Recent Advances in Flavin-Dependent Halogenase Biocatalysis: Sourcing, Engineering, and Application
Abstract
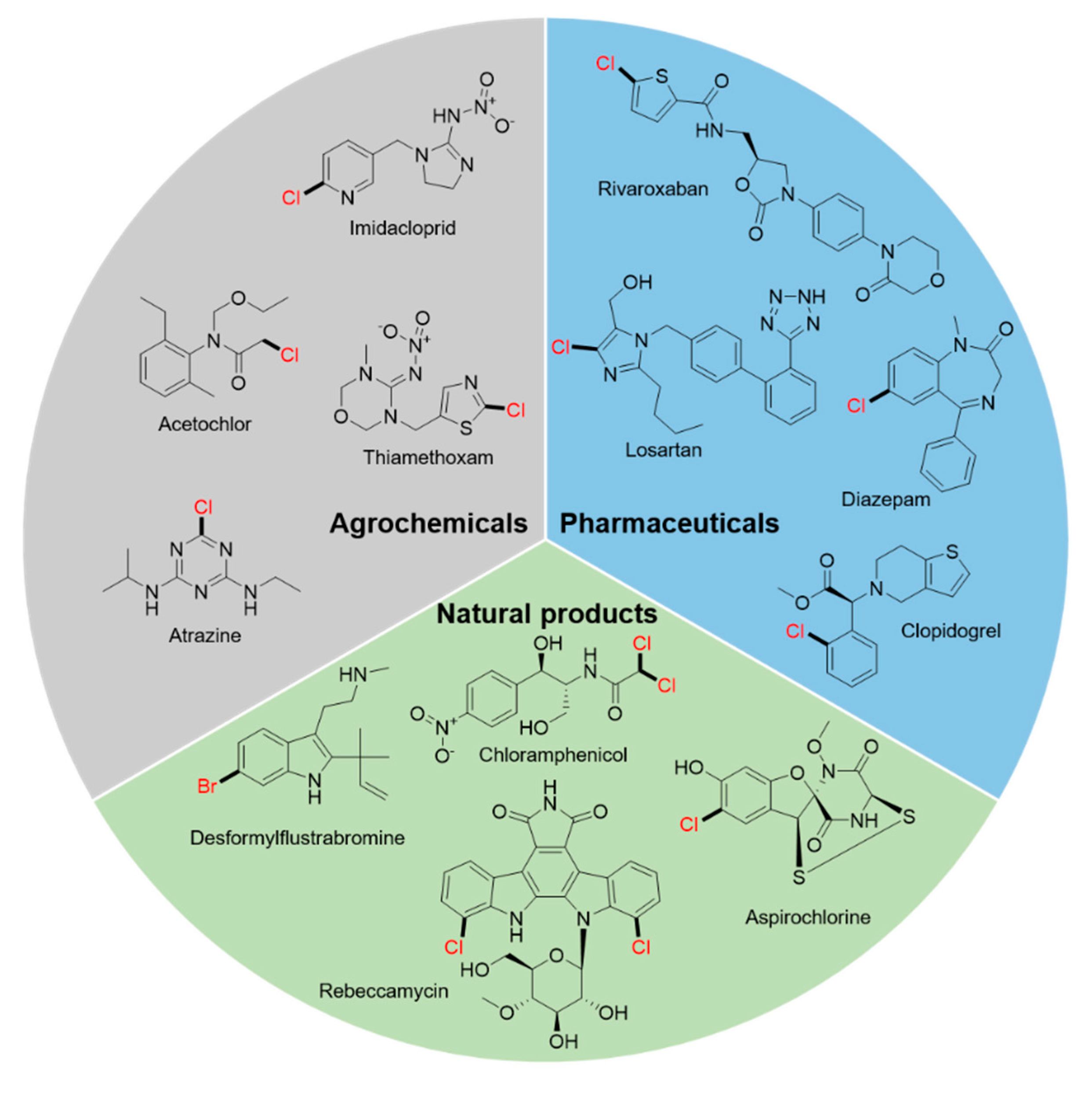
1. Introduction
2. Enzyme Diversity and Substrate Scope
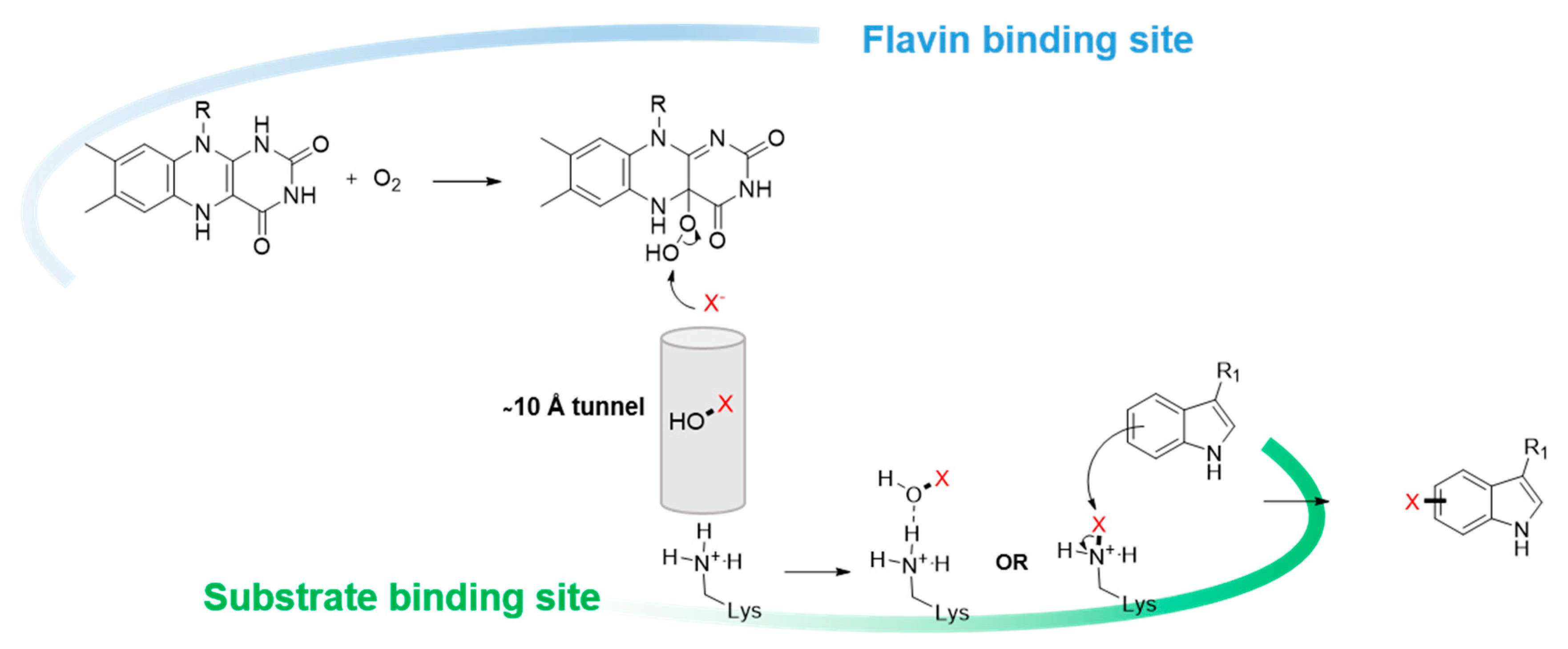
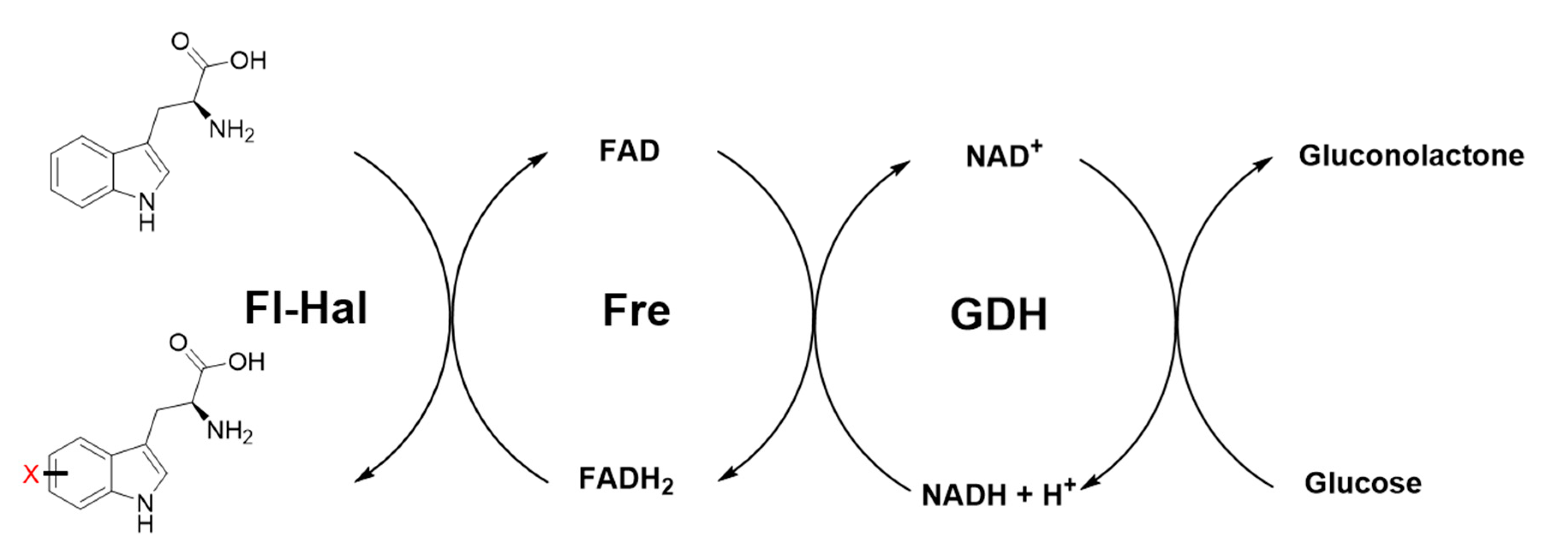
3. Cofactor and Reaction Engineering
4. Strategies to Scale-up Biocatalytic Aromatic Halogenations
4.1. Protein Overexpression and Stabilization
4.2. Substrate Scope and Regioselectivity
4.3. Biosynthetic Pathways
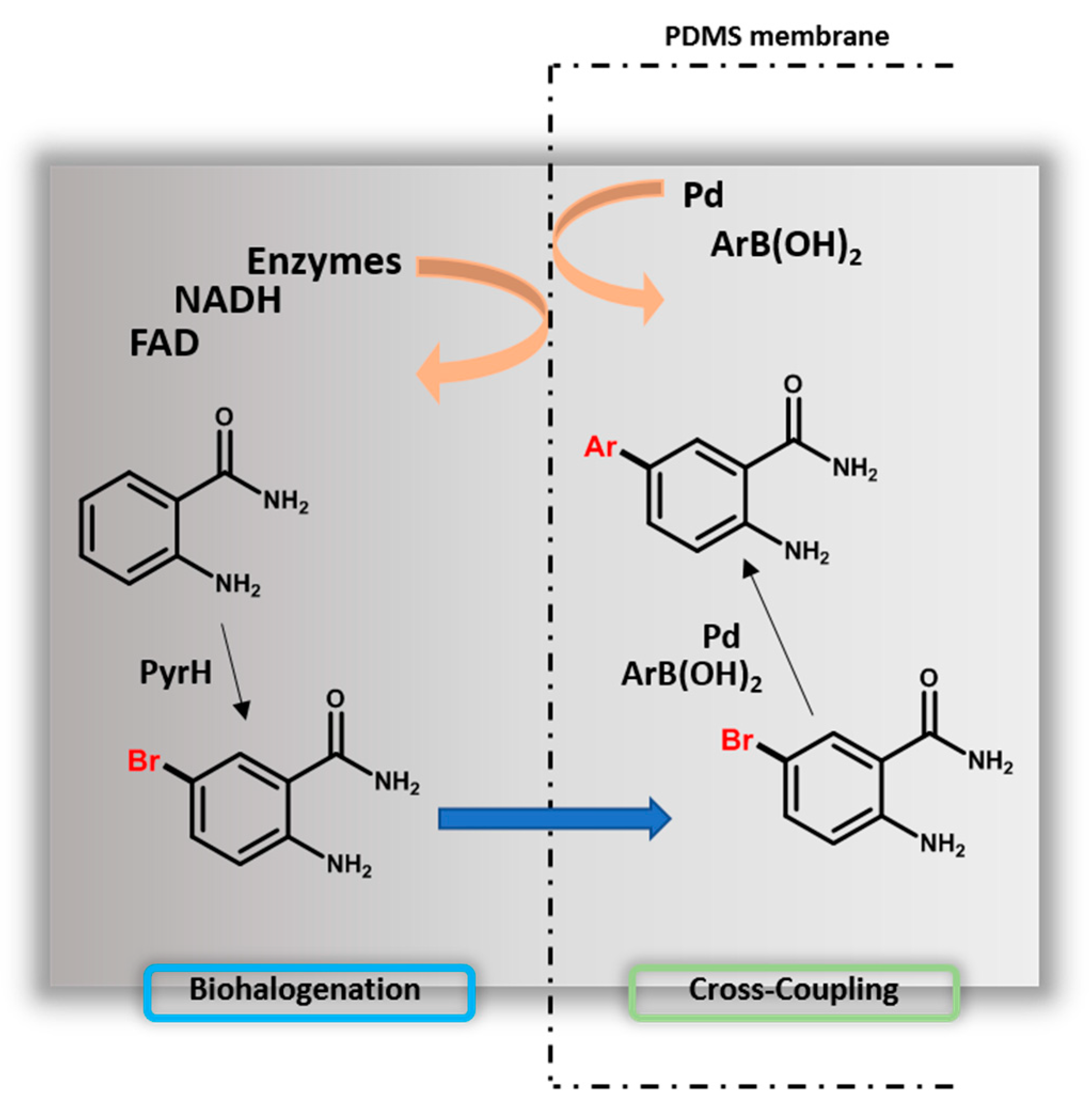
4.4. Chemoenzymatic Synthesis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gribble, G.W. A recent survey of naturally occurring organohalogen compounds. Environ. Chem. 2015, 12, 396–405. [Google Scholar] [CrossRef]
- Latham, J.; Brandenburger, E.; Shepherd, S.A.; Menon, B.R.K.; Micklefield, J. Development of Halogenase Enzymes for Use in Synthesis. Chem. Rev. 2018, 118, 232–269. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Rodriguez, L.; Khan, F.; Robins, K.; Meyer, H.-P. Perspectives on biotechnological halogenation. Chim. Oggi Chem. Today 2011, 29, 31–33. [Google Scholar]
- Njardarson, J.T. Top 200 Small Molecule Pharmaceuticals by Retail Sales in 2018. Available online: https://njardarson.lab.arizona.edu/content/top-pharmaceuticals-poster (accessed on 4 November 2019).
