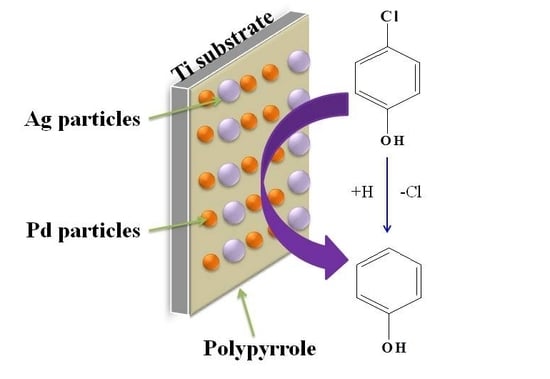
A Polypyrrole-Modified Pd-Ag Bimetallic Electrode for the Electrocatalytic Reduction of 4-Chlorophenol
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Preparation Conditions of Electrodes
2.1.1. Effect of the Composition of the Plating Solutions
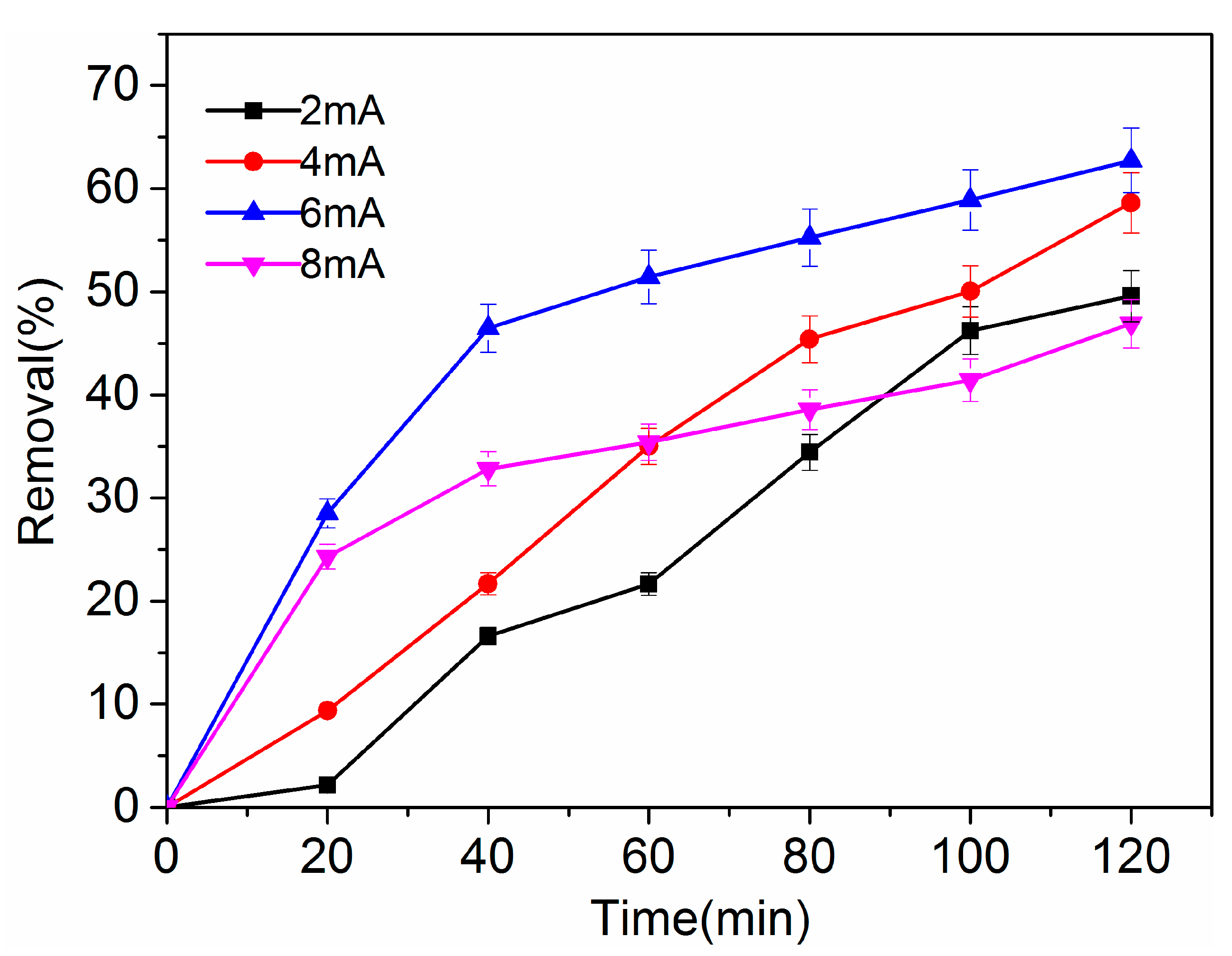
2.1.2. Effect of Reaction Time
2.2. Characterization of Electrodes
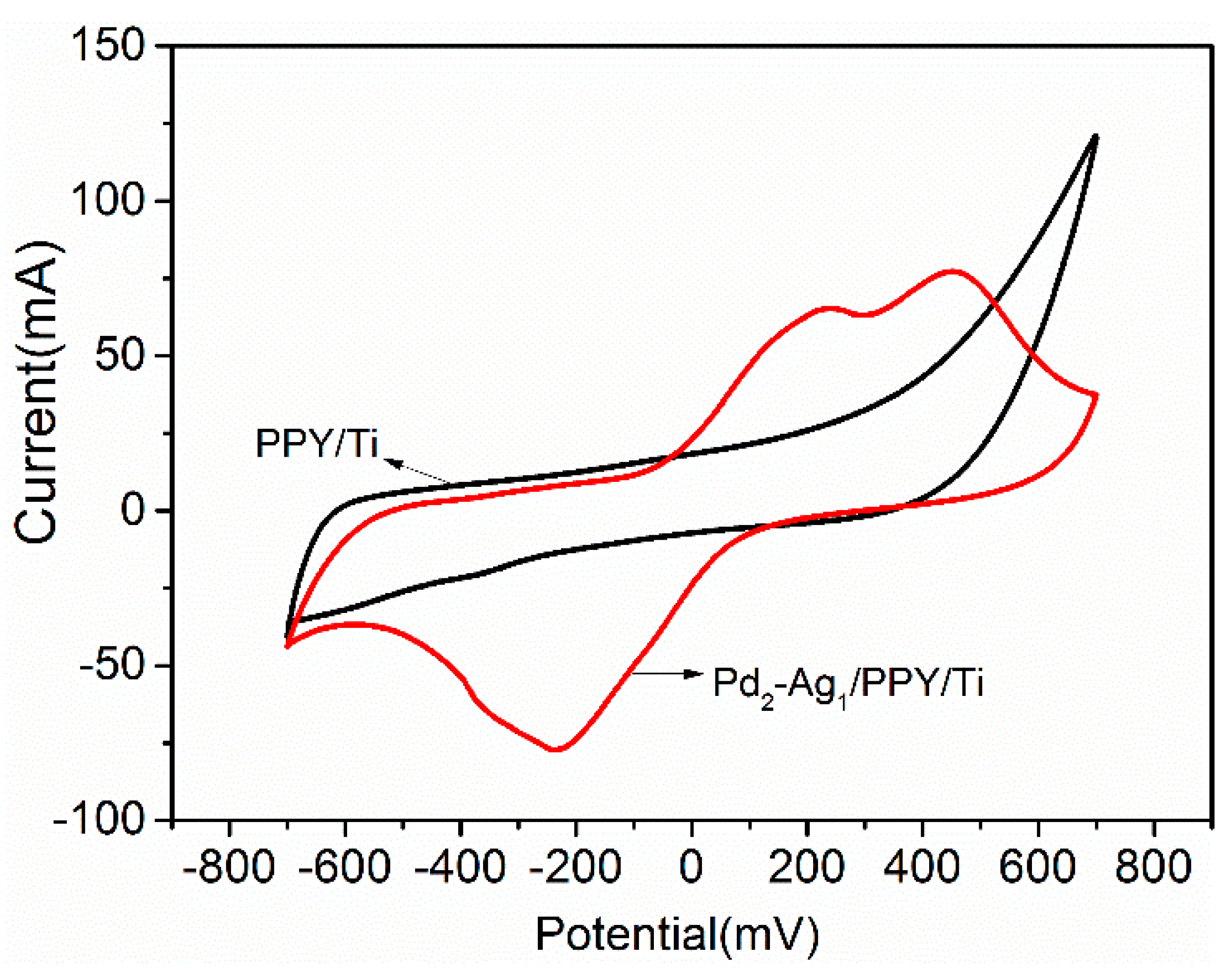
2.2.1. Cyclic Voltammetry Analysis
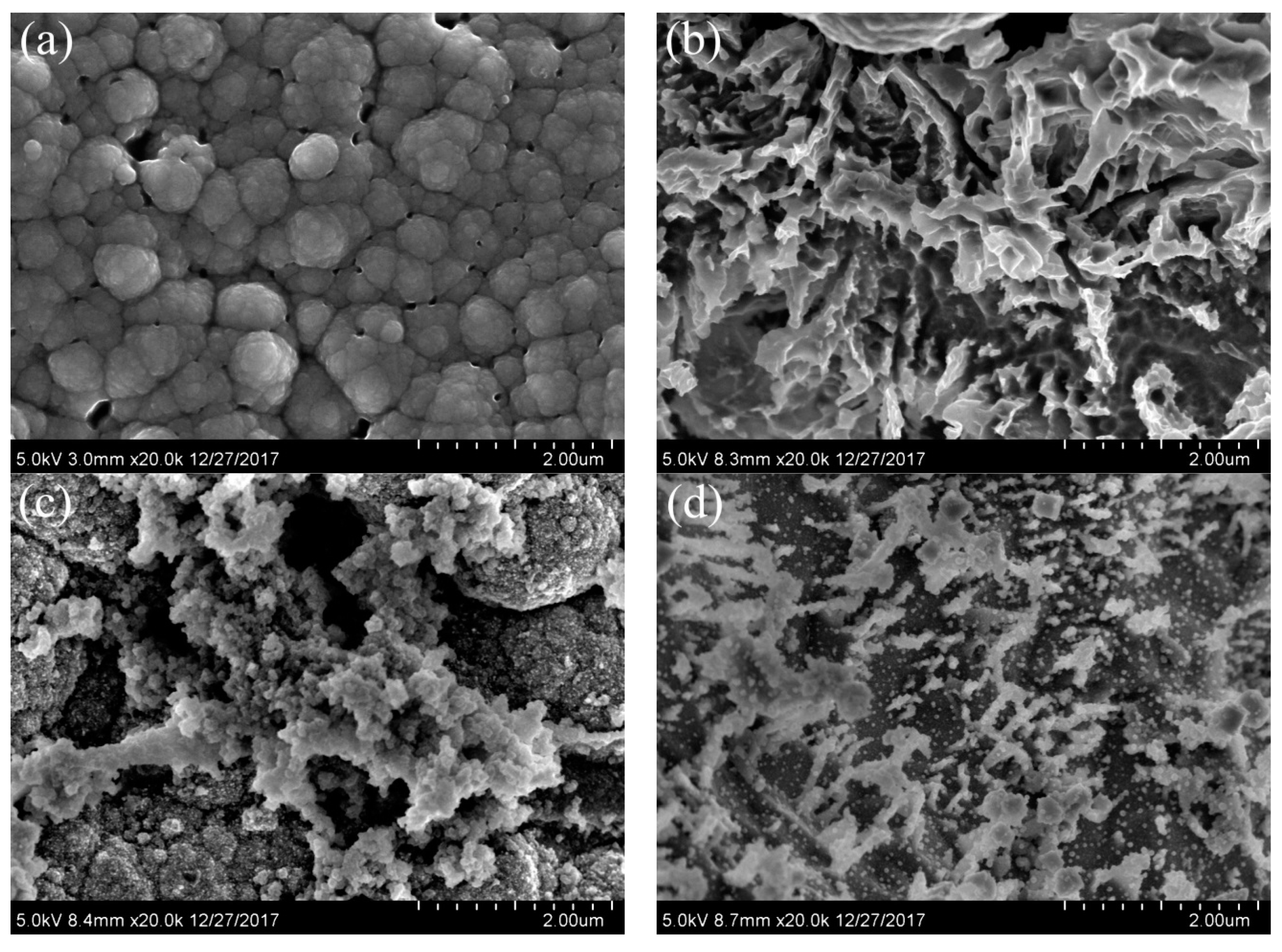
2.2.2. Morphology Analysis
2.2.3. Analysis of Pd Content in the Electrode
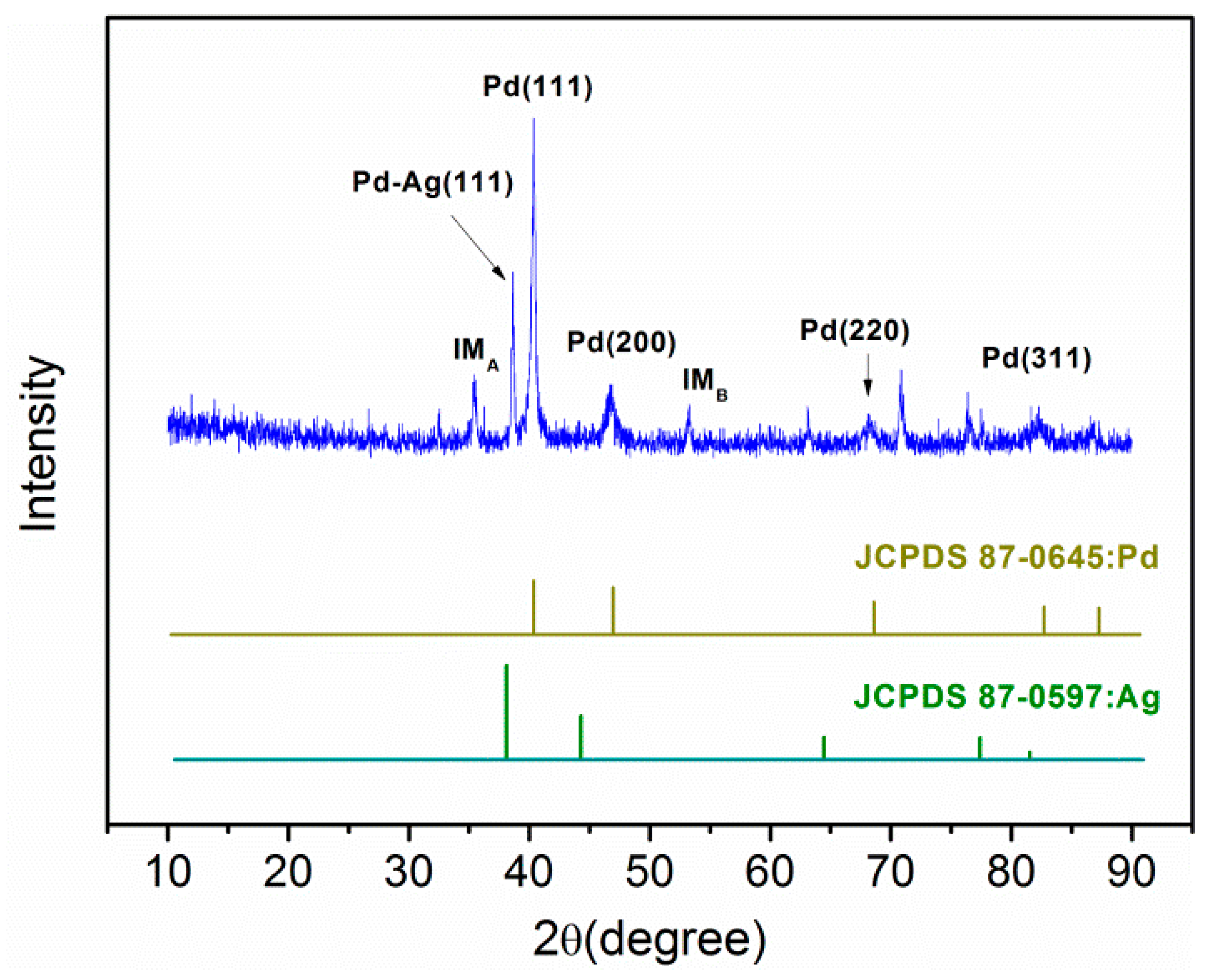
2.2.4. XRD Analysis
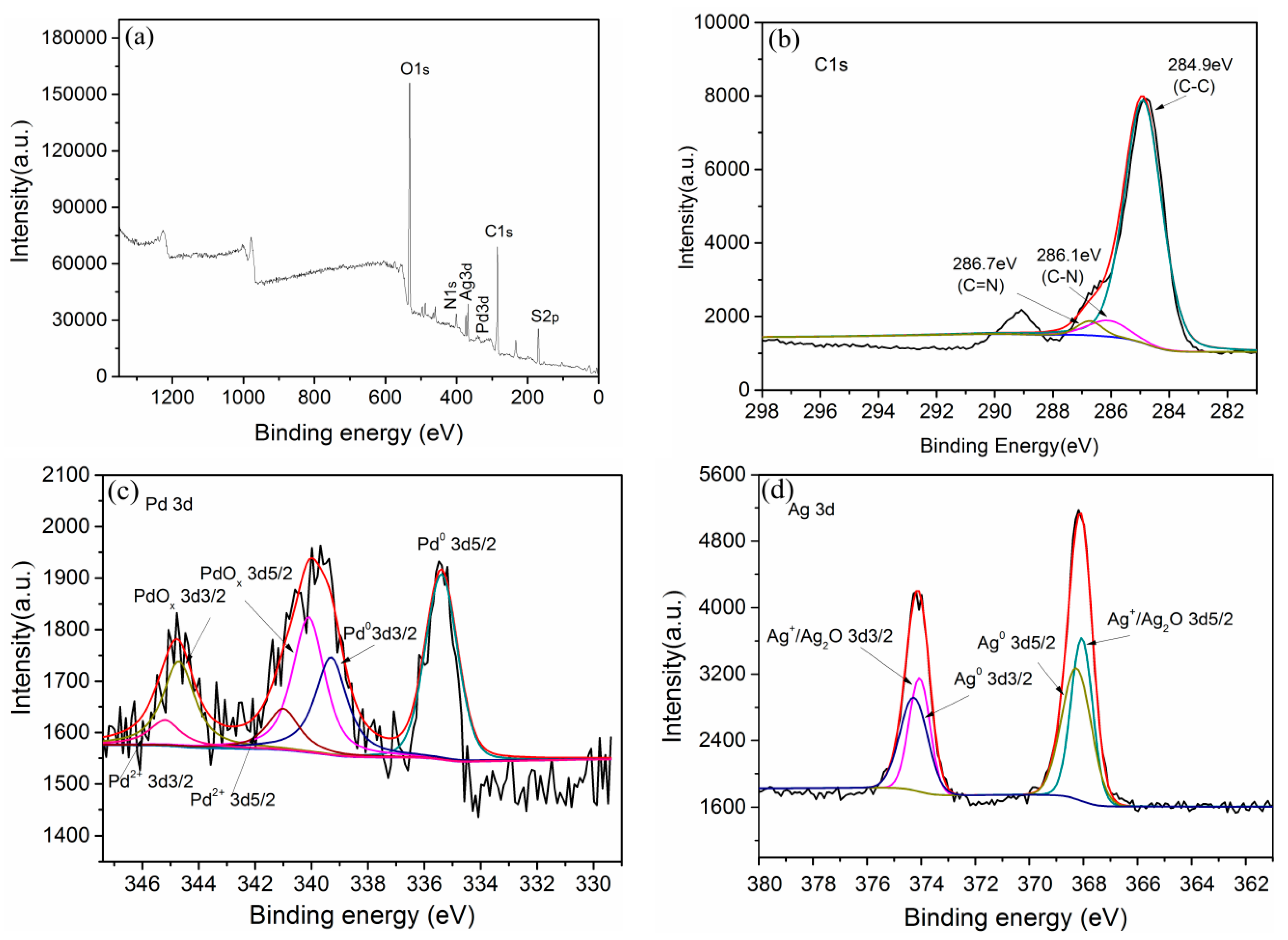
2.2.5. XPS Analysis
2.3. ECH of 4-Chlorophenol
2.3.1. ECH of 4-Chlorophenol
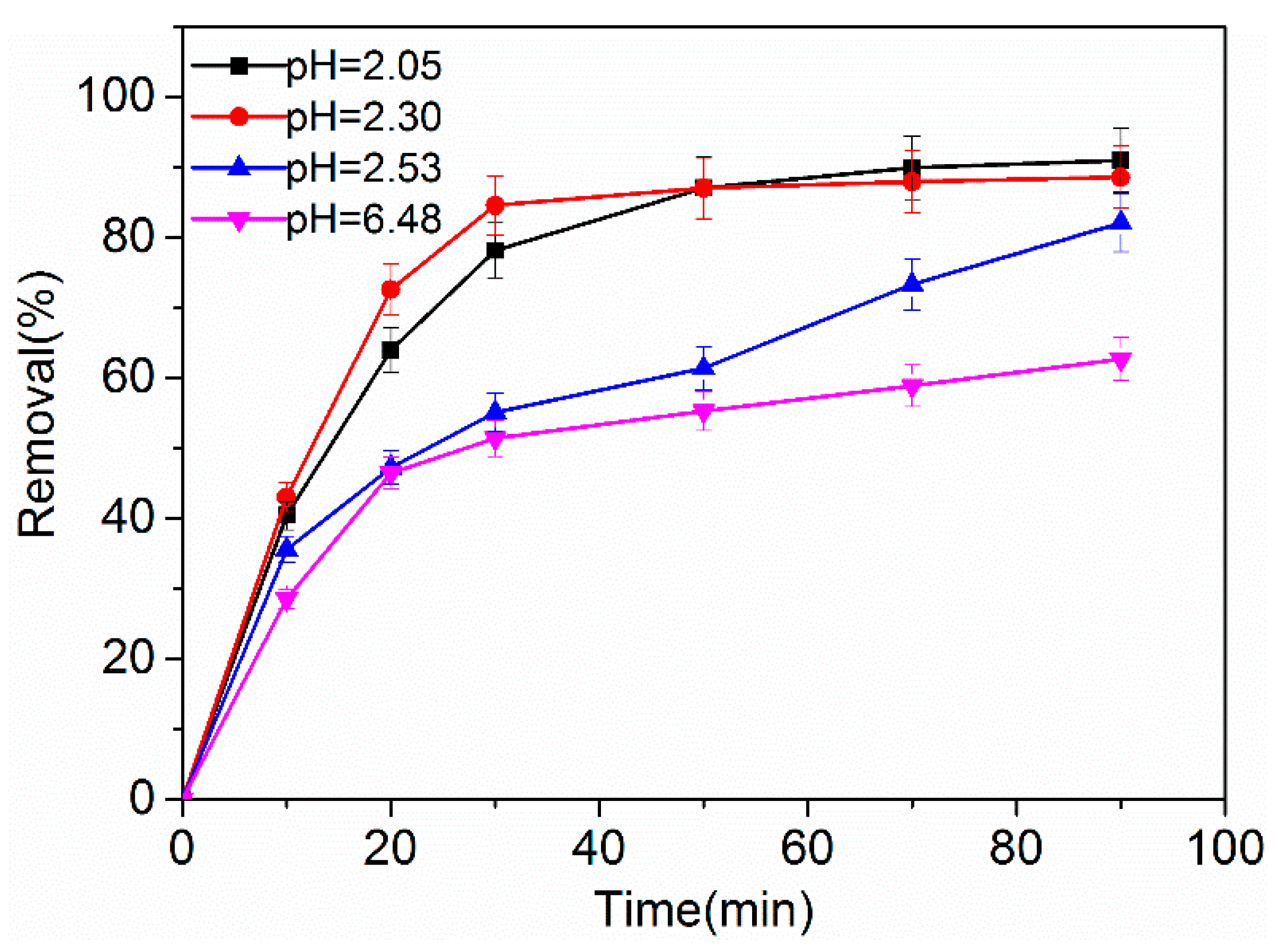
2.3.2. Effect of Initial pH Value
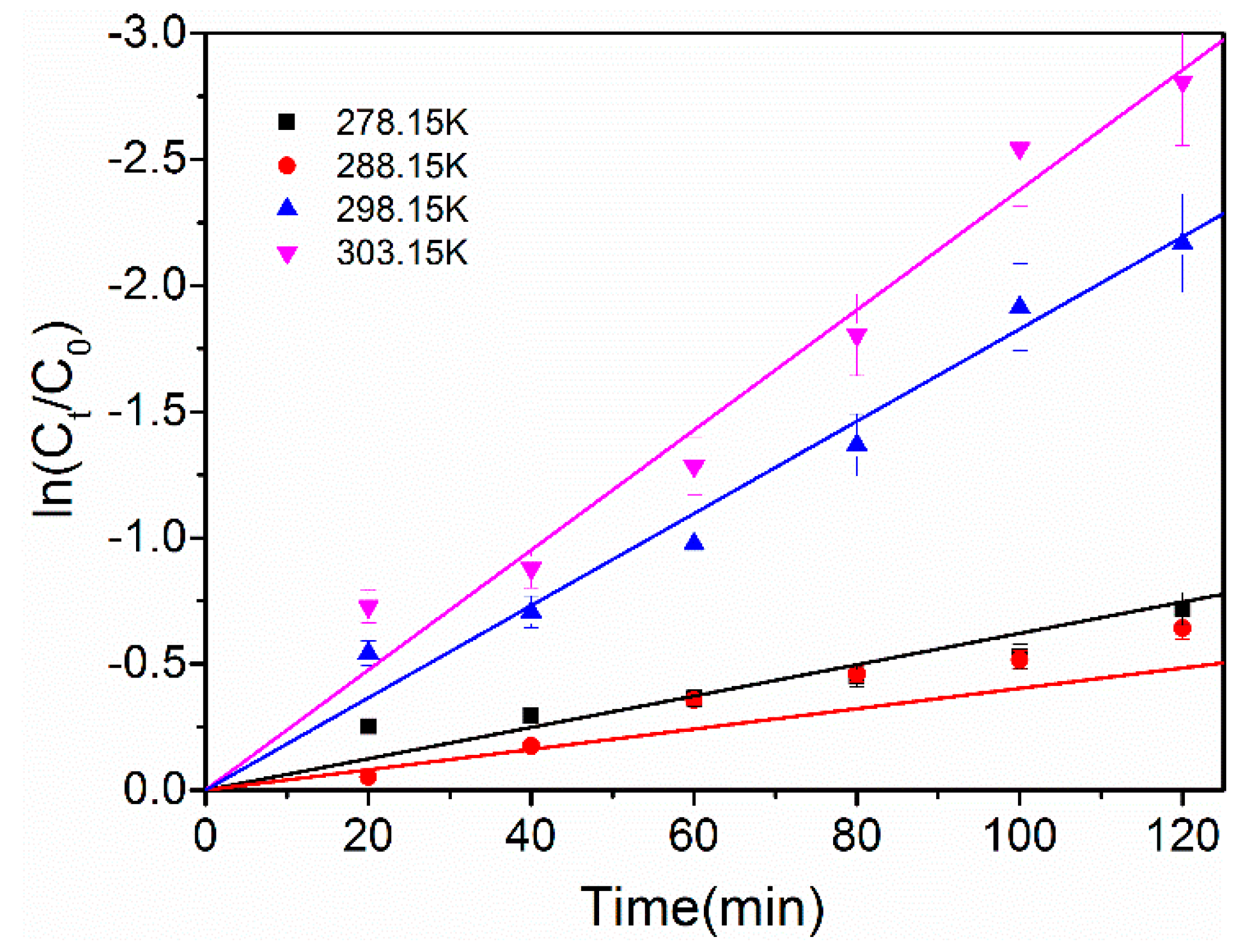
2.3.3. Effect of Temperature
3. Materials and Methods
3.1. Reagents and Materials
3.2. Methods
3.2.1. Preparation of Electrodes
3.2.2. Dechlorination Experiment
3.2.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiong, J.; Ma, Y.; Yang, W.; Zhong, L.S. Rapid, highly efficient and stable catalytic hydrodechlorination of chlorophenols over novel Pd/CNTs-Ni foam composite catalyst in continuous-flow. J. Hazard. Mater. 2018, 355, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.F.; Shen, Z.Y.; Niu, J.F.; Chen, J.; Duan, Y.P. Degradation of pentachlorophenol and 2,4-Dichlorophenol by sequential visible-light driven photocatalysis and laccase catalysis. Environ. Sci. Technol. 2010, 44, 9117–9122. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.R.; Wei, X.F.; Shen, H.T.; Hu, X. Preparation of palladium-nickel loaded titanium electrode with surfactant assistance and its application in pentachlorophenol reductive dechlorination. Sep. Purif. Technol. 2014, 124, 224–230. [Google Scholar] [CrossRef]
- Sun, Z.R.; Song, G.; Du, R.; Hu, X. Modification of a Pd-loaded electrode with a carbon nanotubes-polypyrrole interlayer and its dechlorination performance for 2,3-dichlorophenol. RSC Adv. 2017, 7, 22054–22062. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Serestatidou, E.; Louloudi, M.; Konstantinou, I.K.; Milaeva, E.R.; Deligiannakis, Y. Mechanism of catalytic degradation of 2,4,6-trichlorophenol by a Fe-porphyrin catalyst. Appl. Catal. B-Environ. 2011, 101, 417–424. [Google Scholar] [CrossRef]
