Ga/HZSM-5 Catalysed Acetic Acid Ketonisation for Upgrading of Biomass Pyrolysis Vapours
Abstract
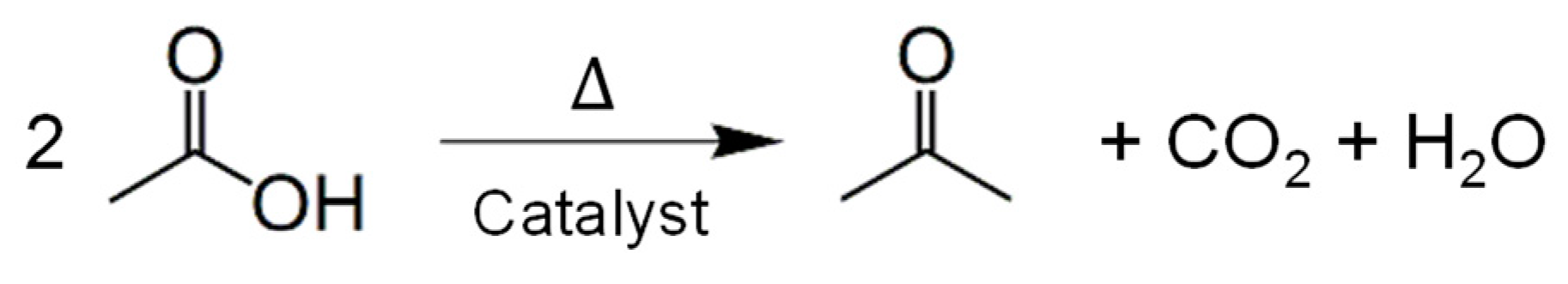
1. Introduction
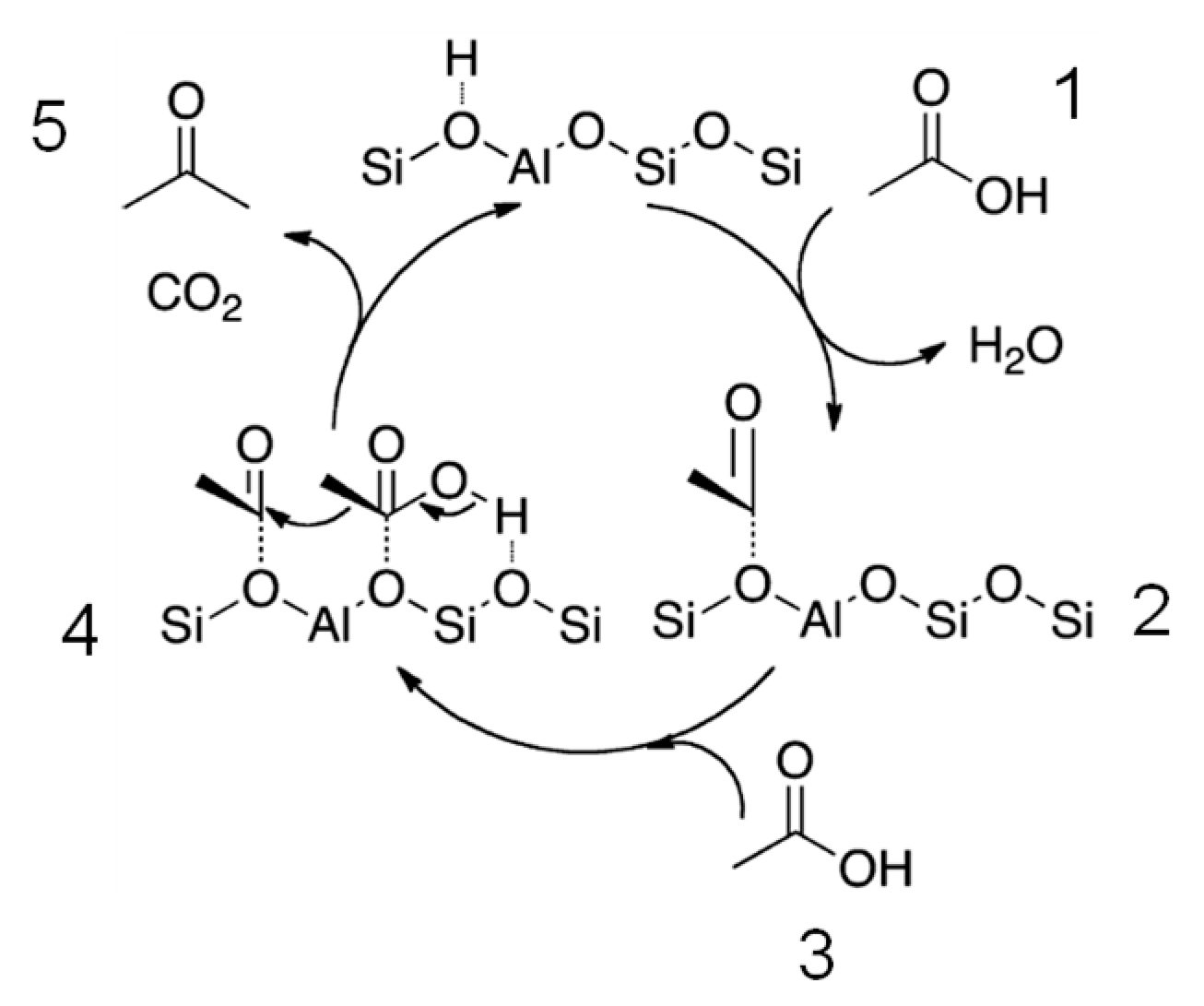
- Adsorption of carboxylic acid with elimination of water,
- Surface acyl formation,
- Surface adsorption of second carboxylic acid,
- Coupling of adsorbed carboxylic acid with a surface acyl group to form an acid anhydride,
- Adsorbed anhydride decomposition to form CO2 and acetone.
