Abstract
This paper describes work to assess the possibility of a modified Fenton process being used to remove the hard-to-degrade plasticizer di(2-ethylhexyl) phthalate (DEHP) from the bottom sediments of a reservoir. The modifications in question entail iron(II) ions being replaced by iron(III), as well as facilitation of the process using a chelating agent. Analysis further revolved around the impact of such factors as amounts of reagents, reaction of the environment, initial contents of the contaminant, and the presence of other “competing” contaminants also of a hard-to-decompose nature. As the maximum efficiency of DEHP removal obtained did not exceed 30%, the low susceptibility to degradation is made clear, as is the need for earlier desorption of the contaminant from the matrix. The effect of the modified Fenton process on the content of organic matter and dissolved organic carbon was also considered, as was the tendency to cause selected metals and plant nutrients to leach from bottom sediments.
1. Introduction
In recent years, special attention has been paid to researching and deploying so-called advanced oxidation processes (AOPs). While these are varied, a common feature is their generation of hydroxyl radicals (HO·) capable of reacting with almost all organic compounds. Due to the high efficiency they achieve in degrading most organic pollutants, oxidation methods are now seen increasingly as the most promising alternative methods of purification or decontamination to be set against so-called conventional methods [1,2,3,4,5,6].
The Fenton process is one of the advanced methods of oxidation, whereby a reaction between hydrogen peroxide (H2O2) and catalyst Fe2+ generates the aforesaid hydroxyl radicals, which manifest marked reactivity and strong oxidizing properties arising out of their high oxidizing potential. The radicals react non-selectively with organic impurities, oxidizing them to such intermediates as alcohols or carboxylic acids, and then to water and carbon dioxide [4].
While the classic Fenton process already has many advantages—not least high efficiency, a lack of exacting requirements where equipment is concerned, wide availability of the reagents, and a lack of harmful by-products given the capacity to degrade organic compounds to H2O and CO2—an array of further modifications has arisen, to increase effectiveness and reduce costs still further. These modifications include the use of alternative catalysts and sources of hydrogen peroxide, a more heterogeneous process, and deployment in combination with other processes [7,8].
In the work described here, the aim was to modify the Fenton process to achieve optimal removal of di(2-ethylhexyl) phthalate (DEHP) from bottom sediments. The modification we arrived at used Fe3+ ions instead of Fe2+ in the reaction environment, with the result being a steadier formation of hydroxyl radicals that raised the level of process efficiency. Degradation of organic compounds in the presence of Fe3+ ions was slower than with Fe2+, and this was seen as advisable in bottom-sediment matrix and soil, in line with the need for earlier desorption of pollutants into the aqueous phase [7].
Di(2-ethylhexyl) phthalate is an organic compound from the group of phthalic acid esters (PAEs), which are regarded as hazardous, given their capacity to cause genetic aberrations, affect reproduction and further development negatively, and give rise to endocrine disorders [9,10,11]. DEHP is the most common compound of the phthalate group to pollute the natural environment, and its low level of solubility in water combines with its unfortunate stability in the environment to ensure accumulation in bottom sediments, where the content may even reach 322 mg/kg d.w. Its high content in the aquatic environment is associated with leaching from plastics, and in particular from microplastics, where it acts as a plasticizer [9,12]. This phenomenon accounts for the urgency of the search for means of degradation, including the modified Fenton process detailed here.
The purpose of the work detailed here was thus to apply a modification of the Fenton process in removing high contents of DEHP from bottom sediments. Research encompassed the possibility of the reaction being conducted in a natural reaction environment with no need for acidification (bearing in mind the negative effects on bottom sediments or soil), in the presence of the chelating agent sodium pyrophosphate Na4P2O7 (PS). The latter represents the group of inorganic chelating substances that differ from their organic counterparts in competing less for access to hydroxyl radicals.
Analysis concerned the impact of the process applied on the contents of soil organic matter (SOM) and dissolved organic carbon (DOC). The possibility of various components in bottom sediments (including plant nutrients) being leached was also investigated, as was competition for access to hydroxyl radicals in the presence of other polycyclic aromatic hydrocarbon (PAH) pollutants and organochlorine pesticides. The work also sought to check the suitability of the process developed in the presence of various initial contents of DEHP.
