Comparative Analysis and Biochemical Characterization of Two Endo-β-1,3-Glucanases from the Thermophilic Bacterium Fervidobacterium sp.
Abstract
1. Introduction
2. Results
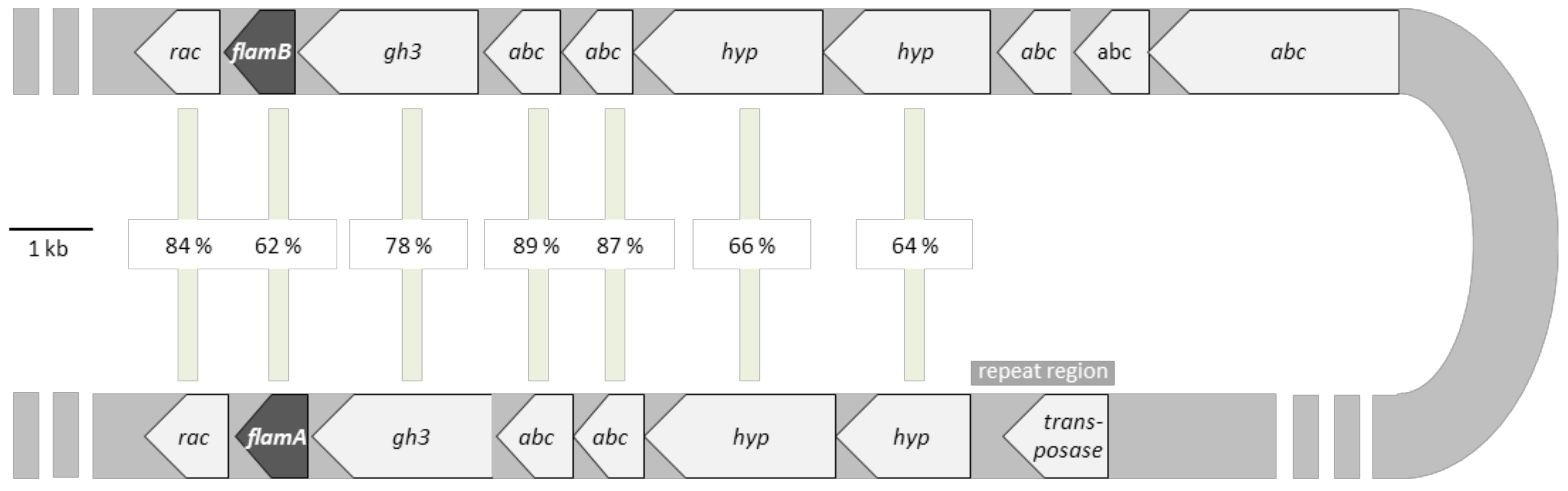
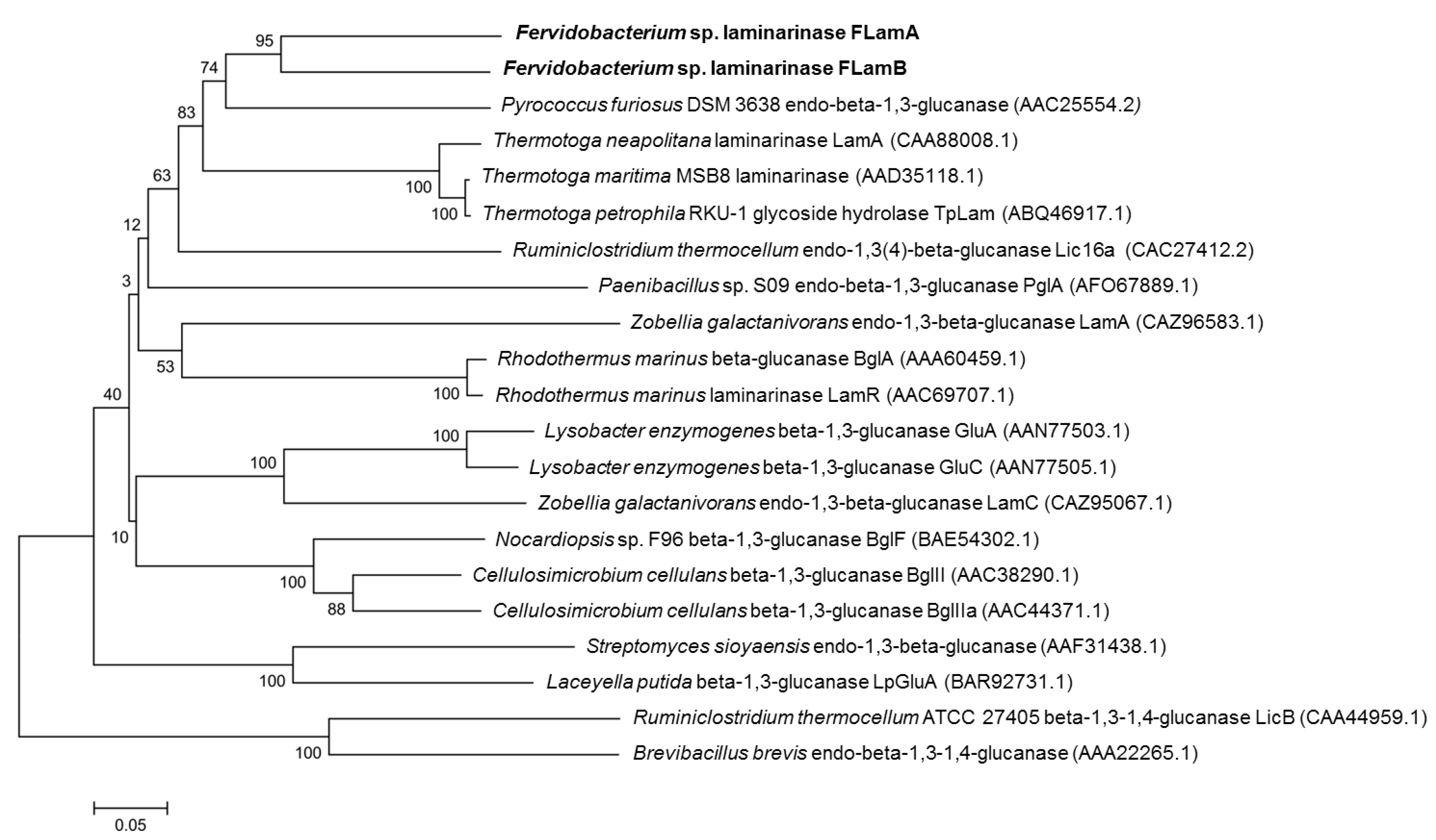
2.1. Sequence Analysis of FLamA and FLamB
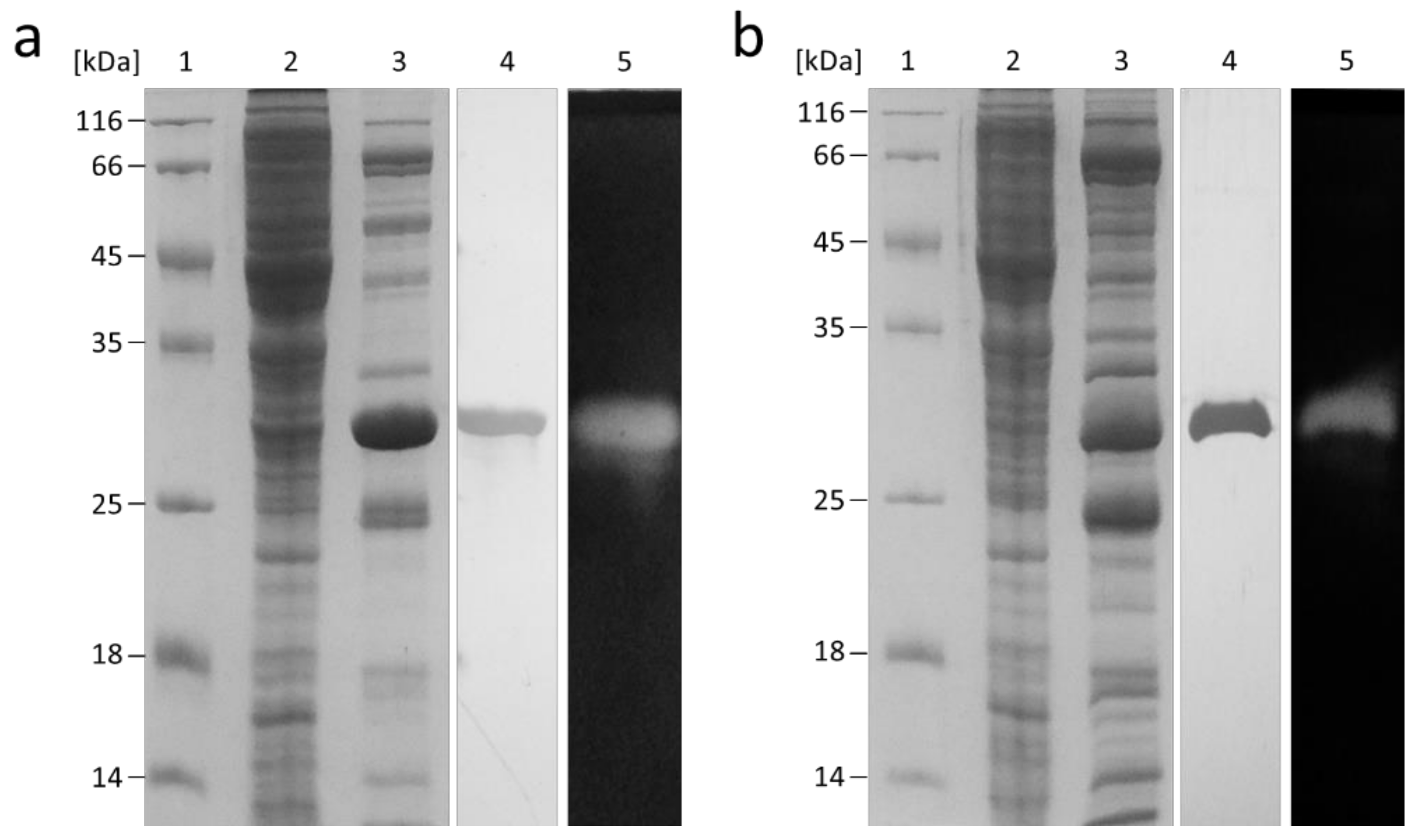
2.2. Recombinant Production of FLamA and FLamB
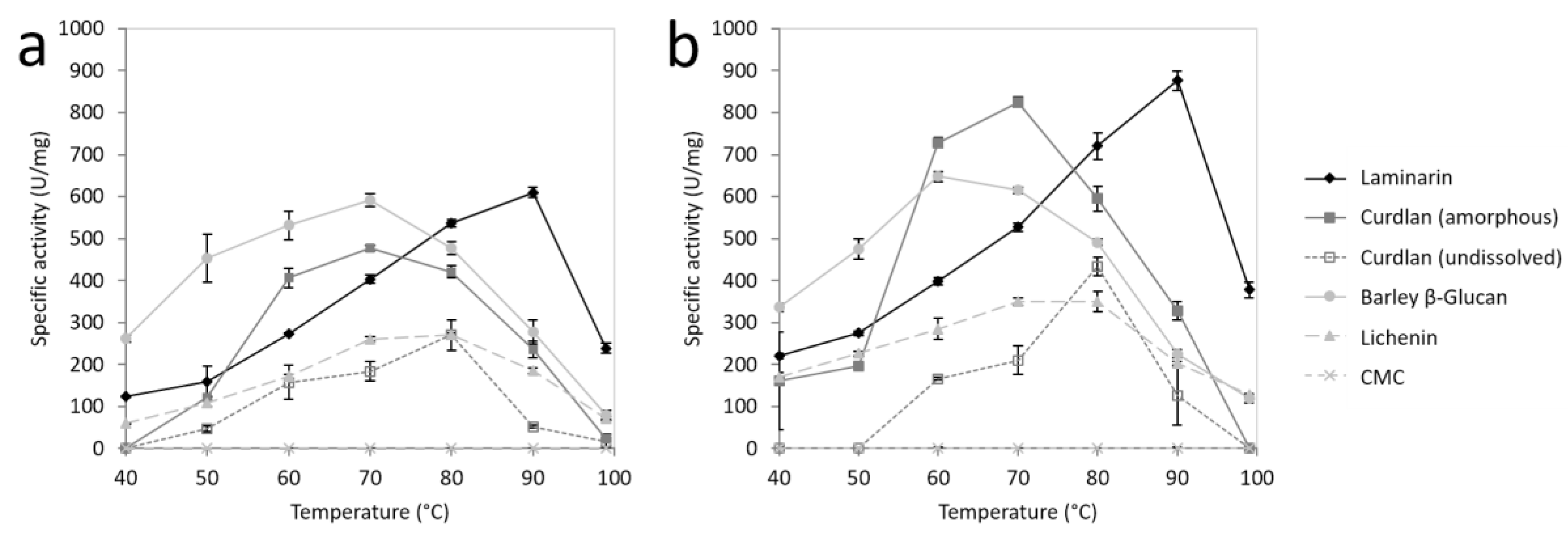
2.3. Substrate Specificity of FLamA and FLamB
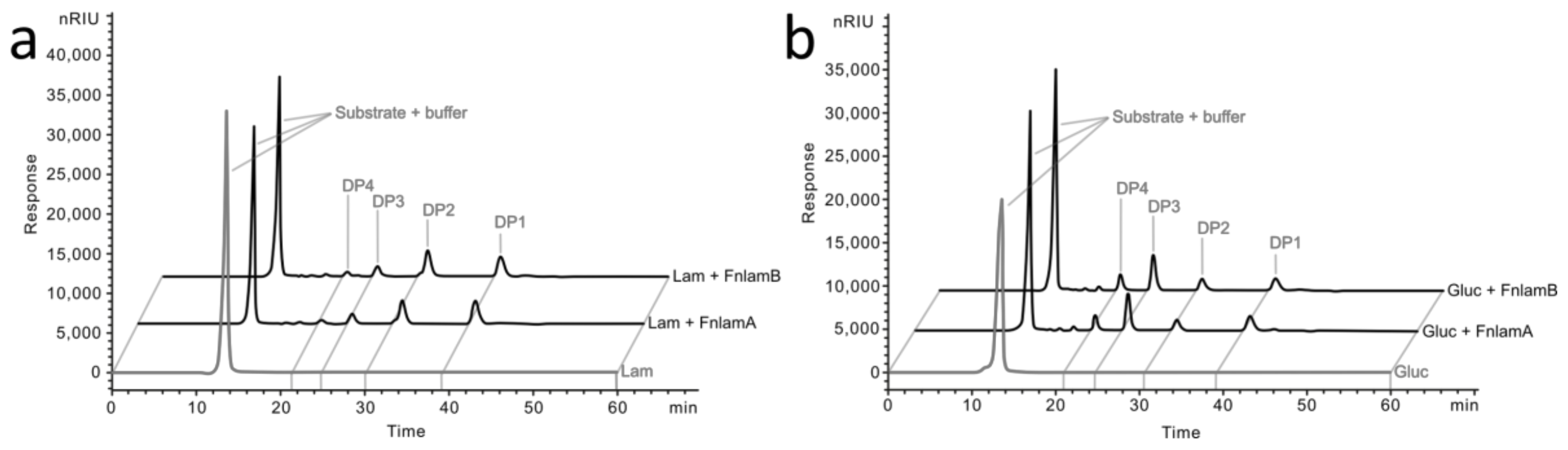
2.4. Degradation Pattern and Enzyme Kinetics
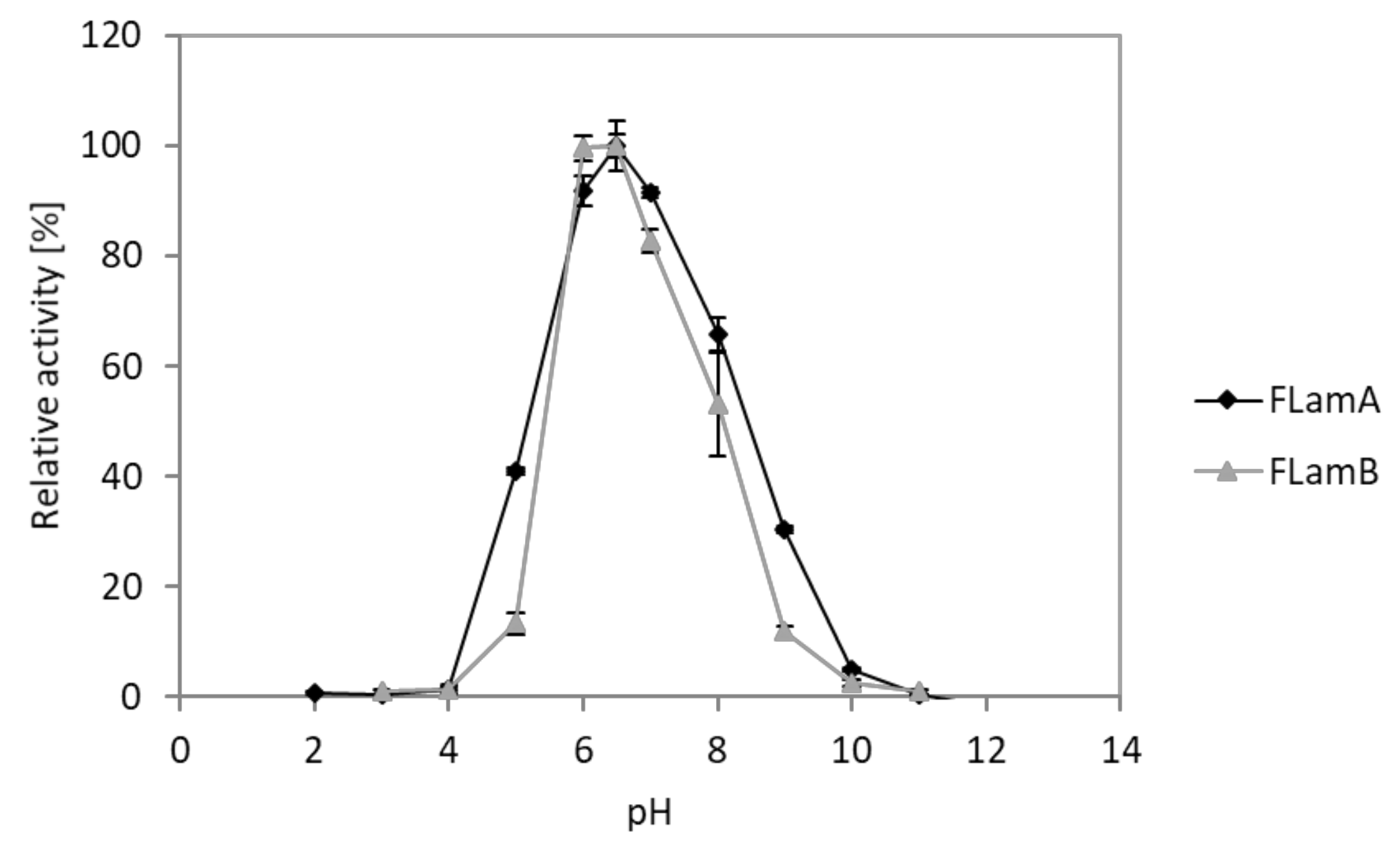
2.5. Effects of pH and Temperature
2.6. Effects of Metal Ions and Chemical Additives
3. Discussion
4. Materials and Methods
4.1. Cloning of the Endo-β-1,3-Glucanase Encoding Genes flamA and flamB
- flamA-for: AGCGGCTCTTCAATGAAAGTTAAATATTTCTCAAATATT
- flamA-rev: AGCGGCTCTTCTCCCCTCATTTTCAAGCTTGTATAC
- flamB-for: AGCGGCTCTTCAATGAGAGAAAAGTTGCTGT
- flamB-rev: AGCGGCTCTTCTCCCCTCTTCATCTAATGTATACAC
4.2. Sequence Comparison and Phylogenetic Analysis
4.3. Heterologous Expression of the flamA and flamB Genes and Purification of the Endo-β-1,3-Glucanases
4.4. β-Glucanase Activity Assay
4.5. Determination of the Hydrolysis Products
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stone, B.A. Chemistry, biochemistry, and biology of (1-3)-β-glucans and related polysaccharides. In Chemistry of β-Glucans, 1st ed.; Bacic, A., Fincher, G.B., Stone, B.A., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2009; pp. 5–46. [Google Scholar]
- Ebringerová, A.; Hromádková, Z.; Heinze, T. Hemicellulose. In Polysaccharides, I. Structure, Characterisation and Use; Heinze, T.T., Ed.; Springer-Verlag GmbH: Berlin/Heidelberg, Germany, 2005; pp. 1–67. [Google Scholar]