2. Results and Discussion
2.1. Catalyst Characterisation
2.2. Catalytic Activity in Ketonisation
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterisation
3.3. Catalytic Ketonisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wilson, K.; Lee, A.F. Catalyst design for biorefining. Philos. Trans. R. Soc. A 2016, 374, 20150081. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Leitner, W.; Klankermayer, J.; Pischinger, S.; Pitsch, H.; Kohse-Höinghaus, K. Advanced Biofuels and Beyond: Chemistry Solutions for Propulsion and Production. Angew. Chem. Int. Ed. 2017, 56, 5412–5452. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Santos, J.; Ouadi, M.; Jahangiri, H.; Hornung, A. Integrated intermediate catalytic pyrolysis of wheat husk. Food Bioprod. Process. 2019, 114, 23–30. [Google Scholar] [CrossRef]
- Yung, M.M.; Jablonski, W.S.; Magrini-Bair, K.A. Review of Catalytic Conditioning of Biomass-Derived Syngas. Energy Fuels 2009, 23, 1874–1887. [Google Scholar] [CrossRef]
- Ouadi, M.; Fivga, A.; Jahangiri, H.; Saghir, M.; Hornung, A. A Review of the Valorization of Paper Industry Wastes by Thermochemical Conversion. Ind. Eng. Chem. Res. 2019, 58, 15914–15929. [Google Scholar] [CrossRef]
- Jahangiri, H.; Bennett, J.; Mahjoubi, P.; Wilson, K.; Gu, S. A review of advanced catalyst development for Fischer-Tropsch synthesis of hydrocarbons from biomass derived syn-gas. Catal. Sci. Technol. 2014, 4, 2210–2229. [Google Scholar] [CrossRef]
- Sartipi, S.; Makkee, M.; Kapteijn, F.; Gascon, J. Catalysis engineering of bifunctional solids for the one-step synthesis of liquid fuels from syngas: A review. Catal. Sci. Technol. 2014, 4, 893–907. [Google Scholar] [CrossRef]
- Hassan, H.; Lim, J.K.; Hameed, B.H. Recent progress on biomass co-pyrolysis conversion into high-quality bio-oil. Bioresour. Technol. 2016, 221, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Papari, S.; Hawboldt, K. A review on the pyrolysis of woody biomass to bio-oil: Focus on kinetic models. Renew. Sustain. Energy Rev. 2015, 52, 1580–1595. [Google Scholar] [CrossRef]
- Sfetsas, T.; Michailof, C.; Lappas, A.; Li, Q.; Kneale, B. Qualitative and quantitative analysis of pyrolysis oil by gas chromatography with flame ionization detection and comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 3317–3325. [Google Scholar] [CrossRef]
- Ciddor, L.; Bennett, J.A.; Hunns, J.A.; Wilson, K.; Lee, A.F. Catalytic upgrading of bio-oils by esterification. J. Chem. Technol. Biotechnol. 2015, 90, 780–795. [Google Scholar] [CrossRef]
