Formation of a Pd/MgO Structured Catalyst for the Aqueous Oxidation of Silane to Silanol
Abstract
1. Introduction
2. Results and Discussion
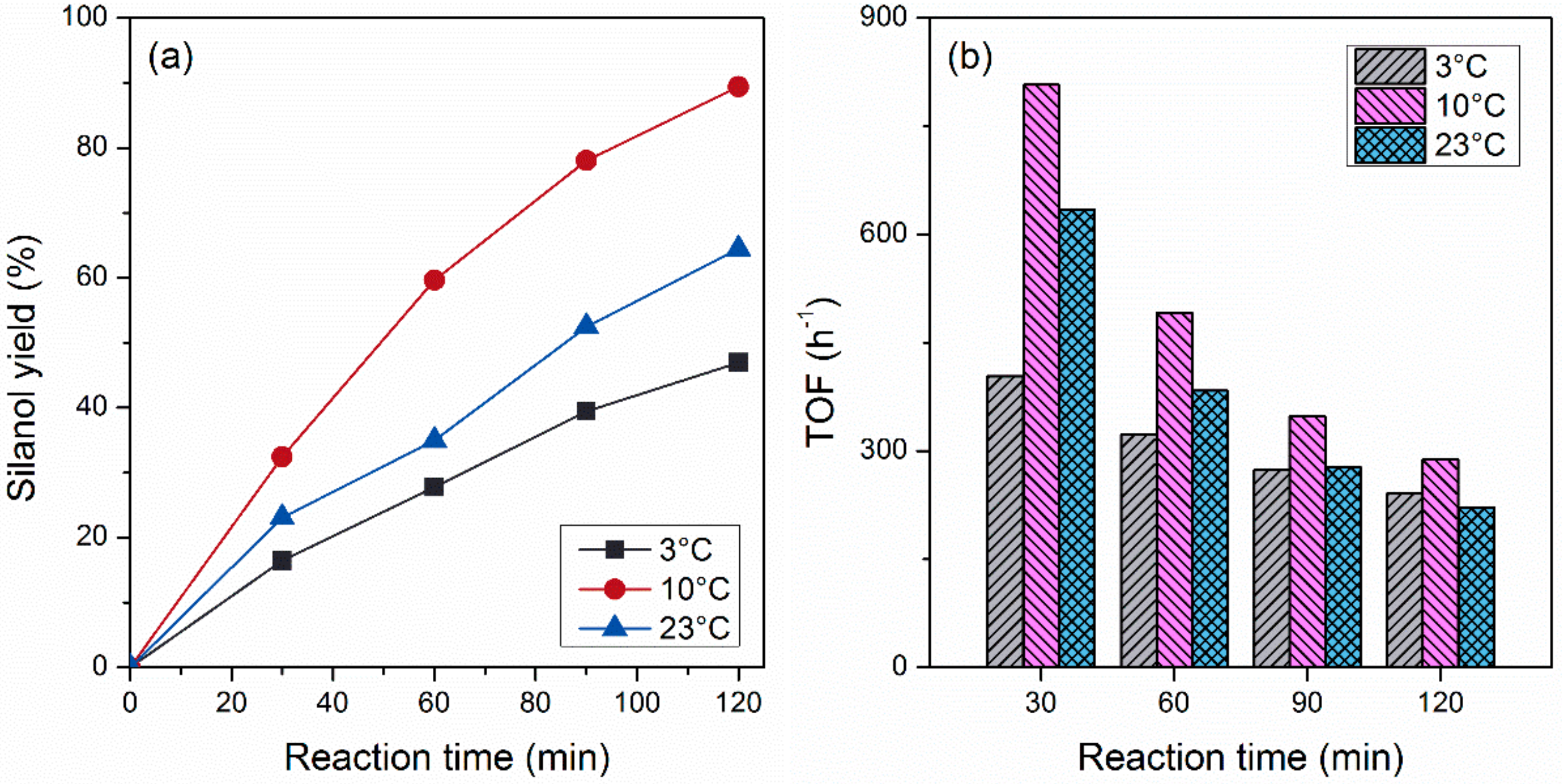
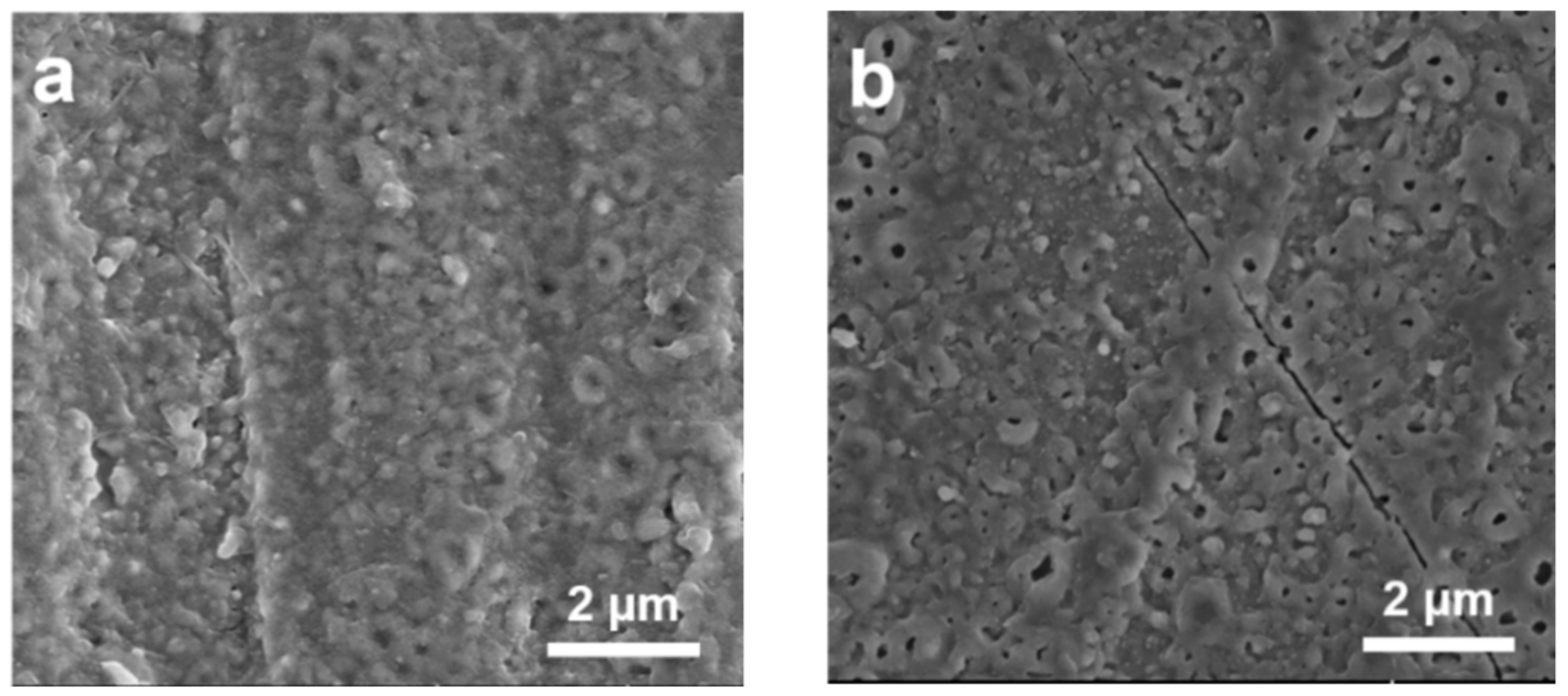
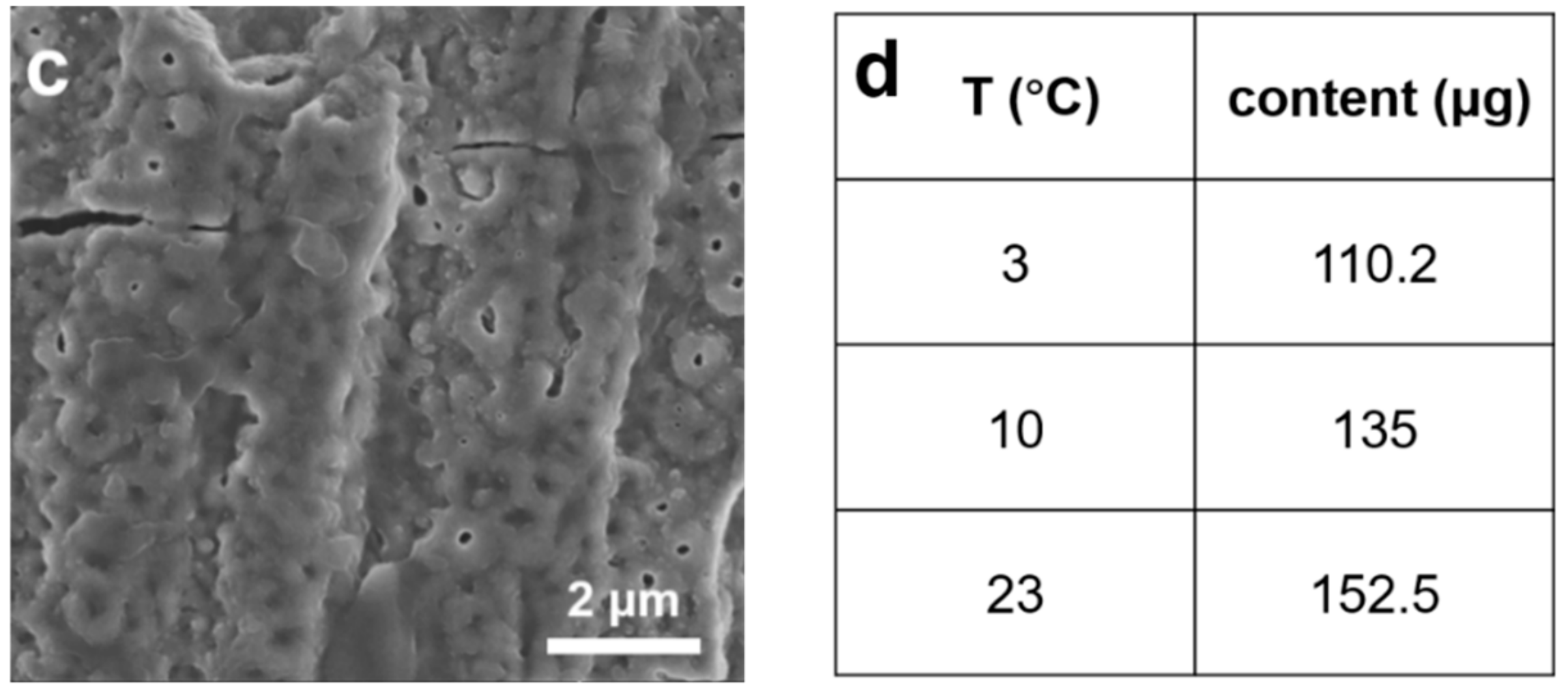
2.1. Effects of Electrolyte Temperature on Reactivity of Pd Nanocatalysts
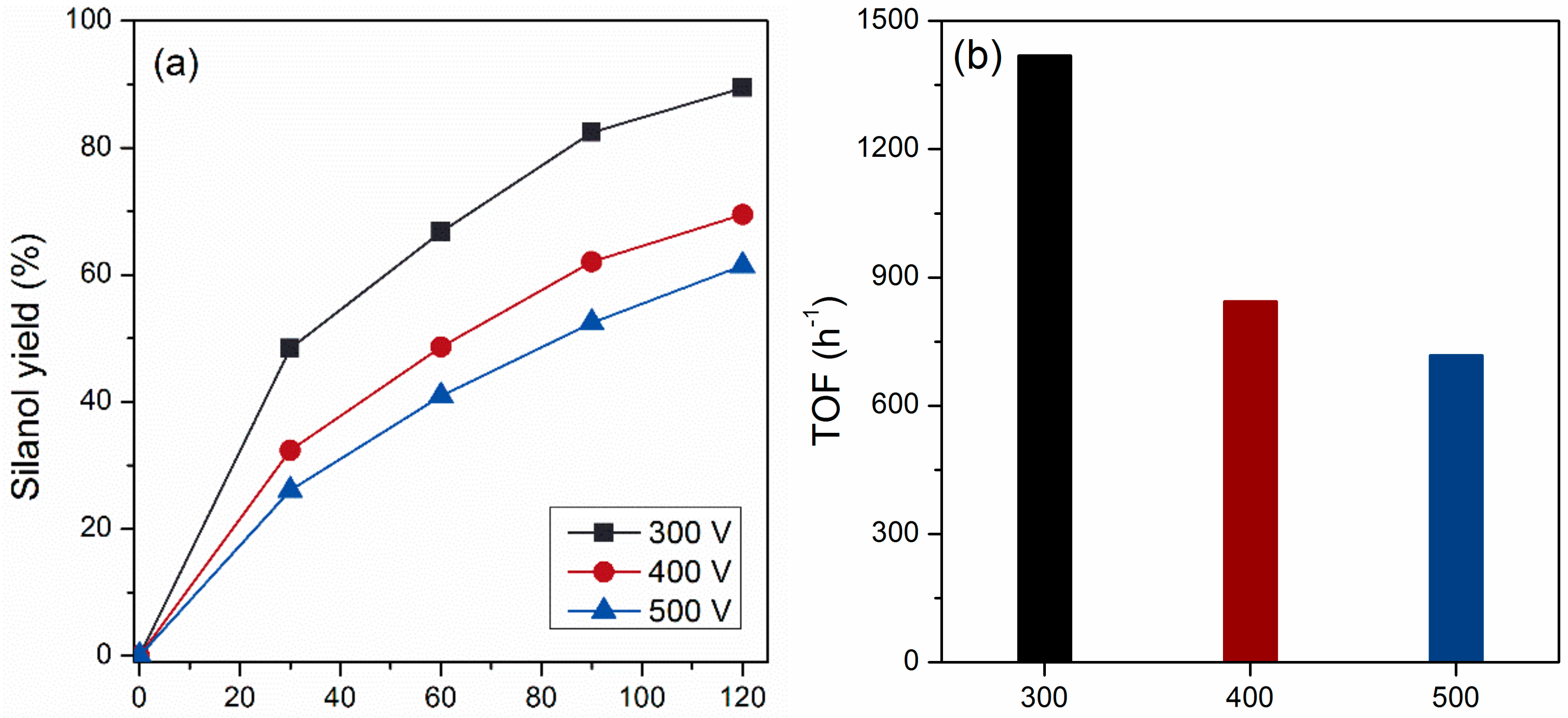
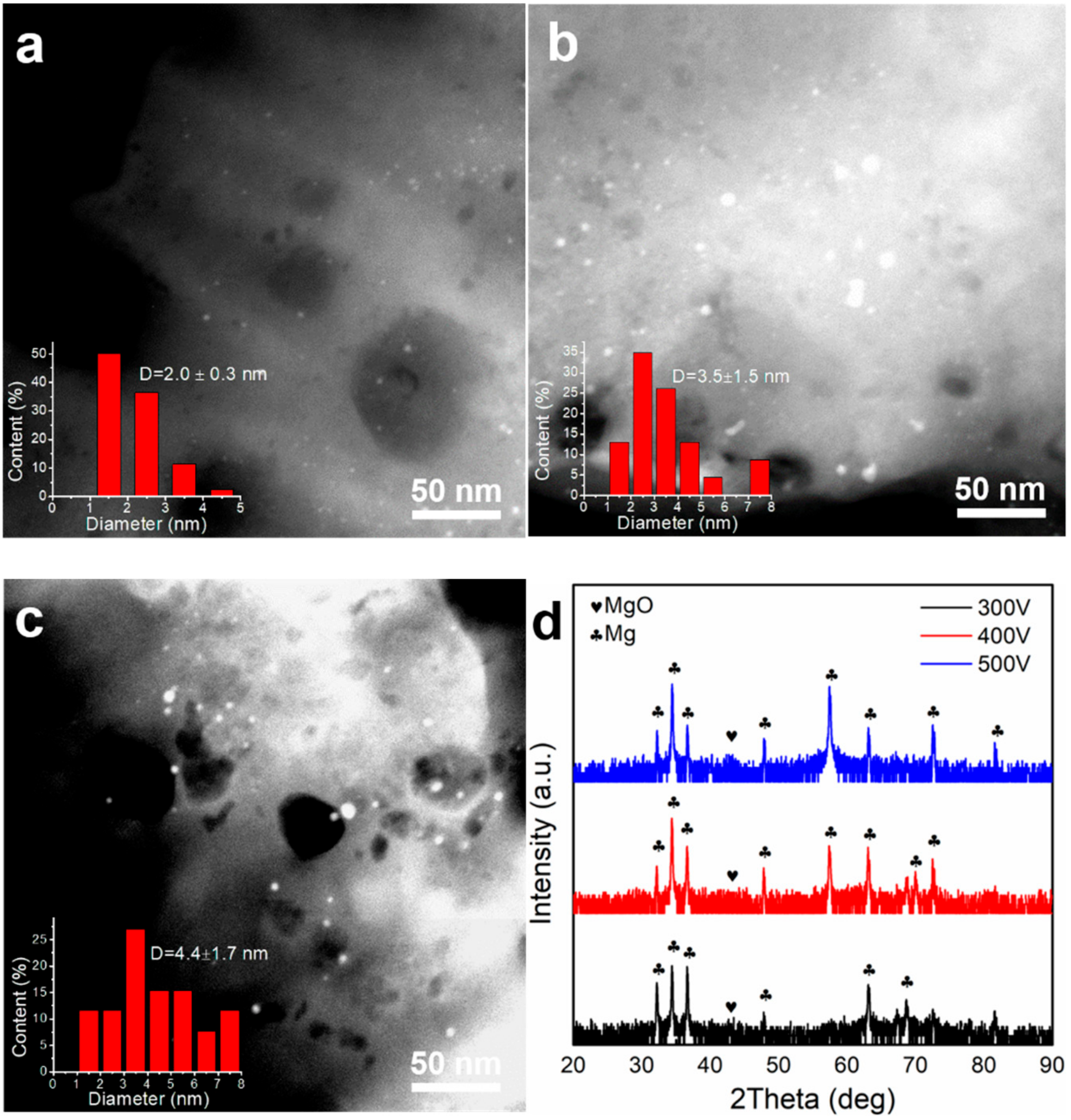
2.2. Influence of Applied Voltage on the Performance of PEO Prepared Nanocatalysts
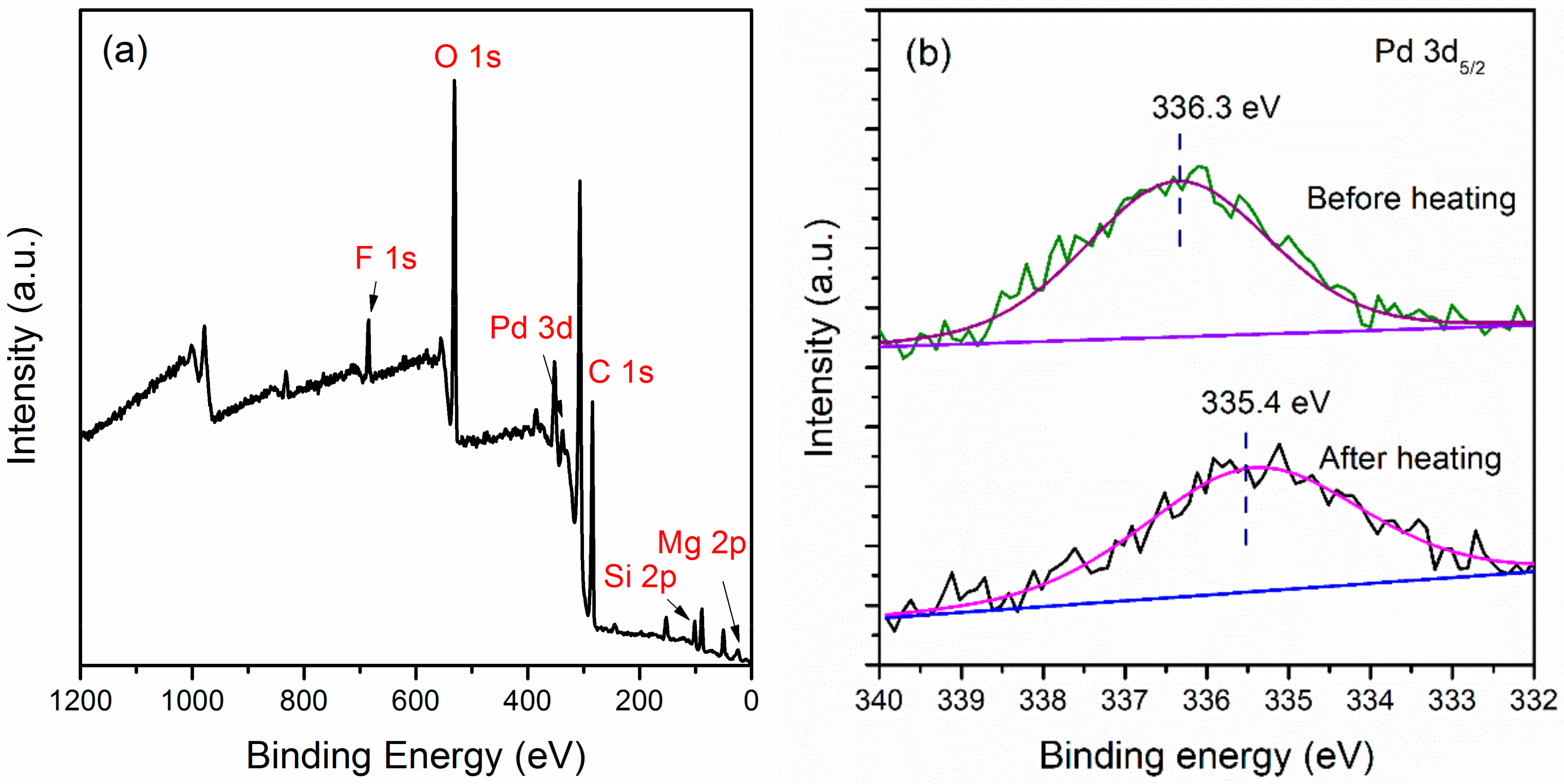
2.3. Influence of the Chemical States of Pd
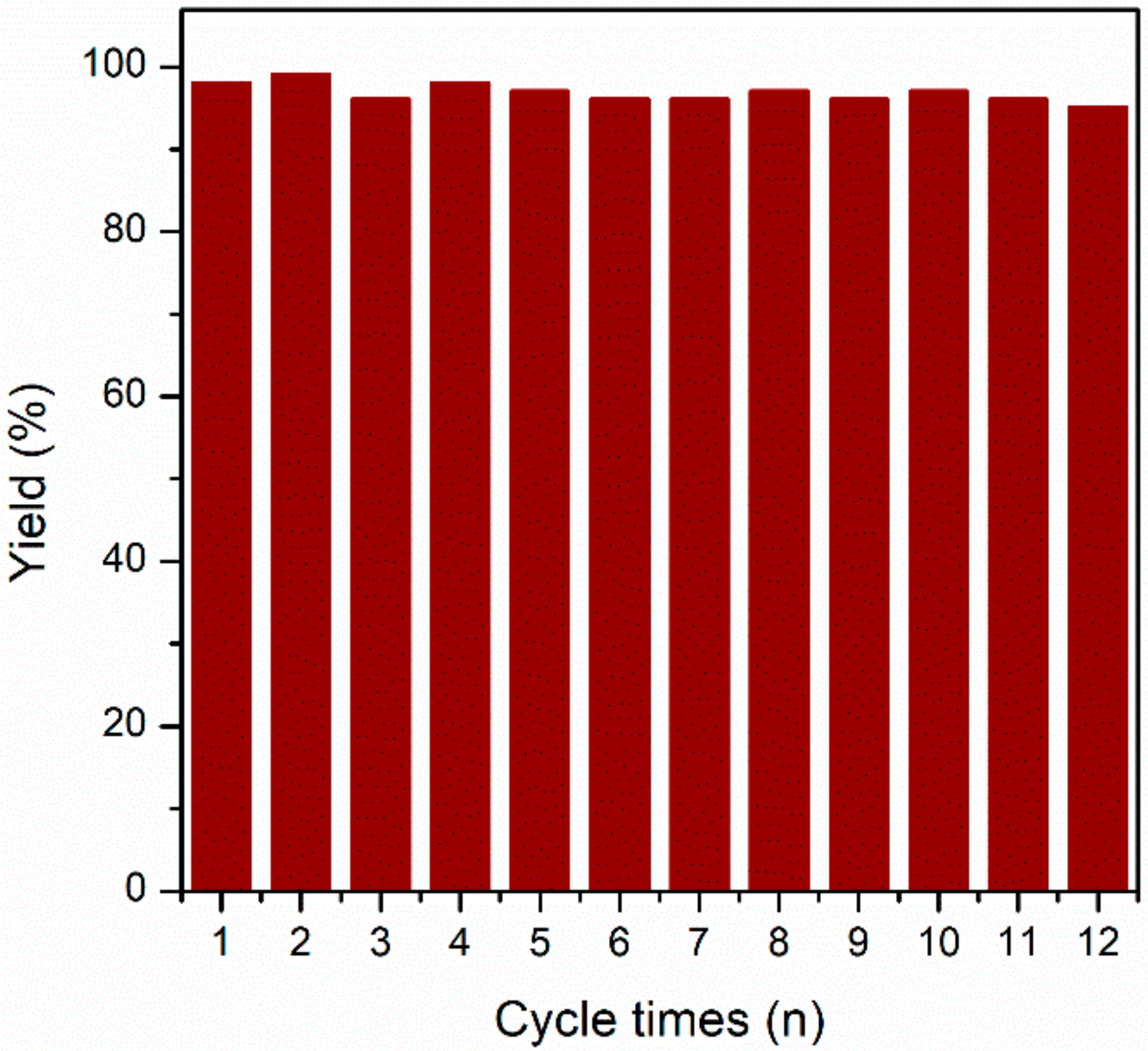
2.4. Cycling Stability and Convenience
3. Experimental Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of the Pd/MgO Catalyst Using Different Electrolyte Temperatures
3.2.2. Preparation of the Pd/MgO Catalyst Using Different Applied Voltages
3.2.3. Sample Characterization