- Painter, T.J. Algal polysaccharides. In The Polysaccharides; Aspinall, G.O., Ed.; Academic Press (Molecular biology): New York, NY, USA, 1983; pp. 195–285. Volume 2. [Google Scholar]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine macroalgae: an untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Lim, S.-R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Hehemann, J.-H.; Boraston, A.B.; Czjzek, M. A sweet new wave: structures and mechanisms of enzymes that digest polysaccharides from marine algae. Curr. Opin. Struct. Biol. 2014, 28, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Yao, G.; Yang, P.; Li, N.; Luo, H.; Bai, Y.; Wang, Y.; Yao, B. Cloning, characterization, and antifungal activity of an endo-1,3-β-D-glucanase from Streptomyces sp. S27. Appl. Microbiol. Biotechnol. 2010, 85, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V. Glucan-immunostimulant, adjuvant, potential drug. World J. Clin. Oncol. 2011, 2, 115–119. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and Hot Extremozymes: Industrial Relevance and Current Trends. Front. Bioeng. Biotechnol. 2015, 3, 348. [Google Scholar] [CrossRef]
- Krahe, M.; Markl, H.; Antranikian, G. Fermentation of extremophilic microorganisms. FEMS Microbiol. Rev. 1996, 18, 271–285. [Google Scholar] [CrossRef]
- Schroeder, C.; Elleuche, S.; Blank, S.; Antranikian, G. Characterization of a heat-active archaeal beta-glucosidase from a hydrothermal spring metagenome. Enzyme Microb. Technol. 2014, 57, 48–54. [Google Scholar] [CrossRef]
- Zhaxybayeva, O.; Swithers, K.S.; Lapierre, P.; Fournier, G.P.; Bickhart, D.M.; DeBoy, R.T.; Nelson, K.E.; Nesbø, C.L.; Doolittle, W.F.; Gogarten, J.P.; et al. On the chimeric nature, thermophilic origin, and phylogenetic placement of the Thermotogales. Proc. Natl. Acad. Sci. USA 2009, 106, 5865–5870. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.C.; Morgan, H.W.; Daniel, R.M. Fervidobacterium nodosum gen. nov. and spec. nov., a new chemoorganotrophic, caldoactive, anaerobic bacterium. Arch. Microbiol. 1985, 141, 63–69. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Tang, R.; Yu, S.; Zheng, B.; Feng, Y. A novel thermostable cellulase from Fervidobacterium nodosum. J. Mol. Catal. B: Enzym. 2010, 66, 294–301. [Google Scholar] [CrossRef]
