Selective Liquid-Phase Oxidation of Toluene with Molecular Oxygen Catalyzed by Mn3O4 Nanoparticles Immobilized on CNTs under Solvent-Free Conditions
Abstract
1. Introduction
2. Results and Discussion
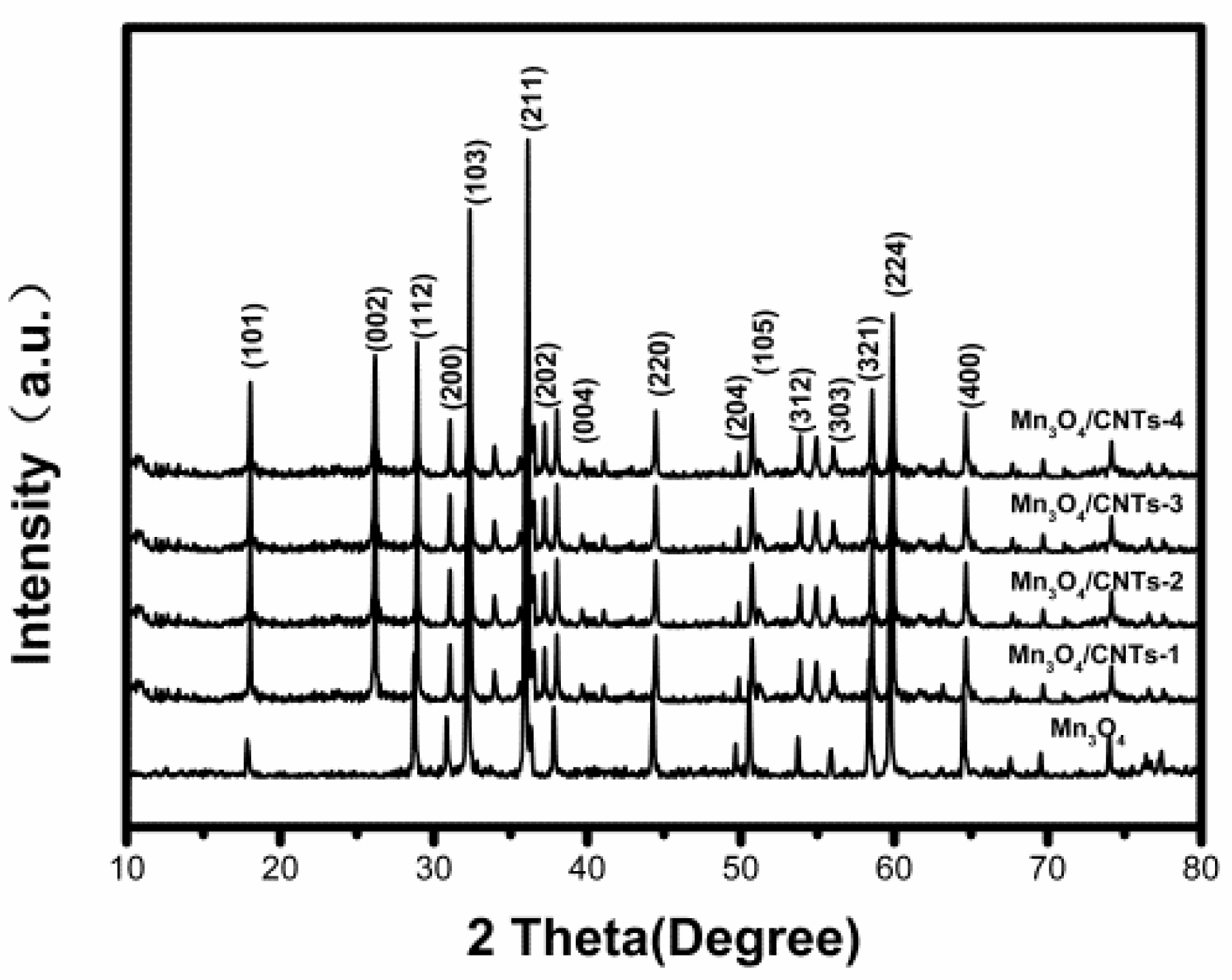
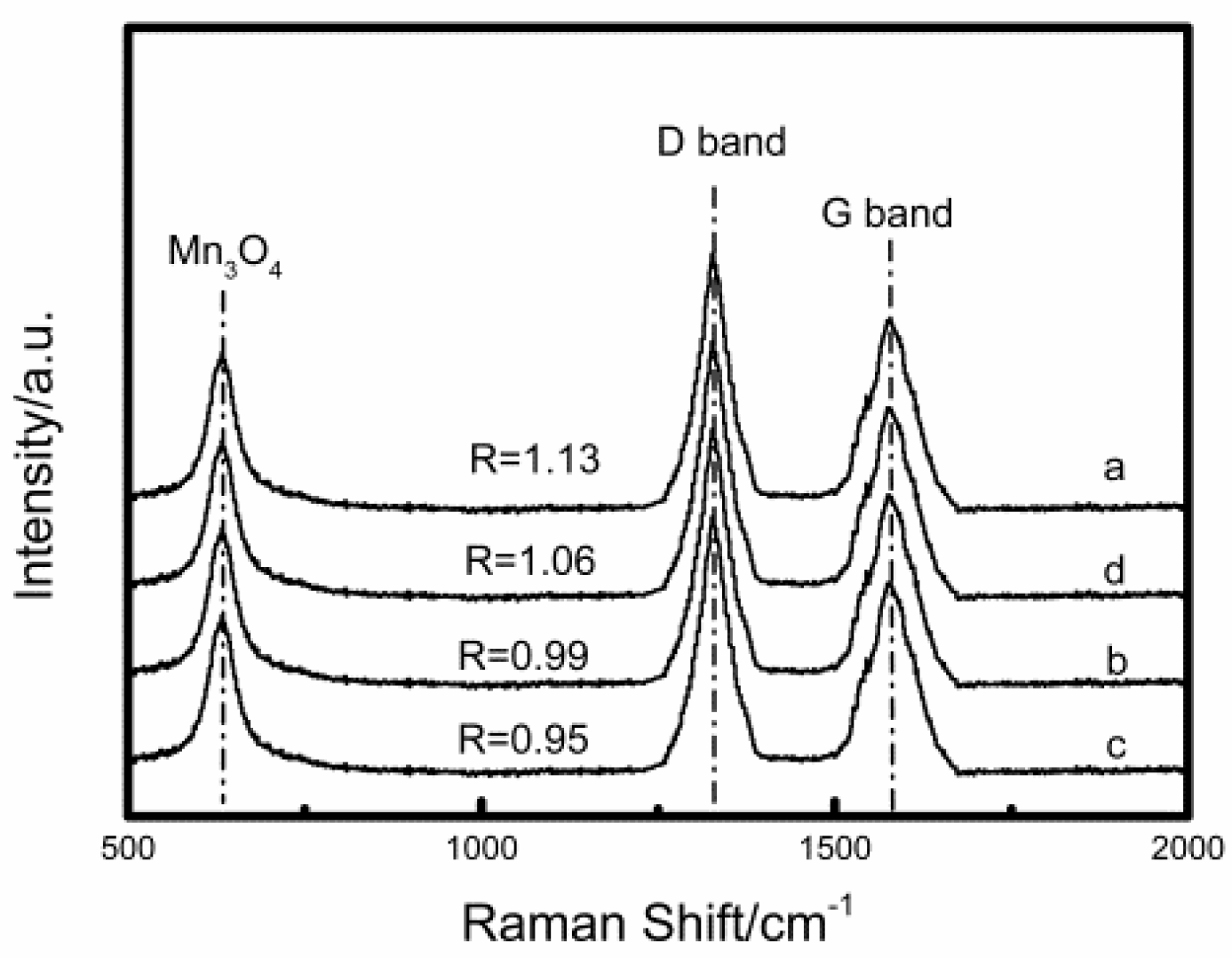
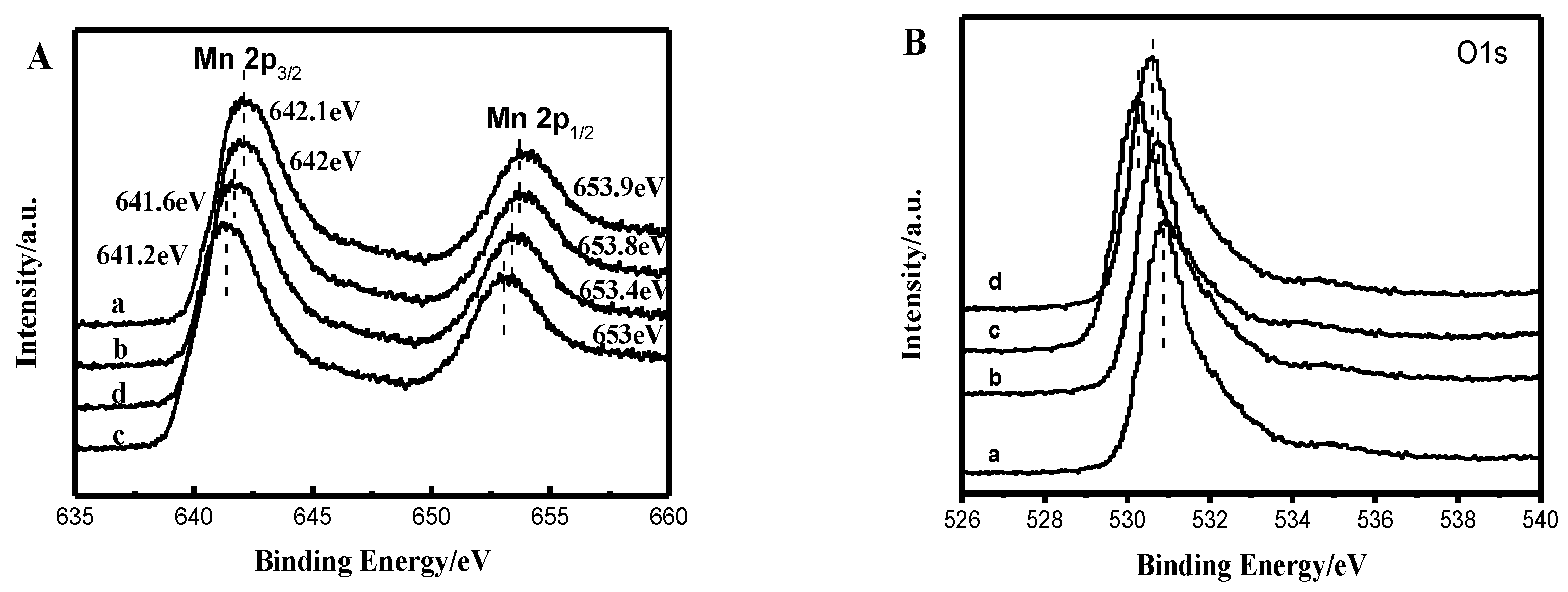
2.1. Catalyst Characterization Analysis
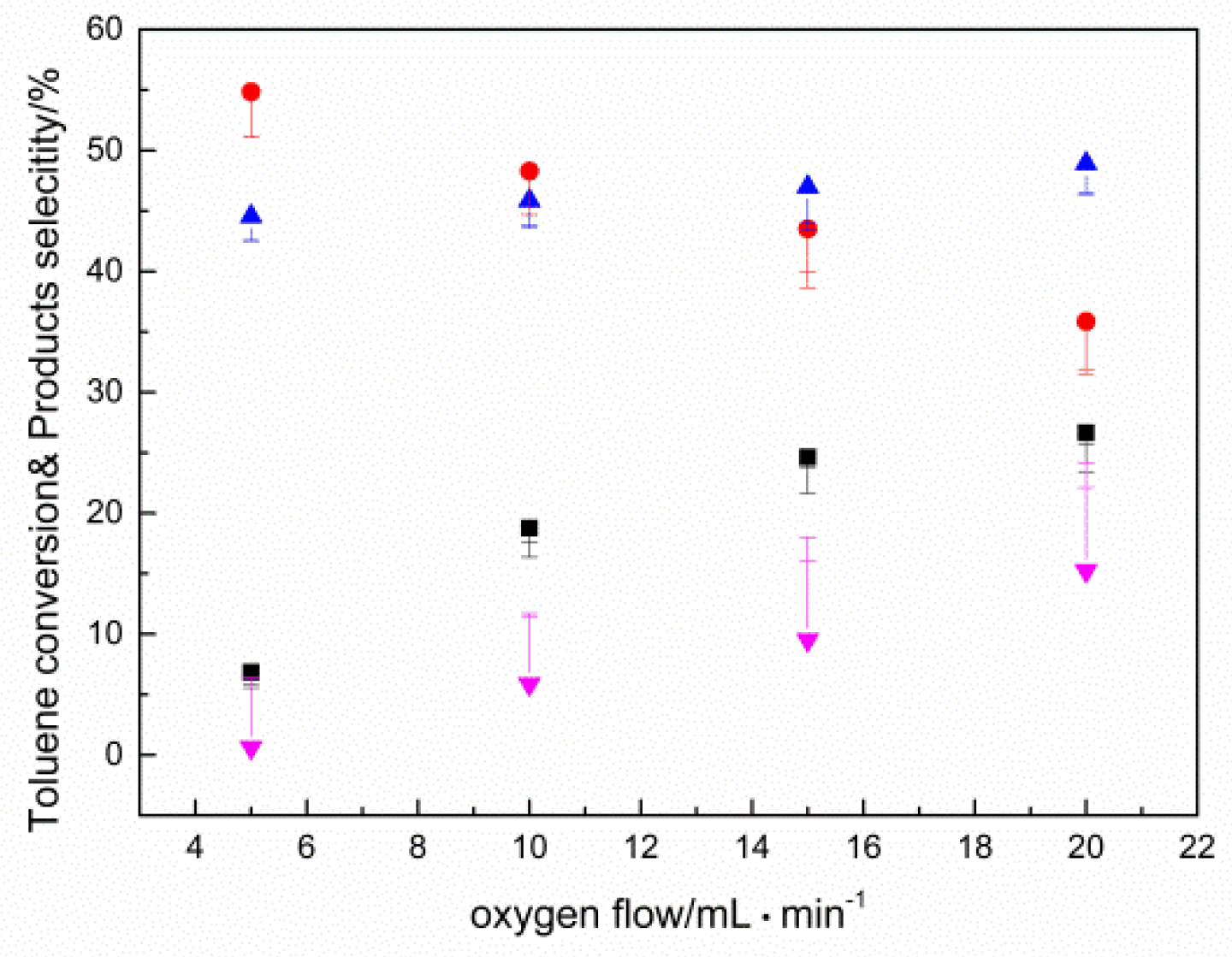
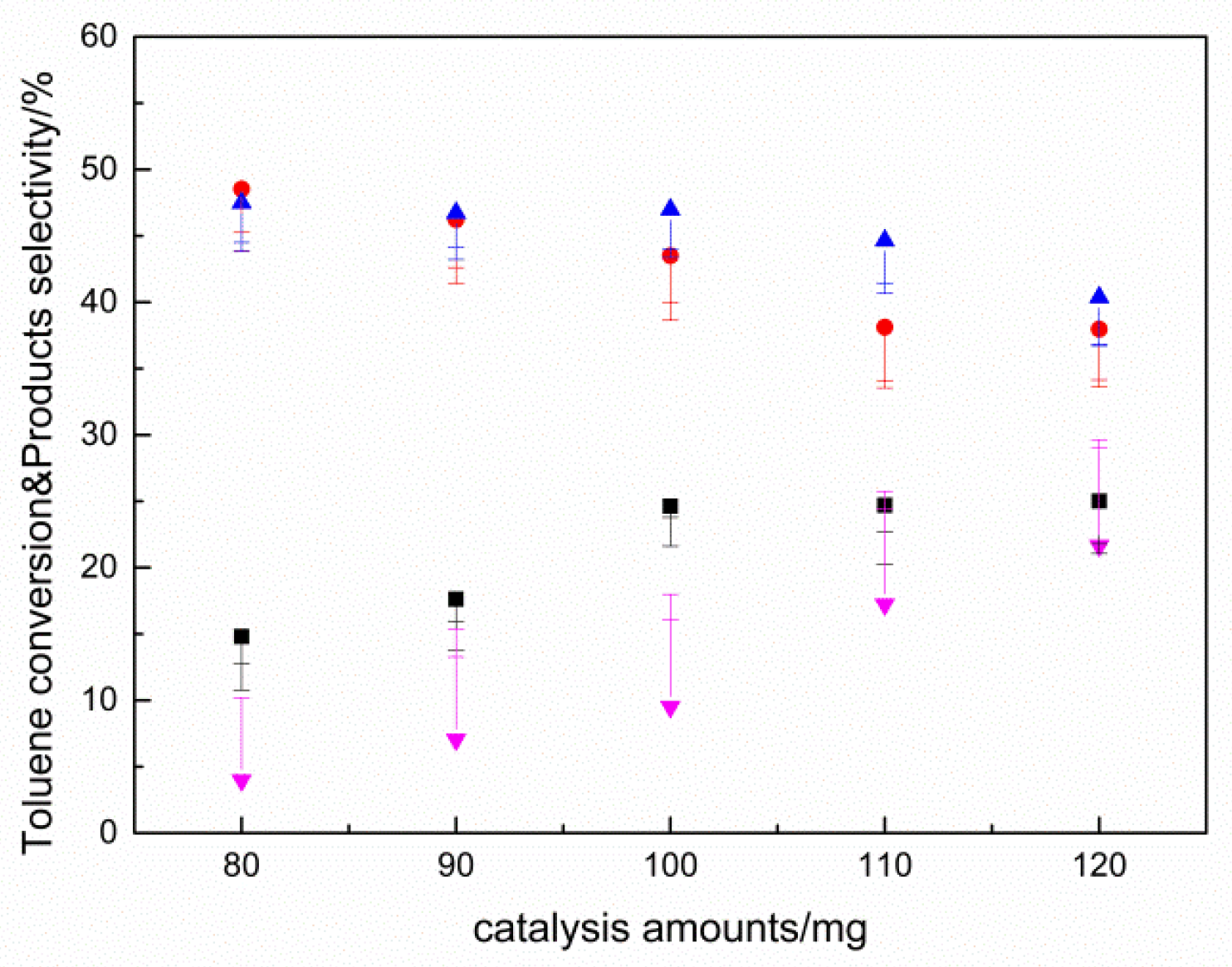
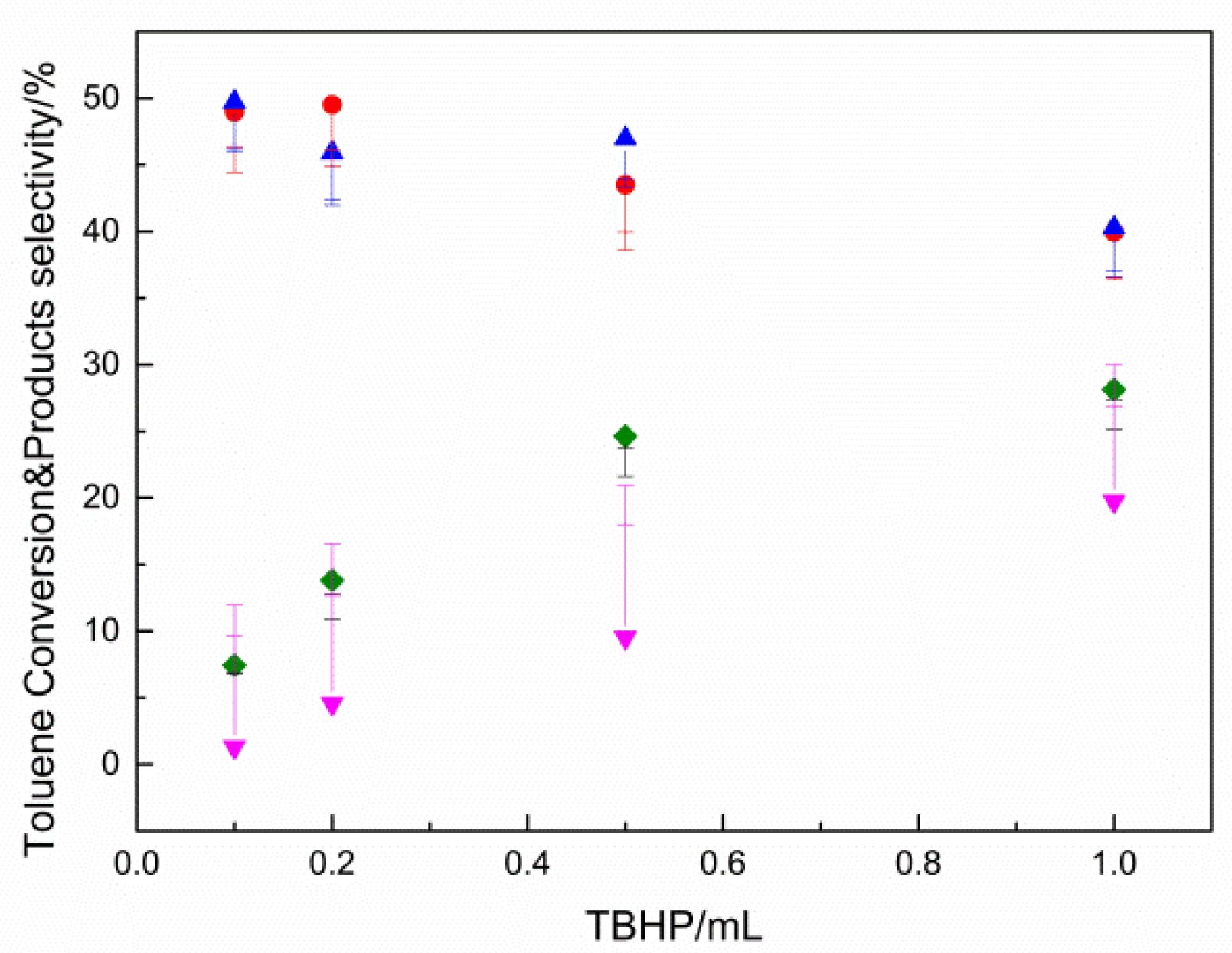
2.2. Results of Catalyst Performance Evaluation of Toluene Liquid-Phase Catalytic Oxidation Reaction
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Evaluation of Catalyst Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, Z.Y. Production and application of benzyl alcohol in China. Fine Special. Chem. 2012, 20, 12–14. [Google Scholar]
- Han, H.; Ding, G.; Wu, T.; Yang, D.; Jiang, T.; Han, B. Cu and boron doped carbon nitride for highly selective oxidation of toluene to benzaldehyde. Molecules 2015, 20, 12686–12697. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.F.; Jiao, N. Oxygenation via C-H/C-C bond activation with molecular oxygen. Acc. Chem. Res. 2017, 50, 1640–1653. [Google Scholar] [CrossRef] [PubMed]
- Gaster, E.; Kozuch, S.; Pappo, D. Selective aerobic oxidation of methylarenes to benzaldehydes catalyzed by N-hydroxyphthalimide and cobalt(II) acetate in hexafluoropropan-2-ol. Angew. Chem. Int. Ed. 2017, 56, 5912–5915. [Google Scholar] [CrossRef]
- Shi, G.; Xu, S.; Bao, Y.; Xu, J.; Liang, Y. Selective aerobic oxidation of toluene to benzaldehyde on immobilized CoOx on SiO2 catalyst in the presence of N-hydroxyphthalimide and hexafluoropropan-2-ol. Catal. Commun. 2019, 123, 73–78. [Google Scholar] [CrossRef]