2. Results and Discussion
2.1. Impact of Reagent Dose on DEHP Degradation
Studies of reagent dose in relation to DEHP distribution in a solid matrix involved DEHP:H2O2:Fe3+ molar ratios of 1:1:1, 1:5:1, 1:28:1, 1:100:1, and 1:1:5, at constant DEHP content equal to 0.13 mM/kg d.w., for times equal to 1, 2, 4, 12, and 24 h, at pH = 3. The results of these tests are presented in Figure 1.
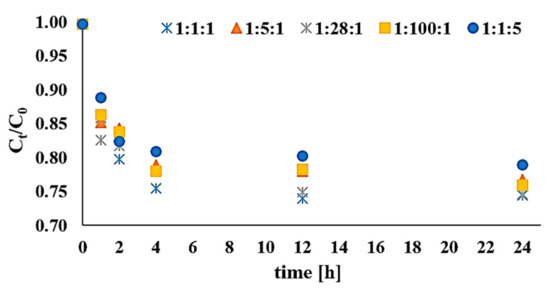
Figure 1.
Impact of reagent doses in the di(2-ethylhexyl) phthalate (DEHP):H2O2:Fe3+ molar ratio on the efficiency of DEHP removal at pH 3. Ct denotes the DEHP content in bottom sediments after time t, and C0 is the initial content of di(2-ethylhexyl) phthalate in bottom sediments.
The impact of hydrogen peroxide dose on the efficiency of DEHP oxidation was tested across a range of oxidant quantities from 0.13 to 13 mM/kg d.w. The maximum observed efficiency of di(2-ethylhexyl) phthalate removal following the introduction of catalytic Fe3+ ions into the reaction environment was 25.49% for t = 24 h and a DEHP:H2O2:Fe3+ molar ratio of 1:1:1. Larger amounts of H2O2 in relation to iron(III) ions did not result in fuller degradation of the contaminant. Furthermore, a relative excess of Fe3+ (with 1:5 H2O2:Fe3+) was associated with effectiveness at just 21.07% on average.
The modified Fenton process proved most effective during the first four hours of reaction. Likewise, in the case of their H2O2/Fe3+ process, Chiou et al. (2006) [11] achieved a 46% reduction in amounts of dibutyl phthalate (DBP) via a reaction involving Fe3+ at 0.36 mM/dm3, H2O2 at 0.032 mM/min·dm3, t = 60 min, and pH = 3. This degree of DBP removal (C0 = 5 mg/dm3) was attributed to the presence of HO· and HO2· formed as H2O2 and Fe3+ interacted (Fe3+ + H2O2 → Fe2+ + HO2· + H+, followed by Fe2+ + H2O2 → Fe3+ + HO· + OH−). Also using an Fe3+ catalyst, Barbusiński (2004) [7] and Jorfi et al. (2011) [13] asserted that reaction rate was limited by Fe2+ formation.
HO· radicals are generated in a two-stage process, via a slow reaction between Fe3+ and H2O2, and a subsequent rapid reaction between generated Fe2+ and H2O2. The H2O2-mediated elimination of hard-to-decompose substances exemplified by the non-ionic surfactant Triton X-114 was studied by Ledakowicz et al. (2001) [14], among others. After 26 hours, they achieved a reduction of 100 mg/dm3 of this substance at a level of just 4%, in the case of a 0.15 mmol/dm3 concentration of hydrogen peroxide. However, a sixfold increase in the amount of oxidant raised this level of removal to 24%.
In research by Goi and Viisimaa (2015) [15], hydrogen peroxide (at a soil:H2O2 ratio of 1:0.0005 w/w) was used to degrade selected polychlorinated biphenyls (PCBs) present at 52 g/kg d.w. The limited level of removal (at just 10% for t = 60 min) in this case too, was probably due to rapid decomposition of the oxidant. For their part, Zhang et al. (2013) [16] applied a Fenton process to reduce levels of petroleum hydrocarbons (PHCs) in oily sludge. The best level of removal they were able to achieve was 13.8%.