- Labourel, A.; Jam, M.; Legentil, L.; Sylla, B.; Hehemann, J.H.; Ferrières, V.; Czjzek, M.; Michel, G. Structural and biochemical characterization of the laminarinase ZgLamCGH16 from Zobellia galactanivorans suggests preferred recognition of branched laminarin. Acta Crystallogr. D 2015, 71 Pt 2, 173–184. [Google Scholar] [CrossRef]
- Palumbo, J.D.; Sullivan, R.F.; Kobayashi, D.Y. Molecular Characterization and Expression in Escherichia coli of Three β-1,3-Glucanase Genes from Lysobacter enzymogenes Strain N4-7. J. Bacteriol. 2003, 185, 4362–4370. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.-Y.; Huang, J.-W.; Meng, M.; Cheng, C.-W. Isolation and biochemical characterization of an endo-1,3-β-glucanase from Streptomyces sioyaensis containing a C-terminal family 6 carbohydrate-binding module that binds to 1,3-β-glucan. Microbiology 2002, 148 Pt 4, 1151–1159. [Google Scholar] [CrossRef]
- Qian, W.; Zhang, J. Gene dosage and gene duplicability. Genetics 2008, 179, 2319–2324. [Google Scholar] [CrossRef]
- Wagner, A. Energy Constraints on the Evolution of Gene Expression. Mol. Boil. Evol. 2005, 22, 1365–1374. [Google Scholar] [CrossRef]
- Francino, M.P. An adaptive radiation model for the origin of new gene functions. Nat. Genet. 2005, 37, 573–578. [Google Scholar] [CrossRef]
- Bratlie, M.S.; Johansen, J.; Sherman, B.T.; Huang, D.W.; Lempicki, R.A.; Drabløs, F. Gene duplications in prokaryotes can be associated with environmental adaptation. BMC Genom. 2010, 11, 588. [Google Scholar] [CrossRef]