- Latham, J.; Henry, J.M.; Sharif, H.H.; Menon, B.R.K.; Shepherd, S.A.; Greaney, M.F.; Micklefield, J. Integrated catalysis opens new arylation pathways via regiodivergent enzymatic C-H activation. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V.; Tong, X.; Pubill-Ulldemolins, C.; Cartmell, C.; Bogosyan, E.J.A.; Rackham, E.J.; Marelli, E.; Hamed, R.B.; Goss, R.J.M. Living GenoChemetics by hyphenating synthetic biology and synthetic chemistry in vivo. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.K.S.; Mathew, T.; Hoole, D.; Esteves, P.M.; Wang, Q.; Rasul, G.; Olah, G.A. N-halosuccinimide/BF3-H2O, efficient electrophilic halogenating systems for aromatics. J. Am. Chem. Soc. 2004, 126, 15770–15776. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Metal-mediated reductive hydrodehalogenation of organic halides. Chem. Rev. 2002, 102, 4009–4091. [Google Scholar] [CrossRef]
- Chung, W.J.; Vanderwal, C.D. Stereoselective Halogenation in Natural Product Synthesis. Angew. Chem. Int. Ed. 2016, 55, 4396–4434. [Google Scholar] [CrossRef]
- Timmins, A.; De Visser, S.P. A comparative review on the catalytic mechanism of nonheme iron hydroxylases and halogenases. Catalysts 2018, 8, 1–25. [Google Scholar] [CrossRef]
- Yeh, E.; Cole, L.J.; Barr, E.W.; Bollinger, J.M.; Ballou, D.P.; Walsh, C.T. Flavin redox chemistry precedes substrate chlorination during the reaction of the flavin-dependent halogenase RebH. Biochemistry 2006, 45, 7904–7912. [Google Scholar] [CrossRef]
- Dong, C. Tryptophan 7-Halogenase (PrnA) Structure Suggests a Mechanism for Regioselective Chlorination. Science 2005, 309, 2216–2219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; De Laurentis, W.; Leang, K.; Herrmann, J.; Ihlefeld, K.; van Pée, K.H.; Naismith, J.H. Structural Insights into Regioselectivity in the Enzymatic Chlorination of Tryptophan. J. Mol. Biol. 2009, 391, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Kotzsch, A.; Dorward, M.; Van Pée, K.H.; Naismith, J.H. Crystallization and X-ray diffraction of a halogenating enzyme, tryptophan 7-halogenase, from Pseudomonas fluorescens. Acta Cryst. 2004, 60, 1438–1440. [Google Scholar]
- Bitto, E.; Huang, Y.; Bingman, C.A.; Singh, S.; Thorson, J.S.; Phillips, G.N., Jr. The structure of flavin-dependent tryptophan 7-halogenase RebH. Proteins 2007, 70, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.; Blasiak, L.C.; Koglin, A.; Drennan, C.L.; Walsh, C.T. Chlorination by a long-lived intermediate in the mechanism of flavin-dependent halogenases. Biochemistry 2007, 46, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Flecks, S.; Patallo, E.P.; Zhu, X.; Ernyei, A.J.; Seifert, G.; Schneider, A.; Dong, C.; Naismith, J.H.; Van Pée, K.H. New insights into the mechanism of enzymatic chlorination of tryptophan. Angew. Chem. Int. Ed. 2008, 47, 9533–9536. [Google Scholar] [CrossRef]
- Andorfer, M.C.; Lewis, J.C. Understanding and Improving the Activity of Flavin-Dependent Halogenases via Random and Targeted Mutagenesis. Annu. Rev. Biochem. 2018, 87, 159–185. [Google Scholar] [CrossRef]
- Altmann, A.; Fischer, I.; Weislo, L.J.; Lanahan, M.; Ligon, J.M. Functions Encoded by Pyrrolnitrin Biosynthetic Genes from Pseudomonas fluorescens. J. Bacteriol. 1998, 180, 1939–1943. [Google Scholar]
- Dorrestein, P.C.; Yeh, E.; Garneau-Tsodikova, S.; Kelleher, N.L.; Walsh, C.T. Dichlorination of a pyrrolyl-S-carrier protein by FADH2-dependent halogenase PltA during pyoluteorin biosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 13843–13848. [Google Scholar] [CrossRef]