- Deng, C.S.; Xu, M.X.; Dong, Z.; Li, L.; Yang, J.Y.; Guo, X.F.; Peng, L.M.; Xue, N.H.; Zhu, Y.; Ding, W.P. Exclusively catalytic oxidation of toluene to benzaldehyde in an O/W emulsion stabilized by hexadecylphosphate acid terminated mixed-oxide nanoparticles. Chin. J. Catal. 2020, 41, 341–349. [Google Scholar] [CrossRef]
- Guo, C.-C.; Liu, Q.; Wang, X.-T.; Hu, H.-Y. Selective liquid phase oxidation of toluene with air. Appl. Catal. A Gen. 2005, 282, 55–59. [Google Scholar] [CrossRef]
- Wen, S.; Xiaoli, W.; Zhenhua, Z.; Keqiang, D.; Xianfeng, L.; Gongde, W. Selective oxidation of toluene to benzyl alcohol catalyzed by F-modified cunial hydrotalcite compounds. J. Mol. Catal. 2013, 27, 37–42. [Google Scholar]
- Biswas, S.; Dutta, A.; Debnath, M.; Dolai, M.; Das, K.K.; Ali, M. A novel thermally stable hydroperoxo-copper(II) complex in a Cu(N2O2) chromophore of a potential N4O2 donor Schiff base ligand: Synthesis, structure and catalytic studies. Dalton Trans. 2013, 42, 13210–13219. [Google Scholar] [CrossRef]
- Biswas, S.; Dutta, A.; Dolai, M.; Debnath, M.; Jana, A.D.; Ali, M. Copper(II) induced oxidative modification and complexation of a Schiff base ligand: Synthesis, crystal structure, catalytic oxidation of aromatic hydrocarbons and DFT calculation. RSC Adv. 2014, 4, 34248–34256. [Google Scholar] [CrossRef]
- Mondal, J.; Borah, P.; Sreejith, S.; Nguyen, K.T.; Han, X.; Ma, X.; Zhao, Y. Morphology-tuned exceptional catalytic activity of porous-polymer-supported Mn3O4 in aerobic sp3 C-H bond oxidation of aromatic hydrocarbons and alcohols. ChemCatChem 2014, 6, 3518–3529. [Google Scholar] [CrossRef]