3.2.4. Catalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Wang, L.; Zhang, B.; Zhao, H.; Kolb, U.; Zhu, Y.; Liu, L.; Han, Y.; Wang, G.; Wang, C.; et al. Sinter-resistant metal nanoparticle catalysts achieved by immobilization within zeolite crystals via seed-directed growth. Nat. Catal. 2018, 1, 540–546. [Google Scholar] [CrossRef]
- Campbell, C.T.; Mao, Z. Chemical Potential of Metal Atoms in Supported Nanoparticles: Dependence upon Particle Size and Support. ACS Catal. 2017, 7, 8460–8466. [Google Scholar] [CrossRef]
- Grillo, F.; Moulijn, J.A.; Kreutzer, M.T.; van Ommen, J.R. Nanoparticle sintering in atomic layer deposition of supported catalysts: Kinetic modeling of the size distribution. Catal. Today 2018, 316, 51–61. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Xu, C.; Sun, Y.; Li, S.; Jiang, M.; Qin, G. Control of Catalytic Activity of Nano-Au through Tailoring the Fermi Level of Support. Small 2019, 15, 1901789. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, F.; Lin, W.; Zhao, F.; Zhou, J.; Li, S.; Qin, G. In situ synthesis of Ni/NiO composites with defect-rich ultrathin nanosheets for highly efficient biomass-derivative selective hydrogenation. J. Mater. Chem. A 2019, 7, 17834–17841. [Google Scholar] [CrossRef]
- Suchorski, Y.; Kozlov, S.M.; Bespalov, I.; Datler, M.; Vogel, D.; Budinska, Z.; Neyman, K.M.; Rupprechter, G. The role of metal/oxide interfaces for long-range metal particle activation during CO oxidation. Nat. Mater. 2018, 17, 519–522. [Google Scholar] [CrossRef]
- Li, J.; Song, S.; Long, Y.; Wu, L.; Wang, X.; Xing, Y.; Jin, R.; Liu, X.; Zhang, H. Investigating the hybrid-structure-effect of CeO2-encapsulated Au nanostructures on the transfer coupling of nitrobenzene. Adv. Mater. 2018, 30, 1704416. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, J.; Wang, H.; Li, S.; Wang, W.; Qin, G. Abnormal thermal stability of sub-10 nm Au nanoparticles and their high catalytic activity. J. Mater. Chem. A 2019, 7, 10980–10987. [Google Scholar] [CrossRef]
- Murugavel, R.; Walawalkar, M.G.; Dan, M.; Roesky, H.W.; Rao, C.N.R. Transformations of Molecules and Secondary Building Units to Materials: A Bottom-Up Approach. Acc. Chem. Res. 2004, 37, 763–774. [Google Scholar] [CrossRef]
- Denmark, S.E.; Werner, N.S. Cross-Coupling of Aromatic Bromides with Allylic Silanolate Salts. J. Am. Chem. Soc. 2008, 130, 16382–16393. [Google Scholar] [CrossRef]
- Ishimoto, R.; Kamata, K.; Mizuno, N. Highly Selective Oxidation of Organosilanes to Silanols with Hydrogen Peroxide Catalyzed by a Lacunary Polyoxotungstate. Angew. Chem. Int. Ed. 2009, 48, 8900–8904. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Han, J.; Park, J. Transformation of Silanes into Silanols using Water and Recyclable Metal Nanoparticle Catalysts. ChemCatChem 2012, 4, 521–524. [Google Scholar] [CrossRef]
- Da Silva, A.G.M.; Kisukuri, C.M.; Rodrigues, T.S.; Candido, E.G.; de Freitas, I.C.; da Silva, A.H.M.; Assaf, J.M.; Oliveira, D.C.; Andrade, L.H.; Camargo, P.H.C. MnO2 nanowires decorated with Au ultrasmall nanoparticles for the green oxidation of silanes and hydrogen production under ultralow loadings. Appl. Catal. B Environ. 2016, 184, 35–43. [Google Scholar] [CrossRef]
- Porsin, A.V.; Kulikov, A.V.; Rogozhnikov, V.N.; Serkova, A.N.; Salanov, A.N.; Shefer, K.I. Structured reactors on a metal mesh catalyst for various applications. Catal. Today 2016, 273, 213–220. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Cheng, Y.; Qin, T.; Li, L.; Wang, H.; Zhang, Z. Comparison of corrosion resistance of microarc oxidation coatings prepared with different electrolyte concentrations on AM60 magnesium alloy. Corros. Eng. Sci. Technol. 