The degradation of organic compounds was slower in the presence of Fe3+ as opposed to Fe2+, and—where pollutants are present in bottom sediments and soil—it is advisable that they be subject to earlier desorption into the water phase, followed by rapid decomposition of hydrogen peroxide to oxygen and water [17,18].
2.2. Impact of pH on DEHP Degradation
The process was investigated in a reaction environment with pH values of 3, 5, 7.95, or 10, where the dose of hydrogen peroxide was equal to 0.13 mM/kg d.w., and the amount of catalyst was set at 0.13 mM/kg d.w., with t = 1, 2, 4, 12, and 24 h (Figure 2a).
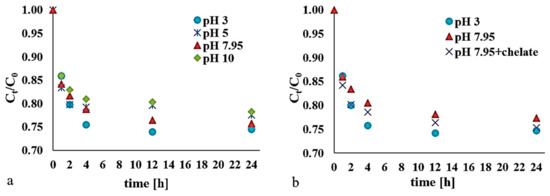
Figure 2.
Impact on the decomposition of DEHP using a modified Fenton process due to: (a) pH (with H2O2:Fe3+ at 1:1); (b) a sodium pyrophosphate (PS) chelating agent (with H2O2:Fe3+:PS at 1:1:1).
Oxidation of the DEHP in bottom sediments proved least effective where the tested reaction environment was alkaline. Slightly higher values for removal (16.27%–22.10% over 1–24 h time intervals) were obtained for pH = 5. However, across the pH range analyzed, the best effects obtained were at pH = 3, with 25.49% removal on average achieved after 24 hours. However, the reaction in fact proved effective over the first four hours of the process only, with no significant further change thereafter.
The process taking place in medium with a natural (unmodified) reaction was found to be comparable to that taking place in an acid reaction medium. Compared with pH = 3 (depending on the duration of the reaction), differences in the efficiency of removal of DEHP were just 0.1%–4.96%. This is an encouraging finding in the light of the more typical situation, whereby proven higher efficiency of removal of impurities at pH = 3 motivates soil acidification in support of the Fenton process, in spite of the negative impact on soil properties and quality.
According to Bokare and Choi (2014) [19], at pH = 6, most Fe3+ ions are in the form of Fe(OH)3 and Fe(OH)2+. The presence of a chelating agent allows for retention of the Fe3+ ions formed in the course of the Fenton reaction, or introduced in a dissolved form at a higher pH. Under such conditions, the effects are usually less favorable than in an acidic environment [7]. This compares with our own research, in which only slight differences were noted in the degradation of DEHP achieved in the natural or acidified reaction environments (Figure 2b).
By adding sodium pyrophosphate (at 0.13 mM/kg d.w.) as a chelating agent in the modified Fenton process (at pH = 7.95), it proved possible to obtain an efficiency higher by 1.69%–3.89% than at pH = 7.95, and lower by a maximum of 3.04% compared with pH = 3. The chelating agent stabilized the hydrogen peroxide, enhanced desorption of the entrapped pollutant, and solubilized part of the iron from the bottom sediments. The presumed mechanism of action of the chelating substance in the process is shown in Figure 3.
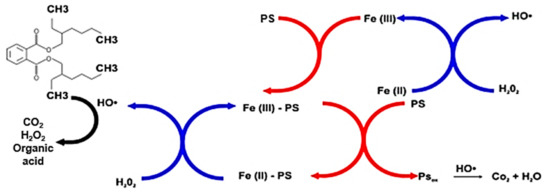
Figure 3.
Mechanism of action of PS in the modified Fenton process [developed on the basis of Zhao et al. (2018) [20]].
On the one hand, sodium pyrophosphate can inhibit iron precipitation by forming stable complexes with it, while on the other it can accelerate the Fe3+/Fe2+ cycle, with the effect that more hydroxyl radicals are produced. Under their optimal process conditions (i.e., 300 mM of H2O2, 30 mM of Fe3+, pH = 3, t = 6 h), Jorfi et al. (2013) [13] achieved 99% removal of pyrene from soil (C0 = 100 mg/kg). At pH 7, the corresponding figure was 93% in the presence of sodium pyrophosphate, which proved the most useful of the chelating agents they analyzed (alongside ethylenediaminetetraacetic acid (EDTA), sodium citrate, humic acids, and fulvic acids). In turn, in PAH-contaminated soils of neutral pH studied by Gan et al. (2012) [21], sodium pyrophosphate as a chelating agent was also found to enhance a modified Fenton (H2O2/Fe3+) method of removal.