- Pirez, C.; Caderon, J.-M.; Dacquin, J.-P.; Lee, A.F.; Wilson, K. Tunable KIT-6 Mesoporous Sulfonic Acid Catalysts for Fatty Acid Esterification. ACS Catal. 2012, 2, 1607–1614. [Google Scholar] [CrossRef]
- Zacher, A.H.; Olarte, M.V.; Santosa, D.M.; Elliott, D.C.; Jones, S.B. A review and perspective of recent bio-oil hydrotreating research. Green Chem. 2014, 16, 491–515. [Google Scholar] [CrossRef]
- Snell, R.W.; Combs, E.; Shanks, B.H. Aldol Condensations Using Bio-oil Model Compounds: The Role of Acid–Base Bi-functionality. Top. Catal. 2010, 53, 1248–1253. [Google Scholar] [CrossRef]
- Jahangiri, H.; Osatiashtiani, A.; Bennett, J.A.; Isaacs, M.A.; Gu, S.; Lee, A.F.; Wilson, K. Zirconia catalysed acetic acid ketonisation for pre-treatment of biomass fast pyrolysis vapours. Catal. Sci. Technol. 2018, 8, 1134–1141. [Google Scholar] [CrossRef]
- Manayil, J.C.; Inocencio, C.V.; Lee, A.F.; Wilson, K. Mesoporous sulfonic acid silicas for pyrolysis bio-oil upgrading via acetic acid esterification. Green Chem. 2016, 18, 1387–1394. [Google Scholar] [CrossRef]
- Marker, T.L.; Felix, L.G.; Linck, M.B.; Roberts, M.J. Integrated hydropyrolysis and hydroconversion (IH 2) for the direct production of gasoline and diesel fuels or blending components from biomass, part 1: Proof of principle testing. Environ. Prog. Sustain. Energy 2012, 31, 191–199. [Google Scholar] [CrossRef]
- Bennett, J.A.; Parlett, C.M.A.; Isaacs, M.A.; Durndell, L.J.; Olivi, L.; Lee, A.F.; Wilson, K. Acetic Acid Ketonization over Fe3O4/SiO2 for Pyrolysis Bio-Oil Upgrading. ChemCatChem 2017, 9, 1648–1654. [Google Scholar] [CrossRef]
- Pham, T.N.; Shi, D.; Resasco, D.E. Kinetics and Mechanism of Ketonization of Acetic Acid on Ru/TiO2 Catalyst. Top. Catal. 2014, 57, 706–714. [Google Scholar] [CrossRef]
- Heracleous, E.; Gu, D.; Schüth, F.; Bennett, J.A.; Isaacs, M.A.; Lee, A.F.; Wilson, K.; Lappas, A.A. Bio-oil upgrading via vapor-phase ketonization over nanostructured FeO x and MnO x: Catalytic performance and mechanistic insight. Biomass Convers. Biorefinery 2017, 7, 319–329. [Google Scholar] [CrossRef]
- Pham, T.N.; Sooknoi, T.; Crossley, S.P.; Resasco, D.E. Ketonization of Carboxylic Acids: Mechanisms, Catalysts, and Implications for Biomass Conversion. ACS Catal. 2013, 3, 2456–2473. [Google Scholar] [CrossRef]
- Gaertner, C.A.; Serrano-Ruiz, J.C.; Braden, D.J.; Dumesic, J.A. Ketonization Reactions of Carboxylic Acids and Esters over Ceria−Zirconia as Biomass-Upgrading Processes. Ind. Eng. Chem. Res. 2010, 49, 6027–6033. [Google Scholar] [CrossRef]
