Developmental Study of Soot-Oxidation Catalysts for Fireplaces: The Effect of Binder and Preparation Techniques on Catalyst Texture and Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effect of the Binder on the Textural Properties of the Catalyst
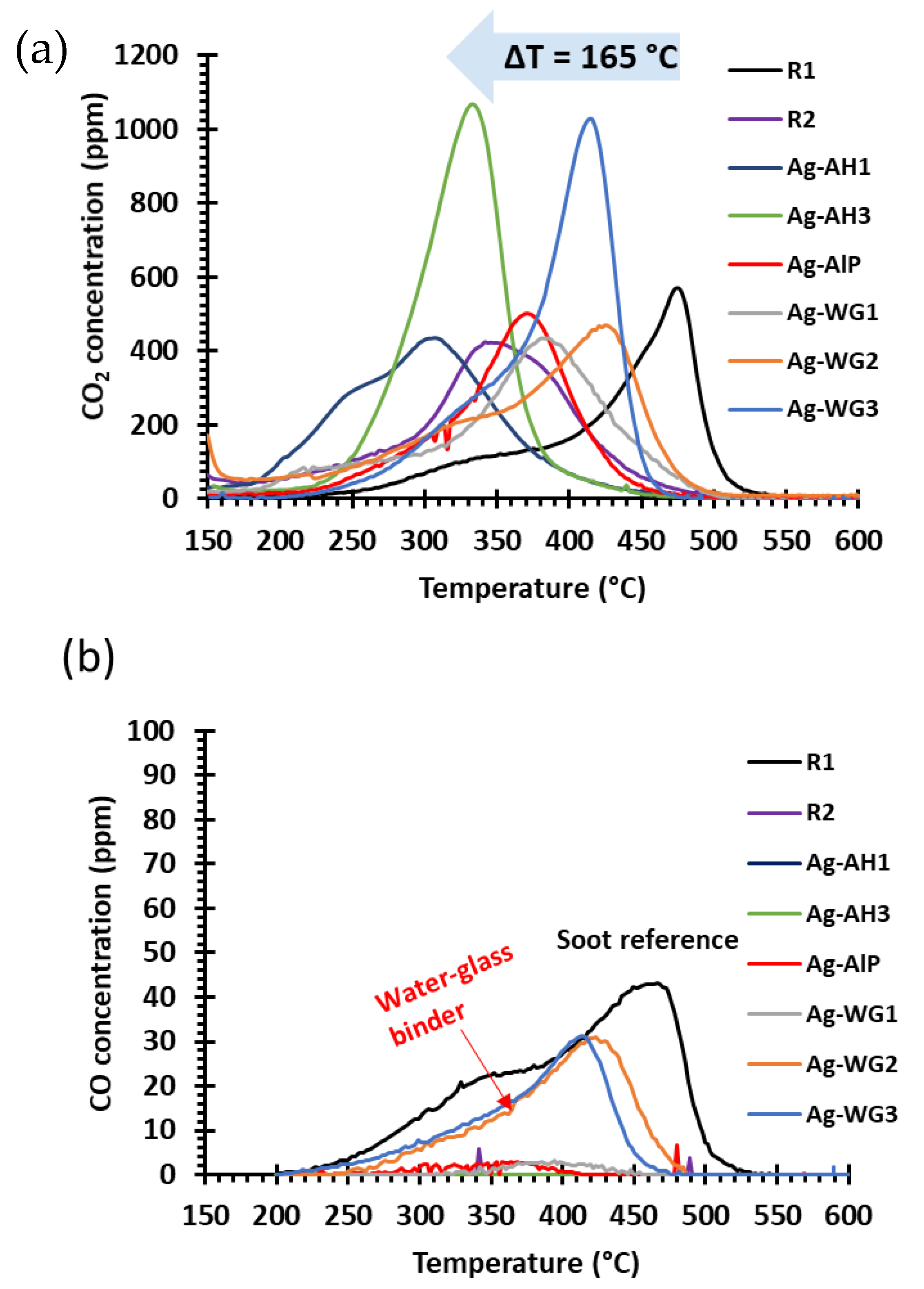
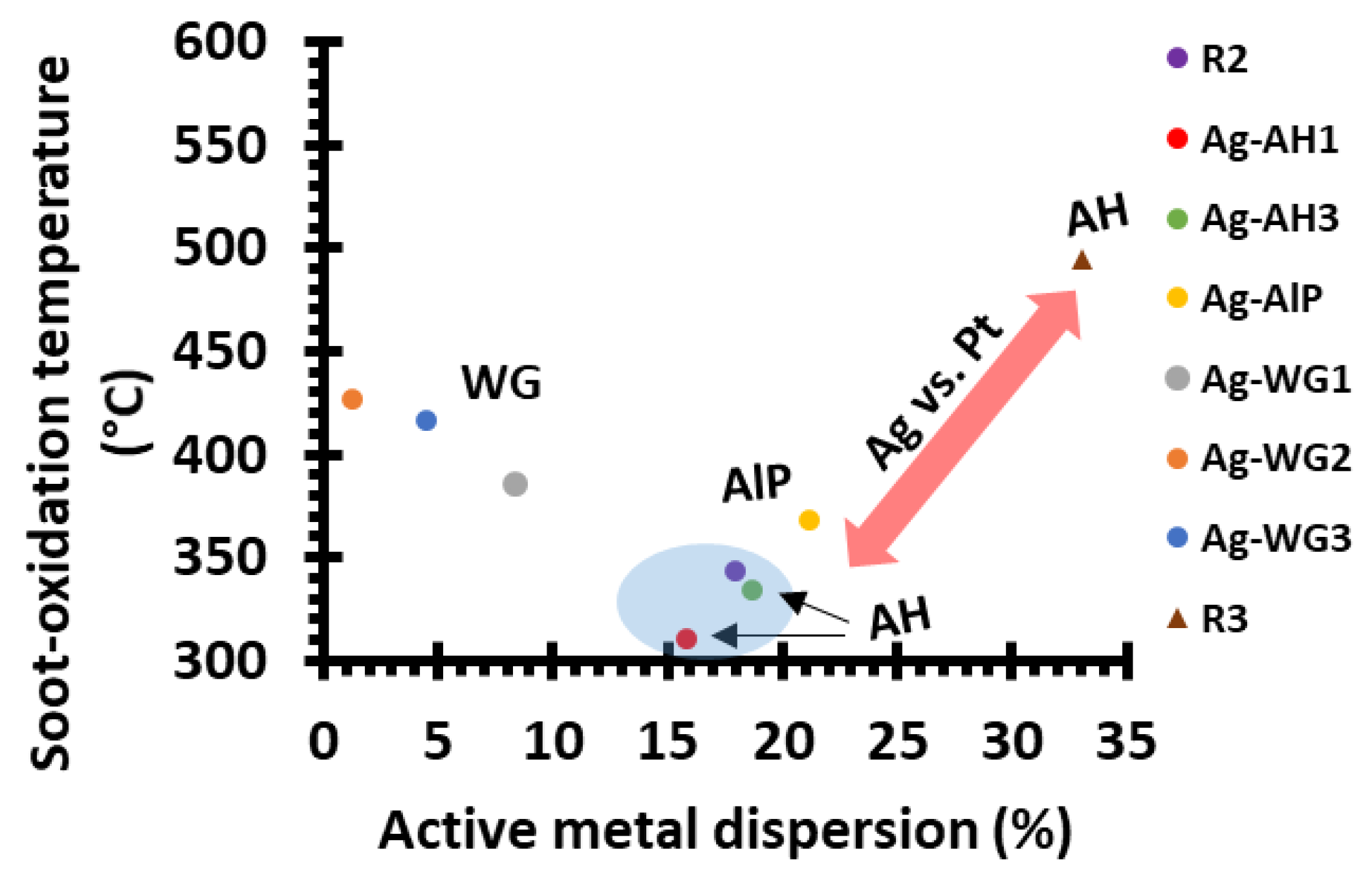
2.2. Soot-Oxidation Activities of the Catalysts
2.2.1. Effect of the Binder on Activity
2.2.2. Steady-State Soot-Oxidation Activity
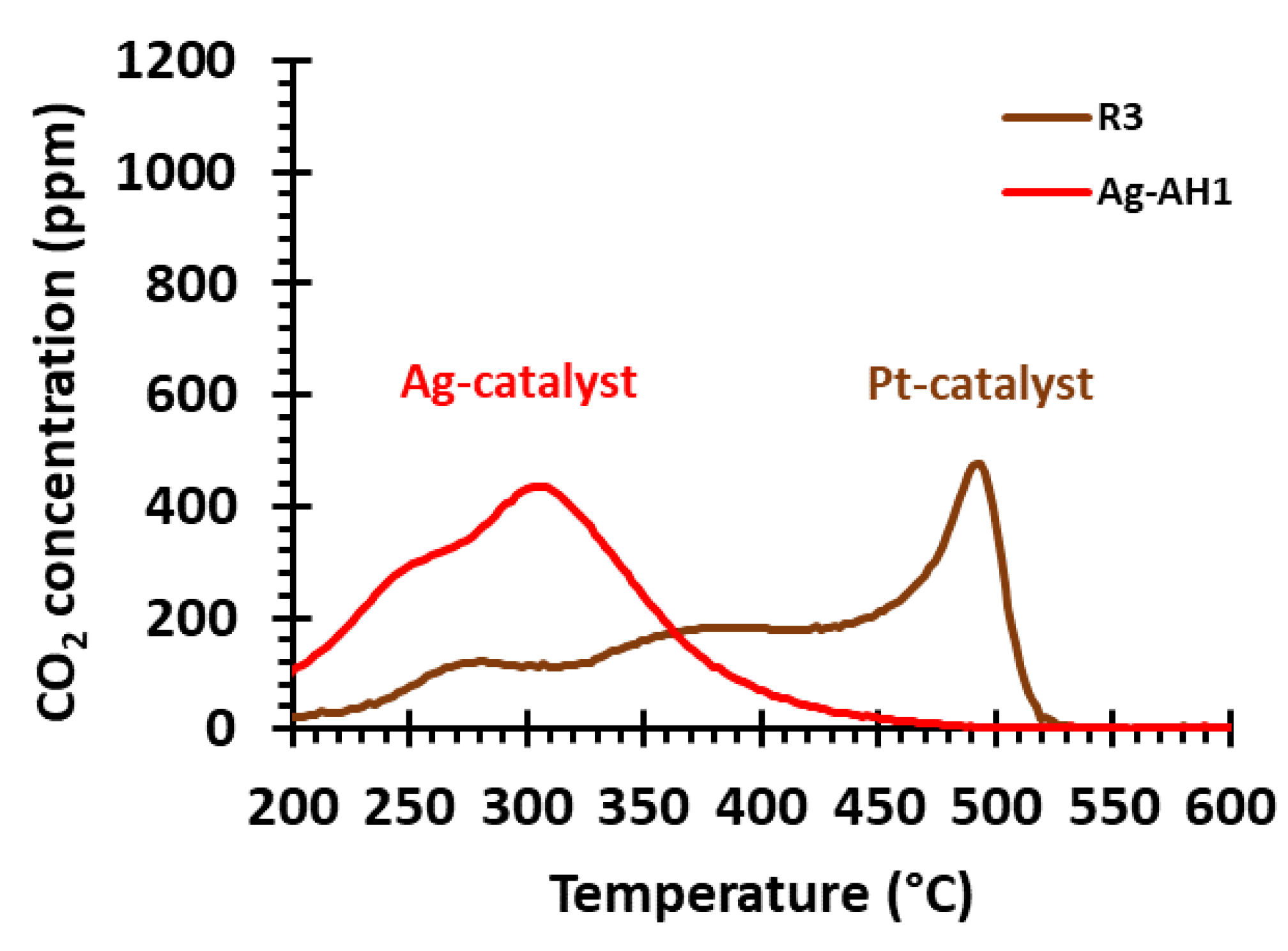
2.2.3. Comparing the Ag and Pt Soot-Oxidation Catalysts
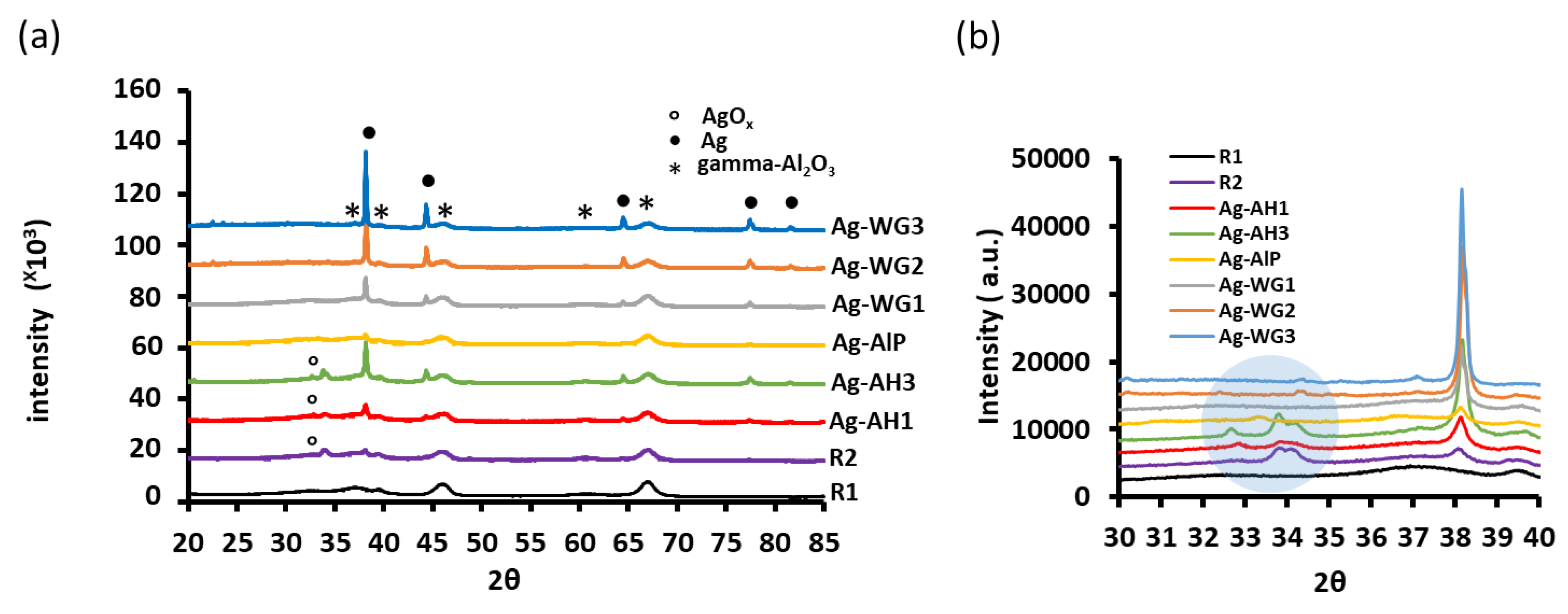
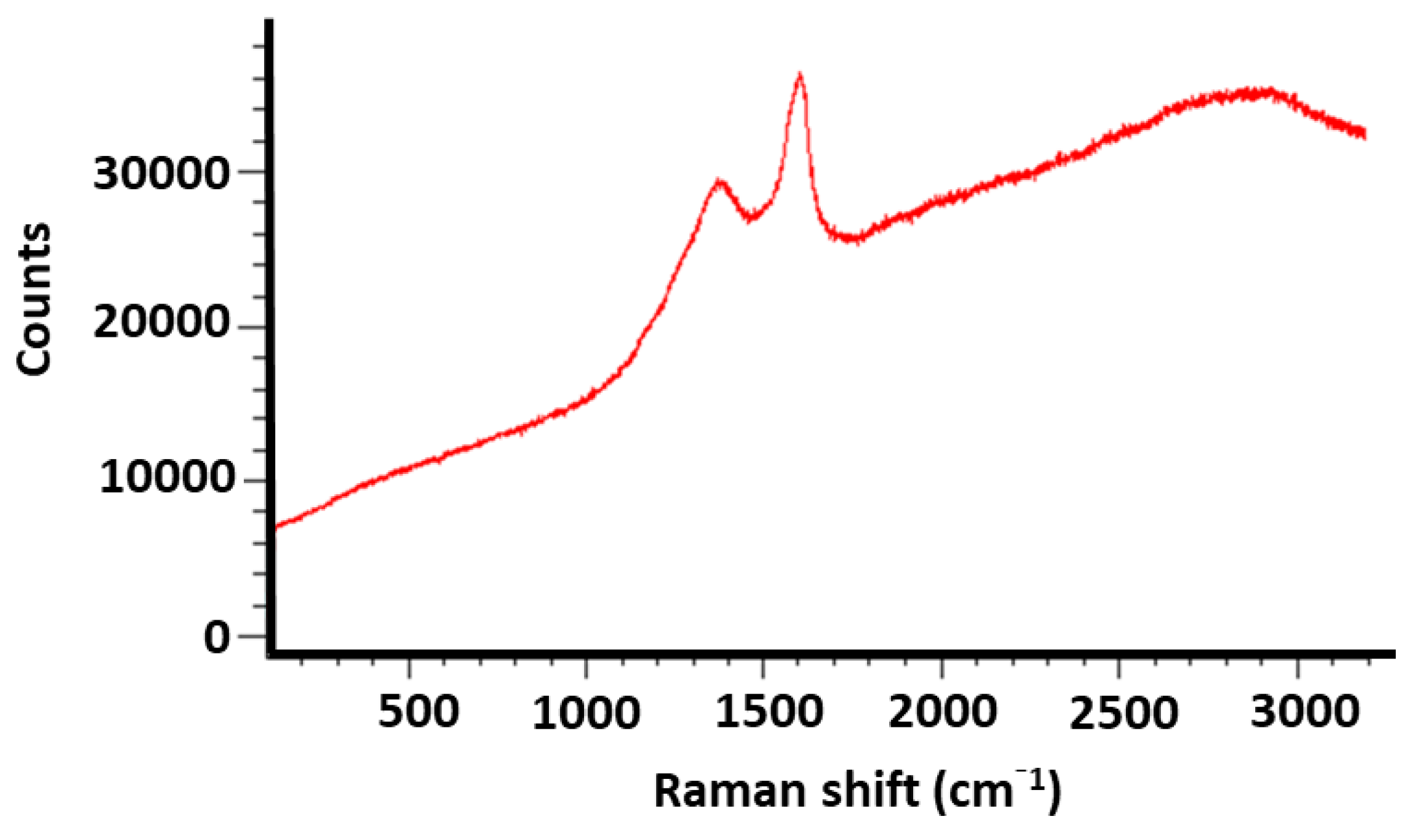
2.3. Powder X-ray Diffraction Patterns of the Catalysts
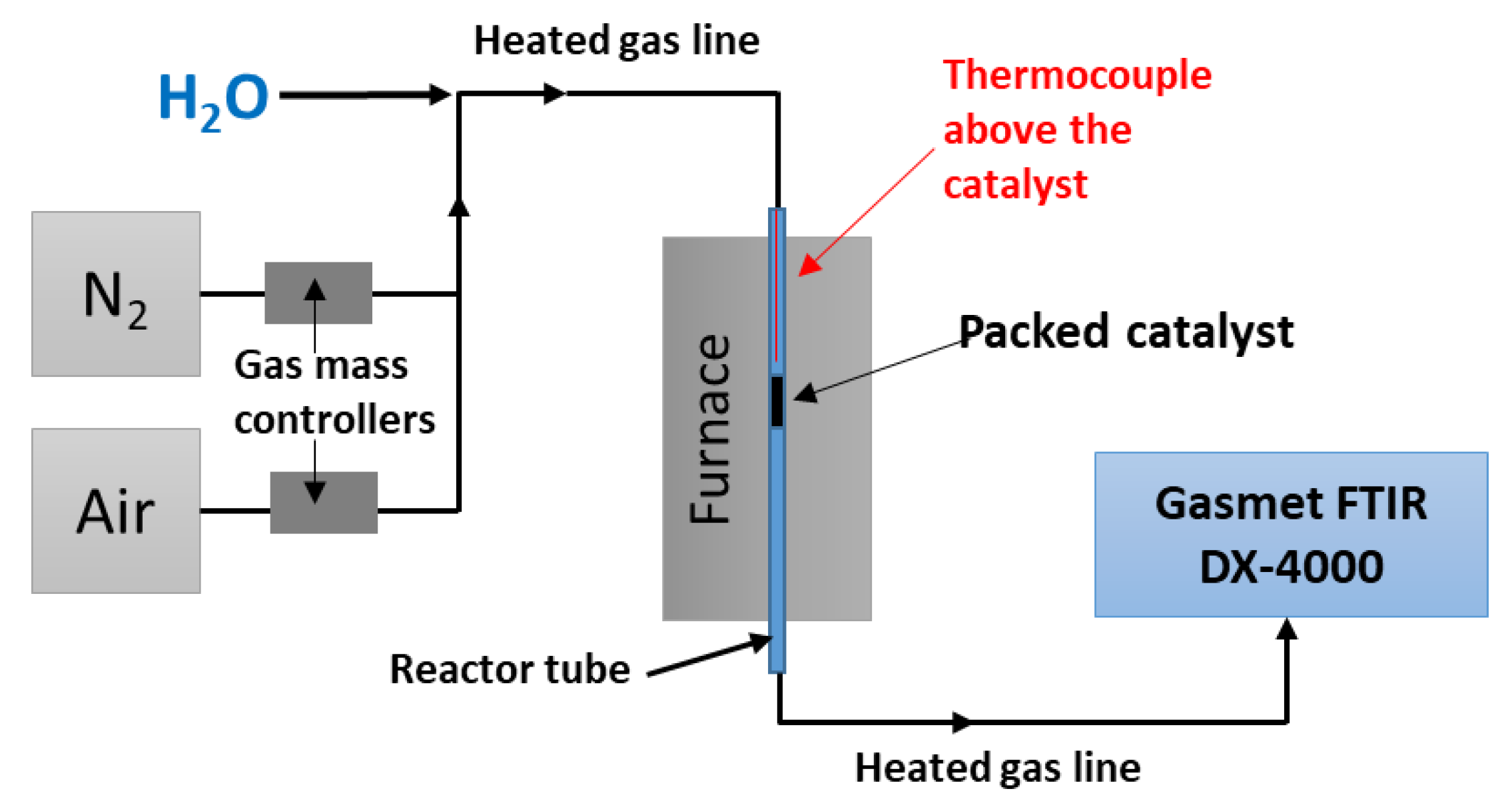
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Puri, I.K. Environmental Implications of Combustion Processes, 2nd ed.; CRC Press Inc.: Boca Raton, FL, USA, 1993. [Google Scholar]
- Richter, H.; Howard, J. Formation of polycyclic aromatic hydrocarbons and their growth to soot—A review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Pope, C.A.; Dockery, D.W. Health Effects of Fine Particulate Air Pollution: Lines that Connect. J. Air Waste Manage. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Kocbach Bølling, A.; Pagels, J.; Yttri, K.; Barregard, L.; Sallsten, G.; Schwarze, P.E.; Boman, C. Health effects of residential wood smoke particles: The importance of combustion conditions and physicochemical particle properties. Part. Fibre Toxicol. 2009, 6, 29. [Google Scholar] [CrossRef]