- She, X.Y.; Yang, Q.; Yao, F.B.; Zhong, Y.; Ren, W.C.; Chen, F.; Sue, J.; Ma, Y.H.; Fe, Z.Y.; Wang, D.B.; et al. Electrocatalytic hydrodechlorination of 4-chlorophenol on Pd supported multi-walled carbon nanotubes particle electrodes. Chem. Eng. J. 2019, 358, 903–911. [Google Scholar] [CrossRef]
- Abeish, A.M.; Ang, M.; Znad, H. Enhanced solar-photocatalytic degradation of combined chlorophenols using ferric ions and hydrogen peroxide. Ind. Eng. Chem. Res. 2014, 53, 10583–10589. [Google Scholar] [CrossRef]
- Suryaman, D.; Hasegawa, K. Biological and photocatalytic treatment integrated with separation and reuse of titanium dioxide on the removal of chlorophenols in tap water. J. Hazard. Mater. 2010, 183, 490–496. [Google Scholar] [CrossRef]
- Sun, C.; Baig, S.A.; Lou, Z.M.; Zhu, J.; Wang, Z.X.; Li, X.; Wu, J.H.; Zhang, Y.F.; Xu, X.H. Electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid using nanosized titanium nitride doped palladium/nickel foam electrodes in aqueous solutions. Appl. Catal. B-Environ. 2014, 158, 38–47. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, J.M.; Xu, Y.H.; Wang, J.D.; Ying, L.; Song, X.X.; Zhou, G.D.; Chen, J.M. Bioelectrocatalytic dechlorination of trichloroacetic acid at gel-immobilized hemoglobin on multiwalled carbon nanotubes modified graphite electrode: Kinetic modeling and reaction pathways. Electrochim. Acta 2013, 92, 153–160. [Google Scholar] [CrossRef]
- Jiang, G.M.; Wang, K.F.; Li, J.Y.; Fu, W.Y.; Zhang, Z.Y.; Johnson, G.; Lv, X.S.; Zhang, Y.X.; Zhang, S.; Dong, F. Electrocatalytic hydrodechlorination of 2,4-dichlorophenol over palladium nanoparticles and its pH-mediated tug-of-war with hydrogen evolution. Chem. Eng. J. 2018, 348, 26–34. [Google Scholar] [CrossRef]
- Maddila, S.; Dasireddy, V.; Oseghe, E.O.; Jonnalagadda, S.B. Ozone initiated dechlorination and degradation of trichlorophenol using Ce-Zr loaded metal oxides as catalysts. Appl. Catal. B-Environ. 2013, 142, 129–141. [Google Scholar] [CrossRef]
- Wan, J.J.; Wan, J.Q.; Ma, Y.W.; Huang, M.Z.; Wang, Y.; Ren, R. Reactivity characteristics of SiO2-coated zero-valent iron nanoparticles for 2,4-dichlorophenol degradation. Chem. Eng. J. 2013, 221, 300–307. [Google Scholar] [CrossRef]
- Blanco, M.; Pizzio, L. Influence of the thermal treatment on the physicochemical properties and photocatalytic degradation of 4-chlorophenol in aqueous solutions with tungstophosphoric acid-modified mesoporous titania. Appl. Catal. A-Gen. 2011, 405, 69–78. [Google Scholar] [CrossRef]