The authors analyzed the usefulness of five chelating substances before homing in on sodium pyrophosphate as the most effective at removing selected substances of the PAH group. According to those authors, inorganic chelating substances differ from organic ones in competing less for access to hydroxyl radicals, while the overall carbon content in soil does not increase, and the phosphate ions can represent an additional source of soil nutrients. A positive effect of the chelating agent was also confirmed by Pardo et al. (2014) [22], Qin et al. (2015) [23], and Wang et al. (2017) [24], among others.
In situ chemical oxidation (ISCO) is a powerful technology for soil remediation. However, one of the main drawbacks of the Fenton process lies in the instability of H2O2 when in contact with soil. In addition, organic pollutants can be entrapped in soil organic matter so strongly that the efficiency of ISCO technology is impaired. However, an H2O2 stabilization effect is also noted where sodium pyrophosphate is applied.
Fenton processes are relatively inexpensive and easy to operate without any further energy requirements. It is therefore reasonable to combine a modified Fenton reaction with other technologies to improve radical-leading oxidation.
The content of organic matter decreased gradually as the reaction proceeded, from an average value of 7.86% to 6.82%. The value of DOC in the first hour of the process almost doubled, while subsequently there was a drop back to almost the initial level (Figure 4).
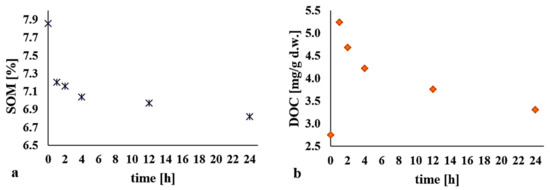
Figure 4.
Impact of the H2O2/Fe3+ process (H2O2:Fe3+ 1:1, pH = 3) on (a) soil organic matter (SOM) depending on t; (b) dissolved organic carbon (DOC) depending on t.
The use of a modified Fenton process resulted in a fourfold increase in the value noted for DOC (to 1.2 mg/g) in trials run by Cheng et al. (2016) [25] seeking to remove atrazine (at 617.5 mg/kg) from soil. Those authors noted that oxidation with Fenton’s reagent resulted in a slight decrease in SOM content, usually by about 10% of the initial value, as the humic acids which are the main components of SOM prove resistant to oxidation. In turn, in research by Zhao et al. (2018) [20], a DOC content four times higher was also obtained as DEHP was being removed from bottom sediments in an H2O2/Fe3+ system. Organic matter content decreased slightly—from 8.51% on average to around 7.70%.
2.3. Impact of Initial DEHP Content and the Presence of Other Impurities on DEHP Degradation
The impact of the initial content of DEHP on the efficiency of removal from bottom sediments was as presented in Figure 5. C0 was analyzed across the 10–100 mg/kg d.w. range, while reaction environment conditions were pH = 3, DEHP:H2O2:Fe3+ 1:1:1, and reaction time in the range 1–24 h.
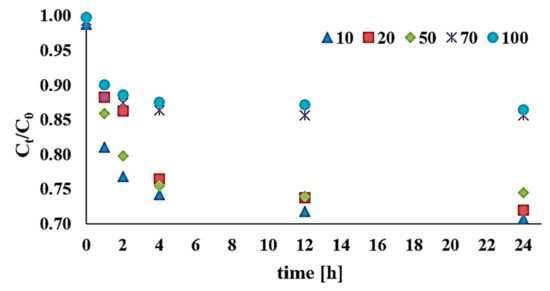
Figure 5.
Influence of initial content (C0 = 10–100 mg/kg d.w.) on DEHP decomposition via a modified Fenton process (H2O2:Fe3+ 1:1, pH 3).