- Cota, J.; Alvarez, T.M.; Citadini, A.P.; Santos, C.R.; Neto, M.D.O.; Oliveira, R.R.; Pastore, G.M.; Ruller, R.; Prade, R.A.; Murakami, M.T.; et al. Mode of operation and low-resolution structure of a multi-domain and hyperthermophilic endo-β-1,3-glucanase from Thermotoga petrophila. Biochem. Biophys. Res. Commun. 2011, 406, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Zverlov, V.V.; Volkov, I.Y.; Velikodvorskaya, T.V.; Schwarz, W.H. Thermotoga neapolitana bglB gene, upstream of lamA, encodes a highly thermostable beta-glucosidase that is a laminaribiase. Microbiology 1997, 143 Pt 11, 3537–3542. [Google Scholar] [CrossRef]
- Gueguen, Y.; Voorhorst, W.G.B.; van der Oost, J.; de Vos, W.M. Molecular and biochemical characterization of an endo-β -1,3-glucanase of the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 1997, 272, 31258–31264. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, W.H.; Schimming, S.; Staudenbauer, W.L. Isolation of a Clostridium thermocellum gene encoding a thermostable β-1,3-glucanase (laminarinase). Biotechnol. Lett. 1988, 10, 225–230. [Google Scholar] [CrossRef]
- Kasai, N.; Harada, T. Ultrastructure of Curdlan. In Fiber Diffraction Methods; French, A.D., Ed.; American Chemical Society (ACS symposium series): Washington, DC, USA, 1980; pp. 363–383. Volume 141. [Google Scholar]
- Meng, D.D.; Wang, B.; Ma, X.Q.; Ji, S.Q.; Lu, M.; Li, F.L. Characterization of a thermostable endo-1,3(4)-beta-glucanase from Caldicellulosiruptor sp. strain F32 and its application for yeast lysis. Appl. Microbiol. Biotechnol. 2016, 100, 4923–4934. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Uchimura, K.; Kubota, T.; Nunoura, T.; Deguchi, S. Biochemical and genetic characterization of beta-1,3 glucanase from a deep subseafloor Laceyella putida. Appl. Microbiol. Biotechnol. 2016, 100, 203–214. [Google Scholar] [CrossRef]