- Olu-Owolabi, B.I.; Alabi, A.H.; Diagboya, P.N.; Unuabonah, E.I.; Duering, R.A. Adsorptive removal of 2,4,6-trichlorophenol in aqueous solution using calcined kaolinite-biomass composites. J. Environ. Manag. 2017, 192, 94–99. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, H.; Wang, L.; Ma, C.; Luan, C.; Zhao, B.; Zhang, Z.H.; Zhang, H.W.; Cheng, X.W.; Liu, J.L. The preparation of Pd/foam-Ni electrode and its electrocatalytic hydrodechlorination for monochlorophenol isomers. Catalysts 2018, 8, 378. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Shan, J.; Zhang, J.D. Electrodeposition of palladium and reduced graphene oxide nanocomposites on foam-nickel electrode for electrocatalytic hydrodechlorination of 4-chlorophenol. J. Hazard. Mater. 2015, 290, 1–8. [Google Scholar] [CrossRef]
- Sun, Z.R.; Wang, K.; Wei, X.F.; Tong, S.; Hu, X. Electrocatalytic hydrodehalogenation of 2,4-dichlorophenol in aqueous solution on palladium-nickel bimetallic electrode synthesized with surfactant assistance. Int. J. Hydrog. Energy 2012, 37, 17862–17869. [Google Scholar] [CrossRef]
- Li, A.Z.; Zhao, X.; Hou, Y.N.; Liu, H.J.; Wu, L.Y.; Qu, J.H. The electrocatalytic dechlorination of chloroacetic acids at electrodeposited Pd/Fe-modified carbon paper electrode. Appl. Catal. B-Environ. 2012, 111, 628–635. [Google Scholar] [CrossRef]
- Mao, R.; Li, N.; Lan, H.C.; Zhao, X.; Liu, H.J.; Qu, J.H.; Sun, M. Dechlorination of trichloroacetic acid using a noble metal-free graphene-Cu foam electrode via direct cathodic reduction and atomic H. Environ. Sci. Technol. 2016, 50, 3829–3837. [Google Scholar] [CrossRef]
- Jiang, G.M.; Lan, M.N.; Zhang, Z.Y.; Lv, X.S.; Lou, Z.M.; Xu, X.H.; Dong, F.; Zhang, S. Identification of active hydrogen species on palladium nanoparticles for an enhanced electrocatalytic hydrodechlorination of 2,4-dichlorophenol in water. Environ. Sci. Technol. 2017, 51, 7599–7605. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.B.; Zhu, Y.Y.; Li, J.; Zeng, G.M.; Lei, C. Uncovering the intrinsic relationship of electrocatalysis and molecular electrochemistry for dissociative electron transfer to polychloroethanes at silver cathode. Electrochim. Acta 2017, 231, 590–600. [Google Scholar] [CrossRef]
- Ma, H.X.; Xu, Y.H.; Ding, X.F.; Liu, Q.; Ma, C.A. Electrocatalytic dechlorination of chloropicolinic acid mixtures by using palladium-modified metal cathodes in aqueous solutions. Electrochim. Acta 2016, 210, 762–772. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, H.; Liu, S.L.; Luo, Q.; Bian, Z.Y. Optimization of a three-electrode system for electrochemical reduction-oxidation of 4-chlorophenol with response surface methodology. Toxicol. Environ. Chem. 2016, 98, 327–344. [Google Scholar] [CrossRef]