Work starting with C0 = 10 mg/kg d.w. demonstrated a decrease in DEHP content to an average of 7.07 mg/kg d.w. after 24 hours (the removal efficiency was 28.5%). In the first two hours, efficacy for C0 = 20 mg/kg d.w. was comparable with the higher DEHP contents tested (70 and 100 mg/kg d.w.) and did not exceed 14.05% on average. Changes in amounts of di(2-ethylhexyl) phthalate decomposed via the modified Fenton process for C0 = 70 mg/kg d.w. and C0 = 100 mg/kg d.w. depended on reaction time, but were anyway only in the 11.01%–13.73% and 8.73%–13.32% ranges respectively. The research analyzed high initial contents of the pollutant, showing how efficiency was greater where the initial content of DEHP was lower.
A further important factor found to determine the efficiency of DEHP removal from bottom sediments via the above process was the presence of selected PAH-group contaminants at 3.2 mg/kg d.w., and of pesticides at 4 mg/kg d.w. (for C0 = 10 and 50 mg/kg d.w.) (Figure 6). The efficiency of removal of di(2-ethylhexyl) phthalate in the presence of other impurities was lower by a maximum of 16.32% for C0 = 10 mg/kg d.w. (t = 1 h) (Figure 6a). Where the initial DEHP content was higher (at C0 = 50 mg/kg d.w.), no significant differences in obtained values were observed (Figure 6b).
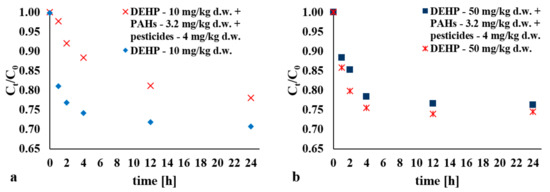
Figure 6.
Impact of other hard-to-degrade impurities in the H2O2/Fe3+ process (H2O2:Fe3+ 1:1, pH = 3) on the DEHP distribution for: (a) C0 = 10 mg/kg d.w.; (b) C0 = 50 mg/kg d.w. PAH: polycyclic aromatic hydrocarbon.
In the presence of additional pollutants, and with a lower initial content of C0 = 10 mg/kg d.w., hard-to-degrade compounds are found to limit access of DEHP to hydroxyl radicals. The presence of other substances, especially in comparable amounts, thus contributes to increased competition for HO∙ radicals, with a consequent reduction in removal efficiency.
The susceptibility to degradation by Fenton’s reagent of PAHs present in soil, sewage sludge, and bottom sediments was investigated by Flotron et al. (2005) [26]. Benzo(a)pyrene (BaP) was oxidized more effectively than fluoranthene, while the process was ineffective with benzo(b)fluoranthene, given its ease of absorption. The level of decomposition of these substances was found to be affected by the initial content of PAHs, as was also the case for the research presented here. Across the analyzed range, the modified Fenton process did not allow for a high degree of DEHP removal from bottom sediments. However, effectiveness was enhanced slightly in comparison with the classic Fenton reagent, as was likewise confirmed by research from Yap et al. (2011) [27], among others.
2.4. Impact of the Process on the Leaching of Selected Metals and Plant Nutrients
Various chemical elements (and especially heavy metals) deposited in bottom sediments constitute permanent and non-decomposable environmental pollution, posing a great secondary risk to water. This reflects the fact that, as many forms of these elements are mobile, transfers deeper or towards the sediment surface are possible, with the threats reflecting ecotoxic and phytotoxic effects [28]. In the research detailed here, it did prove possible to observe leaching of bottom-sediment components, including pollutant lead, copper, zinc, nickel, and aluminum, as well as key plant nutrients magnesium, potassium, and calcium (Table 1).

Table 1.
Leaching elements from sediments during the modified Fenton process (pH = 3, t = 1 h).
A change of bottom-sediment pH to slightly acidic or acidic helped mobilize plant-available heavy metal forms. However, lead and copper were shown to release from bottom sediments to the most limited extent, while Mg was the least-mobilized nutrient, and K the most mobilized. As plants’ absorption of calcium, magnesium, and potassium is reduced in low-pH conditions, the use of a chelating agent seems to be a favorable measure if it permits the process to occur at a natural reaction. The modified Fenton process had a much greater impact on the leaching of Pb, Cu, Zn, Ni, and Al as compared with plant nutrients. The factor determining the degree of elution plant nutrients from the matrix was acidification of the reaction environments.