- Zeng, J.; Zhan, J. A Novel Fungal Flavin-Dependent Halogenase for Natural Product Biosynthesis. ChemBioChem 2010, 11, 2119–2123. [Google Scholar] [CrossRef]
- Neubauer, P.R.; Widmann, C.; Wibberg, D.; Schröder, L.; Frese, M.; Kottke, T.; Kalinowski, J.; Niemann, H.H.; Sewald, N. A flavin-dependent halogenase from metagenomic analysis prefers bromination over chlorination. PLoS ONE 2018, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Dairi, T.; Nakano, T.; Aisaka, K.; Katsumata, R.; Hasegawa, M. Cloning and Nucleotide Sequence of the Gene Responsible for Chlorination of Tetracycline. Biosci. Biotech. Biochem. 1995, 59, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.; Garneau, S.; Walsh, C.T. Robust in vitro activity of RebF and RebH, a two-component reductase/halogenase, generating 7-chlorotryptophan during rebeccamycin biosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 3960–3965. [Google Scholar] [CrossRef] [PubMed]
- Seibold, C.; Schnerr, H.; Rumpf, J.; Kunzendorf, A.; Hatscher, C.; Wage, T.; Ernyei, A.J.; Dong, C.; Naismith, J.H.; van Pée, K.H. A flavin-dependent tryptophan 6-halogenase and its use in modification of pyrrolnitrin biosynthesis. Biocatal. Biotransformation 2006, 24, 401–408. [Google Scholar] [CrossRef]
- Heemstra, J.R.; Walsh, C.T. Tandem action of the O2-and FADH2-dependent halogenases KtzQ and KtzR produce 6,7-dichlorotryptophan for kutzneride assembly. J. Am. Chem. Soc. 2008, 130, 14024–14025. [Google Scholar] [CrossRef]
- Zehner, S.; Kotzsch, A.; Bister, B.; Süssmuth, R.D.; Méndez, C.; Salas, J.A.; Van Pée, K.H. A regioselective tryptophan 5-halogenase is involved in pyrroindomycin biosynthesis in Streptomyces rugosporus LL-42D005. Chem. Biol. 2005, 12, 445–452. [Google Scholar] [CrossRef]
- Bennett, M.R.; Thompson, M.L.; Shepherd, S.A.; Dunstan, M.S.; Herbert, A.J.; Smith, D.R.M.; Cronin, V.A.; Menon, B.R.K.; Levy, C.; Micklefield, J. Structure and Biocatalytic Scope of Coclaurine N-Methyltransferase. Angew. Chem. Int. Ed. 2018, 57, 10600–10604. [Google Scholar] [CrossRef]
- Zeng, J.; Zhan, J. Characterization of a tryptophan 6-halogenase from Streptomyces toxytricini. Biotechnol. Lett. 2011, 33, 1607–1613. [Google Scholar] [CrossRef]
- Luhavaya, H.; Sigrist, R.; Chekan, J.R.; McKinnie, S.M.K.; Moore, B.S. Biosynthesis of L-4-Chlorokynurenine, an Antidepressant Prodrug and a Non-Proteinogenic Amino Acid Found in Lipopeptide Antibiotics. Angew. Chem. Int. Ed. 2019, 58, 8394–8399. [Google Scholar] [CrossRef]
- Lingkon, K.; Bellizi, J. Structure and activity of the thermophilic tryptophan-6 halogenase. ChemBioChem 2019. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.; Zhang, L.; Zhang, W.; Zhu, Y.; Chen, Y.; Zhang, W.; Zhang, C. Functional characterization of the halogenase SpmH and discovery of new deschloro-tryptophan dimers. Org. Biomol. Chem. 2019, 17, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Domergue, J.; Erdmann, D.; Jouenne, A.F.; Petit, J.L.; Debard, A.; de Berardinis, V.; Vaxelaire, C.V.; Zaparucha, A. Xszen FHal, a novel tryptophan 5—halogenase from Xenorhabdus szentirmaii. AMB Express 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Frese, M.; Patschkowski, T.; Ortseifen, V.; Niehaus, K.; Sewald, N. Flavin-Dependent Halogenases from Xanthomonas campestris pv. campestris B100 Prefer Bromination over Chlorination. Adv. Synth. Catal. 2019, 361, 2475–2486. [Google Scholar] [CrossRef]