2011, 46, 17–23. [Google Scholar] [CrossRef]
- Shi, L.; Xu, Y.; Li, K.; Yao, Z.; Wu, S. Effect of additives on structure and corrosion resistance of ceramic coatings on Mg–Li alloy by micro-arc oxidation. Curr. Appl. Phys. 2010, 10, 719–723. [Google Scholar] [CrossRef]
- Khaselev, O.; Weiss, D.; Yahalom, J. Structure and composition of anodic films formed on binary Mg-Al alloys in KOH-aluminate solutions under continuous sparking. Corros. Sci. 2001, 43, 1295–1307. [Google Scholar] [CrossRef]
- Haruta, M. Chance and necessity: My encounter with gold catalysts. Angew. Chem. Int. Ed. 2014, 53, 52–56. [Google Scholar] [CrossRef]
- Ishida, T.; Kinoshita, N.; Okatsu, H.; Akita, T.; Takei, T.; Haruta, M. Influence of the Support and the Size of Gold Clusters on Catalytic Activity for Glucose Oxidation. Angew. Chem. Int. Ed. 2008, 47, 9265–9268. [Google Scholar] [CrossRef]
- Lopez, N. On the origin of the catalytic activity of gold nanoparticles for low-temperature CO oxidation. J. Catal. 2004, 223, 232–235. [Google Scholar] [CrossRef]
- Taketoshi, A.; Haruta, M. Size- and Structure-specificity in Catalysis by Gold Clusters. Chem. Lett. 2014, 43, 380–387. [Google Scholar] [CrossRef]
- Ling, T.; Yan, D.-Y.; Wang, H.; Jiao, Y.; Hu, Z.; Zheng, Y.; Zheng, L.; Mao, J.; Liu, H.; Du, X.-W.; et al. Activating cobalt(II) oxide nanorods for efficient electrocatalysis by strain engineering. Nat. Commun. 2017, 8, 1509. [Google Scholar] [CrossRef] [PubMed]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef]
- Shimizu, K.; Kubo, T.; Satsuma, A. Surface Oxygen-Assisted Pd Nanoparticle Catalysis for Selective Oxidation of Silanes to Silanols. Chem. A Eur. J. 2012, 18, 2226–2229. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Cao, X.; Wang, H.; Li, S. Formation of a Pd/MgO Structured Catalyst for the Aqueous Oxidation of Silane to Silanol. Catalysts 2019, 9, 834. https://doi.org/10.3390/catal9100834
Zhou J, Cao X, Wang H, Li S. Formation of a Pd/MgO Structured Catalyst for the Aqueous Oxidation of Silane to Silanol. Catalysts. 2019; 9(10):834. https://doi.org/10.3390/catal9100834
Chicago/Turabian StyleZhou, Jun, Xiaoqing Cao, Hongna Wang, and Song Li. 2019. "Formation of a Pd/MgO Structured Catalyst for the Aqueous Oxidation of Silane to Silanol" Catalysts 9, no. 10: 834. https://doi.org/10.3390/catal9100834
APA StyleZhou, J., Cao, X., Wang, H., & Li, S. (2019). Formation of a Pd/MgO Structured Catalyst for the Aqueous Oxidation of Silane to Silanol. Catalysts, 9(10), 834. https://doi.org/10.3390/catal9100834