3. Materials and Methods
3.1. Reagents
Di(2-ethylhexyl) phthalate (DEHP) and di(2-ethylhexyl) phthalate-3,4,5,6-d4 (DEHP-3,4,5,6-d4) were purchased from Sigma-Aldrich (Darmstadt, Germany). Standard solutions of the 16 PAHs (polycyclic aromatic hydrocarbons) were purchased from Reagecon (Shannon, Ireland), while a standard solution of the organochlorine pesticides was obtained from Sigma-Aldrich. Analytical-grade n-hexane, methanol, acetone, NaOH, HCl, H2O2 solution (30%), Na4P2O7·10H2O, and Fe(NO3)3·9H2O were obtained from POCH (Gliwice, Poland). Cellulose acetate membrane filters of 0.20 and 0.45 µm pore size and a syringe filter of 0.22 µm pore size were both purchased from LaboPlus (Warsaw, Poland).
3.2. Experimental Procedures
The bottom sediments used in the research were collected from Rzeszow Reservoir in Poland, and dried in air and then at 105 °C to constant weight, before being ground up and passed through a 1.0-mm sieve.
This sediment washed with acetone was allotted to 1 g samples, then placed in glass reaction vessels. DEHP was then introduced in the form of a solution in acetone, in amounts ranging between 10 and 100 mg/kg d.w. The obtainment of homogeneous samples further entailed shaking for one hour, in advance of a 24-hour set-aside period during which the solvent evaporated.
To check for effects of the presence of other hard-to-degrade contaminants, substances from the PAH group (at 3.2 mg/kg d.w.) and organochlorine pesticides (at 4 mg/kg d.w.) were added to samples. Distilled water was introduced into the contaminated sediments to obtain a suspension (1 g sample + 3 mL H2O) before DEHP oxidation proceeded, using a 30% solution of hydrogen peroxide plus an Fe(NO3)3·9H2O catalyst. Reactions progressed at room temperature, but with solution pH varied by adding HCl and NaOH, with different durations of oxidation in the range 1–24 h, and with different initial contents of both DEHP itself, as well as other barely degradable contaminants potentially “in competition” with it.
Separate steps of the work aimed to determine what doses of hydrogen peroxide and catalyst were optimal in relation to the DEHP present in bottom sediments. At this stage we also studied the impact on the removal of DEHP exerted by addition to the reaction mixture (prior to the introduction of the catalyst, though following Fe3+) of the chelating agent sodium pyrophosphate (Na4P2O7·10H2O). Reaction initiation entailed the addition to the sample of the appropriate amount of 30% hydrogen peroxide.
Elution of Ni, Pb, Al, Cu, and Zn from bottom sediments as a result of the applied processes was also evaluated. The samples were mineralized in the presence of 10 cm3 HNO3 in a MARS 6 microwave mineralizer (CEM, Matthews, USA). The obtained aqueous extracts were then filtered through a membrane filter of 0.20 μm pore size. A GBC Quantima E 1330 ICP-OES optical emission spectrometer (GBC, Melbourne, Australia) was used in analysis.
For the determinations of calcium, magnesium, and potassium ions, aqueous extracts obtained were filtered through 0.22-μm syringe filters (LaboPlus, Warsaw, Poland) and then purified on OnGuard II filters with the aim of removing organic substances. Analysis for the presence of calcium, magnesium, and potassium ions was carried out using a DIONEX DC ICS—5000 ion chromatograph (Thermo Scientific, Waltham, USA).
3.3. Analytical Methods of DEHP Determination
Quantitative determination of DEHP entailed microwave-assisted extraction in the presence of methanol, as well as the addition of an internal standard in the form of DEHP-3,4,5,6-d4. The samples were then filtered, and the organic and aqueous layers separated. The extract was dried over anhydrous sodium sulfate, concentrated to 1 mL volume, and subjected to chromatographic analysis. Quantitative DEHP determinations were made by capillary gas chromatography, using a gas chromatograph coupled with a mass detector (Thermo Scientific, Waltham, USA) (GC-MS).