- Gkotsi, D.S.; Ludewig, H.; Sharma, S.V.; Connolly, J.A.; Dhaliwal, J.; Wang, Y.; Unsworth, W.P.; Taylor, R.J.K.; Mclachlan, M.M.W.; Shanahan, S.; et al. A marine viral halogenase that iodinates diverse substrates. Nat. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Reeves, C.D.; Hu, Z.; Reid, R.; Kealey, J.T. Genes for the biosynthesis of the fungal polyketides hypothemycin from Hypomyces subiculosus and radicicol from Pochonia chlamydosporia. Appl. Environ. Microbiol. 2008, 74, 5121–5129. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Maine, E.A.; Wijeratne, E.M.K.; Espinosa-Artiles, P.; Gunatilaka, A.A.L.; Molnár, I. Functional Characterization of the Biosynthesis of Radicicol, an Hsp90 Inhibitor Resorcylic Acid Lactone from Chaetomium chiversii. Chem. Biol. 2008, 15, 1328–1338. [Google Scholar] [CrossRef]
- Agarwal, V.; El Gamal, A.A.; Yamanaka, K.; Poth, D.; Kersten, R.D.; Schorn, M.; Allen, E.E.; Moore, B.S. Biosynthesis of polybrominated aromatic organic compounds by marine bacteria. Nat. Chem. Biol. 2014, 10, 640–647. [Google Scholar] [CrossRef]
- Neumann, C.S.; Walsh, C.T.; Kay, R.R. A flavin-dependent halogenase catalyzes the chlorination step in the biosynthesis of Dictyostelium differentiation-inducing factor 1. Proc. Natl. Acad. Sci. USA 2010, 107, 5798–5803. [Google Scholar] [CrossRef]
- Nowak-Thompson, B.; Chaney, N.; Wing, J.S.; Gould, S.J.; Loper, J.E. Characterization of the pyoluteorin biosynthetic gene cluster of Pseudomonas fluorescens Pf-5. J. Bacteriol. 1999, 181, 2166–2174. [Google Scholar] [PubMed]
- Yan, Q.; Philmus, B.; Chang, J.H.; Loper, J.E. Novel mechanism of metabolic co-regulation coordinates the biosynthesis of secondary metabolites in Pseudomonas protegens. Elife 2017, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Fraley, A.E.; Garcia-Borra, M.; Tripathi, A.; Khare, D.; Mercado-Marin, E.V.; Tran, H.; Dan, Q.; Webb, G.P.; Watts, K.R.; Crews, P.; et al. Function and Structure of MalA/MalA’, Iterative Halogenases for Late-Stage C-H Functionalization of Indole Alkaloids. J. Am. Chem. Soc. 2017, 139, 12060–12068. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Pang, A.H.; Chandrika, N.T.; Garneau-Tsodikova, S.; Tsodikov, O.V. Unusual substrate and halide versatility of phenolic halogenase PltM. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.F.; Snodgrass, H.M.; Jones, K.A.; Andorfer, M.C.; Lewis, J.C. Site-Selective C-H Halogenation using Flavin-Dependent Halogenases Identified via Family-Wide Activity Profiling. ChemRxiv 2019, 5, 1844–1856. [Google Scholar] [CrossRef]
- Menon, B.R.K.; Latham, J.; Dunstan, M.S.; Brandenburger, E.; Klemstein, U.; Leys, D.; Karthikeyan, C.; Greaney, M.F.; Shepherd, S.A.; Micklefield, J. Structure and biocatalytic scope of thermophilic flavin-dependent halogenase and flavin reductase enzymes. Org. Biomol. Chem. 2016, 14, 9354–9361. [Google Scholar] [CrossRef]
- Andorfer, M.C.; Belsare, K.D.; Girlich, A.M.; Lewis, J.C. Aromatic Halogenation by Using Bifunctional Flavin Reductase–Halogenase Fusion Enzymes. ChemBioChem 2017, 18, 2099–2103. [Google Scholar] [CrossRef]
- Frese, M.; Sewald, N. Enzymatic halogenation of tryptophan on a gram scale. Angew. Chem. Int. Ed. 2015, 54, 298–301. [Google Scholar] [CrossRef]
- Schroeder, L.; Frese, M.; Müller, C.; Sewald, N.; Kottke, T. Photochemically Driven Biocatalysis of Halogenases for the Green Production of Chlorinated Compounds. ChemCatChem 2018, 10, 3336–3341. [Google Scholar] [CrossRef]