- European Environment Agency. Air Quality in Europe—2017; Report No 13/2017; European Environment Agency: Copenhagen, Denmark, 2017. [Google Scholar] [CrossRef]
- Fuller, G.W.; Sciare, J.; Lutz, M.; Moukhtar, S.; Wagener, S. New Directions: Time to tackle urban wood burning? Atmos. Environ. 2013, 68, 295–296. [Google Scholar] [CrossRef]
- Salthammer, T.; Schripp, T.; Wientzek, S.; Wensing, M. Impact of operating wood-burning fireplace ovens on indoor air quality. Chemosphere 2014, 103, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Semmens, E.O.; Noonan, C.W.; Allen, R.W.; Weiler, E.C.; Ward, T.J. Indoor particulate matter in rural, wood stove heated homes. Environ. Res. 2015, 138, 93–100. [Google Scholar] [CrossRef]
- Omidvarborna, H.; Kumar, A.; Kim, D.-S. Recent studies on soot modeling for diesel combustion. Renew. Sustain. Energy Rev. 2015, 48, 635–647. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Makkee, M.; Moulijn, J.A. Diesel particulate emission control. Fuel Process. Technol. 1996, 47, 1–69. [Google Scholar] [CrossRef]
- Russell, A.; Epling, W.S. Diesel oxidation catalysts. Catal. Rev. Sci. Eng. 2011, 53, 337–423. [Google Scholar] [CrossRef]
- Heck, R.; Farrauto, R.; Gulati, S. Catalytic Air Pollution Control Commercial Technology, 3rd ed.; A John Wiley & Sons Inc. Publication: Hoboken, NJ, USA, 2009; p. 257. [Google Scholar]