- Huang, B.B.; Isse, A.A.; Durante, C.; Wei, C.H.; Gennaro, A. Electrocatalytic properties of transition metals toward reductive dechlorination of polychloroethanes. Electrochim. Acta 2012, 70, 50–61. [Google Scholar] [CrossRef]
- Isse, A.A.; Huang, B.; Durante, C.; Gennaro, A. Electrocatalytic dechlorination of volatile organic compounds at a copper cathode. Part I: Polychloromethanes. Appl. Catal. B-Environ. 2012, 126, 347–354. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, X.; Hu, X.; Wang, K.; Shen, H. Electrocatalytic dechlorination of 2,4-dichlorophenol in aqueous solution on palladium loaded meshed titanium electrode modified with polymeric pyrrole and surfactant. Colloids Surf. A 2012, 414, 314–319. [Google Scholar] [CrossRef]
- Xu, Y.H.; Cai, Q.Q.; Ma, H.X.; He, Y.; Zhang, H.; Ma, C.A. Optimisation of electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid on a roughened silver-palladium cathode. Electrochim. Acta 2013, 96, 90–96. [Google Scholar] [CrossRef]
- Jiang, C.S.; Yu, H.B.; Wang, X.H.; Lu, Y.; Luo, X.B. Preparation of the palladium/polymeric pyrrole-multi-walled carbon nanotubes film/titanium electrode and its performance for the dechlorination of 4-chlorophenol. Int. J. Electrochem. Sci. 2017, 12, 5208–5219. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Bosko, M.L.; Cornaglia, L.M. Alloying and surface composition of model Pd-Ag films synthesized by electroless deposition. Int. J. Hydrog. Energy 2012, 37, 6020–6029. [Google Scholar] [CrossRef]
- Ge, C.; Wang, Z.; Xia, D. Electrochemically codeposited palladium/molybdenum oxide electrode for electrocatalytic reductive dechlorination of 4-chlorophenol. Electrochem. Commun. 2004, 6, 268–272. [Google Scholar]
- Arellano-Gonzalez, M.A.; Texier, A.C.; Lartundo-Rojas, L.; Gonzalez, I. Electrochemical dechlorination of 2-chlorophenol on Pd/Ti, Ni/Ti and Pd-Ni alloy/Ti electrodes. J. Electrochem. Soc. 2015, 162, E223–E230. [Google Scholar] [CrossRef]
- Sun, Z.; Shen, H.; Wei, X.; Hu, X. Electrocatalytic hydrogenolysis of chlorophenols in aqueous solution on Pd58Ni42 cathode modified with PPy and SDBS. Chem. Eng. J. 2014, 241, 433–442. [Google Scholar] [CrossRef]