- Wortz, C.G. Process for the Production of Ketones. U.S. Patent 2,108,156, 15 February 1938. [Google Scholar]
- Nagashima, O.; Sato, S.; Takahashi, R.; Sodesawa, T. Ketonization of carboxylic acids over CeO2-based composite oxides. J. Mol. Catal. A Chem. 2005, 227, 231–239. [Google Scholar] [CrossRef]
- Pestman, R.; Koster, R.M.; van Duijne, A.; Pieterse, J.A.Z.; Ponec, V. Reactions of carboxylic acids on oxides. 2. Bimolecular reaction of aliphatic acids to ketones. J. Catal. 1997, 168, 265–272. [Google Scholar] [CrossRef]
- Gliński, M.; Kijeński, J. Catalytic Ketonization of Carboxylic Acids Synthesis of Saturated and Unsaturated Ketones. React. Kinet. Catal. Lett. 2000, 69, 123–128. [Google Scholar] [CrossRef]
- Parida, K.M.; Samal, A.; Das, N.N. Catalytic ketonization of monocarboxylic acids over Indian Ocean manganese nodules. Appl. Catal. A Gen. 1998, 166, 201–205. [Google Scholar] [CrossRef]
- Martinez, R.; Huff, M.C.; Barteau, M.A. Ketonization of acetic acid on titania-functionalized silica monoliths. J. Catal. 2004, 222, 404–409. [Google Scholar] [CrossRef]
- Kim, K.S.; Barteau, M.A. Structure and Composition Requirements for Deoxygenation, Dehydration, and Ketonization Reactions of Carboxylic-Acids on Tio2 (001) Single-Crystal Surfaces. J. Catal. 1990, 125, 353–375. [Google Scholar] [CrossRef]
- Pestman, R.; Koster, R.M.; Pieterse, J.A.Z.; Ponec, V. Reactions of carboxylic acids on oxides. 1. Selective hydrogenation of acetic acid to acetaldehyde. J. Catal. 1997, 168, 255–264. [Google Scholar] [CrossRef]
- Randery, S.D.; Warren, J.S.; Dooley, K.M. Cerium oxide-based catalysts for production of ketones by acid condensation. Appl. Catal. A Gen. 2002, 226, 265–280. [Google Scholar] [CrossRef]
- Dooley, K.M.; Bhat, A.K.; Plaisance, C.P.; Roy, A.D. Ketones from acid condensation using supported CeO2 catalysts: Effect of additives. Appl. Catal. A Gen. 2007, 320, 122–133. [Google Scholar] [CrossRef]
- Gliński, M.; Kijeński, J. Decarboxylative coupling of heptanoic acid. Manganese, cerium and zirconium oxides as catalysts. Appl. Catal. A Gen. 2000, 190, 87–91. [Google Scholar]
- Okumura, K.; Iwasawa, Y. Zirconium Oxides Dispersed on Silica Derived from Cp2ZrCl2, [(i-PrCp)2ZrH(μ-H)]2, and Zr(OEt)4Characterized by X-Ray Absorption Fine Structure and Catalytic Ketonization of Acetic Acid. J. Catal. 1996, 164, 440–448. [Google Scholar] [CrossRef]
- Kistler, S.S.; Swann, S.; Appel, E.G. Aërogel Catalysts—Thoria: Preparation of Catalyst and Conversions of Organic Acids to Ketones. Ind. Eng. Chem. 1934, 26, 388–391. [Google Scholar] [CrossRef]