- Heck, R.; Farrauto, R.; Gulati, S. Catalytic Air Pollution Control Commercial Technology, 3rd ed.; A John Wiley & Sons Inc. Publication: Hoboken, NJ, USA, 2009; p. 392. [Google Scholar]
- Ferrandon, M.; Berg, M.; Björnbom, E. Thermal stability of metal-supported catalysts for reduction of cold-start emissions in a wood-fired domestic boiler. Catal. Today 1999, 53, 647–659. [Google Scholar] [CrossRef]
- Pieber, S.M.; Kambolis, A.; Ferri, D.; Bhattu, D.; Bruns, E.A.; Elsener, M.; Kröcher, O.; Prevot, A.S.H.H.; Baltensperger, U.; Emily, A.; et al. Remediation and Control Technologies Mitigation of secondary organic aerosol formation of log wood burning emissions by catalytic removal of aromatic hydrocarbons. Environ. Sci. Technol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Carnö, J.; Berg, M.; Järås, S. Catalytic abatement of emissions from small-scale combustion of wood: A comparison of the catalytic effect in model and real flue gases. Fuel 1996, 75, 959–965. [Google Scholar] [CrossRef]
- Ozil, F.; Tschamber, V.; Haas, F.; Trouvé, G. Efficiency of catalytic processes for the reduction of CO and VOC emissions from wood combustion in domestic fireplaces. Fuel Process. Technol. 2009, 90, 1053–1061. [Google Scholar] [CrossRef]