- Chen, B.F.; Li, S.P.; Liu, S.J.; Dong, M.H.; Han, B.X.; Liu, H.Z.; Zheng, L.R. Aerobic selective oxidation of methylaromatics to benzoic acids over Co@N/Co-CNTs with high loading CoN4 species. J. Mater. Chem. A 2019, 7, 27212–27216. [Google Scholar] [CrossRef]
- Gao, J.; Tong, X.; Li, X.; Miao, H.; Xu, J. The efficient liquid-phase oxidation of aromatic hydrocarbons by molecular oxygen in the presence of MnCO3. J. Chem. Technol. Biotechnol. 2007, 82, 620–625. [Google Scholar] [CrossRef]
- Skliri, E.; Papadogiorgakis, S.; Lykakis, I.N.; Armatas, G.S. Mesoporous assembled Mn3O4 nanoparticle networks as efficient catalysts for selective oxidation of alkenes and aryl alkanes. ChemPlusChem 2017, 82, 136–143. [Google Scholar] [CrossRef]
- Li, X.Q.; Yang, L.F. Liquid-phase oxidation of toluene to benzoic acid over manganese oxide catalyst. Adv. Mater. Res. 2013, 750–752, 1822–1825. [Google Scholar] [CrossRef]
- Biswal, M.; Dhas, V.V.; Mate, V.R.; Banerjee, A.; Pachfule, P.; Agrawal, K.L.; Ogale, S.B.; Rode, C.V. Selectivity tailoring in liquid phase oxidation over MWNT-Mn3O4 nanocomposite catalysts. J. Phys. Chem. 2011, 115, 15440–15448. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, Y.; Pan, F.; Li, Z.; Zhang, W.; Wu, F.; Chen, D.; Wen, W.; Li, J. Mesoporous silica-supported manganese oxides for complete oxidation of volatile organic compounds: Influence of mesostructure, redox properties, and hydrocarbon dimension. Ind. Eng. Chem. Res. 2018, 57, 7374–7382. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, R.H.; Liang, G.M.; Feng, J.K.; Lu, L.; Qian, Y.T. Carboxylated carbon nanotube anchored MnCO3 nanocomposites as anode materials for advanced lithium-ion batteries. Mater. Lett. 2013, 111, 165–168. [Google Scholar] [CrossRef]