- Pacchioni, G. Ketonization of Carboxylic Acids in Biomass Conversion over TiO2 and ZrO2 Surfaces: A DFT Perspective. ACS Catal. 2014, 4, 2874–2888. [Google Scholar] [CrossRef]
- Patil, K.C.; Chandrashekhar, G.V.; George, M.V.; Rao, C.N.R. Infrared spectra and thermal decompositions of metal acetates and dicarboxylates. Can. J. Chem. 1968, 46, 257–265. [Google Scholar] [CrossRef]
- Vervecken, M.; Servotte, Y.; Wydoodt, M.; Jacobs, L.; Martens, J.A.; Jacobs, P.A. Zeolite-Induced Selectivity in the Conversion of the Lower Aliphatic Carboxylic Acids. In Chemical Reactions in Organic and Inorganic Constrained Systems; Setton, R., Ed.; Springer: Dordrecht, The Netherlands, 1986; pp. 95–114. [Google Scholar]
- Iliopoulou, E.F.; Stefanidis, S.D.; Kalogiannis, K.G.; Delimitis, A.; Lappas, A.A.; Triantafyllidis, K.S. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B-Environ. 2012, 127, 281–290. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Neumann, G.T.; Hicks, J.C. Effects of Cerium and Aluminum in Cerium-Containing Hierarchical HZSM-5 Catalysts for Biomass Upgrading. Top. Catal. 2012, 55, 196–208. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Jae, J.; Shi, J.; Fan, W.; Huber, G.W. Production of Renewable Aromatic Compounds by Catalytic Fast Pyrolysis of Lignocellulosic Biomass with Bifunctional Ga/ZSM-5 Catalysts. Angew. Chem. Int. Ed. 2012, 51, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Gumidyala, A.; Sooknoi, T.; Crossley, S. Selective ketonization of acetic acid over HZSM-5: The importance of acyl species and the influence of water. J. Catal. 2016, 340, 76–84. [Google Scholar] [CrossRef]
- Chang, C.D.; Chen, N.Y.; Koenig, L.R.; Walsh, D.E. Synergism in Acetic-Acid Methanol Reactions over Zsm-5 Zeolites. Abstr. Pap. Am. Chem. Soc. 1983, 185, 49-Fuel. [Google Scholar]
- Tessonnier, J.-P.; Louis, B.; Walspurger, S.; Sommer, J.; Ledoux, M.-J.; Pham-Huu, C. Quantitative Measurement of the Brönsted Acid Sites in Solid Acids: Toward a Single-Site Design of Mo-Modified ZSM-5 Zeolite. J. Phys. Chem. B 2006, 110, 10390–10395. [Google Scholar] [CrossRef]
- Li, B.; Li, S.; Li, N.; Chen, H.; Zhang, W.; Bao, X.; Lin, B. Structure and acidity of Mo/ZSM-5 synthesized by solid state reaction for methane dehydrogenation and aromatization. Microporous Mesoporous Mater. 2006, 88, 244–253. [Google Scholar] [CrossRef]
- Fang, Y.; Su, X.; Bai, X.; Wu, W.; Wang, G.; Xiao, L.; Yu, A. Aromatization over nanosized Ga-containing ZSM-5 zeolites prepared by different methods: Effect of acidity of active Ga species on the catalytic performance. J. Energy Chem. 2017, 26, 768–775. [Google Scholar] [CrossRef]