- Kaivosoja, T.; Virén, A.; Tissari, J.; Ruuskanen, J.; Tarhanen, J.; Sippula, O.; Jokiniemi, J. Effects of a catalytic converter on PCDD/F, chlorophenol and PAH emissions in residential wood combustion. Chemosphere 2012, 88, 278–285. [Google Scholar] [CrossRef]
- Corro, G.; Pal, U.; Ayala, E.; Vidal, E. Diesel soot oxidation over silver-loaded SiO2 catalysts. Catal. Today 2013, 212, 63–69. [Google Scholar] [CrossRef]
- Shimizu, K.; Kawachi, H.; Satsuma, A. Study of active sites and mechanism for soot oxidation by silver-loaded ceria catalyst. Appl. Catal. B Environ. 2010, 96, 169–175. [Google Scholar] [CrossRef]
- Nossova, L.; Caravaggio, G.; Couillard, M.; Ntais, S. Effect of preparation method on the performance of silver-zirconia catalysts for soot oxidation in diesel engine exhaust. Appl. Catal. B Environ. 2018, 225, 538–549. [Google Scholar] [CrossRef]
- Aneggi, E.; Llorca, J.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Soot combustion over silver-supported catalysts. Appl. Catal. B Environ. 2009, 91, 489–498. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kayama, T.; Dong, F.; Shinjoh, H. A mechanistic study on soot oxidation over CeO2–Ag catalyst with ‘rice-ball’ morphology. J. Catal. 2011, 282, 289–298. [Google Scholar] [CrossRef]
- Castro, A.; Calvo, A.I.; Blanco-Alegre, C.; Oduber, F.; Alves, C.; Coz, E.; Amato, F.; Querol, X.; Fraile, R. Impact of the wood combustion in an open fireplace on the air quality of a living room: Estimation of the respirable fraction. Sci. Total Environ. 2018, 628–629, 169–176. [Google Scholar] [CrossRef]
