch, D.; Staffel, C.; Ott, D.; Bensaid, S.; Saracco, G.; Bellantonic, P.; Loebd, P. Process design accompanying life cycle management and risk analysis as a decision support tool for sustainable biodiesel production. Green Chem. 2013, 15, 463–477. [Google Scholar] [CrossRef]
- Yusof, Z. An overview of current issues on fabrication, application and metallic corrosion of biodiesel. J. Petrochem. Eng. Dep. 2015, 2, 25–55. [Google Scholar]
- Li, Y.; Zhao, X.; Wang, Y. Synthesis of dimethyl carbonate from methanol, propylene oxide and carbon dioxide over KOH/4A molecular sieve catalyst. Appl. Catal. A 2005, 279, 205–208. [Google Scholar] [CrossRef]
- Choi, J.C.; He, L.N.; Yasuda, H.; Sakakura, T. Selective and high yield synthesis of dimethyl carbonate directly from carbon dioxide and methanol. Green Chem. 2002, 4, 230–234. [Google Scholar] [CrossRef]
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. A review on the performance of glycerol carbonate production via catalytic transesterification: Effects of influencing parameters. Energy Convers. Manag. 2014, 88, 484–497. [Google Scholar] [CrossRef]
- Tudorache, M.; Negoi, A.; Tudora, B.; Parvulescu, V.I. Environmental-friendly strategy for biocatalytic conversion of waste glycerol to glycerol carbonate. Appl. Catal. B Environ. 2014, 146, 274–278. [Google Scholar] [CrossRef]
- Tudorache, M.; Nae, A.; Coman, S.; Parvulescu, V.I. Strategy of cross-linked enzyme aggregates ontomagnetic particles adapted to the green design of biocatalytic synthesis of glycerol carbonate. RSC Adv. 2013, 3, 4052–4058. [Google Scholar] [CrossRef]
- Lanjekar, K.; Rathod, V.K. Utilization of glycerol for the production of glycerol carbonate through greener route. J. Environ. Chem. Eng. 2013, 1, 1231–1236. [Google Scholar] [CrossRef]
- Ji, Y. Review. Recent Development of Heterogeneous Catalysis in the Transesterification of Glycerol to Glycerol Carbonate. Catalysts 2019, 9, 581. [Google Scholar] [CrossRef]
- Algoufi, Y.T.; Kabir, G.; Hameed, B.H. Synthesis of glycerol carbonate from biodiesel by-product glycerol over calcined dolomite. J. Taiwan Inst. Chem. Eng. 2017, 70, 179–187. [Google Scholar] [CrossRef]
- Algoufi, Y.T.; Akpan, U.G.; Kabir, G.; Asif, B.M.; Hameed, H. Upgrading of glycerol from biodiesel synthesis with dimethyl carbonate on reusable Sr–Al mixed oxide catalysts. Energy Convers. Manag. 2017, 138, 183–189. [Google Scholar] [CrossRef]
- Parameswaram, G.; Srinivas, M.; Babu, B.H.; Prasad, P.S.S.; Lingaiah, N. Transesterification of glycerol with dimethyl carbonate for the synthesis of glycerol carbonate over Mg/Zr/Sr mixed oxide base catalysts. Catal. Sci. Technol. 2013, 3, 3242–3249. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Stabilized ladle furnace steel slag for glycerol carbonate synthesis via glycerol transesterification reaction with dimethyl carbonate. Energy Convers. Manag. 2017, 133, 477–485. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Glycerol carbonate synthesis from glycerol and dimethyl carbonate using trisodium phosphate. J. Taiwan Inst. Chem. Eng. 2016, 68, 51–58. [Google Scholar] [CrossRef]
- Wang, S.; Hao, P.; Li, S.; Zhang, A.; Guan, Y.; Zhang, L. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate catalyzed by calcined silicates. Appl. Catal. A Gen. 2017, 542, 174–181. [Google Scholar] [CrossRef]
- Wan, Y.; Lei, Y.; Lan, G.; Liu, D.; Li, G.; Bai, R. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate over DABCO embedded porous organic polymer as a bifunctional and robust catalyst. Appl. Catal. A Gen. 2018, 562, 267–275. [Google Scholar] [CrossRef]
- Fabbri, D.; Bevoni, V.; Notari, M.; Rivetti, F. Properties of a potential biofuel obtained from soybean oil by transmethylation with dimethyl carbonate. Fuel 2007, 86, 690–697. [Google Scholar] [CrossRef]
- Notari, M.; Rivetti, F. Use of a Mixture of Esters of Fatty Acids as Fuel or Solvent. Patent No. WO2004/052874, 24 March 2004. [Google Scholar]
- Islam, M.R.; Kurle, Y.M.; Gossage, J.L.; Benson, T.J. Kinetics of Triazabicyclodecene-Catalyzed Canola Oil Conversion to Glycerol-free Biofuel Using Dimethyl Carbonate. Energy Fuels 2013, 27, 1564–1569. [Google Scholar] [CrossRef]
- Zhang, L.; Sheng, B.; Xin, Z.; Liu, Q.; Sun, S. Kinetics of transesterification of palm oil and dimethyl carbonate for biodiesel production at the catalysis of homogeneous base catalyst. Bioresour. Technol. 2010, 101, 8144–8150. [Google Scholar] [CrossRef]
- Celante, D.; Schenkel, J.V.D.; Castilhos, F. Biodiesel production from soybean oil and dimethyl carbonate catalyzed by potassium methoxide. Fuel 2018, 212, 101–107. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Ayodele, O.O.; Xin, J.; Zhang, S. Dimethyl carbonate mediated production of biodiesel at different reaction temperatures. Renew. Energy 2014, 68, 581–587. [Google Scholar] [CrossRef]
- Panchal, B.M.; Deshmukh, S.A.; Sharma, M.R. Kinetic and effective production of DMC-Sm-BioDs from Sapindus mukorossi seed kernel powder using dimethyl carbonate with KOH–Folch mixture solution as a catalyst. Biofuels 2015, 6, 305–312. [Google Scholar] [CrossRef]
- Kai, T.; Mak, G.L.; Wada, S.; Nakazato, T.; Takanashi, H.; Uemura, Y. Production of biodiesel fuel from canola oil with dimethyl carbonate using an active sodium methoxide catalyst prepared by crystallization. Bioresour. Technol. 2014, 163, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Syamsuddin, Y.; Murat, M.N.; Hameed, B.H. Transesterification of Jatropha oil with dimethyl carbonate to produce fatty acid methyl ester over reusable Ca–La–Al mixed-oxide catalyst. Energy Convers. Manag. 2015, 106, 1356–1361. [Google Scholar] [CrossRef]
- Panchal, B.M.; Deshmukh, S.A.; Sharma, M.R. Biodiesel from Thevetia peruviana Seed Oil with Dimethyl Carbonate Using as an Active Catalyst Potassium-Methoxide. Sains Malays. 2016, 45, 1461–1468. [Google Scholar]
- Al-Saadi, L.S.; Eze, V.C.; Harvey, A.P. Experimental Determination of Optimal Conditions for Reactive Coupling of Biodiesel Production With in situ Glycerol Carbonate Formation in a Triglyceride Transesterification Process. Front. Chem. 2018, 6, 625. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Tyagi, S.; Newalkar, B.; Badoni, R.P. Jatropha and Karanja oil derived DMC–biodiesel synthesis: A kinetics study. Fuel 2015, 140, 597–608. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.M.; Oh, J.I.; Ok, Y.S.; Lee, S.R.; Kwon, E.E. Evaluating the effectiveness of various biochars as porous media for biodiesel synthesis via pseudo-catalytic transesterification. Bioresour. Technol. 2017, 231, 59–64. [Google Scholar] [CrossRef]
- Tang, Y.; Cheng, Q.; CaO, H.; Zhang, L.; Zhang, J.; Li, H. Coupling transesterifications for no-glycerol biodiesel production catalyzed by calcium oxide. C. R. Chim. 2015, 18, 1328–1334. [Google Scholar] [CrossRef]
- Panchal, B.M.; Kalaji, H.M. Synthesis and use of a catalyst in the production of biodiesel from Pongamia Pinnata seed oil with dimethyl carbonate. Int. J. Green Energy 2017, 14, 624–631. [Google Scholar] [CrossRef]
- Tang, Y.; Yan, T.; Shen, B.; Li, H.; Jeje, A. nthesis of no-glycerol biodiesel through transesterification catalyzed by CaO from different precursors. Can. J. Chem. Eng. 2016, 94, 1466–1471. [Google Scholar] [CrossRef]
- Tang, Y.; Ren, H.; Chang, F.; Gu, X.; Zhang, J. Nano KF/Al2O3 particles as an efficient catalyst for no-glycerol biodiesel production by coupling transesterification. RSC Adv. 2017, 7, 5694–5700. [Google Scholar] [CrossRef]
- Panchal, B.; Shenjun, Q.; Jinxi, W.; Kai, B.; Chang, T. Biodiesel Synthesis with Iron Oxide Nano-Catalyst Catalyzed Pongamia Pinnata Seed Oil and Dimethyl Carbonate. Am. J. Energy Eng. 2018, 6, 21–28. [Google Scholar] [CrossRef]
- Ali, N.A.M.; Aziz, N. Optimization of DMC Transesterification Based Biodiesel Production. Adv. Mater. Res. 2015, 1113, 370–375. [Google Scholar] [CrossRef]
- Dhawan, M.S.; Yadav, G.D. Insight into a catalytic process for simultaneous production of biodiesel and glycerol carbonate from triglycerides. Catal. Today 2018, 309, 161–171. [Google Scholar] [CrossRef]
- Syamsuddin, Y.; Hameed, B.H. Synthesis of glycerol free-fatty acid methyl esters from Jatropha oil over Ca–La mixed-oxide catalyst. J. Taiwan Inst. Chem. Eng. 2016, 58, 181–188. [Google Scholar] [CrossRef]