- Masuda, S.; Endo, K.; Koizumi, N.; Hayami, T.; Fukazawa, T.; Yatsunami, R.; Fukui, T.; Nakamura, S. Molecular identification of a novel beta-1,3-glucanase from alkaliphilic Nocardiopsis sp. strain F96. Extremophiles 2006, 10, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Spilliaert, R.; Hreggvidsson, G.O.; Kristjansson, J.K.; Eggertsson, G.; Palsdottir, A. Cloning and Sequencing of a Rhodothermus marinus Gene, bglA, Coding for a Thermostable beta-Glucanase and its Expression in Escherichia coli. JBIC J. Boil. Inorg. Chem. 1994, 224, 923–930. [Google Scholar] [CrossRef]
- Krah, M.; Misselwitz, R.; Politz, O.; Thomsen, K.K.; Welfle, H.; Borriss, R. The laminarinase from thermophilic eubacterium Rhodothermus marinus. Conformation, stability, and identification of active site carboxylic residues by site-directed mutagenesis. Eur. J. Biochem. 1998, 257, 101–111. [Google Scholar] [CrossRef]
- Fuchs, K.-P.; Zverlov, V.V.; Velikodvorskaya, G.A.; Lottspeich, F.; Schwarz, W.H. Lic16A of Clostridium thermocellum, a non-cellulosomal, highly complex endo-beta-1,3-glucanase bound to the outer cell surface. Microbiology 2003, 149 Pt 4, 1021–1031. [Google Scholar] [CrossRef]
- Woo, C.-B.; Kang, H.-N.; Lee, S.-B. Molecular cloning and anti-fungal effect of endo-β-1,3-glucanase from Thermotoga maritima. Food Sci. Biotechnol. 2014, 23, 1243–1246. [Google Scholar] [CrossRef]
- Ilari, A.; Fiorillo, A.; Angelaccio, S.; Florio, R.; Chiaraluce, R.; Van Der Oost, J.; Consalvi, V. Crystal structure of a family 16 endoglucanase from the hyperthermophile Pyrococcus furiosus- structural basis of substrate recognition. FEBS J. 2009, 276, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, O.J.; Piotukh, K.; Ponnuswamy, M.N.; Planas, A.; Borriss, R.; Heinemann, U. Structural basis for the substrate specificity of a Bacillus 1,3-1,4-beta-glucanase. J. Mol. Biol. 2006, 357, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.D.C.; Giese, E.C.; Moretti, M.M.D.S.; Gomes, A.C.D.S.; Perrone, M.B.O.M.; Da Silva, R.; Martins, D.A.B. Effect of Metal Ions, Chemical Agents and Organic Compounds on Lignocellulolytic Enzymes Activities. In Enzyme Inhibitors and Activators; IntechOpen: London, UK, 2017; pp. 139–164. [Google Scholar]