4. Conclusions
It can be concluded that:
- The modified Fenton process described here requires the selection of optimal reagent doses if high-efficiency di(2-ethylhexyl) phthalate removal is to be achieved, as excesses of either iron ions or hydrogen peroxide can result in a hydroxyl scavenging capable of inhibiting the process;
- The process removing di(2-ethylhexyl) phthalate is dependent on reaction-mixture pH, with physico-chemical properties of the analyzed bottom-sediment matrix determining the effectiveness of removal at a given environmental reaction;
- The process of DEHP removal is hindered by the presence of other hard-to-degrade impurities competing for access to highly reactive hydroxyl radicals, given their presence in amounts comparable with those of the analyzed substance;
- A chelating agent present in a properly-selected dose allows for the removal of DEHP in circumstances of non-modified pH, at a level of efficiency similar to that otherwise only obtainable under acidified conditions;
- The use of the modified Fenton process results in the leaching of both plant nutrients and metals from bottom sediments, probably on account of the reduced pH;
- As the modified Fenton reaction process failed to achieve sufficiently effective removal of DEHP from bottom sediments even under the most favorable conditions noted, prior desorption of bottom-sediment contaminants has to be advocated, along with further research to look for other solutions that include combined processes.
Author Contributions
Conceptualization, M.K., S.Z. and P.K; Formal analysis, M.K. and S.Z.; Investigation, M.K. and S.Z.; Methodology, M.K., S.Z. and P.K.; Project administration, P.K.; Writing—original draft, M.K. and S.Z.; Writing—eview & editing, P.K.
Funding
This research received external funding from the Polish Ministry of Science and Higher Education.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kudlek, E.; Dudziak, M. Degradation pathways of pentachlorophenol and benzo(a)pyrene during heterogeneous photocatalysis. Water Sci. Technol. 2018, 77, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Kida, M.; Ziembowicz, S.; Koszelnik, P. Study on the suitability of using low-frequency ultrasonic field for removing di(2-ethylhexyl) phthalate from bottom sediments. Sep. Purif. Technol. 2020, 233, 116010. [Google Scholar] [CrossRef]
- Pochwat, K.; Kida, M.; Ziembowicz, S.; Koszelnik, P. Odours in Sewerage—A Description of Emissions and of Technical Abatement Measures. Environments 2019, 6, 89. [Google Scholar] [CrossRef]
- Kozak, J.; Włodarczyk-Makuła, M. Comparison of the PAHs degradation effectiveness using CaO2 or H2O2 under the photo-Fenton reaction. Desalin. Water Treat. 2018, 134, 57–64. [Google Scholar] [CrossRef]
- Pochwat, K.; Iličić, K. A simplified dimensioning method for high-efficiency retention tanks. In Proceedings of the E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 45, p. 65. [Google Scholar]
- Gajewska, M.; Kopeć, Ł.; Obarska-Pempkowiak, H. Operation of small wastewater treatment facilities in a scattered settlement. Rocz. Ochr. Srod. 2011, 13, 207–225. [Google Scholar]
- Barbusiński, K. Intensyfikacja Procesu Oczyszczania Ścieków i Stabilizacji Osadów Nadmiernych z Wykorzystaniem Odczynnika Fentona; Zeszyty Naukowe: Inżynieria Środowiska/Politechnika Śląska; Wydawnictwo Politechniki Śląskiej: Gliwice, Polska, 2004; p. 50. [Google Scholar]
- Ziembowicz, S.; Kida, M.; Koszelnik, P. The use of alternative catalysts in processes of the chemical degradation of di-n-butyl phthalate in aqueous solutions. Chemosphere 2019, 237, 124450. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, M.; Drogui, P.; Seyhi, B.; Brar, S.K.; Buelna, G.; Dubé, R. Occurrence, fate and effects of Di (2-ethylhexyl) phthalate in wastewater treatment plants: A review. Environ. Pollut. 2014, 194, 281–293. [Google Scholar] [CrossRef]