- Wu, Y.F.; Gan, L.; Zhang, S.P.; Song, H.O.; Lu, C.; Li, W.T.; Wang, Z.; Jiang, B.C.; Li, A.M. Carbon-nanotube-doped Pd-Ni bimetallic three-dimensional electrode for electrocatalytic hydrodechlorination of 4-chlorophenol: Enhanced activity and stability. J. Hazard. Mater. 2018, 356, 17–25. [Google Scholar] [CrossRef]
- He, Z.Q.; Sun, J.J.; Wei, J.; Wang, Q.; Huang, C.X.; Chen, J.M.; Song, S. Effect of silver or copper middle layer on the performance of palladium modified nickel foam electrodes in the 2-chlorobiphenyl dechlorination. J. Hazard. Mater. 2013, 250, 181–189. [Google Scholar] [CrossRef]
- Ahmad, F.; Luo, L.H.; Li, X.; Huang, H.W.; Zeng, J. Boosting fuel cell catalysis by surface doping of W on Pd nanocubes. Chin. J. Catal. 2018, 39, 1202–1209. [Google Scholar] [CrossRef]
- Yao, F.B.; Zhong, Y.; Yang, Q.; Wang, D.B.; Chen, F.; Zhao, J.W.; Xie, T.; Jiang, C.; An, H.X.; Zeng, G.M.; et al. Effective adsorption/electrocatalytic degradation of perchlorate using Pd/Pt supported on N-doped activated carbon fiber cathode. J. Hazard. Mater. 2017, 323, 602–610. [Google Scholar] [CrossRef]
- Rajic, L.; Fallahpour, N.; Podlaha, E.; Alshawabkeh, A. The influence of cathode material on electrochemical degradation of trichloroethylene in aqueous solution. Chemosphere 2016, 147, 98–104. [Google Scholar] [CrossRef]
- Rajesh, D.; Neel, P.I.; Pandurangan, A.; Mahendiran, C. Pd-NiO decorated multiwalled carbon nanotubes supported on reduced graphene oxide as an efficient electrocatalyst for ethanol oxidation in alkaline medium. Appl. Surf. Sci. 2018, 442, 787–796. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, H.; Liu, S.L.; Pang, L.; Bian, Z.Y. Electrocatalytic reduction-oxidation of chlorinated phenols using a nanostructured Pd-Fe modified graphene catalyst. Electrochim. Acta 2015, 178, 92–100. [Google Scholar] [CrossRef]
- Ilieva, M.; Tsakova, V.; Erfurth, W. Electrochemical formation of bi-metal (copper–palladium) electrocatalyst supported on poly-3,4-ethylenedioxythiophene. Electrochim. Acta 2006, 52, 816–824. [Google Scholar] [CrossRef]
- Mourato, A.; Wong, S.M.; Siegenthaler, H.; Abrantes, L.M. Polyaniline films containing palladium microparticles for electrocatalytic purposes. J. Solid State Electron. 2006, 10, 140–147. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, H.L.; Cheng, X.W.; Chen, Q.H.; Xin, Y.J.; Ma, Z.P.; Xu, W.X.; Ma, J.; Ren, N.Q. Preparation and characterization of palladium/polypyrrole/foam nickel electrode for electrocatalytic hydrodechlorination. Chem. Eng. J. 2013, 225, 489–498. [Google Scholar] [CrossRef]