- Labourel, A.; Jam, M.; Jeudy, A.; Hehemann, J.-H.; Czjzek, M.; Michel, G. The β-glucanase ZgLamA from Zobellia galactanivorans evolved a bent active site adapted for efficient degradation of algal laminarin. J. Biol. Chem. 2014, 289, 2027–2042. [Google Scholar] [CrossRef] [PubMed]
- Jeng, W.-Y.; Wang, N.-C.; Lin, C.-T.; Shyur, L.-F.; Wang, A.H.-J. Crystal structures of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with inhibitors: essential residues for β-1,3- and β-1,4-glucan selection. J. Biol. Chem. 2011, 286, 45030–45040. [Google Scholar] [CrossRef] [PubMed]
- Sahm, K.; John, P.; Nacke, H.; Wemheuer, B.; Grote, R.; Daniel, R.; Antranikian, G. High abundance of heterotrophic prokaryotes in hydrothermal springs of the Azores as revealed by a network of 16S rRNA gene-based methods. Extremophiles 2013, 17, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Robinson, R.A. CXCVIII.—Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]

| Substrate | FLamA | FLamB | ||||
|---|---|---|---|---|---|---|
| T (°C) | Specific Activity (U/mg) | Relative Activity (%) | T (°C) | Specific Activity (U/mg) | Relative Activity (%) | |
| Laminarin | 90 | 609 ± 12 | 100 | 90 | 876 ± 23 | 100 |
| Curdlan * | 70 | 478 ± 07 | 78 | 70 | 825 ± 13 | 94 |
| Curdlan | 80 | 270 ± 37 | 44 | 80 | 434 ± 22 | 50 |
| Barley β-glucan | 70 | 592 ± 16 | 97 | 60 | 648 ± 12 | 74 |
| Lichenin | 80 | 271 ± 5 | 45 | 70 | 350 ± 8 | 40 |
| CMC + | - | 0 | 0 | - | 0 | 0 |
| a | Relative Residual Activity (%) * | b | Relative Residual Activity (%) * | ||
|---|---|---|---|---|---|
| Metal ion | FLamA | FLamB | Reagent | FLamA | FLamB |