- Czarny, K.; Szczukocki, D.; Krawczyk, B.; Zieliński, M.; Miękoś, E.; Gadzała-Kopciuch, R. The impact of estrogens on aquatic organisms and methods for their determination. Crit. Rev. Environ. Sci. Technol. 2017, 47, 909–963. [Google Scholar] [CrossRef]
- Chiou, C.S.; Chen, Y.H.; Chang, C.T.; Chang, C.Y.; Shie, J.L.; Li, Y.S. Photochemical mineralization of di-n-butyl phthalate with H2O2/Fe3+. J. Hazard. Mater. 2006, 135, 344–349. [Google Scholar] [CrossRef]
- Wang, F.; Xia, X.; Sha, Y. Distribution of phthalic acid esters in Wuhan section of the Yangtze River, China. J. Hazard. Mater. 2008, 154, 317–324. [Google Scholar] [CrossRef]
- Jorfi, S.; Rezaee, A.; Moheb–ali, G.A.; alah Jaafarzadeh, N. Pyrene removal from contaminated soils by modified Fenton oxidation using iron nano particles. J. Environ. Health Sci. Eng. 2013, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Ledakowicz, S.; Olejnik, D.; Perkowski, J.; Żegota, H. The use of in-depth oxidation processes to break down the non-ionic Triton X-114 surfactant. Przem. Chem. 2001, 80, 453–459. [Google Scholar]
- Goi, A.; Viisimaa, M. Integration of ozonation and sonication with hydrogen peroxide and persulfate oxidation for polychlorinated biphenyls-contaminated soil treatment. J. Environ. Chem. Eng. 2015, 3, 2839–2847. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Thring, R.; Liu, L. Application of ultrasound and Fenton’s reaction process for the treatment of oily sludge. Proced. Environ. Sci. 2013, 18, 686–693. [Google Scholar] [CrossRef]
- Yim, B.; Yoo, Y.; Maeda, Y. Sonolysis of alkylphenols in aqueous solution with Fe (II) and Fe (III). Chemosphere 2003, 50, 1015–1023. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M.; Koszelnik, P. The impact of selected parameters on the formation of hydrogen peroxide by sonochemical process. Sep. Purif. Technol. 2018, 204, 149–153. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, M.; Zeng, G.; Zhang, C. Degradation of di(2-ethylhexyl) phthalate in sediment by a surfactant-enhanced Fenton-like process. Chemosphere 2018, 198, 327–333. [Google Scholar] [CrossRef]
- Gan, S.; Ng, H.K. Modified Fenton oxidation of polycyclic aromatic hydrocarbon (PAH)-contaminated soils and the potential of bioremediation as post–treatment. Sci. Total Environ. 2012, 419, 240–249. [Google Scholar]
- Pardo, F.; Rosas, J.M.; Santos, A.; Romero, A. Remediation of a biodiesel blend-contaminated soil by using a modified Fenton process. Environ. Sci. Pollut. Res. 2014, 21, 12198–12207. [Google Scholar] [CrossRef]
- Qin, Y.; Song, F.; Ai, Z.; Zhang, P.; Zhang, L. Protocatechuic acid promoted alachlor degradation in Fe (III)/H2O2 Fenton system. Environ. Sci. Technol. 2015, 49, 7948–7956. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, H.; Gao, D.W. Occurrence and risk assessment of phthalate esters (PAEs) in agricultural soils of the Sanjiang Plain, Northeast China. Environ. Sci. Pollut. Res. 2017, 24, 19723–19732. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y.; Wan, J.; Gong, X.; Zhu, Y. Degradation of atrazine by a novel Fenton-like process and assessment the influence on the treated soil. J. Hazard. Mater. 2016, 312, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Flotron, V.; Delteil, C.; Padellec, Y.; Camel, V. Removal of sorbed polycyclic aromatic hydrocarbons from soil, sludge and sediment samples using the Fenton’s reagent process. Chemosphere 2005, 59, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.L.; Gan, S.; Ng, H.K. Fenton based remediation of polycyclic aromatic hydrocarbons-contaminated soils. Chemosphere 2011, 83, 1414–1430. [Google Scholar] [CrossRef]
- Bogacki, J.; Naumczyk, J. Ex-situ physicochemical methods for the treatment of heavy metal bottom sediments. Gaz Woda i Technika Sanitarna 2009, 11, 3–42. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).