- Syamsuddin, Y.; Murat, M.N.; Hameed, B.H. Synthesis of fatty acid methyl ester from the transesterification of high- and low-acid-content crude palm oil (Elaeis guineensis) and karanj oil (Pongamia pinnata) over a calcium–lanthanum–aluminum mixed-oxides catalyst. Bioresour. Technol. 2016, 214, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Panchal, B.; Kalaji, H.M.; Deshmukh, S.; Sharma, M.; Strobel, W.R. Synthesis of Methyl Esters from Silk Cotton Tree Seed Kernel Oil Using Dimethyl Carbonate and KOH Catalysis. Eur. J. Sustain. Dev. Res. 2018, 2, 20. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Dimethyl carbonate as potential reactant in non-catalytic biodiesel production by supercritical method. Bioresour. Technol. 2009, 100, 1793–1796. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Production of biodiesel with glycerol carbonate by non-catalytic supercritical dimethyl carbonate. Lipid Technol. 2011, 23, 10–13. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T. A review on supercritical fluids (SCF) technology in sustainable biodiesel production: Potential and challenges. Renew. Sustain. Energy Rev. 2011, 15, 2452–2456. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Optimization of supercritical dimethyl carbonate method for biodiesel production. Fuel 2012, 97, 670–677. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Two-step supercritical dimethyl carbonate method for biodiesel production from Jatropha curcas oil. Bioresour. Technol. 2010, 101, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. Optimization of supercritical dimethyl carbonate (SCDMC) technology for the production of biodiesel and value-added glycerol carbonate. Fuel 2010, 89, 3833–3839. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Esterification of glycerol from biodiesel production to glycerol carbonate in non-catalytic supercritical dimethyl. SpringerPlus 2016, 5, 923. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Tyagi, S.; Newalkar, B.; Badoni, R.P. Glycerin-Free Synthesis of Jatropha and Pongamia Biodiesel in Supercritical Dimethyl and Diethyl Carbonate. Ind. Eng. Chem. Res. 2014, 53, 10525–10533. [Google Scholar] [CrossRef]
- Seong, P.J.; Jeon, B.W.; Lee, M.; Cho, D.H.; Kim, D.K.; Jung, K.S.; Kim, S.W.; Han, S.O.; Kim, Y.H.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil and dimethyl carbonate. Enzym. Microb. Technol. 2011, 48, 505–509. [Google Scholar] [CrossRef]
- Su, E.; You, P.; Wei, D. In situ lipase-catalyzed reactive extraction of oilseeds with short-chained dialkyl carbonates for biodiesel production. Bioresour. Technol. 2009, 100, 5813–5817. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, S.; Xin, Z.; Sheng, B.; Liu, Q. Synthesis and component confirmation of biodiesel from palm oil and dimethyl carbonate catalyzed by immobilized-lipase in solvent-free system. Fuel 2010, 89, 3960–3965. [Google Scholar] [CrossRef]
- Min, J.Y.; Lee, E.Y. Lipase-catalyzed simultaneous biosynthesis of biodiesel and glycerol carbonate from corn oil in dimethyl carbonate. Biotechnol. Lett. 2011, 33, 1789–1796. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, L.; Meng, X.; Xin, Z. Kinetic study on lipase catalyzed trans-esterification of palm oil and dimethyl carbonate for biodiesel production. J. Renew. Sustain. Energy 2013, 5, 033127. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X. Enzymatic synthesis of fatty acid ethyl esters by utilizing camellia oil soapstocks and diethyl carbonate. Bioresour. Technol. 2011, 102, 10173–10179. [Google Scholar] [CrossRef] [PubMed]
- Su, E.; Du, L.; Gong, X.; Wang, P. Lipase-Catalyzed Irreversible Transesterification of Jatropha Curcas L. Seed Oil to Fatty Acid Esters: An Optimization Study. J. Am. Oil Chem. Soc. 2011, 88, 793–800. [Google Scholar] [CrossRef]
- Su, E.Z.; Zhang, M.J.; Zhang, J.G.; Gao, J.F.; Wei, D.Z. Lipase-catalyzed irreversible transesterification of vegetable oils for fatty acid methyl esters production with dimethyl carbonate as the acyl acceptor. Biochem. Eng. J. 2007, 36, 167–173. [Google Scholar] [CrossRef]
- Goa, A.R.; Lee, Y.; Kim, Y.H.; Park, S.; Choi, J.; Lee, J.; Han, S.O.; Kim, S.W.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil in solvent-free system. Enzym. Microb. Technol. 2013, 53, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Gharat, N.; Rathod, V.K. Ultrasound assisted enzyme catalyzed transesterification of waste cooking oil with dimethyl carbonate. Ultrason. Sonochem. 2013, 20, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, Y.; Kim, D.; Han, S.O.; Kim, S.W.; Lee, J.; Kim, Y.H.; Park, C. Enzymatic production of glycerol carbonate from by-product after biodiesel manufacturing process. Enzym. Microb. Technol. 2012, 51, 143–147. [Google Scholar] [CrossRef]
- Gharat, N.; Rathod, V.K. Enzyme catalyzed transesterification of waste cooking oil with dimethyl carbonate. J. Mol. Catal. B Enzym. 2013, 88, 36–40. [Google Scholar] [CrossRef]
- Panadare, D.C.; Rathod, V.K. Microwave assisted enzymatic synthesis of biodiesel with waste cooking oil and dimethyl carbonate. J. Mol. Catal. B Enzym. 2016, 133, S518–S524. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, O.K.; Kim, C.H.; Seo, J.W.; Oh, B.R.; Lee, E.Y. Lipase-catalyzed in-situ biosynthesis of glycerol-free biodiesel from heterotrophic microalgae, Aurantiochytrium sp. KRS101 biomass. Bioresour. Technol. 2016, 211, 472–477. [Google Scholar] [CrossRef]
- Jo, Y.J.; Lee, O.K.; Lee, E.Y. Dimethyl carbonate-mediated lipid extraction and lipase-catalyzed in situ transesterification for simultaneous preparation of fatty acid methyl esters and glycerol carbonate from Chlorella sp. KR-1 biomass. Bioresour. Technol. 2014, 158, 105–110. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, E.Y. Simultaneous Production of Transformer Insulating Oil and Value-Added Glycerol Carbonates from Soybean Oil by Lipase-Catalyzed Transesterification in Dimethyl Carbonate. Energies 2018, 11, 82. [Google Scholar] [CrossRef]
- Leão, R.A.C.; Souza, S.P.; Nogueira, D.O.; Silva, G.M.A.; Silva, M.V.; Gutarra, M.L.E.; Miranda, L.S.M.; Castro, A.M.; Junior, I.I.; Souza, R.O.M.A. Consecutive lipase immobilization and glycerol carbonate production under continuous-flow conditions. Catal. Sci. Technol. 2016, 6, 4743–4748. [Google Scholar] [CrossRef]
- Ishak, Z.H.; Sairi, N.A.; Alias, Y.; Aroua, M.K.T.; Yusoff, R. A review of ionic liquids as catalysts for transesterification reactions of biodiesel and glycerol carbonate production. Catal. Rev. Sci. Eng. 2017, 59, 44–93. [Google Scholar] [CrossRef]
- Fan, P.; Wang, J.; Xing, S.; Yang, L.; Yang, G.; Fu, J.; Miao, C.; Lv, P. Synthesis of Glycerol-Free Biodiesel with Dimethyl Carbonate over Sulfonated Imidazolium Ionic Liquid. Energy Fuels 2017, 31, 4090–4095. [Google Scholar] [CrossRef]
- Jung, J.M.; Oh, J.I.; Kwon, D.K.; Park, Y.K.; Zhang, M.; Lee, J.; Kwon, E.E. Synthesis of fatty acid methyl esters via non-catalytic transesterification of avocado oil with dimethyl carbonate. Energy Convers. Manag. 2019, 195, 1–6. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, E.Y. Environmentally-Benign Dimethyl Carbonate-Mediated Production of Chemicals and Biofuels from Renewable Bio-Oil. Review. Energies 2017, 10, 1790. [Google Scholar] [CrossRef]
- Pyo, S.H.; Park, J.H.; Chang, T.S.; Hatti-Kaul, R. Dimethyl carbonate as a Green Chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl carbonate: A versatile reagent for a sustainable valorization of renewables. Green Chem. 2018, 20, 288–322. [Google Scholar] [CrossRef]
- Szőri1, M.; Giri, B.R.; Wang, Z.; Dawood, A.E.; Viskolcz, B.; Farooq, A. Glycerol Carbonate as Fuel Additive for Sustainable Future. Sustain. Energy Fuels 2018, 2, 2171–2178. [Google Scholar] [CrossRef]
- Sonnati, M.O.; Amigoni, S.; de Givenchy, P.T.; Darmanin, T.; Choulet, O.; Guittard, F. Glycerol carbonate as a versatile building block for tomorrow: Synthesis, reactivity, properties and applications. Green Chem. 2013, 15, 283–306. [Google Scholar] [CrossRef]
- Rounce, P.; Tsolakis, A.; Leung, P.; York, A.P.E. A Comparison of Diesel and Biodiesel Emissions Using Dimethyl Carbonate as an Oxygenated Additive. Energy Fuels 2010, 24, 4812–4819. [Google Scholar] [CrossRef]
- Kumar, R.; Saravanan, S. Partially premixed low temperature combustion using dimethyl carbonate (DMC) in a DI diesel engine for favorable smoke/NOx emissions. Fuel 2016, 180, 396–406. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Balasubramanian, R. Efects of oxygenated fuel blends on carbonaceous particulate composition and particle size distributions from a stationary diesel engine. Fuel 2015, 141, 1–8. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, Y.; Karavalakis, G.; Johnson, K.C.; Kumar, S.; Cocker, D.R.; Durbin, T.D. Impacts of dimethyl carbonate blends on gaseous and particulate emissions from a heavy-duty diesel engine. Fuel 2016, 184, 681–688. [Google Scholar] [CrossRef]