- Ismail, M.; Schroeder, L.; Frese, M.; Kottke, T.; Hollmann, F.; Paul, C.E.; Sewald, N. Straightforward regeneration of FADH2 required for enzymatic tryptophan halogenation. ACS Catal. 2019, 9, 1389–1395. [Google Scholar] [CrossRef]
- Payne, J.T.; Andorfer, M.C.; Lewis, J.C. Regioselective arene halogenation using the FAD-dependent halogenase RebH. Angew. Chem. Int. Ed. 2013, 52, 5271–5274. [Google Scholar] [CrossRef] [PubMed]
- Poor, C.B.; Andorfer, M.C.; Lewis, J.C. Improving the stability and catalyst lifetime of the halogenase RebH by directed evolution. ChemBioChem 2014, 15, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Schnepel, C.; Minges, H.; Frese, M.; Sewald, N. A High-Throughput Fluorescence Assay to Determine the Activity of Tryptophan Halogenases. Angew. Chem. Int. Ed. 2016, 55, 14159–14163. [Google Scholar] [CrossRef] [PubMed]
- Minges, A.H.; Schnepel, C.; Böttcher, D.; Weiß, M.S.; Sproß, J.; Bornscheuer, U.T. Targeted Enzyme Engineering Unveiled Unexpected Patterns of. ChemCatChem 2019. [Google Scholar] [CrossRef]
- Frese, M.; Guzowska, P.H.; Voß, H.; Sewald, N. Regioselective Enzymatic Halogenation of Substituted Tryptophan Derivatives using the FAD-Dependent Halogenase RebH. ChemCatChem 2014, 6, 1270–1276. [Google Scholar]
- Shepherd, S.A.; Karthikeyan, C.; Latham, J.; Struck, A.W.; Thompson, M.L.; Menon, B.R.K.; Styles, M.Q.; Levy, C.; Leys, D.; Micklefield, J. Extending the biocatalytic scope of regiocomplementary flavin-dependent halogenase enzymes. Chem. Sci. 2015, 6, 3454–3460. [Google Scholar] [CrossRef]
- Payne, J.T.; Poor, C.B.; Lewis, J.C. Directed Evolution of RebH for Site-Selective Halogenation of Large Biologically Active Molecules. Angew. Chem. Int. Ed. 2015, 54, 4226–4230. [Google Scholar] [CrossRef]
- Lang, A.; Polnick, S.; Nicke, T.; William, P.; Patallo, E.P.; Naismith, J.H.; Van Pée, K.H. Changing the regioselectivity of the tryptophan 7-halogenase PrnA by site-directed mutagenesis. Angew. Chem. Int. Ed. 2011, 50, 2951–2953. [Google Scholar] [CrossRef]
- Shepherd, S.A.; Menon, B.R.K.; Fisk, H.; Struck, A.; Levy, C.; Leys, D.; Micklefield, J. A Structure-Guided Switch in the Regioselectivity of a Tryptophan Halogenase. ChemBioChem 2016, 17, 821–824. [Google Scholar] [CrossRef]
- Andorfer, M.C.; Park, H.J.; Vergara-Coll, J.; Lewis, J.C. Directed evolution of RebH for catalyst-controlled halogenation of indole C–H bonds. Chem. Sci. 2016, 7, 3720–3729. [Google Scholar] [CrossRef]
- Moritzer, A.-C.; Minges, H.; Prior, T.; Frese, M.; Sewald, N.; Niemann, H.H. Structure-based switch of regioselectivity in the flavin-dependent tryptophan 6-halogenase Thal. J. Biol. Chem. 2019, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.T.; Butkovich, P.H.; Gu, Y.; Kunze, K.N.; Park, H.J.; Wang, D.S.; Lewis, J.C. Enantioselective Desymmetrization of Methylenedianilines via Enzyme-Catalyzed Remote Halogenation. J. Am. Chem. Soc. 2018, 140, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.D.; Grüschow, S.; Cairns, N.; Goss, R.J.M. Gene expression enabling synthetic diversification of natural products: Chemogenetic generation of pacidamycin analogs. J. Am. Chem. Soc. 2010, 132, 12243–12245. [Google Scholar] [CrossRef] [PubMed]
- Runguphan, W.; Qu, X.; O’Connor, S.E. Integrating carbon-halogen bond formation into medicinal plant metabolism. Nature 2010, 468, 461–467. [Google Scholar] [CrossRef]
- Glenn, W.S.; Nims, E.; Connor, S.E.O. Reengineering a Tryptophan Halogenase to Preferentially Chlorinate a Direct Alkaloid Precursor. J. Am. Chem. Soc. 2011, 133, 19346–19349. [Google Scholar] [CrossRef]