- Shromova, O.A.; Kinnunen, N.M.; Pakkanen, T.A.; Suvanto, M. Promotion effect of water in catalytic fireplace soot oxidation over silver and platinum. RSC Adv. 2017, 7, 46051–46059. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Ran, R. Ceria-based catalysts for soot oxidation: A review. J. Rare Earths 2015, 33, 567–590. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Pérez-Cadenas, A.; Moreno-Castilla, C.; Pérez-Cadenas, A.F. Carbon-Based Honeycomb Monoliths for Environmental Gas-Phase Applications. Materials 2010, 3, 1203–1227. [Google Scholar] [CrossRef]
- Gatica, J.M.; Rodríguez-Izquierdo, J.M.; Sánchez, D.; Ania, C.; Parra, J.B.; Vidai, H. Extension of preparation methods employed with ceramic materials to carbon honeycomb monoliths. Carbon 2004, 42, 3251–3254. [Google Scholar] [CrossRef]
- Ridha, F.N.; Manovic, V.; Macchi, A.; Anthony, E.J. The effect of SO2 on CO2 capture by CaO-based pellets prepared with a kaolin derived Al(OH)3 binder. Appl. Energy 2012, 92, 415–420. [Google Scholar] [CrossRef]
- Medri, V.; Fabbri, S.; Ruffini, A.; Dedecek, J.; Vaccari, A. SiC-based refractory paints prepared with alkali aluminosilicate binders. J. Eur. Ceram. Soc. 2011, 31, 2155–2165. [Google Scholar] [CrossRef]
- Kim, E.-H.; Lee, J.-H.; Jung, Y.-G.; Jang, J.-C.; Paik, U. Control of H2O generated during the CO2 hardening process in a casting mold. Ceram. Int. 2013, 39, 3993–3998. [Google Scholar] [CrossRef]