- Sun, Z.R.; Wei, X.F.; Han, Y.B.; Tong, S.; Hu, X. Complete dechlorination of 2,4-dichlorophenol in aqueous solution on palladium/polymeric pyrrole-cetyl trimethyl ammonium bromide/foam-nickel composite electrode. J. Hazard. Mater. 2013, 244, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.R.; Wei, X.F.; Shen, H.T.; Hu, X. Preparation and evaluation of Pd/polymeric pyrrole-sodium lauryl sulfonate/foam-Ni electrode for 2,4-dichlorophenol dechlorination in aqueous solution. Electrochim. Acta 2014, 129, 433–440. [Google Scholar] [CrossRef]
- Mahdavi, B.; Los, P.; Lessard, M.J.; Lessard, J. A comparison of nickel boride and raney nickel electrode activity in the electrocatalytic hydrogenation of phenanthrene. Can. J. Chem. 2011, 72, 2268–2277. [Google Scholar] [CrossRef]
- Majeed, I.; Manzoor, U.; Kanodarwala, F.K.; Nadeem, M.A.; Hussain, E.; Ali, H.; Badshah, A.; Stride, J.A.; Nadeem, M.A. Pd-Ag decorated g-C3N4 as an efficient photocatalyst for hydrogen production from water under direct solar light irradiation. Catal. Sci. Technol. 2018, 8, 1183–1193. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Wu, Z.L.; Zong, X.Q.; Zhang, Y. Fabrication of polypyrrole/Zn2SnO4 nanofilm for ultra-highly sensitive ammonia sensing application. Sens. Actuators B-Chem. 2018, 274, 575–586. [Google Scholar] [CrossRef]
- Deng, Z.H.; Wang, J.; Nie, Y.; Wei, Z.D. Tuning the interface of Ni@Ni(OH)2/Pd/rGO catalyst to enhance hydrogen evolution activity and stability. J. Power Sources 2017, 352, 26–33. [Google Scholar] [CrossRef]
- Casella, I.G.; Contursi, M. Pulsed electrodeposition of nickel/palladium globular particles from an alkaline gluconate bath. An electrochemical, XPS and SEM investigation. J. Electroanal. Chem. 2013, 692, 80–86. [Google Scholar] [CrossRef]
- Zhou, J.S.; Lou, Z.M.; Yang, K.L.; Xu, J.; Li, Y.Z.; Liu, Y.L.; Baig, S.A.; Xu, X.H. Electrocatalytic dechlorination of 2,4-dichlorobenzoic acid using different carbon-supported palladium moveable catalysts: Adsorption and dechlorination activity. Appl. Catal. B-Environ. 2019, 244, 215–224. [Google Scholar] [CrossRef]
- Yang, H.Y.; Tang, Z.H.; Wang, K.; Wu, W.; Chen, Y.H.; Ding, Z.Q.; Liu, Z.; Chen, S.W. Co@Pd core-shell nanoparticles embedded in nitrogen-doped porous carbon as dual functional electrocatalysts for both oxygen reduction and hydrogen evolution reactions. J. Colloid Interface Sci. 2018, 528, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.X.; Liu, X.Y.; Wang, A.Q.; Lee, A.F.; Isaacs, M.A.; Li, L.; Pan, X.L.; Yang, X.F.; Wang, X.D.; Tai, Z.J.; et al. Ag Alloyed Pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene. ACS Catal. 2015, 5, 3717–3725. [Google Scholar] [CrossRef]