- Fräbel, S.; Krischke, M.; Staniek, A.; Warzecha, H. Recombinant flavin-dependent halogenases are functional in tobacco chloroplasts without co-expression of flavin reductase genes. Biotechnol. J. 2016, 11, 1586–1594. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Xiao, A.; Rasmussen, M.; Skidmore, C.; Zhan, J. Metabolic engineering of Escherichia coli for the biosynthesis of various phenylpropanoid derivatives. Metab. Eng. 2015, 29, 153–159. [Google Scholar] [CrossRef]
- Rudroff, F.; Mihovilovic, M.D.; Gröger, H.; Snajdrova, R.; Iding, H.; Bornscheuer, U.T. Opportunities and challenges for combining chemo- and biocatalysis. Nat. Catal. 2018, 1, 12–22. [Google Scholar] [CrossRef]
- Runguphan, W.; O’Connor, S.E. Diversification of monoterpene indole alkaloid analogs through cross-coupling. Org. Lett. 2013, 15, 2850–2853. [Google Scholar] [CrossRef]
- Durak, L.J.; Payne, J.T.; Lewis, J.C. Late-Stage Diversification of Biologically Active Molecules via Chemoenzymatic C-H Functionalization. ACS Catal. 2016, 6, 1451–1454. [Google Scholar] [CrossRef]
- Frese, M.; Schnepel, C.; Minges, H.; Voß, H.; Feiner, R.; Sewald, N. Modular Combination of Enzymatic Halogenation of Tryptophan with Suzuki-Miyaura Cross-Coupling Reactions. ChemCatChem 2016, 8, 1799–1803. [Google Scholar] [CrossRef]
- Steinkellner, G.; Gruber, C.C.; Pavkov-Keller, T.; Binter, A.; Steiner, K.; Winkler, C.; Łyskowski, A.; Schwamberger, O.; Oberer, M.; Schwab, H. Identification of promiscuous ene-reductase activity by mining structural databases using active site constellations. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yan, Y.; Zhang, C.; Dalby, P.A. Two strategies to engineer flexible loops for improved enzyme thermostability. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, E.H.; Leaver-Fay, A.; Baker, D. Role of conformational sampling in computing mutation-induced changes in protein structure and stability. Proteins 2011, 79, 830–838. [Google Scholar] [CrossRef]
- Bednar, D.; Beerens, K.; Sebestova, E.; Bendl, J.; Khare, S.; Chaloupkova, R.; Prokop, Z.; Brezovsky, J.; Baker, D.; Damborsky, J. FireProt: Energy-and Evolution-Based Computational Design of Thermostable Multiple-Point Mutants. PLoS Comput. Biol. 2015, 11. [Google Scholar] [CrossRef]
- Wijma, H.J.; Floor, R.J.; Jekel, P.A.; Baker, D.; Marrink, S.J.; Janssen, D.B. Computationally designed libraries for rapid enzyme stabilization. Protein Eng. Des. Sel. 2014, 27, 49–58. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Q.; Qu, G.; Feng, Y.; Reetz, M.T. Utility of B-Factors in Protein Science: Interpreting Rigidity, Flexibility, and Internal Motion and Engineering Thermostability. Chem. Rev. 2019, 119, 1626–1665. [Google Scholar] [CrossRef]
- Andorfer, M.C.; Grob, J.E.; Hajdin, C.E.; Chael, J.R.; Siuti, P.; Lilly, J.; Tan, K.L.; Lewis, J.C. Understanding Flavin-Dependent Halogenase Reactivity via Substrate Activity Profiling. ACS Catal. 2017, 7, 1897–1904. [Google Scholar] [CrossRef]




| Enzyme | Halogen (X) | Literature | ||
|---|---|---|---|---|
| RebH | Cl > Br | [24] |  | |
| Thal | Cl > Br | [25] | ||
| PyrH | Cl > Br | [27] | ||
| KtzQ | Cl | [26] | ||
| PrnA | Cl | [19] | ||
| Th-Hal | Cl, Br | [47] | ||
| KtzR | Cl | [26] | Tryptophan-Fl-Hal  | |
| SttH | Cl, Br | [29] | ||
| Tar14 | Cl, Br | [30] | ||
| SpmH | Cl, Br | [32] | ||
| XszenFHal | Cl, Br | [33] | ||
| BorH | Cl, Br | [31] | ||
| BrvH | Cl < Br | [22] |  | |