- Pinilla, J.L.; Puron, H.; Torres, D.; Suelves, I.; Millan, M. Ni-MoS2 supported on carbon nanofibers as hydrogenation catalysts: Effect of support functionalisation. Carbon 2015, 81, 574–586. [Google Scholar] [CrossRef]
- Hita, I.; Palos, R.; Arandes, J.M.; Hill, J.M.; Castano, P. Petcoke-derived functionalized activated carbon as support in a bifunctional catalyst for tire oil hydroprocessing. Fuel Process. Technol. 2016, 144, 239–247. [Google Scholar] [CrossRef]
- Li, W.Z.; Liang, C.H.; Qiu, J.S.; Zhou, W.J.; Han, H.M.; Wei, Z.B.; Sun, G.Q.; Xin, Q. Carbon nanotubes as support for cathode catalyst of a direct methanol fuel cell. Carbon 2002, 40, 791–794. [Google Scholar] [CrossRef]
- Pinilla, J.L.; Puron, H.; Torres, D.; de Llobet, S.; Moliner, R.; Suelves, I.; Millan, M. Carbon nanofibres coated with Ni decorated MoS2 nanosheets as catalyst for vacuum residue hydroprocessing. Appl. Catal. 2014, 148, 357–365. [Google Scholar] [CrossRef]
- Li, B.; Wang, C.; Yi, G.; Lin, H.; Yuan, Y. Enhanced performance of Ru nanoparticles confined in carbon nanotubes for CO preferential oxidation in a H2-rich stream. Catal. Today 2011, 164, 74–79. [Google Scholar] [CrossRef]
- Yang, X.; Yu, D.H.; Peng, P.F.; Wang, D.H. Confined iron nanowires enhance the catalytic activity of carbon nanotubes in the aerobic oxidation of cyclohexane. ChemSusChem 2012, 5, 1213–1217. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Wu, C.; Xie, J.; Gu, X.; Yu, P.; Zong, M.; Gates, I.D.; Liu, H.; Rong, J. Electrophilic oxygen on defect-rich carbon nanotubes for selective oxidation of cyclohexane. Catal. Sci. Technol. 2020, 10, 332–336. [Google Scholar] [CrossRef]