- Luz, A.P.; Gomes, D.T.; Pandolfelli, V.C. High-alumina phosphate-bonded refractory castables: Al(OH)3 sources and their effects. Ceram. Int. 2015, 41, 9041–9050. [Google Scholar] [CrossRef]
- Vippola, M.; Keränen, J.; Zou, X.; Hovmöller, S.; Lepistö, T.; Mäntylä, T. Structural Characterization of Aluminum Phosphate Binder. J. Am. Ceram. Soc. 2004, 83, 1834–1836. [Google Scholar] [CrossRef]
- Chung, D.D.L.L. Review: Acid aluminum phosphate for the binding and coating of materials. J. Mater. Sci. 2003, 38, 2785–2791. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Richardson, J.T. Principles of Catalyst Development, 1st ed.; Plenum Press: New York, NY, USA, 1989; p. 28. [Google Scholar]
- Hagen, J. Industrial Catalysis a Practical Approach, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; p. 179. [Google Scholar]
- Richardson, J.T. Principles of Catalyst Development, 1st ed.; Plenum Press: New York, NY, USA, 1989; p. 162. [Google Scholar]
- Gardini, D.; Christensen, J.M.; Damsgaard, C.D.; Jensen, A.D.; Wagner, J.B. Visualizing the mobility of silver during catalytic soot oxidation. Appl. Catal. B Environ. 2016, 183, 28–36. [Google Scholar] [CrossRef]
- Souza, A.D.V.; Arruda, C.C.; Fernandes, L.; Antunes, M.L.P.; Kiyohara, P.K.; Salomão, R. Characterization of aluminum hydroxide (Al(OH)3) for use as a porogenic agent in castable ceramics. J. Eur. Ceram. Soc. 2015, 35, 803–812. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Makkee, M.; Moulijn, J.A. Metal oxides as catalysts for the oxidation of soot. Chem. Eng. J. Biochem. Eng. J. 1996, 64, 295–302. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Haneda, M.; Watanabe, T.; Kamiuchi, N.; Ozawa, M. Effect of platinum dispersion on the catalytic activity of Pt/Al2O3 for the oxidation of carbon monoxide and propene. Appl. Catal. B Environ. 2013, 142, 8–14. [Google Scholar] [CrossRef]
- Hoost, T.E.; Kudla, R.J.; Collins, K.M.; Chattha, M.S. Characterization of Ag/γ-Al2O3 catalysts and their lean-NOx properties. Appl. Catal. B Environ. 1997, 13, 59–67. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Makkee, M.; Moulijn, J.A. Catalysts for the oxidation of soot from diesel exhaust gases. I. An exploratory study. Appl. Catal. B Environ. 1996, 8, 57–78. [Google Scholar] [CrossRef]
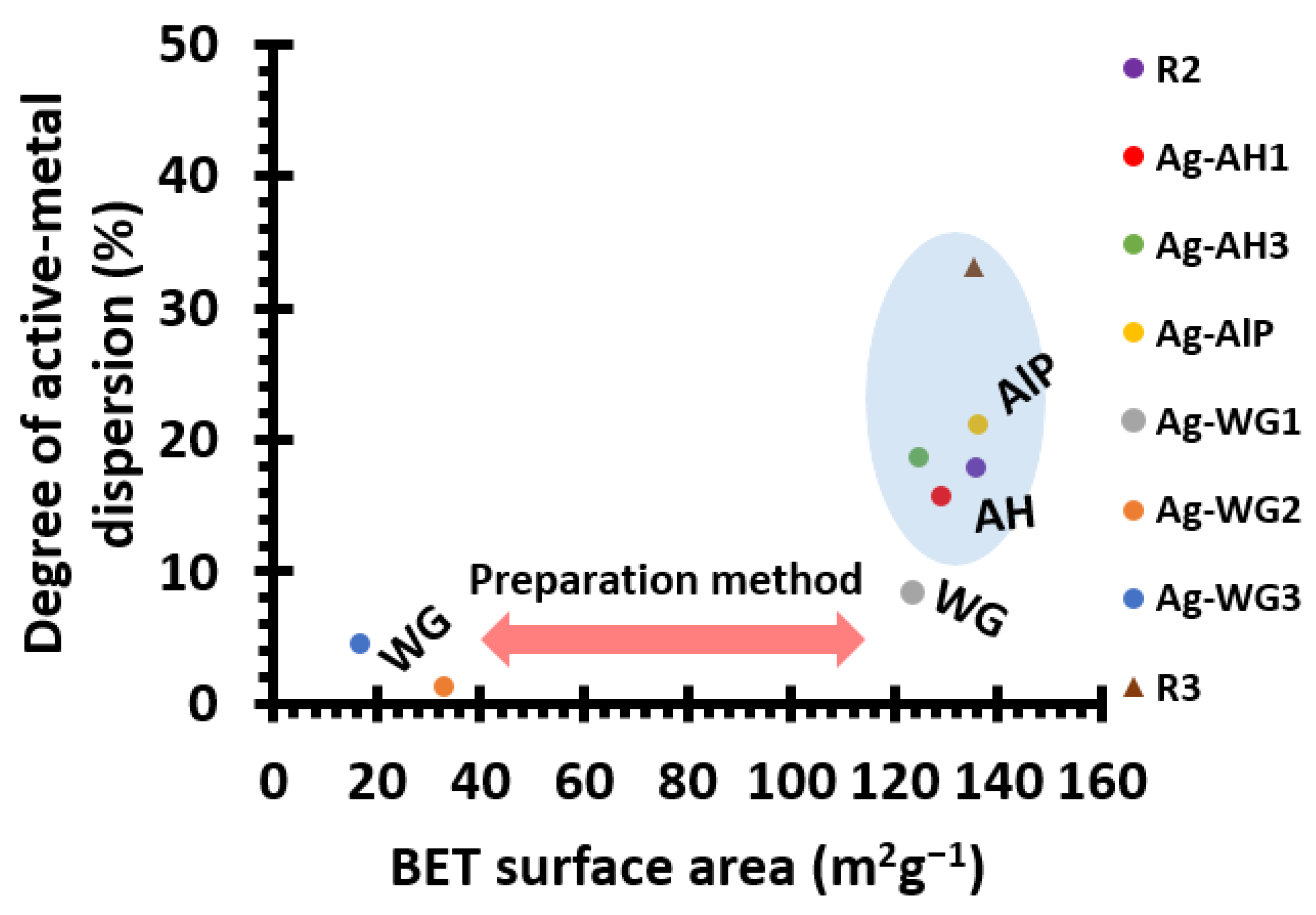
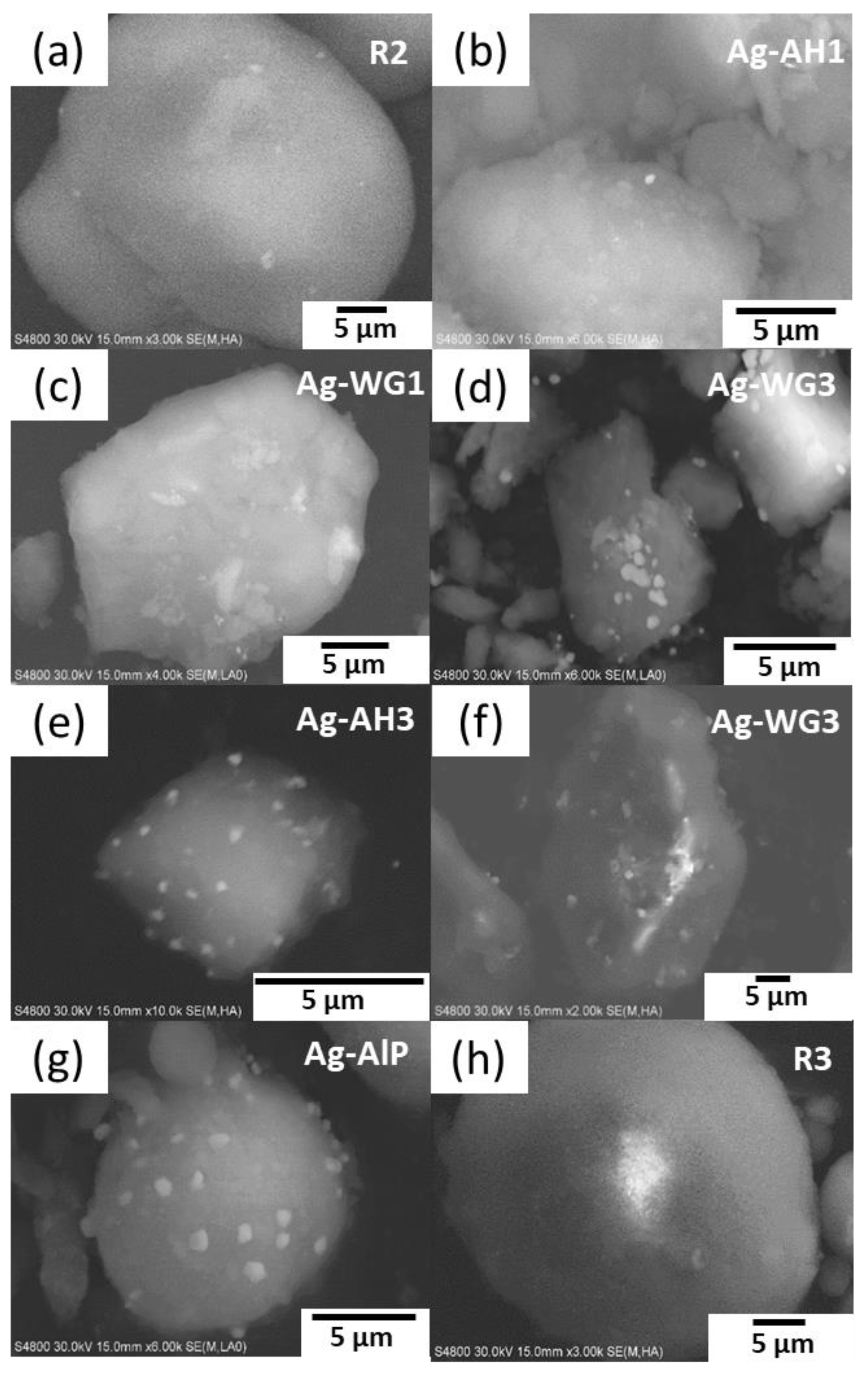
| Catalyst | Support Material | Active Metal | Binder Material | BET Surface Area (m2g−1) | Active-Metal Dispersion (%) |
|---|---|---|---|---|---|
| R1 | La-Al2O3 | - | - | 157.1 | - |
| R2 | La-Al2O3 | Ag | - | 135.6 | 17.9 |
| Ag-AH1 | La-Al2O3 | Ag | Al(OH)3 | 129.0 | 15.8 |
| Ag-AH3 a | La-Al2O3 | Ag | Al(OH)3 | 124.8 | 18.7 |
| Ag-AlP | La-Al2O3 | Ag | Acid aluminium phosphate | 136.2 | 21.2 |
| Ag-WG1 | La-Al2O3 | Ag | Water glass | 123.6 | 8.4 |