- Amin, N.A.S.; Ali, A. Characterization of Modified HZSM–5 with Gallium and its Reactivity in Direct Conversion of Methane to Liquid Hydrocarbons. J. Teknol. 2001, 35, 21–30. [Google Scholar]
- Li, J.Y.; Chen, X.L.; Qiao, Z.Y.; He, M.; Li, H. Large-scale synthesis of single-crystalline beta-Ga2O3 nanoribbons, nanosheets and nanowires. J. Phys. Condens. Matter 2001, 13, L937–L941. [Google Scholar] [CrossRef]
- Huang, C.-C.; Yeh, C.-S. GaOOH, and [small beta]- and [gamma]-Ga2O3 nanowires: Preparation and photoluminescence. New J. Chem. 2010, 34, 103–107. [Google Scholar] [CrossRef]
- Wang, S.; Yin, Q.; Guo, J.; Ru, B.; Zhu, L. Improved Fischer—Tropsch synthesis for gasoline over Ru, Ni promoted Co/HZSM-5 catalysts. Fuel 2013, 108, 597–603. [Google Scholar] [CrossRef]
- Rodrigues, V.D.; Eon, J.G.; Faro, A.C. Correlations between Dispersion, Acidity, Reducibility, and Propane Aromatization Activity of Gallium Species Supported on HZSM5 Zeolites. J. Phys. Chem. C 2010, 114, 4557–4567. [Google Scholar] [CrossRef]
- Tamba, D.; Kubo, O.; Oda, M.; Osaka, S.; Takahashi, K.; Tabata, H.; Kaneko, K.; Fujita, S.; Katayama, M. Surface termination structure of α-Ga2O3 film grown by mist chemical vapor deposition. Appl. Phys. Lett. 2016, 108, 251602. [Google Scholar] [CrossRef]
- Grunert, W.; Muhler, M.; Schroder, K.P.; Sauer, J.; Schlogl, R. Investigations of Zeolites by Photoelectron and Ion-Scattering Spectroscopy.2. A New Interpretation of Xps Binding-Energy Shifts in Zeolites. J. Phys. Chem. 1994, 98, 10920–10929. [Google Scholar] [CrossRef]
- Borade, R.B.; Clearfield, A. Characterization of Acid Sites in Beta and Zsm-20 Zeolites. J. Phys. Chem. 1992, 96, 6729–6737. [Google Scholar] [CrossRef]
- Guo, D.; Wu, Z.; An, Y.; Li, P.; Wang, P.; Chu, X.; Guo, X.; Zhi, Y.; Lei, M.; Li, L. Unipolar resistive switching behavior of amorphous gallium oxide thin films for nonvolatile memory applications. Appl. Phys. Lett. 2015, 106, 042105. [Google Scholar] [CrossRef]
- Wei, W.; Qin, Z.; Fan, S.; Li, Z.; Shi, K.; Zhu, Q.; Zhang, G. Valence band offset of β-Ga2O3/wurtzite GaN heterostructure measured by X-ray photoelectron spectroscopy. Nanoscale Res. Lett. 2012, 7, 1–5. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.F.; Wang, X.X.; Zhang, Q.D.; Xie, H.J.; Han, Y.Z.; Tan, Y.S. A highly efficient Ga/ZSM-5 catalyst prepared by formic acid impregnation and in situ treatment for propane aromatization. Catal. Sci. Technol. 2015, 5, 4081–4090. [Google Scholar] [CrossRef]