- Luo, J.; Yu, H.; Wang, H.J.; Wang, H.H.; Peng, F. Aerobic oxidation of benzyl alcohol to benzaldehyde catalyzed by carbon nanotubes without any promoter. Chem. Eng. J. 2014, 240, 434–442. [Google Scholar] [CrossRef]
- Song, S.Q.; Jiang, S.J. Selective catalytic oxidation of ammonia to nitrogen over CuO/CNTs: The promoting effect of the defects of CNTs on the catalytic activity and selectivity. Appl. Catal. 2012, 117, 346–350. [Google Scholar] [CrossRef]
- Rodríguez-Manzo, J.A.; Cretu, O.; Banhart, F. Trapping of metal atoms in vacancies of carbon nanotubes and graphene. ACS Nano 2010, 4, 3422–3428. [Google Scholar]
- Luo, J.; Peng, F.; Yu, H.; Wang, H.J.; Zheng, W.X. Aerobic liquid-phase oxidation of ethylbenzene to acetophenone catalyzed by carbon nanotubes. ChemCatChem 2013, 5, 1578–1586. [Google Scholar] [CrossRef]
- Chen, W.; Fan, Z.; Pan, X.; Bao, X. Effect of confinement in carbon nanotubes on the activity of fischer-tropsch iron catalyst. J. Am. Chem. Soc. 2008, 130, 9414–9419. [Google Scholar] [CrossRef]
- Su, D.S.; Bao, X.; Chen, W.; Pan, X.; Willinger, M.-G. Facile autoreduction of iron oxide/carbon nanotube encapsulates. J. Am. Chem. Soc. 2006, 128, 3136–3137. [Google Scholar]
- Chen, W.; Pan, X.; Bao, X. Tuning of redox properties of iron and iron oxides via encapsulation within carbon nanotubes. J. Am. Chem. Soc. 2007, 129, 7421–7426. [Google Scholar] [CrossRef]
- An, G.M.; Yu, P.; Xiao, M.J.; Liu, Z.M.; Miao, Z.J.; Ding, K.L.; Mao, L.Q. Low-temperature synthesis of Mn3O4 nanoparticles loaded on multi-walled carbon nanotubes and their application in electrochemical capacitors. Nanotechnology 2008, 19, 275709. [Google Scholar] [CrossRef]