- Verma, P.; Stevanovic, S.; Zare, A.; Dwivedi, G.; Van, T.C.; Davidson, M.; Rainey, T.; Brown, R.J.; Ristovski, Z.D. An Overview of the Influence of Biodiesel, Alcohols, and Various Oxygenated Additives on the Particulate Matter Emissions from Diesel Engines. Energies 2019, 12, 1987. [Google Scholar] [CrossRef]
- Kumar, B.R.; Saravanan, S.; Rana, R.; Nagendran, A. Combined effect of injection timing and exhaust gas recirculation (EGR) on performance and emissions of a DI diesel engine fuelled with nextgeneration advanced biofuel-diesel blends using response surface methodology. Energy Convers. Manag. 2016, 123, 470–486. [Google Scholar] [CrossRef]
- Srihari, S.; Thirumalini, S. Investigation on reduction of emission in PCCI-DI engine with biofuel blends. Renew. Energy 2017, 114, 1232–1237. [Google Scholar] [CrossRef]
- Khalife, E.; Tabatabaei, M.; Demirbas, A.; Aghbashlo, M. Impacts of additives on performance and emission characteristics of diesel engines during steady state operation. Prog. Energy Combust. Sci. 2017, 59, 32–78. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, Z.; Miao, H.; Di, Y.; Jiang, D.; Zeng, K.; Liu, B.; Wang, X. Combustion and emissions of a DI diesel engine fuelled with diesel-oxygenate blends. Fuel 2008, 87, 2691–2697. [Google Scholar] [CrossRef]
- Arteconi, A.; Mazzarini, A.; Nicola, G. Emissions from Ethers and Organic Carbonate Fuel Additives: A Review. Water Air Soil Pollut. 2011, 221, 405–423. [Google Scholar] [CrossRef]
- Bruno, T.J.; Arron Wolk, A.; Naydich, A.; Huber, M.L. Composition-explicit Distillation Curves for Mixtures of Diesel Fuel with Dimethyl Carbonate and Diethyl Carbonate. Energy Fuels 2009, 23, 3989–3997. [Google Scholar] [CrossRef]
- Nakamura, H.; Curran, H.J.; Córdoba, A.P.; Pitz, W.J.; Dagaut, P.; Togbé, C.; Sarathy, S.M.; Mehl, M.; Agudelo, J.R.; Bustamante, F. An experimental and modeling study of diethyl carbonate oxidation. Combust. Flame 2015, 162, 1395–1405. [Google Scholar] [CrossRef]
- Shahl, R.; Togbé, C.; Thion, S.; Timothée, R.; Lailliau, M.; Halter, F.; Chauveau, C.; Dayma, G.; Dagaut, P. Burning velocities and jet-stirred reactor oxidation of diethyl carbonate. Proc. Combust. Inst. 2017, 36, 553–560. [Google Scholar] [CrossRef]
- Kozak, M.; Merkisz, J.; Bielaczyc, P.; Szczotka, A. The Influence of Oxygenated Diesel Fuels on a Diesel Vehicle PM/NOx Emission Trade-Off; SAE Technical Paper 2009; SAE International: Detroit, MI, USA, 2009. [Google Scholar] [CrossRef]
- Kozak, M.; Merkisz, J.; Bielaczyc, P.; Szczotka, A. The Influence of Synthetic Oxygenates on Euro IV Diesel Passenger Car Exhaust Emissions—Part 3; SAE Technical Paper 2008; SAE International: Shanghai, China, 2008. [Google Scholar] [CrossRef]
- Wenyu, S.; Can, H.; Tao, T.; Feng, Z.; Wei, L.; Hansen, N.; Bin, Y. Exploring the high-temperature kinetics of diethyl carbonate (DEC) under pyrolysis and flame conditions. Combust. Flame 2017, 181, 71–81. [Google Scholar] [CrossRef]
- Kumaresan, M.; Devaradjane, G. Effect of two-springs split injection on the performance and emission characteristics of diesel-oxygenated blends in a DI diesel engine. Indian J. Eng. Mater. Sci. 2012, 19, 411–416. [Google Scholar]
- Mei, D.; Hielscher, K.; Baar, R. Study on Combustion Process and Emissions of a Single-Cylinder Diesel Engine Fueled with DMC/Diesel Blend. J. Energy Eng. 2014, 140, 04013004. [Google Scholar] [CrossRef]
- Caballero, V.; Bautista, F.M.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A.; Hidalgo, J.M.; Luque, R.; Macario, A.; Giordano, G. Sustainable preparation of a novel glycerol-free biofuel by using pig pancreatic lipase: Partial 1,3-regiospecific alcoholysis of sunflower oil. Process. Biochem. 2009, 44, 334–342. [Google Scholar] [CrossRef]
- Verdugo, C.; Luque, R.; Luna, D.; Hidalgo, J.M.; Posadillo, A.; Sancho, E.; Rodríguez, S.; Ferreira-Días, S.; Bautista, F.M.; Romero, A.A. A comprehensive study of reaction parameters in the enzymatic production of novel biofuels integrating glycerol into their composition. Bioresour. Technol. 2010, 101, 6657–6662. [Google Scholar] [CrossRef]
- Luna, D.; Posadillo, A.; Caballero, V.; Verdugo, C.; Bautista, F.M.; Romero, A.A.; Sancho, E.D.; Luna, C.; Calero, J. New Biofuel Integrating Glycerol into Its Composition Through the Use of Covalent Immobilized Pig Pancreatic Lipase. Inter. J. Mol. Sci. 2012, 13, 10091–10112. [Google Scholar] [CrossRef]
- Luna, C.; Sancho, E.D.; Luna, D.; Caballero, V.; Calero, J.; Posadillo, A.; Verdugo, C.; Bautista, F.M.; Romero, A.A. Biofuel that Keeps Glycerol as Monoglyceride by 1,3-Selective Ethanolysis with Pig Pancreatic Lipase Covalently Immobilized on AlPO4 Support. Energies 2013, 6, 3879–3900. [Google Scholar] [CrossRef]
- Luna, D.; Bautista, F.M.; Caballero, V.; Campelo, J.M.; Marinas, J.M.; Romero, A.A. Method for Producing Biodiesel Using Porcine Pancreatic Lipase as An Enzymatic Catalyst. European Patent EP 2050823A1, 22 April 2009. [Google Scholar]
- Verdugo, C.; Luna, D.; Posadillo, A.; Sancho, E.D.; Rodríguez, S.; Bautista, F.M.; Luque, R.; Marinas, J.M.; Romero, A.A. Production of a new second generation biodiesel with a low cost lipase derived from Thermomyces lanuginosus: Optimization by response surface methodology. Catal. Today 2011, 167, 107–112. [Google Scholar] [CrossRef]
- Luna, C.; Verdugo, C.; Sancho, E.D.; Luna, D.; Calero, J.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Production of a biodiesel-like biofuel without glycerol generation, by using Novozym 435, an immobilized Candida antarctica lipase. Bioresour. Bioprocess. 2014, 1, 11. [Google Scholar] [CrossRef]
- Luna, C.; Verdugo, C.; Sancho, E.D.; Luna, D.; Calero, J.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Biocatalytic behaviour of immobilized Rhizopus oryzae lipase in the 1, 3-selective ethanolysis of sunflower oil to obtain a biofuel similar to biodiesel. Molecules 2014, 19, 11419–11439. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Niño, A.; Luna, C.; Luna, D.; Marcos, A.T.; Cánovas, D.; Mellado, E. Selection and Characterization of Biofuel-Producing Environmental Bacteria Isolated from Vegetable Oil Rich Wastes. PLoS ONE 2014, 9, e104063. [Google Scholar] [CrossRef]
- Luna, C.; Verdugo-Escamilla, C.; Sancho, E.D.; Luna, D.; Calero, J.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Achievement of a biofuel-like biodiesel by regioselective transesterification of sunflower oil with mucor miehei lipase. New Biotechnol. 2014, 31, S95. [Google Scholar] [CrossRef]
- Luna, C.; Verdugo-Escamilla, C.; Sancho, E.D.; Luna, D.; Calero, J.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Enzymatic production of biodiesel that avoids glycerol as byproduct, by using immobilized rhizopus oryzae lipase. New Biotechnol. 2014, 31, 594. [Google Scholar] [CrossRef]
- Calero, J.; Verdugo-Escamilla, C.; Luna, D.; Sancho, E.D.; Luna, C.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Selective ethanolysis of sunflower oil with lipozyme rm im, an immobilized rhizomucor miehei lipase, to obtain a biodiesel-like biofuel, which avoids glycerol production through the monoglyceride formation. New Biotechnol. 2014, 31, 596–601. [Google Scholar] [CrossRef]
- Luna, C.; Verdugo-Escamilla, C.; Sancho, E.D.; Luna, D.; Calero, J.; Posadillo, A.; Bautista, F.M.; Romero, A.A. A biofuel similar to biodiesel obtained by using a lipase from rhizopus oryzae, optimized by response surface methodology. Energies 2014, 7, 3383–3399. [Google Scholar] [CrossRef]
- Luna, C.; Sancho, E.D.; Luna, D.; Calero, J.; Verdugo-Escamilla, C.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Production of a biofuel similar to conventional biodiesel that avoids residual glycerol by using lipopan 50BG, a low cost commercial thermomyces lanuginosus lipase. Récents Progrès En Génie Des. Procédés 2014, 106, 641. [Google Scholar] [CrossRef]
- Luna, C.; Luna, D.; Bautista, F.M.; Estevez, R.; Calero, J.; Posadillo, A.; Romero, A.A.; Sancho, E.D. Application of Enzymatic Extracts from a CALB Standard Strain as Biocatalyst within the Context of Conventional Biodiesel Production Optimization. Molecules 2017, 22, 2025. [Google Scholar] [CrossRef]