- Witonska, I.; Krolak, A.; Karski, S. Bi modified Pd/support (SiO2, Al2O3) catalysts for hydrodechlorination of 2,4-dichlorophenol. J. Mol. Catal. A-Chem. 2010, 331, 21–28. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Yue, P.L.; Chen, G. Catalytic dechlorination of chlorophenols in water by palladium/iron. Water Res. 2001, 35, 1887–1890. [Google Scholar] [CrossRef]
- Zhu, K.R.; Baig, S.A.; Xu, J.; Sheng, T.T.; Xu, X.H. Electrochemical reductive dechlorination of 2,4-dichlorophenoxyacetic acid using a palladium/nickel foam electrode. Electrochim. Acta 2012, 69, 389–396. [Google Scholar] [CrossRef]
- Lou, Z.M.; Zhou, J.S.; Sun, M.; Xu, J.; Yang, K.L.; Lv, D.; Zhao, Y.P.; Xu, X.H. MnO2 enhances electrocatalytic hydrodechlorination by Pd/Ni foam electrodes and reduces Pd needs. Chem. Eng. J. 2018, 352, 549–557. [Google Scholar] [CrossRef]
- Lou, Z.M.; Li, Y.Z.; Zhou, J.S.; Yang, K.L.; Liu, Y.L.; Baig, S.A.; Xu, X.H. TiC doped palladium/nickel foam cathode for electrocatalytic hydrodechlorination of 2,4-DCBA: Enhanced electrical conductivity and reactive activity. J. Hazard. Mater. 2019, 362, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.J.; Yuan, S.H.; Mao, X.H.; Hu, W.; Liao, P.; Tong, M.; Alshaulabkeh, A.N. Electrocatalytic activity of Pd-loaded Ti/TiO2 nanotubes cathode for TCE reduction in groundwater. Water Res. 2013, 47, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.F.; Wan, X.Y.; Miao, J.; Zhang, R.C.; Zhang, J.; Niu, Q.J. Hydrodechlorination of p-Chlorophenol on Pd-coated Fe3O4@polypyrrole catalyst with ammonia borane as hydrogen donor. Catal. Lett. 2019, 149, 823–830. [Google Scholar] [CrossRef]
- Liu, Y.S.; Li, X.L.; Le, X.D.; Zhang, W.; Gu, H.; Xue, R.W.; Ma, J.T. Catalysis of the hydro-dechlorination of 4-chlorophenol by Pd-(0)-modified MCM-48 mesoporous microspheres with an ultra-high surface area. New J. Chem. 2015, 39, 4519–4525. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A. Reductive destruction and decontamination of aqueous solutions of chlorinated antimicrobial agent using bimetallic systems. J. Hazard. Mater. 2009, 164, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.F.; Long, G.Y.; Luo, Y.; Sun, R.Z.; Chen, M.X.; Li, Y.J.; Zhou, Y.F.; Xu, X.H.; Zhao, W.R. Photocatalytic reductive dechlorination of 2-chlorodibenzo-p-dioxin by Pd modified g-C3N4 photocatalysts under UV-vis irradiation: Efficacy, kinetics and mechanism. J. Hazard. Mater. 2018, 355, 74–81. [Google Scholar] [CrossRef] [PubMed]