- Ma, S.B.; Ahn, K.Y.; Lee, E.S.; Oh, K.H.; Kim, K.B. Synthesis and characterization of manganese dioxide spontaneously coated on carbon nanotubes. Carbon 2007, 45, 375–382. [Google Scholar] [CrossRef]
- Garcês Gonçalves, P.R.; De Abreu, H.A.; Duarte, H.A. Stability, Structural, and Electronic Properties of Hausmannite (Mn3O4) Surfaces and Their Interaction with Water. J. Phys. Chem. C 2018, 122, 20841–20849. [Google Scholar] [CrossRef]
- Lv, J.; Shen, Y.; Peng, L.; Guo, X.; Ding, W. Exclusively selective oxidation of toluene to benzaldehyde on ceria nanocubes by molecular oxygen. Chem. Commun. 2010, 46, 5909–5911. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Wang, G. Preparation of Mn3O4 nanowires by calcining the precursor powders synthesized in a novel inverse microemulsion. Appl. Phys. A Mater. 2003, 76, 1117–1120. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mat. Sci. Eng. B Adv. 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.Q.; Chen, Z.; Zhou, H.H.; Xu, Q.Y.; Sun, P.P.; Zhou, J.; Xia, L.; Sun, Y.M.; Lu, Y.F. Preparation of pompon-like MnO/carbon nanotube composite microspheres as anodes for lithium ion batteries. Electrochim. Acta 2015, 180, 858–865. [Google Scholar] [CrossRef]
- Peng, Y.Q.; Du, C.C.; Lu, S.Y.; Zhao, R.X. Catalytic oxidation of chlorobenzene over V2O5/TiO2-CNTs Nano Composites. In Proceedings of the 2016 International Conference on Environmental Science and Engineering (ESE 2016), Guilin, China, 15–17 April 2016; pp. 499–505. [Google Scholar]
- Yang, D.Q.; Hennequin, B.; Sacher, E. XPS demonstration of p-p interaction between benzyl mercaptan and multiwalled carbon nanotubes and their use in the adhesion of Pt nanoparticles. Chem. Mater. 2006, 18, 5033–5038. [Google Scholar] [CrossRef]