- Luna, C.; Luna, D.; Bautista, F.M.; Calero, J.; Romero, A.A.; Posadillo, A.; Sancho, E.D.; Estevez, R. Evaluation of Lipases fromWild Microbial Strains as Biocatalysts in Biodiesel Production. Separations 2018, 5, 53. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Luna, C.; Bautista, F.M.; Hurtado, B.; Romero, A.A.; Posadillo, A.; Estevez, R. Rhizomucor miehei Lipase Supported on Inorganic Solids, as Biocatalyst for the Synthesis of Biofuels: Improving the Experimental Conditions by Response Surface Methodology. Energies 2019, 12, 831. [Google Scholar] [CrossRef]
- Calero, J.; Cumplido, G.; Luna, D.; Sancho, E.D.; Luna, C.; Posadillo, A.; Bautista, F.M.; Romero, A.A.; Verdugo-Escamilla, C. Production of a Biofuel that Keeps the Glycerol as a Monoglyceride by Using Supported KF as Heterogeneous Catalyst. Energies 2014, 7, 3764–3780. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Verdugo, C. development of a new biodiesel that integrates glycerol, by using CaO as heterogeneous catalyst, in the partial methanolysis of sunflower oil. Fuel 2014, 7, 94–102. [Google Scholar] [CrossRef]
- Hurtado, B.; Posadillo, A.; Luna, D.; Bautista, F.; Hidalgo, J.; Luna, C.; Calero, J.; Romero, A.; Estevez, R. Synthesis, Performance and Emission Quality Assessment of Ecodiesel from Castor Oil in Diesel/Biofuel/Alcohol Triple Blends in a Diesel Engine. Catalysts 2019, 9, 40. [Google Scholar] [CrossRef]
- Hu, J.; Du, Z.; Li, C.; Min, E. Study on the lubrication properties of biodiesel as fuel lubricity enhancers. Fuel 2005, 84, 1601–1606. [Google Scholar] [CrossRef]
- Hazrat, M.A.; Rasul, M.G.; Khan, M.M.K. Lubricity Improvement of the Ultra-low Sulfur Diesel Fuel with the Biodiesel. Energy Procedia 2015, 75, 111–117. [Google Scholar] [CrossRef]
- Paryanto, I.; Prakoso, T.; Susanto, B.H.; Gozan, M. The Effect of Outdoor Temperature Conditions and Monoglyceride Content on the Precipitate Formation of Biodiesel-Petrodiesel Blended Fuel (BXX). Evergr. Jt. J. Nov. Carbon Resour. Sci. Green Asia Strategy 2019, 6, 59–64. [Google Scholar] [CrossRef]
- Haseeb, A.; Sia, S.; Fazal, M.; Masjuki, H. Effect of temperature on tribological properties of palm biodiesel. Energy 2010, 35, 1460–1464. [Google Scholar] [CrossRef]
- Paryanto, I.; Prakoso, T.; Suyono, E.A.; Gozan, M. Determination of the upper limit of monoglyceride content in biodiesel for B30 implementation based on the measurement of the precipitate in a Biodiesel–Petrodiesel fuel blend (BXX). Fuel 2019, 258, 116104. [Google Scholar] [CrossRef]
- Tongroon, M.; Suebwong, A.; Kananont, M.; Aunchaisri, J.; Chollacoop, N. High quality jatropha biodiesel (H-FAME) and its application in a common rail diesel engine. Renew. Energy 2017, 113, 660–668. [Google Scholar] [CrossRef]
- Coniglio, L.; Bennadji, H.H.; Glaude, P.A.; Herbinet, O.; Billaud, F. Combustion chemical kinetics of biodiesel and related compounds (methyl and ethyl esters): Experiments and modeling—Advances and future refinements. Prog. Energy Combust. Sci. 2013, 39, 340–382. [Google Scholar] [CrossRef]
- Fernando, S.; Hanna, M.; Adhikari, S. Lubricity characteristics of selected vegetable oils, animal fats, and their derivatives. Appl. Eng. Agric. 2007, 23, 5–11. [Google Scholar] [CrossRef]
- Ilves, R.; Kuut, A.; Kuut, K.; Olt, J. Impact of multifunctional biodiesel fuel additive on diesel engine combustion process. Eng. Rural Dev. 2017, 24, 369–377. [Google Scholar] [CrossRef]
- Estévez, C.; Bayarri, N.; Castell, J. Preparation of Fatty Acid Esters of Glycerol Formal and Its Use as Biofuel. Patent WO2008006860, 8 May 2008. [Google Scholar]
- Ferrer, N.B.; Boliart, J.C. Process for Manufacturing Biofuels. Patent US9157039B1, 13 October 2015. [Google Scholar]
- Lapuerta, M.; Rodríguez-Fernández, J.; Estevez, C.; Bayarri, N. Properties of fatty acid glycerol formal ester (FAGE) for use as a component in blends for diesel engines. Biomass Bioenergy 2015, 76, 130–140. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; García-Contreras, R. Effect of a glycerol-derived advanced biofuel—FAGE (fatty acid formal glycerol ester)–on the emissions of a diesel engine tested under the New European Driving Cycle. Energy 2015, 93, 568–579. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, J.; Hernández, J.; Calle-Asensio, A.; Ramos, A.; Barba, J. Selection of Blends of Diesel Fuel and Advanced Biofuels Based on Their Physical and Thermochemical Properties. Energies 2019, 12, 2034. [Google Scholar] [CrossRef]
- Knothe, G. Biodiesel and renewable diesel: A comparison. Prog. Energy Combust. Sci. 2010, 36, 364–373. [Google Scholar] [CrossRef]
- Chang, C.C.; Wan, S.W. China’s motor fuels from tung oil. Ind. Eng. Chem. 1947, 39, 1543–1548. [Google Scholar] [CrossRef]
- Lapuerta, M.; Villajos, M.; Agudelo, J.M.; Boehman, A.L. Key properties and blending strategies of hydrotreated vegetable oil as biofuel for diesel engines. Fuel Proc. Technol. 2011, 92, 2406–2411. [Google Scholar] [CrossRef]
- Veriansyah, B.; Han, J.Y.; Kim, S.K.; Hong, S.A.; Kim, Y.J.; Lim, J.; Shu, Y.W.; Oh, S.G.; Kim, J. Production of renewable diesel by hydroprocessing of soybean oil: Effect of catalysts. Fuel 2012, 94, 578–585. [Google Scholar] [CrossRef]
- Sotelo-Boyás, R.; Liu, Y.; Minowa, T. Renewable Diesel Production from the Hydrotreating of Rapeseed Oil with Pt/Zeolite and NiMo/Al2O3 Catalysts. Ind. Eng. Chem. Res. 2011, 50, 2791–2799. [Google Scholar] [CrossRef]
- Liu, Y.; Sotelo-Boyás, R.; Murata, K.; Minowa, T.; Sakanishi, K. Hydrotreatment of Vegetable Oils to Produce Bio-Hydrogenated Diesel and Liquefied Petroleum Gas Fuel over Catalysts Containing Sulfided Ni–Mo and Solid Acids. Energy Fuels 2011, 25, 4675–4685. [Google Scholar] [CrossRef]
- Templis, C.; Vonortas, A.; Sebos, I.; Papayannakos, N. Vegetable oil effect on gasoil HDS in their catalytic co-hydroprocessing. Appl. Catal. B Enviton. 2011, 104, 324–329. [Google Scholar] [CrossRef]
- Shi, N.; Liu, Q.; Jiang, T.; Wang, T.; Ma, L.; Zhang, Q.; Zhang, X. Hydrodeoxygenation of vegetable oils to liquid alkane fuels over Ni/HZSM-5 catalysts: Methyl hexadecanoate as the model compound. Catal. Commun. 2012, 20, 80–84. [Google Scholar] [CrossRef]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic Applications in the Production of Biodiesel from Vegetable Oils. Chemsuschem Chem. Sustain. Energy Mater. 2009, 2, 278–300. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Rana, R.S.; Kumar, R.; Verma, D.; Kumar, R.; Joshi, R.K.; Garg, M.O.; Sinha, A.K. Hydrotreating and hydrocracking catalysts for processing of waste soya-oil and refinery-oil mixtures. Catal. Commun. 2011, 12, 559–562. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Ramos-Fernández, E.V.; Sepúlveda-Escribano, A. From biodiesel and bioethanol to liquid hydrocarbon fuels: New hydrotreating and advanced microbial technologies. Energy Environ. Sci. 2012, 5, 5638–5652. [Google Scholar] [CrossRef]
- Gong, S.; Shinozaki, A.; Shi, M.; Qian, E.W. Hydrotreating of Jatropha Oil over Alumina Based Catalysts. Energy Fuels 2012, 26, 2394–2399. [Google Scholar] [CrossRef]
- Šimáček, P.; Kubička, D.; Kubičková, I.; Homola, F.; Pospíšil, M.; Chudoba, J. Premium quality renewable diesel fuel by hydroprocessing of sunflower oil. Fuel 2011, 90, 2473–2479. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Zhou, G.; Shen, S.; Rong, L. Hydrotreatment of Jatropha oil over NiMoLa/Al2O3 catalyst. Green Chem. 2012, 14, 2499–2505. [Google Scholar] [CrossRef]
- Liu, J.; Fan, K.; Tian, W.; Liu, C.; Rong, L. Hydroprocessing of Jatropha oil over NiMoCe/Al2O3 catalyst. Int. J. Hydrogen Energy 2012, 37, 17731–17737. [Google Scholar] [CrossRef]
- Sebos, I.; Matsoukas, A.; Apostolopoulos, V.; Papayannakos, N. Catalytic hydroprocessing of cottonseed oil in petroleum diesel mixtures for production of renewable diesel. Fuel 2009, 88, 145–149. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Singh, D.; Subramanian, K.A.; Singal, S.K. Emissions and fuel consumption characteristics of a heavy duty diesel engine fueled with Hydroprocessed Renewable Diesel and Biodiesel. Appl. Energy 2015, 155, 440–446. [Google Scholar] [CrossRef]
- Ogunkoya, D.; Roberts, W.L.; Fang, T.; Thapaliya, N. Investigation of the effects of renewable diesel fuels on engine performance, combustion, and emissions. Fuel 2015, 140, 541–554. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A. Comparison between different types of renewable diesel. Renew. Sustain. Energy Rev. 2013, 21, 110–116. [Google Scholar] [CrossRef]
- Guzman, A.; Torres, J.E.; Prada, L.P.; Nunñez, M.L. Hydroprocessing of crude palm oil at pilot plant scale. Catal. Today 2010, 156, 38–43. [Google Scholar] [CrossRef]