| Ag-WG2 b | La-Al2O3 | Ag | Water glass | 32.7 | 1.3 |
| Ag-WG3 a | La-Al2O3 | Ag | Water glass | 16.7 | 4.5 |
| R3 | La-Al2O3 | Pt | Al(OH)3 | 135.4 | 33.1 |
| Temperature (°C) | Initial Carbon Content (%) | Carbon Content After Heating (%) |
|---|---|---|
| 330 | 3.91 | 0.18 |
| 350 | 4.49 | 0.15 |
| 370 | 4.26 | 0.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevalainen, P.; Kinnunen, N.; Suvanto, M. Developmental Study of Soot-Oxidation Catalysts for Fireplaces: The Effect of Binder and Preparation Techniques on Catalyst Texture and Activity. Catalysts 2019, 9, 957. https://doi.org/10.3390/catal9110957
Nevalainen P, Kinnunen N, Suvanto M. Developmental Study of Soot-Oxidation Catalysts for Fireplaces: The Effect of Binder and Preparation Techniques on Catalyst Texture and Activity. Catalysts. 2019; 9(11):957. https://doi.org/10.3390/catal9110957
Chicago/Turabian StyleNevalainen, Pauliina, Niko Kinnunen, and Mika Suvanto. 2019. "Developmental Study of Soot-Oxidation Catalysts for Fireplaces: The Effect of Binder and Preparation Techniques on Catalyst Texture and Activity" Catalysts 9, no. 11: 957. https://doi.org/10.3390/catal9110957
APA StyleNevalainen, P., Kinnunen, N., & Suvanto, M. (2019). Developmental Study of Soot-Oxidation Catalysts for Fireplaces: The Effect of Binder and Preparation Techniques on Catalyst Texture and Activity. Catalysts, 9(11), 957. https://doi.org/10.3390/catal9110957