- Brenner, M.W.; Boddu, V.M.; Kumar, A. Control of morphology for energy dissipation in carbon nanotube forests. Appl. Phys. A Mater. Sci. Process. 2014, 117, 1849–1857. [Google Scholar] [CrossRef]
- Fan, S.; Pan, Y.; Wang, H.; Lu, B.; Zhao, J.; Cai, Q. Effect of acidic and red-ox sites over modified ZSM-5 surface on selectivity in oxidation of toluene. Mol. Catal. 2017, 442, 20–26. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Q.; Zeng, A. Controlled synthesis of CuxMn3.66-xMo3O12 with the citrate sol-gel method for the selective liquid-phase toluene oxidation to benzaldehyde by air. React. Kinet. Mech. Catal. 2018, 125, 707–731. [Google Scholar] [CrossRef]
- Hoorn, J.A.A.; Van Soolingen, J.; Versteeg, G.F. Modelling toluene oxidation—Incorporation of mass transfer phenomena. Chem. Eng. Res. Des. 2005, 83, 187–195. [Google Scholar] [CrossRef][Green Version]
- Takenaka, S.; Mikami, D.; Tanabe, E.; Matsune, H.; Kishida, M. Modification of carbon nanotube surfaces with precious metal and transition metal oxide nanoparticles using thin silica layers. Appl. Catal. A Gen. 2015, 492, 60–67. [Google Scholar] [CrossRef]



| Catalyst Composites a | Notation | CNTs b | BET Surface Area (m2/g) | Pore Volume (cm3/g) c | Pore Diameter (nm) d |
|---|---|---|---|---|---|
| CNTs | CNTs | - | 341 | 1.2321 | 14.5 |
| Mn3O4/CNTs(1:0.8) | Mn3O4/CNTs-1 | 0.4 | 251 | 0.7842 | 12.0 |
| Mn3O4/CNTs(1:1.2) | Mn3O4/CNTs-2 | 0.6 | 268 | 0.8136 | 12.1 |
| Mn3O4/CNTs(1:1.6) | Mn3O4/CNTs-3 | 0.8 | 269 | 0.8421 | 12.5 |
| Mn3O4/CNTs(1:2.1) | Mn3O4/CNTs-4 | 1 | 272 | 0.9031 | 13.2 |
| Mn3O4/CNTs-run | Mn3O4/CNTs-3-run4 | - | 270 | 0.8532 | 12.6 |
| Catalysis | Conversion/% | Selectivity/% | ||