- Fu, J.; Turn, S.Q. Characteristics and Stability of Neat and Blended Hydroprocessed Renewable Diesel. Energy Fuels 2014, 28, 3899–3907. [Google Scholar] [CrossRef]
- Singh, D.; Subramanian, K.A.; Garg, M.O. Comprehensive review of combustion, performance and emissions characteristics of a compression ignition engine fueled with hydroprocessed renewable diesel. Renew. Sustain. Energy Rev. 2018, 81, 2947–2954. [Google Scholar] [CrossRef]
- Cadrazco, M.; Santamaría, A.; Agudelo, J.R. Chemical and nanostructural characteristics of the particulate matter produced by renewable diesel fuel in an automotive diesel engine. Combust. Flame 2019, 203, 130–142. [Google Scholar] [CrossRef]
- Singh, D.; Subramanian, K.A.; Bal, R.; Singh, S.P.; Badola, R. Combustion and emission characteristics of a light duty diesel engine fueled with hydro-processed renewable diesel. Energy 2018, 154, 498–507. [Google Scholar] [CrossRef]
- Srifa, A.; Faungnawakij, K.; Itthibenchapong, V.; Assabumrungrat, S. Roles of monometallic catalysts in hydrodeoxygenation of palm oil to green diesel. Chem. Eng. J. 2015, 278, 249–258. [Google Scholar] [CrossRef]
- Zarchin, R.; Rabaev, M.; Vidruk-Nehemya, R.; Landau, M.V.; Herskowitz, M. Hydroprocessing of soybean oil on nickel-phosphide supported catalysts. Fuel 2015, 139, 684–691. [Google Scholar] [CrossRef]
- Chu, P.L.; Vanderghem, C.; MacLean, H.L.; Saville, B.A. Financial analysis and risk assessment of hydroprocessed renewable jet fuel production from camelina, carinata and used cooking oil. Appl. Energy 2017, 198, 401–409. [Google Scholar] [CrossRef]
- Wang, W.C. Techno-economic analysis of a bio-refinery process for producing Hydro-processed Renewable Jet fuel from Jatropha. Renew. Energy 2016, 95, 63–73. [Google Scholar] [CrossRef]
- Kruger, J.S.; Christensen, E.D.; Dong, T.; Wychen, S.V.; Fioroni, G.M.; Pienkos, P.T.; McCormick, R.L. Bleaching and Hydroprocessing of Algal Biomass-Derived Lipids to Produce Renewable Diesel Fuel. Energy Fuels 2017, 31, 10946–10953. [Google Scholar] [CrossRef]
- Gutiérrez-Antonio, C.; Gómez-De la Cruz, A.; Romero-Izquierdo, A.G.; Gómez-Castro, F.I.; Hernández, S. Modeling, simulation and intensification of hydroprocessing of micro-algae oil to produce renewable aviation fuel. Clean Technol. Environ. Policy 2018, 20, 1589–1598. [Google Scholar] [CrossRef]
- Dang, Q.; Yu, C.; Luo, Z. Environmental life cycle assessment of bio-fuel production via fast pyrolysis of corn stover and hydroprocessing. Fuel 2014, 131, 36–42. [Google Scholar] [CrossRef]
- Hari, T.K.; Yaakob, Z.; Binitha, N.N. Aviation biofuel from renewable resources: Routes, opportunities and challenges. Renew. Sustain. Energy Rev. 2015, 42, 1234–1244. [Google Scholar] [CrossRef]
- Miller, P.; Kumar, A. Techno-economic assessment of hydrogenation-derived renewable diesel production from canola and camelina. Sustain. Energy Technol. Assess. 2014, 6, 105–115. [Google Scholar] [CrossRef]
- Xu, Y.; Keresztes, I.; Condo, A.M., Jr.; Phillips, D.; Pepiot, P.; Avedisian, C.T. Droplet combustion characteristics of algae-derived renewable diesel, conventional #2 diesel, and their mixtures. Fuel 2016, 167, 295–305. [Google Scholar] [CrossRef]
- Chu, P.L.; Vanderg, C.; MacLean, H.L.; Saville, B.A. Process modeling of hydrodeoxygenation to produce renewable jet fuel and other hydrocarbon fuels. Fuel 2017, 196, 298–305. [Google Scholar] [CrossRef]
- Patel, M.; Oyedun, A.O.; Kumar, A.; Gupta, R. A Techno-Economic Assessment of Renewable Diesel and Gasoline Production from Aspen Hardwood. Waste Biomass Valor. 2019, 10, 2745–2760. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Cui, C.; Liu, S.; Qiu, Q.; Ding, Y.; Wu, Y.; Zhang, B. Catalytic hydroprocessing of microalgae-derived biofuels: A review. Green Chem. 2016, 18, 3684–3699. [Google Scholar] [CrossRef]
- Mangus, M.; Mattson, J.; Depcik, C. Performance and Emissions Characteristics of Hydroprocessed Renewable Jet Fuel Blends in a Single-Cylinder Compression Ignition Engine with Electronically Controlled Fuel Injection. Combust. Sci. Technol. 2015, 187, 857–873. [Google Scholar] [CrossRef]
- Hengsawad, T.; Srimingkwanchai, C.; Butnark, S.; Resasco, D.E.; Jongpatiwut, S. Effect of Metal–Acid Balance on Hydroprocessed Renewable Jet Fuel Synthesis from Hydrocracking and Hydroisomerization of Biohydrogenated Diesel over Pt-Supported Catalysts. Ind. Eng. Chem. Res. 2018, 57, 1429–1440. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Ramli, A.; Yusup, S.; Sahban, M.; Alnarabiji, M. Catalytic hydrodeoxygenation of rubber seed oil over sonochemically synthesized Ni-Mo/γ-Al2O3 catalyst for green diesel production. Ultrason. Sonochem. 2019, 51, 90–102. [Google Scholar] [CrossRef]
- Douvartzides, S.L.; Charisiou, N.D.; Papageridis, K.N.; Goula, M.A. Green Diesel: Biomass Feedstocks, Production Technologies, Catalytic Research, Fuel Properties and Performance in Compression Ignition Internal Combustion Engines. Review. Energies 2019, 12, 809. [Google Scholar] [CrossRef]
- Taromi, A.A.; Kaliaguine, S. Green diesel production via continuous hydrotreatment of triglycerides over mesostructured γ-alumina supported NiMo/CoMo catalysts. Fuel Process. Technol. 2018, 171, 20–30. [Google Scholar] [CrossRef]
- Scaldaferri, C.A.; Pasa, V.M.D. Hydrogen-free process to convert lipids into bio-jet fuel and green diesel over niobium phosphate catalyst in one-step. Chem. Eng. J. 2019, 370, 98–109. [Google Scholar] [CrossRef]
- Romero-Izquierdo, A.G.; Gutiérrez-Antonio, C.; Gómez-Castro, F.I.; Hernández, S. Hydrotreating of Triglyceride Feedstock to Produce Renewable Aviation Fuel. Recent Innov. Chem. Eng. 2018, 11, 77–89. [Google Scholar] [CrossRef]
- Mohd, F.O.; Adam, A.; Najafi, G.; Mamat, R. Green fuel as alternative fuel for diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 80, 694–709. [Google Scholar] [CrossRef]
- Orozco, L.M.; Echeverri, D.A.; Sánchez, L.; Rios, L.A. Second-generation green diesel from castor oil: Development of a new and efficient continuous-production process. Chem. Eng. J. 2017, 322, 149–156. [Google Scholar] [CrossRef]
- Kordouli, E.; Pawelec, B.; Bourikas, K.; Kordulis, C.; Fierro, J.L.G.; Lycourghiotis, A. Mo promoted Ni-Al2O3 co-precipitated catalysts for green diesel production. Appl. Catal. B Environ. 2018, 229, 139–154. [Google Scholar] [CrossRef]
- Kordouli, E.; Sygellou, L.; Kordulis, C.; Bourikas, K.; Lycourghiotis, A. Probing the synergistic ratio of the NiMo/-Al2O3 reduced catalysts for the transformation of natural triglycerides into green diesel. Appl. Catal. B Environ. 2017, 209, 12–22. [Google Scholar] [CrossRef]
- Kordouli, E.; Kordulis, C.; Lycourghiotis, A.; Cole, R.; Vasudevan, P.T.; Pawelec, B.; Fierro, J.L.G. HDO activity of carbon-supported Rh, Ni and Mo-Ni catalysts. Mol. Catal. 2017, 441, 209–220. [Google Scholar] [CrossRef]
- Gousi, M.; Andriopoulou, C.; Bourikas, K.; Ladas, S.; Sotiriou, M.; Korduli, C.; Lycourghiotis, A. Green diesel production over nickel-alumina co-precipitated catalysts. Appl. Catal. A Gen. 2017, 536, 45–56. [Google Scholar] [CrossRef]
- Gousi, M.; Kordulis, E.; Bourikas, K.; Simianakis, E.; Ladas, S.; Panagiotou, G.D.; Kordulis, C.; Lycourghiotis, A. Green diesel production over nickel-alumina nanostructured catalysts promoted by zinc. Catal. Today 2019. [Google Scholar] [CrossRef]
- Mwangi, K.J.; Lee, W.J.; Chang, Y.C.; Chen, C.Y.; Wang, L.C. An overview: Energy saving and pollution reduction by using green fuel blends in diesel engines. Appl. Energy 2015, 159, 214–236. [Google Scholar] [CrossRef]
- Sahu, R.; Song, B.J.; Im, J.S.; Jeon, Y.P.; Lee, C.W. A review of recent advances in catalytic hydrocracking of heavy residues. J. Ind. Eng. Chem. 2015, 27, 12–24. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A.; Karonis, D. Diesel decarbonization via effective catalytic Co-hydroprocessing of residual lipids with gas–oil. Fuel 2014, 136, 366–373. [Google Scholar] [CrossRef]
- Huber, G.W.; O’Connor, P.; Corma, A. Processing biomass in conventional oil refineries: Production of high quality diesel by hydrotreating vegetable oils in heavy vacuum oil mixtures. Appl. Catal. A Gen. 2007, 329, 120–129. [Google Scholar] [CrossRef]