| xcc-b100-4156 | Br | [34] | ||
| xcc-b100-1333 | Br | [34] | Indole-Fl-Hal  | |
| xcc-b100-4345 | Br | [34] | ||
| VirX1 | Cl < Br < I | [35] | ||
| Bmp5 | Br, I | [40] |  | |
| ChlA | Cl | [41] | ||
| PltM | Cl, Br, I | [45] | Phenolic-Fl-Hal  | |
| RadH | Cl | [39] | ||
| Rdc2 | Cl, Br | [21] | ||
| PrnC | Cl | [19] |  | |
| PltA | Cl, Br | [20] | Pyrrole-Fl-Hal  | |
| MalA | Cl, Br | [44] | ||
| Enzyme | Scale (mg) | Substrate | Halogen Insertion | Conversion Yield %/Isolated Product Yield % | Ref. |
|---|---|---|---|---|---|
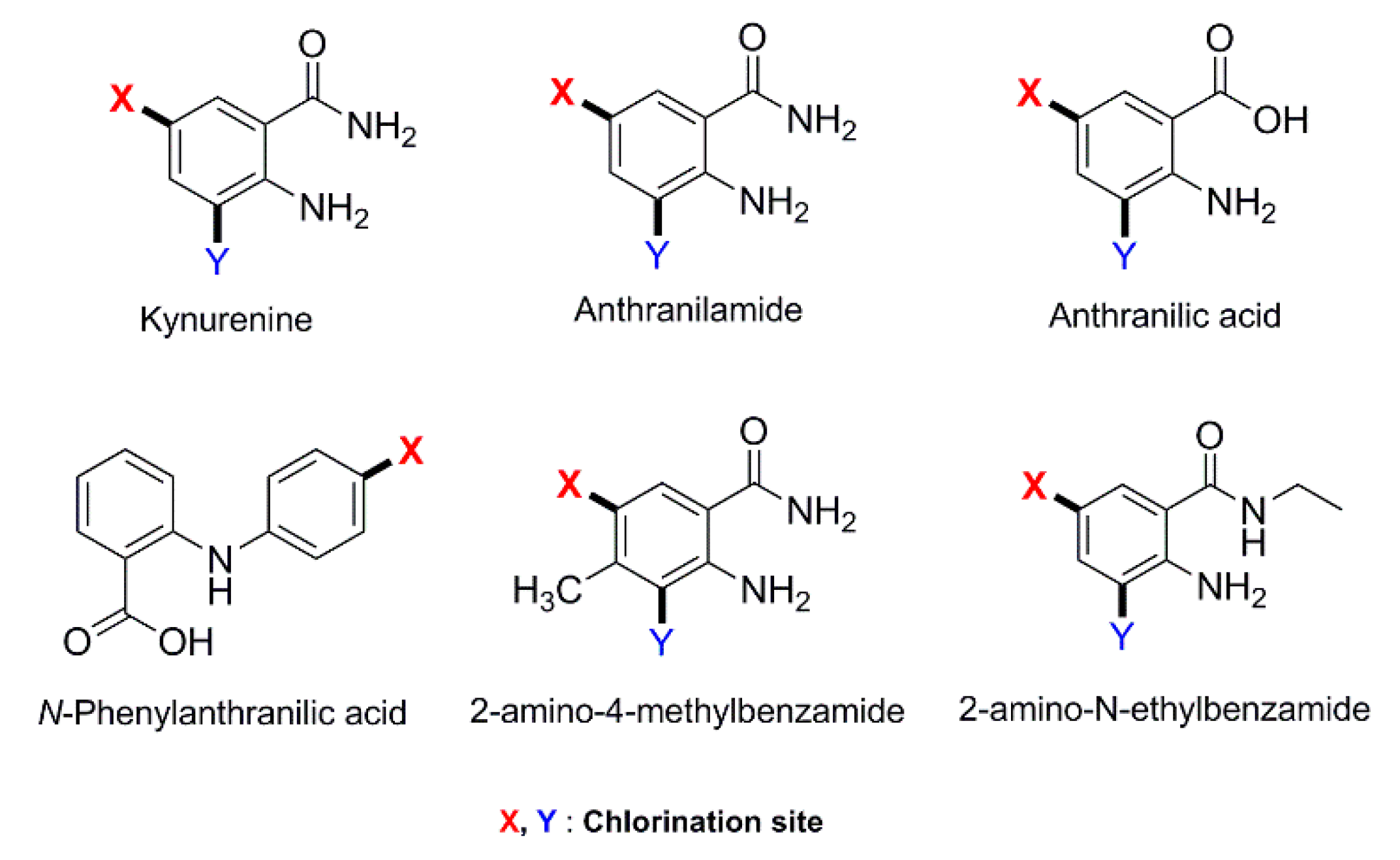
| PyrH (combiCLEAs) PyrH and SttH (combiCLEAs) RebH (combiCLEAs) | 136 15 24 | Anthranilamide 3-Indolepropionic acid Tryptophol | Br | n.d. [a] | [5] |
| Th-Hal (purified) | 16 18 17 16 11 11 | L-tryptophan 1-methyl-L-tryptophan 5-hydroxy-L-tryptophan L-kynurenine, Anthranilic acid Anthranilamide | Cl | -/62.5 -/12 -/16 -/21 -/8 -/19 | [47] |
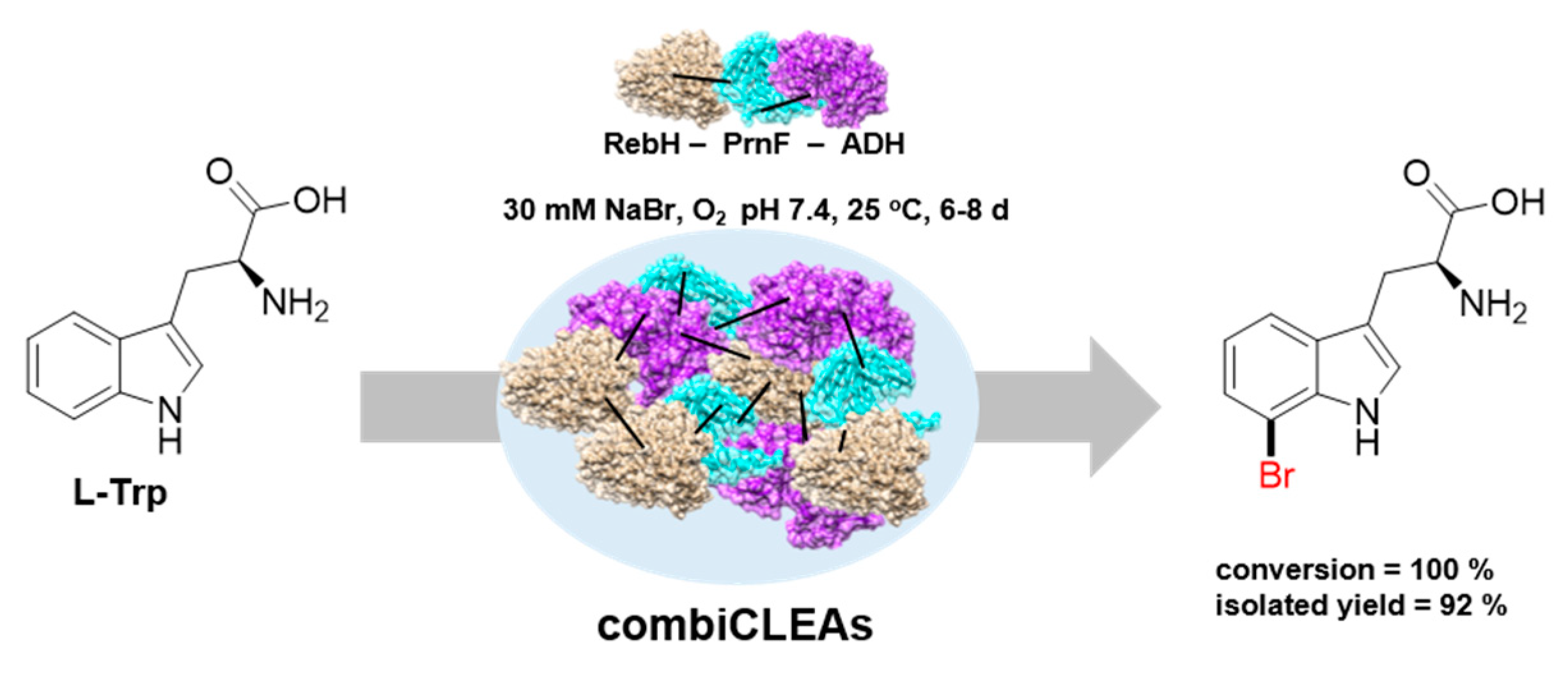
| RebH (combiCLEAs) | 1000 40 40 | L-tryptophan D-tryptophan L-5-hydroxytryptophan | Br | 100/- 57 / 53 / | [49] |
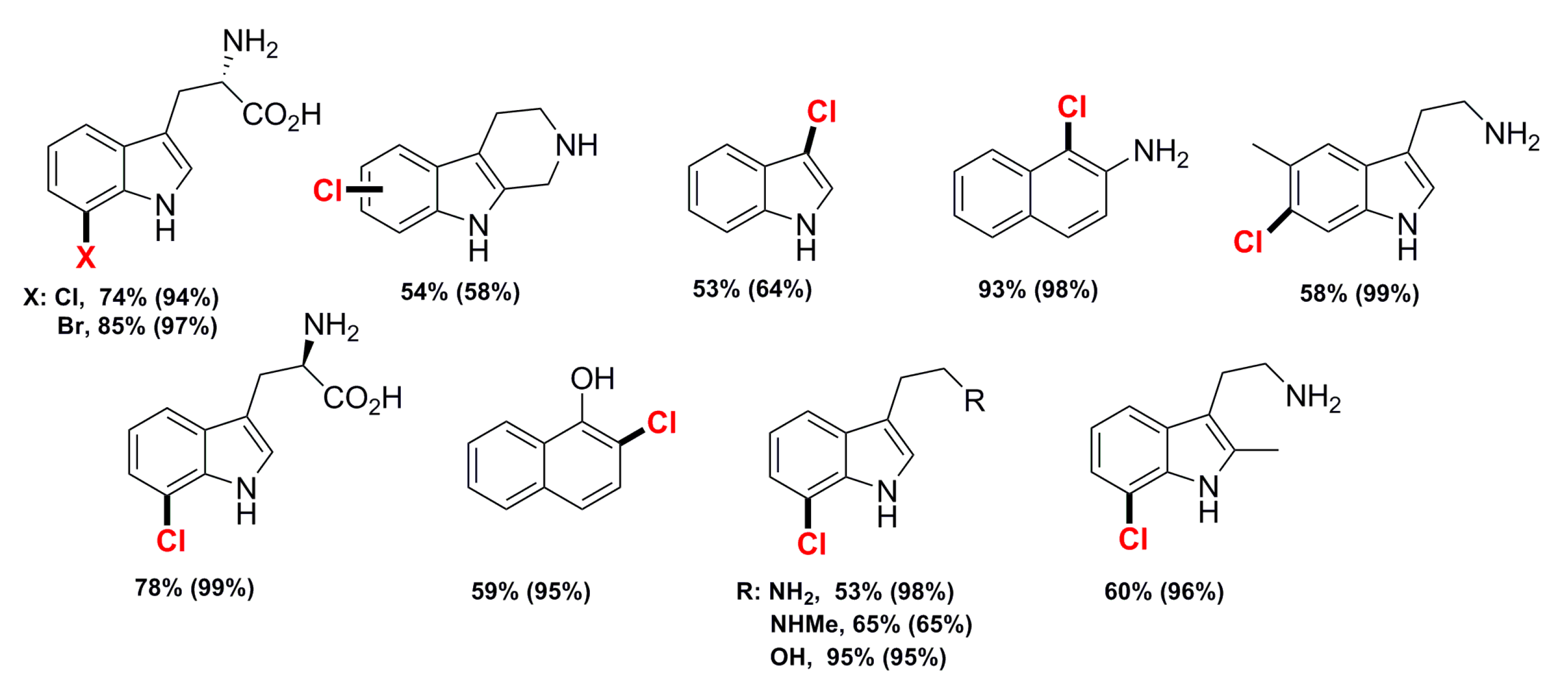
| RebH (purified) RebH (purified) RebH (lysate) | 10 10 100 | L-tryptophan D-tryptophan Tryptamine N-Ω-methyltryptamine Tryptophol 2-methyltryptamine 5-methyltryptamine 2,3-disubstituted indole tryptoline Indole 2-aminonaphthalene 1-naphthol L-tryptophan | Cl Br Cl Cl | 94/74 97/85 99/78 98/53 65/65 95/95 96/60 99/58 58/54 64/53 98/93 95/59 74/69 | [52] |
| RebH (purified) | 10 | L-tryptophan 2-methyltryptamine 2-aminonaphthalene Tryptoline | Cl | -/69 -/56 -/62 -/67 | [53] |
| PyrH (combiCLEAs) Thal (combiCLEAs) RebH (combiCLEAs) | 50 50 150 | L-tryptophan L-tryptophan L-tryptophan | Br | -/76 -/61 -/84 | [54] |
| SttH (combiCLEAs) | 100 | Anthranilamide | Cl | -/25 | [60] |
| RebH variants 0S/8F/10S (purified) | 10 | Tryptamine | Cl | 98/73/78 [b] | [61] |
| Thal-RebH5 variant (lysate) | 30 | L-tryptophan | Cl Br | 30/- 15/- | [62] |
| RebH variant 4-V (purified) | 10 | t-Bu dianiline methane | Cl | 80/- | [63] |
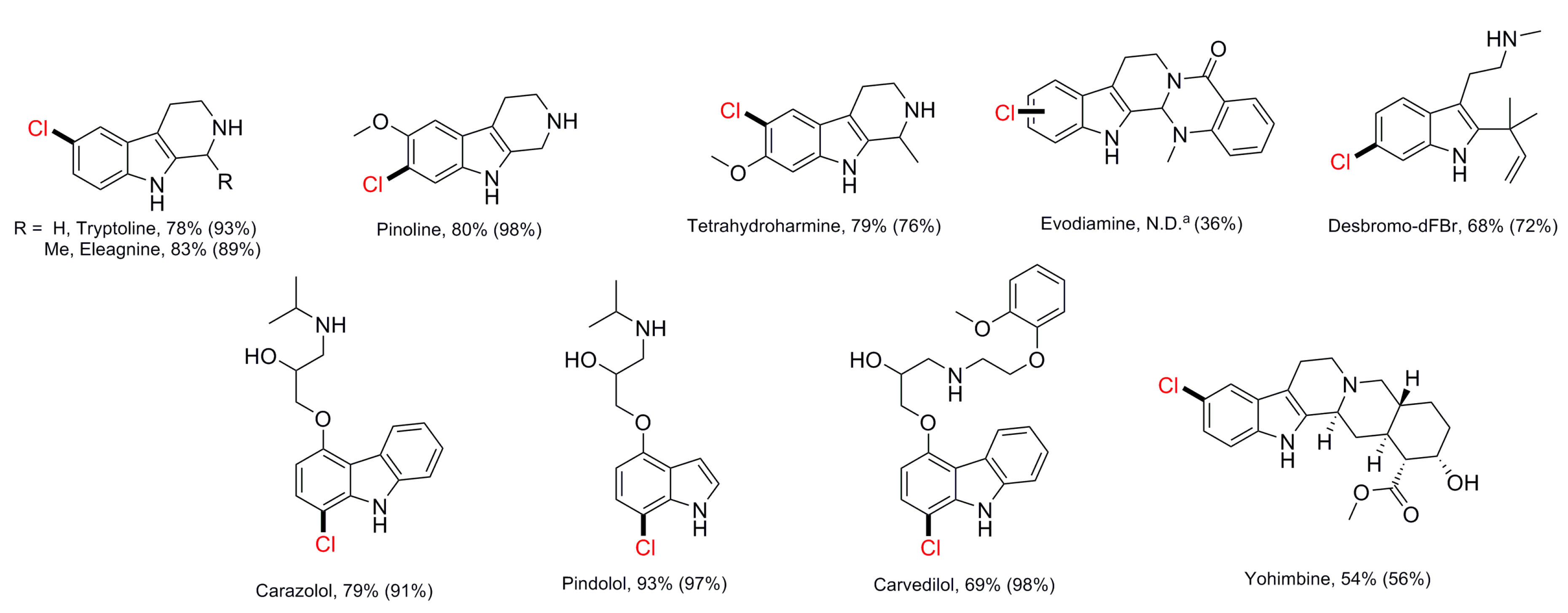
| RebH variant 3-SS (purified) RebH variant 4-V (purified) | 10 10 | Tryptoline Eleagnine Pinoline Tetrahydroharmine Debromodeformylflustrabromine Yohimbine Pindolol Carazolol Carvedilol | Cl Cl | -/78 -/83 -/80 -/79 -/68 -/54 -/93 -/79 -/69 | [58] |
| RebH variant 3-SS (purified) RebH variant 4-V (purified) | 10 10 | Tryptoline Carvedilol Pindolol Thenalidine | Br Cl Br | n.d. [a] | [71] |
| RebH/ThaI/PyrH (combiCLEAs) | 150 | L-tryptophan | Br | 100 [c] | [72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büchler, J.; Papadopoulou, A.; Buller, R. Recent Advances in Flavin-Dependent Halogenase Biocatalysis: Sourcing, Engineering, and Application. Catalysts 2019, 9, 1030. https://doi.org/10.3390/catal9121030
Büchler J, Papadopoulou A, Buller R. Recent Advances in Flavin-Dependent Halogenase Biocatalysis: Sourcing, Engineering, and Application. Catalysts. 2019; 9(12):1030. https://doi.org/10.3390/catal9121030
Chicago/Turabian StyleBüchler, Johannes, Athena Papadopoulou, and Rebecca Buller. 2019. "Recent Advances in Flavin-Dependent Halogenase Biocatalysis: Sourcing, Engineering, and Application" Catalysts 9, no. 12: 1030. https://doi.org/10.3390/catal9121030
APA StyleBüchler, J., Papadopoulou, A., & Buller, R. (2019). Recent Advances in Flavin-Dependent Halogenase Biocatalysis: Sourcing, Engineering, and Application. Catalysts, 9(12), 1030. https://doi.org/10.3390/catal9121030