|---|---|---|---|---|
| Benzyl Alcohol | Benzaldehyde | Other Products | ||
| Mn3O4 | 3.54 | 40.47 | 58.51 | 1.02 |
| Mn3O4 a | 1.67 | 38.57 | 61.19 | 0.24 |
| CNTs | 0.65 | 41.56 | 58.44 | 0 |
| Mn3O4/CNTs-1 | 7.00 | 58.14 | 40.59 | 1.27 |
| Mn3O4/CNTs-2 | 15.10 | 53.06 | 43.58 | 3.36 |
| Mn3O4CNTs-3 | 24.63 | 43.51 | 46.98 | 9.51 |
| Mn3O4/CNTs-4 | 18.56 | 50.52 | 44.03 | 5.45 |
| Run | Conversion/% | Selectivity/% | ||
|---|---|---|---|---|
| Benzyl Alcohol | Benzaldehyde | Other Products | ||
| 1 | 24.63 | 43.51 | 46.98 | 9.51 |
| 2 | 23.76 | 44.53 | 46.72 | 8.75 |
| 3 | 23.53 | 42.56 | 48.41 | 9.03 |
| 4 | 22.97 | 42.65 | 48.39 | 8.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Zeng, A. Selective Liquid-Phase Oxidation of Toluene with Molecular Oxygen Catalyzed by Mn3O4 Nanoparticles Immobilized on CNTs under Solvent-Free Conditions. Catalysts 2020, 10, 623. https://doi.org/10.3390/catal10060623
Feng Y, Zeng A. Selective Liquid-Phase Oxidation of Toluene with Molecular Oxygen Catalyzed by Mn3O4 Nanoparticles Immobilized on CNTs under Solvent-Free Conditions. Catalysts. 2020; 10(6):623. https://doi.org/10.3390/catal10060623
Chicago/Turabian StyleFeng, Yuwei, and Aiwu Zeng. 2020. "Selective Liquid-Phase Oxidation of Toluene with Molecular Oxygen Catalyzed by Mn3O4 Nanoparticles Immobilized on CNTs under Solvent-Free Conditions" Catalysts 10, no. 6: 623. https://doi.org/10.3390/catal10060623
APA StyleFeng, Y., & Zeng, A. (2020). Selective Liquid-Phase Oxidation of Toluene with Molecular Oxygen Catalyzed by Mn3O4 Nanoparticles Immobilized on CNTs under Solvent-Free Conditions. Catalysts, 10(6), 623. https://doi.org/10.3390/catal10060623