- Corma, A.; Renz, M.; Schaverien, C. Coupling Fatty acids by Ketonic Decarboxylation Using Solid Catalysts for the Direct Production of Diesel, Lubricants and Chemicals. Chemsuschem Chem. Sustain. Energy Mater. 2008, 1, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Clavero, M.A.; Calleja, G.; García, A.; Miravalles, R.; Galindo, T. Production of Biofuels via the Catalytic Cracking of Mixtures of Crude Vegetable Oils and Nonedible Animal Fats with Vacuum Gas Oil. Energy Fuels 2010, 24, 707–717. [Google Scholar] [CrossRef]
- Tóth, C.; Baladincz, P.; Kovács, S.; Hancsók, J. Producing clean diesel fuel by co-hydrogenation of vegetable oil with gas oil. Clean Technol. Environ. Policy 2011, 13, 581–585. [Google Scholar] [CrossRef]
- Rana, B.S.; Kumar, R.; Tiwari, R.; Kumar, R.; Joshi, R.K.; Garg, M.O.; Sinha, A.K. Transportation fuels from co-processing of waste vegetable oil and gas oil mixtures. Biomass Bioenergy 2013, 56, 43–52. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A.; Kikhtyanin, O.; Kubičk, D. Refinery co-processing of renewable feeds. Prog. Energy Combust. Sci. 2018, 68, 29–64. [Google Scholar] [CrossRef]
- Perez-Cisneros, E.S.; Sales-Cruz, M.; Lobo-Oehmichen, R.; Viveros-García, T. A reactive distillation process for co-hydrotreating of non-edible vegetable oils and petro-diesel blends to produce green diesel fuel. Comput. Chem. Eng. 2017, 105, 105–112. [Google Scholar] [CrossRef]
- Beims, R.F.; Bertoli, S.L.; Botton, V.; Ender, L.; Simionatto, E.L.; Meier, H.F.; Wiggers, V.R. Co-processing of thermal cracking bio-oil at petroleum refineries. Braz. J. Pet. Gas 2017, 11, 99–113. [Google Scholar] [CrossRef][Green Version]
- Sági, D.; Baladincz, P.; Varga, Z.; Hancsók, J. Co-processing of FCC light cycle oil and waste animal fats with straight run gas oil fraction. J. Clean. Prod. 2016, 111, 34–41. [Google Scholar] [CrossRef]
- Boonyasuwat, S.; Tscheikuna, J. Co-processing of palm fatty acid distillate and light gas oil in pilot-scale hydrodesulfurization unit over commercial CoMo/Al2O3. Fuel 2017, 199, 115–124. [Google Scholar] [CrossRef]
- Carmona, H.P.; Horáček, J.; Alayón, A.B.; Hernández, J.J.M. Suitability of used frying oil for co-processing with atmospheric gas oil. Fuel 2018, 214, 165–173. [Google Scholar] [CrossRef]
- Carmona, H.P.; Alfaro, O.T.; Alayón, A.B.; Vázquez, M.A.R.; Hernández, J.J.M. Co-processing of straight run gas oil with used cooking oil and animal fats. Fuel 2019, 254, 115583. [Google Scholar] [CrossRef]
- Hidalgo-Herrador, J.M.; Psenička, M.; Horaček, J.; Tišler, Z.; Vráblík, A.; Černý, R.; Murat, M. Co-processing of Waste Cooking Oil and Light Cycle Oil with NiW/(Pseudoboehmite + SBA-15). Catalyst 2019, 42, 512–517. [Google Scholar] [CrossRef]
- Wang, H.; Farooqi, H.; Chen, J. Co-hydrotreating light cycle oil-canola oil blends. J. Front. Chem. Sci. Eng. 2015, 9, 64–76. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Bezergianni, S. Co-hydroprocessing gas-oil with residual lipids: Effect of residence time and H2/Oil ratio. J. Clean. Prod. 2016, 131, 321–326. [Google Scholar] [CrossRef]
- Poddar, M.K.; Rai, A.; Maury, M.R.; Sinh, A.K. Co-processing of bio-oil from de-oiled Jatropha curcas seed cake with refinery gas–oil over sulfided CoMoP/Al2O3 catalyst. RSC Adv. 2016, 6, 113720–113726. [Google Scholar] [CrossRef]
- Ng, S.H.; Al-Sabawi, M.A.; Wang, J.; Ling, H.; Zheng, Y.; Wei, Q.; Ding, F.; Little, E. FCC coprocessing oil sands heavy gas oil and canola oil. 1. Yield structure. Fuel 2015, 156, 163–176. [Google Scholar] [CrossRef]
- Tóth, O.; Holló, A.; Hancsók, J. Co-processing a waste fatty acid mixture and unrefined gas oil to produce renewable diesel fuel-blending components. Energy Convers. Manag. 2019, 185, 304–312. [Google Scholar] [CrossRef]
- Tóth, O.; Holló, A.; Hancsók, J. Co-processing of high-sulphur gas oil and waste with high fatty acid content. Chem. Eng. Trans. 2018, 70, 1639–1644. [Google Scholar] [CrossRef]
- Chen, S. Green Oil Production by Hydroprocessing. Int. J. Clean Coal Energy 2012, 1, 43–55. [Google Scholar] [CrossRef]
- Satyarthi, J.K.; Chiranjeevi, T.; Gokak, D.T.; Viswanathan, P.S. An overview of catalytic conversion of vegetable oils/fats into middle distillates. Catal. Sci. Technol. 2013, 3, 70–80. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Garcia, A. Biomass as renewable feedstock in standard refinery units. Feasibility, opportunities and challenges. Energy Environ. Sci. 2012, 5, 7393–7420. [Google Scholar] [CrossRef]
- Hachemi, I.; Kumar, N.; Mäki-Arvela, P.; Roine, J.; Peurla, M.; Hemming, J.; Salonen, J.; Murzin, D.Y. Sulfur-535 free Ni catalyst for production of green diesel by hydrodeoxygenation. J. Catal. 2017, 347, 205–221. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M. Catalytic hydrodeoxygenation of triglycerides: An 495 approach to clean diesel fuel production. Renew. Sustain. Energy Rev. 2017, 80, 1072–1088. [Google Scholar] [CrossRef]
- Huynh, T.M.; Armbruster, U.; Atia, H.; Bentrup, U.; Phan, B.M.Q.; Eckelt, R.; Nguyen, L.H.; Nguyen, D.A.; Martin, A. Upgrading of bio-oil and subsequent co-processing under fcc conditions for fuel production. React. Chem. Eng. 2016, 1, 239–251. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, L.; Cheng, S.; Julson, J. Review of Heterogeneous Catalysts for Catalytically Upgrading Vegetable Oils into Hydrocarbon Biofuels. Catalysts 2017, 7, 83. [Google Scholar] [CrossRef]
- Aslam, M.; Konwar, L.J.; Sarma, A.K.; Kothiyal, N.C. An investigation of catalytic hydrocracking of high FFA vegetable oils to liquid hydrocarbons using biomass derived heterogeneous catalysts. J. Anal. Appl. 2015, 115, 401–409. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Afandi, A.; Abdulkareem-Alsultan, G.; Lee, H.V.; Taufiq-Yap, Y.H. Production of green diesel via cleaner catalytic deoxygenation of Jatropha curcas oil. J. Clean. Prod. 2017, 167, 1048–1059. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Mansir, N.; Lee, H.V.; Zainal, Z.; Islam, A.; Taufiq-Yap, Y.H. Pyrolytic deoxygenation of waste cooking oil for green diesel production over Ag2O3-La2O3/AC nanocatalyst. J. Anal. Appl. Pyrolysis 2019, 137, 171–184. [Google Scholar] [CrossRef]
- Arun, N.; Sharma, R.V.; Dalai, A.K. Green diesel synthesis by hydrodeoxygenation of bio-based feedstocks: Strategies for catalyst design and development. Renew. Sustain. Energy Rev. 2015, 48, 240–255. [Google Scholar] [CrossRef]
- Pattanaik, B.P.; Misra, R.D. Effect of reaction pathway and operating parameters on the deoxygenation of vegetable oils to produce diesel range hydrocarbon fuels: A review. Renew. Sustain. Energy Rev. 2017, 73, 545–557. [Google Scholar] [CrossRef]
- Sousa, F.P.; Silva, L.N.; De Rezende, D.B.; De Oliveira, L.C.A.; Pasa, V.M. Simultaneous deoxygenation, cracking and isomerization of palm kernel oil and palm olein over beta zeolite to produce biogasoline, green diesel and biojet-fuel. Fuel 2018, 223, 149–156. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Lee, H.V.; Juan, J.C.; Noorsaadah, A.R.; Ong, H.C.; Razali, S.M.; Taufiq-Yap, Y.H. Promoting deoxygenation of triglycerides via Co-Ca loaded SiO2-Al2O3 catalyst. Appl. Catal. A Gen. 2018, 552, 38–48. [Google Scholar] [CrossRef]
- Kordulis, C.; Bourikas, K.; Gousi, M.; Kordouli, E.; Lycourghiotis, A. Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel: A critical review. Appl. Catal. B Environ. 2016, 181, 156–196. [Google Scholar] [CrossRef]
- Arbogast, S.; Bellman, D.; Paynter, J.D.; Wykowski, J. Advanced bio-fuels from pyrolysis oil: The impact of economies of scale and use of existing logistic and processing capabilities. Fuel Process. Technol. 2012, 104, 121–127. [Google Scholar] [CrossRef]
- Kumar, V.; Sindhu, R.K.; Kumar, S. Comparative analysis of green diesel versus petro-diesel in compression ignition engine. Biosci. Biotechnol. Res. Commun. 2018, 11, 128–135. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.; Roberts, W.L.; Dibble, R.W. Feasibility of using less viscous and lower cetane (LVLC) fuels in a diesel engine: A review. Renew. Sustain. Energy Rev. 2015, 51, 1166–1190. [Google Scholar] [CrossRef]
- Kasiraman, G.; Nagalingam, B.; Balakrishnan, M. Performance, emission and combustion improvements in a direct injection diesel engine using cashew nut shell oil as fuel with camphor oil blending. Energy 2012, 47, 116–124. [Google Scholar] [CrossRef]
- Martin, M.L.J.; Geo, V.E.; Singh, D.K.J.; Nagalingam, B. A comparative analysis of different methods to improve the performance of cotton seed oil fuelled diesel engine. Fuel 2012, 102, 372–378. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.M.; Lee, P.S.; Chua, K.J.E.; Chou, S.K. Pine oil–biodiesel blends: A double biofuel strategy to completely eliminate the use of diesel in a diesel engine. Appl. Energy 2014, 130, 466–473. [Google Scholar] [CrossRef]