| AgNO3 | 0.65 ± 0.00 | 3.29 ± 0.58 | CHAPS | 78.95 ± 3.64 | 80.11 ± 2.36 |
| AlCl3 | 4.98 ± 2.31 | 4.73 ± 0.27 | SDS | 29.10 ± 1.08 | 36.70 ± 6.61 |
| CaCl2 | 89.85 ± 8.46 | 87.79 ± 1.60 | Triton X-100 | 89.42 ± 4.03 | 89.77 ± 0.61 |
| CoCl2 | 9.31 ± 0.33 | 16.64 ± 1.30 | Tween 20 | 91.83 ± 1.67 | 91.18 ± 2.20 |
| CrCl3 | 2.50 ± 0.04 | 1.32 ± 4.80 | Tween 80 | 95.41 ± 3.85 | 92.52 ± 1.60 |
| CuCl2 | 0.66 ± 0.40 | 0.84 ± 4.18 | Guanidine-HCl | 91.14 ± 7.34 | 84.70 ± 0.68 |
| FeCl2 | 1.05 ± 0.17 | 3.29 ± 1.14 | Urea | 100.14 ± 1.41 | 93.30 ± 1.06 |
| KCl | 93.78 ± 1.00 | 91.08 ± 1.39 | DTT | 119.04 ± 1.16 | 124.61 ± 1.88 |
| MgCl2 | 92.48 ± 2.42 | 95.15 ± 1.57 | β-Mercaptoethanol | 125.03 ± 2.88 | 124.12 ± 3.42 |
| NaCl | 92.41 ± 1.53 | 86.42 ± 1.17 | EDTA | 109.32 ± 3.01 | 95.28 ± 2.20 |
| NiCl2 | 4.67 ± 0.16 | 7.06 ± 0.10 | Na-Iodoacetate | 105.34 ± 1.19 | 92.45 ± 2.57 |
| RbCl | 90.79 ± 1.76 | 97.47 ± 1.01 | Pefabloc | 37.79 ± 5.67 | 76.94 ± 12.14 |
| SrCl2 | 93.15 ± 1.84 | 96.20 ± 2.63 | CTAB | 24.85 ± 1.69 | 30.68 ± 2.60 |
| ZnCl2 | 1.85 ± 0.20 | 3.67 ± 0.29 | Na-Azide | 102.37 ± 2.14 | 103.81 ± 6.97 |
| Organism and Enzyme | Topt (°C) | Thermal Stability | pHopt | Activity (U/mg) + | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | t (h) | A * (%) | Lam | Curd | Glu | Lich | ||||
| Caldicellulosiruptor sp. F32, Lam16A-GH | 75 | 65 | 42 | 72 | 6.5 | 172 | ND | 2961 | ND | [29] |
| Fervidobacterium sp., FLamA | 90 | 80 | 5 | 50 | 6.5 | 609 | 270 | 592 | 271 | Present study |
| Fervidobacterium sp., FLamB | 90 | 80 | 1 | 50 | 6.5 | 876 | 434 | 648 | 350 | Present study |
| Laceyella putida, LpGluA | 80 | 75 | 0.5 | 45 | 4.2 | 48% | 100% | ND | ND | [30] |
| Nocardiopsis sp. F96, BglF | 70 | ND | ND | ND | 9.0 | 100% | 159% | ND | 815% | [31] |
| Pyrococcus furiosus, LamA | 100/105 | 80 | 80 | 100 | 6–6.5 | 922 | ND | 99 | 95 | [26] |