- Carli, R.; Bianchi, C.L. Xps Analysis of Gallium Oxides. Appl. Surf. Sci. 1994, 74, 99–102. [Google Scholar] [CrossRef]
- Altwasser, S.; Raichle, A.; Traa, Y.; Weitkamp, J. Preparation of gallium-containing catalysts by solid-state reaction of acidic Zeolites with elemental gallium. Chem. Eng. Technol. 2004, 27, 1262–1265. [Google Scholar] [CrossRef]
- Anunziata, O.A.; Pierella, L.B. Nature of the Active-Sites in H-Zsm-11 Zeolite Modified with Zn(2+) and Ga(3+). Catal. Lett. 1993, 19, 143–151. [Google Scholar] [CrossRef]
- Lavalley, J.C.; Daturi, M.; Montouillout, V.; Clet, G.; Arean, C.O.; Delgado, M.R.; Sahibed-dine, A. Unexpected similarities between the surface chemistry of cubic and hexagonal gallia polymorphs. Phys. Chem. Chem. Phys. 2003, 5, 1301–1305. [Google Scholar] [CrossRef]
- Vimont, A.; Lavalley, J.C.; Sahibed-Dine, A.; Arean, C.O.; Delgado, M.R.; Daturi, M. Infrared spectroscopic study on the surface properties of gamma-gallium oxide as compared to those of gamma-alumina. J. Phys. Chem. B 2005, 109, 9656–9664. [Google Scholar] [CrossRef] [PubMed]
- Phumman, P.; Niamlang, S.; Sirivat, A. Fabrication of Poly(p-Phenylene)/Zeolite Composites and Their Responses Towards Ammonia. Sensors 2009, 9, 8031–8046. [Google Scholar] [CrossRef]
- Morterra, C.; Cerrato, G.; Ferroni, L.; Negro, A.; Montanaro, L. Surface characterization of tetragonal ZrO2. Appl. Surf. Sci. 1993, 65, 257–264. [Google Scholar] [CrossRef]
- Dompas, D.H.; Mortier, W.J.; Kenter, O.C.H.; Janssen, M.J.G.; Verduijn, J.P. The influence of framework-gallium in zeolites: Electronegativity and infrared spectroscopic study. J. Catal. 1991, 129, 19–24. [Google Scholar] [CrossRef]
- Pulido, A.; Oliver-Tomas, B.; Renz, M.; Boronat, M.; Corma, A. Ketonic Decarboxylation Reaction Mechanism: A Combined Experimental and DFT Study. ChemSusChem 2013, 6, 141–151. [Google Scholar] [CrossRef]
- Wang, S.; Iglesia, E. Experimental and Theoretical Evidence for the Reactivity of Bound Intermediates in Ketonization of Carboxylic Acids and Consequences of Acid–Base Properties of Oxide Catalysts. J. Phys. Chem. C 2017, 121, 18030–18046. [Google Scholar] [CrossRef]
- Schreiber, M.W.; Plaisance, C.P.; Baumgartl, M.; Reuter, K.; Jentys, A.; Bermejo-Deval, R.; Lercher, J.A. Lewis-Bronsted Acid Pairs in Ga/H-ZSM-5 To Catalyze Dehydrogenation of Light Alkanes. J. Am. Chem. Soc. 2018, 140, 4849–4859. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, A.; Österlund, L. Adsorption and Photoinduced Decomposition of Acetone and Acetic Acid on Anatase, Brookite, and Rutile TiO2 Nanoparticles. J. Phys. Chem. C 2010, 114, 14121–14132. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Y.; Liu, C.; He, H. Heterogeneous reaction of acetic acid on MgO, [small alpha]-Al2O3, and CaCO3 and the effect on the hygroscopic behaviour of these particles. Phys. Chem. Chem. Phys. 2012, 14, 8403–8409. [Google Scholar] [CrossRef] [PubMed]