- Purushothaman, K.; Nagarajan, G. Performance, emission and combustion characteristics of a compression ignition engine operating on neat orange oil. Renew. Energy 2009, 34, 242–245. [Google Scholar] [CrossRef]
- Subramanian, T.; Varuvel, E.G.; Martin, L.J.; Beddhannan, N. Effect of lower and higher alcohol fuel synergies in biofuel blends and exhaust treatment system on emissions from CI engine. Environ. Sci. Pollut. Res. 2017, 24, 25103–25113. [Google Scholar] [CrossRef]
- Panneerselvam, N.; Ramesh, M.; Murugesan, A.; Vijayakumar, C.; Subramaniam, D.; Kumaravel, A. Effect on direct injection naturally aspirated diesel engine characteristics fuelled by pine oil, Ceiba pentandra methyl ester compared with diesel. Transp. Res. Part. D Transp. Environ. 2016, 48, 225–234. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Geo, V.E.; Martin, L.J.; Nagalingam, B. Effects of low carbon biofuel blends with Karanja (Pongamia pinnata) oil methyl ester in a single cylinder CI engine on CO2 emission and other performance and emission characteristics. Nat. Environ. Pollut. Technol. 2016, 15, 1249–1256. [Google Scholar]
- Kommana, S.; Banoth, B.N.; Kadavakollu, K.R. Performance and emission of VCR-CI engine with palm kernel and eucalyptus blends. Perspect. Sci. 2016, 8, 195–197. [Google Scholar] [CrossRef]
- Senthil, R.; Sivakumar, E.; Silambarasan, R. Effect of di ethyl ether on the performance and emission characteristics of a diesel engine using biodiesel–eucalyptus oil blends. RSC Adv. 2015, 5, 54019–54027. [Google Scholar] [CrossRef]
- Senthil, R.; Sivakumar, E.; Silambarasan, R. Exhaust emissions reduction from diesel engine using combined Annona–Eucalyptus oil blends and antioxidant additive. Heat Mass Transf. 2016, 53, 1105–1112. [Google Scholar] [CrossRef]
- Tamilvendhan, D.; Ilangovan, V. A performance, emission and combustion investigation on hot air assisted eucalyptus oil direct injected compression ignition engine. Mod. Appl. Sci. 2011, 5, 53–62. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.M.; Lee, P.S.; Chua, K.J.E.; Chou, S.K. Combustion performance and emission characteristics study of pine oil in a diesel engine. Energy 2013, 57, 344–351. [Google Scholar] [CrossRef]
- Tamilselvan, P.; Nallusamy, N. Performance, combustion andemission characteristics of a compression ignition engine operating on pine oil. Biofuels 2015, 6, 273–281. [Google Scholar] [CrossRef]
- Subramanian, T.; Varuvel, E.G.; Ganapathy, S.; Vedharaj, S.; Vallinayagam, R. Role of fuel additives on reduction of NOX emission from a diesel engine powered by camphor oil biofuel. Environ. Sci. Pollut. Res. 2018, 25, 15368–15377. [Google Scholar] [CrossRef] [PubMed]
- Devan, P.; Mahalakshmi, N. A study of the performance, emission and combustion characteristics of a compression ignition engine using methyl ester of paradise oil–eucalyptus oil blends. Appl. Energy 2009, 86, 675–680. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.; Saravanan, C.; Lee, P.; Chua, K.; Chou, S. Impact of ignition promoting additives on the characteristics of a diesel engine powered by pine oil–diesel blend. Fuel 2014, 117, 278–285. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Geo, V.E.; Martin, L.J.; Nagalingam, B. Simultaneous reduction of NO–smoke–CO2 emission in a biodiesel engine using low-carbon biofuel and exhaust after-treatment system. Clean Technol. Environ. Policy 2017, 19, 1271–1283. [Google Scholar] [CrossRef]
- Ashok, B.; Raj, R.T.K.; Nanthagopal, K.; Krishnan, R.; Subbarao, R. Lemon peel oil—A novel renewable alternative energy source for diesel engine. Energy Convers. Manag. 2017, 139, 110–121. [Google Scholar] [CrossRef]
- Fayyazbakhsh, A.; Pirouzfar, V. Comprehensive overview on diesel additives to reduce emissions, enhance fuel properties and improve engine performance. Renew. Sustain. Energy Rev. 2017, 74, 891–901. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Chen, S.-J.; Huang, K.-L.; Lin, W.-Y.; Lee, W.-J.; Lin, C.-C.; Hsieh, L.-T.; Chiu, J.-Y.; Kuo, W.-C. Emissions from a generator fueled by blends of diesel, biodiesel, acetone, and isopropyl alcohol: Analyses of emitted PM, particulate carbon, and PAHs. Sci. Total Environ. 2014, 466, 195–202. [Google Scholar] [CrossRef]
- Bragadeshwaran, A.; Kasianantham, N.; Balusamy, S.; Muniappan, S.K.; Reddy, D.M.S.; Subhash, R.V.; Pravin, N.A.; Subbarao, R. Mitigation of NOx and smoke emissions in a diesel engine using novel emulsified lemon peel oil biofuel. Environ. Sci. Pollut. Res. 2018, 25, 25098. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Atmanli, A.; Vigil, F.M. Quaternary blends of diesel, biodiesel, higher alcohols and vegetable oil in a compression ignition engine. Fuel 2018, 212, 462–469. [Google Scholar] [CrossRef]
- Mat, S.C.; Idroas, M.Y.; Hamid, M.F.; Zainal, Z.A. Performance and emissions of straight vegetable oils and its blends as a fuel in diesel engine: A review. Renew. Sustain. Energy Rev. 2018, 82, 808–823. [Google Scholar] [CrossRef]
- Coughlin, B.; Hoxie, A. Combustion characteristics of ternary fuel Blends: Pentanol, butanol and vegetable oil. Fuel 2017, 196, 488–496. [Google Scholar] [CrossRef]
- Dunn, R.O.; Bagby, M.A. low-Temperature Phase Behavior of Vegetable Oill Co-solvent Blends as Alternative Diesel Fuel. J. Am. Oil Chem. Soc. 2000, 77, 1315–1323. [Google Scholar] [CrossRef]
- Lujaji, F.; Kristóf, L.; Bereczky, A.; Mbarawa, M. Experimental investigation of fuel properties, engine performance, combustion and emissions of blends containing croton oil, butanol, and diesel on a CI engine. Fuel 2011, 90, 505–510. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakoumis, E.G.; Papagiannakis, R.G.; Kyritsis, D.C. Influence of properties of various common bio-fuels on the combustion and emission characteristics of high-speed DI (direct injection) diesel engine: Vegetable oil, bio-diesel, ethanol, n-butanol, diethyl ether. Energy 2014, 73, 354–366. [Google Scholar] [CrossRef]
- Atmanli, A.; Ileri, E.; Yuksel, B.; Yilmaz, N. Extensive analyses of diesel–vegetable oil–n-butanol ternary blends in a diesel engine. Appl. Energy 2015, 145, 155–162. [Google Scholar] [CrossRef]
- Guarieiro, L.L.N.; Souza, A.F.; Torres, E.A.; Andrade, J.B. Emission profile of 18 carbonyl compounds, CO, CO2, and NOx emitted by a diesel engine fuelled with diesel and ternary blends containing diesel, ethanol and biodiesel or vegetable oils. Atmos. Environ. 2009, 43, 2754–2761. [Google Scholar] [CrossRef]
- Atmanli, A.; Ileri, E.; Yüksel, B. Experimental investigation of engine performance and exhaust emissions of a diesel engine fueled with diesel–n-butanol–vegetable oil blends. Energy Convers. Manag. 2014, 81, 312–321. [Google Scholar] [CrossRef]
- Atmanlı, A.; İleri, E.; Yüksel, B. Effects of higher ratios of n-butanol addition to diesel–vegetable oil blends on performance and exhaust emissions of a diesel engine. J. Energy Inst. 2015, 88, 209–220. [Google Scholar] [CrossRef]
- Zhu, L.; Xiao, Y.; Cheung, C.S.; Guan, C.; Huang, Z. Combustion, gaseous and particulate emission of a diesel engine fueled with n-pentanol (C5 alcohol) blended with waste cooking oil biodiesel. Appl. Therm. Eng. 2016, 102, 73–79. [Google Scholar] [CrossRef]
- Atmanlı, A.; Yüksel, B.; İleri, E. Experimental investigation of the effect of diesel–cotton oil–n-butanol ternary blends on phase stability, engine performance and exhaust emission parameters in a diesel engine. Fuel 2013, 109, 503–511. [Google Scholar] [CrossRef]
- Atmanlı, A.; Yüksel, B.; İleri, E.; Karaoglan, D. Response surface methodology based optimization of diesel–n-butanol–cotton oil ternary blend ratios to improve engine performance and exhaust emission characteristics. Energy Convers. Manag. 2015, 90, 383–394. [Google Scholar] [CrossRef]
- Yilmaz, N.; Vigil, F.M. Potential use of a blend of diesel, biodiesel, alcohols and vegetable oil in compression ignition engines. Fuel 2014, 124, 168–172. [Google Scholar] [CrossRef]
- Atmanli, A.; Ileri, E.; Yilmaz, N. Optimization of diesel–butanol–vegetable oil blend ratios based on engine operating parameters. Energy 2016, 96, 569–580. [Google Scholar] [CrossRef]