| Rhodothermus marinus 21, BglA | 85 | 80 | 16 | 100 | 7.0 | 542 | ND | 1568 | 1445 | [32] |
| Rhodothermus marinus ITI278, LamR | 88 | 90 | 0.45 | 50 | 5.5 | 656 | ND | 2199 | 3111 | [33] |
| Ruminiclostridium thermocellum, CelC | 65 | 70 | 10 | 30 | 6.5 | 86 | ND | 504 | 245 | [27] |
| Ruminiclostridium thermocellum, Lic16A | 70 | 70 | 0.17 | 50 | 6.0 | 340 | 29 | 268 | 2404 | [34] |
| Thermotoga maritima, TmβG | 80 | ND | ND | ND | 5.0 | Efficient on β-1,3-glucans | [35] | |||
| Thermotoga neapolitana, LamA | 95 | 95 | 0.5 | 82 | 6.3 | 3100 | ND | ND | 90 | [25] |
| Thermotoga petrophila, TpLam | 91 | 80 | 16 | 60 | 6.2 | 48 | ND | 41 | 21 | [24] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkhardt, C.; Schäfers, C.; Claren, J.; Schirrmacher, G.; Antranikian, G. Comparative Analysis and Biochemical Characterization of Two Endo-β-1,3-Glucanases from the Thermophilic Bacterium Fervidobacterium sp. Catalysts 2019, 9, 830. https://doi.org/10.3390/catal9100830
Burkhardt C, Schäfers C, Claren J, Schirrmacher G, Antranikian G. Comparative Analysis and Biochemical Characterization of Two Endo-β-1,3-Glucanases from the Thermophilic Bacterium Fervidobacterium sp. Catalysts. 2019; 9(10):830. https://doi.org/10.3390/catal9100830
Chicago/Turabian StyleBurkhardt, Christin, Christian Schäfers, Jörg Claren, Georg Schirrmacher, and Garabed Antranikian. 2019. "Comparative Analysis and Biochemical Characterization of Two Endo-β-1,3-Glucanases from the Thermophilic Bacterium Fervidobacterium sp." Catalysts 9, no. 10: 830. https://doi.org/10.3390/catal9100830
APA StyleBurkhardt, C., Schäfers, C., Claren, J., Schirrmacher, G., & Antranikian, G. (2019). Comparative Analysis and Biochemical Characterization of Two Endo-β-1,3-Glucanases from the Thermophilic Bacterium Fervidobacterium sp. Catalysts, 9(10), 830. https://doi.org/10.3390/catal9100830