- Finocchio, E.; Willey, R.J.; Busca, G.; Lorenzelli, V. FTIR studies on the selective oxidation and combustion of light hydrocarbons at metal oxide surfaces Part 3.-Comparison of the oxidation of C3 organic compounds over Co3O4, MgCr2O4 and CuO, Journal of the Chemical Society. Faraday Trans. 1997, 93, 175–180. [Google Scholar] [CrossRef]
- Geiculescu, A.C.; Spencer, H.G. Thermal Decomposition and Crystallization of Aqueous Sol-Gel Derived Zirconium Acetate Gels: Effects of the Additive Anions. J. Sol-Gel Sci. Technol. 2000, 17, 25–35. [Google Scholar] [CrossRef]

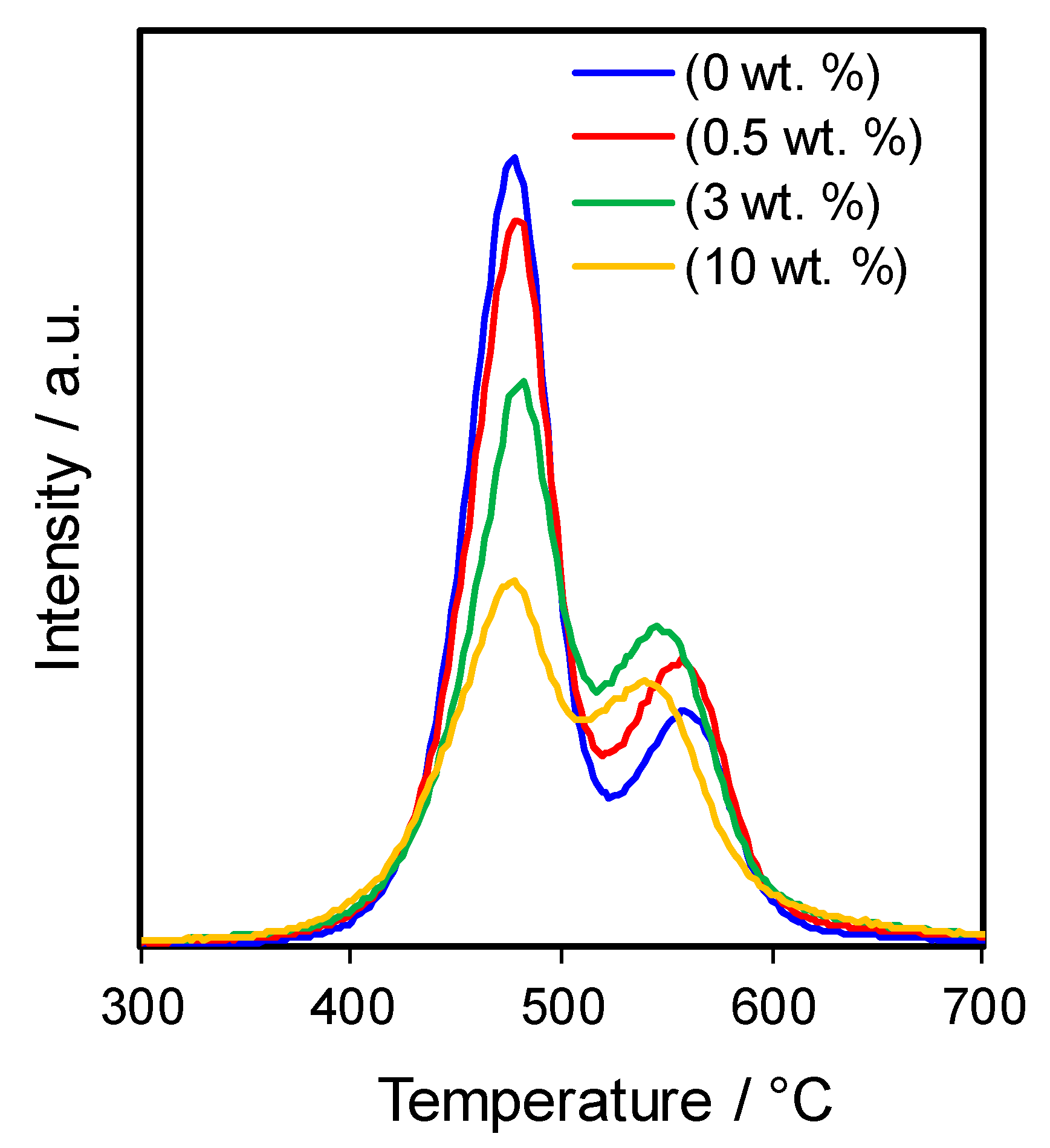
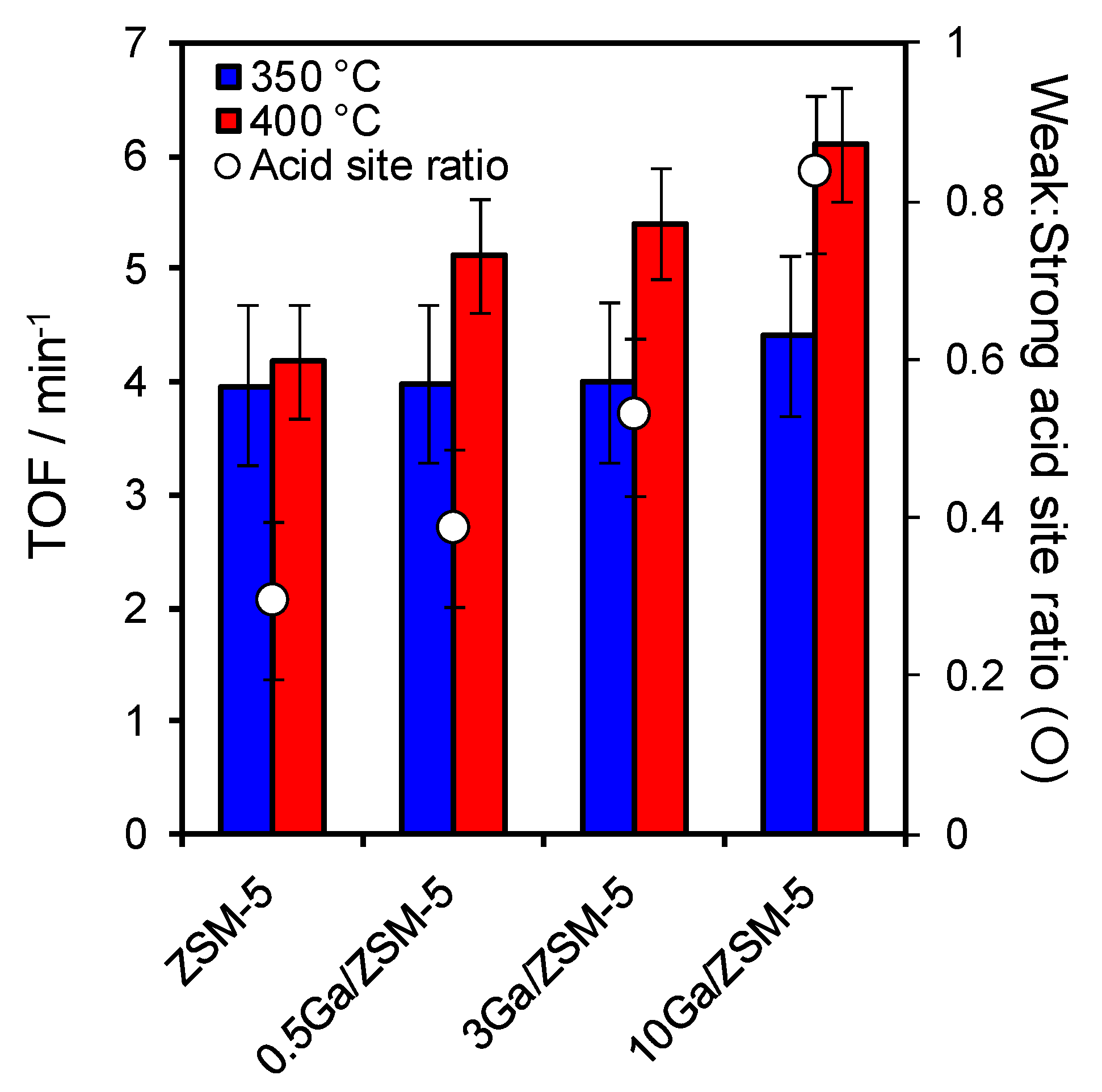
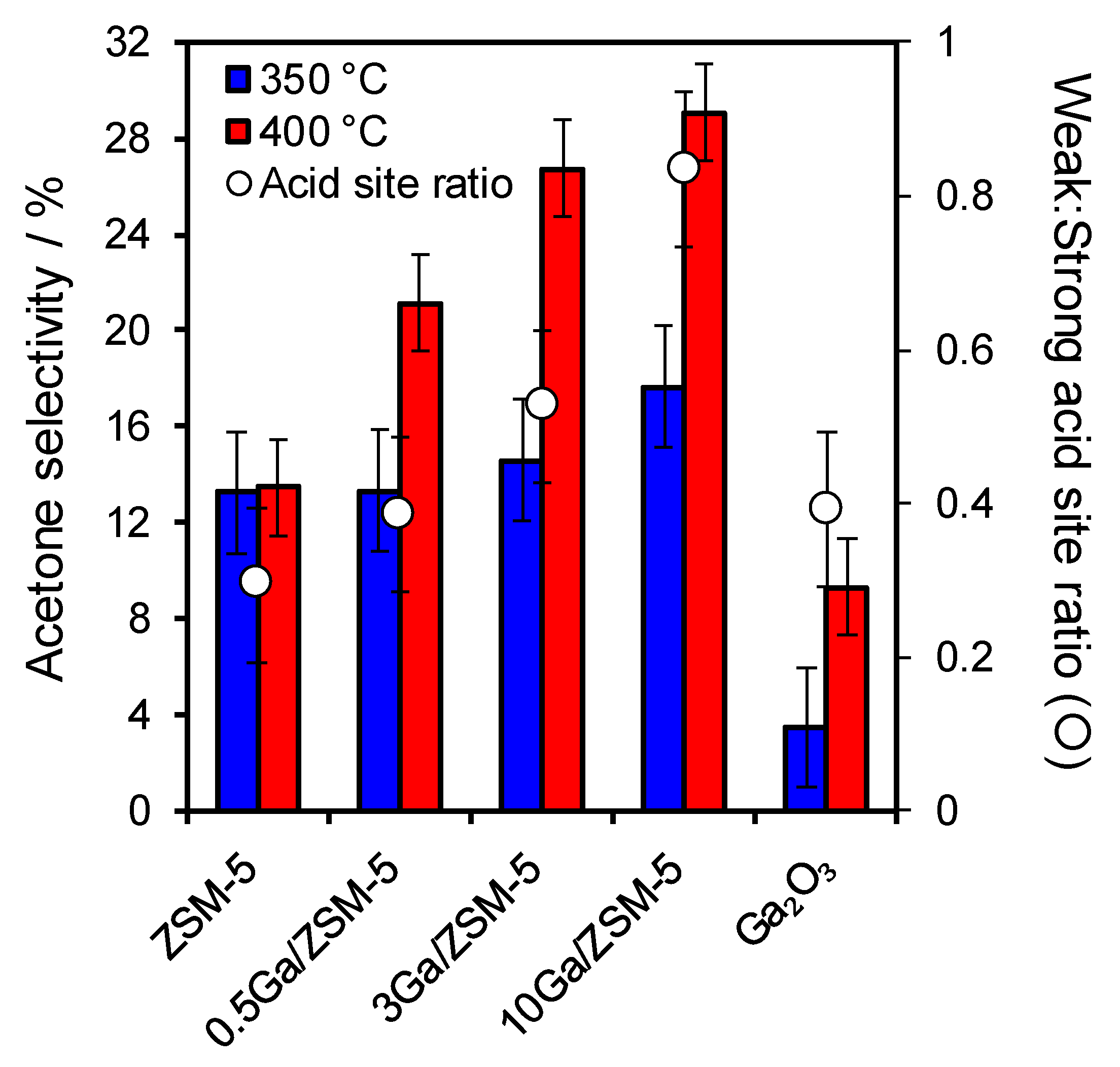
| Catalysts | Ga Loading a /wt% | SBET /m2·g−1 | VTotal b /mL·g−1 | Vmicro c /mL·g−1 | Crystallite Size d /nm | Acid Loading e /mmol.g−1 | Weak:Strong Acid Site Ratio f |
|---|---|---|---|---|---|---|---|
| HZSM-5 | 0 | 427 | 0.294 | 0.141 | 65.7 | 1.13 | 0.29 |
| 0.5 Ga/HZSM-5 | 0.3 | 420 | 0.290 | 0.140 | 55.2 | 1.09 | 0.38 |
| 3 Ga/HZSM-5 | 3.0 | 338 | 0.249 | 0.106 | 50.3 | 1.03 | 0.53 |
| 10 Ga/HZSM-5 | 9.0 | 313 | 0.210 | 0.099 | 62.6 | 0.80 | 0.83 |
| Ga2O3 | 75 | 7.6 | 0.08 | − | 26.4 | 0.14 | 0.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahangiri, H.; Osatiashtiani, A.; Ouadi, M.; Hornung, A.; Lee, A.F.; Wilson, K. Ga/HZSM-5 Catalysed Acetic Acid Ketonisation for Upgrading of Biomass Pyrolysis Vapours. Catalysts 2019, 9, 841. https://doi.org/10.3390/catal9100841
Jahangiri H, Osatiashtiani A, Ouadi M, Hornung A, Lee AF, Wilson K. Ga/HZSM-5 Catalysed Acetic Acid Ketonisation for Upgrading of Biomass Pyrolysis Vapours. Catalysts. 2019; 9(10):841. https://doi.org/10.3390/catal9100841
Chicago/Turabian StyleJahangiri, Hessam, Amin Osatiashtiani, Miloud Ouadi, Andreas Hornung, Adam F. Lee, and Karen Wilson. 2019. "Ga/HZSM-5 Catalysed Acetic Acid Ketonisation for Upgrading of Biomass Pyrolysis Vapours" Catalysts 9, no. 10: 841. https://doi.org/10.3390/catal9100841
APA StyleJahangiri, H., Osatiashtiani, A., Ouadi, M., Hornung, A., Lee, A. F., & Wilson, K. (2019). Ga/HZSM-5 Catalysed Acetic Acid Ketonisation for Upgrading of Biomass Pyrolysis Vapours. Catalysts, 9(10), 841. https://doi.org/10.3390/catal9100841