- Atmanli, A. Effects of a cetane improver on fuel properties and engine characteristics of a diesel engine fueled with the blends of diesel, hazelnut oil and higher carbon alcohol. Fuel 2016, 172, 209–217. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Dhanasekaran, R.; Rana, D.; Saravanan, S.; Kumar, B.R. A comparative assessment of ternary blends of three bio-alcohols with waste cooking oil and diesel for optimum emissions and performance in a CI engine using response surface methodology. Energy Convers. Manag. 2018, 156, 337–357. [Google Scholar] [CrossRef]
- Qi, D.H.; Yang, K.; Zhang, D.; Chen, B.; Wei, Q.; Zhang, C.H. Experimental investigation of a turbocharged CRDI diesel engine fueled with Tung oil-diesel-ethanol microemulsion fuel. Renew. Energy 2017, 113, 1201–1207. [Google Scholar] [CrossRef]
- Schwab, A.W.; Nielsen, H.C.; Brooks, D.D.; Pryde, E.H. Triglyceride/aqueous ethanol/1-butanol microemulsions. J. Dispers. Sci. Technol. 1983, 4, 1–17. [Google Scholar] [CrossRef]
- Singh, P.J.; Khurma, J.; Singh, A. Preparation, characterisation, engine performance and emission characteristicsof coconut oil based hybrid fuels. Renew. Energy 2010, 35, 2065–2070. [Google Scholar] [CrossRef]
- Singh, P.; Khurma, J.; Singh, A. Coconut oil based hybrid fuels as alternative fuel for diesel engines. Am. J. Environ. Sci. 2010, 6, 69–75. [Google Scholar] [CrossRef][Green Version]
- Do, L.D.; Singh, V.; Kibbey, T.; Gollahalli, S.R.; Sabatini, D.A. Algae, canola, or palm oils–diesel microemulsion fuels: Phase behaviors, viscosity and combustion properties. Int. J. Green Energy 2011, 8, 748–767. [Google Scholar] [CrossRef]
- Attaphong, C.; Do, L.; Sabatini, D.A. Vegetable oil-based microemulsions using carboxylate-based extended surfactants and their potential as an alternative renewable biofuel. Fuel 2012, 94, 606–613. [Google Scholar] [CrossRef]
- Ding, X.; Yuan, X.; Leng, L.; Huang, H.; Wang, H.; Shao, J.; Jiang, L.; Chen, X.; Zeng, G. Upgrading Sewage Sludge Liquefaction Bio-Oil by Microemulsification: The Effect of Ethanol as Polar Phase on Solubilization Performance and Fuel Properties. Energy Fuels 2017, 31, 1574–1582. [Google Scholar] [CrossRef]
- Prakash, T.; Geo, V.E.; Martin, L.J.; Nagalingam, B. Effect of ternary blends of bio-ethanol, diesel and castor oil on performance, emission and combustion in a CI engine. Renew. Energy 2018, 122, 301–309. [Google Scholar] [CrossRef]
- Redel-Macíasa, M.D.; Pinzi, S.; Leiva-Candi, D.E.; López, I.; Dorado, M.P. Ternary blends of diesel fuel oxygenated with ethanol and castor oil for diesel engines. Energy Procedia 2017, 142, 855–860. [Google Scholar] [CrossRef]
- Pinzia, S.; López, I.; Leiva-Candia, D.E.; Redel-Macías, M.D.; Herreros, J.M.; Cubero-Atienza, A.; Dorado, M.P. Castor oil enhanced effect on fuel ethanol-diesel fuel blend properties. Appl. Energy 2018, 224, 409–416. [Google Scholar] [CrossRef]
- Rakopoulos, D.C. Combustion and emissions of cottonseed oil and its bio-diesel in blends with either n-butanol or diethyl ether in HSDI diesel engine. Fuel 2013, 105, 603–613. [Google Scholar] [CrossRef]
- Al-Widyan, M.I.; Tashtoush, G.; Abu-Qudais, M. Utilization of ethyl ester of waste vegetable oils as fuel in diesel engines. Fuel Process. Technol. 2002, 76, 91–103. [Google Scholar] [CrossRef]
- Krishna, R.; Bandewar, A.G.; Dongare, V.K. Experimental Investigations of Blending Diethyl Ether in Karanja Vegetable Oil using A Multi-Cylinder Diesel Engine. Int. J. Res. Innov. Technol. 2014, 1, 70–73. [Google Scholar]
- Krishnamoorthi, M.; Malayalamurthi, R. A Review on effect of diethyl ether additive on combustion, performance and emission characteristics of a diesel and biodiesel/vegetable oil fuelled engine. Adv. Nat. Appl. Sci. 2016, 10, 9–18. [Google Scholar]
- Kumar, A.; Rajan, K.; Naraynan, M.R.; Kumar, K.R.S. Performance and Emission Characteristics of a Di Diesel Engine Fuelled with Cashew Nut Shell Oil (CNSO)-Diesel Blends with Diethyl Ether as Additive. Appl. Mech. Mater. 2015, 787, 746–750. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Papagiannakis, R.G.; Giakoumis, E.G. Combustion and Emissions in an HSDI Engine Running on Diesel or Vegetable Oil Base Fuel with n-Butanol or Diethyl Ether as a Fuel Extender. J. Energy Eng. 2016, 142, E4015006. [Google Scholar] [CrossRef]
- Kumar, A.; Rajan, K.; Kumar, K.R.S.; Maiyappan, K.; Rasheed, U.T. Green fuel utilization for diesel engine, combustion and emission analysis fuelled with CNSO diesel blends with Diethyl ether as additive. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Beijing, China, 24–27 October 2017. [Google Scholar]
- Geo, V.E.; Nagarajan, G.; Nagalingam, B. Studies on improving the performance of rubber seed oil fuel for diesel engine with DEE port injection. Fuel 2010, 89, 3559–3567. [Google Scholar] [CrossRef]
- Kumar, M.S.; Ramesh, A.; Nagalingam, B. A Comparison of the Different Methods of Using Jatropha Oil as Fuel in a Compression Ignition Engine. J. Eng. Gas. Turbines Power 2010, 132, 032801. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Kyritsis, D.C. Butanol or DEE blends with either straight vegetable oil or biodiesel excluding fossil fuel: Comparative effects on diesel engine combustion attributes, cyclic variability and regulated emissions trade-off. Energy 2016, 115, 314–325. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakoumis, E.G. Impact of properties of vegetable oil, bio-diesel, ethanol and n-butanol on the combustion and emissions of turbocharged HDDI diesel engine operating under steady and transient conditions. Fuel 2015, 156, 1–19. [Google Scholar] [CrossRef]
- Geo, V.E.; Nagarajan, G.; Kamalakannan, J.; Nagalingam, B. Experimental Investigations to Study the Characteristics of Rubber-Seed-Oil-Fueled Diesel Engine Supplemented with Diethyl Ether. Energy Fuels 2009, 23, 533–538. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Malayalamurthi, R. Experimental investigation on performance, emission behavior and exergy analysis of a variable compression ratio engine fueled with diesel-aegle marmelos oil-diethyl ether blends. Energy 2017, 128, 312–328. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Malayalamurthi, R. Availability analysis, performance, combustion and emission behavior of bael oil-diesel-diethyl ether blends in a variable compression ratio diesel engine. Renew. Energy 2018, 119, 235–252. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Malayalamurthi, R. Experimental investigation on the availability, performance, combustion and emission distinctiveness of bael oil/diesel/diethyl ether blends powered in a variable compression ratio diesel engine. Heat Mass Transf. 2018, 54, 2023–2044. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Malayalamurthi, R. Combined Effect of Compression Ratio, Injection Pressure, and Injection Timing on Performance and Emission of a DI Compression Ignition Engine Fueled with Diesel–Aegle Marmelos Oil–Diethyl Ether Blends Using Response Surface Methodology. Energy Fuels 2017, 31, 11362–11376. [Google Scholar] [CrossRef]
- Venkanna, B.K.; Reddy, C.V. Performance, emission and combustion characteristics of DI diesel engine running on blends of honne oil/diesel fuel/kerosene/DMC. Int. J. Agric. Biol. Eng. 2011, 4, 48–57. [Google Scholar] [CrossRef]
- Kasiramana, G.; Geo, V.E.; Nagalingam, B. Assessment of cashew nut shell oil as an alternate fuel for CI (Compression ignition) engines. Energy 2016, 101, 402–410. [Google Scholar] [CrossRef]
- Lakshminarayanan, A.; Olsen, D.B.; Cabot, P.E. Performance and emission evaluation of triglyceride-gasoline blends in agricultural compression ignition engines. Appl. Eng. Agric. 2014, 30, 523–534. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Posadillo, A.; Hurtado, B.; Bautista, F.; Luna, D.; Hidalgo, J.M.; Luna, C.; Calero, J.; Romero, A.; et al. Performance and Emission Quality Assessment in a Diesel Engine of Straight Castor and Sunflower Vegetable Oils, in Diesel/Gasoline/Oil Triple Blends. Energies 2019, 12, 2181. [Google Scholar] [CrossRef]
- Righi, S.; Bandini, V.; Fabbri, D.; Cordella, M.; Stramigioli, C.; Tugnoli, A. Modelling of an alternative process technology for biofuel production and assessment of its environmental impacts. J. Clean. Prod. 2016, 122, 42–51. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Davis, S.J.; Lewis, N.S.; Shaner, M.; Aggarwal, S.; Arent, D.; Azevedo, I.L.; Benson, S.M.; Bradley, T.; Brouwer, J.; Chiang, Y.M.; et al. Net-zero emissions energy systems. Science 2018, 360, eaas9793. [Google Scholar] [CrossRef]
- Kalghatgi, G. Is it really the end of internal combustion engines and petroleum in transport? Appl. Energy 2018, 225, 965–974. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).