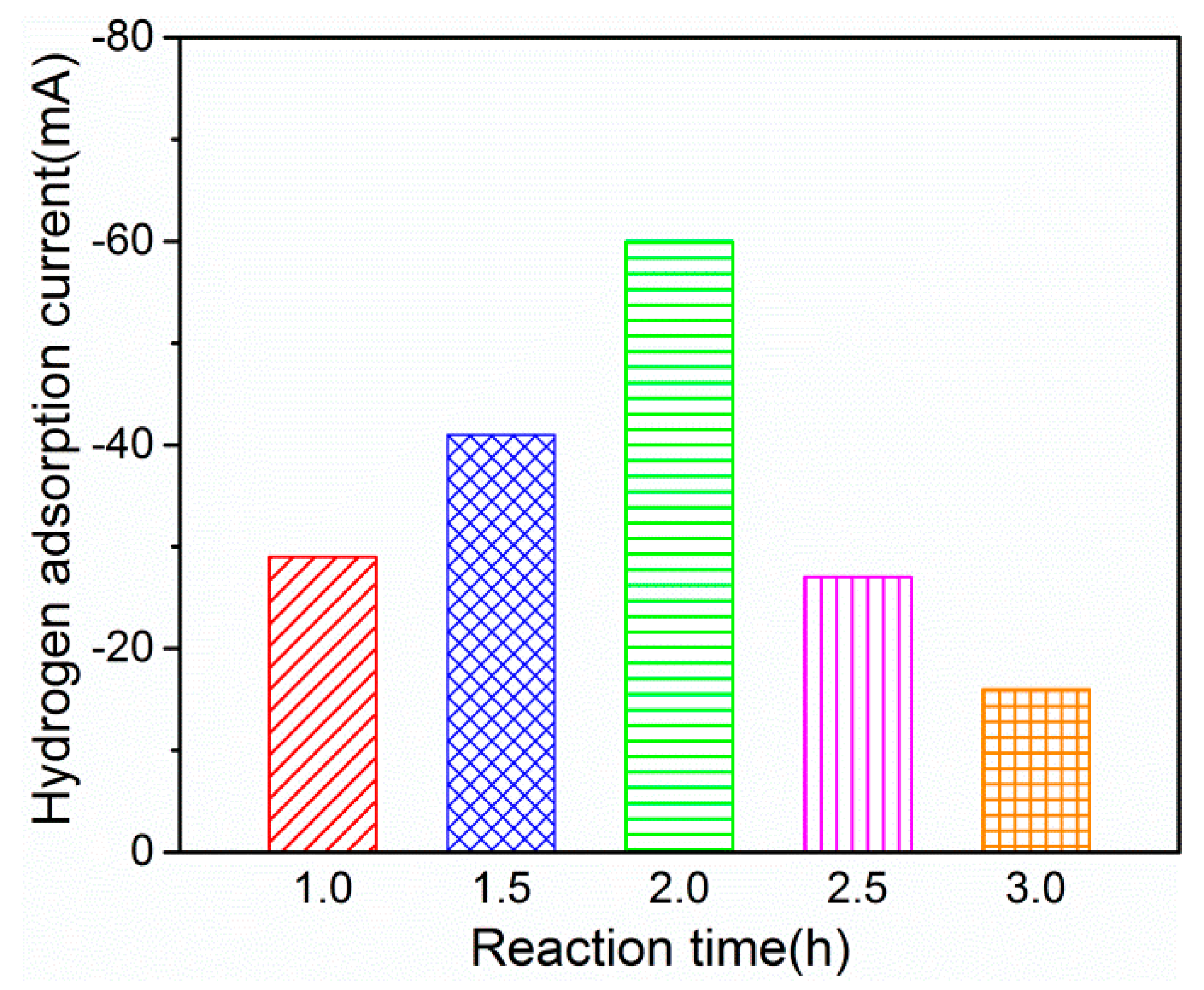
| Composition (mmol/L) | PdCl2 | 2 | 2 | 0 | 1 | 1 | 4 | 2 | 0.5 |
| AgNO3 | 1 | 0 | 1 | 1 | 1.5 | 1 | 2 | 0.5 | |
| Hydrogen Adsorption Current (mA) | 80 | 21 | 30 | 60 | 56 | 75 | 40 | 62 | |
| Technology | Electrode or Catalyst | Model Pollutant | Pd Loading (mg.cm−2) | kobs (min−1) | kpd (L.gPd−1·min−1) | Reference |
|---|---|---|---|---|---|---|
| Electrocatalytic hydrodechlorination | Pd/Ni foam | 2,4-D | 1.78 | 0.5 × 10−2 | 0.019 | [56] |
| Pd/MnO2/Ni foam | 2,4-DCBA | 0.44 | 1.5 × 10−2 | 0.283 | [57] | |
| TiC-Pd/Ni foam | 2,4-DCBA | 0.44 | 3.9 × 10−2 | 0.591 | [58] | |
| Pd-Ti/TiO2NTs | TCE | 3.2 | 1.9 × 10−2 | 0.37 | [59] | |
| Pd-Ag/PPY/Ti | 4-CP | 0.14 | 2.4×10−2 | 0.63 | This work | |
| Catalytic hydrodechlorination | Fe3O4@PPY@Pd nanoparticles | 4-CP | - | 2.3 × 10−2 | 0.43 | [60] |
| Pd/Fe3O4@SiO2@m-SiO2 | 4-CP | - | 3.5 × 10−2 | 0.13 | [61] | |
| Fe/Pd | DCP | - | 4.0 × 10−2 | 0.10 | [62] | |
| Photocatalytic reduction | Pd/g-C3N4 | 2-CDD | - | 2.8 × 10−3 | 0.06 | [63] |
| Step | Reagent | Time (min) |
|---|---|---|
| Fluorination | 0.724 g/L Na2F and 1.5 mL/L HF | 15 |
| Sensibilization | 10 g/L SnCl2 and 12 mL/L 36% HCl | 1 |
| Activation | 0. 25 g/L PdCl2 and 2.5 mL/L 36% HCl | 1 |
| Neutralization | 1% ammonium hydroxide | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Zeng, L.; Lu, W.; Miao, J.; Zhang, R.; Zhou, M.; Zhang, J. A Polypyrrole-Modified Pd-Ag Bimetallic Electrode for the Electrocatalytic Reduction of 4-Chlorophenol. Catalysts 2019, 9, 931. https://doi.org/10.3390/catal9110931
Wei X, Zeng L, Lu W, Miao J, Zhang R, Zhou M, Zhang J. A Polypyrrole-Modified Pd-Ag Bimetallic Electrode for the Electrocatalytic Reduction of 4-Chlorophenol. Catalysts. 2019; 9(11):931. https://doi.org/10.3390/catal9110931
Chicago/Turabian StyleWei, Xuefeng, Laiyuan Zeng, Weiwei Lu, Juan Miao, Ruichang Zhang, Ming Zhou, and Jun Zhang. 2019. "A Polypyrrole-Modified Pd-Ag Bimetallic Electrode for the Electrocatalytic Reduction of 4-Chlorophenol" Catalysts 9, no. 11: 931. https://doi.org/10.3390/catal9110931
APA StyleWei, X., Zeng, L., Lu, W., Miao, J., Zhang, R., Zhou, M., & Zhang, J. (2019). A Polypyrrole-Modified Pd-Ag Bimetallic Electrode for the Electrocatalytic Reduction of 4-Chlorophenol. Catalysts, 9(11), 931. https://doi.org/10.3390/catal9110931